Study of the Direct CO2 Carboxylation Reaction on Supported Metal Nanoparticles
Abstract
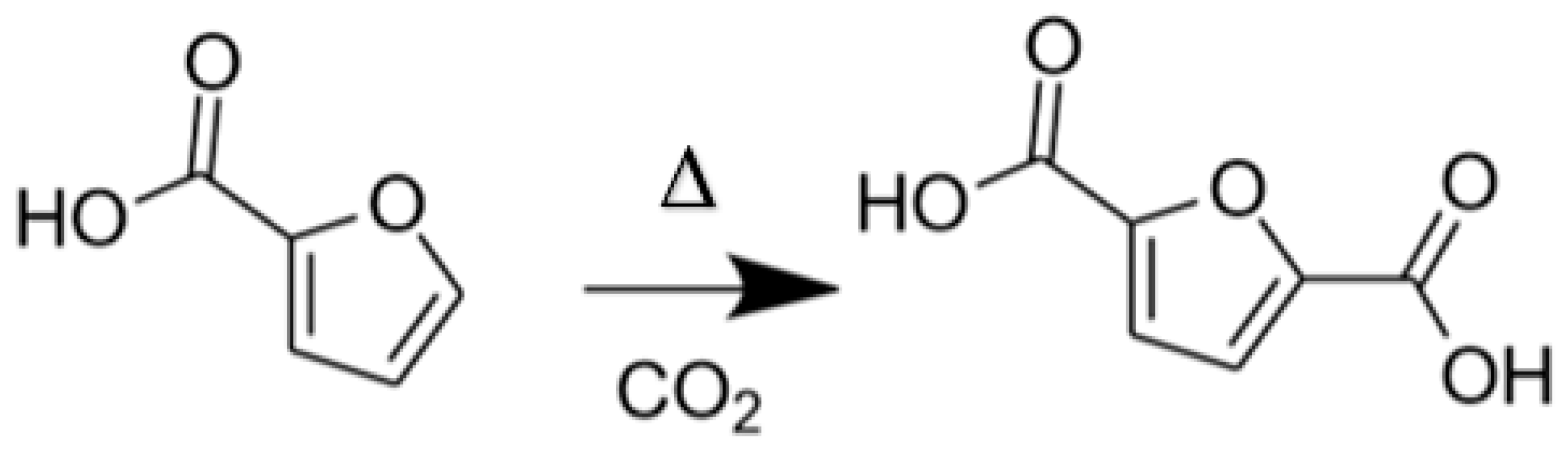
:1. Introduction
2. Results
2.1. Validation of the Reaction Setup
2.2. Dual Catalytic System (Ag/SiO2 + CdI2)
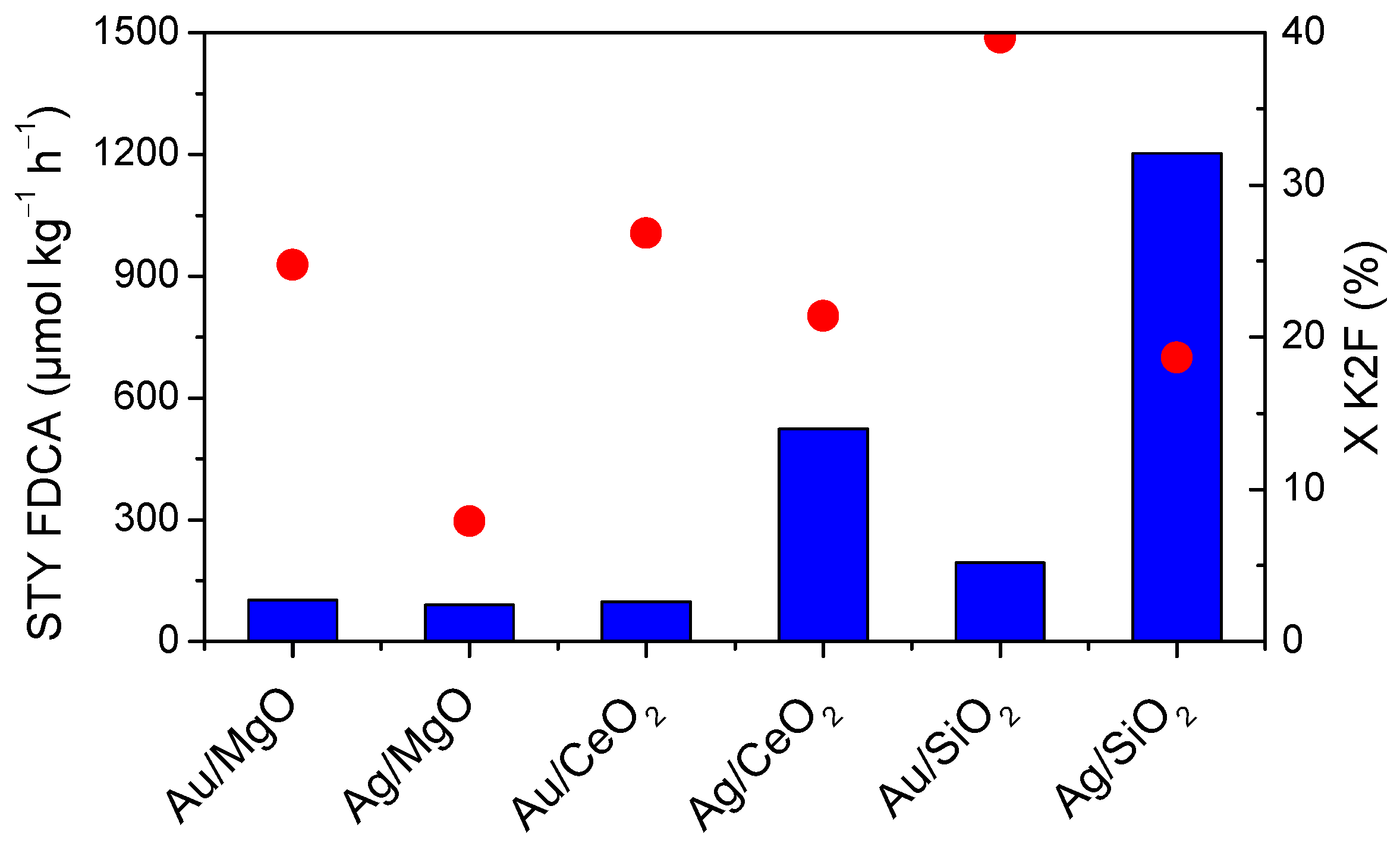
2.3. Effect of the Support
2.4. Effect of the Metal
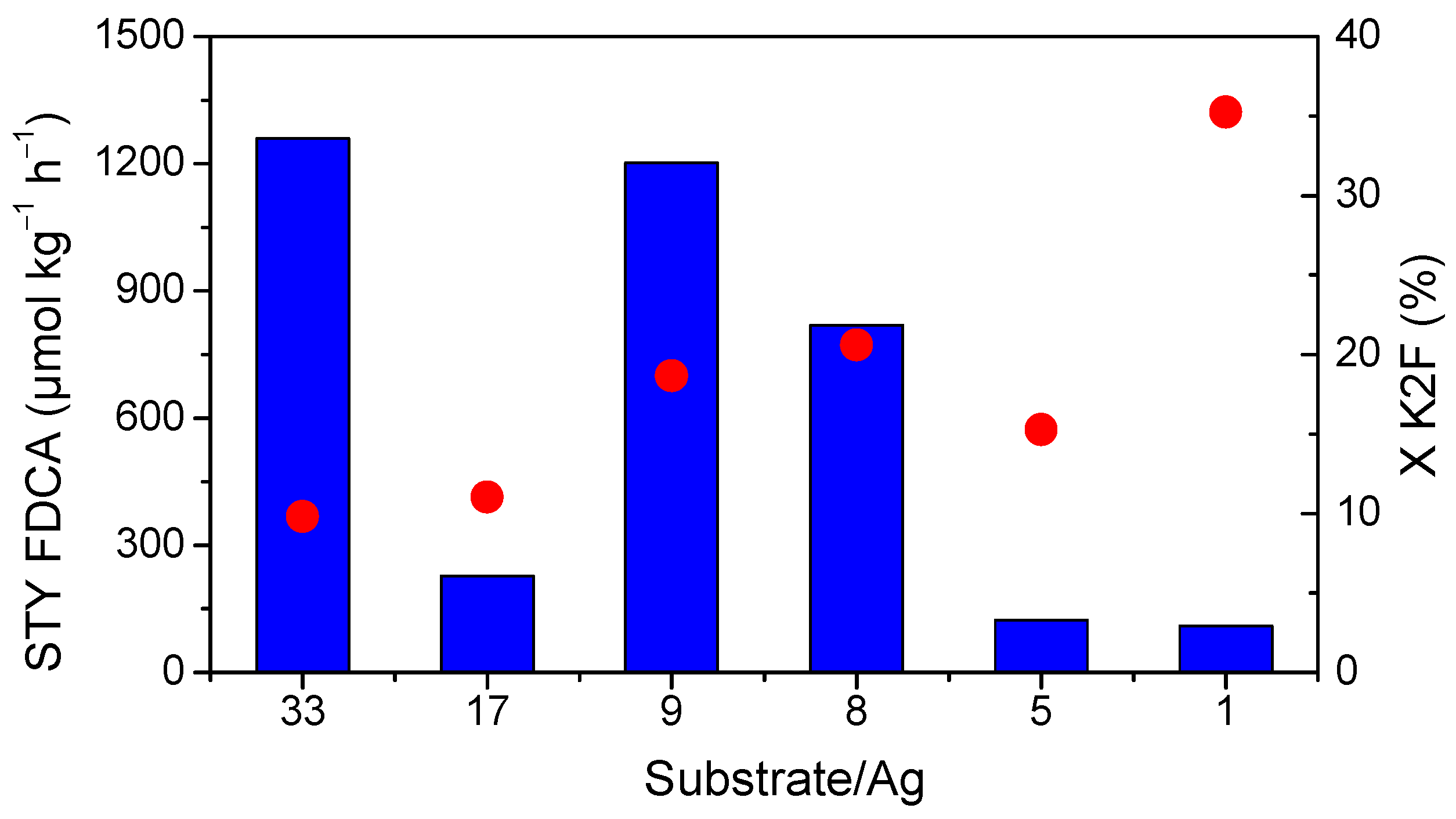
2.5. Effect of the Substrate/Metal Molar Ratio
2.6. Effect of Reaction Temperature
3. Materials and Methods
3.1. Catalysts Preparation
3.2. Catalytic Test
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Corma, A.; Iborra, S.; Velty, A. Chemical Routes for the Transformation of Biomass into Chemicals. Chem. Rev. 2007, 107, 2411–2502. [Google Scholar] [CrossRef]
- Zhou, H.; Xu, H.; Wang, X.; Liu, Y. Convergent Production of 2,5-Furandicarboxylic Acid from Biomass and CO2. Green Chem. 2019, 21, 2923–2927. [Google Scholar] [CrossRef]
- De Jong, E.; Dam, M.A.; Sipos, L.; Gruter, G.-J.M. Furandicarboxylic Acid (FDCA), A Versatile Building Block for a Very Interesting Class of Polyesters. In Biobased Monomers, Polymers, and Materials; Smith, P.B., Gross, R.A., Eds.; American Chemical Society: Washington, DC, USA, 2012; Volume 1105, pp. 1–13. ISBN 978-0-8412-2767-5. [Google Scholar]
- Casanova, O.; Iborra, S.; Corma, A. Mécanisme: Biomass into Chemicals: One Pot-Base Free Oxidative Esterification of 5-Hydroxymethyl-2-Furfural into 2,5-Dimethylfuroate with Gold on Nanoparticulated Ceria. J. Catal. 2009, 265, 109–116. [Google Scholar] [CrossRef]
- Teong, S.P.; Yi, G.; Zhang, Y. Hydroxymethylfurfural Production from Bioresources: Past, Present and Future. Green Chem. 2014, 16, 2015. [Google Scholar] [CrossRef]
- Ferraz, C.P.; Silva, A.G.M.D.; Rodrigues, T.S.; Camargo, P.H.C.; Paul, S.; Wojcieszak, R. Furfural Oxidation on Gold Supported on MnO2: Influence of the Support Structure on the Catalytic Performances. Appl. Sci. 2018, 8, 1246. [Google Scholar] [CrossRef] [Green Version]
