Abstract
Crystalline porous materials (CPM)-200-In and CPM-200-In/Mg metal-organic frameworks (MOFs) were synthesized by a solvothermal method and were characterized by using powder X-ray diffraction (PXRD), FT-IR, Brunauer–Emmett–Teller (BET), temperature programmed desorption (TPD), TGA, XPS, and SEM-EDS. They were used as heterogeneous catalysts for the cycloaddition of CO2 with epoxides and found to be highly efficient toward the cycloaddition reaction at moderate reaction conditions under solvent-free conditions. The catalyst was easily separated by a simple filtration and can be reused up to five consecutive times without any considerable decrease of its initial activity. CPM-200-In/Mg showed excellent catalytic performance in the cycloaddition reaction due to the synergistic role of the acidic sites and basic sites. A plausible reaction mechanism for the CPM-200-In/Mg MOF catalyzed cycloaddition reaction is proposed based on the experimental results and our previously reported DFT (Density Functional Theory) studies.
1. Introduction
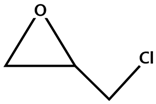
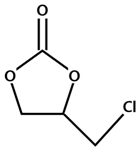
The conversion of carbon dioxide to value-added chemical products has attracted scientists to develop efficient technologies for the reduction of CO2 emission, which is a main cause of global warming [1,2,3,4,5,6,7,8,9]. The production of cyclic carbonates has been considered as a promising way to convert CO2. Cyclic carbonates have a wide range of applications such as solvents, electrolytes, and intermediates for the synthesis of other monomers and pharmaceuticals [10,11,12,13,14,15,16,17]. Since CO2 is a thermally and chemically stable molecule, various homogeneous (e.g., quaternary ammonium and phosphonium salts, Schiff bases, ionic liquids, alkali metal salts, and other organocatalysts) [18,19,20,21,22,23] and heterogeneous (e.g., metal oxides, functional polymers, and immobilized ionic liquids) [24,25,26,27,28,29,30] catalysts have been developed in the synthesis of cyclic carbonates. The heterogeneous catalysts have higher catalytic performance, easier separation, and higher reusability than homogeneous ones [27,28]. It is well reported that heterogeneous catalysts based on metal-organic frameworks (MOFs) can efficiently proceed the topic reaction (Scheme 1) [31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50]. Porous MOFs composed of metal ions and ligands have been widely used as catalysts for many chemical reactions due to their crystalline nature, tunable structure, highly specific surface area, good thermal stability, and easier mass transfer of reactants [45,51,52,53].
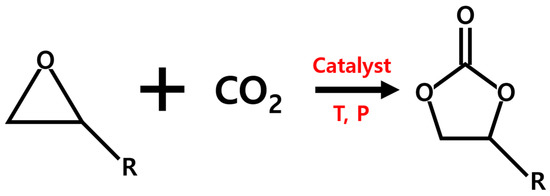
Scheme 1.
Synthesis of cyclic carbonate from epoxide and CO2.
Recent studies on MOF-based materials for CO2 mitigation are focused on the development of MOFs having high adsorption capacity and high catalytic performance at the same time [8]. Metal organic frameworks (MOFs), based on transition metals, are predominant over those based on main-group elements. However, it is reported that the MOF-74-Mg exhibited the highest CO2 uptake capacity over its transition metal analogues such as MOF-74-Co and MOF-74-Ni [54]. The application of MOF-74-Mg as a catalyst for the cycloaddition of CO2 and epoxides has also been reported previously [55,56]. Recently, bimetallic MOFs (Ni-Co and Co-Zn MOF) have been developed as catalysts in the cycloaddition reaction [57,58,59]. They have shown synergistic effects in promoting the catalytic activity by increasing the number of acidic and basic sites. We also reported the synergistic role of Cu-Zr bimetallic MOF, which enhanced the number of basic sites for the cycloaddition reaction [59]. Zhai et al. [60] reported successful preparation of heterometallic MOFs, denoted as crystalline porous materials (CPM-200s) with systematic combinations of trivalent and divalent metals, and their high CO2 adsorption capacity.
In this work, therefore, we selected indium (In) as a second metal to Mg containing MOF and tried to suggest the promotional effect of the bimetallic In-Mg MOF in the cycloaddition reaction. We synthesized CPM-200-In/Mg MOF, and it was used as a catalyst for the first time in the cycloaddition reaction under solvent-free conditions. The bimetallic CPM-200-In/Mg showed better catalytic performance than CPM-200-In since the In-Mg bimetallic MOF exhibited higher CO2 adsorption capacity, higher number of acidic and basic sites, and higher thermal stability. The CPM-200-In/Mg MOF catalyst can be reused up to five consecutive times without a considerable loss of its activity. A reaction mechanism is also proposed based on the experimental results and our previously reported DFT studies on the reaction steps, including reaction intermediates and transition states.
2. Results
2.1. Characterization of Catalysts
The powder X-ray diffraction (PXRD) patterns of the prepared CPM-200 catalysts were compared with the simulated single crystal pattern (Figure 1). The main characteristic peak corresponding to (200) face of CPM-200 catalysts in PXRD matched well with the simulated pattern of the Cambridge Crystallographic Data Center (CCDC) data, confirming the crystalline structure of the prepared catalysts.
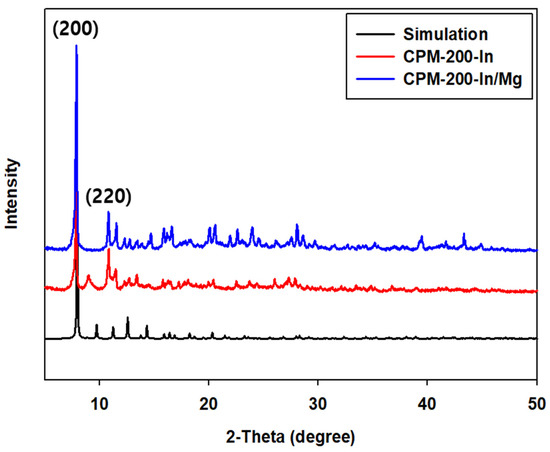
Figure 1.
XRD patterns of crystalline porous materials (CPM)-200-In and CPM-200-In/Mg with simulated CPM-200 (Cambridge Crystallographic Data Center (CCDC) number: 1444375).
Figure 2 show SEM-EDS images of the synthesized CPM-200-In and CPM-200-In/Mg samples. The SEM images reveal that both CPM-200s have cubic like structures. As a result of EDS mapping, only In metal was observed in CPM-200-In, while In and Mg metals are well distributed in CPM-200-In/Mg.
