Natural Rocks–Heterogeneous Catalysts for Oil Transesterification in Biodiesel Synthesis
Abstract
1. Introduction
2. Results and Discussions
2.1. Use of Various Rocks as Catalysts
2.2. Selection of Optimal Conditions for Dolomite Preparation
2.2.1. Fraction Selection
2.2.2. Selection of Heating Temperature
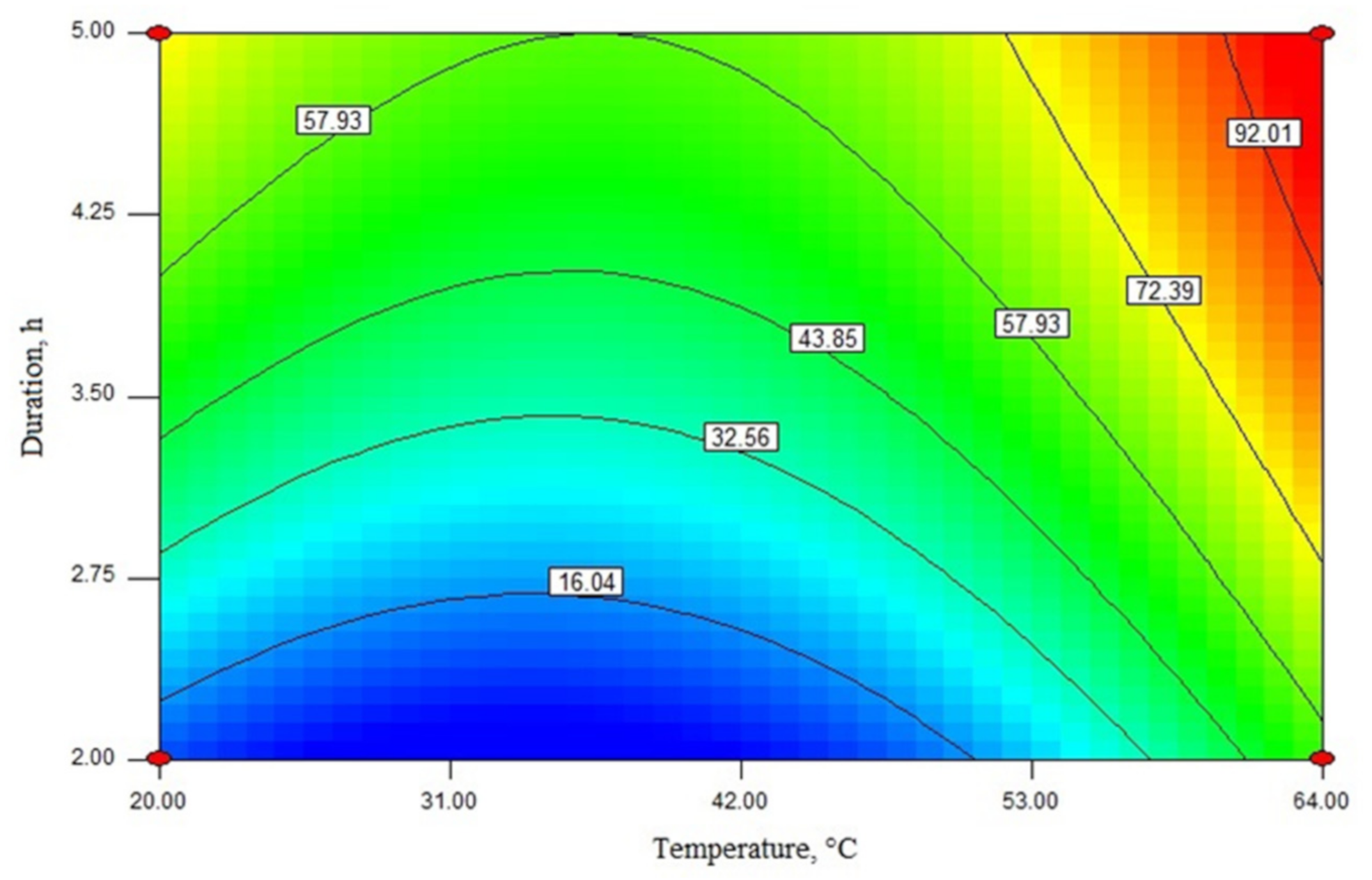
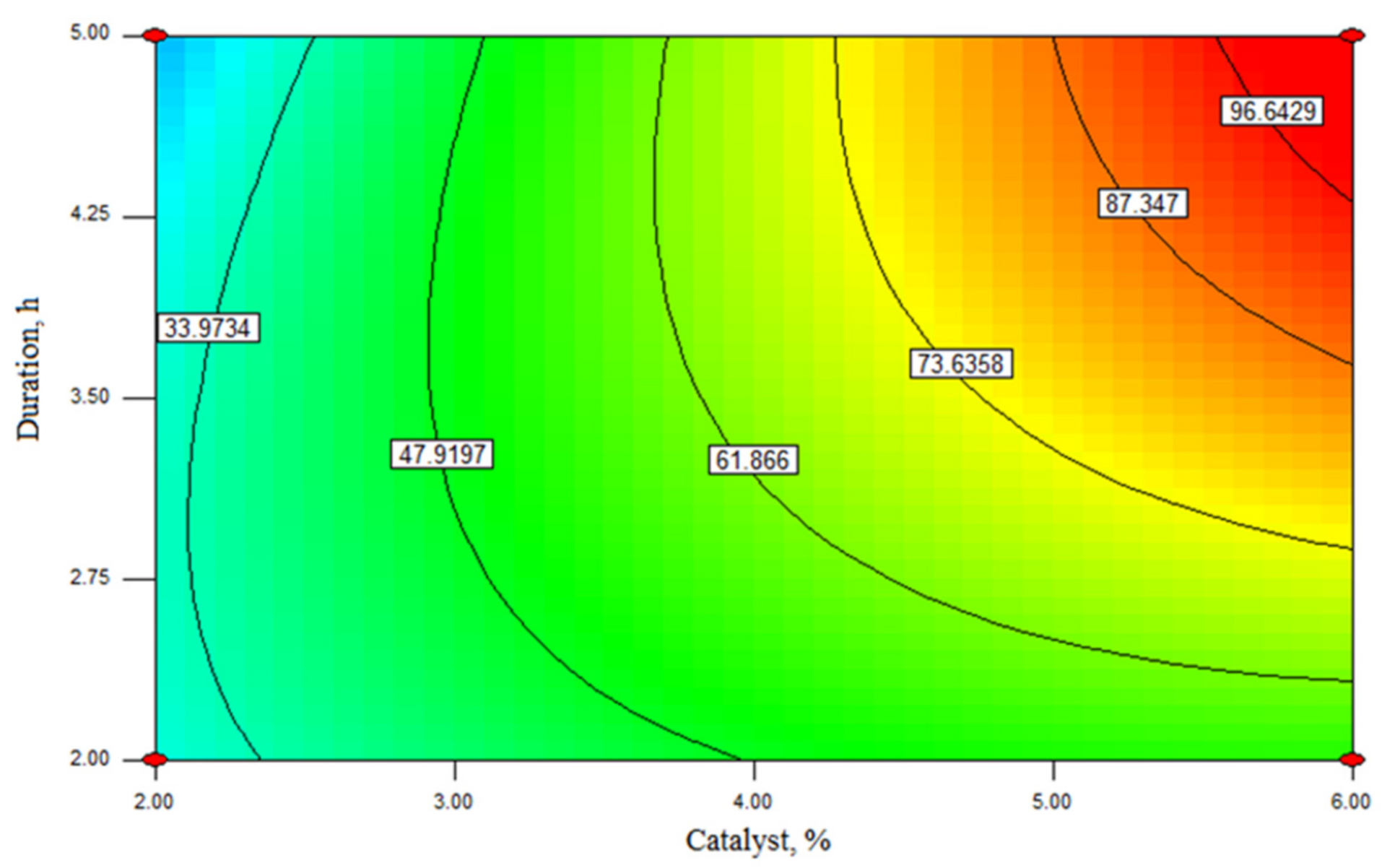
2.3. Modeling and Determination of Optimal Reaction Conditions Using Response Surface Methodology
- EY is the ester yield (%);
- A is the methanol-to-oil molar ratio;
- B is the temperature (°C);
- C is the catalyst amount (%); and
- D is the process duration (h).
2.4. Optimization of Rapeseed Oil Transesterification
3. Materials and Methods
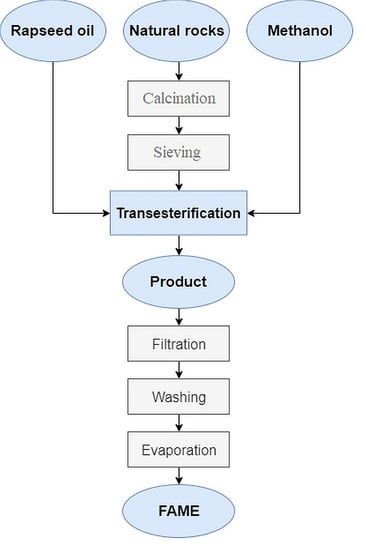
3.1. Preparation of Catalysts
3.2. Determination of CaO and MgO in Catalysts
- V1 is the amount of trilon B used for calcium titration, mL;
- V2 is the amount of trilon B used for the titration of calcium and magnesium, mL;
- m—mass of the sample, g; and
- K—trilon B correction factor.