- Cavani, F.; Teles, J.H. Sustainability in Catalytic Oxidation: An Alternative Approach or a Structural Evolution? ChemSusChem 2009, 2, 508–534. [Google Scholar] [CrossRef]
- Wojcieszak, R.; Itabaiana, I. Engineering the Future: Perspectives in the 2,5-Furandicarboxylic Acid Synthesis. Catal. Today 2019, 354, 211–217. [Google Scholar] [CrossRef]
- Mabee, W.E.; Gregg, D.J.; Saddler, J.N. Assessing the Emerging Biorefinery Sector in Canada. Appl. Biochem. Biotechnol. 2005, 123, 765–778. [Google Scholar] [CrossRef]
- Papageorgiou, G.Z.; Tsanaktsis, V.; Bikiaris, D.N. Synthesis of Poly(Ethylene Furandicarboxylate) Polyester Using Monomers Derived from Renewable Resources: Thermal Behavior Comparison with PET and PEN. Phys. Chem. Chem. Phys. 2014, 16, 7946–7958. [Google Scholar] [CrossRef]
- Van Putten, R.-J.; van der Waal, J.C.; de Jong, E.; Rasrendra, C.B.; Heeres, H.J.; de Vries, J.G. Hydroxymethylfurfural, A Versatile Platform Chemical Made from Renewable Resources. Chem. Rev. 2013, 113, 1499–1597. [Google Scholar] [CrossRef]
- Albonetti, S.; Lolli, A.; Morandi, V.; Migliori, A.; Lucarelli, C.; Cavani, F. Conversion of 5-Hydroxymethylfurfural to 2,5-Furandicarboxylic Acid over Au-Based Catalysts: Optimization of Active Phase and Metal–Support Interaction. Appl. Catal. B Environ. 2015, 163, 520–530. [Google Scholar] [CrossRef]
- Davis, S.E.; Houk, L.R.; Tamargo, E.C.; Datye, A.K.; Davis, R.J. Oxidation of 5-Hydroxymethylfurfural over Supported Pt, Pd and Au Catalysts. Catal. Today 2011, 160, 55–60. [Google Scholar] [CrossRef]
- Miao, Z.; Zhang, Y.; Pan, X.; Wu, T.; Zhang, B.; Li, J.; Yi, T.; Zhang, Z.; Yang, X. Superior Catalytic Performance of Ce1−x Bix O2−δ Solid Solution and Au/Ce1−x Bix O2−δ for 5-Hydroxymethylfurfural Conversion in Alkaline Aqueous Solution. Catal. Sci. Technol. 2015, 5, 1314–1322. [Google Scholar] [CrossRef]
- Tian, Q.; Shi, D.; Sha, Y. CuO and Ag2O/CuO Catalyzed Oxidation of Aldehydes to the Corresponding Carboxylic Acids by Molecular Oxygen. Molecules 2008, 13, 948–957. [Google Scholar] [CrossRef] [PubMed]
