Tuning the Reaction Selectivity over MgAl Spinel-Supported Pt Catalyst in Furfuryl Alcohol Conversion to Pentanediols
Abstract
1. Introduction
2. Results and Discussion
2.1. Catalytic Conversion of FFA over Different Catalysts
2.1.1. Performance of Pt/MgAl2O4 Catalysts in FFA Conversion
2.1.2. Alkali-Modified Pt/MgAl2O4 Catalysts
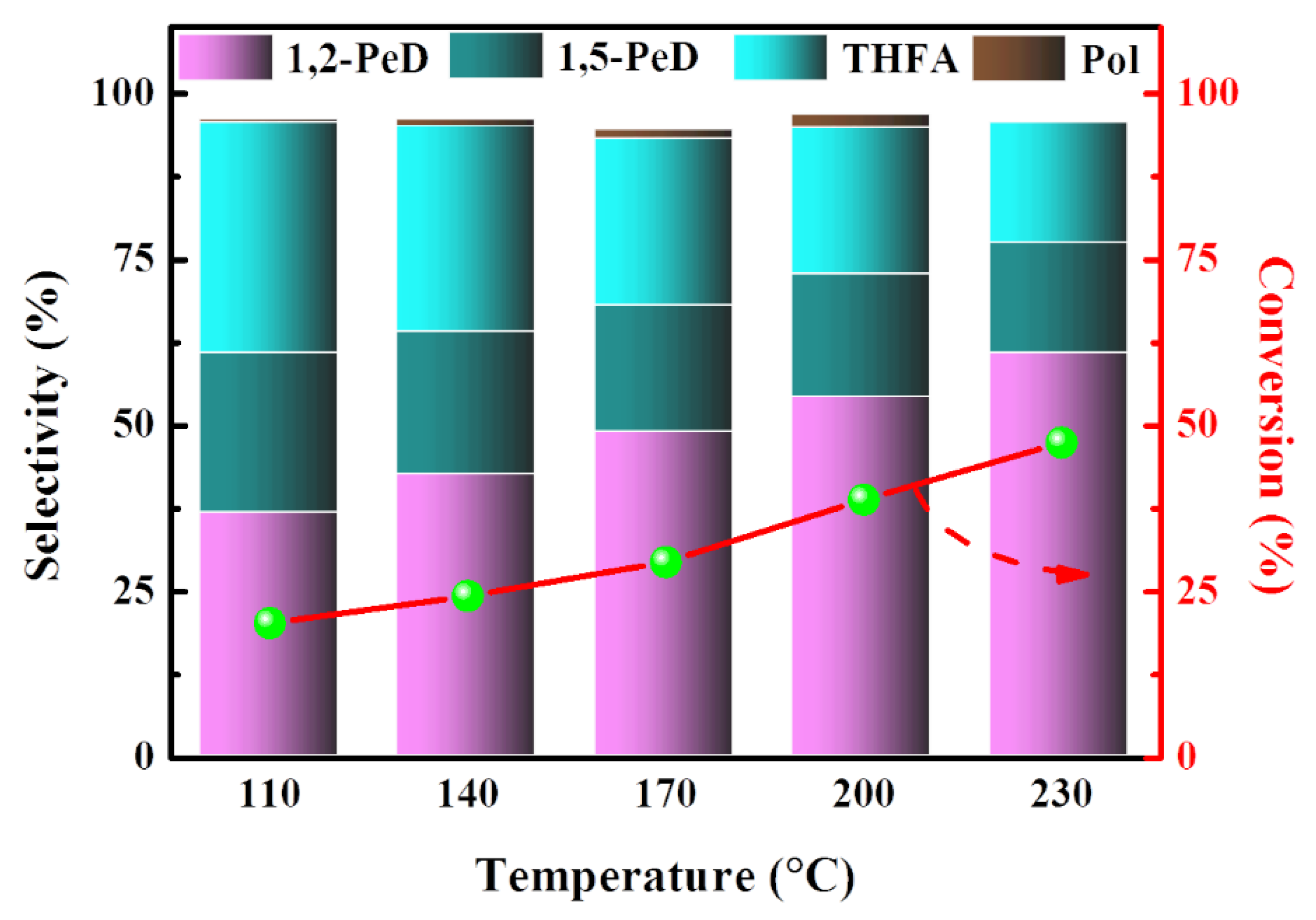
2.1.3. Effects of Reaction Temperature on Product Selectivity
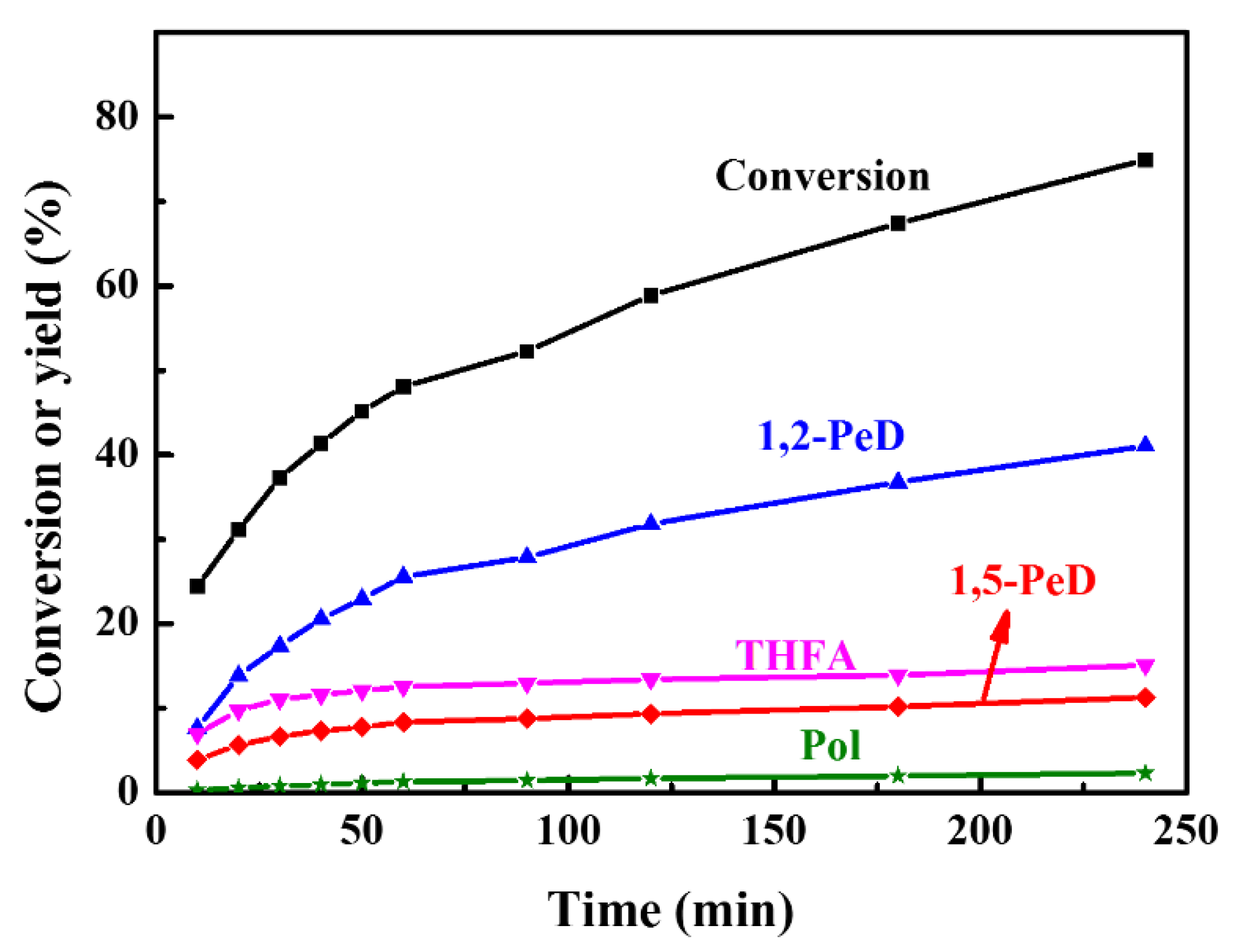
2.1.4. Catalyst Stability
2.2. Physicochemical Properties of Catalysts
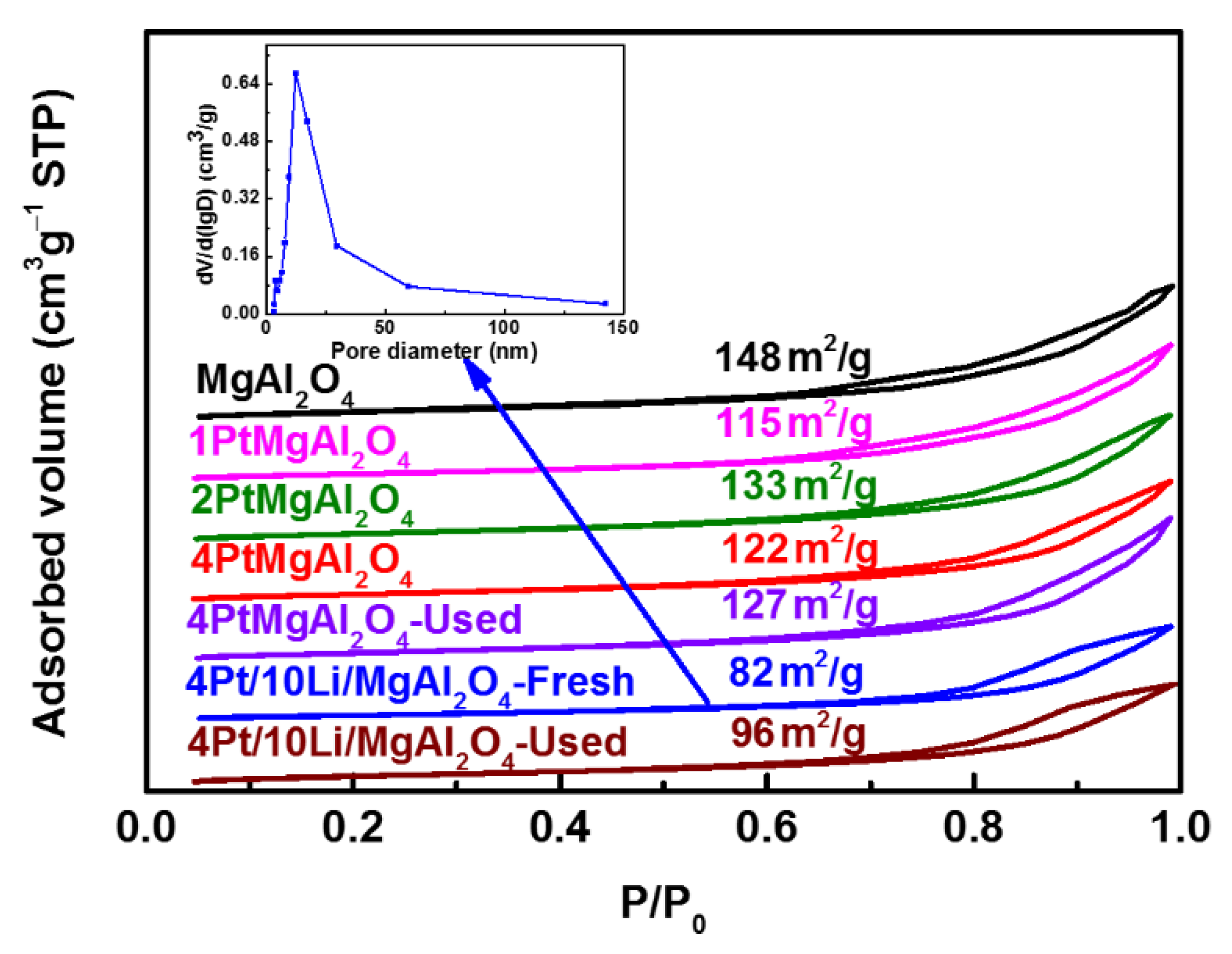
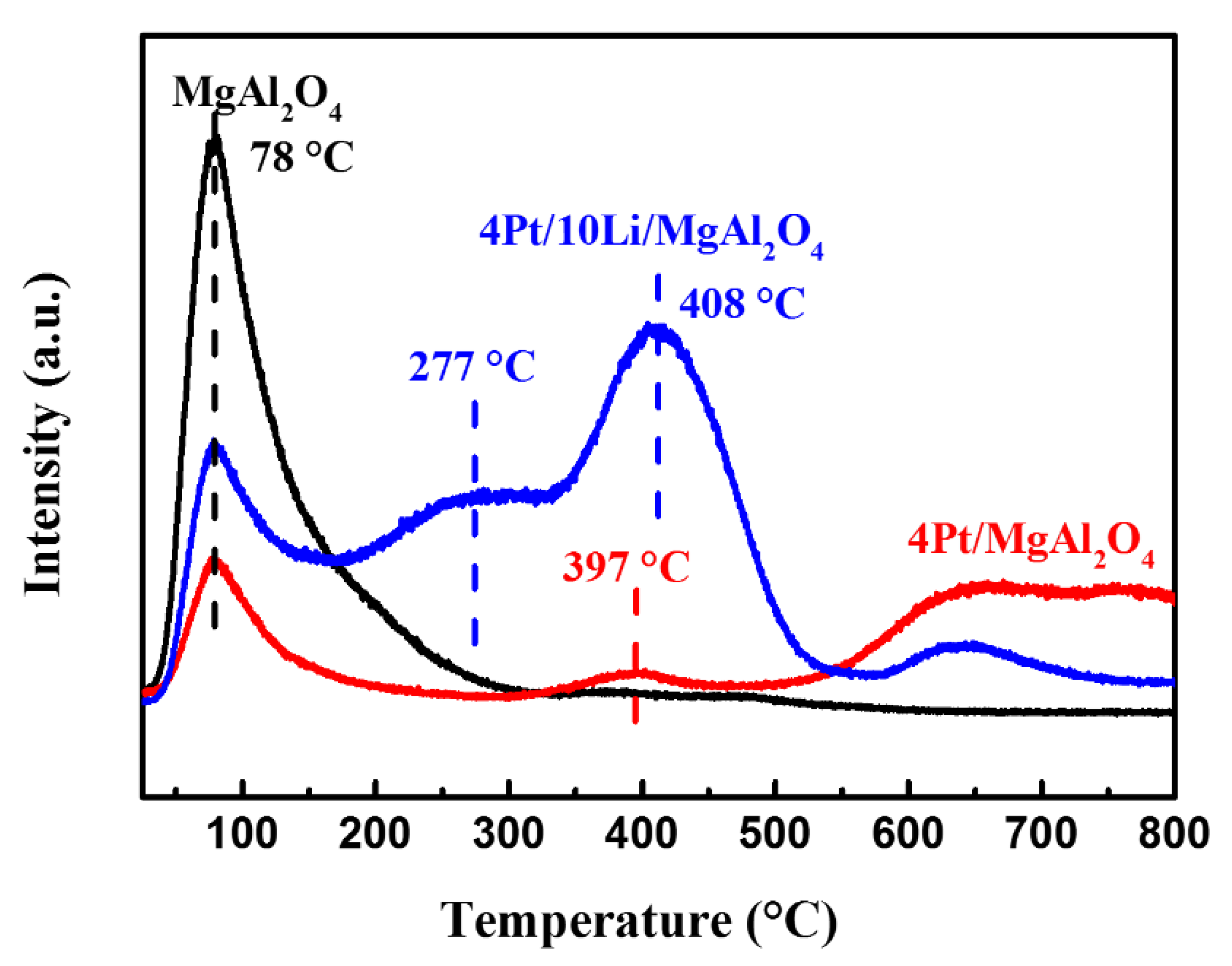
2.2.1. Physical Properties of Catalysts
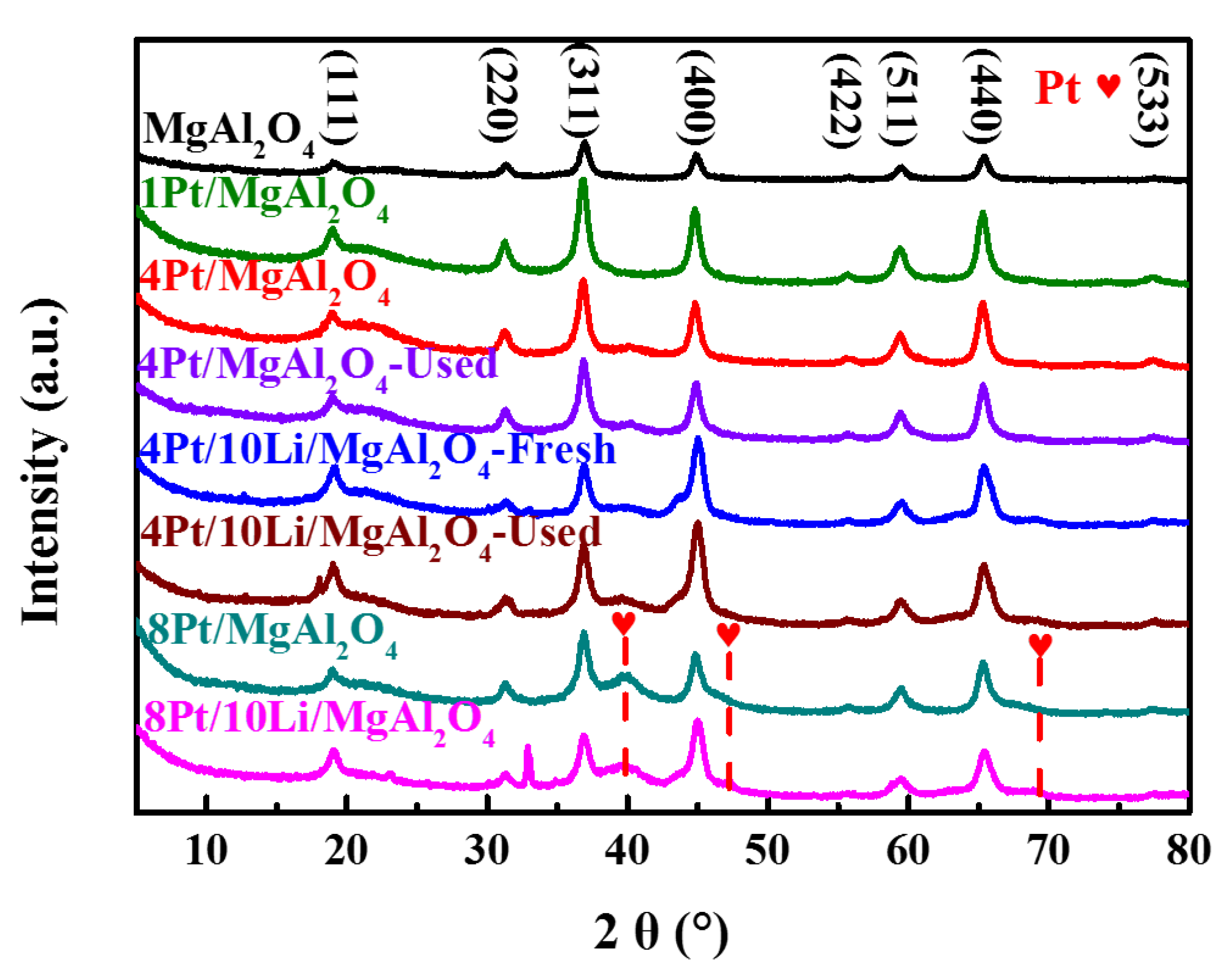
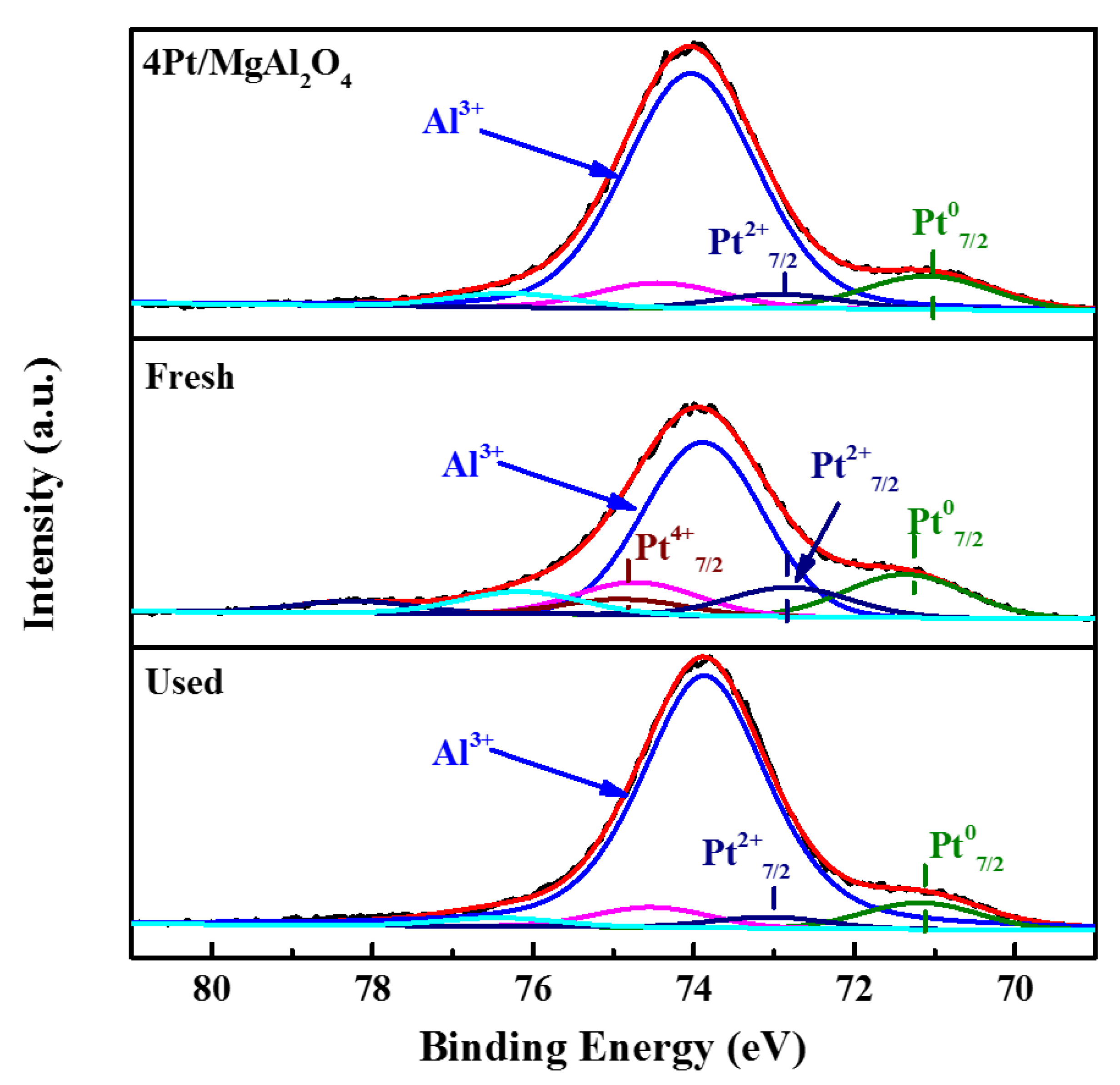
2.2.2. Chemical Properties of Catalysts
2.3. Discussion
3. Materials and Methods
3.1. Catalysts Preparation
3.1.1. Synthesis of MgAl2O4 Support
3.1.2. Synthesis of MgAl2O4-Supported Catalysts
3.2. Catalyst Characterization
3.3. Catalytic Reaction
3.4. Products’ Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gallezot, P. Conversion of biomass to selected chemical products. Chem. Soc. Rev. 2012, 41, 1538–1558. [Google Scholar] [CrossRef]
- Mariscal, R.; Maireles-Torres, P.; Ojeda, M.; Sadaba, I.; Granados, M.L. Furfural: A renewable and versatile platform molecule for the synthesis of chemicals and fuels. Energy Environ. Sci. 2016, 9, 1144–1189. [Google Scholar] [CrossRef]
- Li, X.; Jia, P.; Wang, T. Furfural: A promising platform compound for sustainable production of C4 and C5 chemicals. ACS Catal. 2016, 6, 7621–7640. [Google Scholar] [CrossRef]