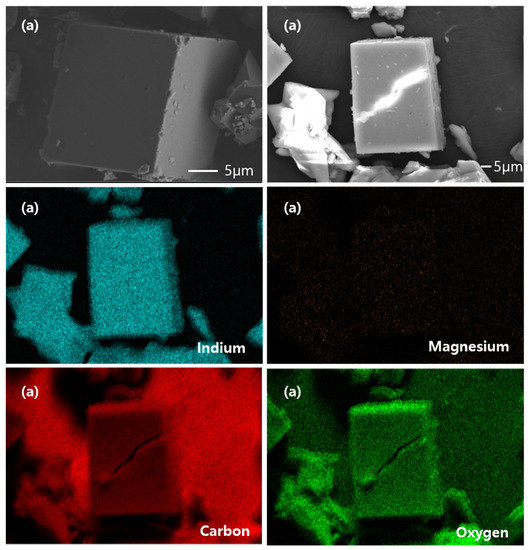
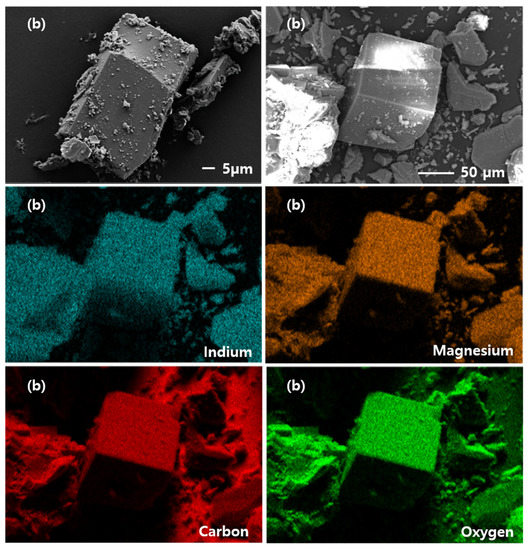
Figure 2.
SEM-EDS image of synthesized catalysts: (a) CPM-200-In and (b) CPM-200-In/Mg.
The FT-IR spectra of the catalysts are shown in Figure 3. The broad peaks in the range of 3400–3450 cm−1 are assigned to the O-H stretching frequency of coordinated water. The big peaks at 1635 and 1430 cm−1 correspond to the symmetric and asymmetric stretching vibration modes of the COOH group. The symmetric stretching vibration of the In-O and Mg-O bond at 419 cm−1 and 548 cm−1 verifies the coordination of metals with the ligands [61,62].
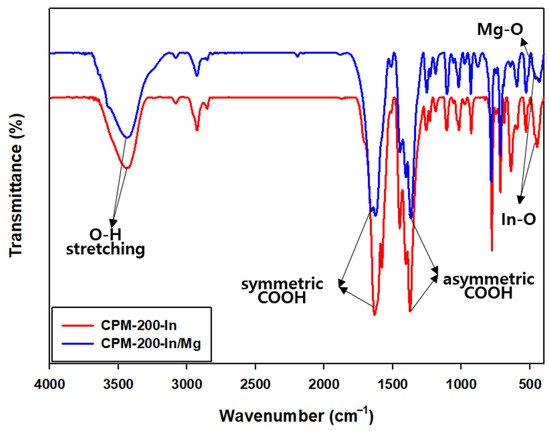
Figure 3.
FT-IR spectrum of CPM-200-In and CPM-200-In/Mg.
TGA results of CPM-200-In and CPM-200-In/Mg (Figure 4) showed an initial weight loss of solvent inside their pores around 130 °C and 200 °C. Even after the initial weight loss, CPM-200-In/Mg exhibited better thermal stability than CPM-200-In, probably due to the better crystallinity from the synergy effect of two metals during CPM-200-In/M crystallization process [60] and/or to the smaller micropore diameter for CPM-200-In/M compared to CPM-200-In.
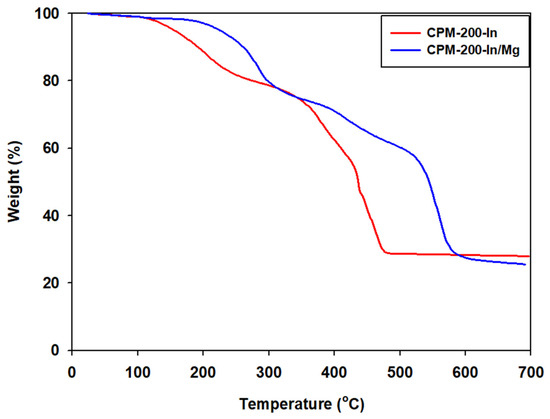
Figure 4.
TGA curve of CPM-200-In and CPM-200-In/Mg.
To verify the elemental composition of both catalysts, elemental analysis (EA) and inductively coupled plasma–optical emission spectrometry (ICP-OES) were conducted, and the results in wt% are given in Table 1.

Table 1.
Elemental analysis (EA) and inductively coupled plasma (ICP) results of CPM-200-In and CPM-200-In/Mg.
X-ray photoelectron spectroscopy (XPS) was also performed (Figure 5) to verify the presence of bimetal in CPM-200-In/Mg. In 3d5 and Mg 1s peaks located at binding energies of 445.26 eV and 1304.30 eV show the presence of indium and magnesium metals in the MOF [63,64,65]. CPM-200-In also exhibits In 3d5 peak at the binding energy of 445.12 eV, demonstrating the presence of the indium atom.
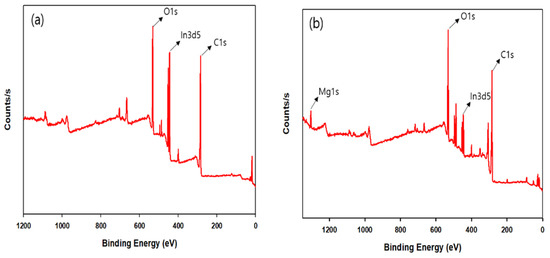
Figure 5.
XPS spectrum of (a) CPM-200-In and (b) CPM-200-In/Mg.
The porosity and surface area of both CPMs were studied by the physical adsorption of N2 at 77 K, and the type I adsorption isotherms revealed the existence of micropores (Figure 6). Brunauer–Emmett–Teller (BET) surface areas of 1231 m2g−1 and 756 m2g−1, and pore volume of 0.50 cm3g−1 and 0.44 cm3g−1, were calculated from N2 adsorption isotherms for CPM-200-In/Mg and CPM-200-In, respectively. The pore size distribution curves confirm the presence of micropores in both catalysts, and the smaller micropore diameter of CPM-200-In/Mg than CPM-200-In. The presence of mesopores is known to be beneficial for promoting mass transfer [51,52,53]; however, no meaningful evidence of mesopore was observed in both CPM-200 catalysts.