3.3. Transesterification of Rapeseed Oil
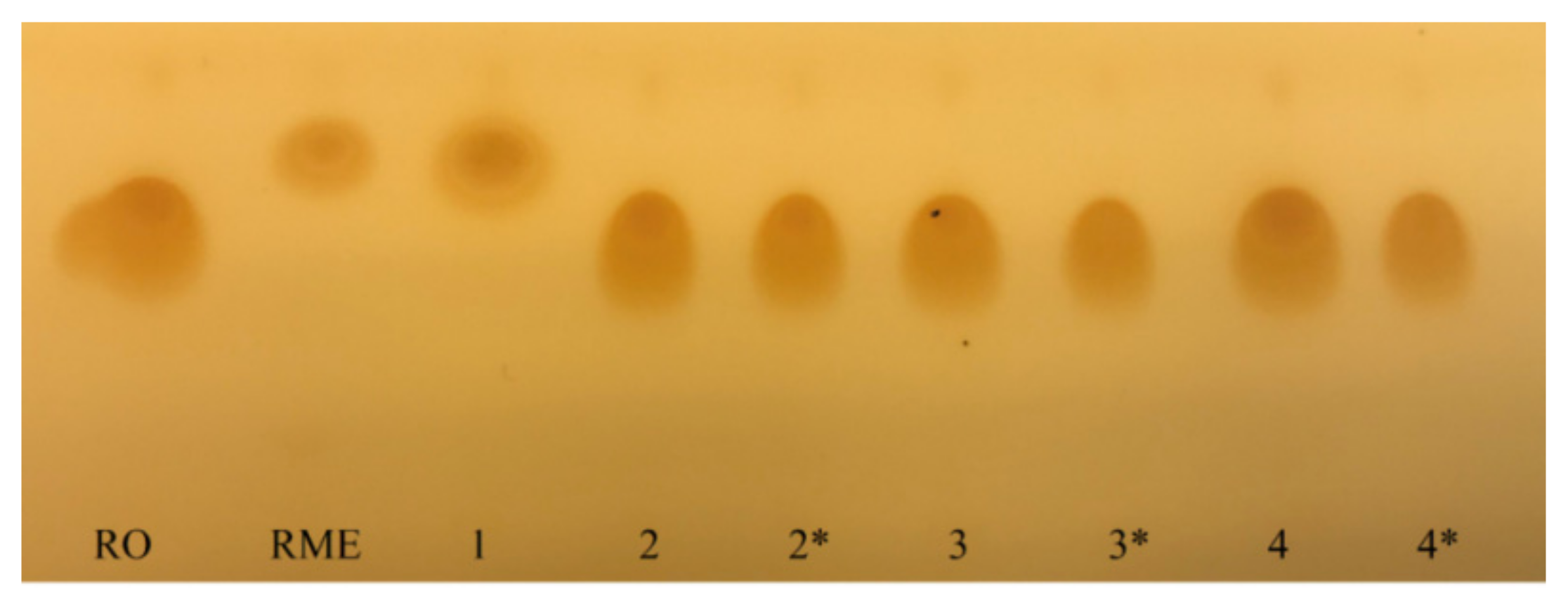
3.4. Thin Layer Chromatography
3.5. Gas Chromatorgraphy
- C—ester content, %;
- ∑A—is the total peak area from methyl ester in C6:0 to that in C24:1;
- AEI—is the peak area corresponding to nonadecanoic acid methyl ester;
- WEI—is the weight (mg) of the nonadecanoic acid methyl ester being used as internal standard; and
- W—is the weight (mg) of the sample.