- Mika, L.T.; Csefalvay, E.; Nemeth, A. Catalytic conversion of carbohydrates to initial platform chemicals: Chemistry and sustainability. Chem. Rev. 2018, 118, 505–613. [Google Scholar] [CrossRef]
- Anselmi, C.; Ettorre, A.; Andreassi, M.; Centini, M.; Neri, P.; Di Stefano, A. In vitro induction of apoptosis vs. necrosis by widely used preservatives: 2-phenoxyethanol, a mixture of isothiazolinones, imidazolidinyl urea and 1,2-pentanediol. Biochem. Pharmacol. 2002, 63, 437–453. [Google Scholar] [CrossRef]
- Schlaf, M. Selective deoxygenation of sugar polyols to α,ω-diols and other oxygen content reduced materials—a new challenge to homogeneous ionic hydrogenation and hydrogenolysis catalysis. Dalton Trans. 2006, 39, 4645–4653. [Google Scholar] [CrossRef]
- Zhang, B.; Zhu, Y.L.; Ding, G.Q.; Zheng, H.Y.; Li, Y.W. Selective conversion of furfuryl alcohol to 1,2-pentanediol over a Ru/MnOx catalyst in aqueous phase. Green Chem. 2012, 14, 3402–3409. [Google Scholar] [CrossRef]
- Ma, R.F.; Wu, X.P.; Tong, T.; Shao, Z.J.; Wang, Y.Q.; Liu, X.H.; Xia, Q.N.; Gong, X.Q. The critical role of water in the ring opening of furfural alcohol to 1,2-pentanediol. ACS Catal. 2017, 7, 333–337. [Google Scholar] [CrossRef]
- Tong, T.; Liu, X.H.; Guo, Y.; Banis, M.N.; Hu, Y.F.; Wang, Y.Q. The critical role of CeO2 crystal-plane in controlling Pt chemical states on the hydrogenolysis of furfuryl alcohol to 1,2-pentanediol. J. Catal. 2018, 365, 420–428. [Google Scholar] [CrossRef]