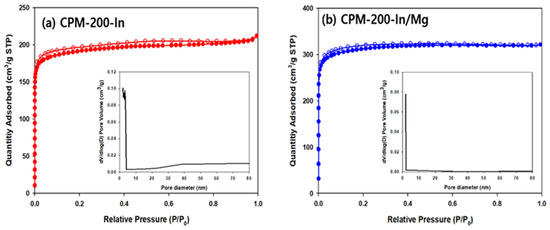
Figure 6.
N2 adsorption-desorption analysis of (a) CPM-200-In and (b) CPM-200-In/Mg.
Temperature-programmed desorption (TPD) of NH3 and CO2 analysis was performed to investigate acid-base nature of the catalysts. As shown in Table 2, CPM-200-In/Mg shows a higher number of basic sites, which is necessary for CO2 adsorption, than CPM-200-In. NH3 adsorption properties of CPM-200 catalyst was studied to explain the presence of acidic sites; these sites are necessary catalytic centers for the activation of epoxide molecules and also for the coordination with CO2 molecules [8]. The number of acidic sites was almost the same in both catalysts.

Table 2.
Number of acidic and basic sites in the catalysts.
The CO2 adsorption experiment at 298 K (Figure 7) showed that CPM-200-In/Mg reached a CO2 uptake capacity of 89.5 cm3g−1, much higher than CPM-200-In, 49.3 cm3g−1 at 298 K, respectively. The increase in the basic sites in CPM-200-In/Mg is responsible to the increase of CO2 uptake capacity, favoring the attraction between CO2 and the catalyst.
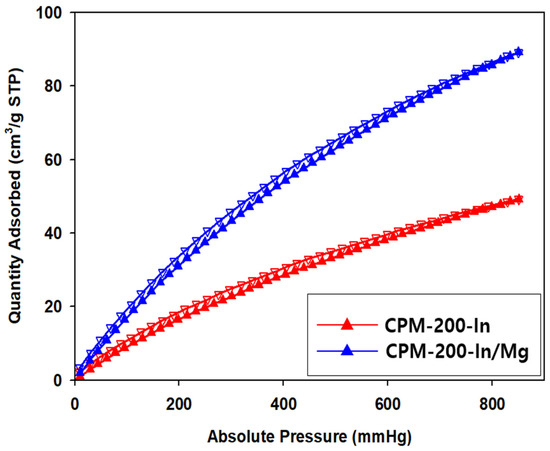
Figure 7.
CO2 adsorption-desorption analysis of CPM-200-In and CPM-200-In/Mg.
2.2. Cycloaddition of CO2 with Epichlorohydrin
The catalytic ability of CPM-200-In/Mg was tested in the cycloaddition of CO2 and epichlorohydrin (ECH). Without catalyst, the reaction did not give any ECH conversion (Table 3, entry 1). CPM-200-In/Mg and CPM-200-In alone without a co-catalyst showed very low ECH conversion at the standard condition (6 h of reaction, 38.3 mmol of ECH, 1.2 MPa of CO2 pressure, 80 °C). They showed an appreciable conversion at high temperature and long reaction time (110 °C and 12 h). The homogeneous catalyst precursors exhibited much lower ECH conversion (Table 3, entries 2-5). In order to promote the cycloaddition reaction, tetrabutylammonium halides (TBAX, X = Cl−, Br−, I−) were used as co-catalysts (Table 3, entries 10, 13, 14). CPM-200-In/Mg catalyst with tetrabutylammonium bromide (TBAB) co-catalyst exhibited very high ECH conversion (90.3%) with >99% (chloromethyl)ethylene carbonate selectivity (Table 3, entry 10). Br− ions showed higher ECH conversion than iodide and chloride ions. Due to the bulky nature of iodide anion, CPM-200-In/Mg with tetrabutylammonium iodide (TBAI) exhibited lower ECH conversion (85.8%) than with TBAB because of mass transfer limitation. The catalytic performance of the CPM-200-In/Mg/TBAB system increased with the increase in temperature from 60 °C to 80 °C (Table 3, entries 10 and 12).

Table 3.
Catalytic activity comparison of catalysts for the cycloaddition reaction of ECH and CO2.
Various reaction parameters such as reaction time, temperature, catalyst amount, and CO2 pressure are optimized for the catalytic activity of CPM-200-In/Mg/TBAB system. As illustrated in Figure 8, with increasing temperature from 40 to 80 °C, ECH conversion increased steadily; however, a small increase in ECH conversion was shown with increasing reaction time from 8 to 10 h. A maximum in ECH conversion occurred at 1.5 MPa CO2 pressure, since excessive pressure decreased the concentration of ECH, disturbing the interaction between CPM-200-In/Mg/TBAB and the ECH [25,43]. The optimized reaction parameters were determined at 80 °C, CO2 pressure of 1.2 MPa, after 6 h, and with 0.6 mol% of CPM-200-In/Mg with TBAB co-catalyst.
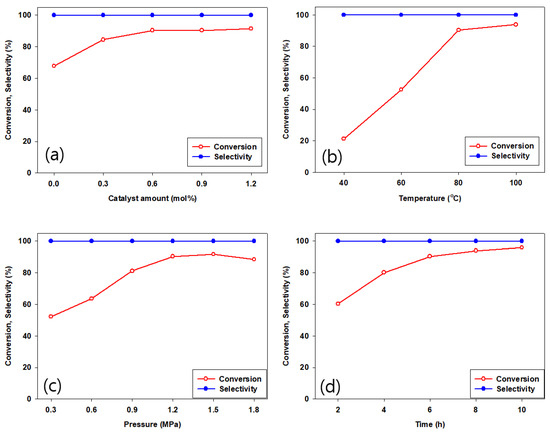
Figure 8.
Effect of various reaction parameter on the cycloaddition of CO2 and ECH with CPM-200-In/Mg catalyst (a) catalyst amount (80 °C, 6 h, 1.2 MPa CO2), (b) temperature (0.6 mol%, 6 h, 1.2 MPa CO2), (c) CO2 pressure (0.6 mol%, 80 °C, 6 h), and (d) time (0.6 mol%, 80 °C, 1.2 MPa CO2).