3.6. Response Surface Methodology
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Lourinho, G.; Brito, P. Advanced biodiesel production technologies: Novel developments. Rev. Environ. Scie. Biotechnol. 2015, 14, 287–316. [Google Scholar] [CrossRef]
- Ismail, S.; Ahmed, A.S.; Reddy, A.; Hamdan, S. Biodiesel production from castor oil by using calcium oxide derived from mud clam shell. J. Renew. Energy 2016. [Google Scholar] [CrossRef]
- Makareviciene, V.; Sendzikiene, E.; Gaide, I. Application of heterogeneous catalysis to biodiesel synthesis using microalgae oil. Front. Environ. Sci. Eng. 2021, 15, 1–21. [Google Scholar] [CrossRef]
- Ilgen, O. Dolomite as a heterogeneous catalyst for transesterification of canola oil. Fuel Process. Technol. 2011, 92, 452–455. [Google Scholar] [CrossRef]
- Zarei, A.; Amin, N.A.S.; Talebian-Kiakalaieh, A.; Zain, N.A.M. Immobilized lipase-catalyzed transesterification of Jatropha curcas oil: Optimization and modeling. J. Taiwan Inst. Chem. Eng. 2014, 45, 444–451. [Google Scholar] [CrossRef]
- Ngamcharussrivichai, C.; Wiwatnimit, W.; Wangnoi, S. Modified dolomites as catalysts for palm kernel oil transesterification. J. Mol. Catal. A Chem. 2007, 276, 24–33. [Google Scholar] [CrossRef]
- Wang, J.; Wang, Z.; Yang, L.; Yang, G.; Miao, C.; Lv, P. Natural albite as a novel solid basic catalyst for the effective synthesis of biodiesel: Characteristics and performance. Energy 2017, 141, 1650–1660. [Google Scholar] [CrossRef]
- Kusuma, R.I.; Hadinoto, J.P.; Ayucitra, A.; Soetaredjo, F.E.; Ismadji, S. Natural zeolite from Pacitan Indonesia, as catalyst support for transesterification of palm oil. Appl. Clay. Sci. 2013, 74, 121–126. [Google Scholar] [CrossRef]
- Syazwani, O.N.; Teo, S.H.; Aminul, I.; Taufiq-Yap, Y.H. Transesterification activity and characterization of natural CaO derived from waste venus clam (Tapes belcheri S.) material for enhancement of biodiesel production. Process Saf. Environ. 2017, 105, 303–315. [Google Scholar] [CrossRef]
- Girish, N.; Niju, S.P.; Begum, M.S.K.M.; Anantharaman, N. Utilization of a cost effective solid catalyst derived from natural white bivalve clam shell for transesterification of waste frying oil. Fuel 2013, 111, 653–658. [Google Scholar] [CrossRef]