- Liu, F.; Liu, Q.Y.; Xu, J.M.; Li, L.; Cui, Y.T.; Lang, R.; Li, L.; Su, Y.; Miao, S.; Sun, H.; et al. Catalytic cascade conversion of furfural to 1,4-pentanediol in a single reactor. Green Chem. 2018, 20, 1770–1776. [Google Scholar] [CrossRef]
- Liu, Q.; Qiao, B.; Liu, F.; Zhang, L.; Su, Y.; Wang, A.; Zhang, T. Correction: Catalytic production of 1,4-pentanediol from furfural in a fixed-bed system under mild conditions. Green Chem. 2020, 22, 3532–3538. [Google Scholar] [CrossRef]
- Nakagawa, Y.; Tomishige, K. Production of 1,5-pentanediol from biomass via furfural and tetrahydrofurfuryl alcohol. Catal. Today 2012, 195, 136–143. [Google Scholar] [CrossRef]
- Liu, S.B.; Amada, Y.; Tamura, M.; Nakagawa, Y.; Tomishige, K. Performance and characterization of rhenium-modified Rh-Ir alloy catalyst for one-pot conversion of furfural into 1,5-pentanediol. Catal. Sci. Technol. 2014, 4, 2535–2549. [Google Scholar] [CrossRef]
- Huang, K.F.; Brentzel, Z.J.; Barnett, K.J.; Dumesic, J.A.; Huber, G.W.; Maravelias, C.T. Conversion of furfural to 1,5-pentanediol: Process synthesis and analysis. ACS Sustain. Chem. Eng. 2017, 5, 4699–4706. [Google Scholar] [CrossRef]
- Brentzel, Z.J.; Barnett, K.J.; Huang, K.; Maravelias, C.T.; Dumesic, J.A.; Huber, G.W. Chemicals from biomass: Combining ring-opening tautomerization and hydrogenation reactions to produce 1,5-pentanediol from furfural. ChemSusChem 2017, 10, 1351–1355. [Google Scholar] [CrossRef]
- Nakagawa, Y.; Tamura, M.; Tomishige, K. Catalytic reduction of biomass-derived furanic compounds with hydrogen. ACS Catal. 2013, 3, 2655–2668. [Google Scholar] [CrossRef]