2.3. Cycloaddition of CO2 with Different Epoxides
We tested various aliphatic and aromatic epoxides to investigate the versatility of the CPM-200-In/Mg catalyst. As shown in Table 4, the CPM-200-In/Mg/TBAB system was efficient with regard to various epoxides in the corresponding cyclic carbonates, except for cyclohexene oxide. High conversion of propylene oxide (87.3%), epichlorohydrin (90.3%), and allylglycidyl ether (82.1%) might be ascribed from the effective size of the epoxides, facilitating their access to the active sites. Very low conversion (14.3%) was obtained for cyclohexene oxide since the sterically hindered cyclohexene ring may prevent its approach to the Lewis acidic site [32,37,66]. In addition, it is well known that CO2-based polycarbonate is easily formed from the reaction of cyclohexene oxide and CO2 [67].

Table 4.
Synthesis of cyclic carbonates from various epoxides.
2.4. Reusability of Catalyst
After easy separation using a centrifuge, the recyclability studies of CPM-200-In/Mg/TBAB catalyst were carried out under optimal reaction conditions (80 °C, 6 h, 0.6 mol% catalyst, and 1.2 MPa of CO2 pressure). As shown in Figure 9, CPM-200-In/Mg/TBAB system can be recycled five times, without considerable loss in its initial activity. The reused CPM-200-In/Mg catalyst was analyzed by FT-IR, PXRD, and TGA to investigate its chemical and thermal stability. PXRD patterns and FT-IR spectra of the fresh and reused CPM-200-In/Mg are identical (Figures S1 and S2). The TGA of the recycled CPM-200-In/Mg catalyst showed no destruction of the original structure of the fresh one (Figure S3).
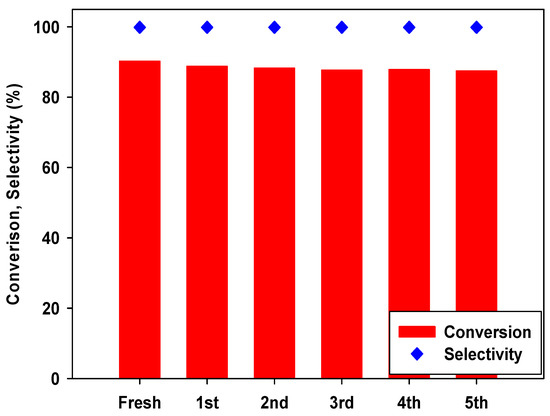
Figure 9.
Reusability test of CPM-200-In/Mg catalyst. (epichlorohydrin (ECH) 38.3 mmol, catalyst 0.6 mol%, 80 °C, 6 h, 1.2 MPa).
2.5. Comparison with Other MOFs
Table 5 shows the catalytic performance of CPM-200-In/Mg together with some other reported MOF catalysts. Compared to Mg or In containing single metal MOFs (Table 5, entries 1, 8-11), CPM-200-In/Mg exhibited similar or higher cyclic carbonate TOF at lower CO2 pressure and shorter reaction time. Other metal containing MOFs (Table 5, entries 2-5) in the cycloaddition of ECH and CO2 are also compared with CPM-200-In/Mg (Table 5, entry 6). It also confirms superior performance of the present work.

Table 5.
Comparison of the catalytic potential of the CPM-200-In/Mg with previously reported MOFs.
2.6. Cycloaddition Reaction Mechanism
A plausible reaction mechanism based on the experimental results and our previous DFT studies [31,33,37] is proposed, for the cycloaddition of epoxide and CO2 catalyzed by CPM-200-In/Mg and co-catalyst (Scheme 2). In the first step, Lewis acidic metal centers interact with the O atom of the epoxide ring. Then, the Br− ion of the co-catalyst attacks the least hindered C atoms of epoxide, resulting in the ring opening of epoxide. Thereafter, the generated partial positive charge on the carbon atom of epoxide polarizes the CO2 molecule. Finally, the cyclic carbonate is generated by the ring closure with the elimination of the bromide ion. The synergistic role of acidic sites (Lewis acidic In and Mg metal sites) and basic sites (Lewis basic motif inside MOF pores) seems essential for the improved catalytic performance of CPM-200-In/Mg.
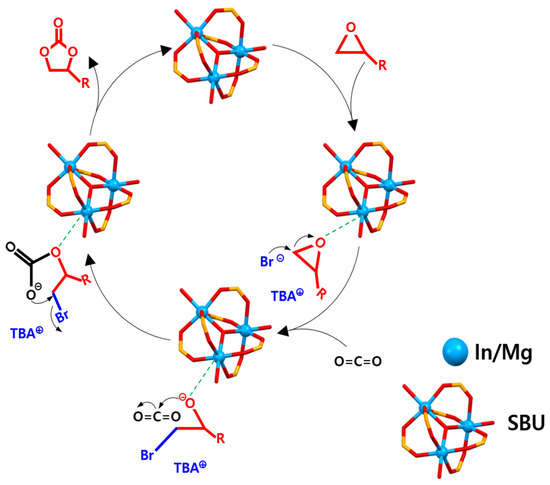
Scheme 2.
Suggested mechanism for the cycloaddition of ECH and CO2 catalyzed by CPM-200-In/Mg.
3. Materials and Methods
3.1. Chemicals
In(NO3)3·xH2O (99.9%), InCl3 (98%), Mg(OAc)2·4H2O (≥99%, ReagentPlus), N,N-dimethylacetamide (DMA. 99%, ReagentPlus), acetonitrile (can, 98%, anhydrous), HNO3 (65%, GR grade), HCl (37%, AR grade), ECH (≥99%, purum), and dichloromethane(≥99.9%, ACS reagent) were purchased from Sigma-Aldrich Chemical Co. (St. Louis, MO, USA) and used as received without further purification. 3,3′,5,5′-azobenzenetetracarboxylic acid (H4ABTC) was prepared according to the methods previously reported [73].
3.2. Preparation of Catalyst
3.2.1. Preparation of CPM-200-In
CPM-200-In was prepared according to the reported method [60]. In a 20 mL glass vial, 22.0 mg of In(NO3)3·xH2O and 16.0 mg of H4ABTC were dissolved in a mixture of 2.0 g of DMA and 1.0 g of ACN. After addition of 120.0 mg HNO3, the vial was sealed and placed in a 90 °C oven for 3 days. After cooling to room temperature, pure yellow cubic crystals were obtained.
3.2.2. Preparation of CPM-200-In/Mg
For the preparation of CPM-200-In/Mg in a 20 mL glass vial, 22.1 mg of InCl3, 86.0 mg of Mg(OAc)2, 35.6 mg of H4ABTC were dissolved in a mixture of 4.0 g of DMA and 0.8 g of H2O. After addition of 120.0 mg HCl, the vial was sealed and placed in a 90 °C oven for 2 days. Pure yellow cubic crystals were obtained.