- Pandit, P.R.; Fulekar, M.H. Biodiesel production from microalgal biomass using CaO catalyst synthesized from natural waste material. Renew. Energy 2019, 136, 837–845. [Google Scholar] [CrossRef]
- Niu, S.L.; Huo, M.J.; Lu, C.M.; Liu, M.Q.; Li, H. An investigation on the catalytic capacity of dolomite in transesterification and the calculation of kinetic parameters. Bioresour. Technol. 2014, 158, 74–80. [Google Scholar] [CrossRef] [PubMed]
- Sendzikiene, E.; Makareviciene, V.; Kazancev, K. Application of dolomite as a heterogeneous catalyst of biodiesel synthesis. Transport 2018, 33, 1155–1161. [Google Scholar] [CrossRef]
- Korkut, I.; Bayramoglu, M. Ultrasound assisted biodiesel production in presence of dolomite catalyst. Fuel 2016, 180, 624–629. [Google Scholar] [CrossRef]
- Correia, L.M.; Campelo, N.S.; Novaes, D.S.; Cavalcante, C.L., Jr.; Cecilia, J.A.; Rodríguez-Castellón, E.; Vieira, R.S. Characterization and application of dolomite as catalytic precursor for canola and sunflower oils for biodiesel production. Chem. Eng. J. 2015, 269, 35–43. [Google Scholar] [CrossRef]
- Zhao, S.; Niu, S.; Yu, H.; Ning, Y.; Zhang, X.; Li, H.; Zhang, Y.; Lu, C.; Han, K. Experimental investigation on biodiesel production through transesterification promoted by the La-dolomite catalyst. Fuel 2019, 257, 116092. [Google Scholar] [CrossRef]
- Choudhury, H.A.; Chakma, S.; Moholkar, V.S. Mechanistic insight into sonochemical biodiesel synthesis using heterogeneous base catalyst. Ultrason. Sonochem. 2014, 21, 169–181. [Google Scholar] [CrossRef]
- Niu, S.; Zhang, X.; Ning, Y.; Zhang, Y.; Qu, T.; Hu, X.; Gong, Z.; Lu, C. Dolomite incorporated with cerium to enhance the stability in catalyzing transesterification for biodiesel production. Renew. Energy 2020, 154, 107–116. [Google Scholar] [CrossRef]
- Niu, S.L.; Yu, H.W.; Ning, Y.L.; Tang, X.C.; Zhang, X.Y.; Zhao, S.; Han, K.H.; Lu, C.M. Synthesis of 4-aminobenzenesulfonic acid functionalized carbon catalyst through diazonium salt reduction for biodiesel production. Energy Convers. Manag. 2018, 173, 753–762. [Google Scholar] [CrossRef]
- Ngamcharussrivichai, C.; Nunthasanti, P.; Tanachai, S.; Bunyakiat, K. Biodiesel production through transesterification over natural calciums. Fuel Process. Technol. 2010, 91, 1409–1415. [Google Scholar] [CrossRef]