- Nakagawa, Y.; Tamura, M.; Tomishige, K. Catalytic conversions of furfural to pentanediols. Catal. Surv. Asia 2015, 19, 249–256. [Google Scholar] [CrossRef]
- Chen, S.; Wojcieszak, R.; Dumeignil, F.; Marceau, E.; Royer, S. How catalysts and experimental conditions determine the selective hydroconversion of furfural and 5-hydroxymethylfurfural. Chem. Rev. 2018, 118, 11023–11117. [Google Scholar] [CrossRef] [PubMed]
- Enjamuri, N.; Darbha, S. Solid catalysts for conversion of furfural and its derivatives to alkanediols. Catal. Rev. 2020, 62, 566–606. [Google Scholar] [CrossRef]
- Homer, A.; Conno, R. The catalytic hydrogenation of organic compounds over copper chromite. JACS 1931, 53, 1091–1095. [Google Scholar]
- Mizugaki, T.; Yamakawa, T.; Nagatsu, Y.; Maeno, Z.; Mitsudome, T.; Jitsukawa, K.; Kaneda, K. Direct transformation of furfural to 1,2-pentanediol using a hydrotalcite-supported platinum nanoparticle catalyst. ACS Sustain. Chem. Eng. 2014, 2, 2243–2247. [Google Scholar] [CrossRef]
- Zhu, Y.R.; Zhao, W.F.; Zhang, J.; An, Z.; Ma, X.D.; Zhang, Z.J.; Jiang, Y.T.; Zheng, L.R.; Shu, X.; Song, H.Y.; et al. Selective activation of C-OH, C-O-C, or C = C in furfuryl alcohol by engineered Pt sites supported on layered double oxides. ACS Catal. 2020, 10, 8032–8041. [Google Scholar] [CrossRef]
- Liu, H.L.; Huang, Z.W.; Zhao, F.; Cui, F.; Li, X.M.; Xia, C.G.; Chen, J. Efficient hydrogenolysis of biomass-derived furfuryl alcohol to 1,2-and 1,5-pentanediols over a non-precious Cu-Mg3AlO4.5 bifunctional catalyst. Catal. Sci. Technol. 2016, 6, 668–671. [Google Scholar] [CrossRef]