3.3. Cycloaddition of CO2 and Epoxide
The cycloaddition reactions were carried out in a semi-batch reactor according to our previously reported method [43]. The detailed procedure is described in the Supplementary Materials. The products were analyzed by using a gas chromatograph (GC, Agilent technologies, HP 7890 A) with a flame ionization detector. Dichloromethane was used as an internal standard.
4. Conclusions
In this work, CPM-200-In and CPM-200-In/Mg MOF were successfully prepared by a solvothermal method and were characterized by using PXRD, SEM-EDS, FT-IR, TGA, XPS, BET, CO2, and NH3 TPD analysis. Both MOFs were used as catalysts for the CO2 fixation with various epoxides, and they were shown to be highly active (TOF of 25.0/h for ECH conversion) for the cycloaddition reaction under moderate operating conditions and solvent-less conditions. Especially, CPM-200-In/Mg revealed superior catalytic performance to CPM-200-In in the cycloaddition process due to higher surface area, higher CO2 adsorption capacity, and higher number of basic sites. CPM-200-In/Mg catalyst could be recycled five times, without considerable loss in its initial activity. Based on the experimental data and our previous DFT studies, a plausible reaction mechanism, including the synergistic role of the acidic sites and basic sites for the bimetallic catalyzed epoxide-CO2 cycloaddition reaction, was also suggested.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/catal11040430/s1: experimental procedure of the cycloaddition reaction, Figure S1: PXRD patterns of reused CPM-200-In/Mg catalyst, Figure S2: FT-IR spectra of reused CPM-200-In/Mg catalyst, Figure S3: TGA curve of reused CPM-200-In/Mg catalyst.
Author Contributions
Conceptualization and writing original draft preparation, Y.G., writing, review, and editing, Y.C. and D.-W.P.; Supervision, D.-W.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by National Research Foundation of Korea through Basic Research Program (2019R1I1A3A01057644).
Data Availability Statement
Data is contained within the article or Supplementary Materials.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Aresta, M.; Dibenedetto, A.; Angelini, A. Catalysis for the valorization of exhaust carbon: From CO2 to chemicals, materials, and fuels. Technological use of CO2. Chem. Rev. 2013, 114, 1709–1742. [Google Scholar] [CrossRef] [PubMed]
- Wilmer, C.E.; Farha, O.K.; Bae, Y.-S.; Hupp, J.T.; Snurr, R.Q. Structure–property relationships of porous materials for carbon dioxide separation and capture. Energy Environ. Sci. 2012, 5, 9849–9856. [Google Scholar] [CrossRef]
- Song, Q.-W.; Zhou, Z.-H.; He, L.-N. Efficient, selective and sustainable catalysis of carbon dioxide. Green Chem. 2017, 19, 3707–3728. [Google Scholar] [CrossRef]
- Mikkelsen, M.; Jørgensen, M.; Krebs, F.C. The teraton challenge. A review of fixation and transformation of carbon dioxide. Energy Environ. Sci. 2010, 3, 43–81. [Google Scholar] [CrossRef]
- Kleij, A.W.; North, M.; Urakawa, A. CO2 catalysis. ChemSusChem 2017, 10, 1036–1038. [Google Scholar] [CrossRef] [PubMed]
- Maeda, C.; Miyazaki, Y.; Ema, T. Recent progress in catalytic conversions of carbon dioxide. Catal. Sci. Technol. 2014, 4, 1482–1497. [Google Scholar] [CrossRef]
- Lang, X.-D.; He, L.-N. Green Catalytic Process for Cyclic Carbonate Synthesis from Carbon Dioxide under Mild Conditions. Chem. Rec. 2016, 16, 1337–1352. [Google Scholar] [CrossRef]
- Ding, M.; Flaig, R.W.; Jiang, H.-L.; Yaghi, O.M. Carbon capture and conversion using metal–organic frameworks and MOF-based materials. Chem. Soc. Rev. 2019, 48, 2783–2828. [Google Scholar] [CrossRef]
- Liu, Q.; Wu, L.; Jackstell, R.; Meller, M. Using carbon dioxide as a building block in organic synthesis. Nat. Commun. 2015, 5, 5933. [Google Scholar] [CrossRef]
- Guo, W.; Martínez-Rodríguez, L.; Martin, E.; Escudero-Adán, E.C.; Kleij, A.W. Highly Efficient Catalytic Formation of (Z)-1,4-But-2-ene Diols Using Water as a Nucleophile. Angew. Chem. Int. Ed. 2016, 55, 11037–11040. [Google Scholar] [CrossRef]
- Khan, A.; Khan, S.; Khan, I.; Zhao, C.; Mao, Y.; Chen, Y.; Zhang, Y.J. Enantioselective Construction of Tertiary C–O Bond via Allylic Substitution of Vinylethylene Carbonates with Water and Alcohols. J. Am. Chem. Soc. 2017, 139, 10733–10741. [Google Scholar] [CrossRef] [PubMed]
- Scrosati, B.; Hassoun, J.; Sun, Y.K. Lithium-ion batteries. A look into the future. Energy Env. Sci. 2011, 4, 3287–3295. [Google Scholar] [CrossRef]
- Pascual, A.; Tan, J.P.K.; Yuen, A.; Chan, J.M.W.; Coady, D.J.; Mecerreyes, D.; Hedrick, J.L.; Yang, Y.Y.; Sardon, H. Broad-Spectrum Antimicrobial Polycarbonate Hydrogels with Fast Degradability. Biomacromolecules 2015, 16, 1169–1178. [Google Scholar] [CrossRef] [PubMed]
- Shi, F.; Deng, Y.; Sima, T.; Peng, J.; Gu, Y.; Qiao, B. Alternatives to Phosgene and Carbon Monoxide: Synthesis of Symmetric Urea Derivatives with Carbon Dioxide in Ionic Liquids. Angew. Chem. Int. Ed. 2003, 42, 3257–3260. [Google Scholar] [CrossRef]