- Wilson, K.; Hardacre, C.; Lee, A.F.; Montero, J.M.; Shellard, L. The application of calcined natural dolomitic rock as a solid base catalyst in triglyceride transesterification for biodiesel synthesis. Green Chem. 2008, 10, 654–659. [Google Scholar] [CrossRef]
- Murguía-Ortizm, D.; Cordova, I.; Manriquez, M.E.; Ortiz-Islas, E.; Cabrera-Sierra, R.; Contreras, J.L.; Alcántar-Vázquez, B.; Trejo-Rubio, M.; Vázquez-Rodríguez, J.T.; Castro, L.V. Na-CaO/MgO dolomites used as heterogeneous catalysts in canola oil transesterification for biodiesel production. Mater. Lett. 2021, 291, 129587. [Google Scholar]
- Nur, Z.A.S.; Taufiq-Yap, Y.H.; Nizah, M.F.R.; Teo, S.H.; Syazwani, O.N.; Islam, A. Production of biodiesel from palm oil using modified Malaysian natural dolomites. Energy Convers. Manag. 2014, 78, 738–744. [Google Scholar]
- Bailer, J.; Hödl, P.; De Hueber, K.; Mitelbach, M.; Plank, C.; Schindlbauer, H. Handbook of Analytical Methods for Fatty Acid Methyl Esters Used as Biodiesel Fuel Substitutes; Fichte, Ed.; Research Institute for Chemistry and Technology of Petroleum Products, University of Technology: Vienna, Austria, 1994; pp. 36–38. [Google Scholar]


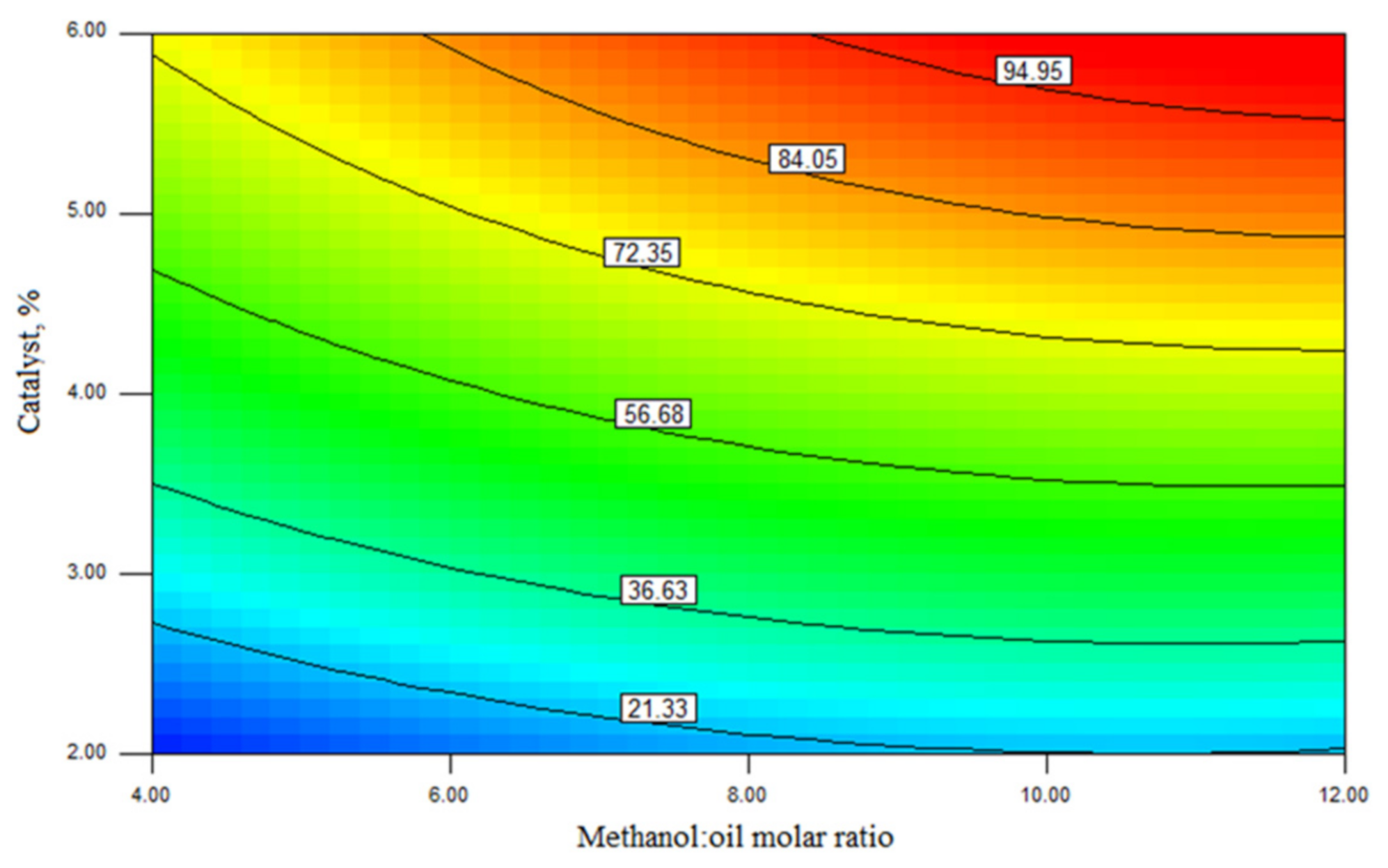
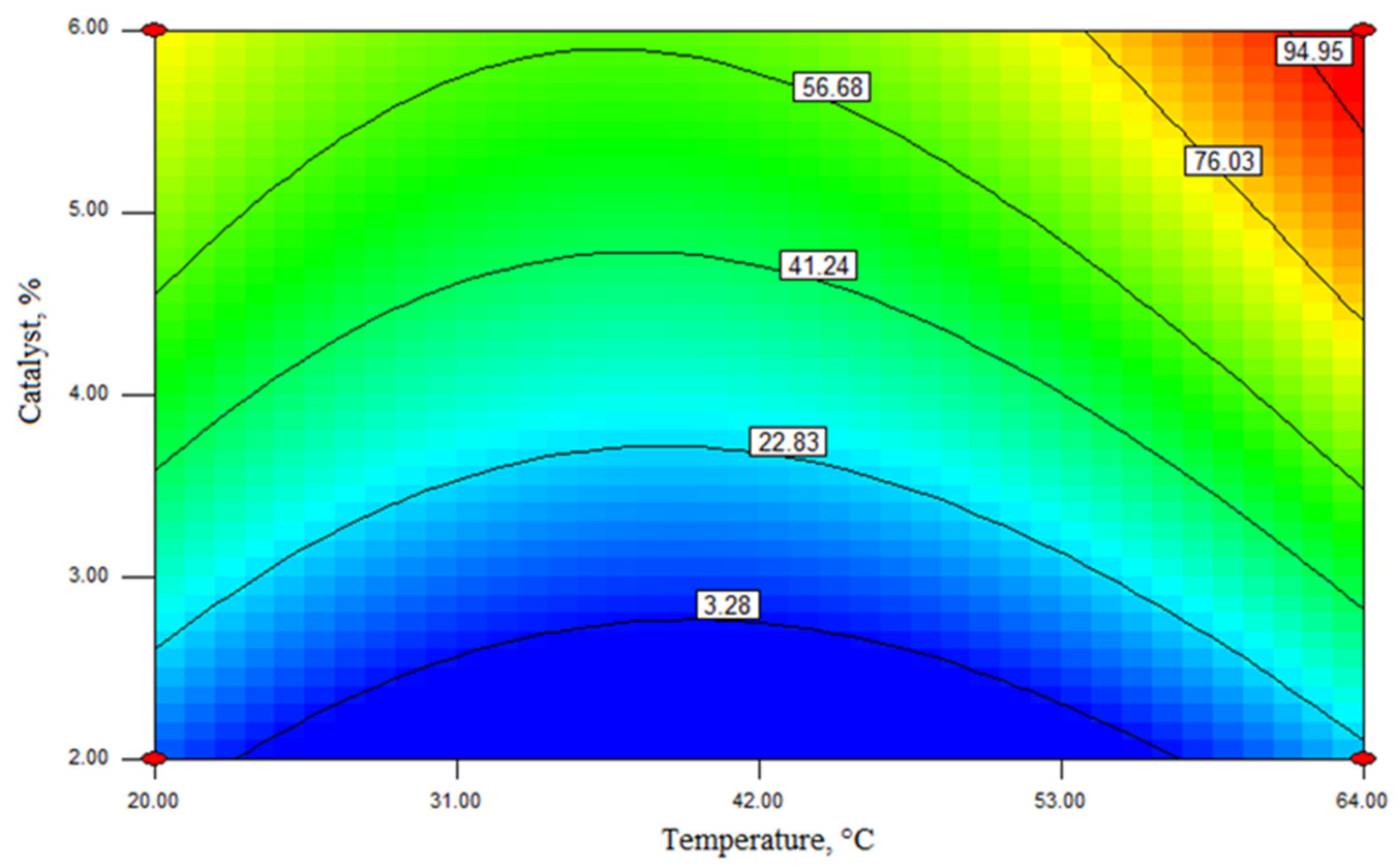
| Independent Variable | Predicted Ester Yield, wt% | Experimental Ester Yield, wt% | ||||
|---|---|---|---|---|---|---|
| A: Methanol to Oil Molar Ratio (mol/mol) | B: Temperature, °C | C: Catalyst Account, wt% | D: Duration, h | |||
| 1 | 4.00 | 20.00 | 2.00 | 2.00 | 1.52 | 1.60 ± 0.32 |
| 2 | 12.00 | 20.00 | 2.00 | 2.00 | 11.20 | 8.78 ± 0.52 |
| 3 | 4.00 | 64.00 | 2.00 | 2.00 | 12.77 | 11.10 ± 0.55 |
| 4 | 12.00 | 64.00 | 2.00 | 2.00 | 28.86 | 17.76 ± 0.45 |
| 5 | 4.00 | 20.00 | 6.00 | 2.00 | 2.32 | 3.50 ± 0.33 |
| 6 | 12.00 | 20.00 | 6.00 | 2.00 | 9.12 | 3.09 ± 0.23 |
| 7 | 4.00 | 64.00 | 6.00 | 2.00 | 28.69 | 18.20 ± 0.66 |
| 8 | 12.00 | 64.00 | 6.00 | 2.00 | 44.77 | 62.29 ± 1.02 |
| 9 | 4.00 | 20.00 | 2.00 | 5.00 | 3.25 | 6.26 ± 0.32 |
| 10 | 12.00 | 20.00 | 2.00 | 5.00 | 15.68 | 11.17 ± 0.46 |
| 11 | 4.00 | 64.00 | 2.00 | 5.00 | 5.30 | 3.33 ± 0.14 |
| 12 | 12.00 | 64.00 | 2.00 | 5.00 | 21.38 | 18.10 ± 0.69 |
| 13 | 4.00 | 20.00 | 6.00 | 5.00 | 58.27 | 65.05 ± 0.85 |
