Morphology Regulation Mechanism and Enhancement of Photocatalytic Performance of BiOX (X = Cl, Br, I) via Mannitol-Assisted Synthesis
Abstract
1. Introduction
2. Results and Discussion
2.1. Characterization
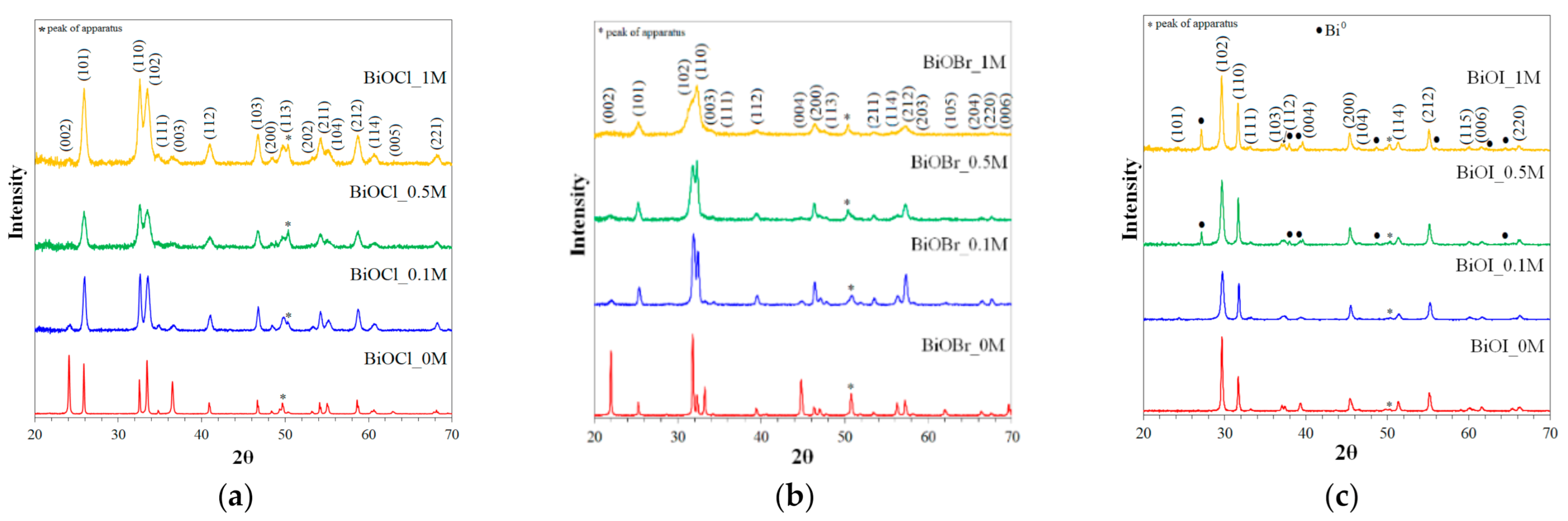
2.1.1. XRD Analysis
2.1.2. SEM Analysis
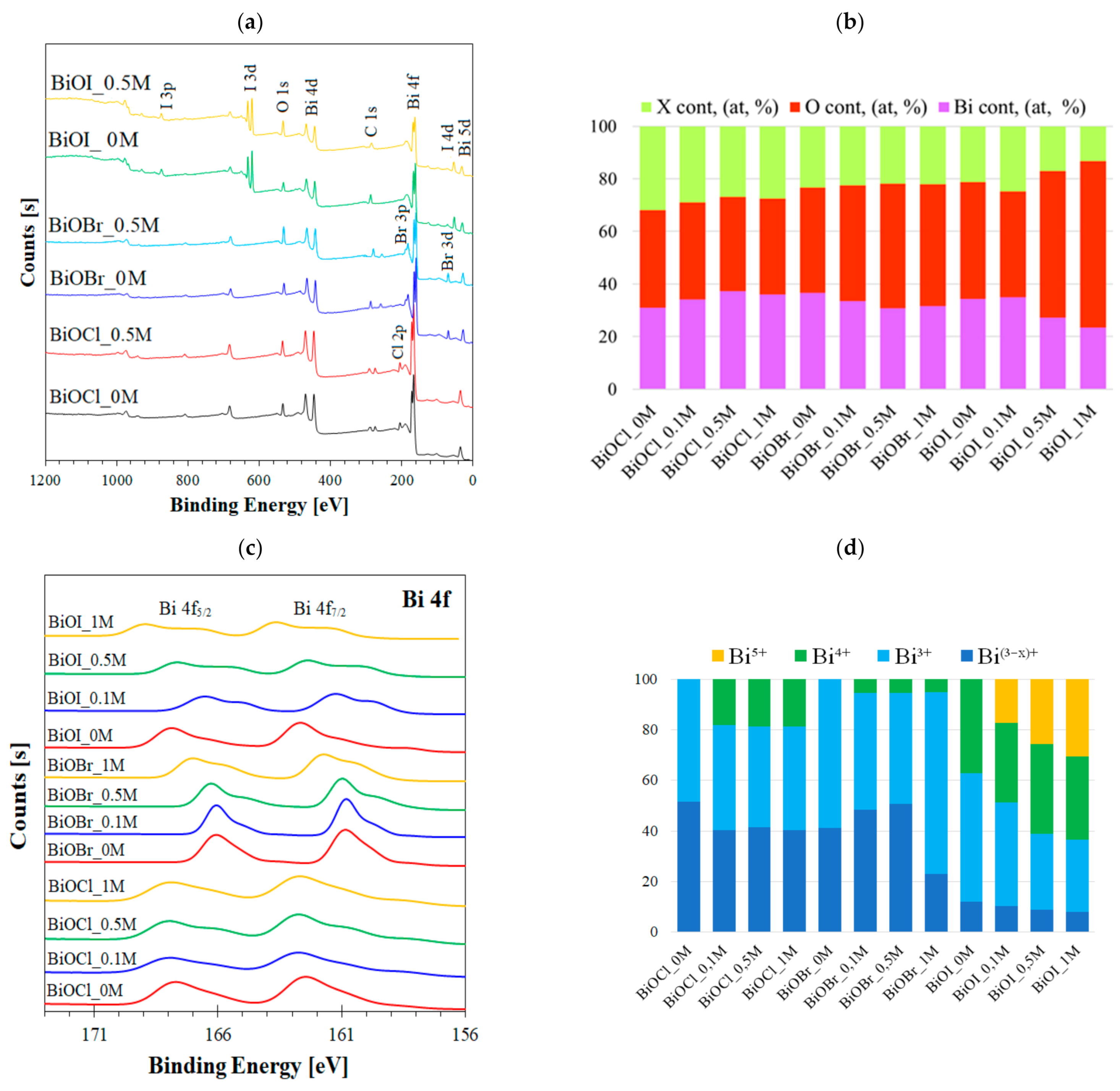
2.1.3. XPS Analysis
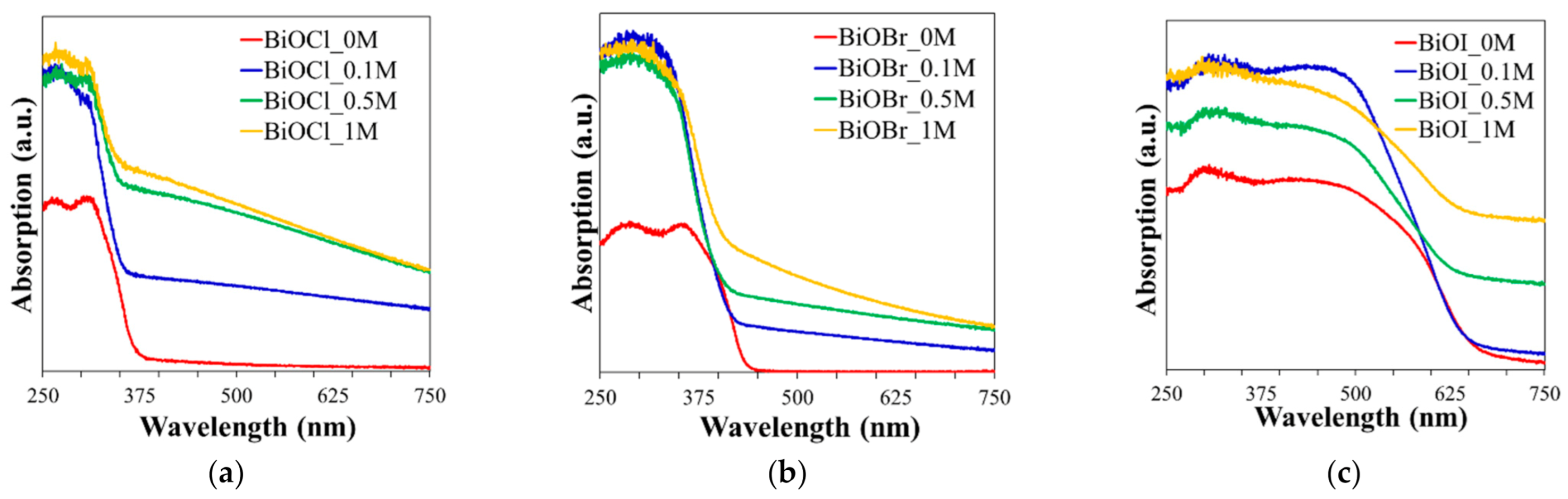
2.1.4. UV–Vis/DRS Analysis
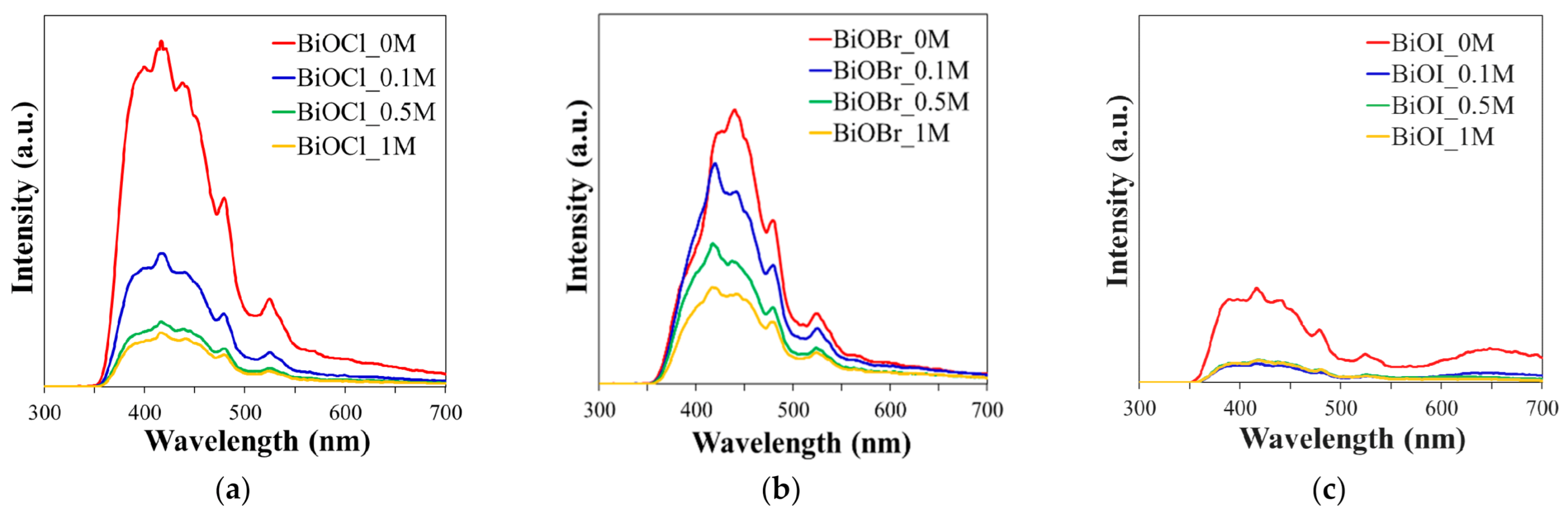
2.1.5. PL Analysis
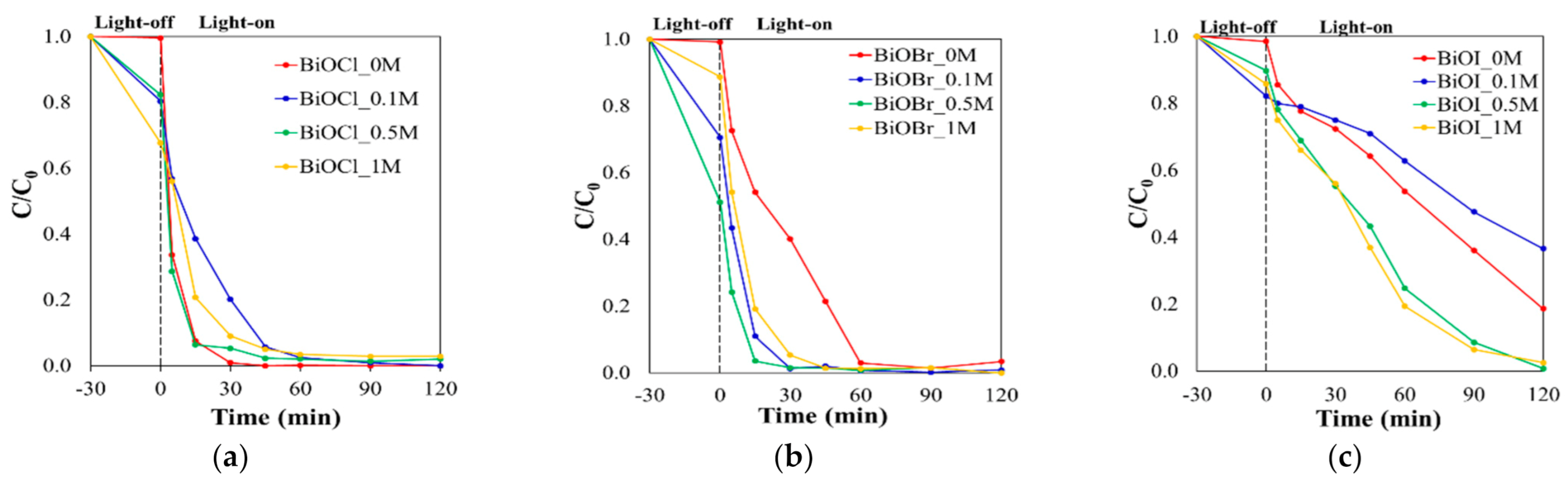
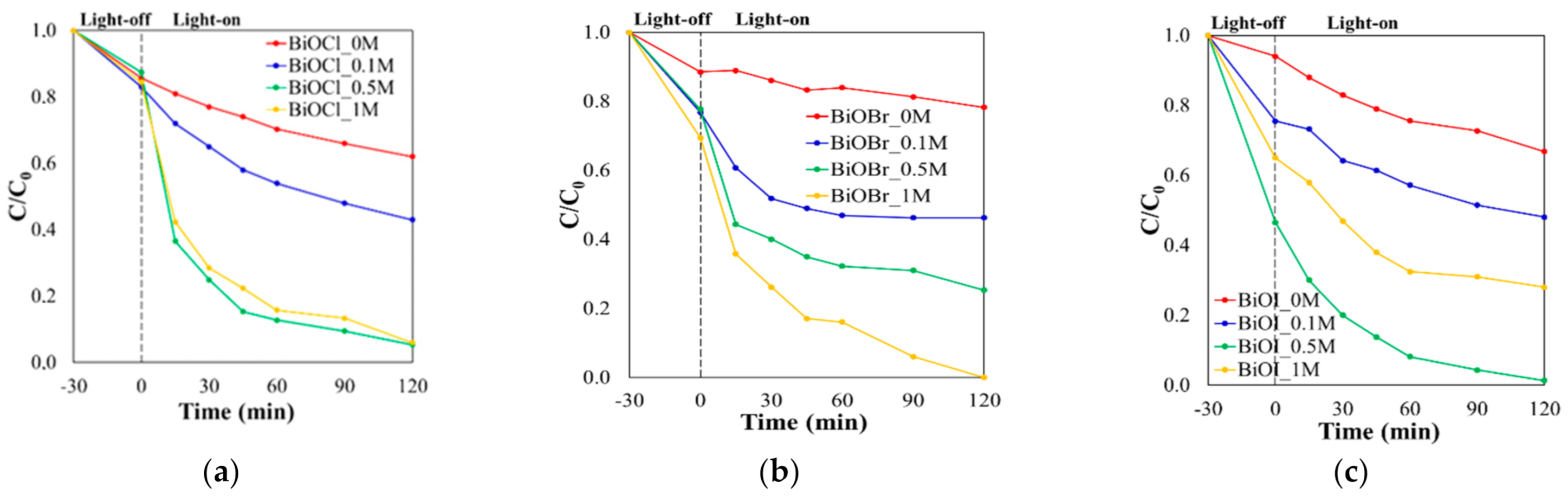
2.2. Photocatalytic Activity
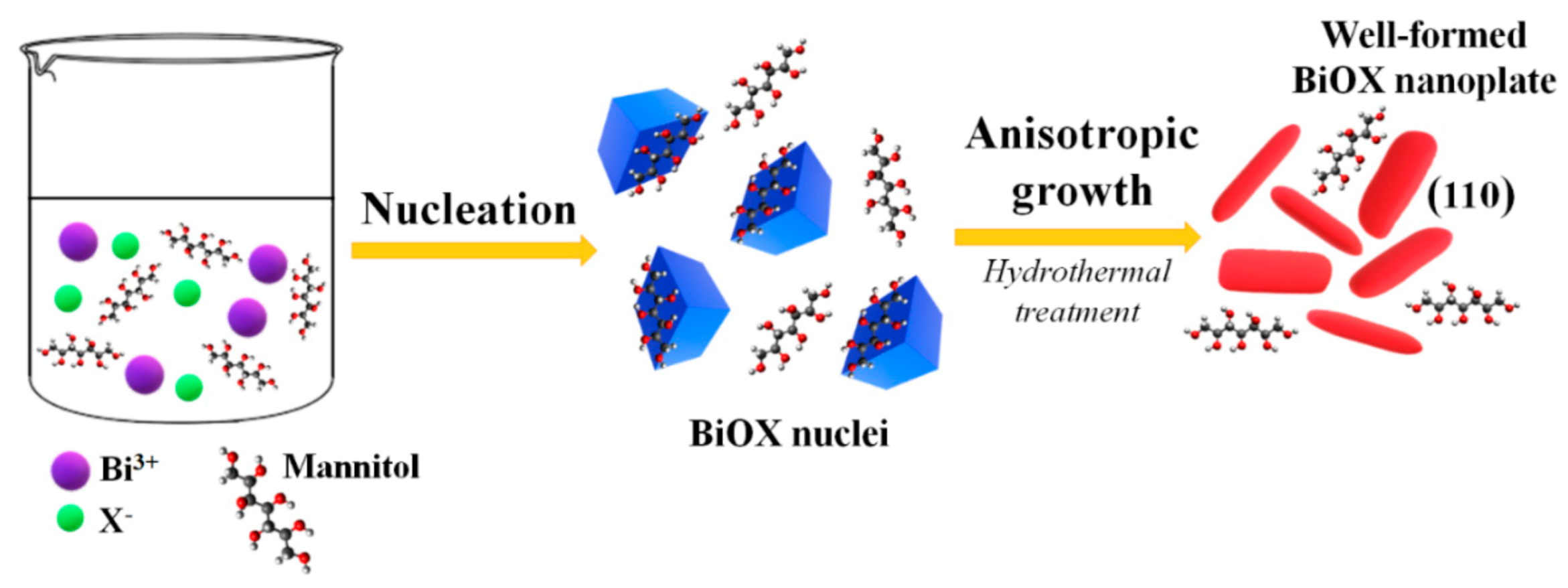
2.3. Mechanism of BiOX Crystallites Formation
3. Materials and Methods
3.1. Synthesis
3.2. Characterization
3.3. Photocatalytic Activity