- Xiong, H.; Wei, X.; Zhou, D.; Qi, Y.; Xie, Z.; Chen, X.; Jing, X.; Huang, Y. Amphiphilic Polycarbonates from Carborane-Installed Cyclic Carbonates as Potential Agents for Boron Neutron Capture Therapy. Bioconjug. Chem. 2016, 27, 2214–2223. [Google Scholar] [CrossRef]
- Gennen, S.; Grignard, B.; Tassaing, T.; Jérôme, C.; Detrembleur, C. CO2-Sourced α-Alkylidene Cyclic Carbonates: A Step Forward in the Quest for Functional Regioregular Poly(urethane)s and Poly(carbonate)s. Angew. Chem. Int. Ed. 2017, 56, 10394–10398. [Google Scholar] [CrossRef] [PubMed]
- Fulfer, K.D.; Kuroda, D.G. Solvation Structure and Dynamics of the Lithium Ion in Organic Carbonate-Based Electrolytes: A Time-Dependent Infrared Spectroscopy Study. J. Phys. Chem. C 2016, 120, 24011–24022. [Google Scholar] [CrossRef]
- Calo, V.; Nacci, A.; Monopoli, A.; Fanizzi, A. Cyclic carbonate formation from carbon dioxide and oxiranes in tetrabu-tylammonium halides as solvents and catalysts. Org. Lett. 2002, 4, 2561–2563. [Google Scholar] [CrossRef] [PubMed]
- Toda, Y.; Komiyama, Y.; Kikuchi, A.; Suga, H. Tetraarylphosphonium Salt-Catalyzed Carbon Dioxide Fixation at Atmospheric Pressure for the Synthesis of Cyclic Carbonates. ACS Catal. 2016, 6, 6906–6910. [Google Scholar] [CrossRef]
- Yang, H.; Zheng, D.; Zhang, J.; Chen, K.; Li, J.; Wang, L.; Zhang, J.; He, H.-Y.; Zhang, S. Protic Quaternary Ammonium Ionic Liquids for Catalytic Conversion of CO2 into Cyclic Carbonates: A Combined Ab Initio and MD Study. Ind. Eng. Chem. Res. 2018, 57, 7121–7129. [Google Scholar] [CrossRef]
- Al-Qaisi, F.M.; Nieger, M.; Kemell, M.L.; Repo, T.J. Catalysis of cycloaddition of carbon dioxide and epoxides using a bifunc-tional schiff base iron(III) catalyst. ChemistrySelect 2016, 3, 545–548. [Google Scholar] [CrossRef]
- Decortes, A.; Belmonte, M.M.; Benet-Buchholz, J.; Kleij, A.W. Efficient carbonate synthesis under mild conditions through cycloaddition of carbon dioxide to oxiranes using a Zn(salphen) catalyst. Chem. Commun. 2010, 46, 4580–4582. [Google Scholar] [CrossRef] [PubMed]
- Sopeña, S.; Martin, E.; Escudero-Adán, E.C.; Kleij, A.W. Pushing the Limits with Squaramide-Based Organocatalysts in Cyclic Carbonate Synthesis. ACS Catal. 2017, 7, 3532–3539. [Google Scholar] [CrossRef]
- Choi, H.-J.; Selvaraj, M.; Park, D.-W. Catalytic performance of immobilized ionic liquid onto PEG for the cycloaddition of carbon dioxide to allyl glycidyl ether. Chem. Eng. Sci. 2013, 100, 242–248. [Google Scholar] [CrossRef]
- Han, L.; Choi, H.-J.; Choi, S.-J.; Liu, B.; Park, D.-W. Ionic liquids containing carboxyl acid moieties grafted onto silica: Synthesis and application as heterogeneous catalysts for cycloaddition reactions of epoxide and carbon dioxide. Green Chem. 2011, 13, 1023–1028. [Google Scholar] [CrossRef]
- Roshith, K.R.; Mathai, G.; Kim, J.; Tharun, J.; Park, G.A.; Park, D.W. A biopolymer mediated efficient synthesis of cyclic car-bonates from epoxides and carbon dioxide. Green Chem. 2012, 14, 2933–2940. [Google Scholar]
- Lee, S.D.; Kim, B.M.; Kim, D.W.; Kim, M.I.; Roshan, K.R.; Kim, M.K.; Won, Y.S.; Park, D.W. Synthesis of cyclic carbonate from carbon dioxide and epoxides with polystyrene-supported quaternary ammonium salt catalysts. Appl. Catal. A Gen. 2014, 486, 69–76. [Google Scholar] [CrossRef]
- Rehman, A.; Saleem, F.; Javed, F.; Qutab, H.; Eze, V.C.; Harvey, A. Kinetic study for styrene carbonate synthesis via CO2 cycloaddition to styrene oxide using silica-supported pyrrolidinopyridinium iodide catalyst. J. CO2 Util. 2021, 43, 101379. [Google Scholar] [CrossRef]
- Janeta, M.; Lis, T.; Szafert, S. Zinc Imine Polyhedral Oligomeric Silsesquioxane as a Quattro-Site Catalyst for the Synthesis of Cyclic Carbonates from Epoxides and Low-Pressure CO2. Chem. Eur. J. 2020, 26, 13686–13697. [Google Scholar] [CrossRef] [PubMed]
- Mirabaud, A.; Mulatier, J.-C.; Martinez, A.; Dutasta, J.-P.; Dufaud, V. Investigating Host–Guest Complexes in the Catalytic Synthesis of Cyclic Carbonates from Styrene Oxide and CO2. ACS Catal. 2015, 5, 6748–6752. [Google Scholar] [CrossRef]
- Rachuri, Y.; Kurisingal, J.F.; Chitumalla, R.K.; Vuppala, S.; Gu, Y.; Jang, J.; Choe, Y.; Suresh, E.; Park, D.-W. Adenine-Based Zn(II)/Cd(II) Metal–Organic Frameworks as Efficient Heterogeneous Catalysts for Facile CO2 Fixation into Cyclic Carbonates: A DFT-Supported Study of the Reaction Mechanism. Inorg. Chem. 2019, 58, 11389–11403. [Google Scholar] [CrossRef]
- Kurisingal, J.F.; Rachuri, Y.; Gu, Y.; Choe, Y.; Park, D.-W. Multi-variate metal organic framework as efficient catalyst for the cycloaddition of CO2 and epoxides in a gas-liquid-solid reactor. Chem. Eng. J. 2020, 386, 121700. [Google Scholar] [CrossRef]
- Kurisingal, J.F.; Rachuri, Y.; Palakkal, A.S.; Pillai, R.S.; Gu, Y.; Choe, Y.; Park, D.-W. Water-Tolerant DUT-Series Metal–Organic Frameworks: A Theoretical–Experimental Study for the Chemical Fixation of CO2 and Catalytic Transfer Hydrogenation of Ethyl Levulinate to γ-Valerolactone. ACS Appl. Mater. Interfaces 2019, 11, 41458–41471. [Google Scholar] [CrossRef] [PubMed]