| 14 | 12.00 | 20.00 | 6.00 | 5.00 | 74.35 | 84.53 ± 1.06 |
| 15 | 4.00 | 64.00 | 6.00 | 5.00 | 81.97 | 76.48 ± 0.96 |
| 16 | 12.00 | 64.00 | 6.00 | 5.00 | 98.05 | 98.66 ± 0.65 |
| 17 | 3.20 | 42.00 | 4.00 | 3.50 | 1.92 | 5.20 ± 0.34 |
| 18 | 12.80 | 42.00 | 4.00 | 3.50 | 21.22 | 20.20 ± 0.23 |
| 19 | 8.00 | 20.00 | 4.00 | 3.50 | 36.83 | 19.21 ± 0.14 |
| 20 | 8.00 | 64.00 | 4.00 | 3.50 | 57.51 | 82.39 ± 0.56 |
| 21 | 8.00 | 42.00 | 1.60 | 3.50 | 2.53 | 2.34 ± 0.14 |
| 22 | 8.00 | 42.00 | 6.40 | 3.50 | 33.31 | 21.80 ± 0.47 |
| 23 | 8.00 | 42.00 | 4.00 | 1.70 | 2.12 | 3.55 ± 0.16 |
| 24 | 8.00 | 42.00 | 4.00 | 5.30 | 28.22 | 20.51 ± 0.34 |
| 25 | 8.00 | 42.00 | 4.00 | 3.50 | 19.43 | 17.10 ± 0.25 |
| 26 | 8.00 | 42.00 | 4.00 | 3.50 | 19.43 | 18.20 ± 0.26 |
| 27 | 8.00 | 42.00 | 4.00 | 3.50 | 19.43 | 16.20 ± 0.19 |
| Source | Sum of Squares | df | Mean Square | F Value | p-Value Prob > F | |
|---|---|---|---|---|---|---|
| Model | 20,598.31 | 14 | 1471.31 | 16.62 | <0.0001 | Significant |
| A-methanol/oil molar ratio | 1144.58 | 1 | 1144.58 | 12.93 | 0.0037 | - |
| B-temperature | 2115.53 | 1 | 2115.53 | 23.89 | 0.0004 | - |
| C-catalyst | 6985.52 | 1 | 6985.52 | 78.90 | <0.0001 | - |
| D-duration | 3001.47 | 1 | 3001.47 | 33.90 | <0.0001 | - |
| AB | 130.25 | 1 | 130.25 | 1.47 | 0.2485 | - |
| AC | 83.22 | 1 | 83.22 | 0.94 | 0.3514 | - |
| AD | 1.66 | 1 | 1.66 | 0.019 | 0.8934 | - |
| BC | 402.91 | 1 | 402.91 | 4.55 | 0.0542 | - |
| BD | 290.11 | 1 | 290.11 | 3.28 | 0.0954 | - |
| CD | 3168.28 | 1 | 3168.28 | 35.78 | <0.0001 | - |
| A2 | 361.68 | 1 | 361.68 | 4.08 | 0.0662 | - |
| B2 | 3168.20 | 1 | 3168.20 | 35.78 | <0.0001 | - |
| C2 | 145.28 | 1 | 145.28 | 1.64 | 0.2244 | - |
| D2 | 224.49 | 1 | 224.49 | 2.54 | 0.1373 | - |
| Residual | 1062.45 | 12 | 88.54 | - | - | - |
| Lack of Fit | 1060.45 | 10 | 46.04 | 3.69 | 0.1194 | Not significant |
| Pure Error | 2.01 | 2 | 1.00 | - | - | - |
| Cor Total | 21,660.76 | 26 | - | - | - | - |
| Variable | Value | Variable | Value |
|---|---|---|---|
| Std. Dev. | 9.41 | R-Squared | 0.9510 |
| Mean | 27.84 | Adj R-Squared | 0.8937 |
| C.V.% | 33.80 | Pred R-Squared | 0.7629 |
| PRESS | 5135.38 | Adeq Precision | 15.981 |