- Rennard, R.J.; Freel, J. The role of sulfur in deactivation of Pt/MgAl2O4 for propane dehydrogenation. J. Catal. 1986, 98, 235–244. [Google Scholar] [CrossRef]
- Li, W.Z.; Kovarik, L.; Mei, D.; Liu, J.; Wang, Y.; Peden, C.H. Stable platinum nanoparticles on specific MgAl2O4 spinel facets at high temperatures in oxidizing atmospheres. Nat. Commun. 2013, 4, 2481. [Google Scholar] [CrossRef]
- Yang, J.; Peng, M.; Ren, G.; Qi, H.; Zhou, X.; Xu, J.; Deng, F.; Chen, Z.; Zhang, J.; Liu, K.; et al. A hydrothermally stable irreducible oxide-modified Pd/MgAl2O4 catalyst for methane combustion. Angew. Chem. Int. Ed. 2020, 59, 18522–18526. [Google Scholar] [CrossRef]
- Liu, K.; Zhao, X.; Ren, G.; Yang, T.; Ren, Y.; Lee, A.F.; Su, Y.; Pan, X.; Zhang, J.; Chen, Z.; et al. Strong metal-support interaction promoted scalable production of thermally stable single-atom catalysts. Nat. Commun. 2020, 11, 1263. [Google Scholar] [CrossRef]
- Gao, F.; Liu, H.; Hu, X.; Chen, J.; Huang, Z.; Xia, C. Selective hydrogenolysis of furfuryl alcohol to 1,5- and 1,2-pentanediol over Cu-LaCoO3 catalysts with balanced Cu0-CoO sites. Chin. J. Catal. 2018, 39, 1711–1723. [Google Scholar] [CrossRef]
- Xu, W.; Wang, H.; Liu, X.; Ren, J.; Wang, Y.; Lu, G. Direct catalytic conversion of furfural to 1,5-pentanediol by hydrogenolysis of the furan ring under mild conditions over Pt/Co2AlO4 catalyst. Chem. Commun. 2011, 47, 3924–3926. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87. [Google Scholar] [CrossRef]