- Hurd, C.D.; Garrett, J.W.; Osborne, E.N. Furan Reactions. IV. Furoic Acid from Furfural. J. Am. Chem. Soc. 1933, 55, 1082–1084. [Google Scholar] [CrossRef]
- Taarning, E.; Nielsen, I.S.; Egeblad, K.; Madsen, R.; Christensen, C.H. Chemicals from Renewables: Aerobic Oxidation of Furfural and Hydroxymethylfurfural over Gold Catalysts. ChemSusChem 2008, 1, 75–78. [Google Scholar] [CrossRef]
- Santarelli, F.; Wojcieszak, R.; Paul, S.; Dumeignil, F.; Cavani, F. Furoic Acid Preparation Method. Patent W02017158106A1, 21 September 2017. [Google Scholar]
- Payne, K.A.P.; Marshall, S.A. Enzymatic Carboxylation of 2-Furoic Acid Yields 2,5-Furandicarboxylic Acid (FDCA). ACS Catal. 2019, 9, 2854–2865. [Google Scholar] [CrossRef]
- Shen, G.; Zhang, S.; Lei, Y.; Chen, Z.; Yin, G. Synthesis of 2,5-Furandicarboxylic Acid by Catalytic Carbonylation of Renewable Furfural Derived 5-Bromofuroic Acid. Mol. Catal. 2018, 455, 204–209. [Google Scholar] [CrossRef]
- Zhang, S.; Lan, J.; Chen, Z.; Yin, G.; Li, G. Catalytic Synthesis of 2,5-Furandicarboxylic Acid from Furoic Acid: Transformation from C5 Platform to C6 Derivatives in Biomass Utilizations. ACS Sustain. Chem. Eng. 2017, 5, 9360–9369. [Google Scholar] [CrossRef]
- Luo, J.; Larrosa, I. C−H Carboxylation of Aromatic Compounds through CO2 Fixation. ChemSusChem 2017, 10, 3317–3332. [Google Scholar] [CrossRef] [Green Version]
- Drault, F.; Snoussi, Y.; Paul, S.; Itabaiana, I.; Wojcieszak, R. Recent Advances in Carboxylation of Furoic Acid into 2,5-Furandicarboxylic Acid: Pathways towards Bio-Based Polymers. ChemSusChem 2020, 13, 5164–5172. [Google Scholar] [CrossRef] [PubMed]
- Dabestani, R.; Britt, P.F.; Buchanan, A.C. Pyrolysis of Aromatic Carboxylic Acid Salts: Does Decarboxylation Play a Role in Cross-Linking Reactions? Energy Fuels 2005, 19, 365–373. [Google Scholar] [CrossRef]
- Lindsey, A.S.; Jeskey, H. The Kolbe-Schmitt Reaction. Chem. Rev. 1957, 57, 583–620. [Google Scholar] [CrossRef]