| Methanol-to-Oil Molar Ratio, mol/mol | Reaction Temperature, °C | Concentration of Catalyst, % (From Oil Mass) | Duration of Reaction, h | Predicted Ester Yield, wt% | Experimental Ester Yield, wt% |
|---|---|---|---|---|---|
| 11.94 | 64.0 | 6.0 | 5.0 | 98.66 | 98.89 ± 0.42 |
| Oil | Dolomite | Methanol-to-Oil Molar Ratio | Catalyst Ammount, % | Temperature, °C | Duration, h | Yield, % | References |
|---|---|---|---|---|---|---|---|
| Calcined dolomites | |||||||
| Palm kernel | Calcined at 800 °C | 30:1 | 6 | 60 | 3 | 98 | [20] |
| Canola | Calcined at 850 °C | 6:1 | 1 | 60 | 4 | 98.81 | [15] |
| Canola | Calcined at 850 °C | 6:1 | 3 | 67.5 | 3 | 91.78 | [4] |
| Canola | Calcined at 840 °C, ultrasound assisted | 9:1 | 5 | 60 | 1.5 | 97.4 | [14] |
| Olive | Calcined at 900 °C | 30:1 | 15.6 | 60 | 3 | >98 | [21] |
| Sunflower | Calcined at 850 °C | 9:1 | 2 | 60 | 4 | 96.52 | [15] |
| Rapeseed | Calcined at 850 °C | 11.94 | 6 | 64 | 5 | 98.66 | Our study |
| Calcined and modified dolomites | |||||||
| Canola | Na-CaO/MgO dolomites | 12:1 | 6 | 65 | 7 | 97.6 | [22] |
| Palm | La-dolomite | 18:1 | 7 | 65 | 3 | 98.7 | [16] |
| Palm | Dolomite incorporated with cerium | 15:1 | 0.05 | 65 | 2 | 97.21 | [18] |
| Palm kernel | Modified dolomite | 15:1 | 10 | 60 | 3 | 99.9 | [6] |
| Palm oil | SnO2 doped dolomite | 15:1 | 1 | 65 | 4 | 99.98 | [23] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gaide, I.; Makareviciene, V.; Sendzikiene, E.; Kazancev, K. Natural Rocks–Heterogeneous Catalysts for Oil Transesterification in Biodiesel Synthesis. Catalysts 2021, 11, 384. https://doi.org/10.3390/catal11030384
Gaide I, Makareviciene V, Sendzikiene E, Kazancev K. Natural Rocks–Heterogeneous Catalysts for Oil Transesterification in Biodiesel Synthesis. Catalysts. 2021; 11(3):384. https://doi.org/10.3390/catal11030384
Chicago/Turabian StyleGaide, Ieva, Violeta Makareviciene, Egle Sendzikiene, and Kiril Kazancev. 2021. "Natural Rocks–Heterogeneous Catalysts for Oil Transesterification in Biodiesel Synthesis" Catalysts 11, no. 3: 384. https://doi.org/10.3390/catal11030384
APA StyleGaide, I., Makareviciene, V., Sendzikiene, E., & Kazancev, K. (2021). Natural Rocks–Heterogeneous Catalysts for Oil Transesterification in Biodiesel Synthesis. Catalysts, 11(3), 384. https://doi.org/10.3390/catal11030384