- Xiang, W.; Ren, J.; Chen, S.; Shen, C.; Chen, Y.; Zhang, M.; Liu, C.-J. The metal–organic framework UiO-66 with missing-linker defects: A highly active catalyst for carbon dioxide cycloaddition. Appl. Energy 2020, 277, 115560. [Google Scholar] [CrossRef]
- Kuruppathparambil, R.R.; Babu, R.; Jeong, H.; Jang, Y.H.; Lee, M.H.; Park, D.-W. Room temperature CO2 fixation via cyclic carbonate synthesis over vanadium-MOF catalysts. Korean J. Chem. Eng. 2019, 36, 643–649. [Google Scholar] [CrossRef]
- He, L.; Nath, J.K.; Lin, Q. Robust multivariate metal–porphyrin frameworks for efficient ambient fixation of CO2 to cyclic carbonates. Chem. Commun. 2018, 55, 412–415. [Google Scholar] [CrossRef]
- Kurisingal, J.F.; Rachuri, Y.; Gu, Y.; Chitumalla, R.K.; Vuppala, S.; Jang, J.; Bisht, K.K.; Suresh, E.; Park, D.-W. Facile Green Synthesis of New Copper-Based Metal–Organic Frameworks: Experimental and Theoretical Study of the CO2 Fixation Reaction. ACS Sustain. Chem. Eng. 2020, 8, 10822–10832. [Google Scholar] [CrossRef]
- Li, P.-Z.; Wang, X.-J.; Liu, J.; Lim, J.S.; Zou, R.; Zhao, Y. A Triazole-Containing Metal–Organic Framework as a Highly Effective and Substrate Size-Dependent Catalyst for CO2 Conversion. J. Am. Chem. Soc. 2016, 138, 2142–2145. [Google Scholar] [CrossRef]
- Liu, L.; Wang, S.-M.; Han, Z.-B.; Ding, M.; Yuan, D.-Q.; Jiang, H.-L. Exceptionally Robust In-Based Metal–Organic Framework for Highly Efficient Carbon Dioxide Capture and Conversion. Inorg. Chem. 2016, 55, 3558–3565. [Google Scholar] [CrossRef] [PubMed]
- Reimer, N.; Reinsch, H.; Inge, A.K.; Stock, N. New Al-MOFs Based on Sulfonyldibenzoate Ions: A Rare Example of Intralayer Porosity. Inorg. Chem. 2014, 54, 492–501. [Google Scholar] [CrossRef]
- Kathalikkattil, A.C.; Roshan, R.; Tharun, J.; Soek, H.-G.; Ryu, H.-S.; Park, D.-W. Pillared Cobalt-Amino Acid Framework Catalysis for Styrene Carbonate Synthesis from CO2 and Epoxide by Metal-Sulfonate-Halide Synergism. ChemCatChem 2014, 6, 284–292. [Google Scholar] [CrossRef]
- Han, L.; Choi, S.J.; Park, M.S.; Kim, Y.J.; Lee, S.M.; Liu, B.; Park, D.W. Carboxylic acid functionalized imidazolium-based ionic liquids: Efficient catalyst for the cycloaddition reaction of CO2 and epoxides. React. Kinet. Mech. Catal. 2016, 106, 25–35. [Google Scholar] [CrossRef]
- Kathalikkattil, A.C.; Kim, D.-W.; Tharun, J.; Soek, H.-G.; Roshan, R.; Park, D.-W. Aqueous-microwave synthesized carboxyl functional molecular ribbon coordination framework catalyst for the synthesis of cyclic carbonates from epoxides and CO2. Green Chem. 2013, 16, 1607–1616. [Google Scholar] [CrossRef]
- De, D.; Pal, T.K.; Neogi, S.; Senthilkumar, S.; Das, D.; Gupta, S.S.; Bharadwaj, P.K. A Versatile Cu (II) metal-organic framework exhibiting high gas storage capacity with selectivity for CO2: Conversion of CO2 to cyclic carbonate and other catalytic abilities. Chem. Eur. J. 2016, 22, 3387–3396. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Perman, J.A.; Zhu, G.; Ma, S. Metal-Organic Frameworks for CO2 Chemical Transformations. Small 2016, 12, 6309–6324. [Google Scholar] [CrossRef]
- Lan, J.; Qu, Y.; Zhang, X.; Ma, H.; Xu, P.; Sun, J. A novel water-stable MOF Zn(Py)(Atz) as heterogeneous catalyst for chemical conversion of CO2 with various epoxides under mild conditions. J. CO2 Util. 2020, 35, 216–224. [Google Scholar] [CrossRef]
- Pal, T.K.; De, D.; Bharadwaj, P.K. Metal–organic frameworks for the chemical fixation of CO2 into cyclic carbonates. Coord. Chem. Rev. 2020, 408, 213173. [Google Scholar] [CrossRef]
- Liang, J.; Xie, Y.Q.; Wu, Q.; Wang, W.Y.; Liu, T.T.; Li, H.F.; Huang, Y.B.; Cao, R. Zinc porphyrin/imidazolium intergrated multivariate zirconium metal-organic frameworks for transformation of CO2 into cyclic carbonates. Inorg. Chem. 2018, 57, 2584–2593. [Google Scholar] [CrossRef]
- Beyzavi, M.H.; Stephenson, C.J.; Liu, Y.; Karagiaridi, O.; Hupp, J.T.; Farha, O.K. Metal-organic framework-based catalysts: Chemical fixation of CO2 with epoxides leading to cyclic organic carbonates. Front. Energy Res. 2015, 2, 63. [Google Scholar] [CrossRef]
- Pander, M.; Janeta, M.; Bury, W. Quest for an Efficient 2-in-1 MOF-Based Catalytic System for Cycloaddition of CO2 to Epoxides under Mild Conditions. ACS Appl. Mater. Interfaces 2021, 13, 8344–8352. [Google Scholar] [CrossRef]
- Duan, C.; Li, F.; Zhang, H.; Li, J.; Wang, X.; Xi, H. Template synthesis of hierarchical porous metal–organic frameworks with tunable porosity. RSC Adv. 2017, 7, 52245–52251. [Google Scholar] [CrossRef]
- Zhang, H.; Huo, J.; Li, J.; Li, F.; Duan, C.; Xi, H. Hierarchically porous metal–organic frameworks with single-crystal structures and their enhanced catalytic properties. CrystEngComm 2018, 20, 5754–5759. [Google Scholar] [CrossRef]
- Ghosh, M.; Lohrasbi, M.; Chuang, S.S.C.; Jana, S.C. Mesoporous Titanium Dioxide Nanofibers with a Significantly Enhanced Photocatalytic Activity. ChemCatChem 2016, 8, 2525–2535. [Google Scholar] [CrossRef]