- Tan, X.H.; Prabhudev, S.; Kohandehghan, A.; Karpuzov, D.; Botton, G.A.; Mitlin, D. Pt-Au-Co alloy electrocatalysts demonstrating enhanced activity and durability toward the oxygen reduction reaction. ACS Catal. 2015, 5, 1513–1524. [Google Scholar] [CrossRef]
- Heyden, B.E.; Bradshaw, A.M. The adsorption of CO on Pt(111) studied by infrared reflection—absortion spectroscopy. Surf. Sci. 1983, 125, 787–802. [Google Scholar] [CrossRef]
- Prieto, P.J.S.; Ferreira, A.P.; Haddad, P.S.; Zanchet, D.; Bueno, J.M.C. Designing Pt nanoparticles supported on CeO2-Al2O3 synthesis, characterization and catalytic properties in the steam reforming and partial oxidation of methane. J. Catal. 2010, 276, 351–359. [Google Scholar] [CrossRef]
- Fanson, P.T.; Delgass, W.N.; Lauterbach, J. Island formation during kinetic rate oscillations in the oxidation of CO over Pt/SiO2: A transient Fourier transform infrared spectrometry study. J. Catal. 2001, 204, 35–52. [Google Scholar] [CrossRef]
- Xu, J.Z.; Yates, J.T. Terrace width effect on adsorbate vibrations—A comparison of Pt(335) and Pt(112) for chemisorption of Co. Surf. Sci. 1995, 327, 193–201. [Google Scholar] [CrossRef]
- Tan, L.; Yang, G.H.; Yoneyama, Y.; Kou, Y.L.; Tan, Y.S.; Vitidsant, T.; Tsubaki, N. Iso-butanol direct synthesis from syngas over the alkali metals modified Cr/ZnO catalysts. Appl. Catal. A Gen. 2015, 505, 141–149. [Google Scholar] [CrossRef]




| Entry | Catalyst | Conversion % | Selectivity/% | C Balance/% | |||
|---|---|---|---|---|---|---|---|
| 1,2-PeD | 1,5-PeD | THFA | Pol | ||||
| 1 | 4Pt/MgAl2O4 | 100 | 19.2 | 29.0 | 45.9 | 0.7 | 94.8 |