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ye, L.; Su, Y.; Jin, X.; Xie, H.; Zhang, C. Recent Advances in BiOX (X = Cl, Br and I) Photocatalysts: Synthesis, Modification, Facet Effects and Mechanisms. Environ. Sci. Nano 2014, 1, 90. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, C.; Lai, C.; Zeng, G.; Huang, D.; Cheng, M.; Wang, J.; Chen, F.; Zhou, C.; Xiong, W. BiOX (X = Cl, Br, I) Photocatalytic Nanomaterials: Applications for Fuels and Environmental Management. Adv. Colloid Interface Sci. 2018, 254, 76–93. [Google Scholar] [CrossRef]
- Liu, Y.; Hu, Z.; Yu, J. Fe Enhanced Visible-Light-Driven Nitrogen Fixation on BiOBr Nanosheets. Chem. Mater. 2020, 32, 1488–1494. [Google Scholar] [CrossRef]
- Wu, S.; Wang, C.; Cui, Y.; Wang, T.; Huang, B.; Zhang, X.; Qin, X.; Brault, P. Synthesis and Photocatalytic Properties of BiOCl Nanowire Arrays. Mater. Lett. 2010, 64, 115–118. [Google Scholar] [CrossRef]
- Mi, Y.; Li, H.; Zhang, Y.; Du, N.; Hou, W. Synthesis and Photocatalytic Activity of BiOBr Nanosheets with Tunable Crystal Facets and Sizes. Catal. Sci. Technol. 2018, 8, 2588–2597. [Google Scholar] [CrossRef]
- Gao, M.; Zhang, D.; Pu, X.; Li, H.; Lv, D.; Zhang, B.; Shao, X. Facile Hydrothermal Synthesis of Bi/BiOBr Composites with Enhanced Visible-Light Photocatalytic Activities for the Degradation of Rhodamine B. Sep. Purif. Technol. 2015, 154, 211–216. [Google Scholar] [CrossRef]
- Cheng, G.; Xiong, J.; Stadler, F.J. Facile Template-Free and Fast Refluxing Synthesis of 3D Desertrose-like BiOCl Nanoarchitectures with Superior Photocatalytic Activity. New J. Chem. 2013, 37, 3207. [Google Scholar] [CrossRef]