- Kolbe, H.; Lautemann, E. Constitution of Salicylic Acid and Its Bascity. Liebigs Ann. Chem. 1860, 157–206. [Google Scholar] [CrossRef] [Green Version]
- Schmitt, R. Beitrag Zur Kenntniss Der Kolbe’schen Salicylsäure Synthese. J. Prakt. Chem. 1885, 1, 397–411. [Google Scholar] [CrossRef] [Green Version]
- Olah, G.A.; Török, B.; Joschek, J.P.; Bucsi, I.; Esteves, P.M.; Rasul, G.; Surya Prakash, G.K. Efficient Chemoselective Carboxylation of Aromatics to Arylcarboxylic Acids with a Superelectrophilically Activated Carbon Dioxide−Al2 Cl6/Al System. J. Am. Chem. Soc. 2002, 124, 11379–11391. [Google Scholar] [CrossRef] [PubMed]
- Dalton, D.M.; Rovis, T. C–H Carboxylation Takes Gold. Nat. Chem. 2010, 2, 710–711. [Google Scholar] [CrossRef] [PubMed]
- Wuensch, C.; Glueck, S.M.; Gross, J.; Koszelewski, D.; Schober, M.; Faber, K. Regioselective Enzymatic Carboxylation of Phenols and Hydroxystyrene Derivatives. Org. Lett. 2012, 14, 1974–1977. [Google Scholar] [CrossRef]
- Banerjee, A.; Dick, G.R.; Yoshino, T.; Kanan, M.W. Carbon Dioxide Utilization via Carbonate-Promoted C–H Carboxylation. Nature 2016, 531, 215–219. [Google Scholar] [CrossRef]
- Tanaka, S.; Watanabe, K.; Tanaka, Y.; Hattori, T. EtAlCl 2 /2,6-Disubstituted Pyridine-Mediated Carboxylation of Alkenes with Carbon Dioxide. Org. Lett. 2016, 18, 2576–2579. [Google Scholar] [CrossRef]
- Raecke, B. Synthese von Di- und Tricarbonsäuren aromatischer Ringsysteme durch Verschiebung von Carboxyl-Gruppen. Angew. Chem. 1958, 70, 1–5. [Google Scholar] [CrossRef]
- Raecke, B. A Process for the Production of Terephthalic Acid or Salts thereof or Derivatives thereof of Potassium Benzoate. Patent DE958920C, 28 February 1957. [Google Scholar]
- Wang, Z. Henkel reaction. In Comprehensive Organic Name Reactions and Reagents; John Wiley & Sons: Hoboken, NJ, USA, 2010; pp. 1379–1382. [Google Scholar]
- McNelis, E. Reactions of Aromatic Carboxylates. II. 1 The Henkel Reaction. J. Org. Chem. 1965, 30, 1209–1213. [Google Scholar] [CrossRef]