| 2 | 4Pd/MgAl2O4 | 67.7 | 2.3 | 2.3 | 70.3 | 0 | 74.9 |
| 3 | 4Ir/MgAl2O4 | 13.6 | 5.4 | 5.4 | 20.5 | 0 | 31.3 |
| 4 | 4Rh/MgAl2O4 | 91.3 | 0.4 | 0.4 | 99.4 | 0 | 100 |
| 5 | 4Ru/MgAl2O4 | 84.2 | 9.5 | 9.5 | 61.0 | 0 | 80.0 |
| 6 | 1Pt/MgAl2O4 | 55.4 | 20.3 | 32.1 | 43.1 | 0.7 | 96.1 |
| 7 | 2Pt/MgAl2O4 | 83.6 | 23.3 | 28.4 | 43.9 | 0.6 | 96.2 |
| 8 | 8Pt/MgAl2O4 | 100 | 22.3 | 24.3 | 46.0 | 0.9 | 93.5 |
| Entry | Catalyst | Conversion % | Selectivity/% | C Balance/% | |||
|---|---|---|---|---|---|---|---|
| 1,2-PeD | 1,5-PeD | THFA | Pol | ||||
| 1 | 4Pt/10Li/MgAl2O4 [b] | 100 | 23.1 | 29.1 | 41.8 | 0 | 94.0 |
| 2 | 4Pt/10Li/MgAl2O4 [c] | 5.6 | - | 30.0 | 63.3 | - | 93.4 |
| 3 | 4Pt/10Li/MgAl2O4 [d] | 12.6 | 37.0 | 25.5 | 32.9 | 1.2 | 96.6 |
| 4 | 4Pt/10Li/MgAl2O4 | 24.4 | 42.4 | 21.6 | 31.0 | 1.0 | 95.9 |
| 5 | 4Pt/10Na/MgAl2O4 | 11.8 | 22.8 | 15.7 | 57.0 | 0.9 | 96.2 |
| 6 | 4Pt/10K/MgAl2O4 | 0 | - | - | - | - | - |
| 7 | 4Pt/10Cs/MgAl2O4 | 0 | - | - | - | - | - |
| 8 | 4Pt/2Li/MgAl2O4 | 54.4 | 36.6 | 20.5 | 41.4 | 0.6 | 99.2 |
| 9 | 4Pt/6Li/MgAl2O4 | 42.1 | 43.0 | 20.4 | 35.9 | 0.4 | 99.7 |
| 10 | 4Pt/MgAl2O4 | 18.7 | 22.0 | 17.2 | 48.5 | 2.2 | 89.9 |
| 11 | 8Pt/10Li/MgAl2O4 | 83.9 | 40.8 | 20.1 | 34.4 | 0.4 | 95.8 |
| 12 | 8Pt/MgAl2O4 | 100 | 11.3 | 24.4 | 50.0 | 5.4 | 91.2 |
| 13 | 4Pt/MgAl2O4+LiOH[e] | 22.8 | 15.1 | 21.4 | 43.3 | 4.0 | 83.7 |
| Entry | Catalyst | Dispersion/% | Pt diameter/nm |
|---|---|---|---|
| 1 | 1Pt/MgAl2O4 | 49.1 | 2.0 |
| 2 | 2Pt/MgAl2O4 | 35.3 | 2.8 |
| 3 | 4Pt/MgAl2O4 | 22.3 | 4.5 |