- Mason, J.A.; Sumida, K.; Herm, Z.R.; Krishna, R.; Long, J.R. Evaluating metal–organic frameworks for post-combustion carbon dioxide capture via temperature swing adsorption. Energy Environ. Sci. 2011, 4, 3030–3040. [Google Scholar] [CrossRef]
- Yang, D.-A.; Cho, H.-Y.; Kim, J.; Yang, S.-T.; Ahn, W.-S. CO2 capture and conversion using Mg-MOF-74 prepared by a sonochemical method. Energy Environ. Sci. 2012, 5, 6465–6473. [Google Scholar] [CrossRef]
- Gu, Y.; Lee, C.-H.; Park, D.-W.; Lim, D.-H. Effect of Water for the Synthesis of Cyclic Carbonate from Epoxide and CO2 Using Mg-MOF-74. Nanosci. Nanotechnol. Lett. 2018, 10, 1088–1094. [Google Scholar] [CrossRef]
- Kurisingal, J.F.; Babu, R.; Kim, S.H.; Li, Y.X.; Cho, S.J.; Chang, J.S.; Park, D.W. Microwave-induced synthesis of a bimetallic charge-transfer metal organic framework: A promising host for the chemical fixation of CO2 via cyclic carbonate synthesis. Catal. Sci. Technol. 2018, 8, 591–600. [Google Scholar] [CrossRef]
- Wu, Y.; Song, X.; Xu, S.; Chen, Y.; Oderinde, O.; Gao, L.; Wei, R.; Xiao, G. Chemical fixation of CO2 into cyclic carbonates catalyzed by bimetal mixed MOFs: The role of the interaction between Co and Zn. Dalton Trans. 2020, 49, 312–321. [Google Scholar] [CrossRef]
- Kurisingal, J.F.; Rachuri, Y.; Gu, Y.; Kim, G.H.; Park, D.W. Binary metal organic frameworks: Catalysts for the efficient sol-vent-free CO2 fixation reaction via cyclic carbonate synthesis. Appl. Catal. A Gen. 2019, 571, 1–11. [Google Scholar] [CrossRef]
- Zhai, Q.-G.; Bu, X.; Mao, C.; Zhao, X.; Feng, P. Systematic and Dramatic Tuning on Gas Sorption Performance in Heterometallic Metal–Organic Frameworks. J. Am. Chem. Soc. 2016, 138, 2524–2527. [Google Scholar] [CrossRef]
- Babu, R.; Kurisingal, J.F.; Chang, J.S.; Park, D.W. Bifunctional pyridinium-based ionic-liquid-immobilized diindium tris(diphenic acid) bis(1,10-phenanthroline) for CO2 fixation. ChemSusChem 2018, 11, 924–932. [Google Scholar] [CrossRef]
- Ranjbar, M.; Taher, M.A.; Sam, A. Mg-MOF-74 nanostructures: Facile synthesis and characterization with aid of 2,6-pyridinedicarboxylic acid ammonium. J. Mater. Sci. Mater. Electron. 2016, 27, 1449–1456. [Google Scholar] [CrossRef]
- Gofer, Y.; Turgeman, R.; Cohen, H.; Aurbach, R. XPS Investigation of Surface Chemistry of Magnesium Electrodes in Contact with Organic Solutions of Organochloroaluminate Complex Salts. Langmuir 2003, 19, 2344–2348. [Google Scholar] [CrossRef]
- Badoga, S.; Kamath, G.; Dalai, A. Effects of promoters (Mn, Mg, Co and Ni) on the Fisher-Tropsch activity and selectivity of KCuFe/mesoporous-alumina catalyst. Appl. Catal. A Gen. 2020, 607, 117861. [Google Scholar] [CrossRef]
- Kong, A.; Lin, Q.; Mao, C.; Bu, X.; Feng, P. Efficient oxygen reduction by nanocomposites of heterometallic carbide and ni-trogen-enriched carbon derived from the cobalt-encapsulated indium-MOF. Chem. Commun. 2014, 50, 15619–15622. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Fu, R.; Liu, F.; Pan, Y.; Ding, X.; He, G. Novel Electron-Rich and Sterically Hindered Phosphonium as a Highly Efficient and Recyclable Heterogeneous Catalyst for CO2 Cycloaddition. Ind. Eng. Chem. Res. 2018, 57, 3195–3203. [Google Scholar] [CrossRef]
- Darensbourg, D.J.; Billodeaux, D.R. Aluminum Salen Complexes and Tetrabutylammonium Salts: A Binary Catalytic System for Production of Polycarbonates from CO2 and Cyclohexene Oxide. Inorg. Chem. 2005, 44, 1433–1442. [Google Scholar] [CrossRef]
- Patel, P.; Parmar, B.; Kureshy, R.I.; Khan, N.H.; Suresh, E. Amine-functionalized Zn(II) MOF as efficient multifunctional cat-alyst for CO2 utilization and sulfoxidation reaction. Dalton Trans. 2018, 47, 8041–8051. [Google Scholar] [CrossRef]
- Parmar, B.; Patel, P.; Kureshy, R.I.; Khan, N.-U.H.; Suresh, E. Sustainable Heterogeneous Catalysts for CO2 Utilization by Using Dual Ligand ZnII/CdII Metal-Organic Frameworks. Chem. Eur. J. 2018, 24, 15831–15839. [Google Scholar] [CrossRef]
- Kim, S.-N.; Kim, J.; Kim, H.-Y.; Cho, H.-Y.; Ahn, W.-S. Adsorption/catalytic properties of MIL-125 and NH2-MIL-125. Catal. Today 2013, 204, 85–93. [Google Scholar] [CrossRef]
- Zhang, S.; Jang, M.-S.; Lee, J.; Puthiaraj, P.; Ahn, W.-S. Zeolite-Like Metal Organic Framework (ZMOF) with a rho Topology for a CO2 Cycloaddition to Epoxides. ACS Sustain. Chem. Eng. 2020, 8, 7078–7086. [Google Scholar] [CrossRef]
- Li, Y.-H.; Wang, S.-L.; Su, Y.-C.; Ko, B.-T.; Tsai, C.-Y.; Lin, C.-H. Microporous 2D indium metal–organic frameworks for selective CO2 capture and their application in the catalytic CO2-cycloaddition of epoxides. Dalton Trans. 2018, 47, 9474–9481. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Wang, X.; Li, L.; Advincula, R.C. Design, Synthesis, and Photochemical Behavior of Poly(benzyl ester) Dendrimers with Azobenzene Groups throughout Their Architecture. J. Org. Chem. 2004, 69, 9073–9084. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).