- Patton, J.W.; Son, M.O. The Synthesis of Naphthalene-2,3-Dicarboxylic Acid by the Henkel Process. J. Org. Chem. 1965, 30, 2869–2870. [Google Scholar] [CrossRef]
- Clayton, T.W.; Britt, P.F.; Buchanan, A.C. Decarboxylation of salts of aromatic carboxylic acids and their role in cross-linking reactions. Prepr. Pap. Am. Chem. Soc. Div. Fuel Chem. 2001, 46, 5. [Google Scholar]
- Ogata, Y.; Tsuchida, M.; Muramoto, A. The Preparation of Terephthalic Acid from Phthalic or Benzoic Acid. J. Am. Chem. Soc. 1957, 79, 6005–6008. [Google Scholar] [CrossRef]
- Thiyagarajan, S.; Pukin, A.; van Haveren, J.; Lutz, M.; van Es, D.S. Concurrent Formation of Furan-2,5- and Furan-2,4-Dicarboxylic Acid: Unexpected Aspects of the Henkel Reaction. RSC Adv. 2013, 3, 15678–15686. [Google Scholar] [CrossRef] [Green Version]
- Dawes, G.J.S.; Scott, E.L.; Le Nôtre, J.; Sanders, J.P.M.; Bitter, J.H. Deoxygenation of Biobased Molecules by Decarboxylation and Decarbonylation—A Review on the Role of Heterogeneous, Homogeneous and Bio-Catalysis. Green Chem. 2015, 17, 3231–3250. [Google Scholar] [CrossRef] [Green Version]
- Van Es, D.S. Rigid Biobased Building Blocks. J. Renew. Mater. 2013, 1, 61–72. [Google Scholar] [CrossRef]
- Van Haveren, J.; Thiyagarajan, S.; Teruo Morita, A. Process for the Production of a Mixture of 2,4- Furandicarboxylic Acid and 2,5- Furandicarboxylic Acid (FDCA) via Disproportionation Reaction, Mixture of 2,4-FDCA and 2,5-FDCA Obtainable Thereby, 2,4-FDCA Obtainable Thereby and Use of 2,4-FDCA 2015. U.S. Patent US9284290B2, 15 March 2016. [Google Scholar]
- Pan, T.; Deng, J.; Xu, Q.; Zuo, Y.; Guo, Q.-X.; Fu, Y. Catalytic Conversion of Furfural into a 2,5-Furandicarboxylic Acid-Based Polyester with Total Carbon Utilization. ChemSusChem 2013, 6, 47–50. [Google Scholar] [CrossRef]
- Nakanishi, K.; Tanaka, A.; Hashimoto, K.; Kominami, H. Photocatalytic Hydrogenation of Furan to Tetrahydrofuran in Alcoholic Suspensions of Metal-Loaded Titanium(IV) Oxide without Addition of Hydrogen Gas. Phys. Chem. Chem. Phys. 2017, 19, 20206–20212. [Google Scholar] [CrossRef]