| 4 | 4Pt/MgAl2O4-Used | 18.1 | 5.5 |
| 5 | 4Pt/10Li/MgAl2O4-Fresh | 39.6 | 2.5 |
| 6 | 4Pt/10Li/MgAl2O4-Used | 31.0 | 3.2 |
| Catalyst | Pt07/2 | P2+7/2 | P4+7/2 | |||
|---|---|---|---|---|---|---|
| B.E./eV | Ratio/% | B.E./eV | Ratio/% | B.E./eV | Ratio% | |
| 4Pt/MgAl2O4 | 71.1 | 70.7 | 72.9 | 29.3 | - | - |
| 4Pt/10Li/ MgAl2O4-Fresh | 71.4 | 49.1 | 72.8 | 66.4 | 74.9 | 17.5 |
| 4Pt/ 10Li/MgAl2O4-Used | 71.2 | 71.2 | 73.1 | 29.5 | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Pang, J.; Zhang, J.; Li, X.; Jiang, Y.; Su, Y.; Li, W.; Zheng, M. Tuning the Reaction Selectivity over MgAl Spinel-Supported Pt Catalyst in Furfuryl Alcohol Conversion to Pentanediols. Catalysts 2021, 11, 415. https://doi.org/10.3390/catal11040415
Li X, Pang J, Zhang J, Li X, Jiang Y, Su Y, Li W, Zheng M. Tuning the Reaction Selectivity over MgAl Spinel-Supported Pt Catalyst in Furfuryl Alcohol Conversion to Pentanediols. Catalysts. 2021; 11(4):415. https://doi.org/10.3390/catal11040415
Chicago/Turabian StyleLi, Xinsheng, Jifeng Pang, Jingcai Zhang, Xianquan Li, Yu Jiang, Yang Su, Weizhen Li, and Mingyuan Zheng. 2021. "Tuning the Reaction Selectivity over MgAl Spinel-Supported Pt Catalyst in Furfuryl Alcohol Conversion to Pentanediols" Catalysts 11, no. 4: 415. https://doi.org/10.3390/catal11040415
APA StyleLi, X., Pang, J., Zhang, J., Li, X., Jiang, Y., Su, Y., Li, W., & Zheng, M. (2021). Tuning the Reaction Selectivity over MgAl Spinel-Supported Pt Catalyst in Furfuryl Alcohol Conversion to Pentanediols. Catalysts, 11(4), 415. https://doi.org/10.3390/catal11040415