- Ma, J.; Yu, X.; Xu, J.; Pang, Y. Synthesis and Crystallinity of Poly(Butylene 2,5-Furandicarboxylate). Polymer 2012, 53, 4145–4151. [Google Scholar] [CrossRef]
- Martin, D.; Duprez, D. Mobility of Surface Species on Oxides. 1. Isotopic Exchange of 18O2 with 16O of SiO2, Al2O3, ZrO2, MgO, CeO2, and CeO2-Al2O3. Activation by Noble Metals. Correlation with Oxide Basicity. J. Phys. Chem. 1996, 100, 9429–9438. [Google Scholar] [CrossRef]
- Yoshikawa, K.; Kaneeda, M.; Nakamura, H. Development of Novel CeO2-Based CO2 Adsorbent and Analysis on Its CO2 Adsorption and Desorption Mechanism. Energy Proc. 2017, 114, 2481–2487. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, R.; Du, L.; Zhang, Q.; Wang, W. Catalytic Mechanism of C–F Bond Cleavage: Insights from QM/MM Analysis of Fluoroacetate Dehalogenase. Catal. Sci. Technol. 2016, 6, 73–80. [Google Scholar] [CrossRef]
- Fukue, Y.; Inoue, Y.; Oi, S. Direct Synthesis of Alkyl2-Alkynoates from Alk-l-Ynes, C02, and Bromoalkanes Catalysed by Copper(1) or Silver(1) Salt. J. Chem. Soc. Chem. Commun. 1994, 18, 2091. [Google Scholar] [CrossRef]
- Sekine, K.; Yamada, T. Silver-Catalyzed Carboxylation. Chem. Soc. Rev. 2016, 45, 4524–4532. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manjolinho, F.; Arndt, M.; Gooßen, K.; Gooßen, L.J. Catalytic C–H Carboxylation of Terminal Alkynes with Carbon Dioxide. ACS Catal. 2012, 2, 2014–2021. [Google Scholar] [CrossRef]
- Liu, X.-H.; Ma, J.-G.; Niu, Z.; Yang, G.-M.; Cheng, P. An Efficient Nanoscale Heterogeneous Catalyst for the Capture and Conversion of Carbon Dioxide at Ambient Pressure. Angew. Chem. 2015, 127, 1002–1005. [Google Scholar] [CrossRef]
- Ye, Y.; Yang, H.; Qian, J.; Su, H.; Lee, K.-J.; Cheng, T.; Xiao, H.; Yano, J.; Goddard, W.A.; Crumlin, E.J. Dramatic Differences in Carbon Dioxide Adsorption and Initial Steps of Reduction between Silver and Copper. Nat. Commun. 2019, 10, 1875. [Google Scholar] [CrossRef]
- Ayastuy, J.L.; Gurbani, A.; Gutiérrez-Ortiz, M.A. Effect of Calcination Temperature on Catalytic Properties of Au/Fe 2 O 3 Catalysts in CO-PROX. Int. J. Hydrog. Energy 2016, 41, 19546–19555. [Google Scholar] [CrossRef]
- Vigneron, F.; Caps, V. Evolution in the Chemical Making of Gold Oxidation Catalysts. C. R. Chim. 2016, 19, 192–198. [Google Scholar] [CrossRef] [Green Version]



| Experiment | XK2F (%) | FDCAs Formation (%) | S2,5-FDCA (%) | S2,4-FDCA (%) |
|---|---|---|---|---|
| Literature [40] | 92 | 91 | 70 | 30 |
| This work | 73 | 69 | 69 | 31 |
| Entry | Catalyst | Temperature (°C) | Conversion (%) | STY2,5-FDCA (µmol kg−1 h−1) | STYDFF (µmol kg−1 h−1) |
|---|---|---|---|---|---|
| 1 | CdI2 | 200 | 0 | - | - |
| 2 | Ag/SiO2/CdI2 | 200 | 51 | 264 | 951 |
| 3 | Ag/SiO2/CdI2 | 230 | 74 | 145 | - |
| 4 | Ag/SiO2/CdI2 | 260 | 69 | 188 | - |
| 5 | Ag/SiO2 | 200 | 20 | 1203 | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Drault, F.; Snoussi, Y.; Thuriot-Roukos, J.; Itabaiana, I., Jr.; Paul, S.; Wojcieszak, R. Study of the Direct CO2 Carboxylation Reaction on Supported Metal Nanoparticles. Catalysts 2021, 11, 326. https://doi.org/10.3390/catal11030326
Drault F, Snoussi Y, Thuriot-Roukos J, Itabaiana I Jr., Paul S, Wojcieszak R. Study of the Direct CO2 Carboxylation Reaction on Supported Metal Nanoparticles. Catalysts. 2021; 11(3):326. https://doi.org/10.3390/catal11030326
Chicago/Turabian StyleDrault, Fabien, Youssef Snoussi, Joëlle Thuriot-Roukos, Ivaldo Itabaiana, Jr., Sébastien Paul, and Robert Wojcieszak. 2021. "Study of the Direct CO2 Carboxylation Reaction on Supported Metal Nanoparticles" Catalysts 11, no. 3: 326. https://doi.org/10.3390/catal11030326
APA StyleDrault, F., Snoussi, Y., Thuriot-Roukos, J., Itabaiana, I., Jr., Paul, S., & Wojcieszak, R. (2021). Study of the Direct CO2 Carboxylation Reaction on Supported Metal Nanoparticles. Catalysts, 11(3), 326. https://doi.org/10.3390/catal11030326