- Wang, D.-H.; Gao, G.-Q.; Zhang, Y.-W.; Zhou, L.-S.; Xu, A.-W.; Chen, W. Nanosheet-Constructed Porous BiOCl with Dominant {001} Facets for Superior Photosensitized Degradation. Nanoscale 2012, 4, 7780. [Google Scholar] [CrossRef]
- Zhang, D.; Li, J.; Wang, Q.; Wu, Q. High {001} Facets Dominated BiOBr Lamellas: Facile Hydrolysis Preparation and Selective Visible-Light Photocatalytic Activity. J. Mater. Chem. A 2013, 1, 8622. [Google Scholar] [CrossRef]
- Hou, L.; Niu, Y.; Yang, F.; Ge, F.; Yuan, C. Facile Solvothermal Synthesis of Hollow BiOBr Submicrospheres with Enhanced Visible-Light-Responsive Photocatalytic Performance. J. Anal. Methods Chem. 2020, 3058621. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Xu, Y.; Zhu, H.; Hua, F.; Zhu, S. Controllable Hydrothermal Synthesis of BiOCl Nanoplates with High Exposed {001} Facets. Mater. Sci. Semicond. Process. 2016, 41, 12–16. [Google Scholar] [CrossRef]
- Jia, M.; Hu, X.; Wang, S.; Huang, Y.; Song, L. Photocatalytic Properties of Hierarchical BiOXs Obtained via an Ethanol-Assisted Solvothermal Process. J. Environ. Sci. 2015, 35, 172–180. [Google Scholar] [CrossRef] [PubMed]
- Tian, F.; Xiong, J.; Zhao, H.; Liu, Y.; Xiao, S.; Chen, R. Mannitol-Assisted Solvothermal Synthesis of BiOCl Hierarchical Nanostructures and Their Mixed Organic Dye Adsorption Capacities. CrystEngComm 2014, 16, 4298–4305. [Google Scholar] [CrossRef]
- Xiong, J.; Cheng, G.; Qin, F.; Wang, R.; Sun, H.; Chen, R. Tunable BiOCl Hierarchical Nanostructures for High-Efficient Photocatalysis under Visible Light Irradiation. Chem. Eng. J. 2013, 220, 228–236. [Google Scholar] [CrossRef]
- Xiong, J.; Cheng, G.; Li, G.; Qin, F.; Chen, R. Well-Crystallized Square-like 2D BiOCl Nanoplates: Mannitol-Assisted Hydrothermal Synthesis and Improved Visible-Light-Driven Photocatalytic Performance. RSC Adv. 2011, 1, 1542. [Google Scholar] [CrossRef]
- Bielicka–Giełdoń, A.; Wilczewska, P.; Malankowska, A.; Szczodrowski, K.; Ryl, J.; Zielińska-Jurek, A.; Siedlecka, E.M. Morphology, Surface Properties and Photocatalytic Activity of the Bismuth Oxyhalides Semiconductors Prepared by Ionic Liquid Assisted Solvothermal Method. Sep. Purif. Technol. 2019, 217, 164–173. [Google Scholar] [CrossRef]
- Wilczewska, P.; Bielicka-Giełdoń, A.; Borzyszkowska, A.F.; Ryl, J.; Klimczuk, T.; Siedlecka, E.M. Photocatalytic Activity of Solvothermal Prepared BiOClBr with Imidazolium Ionic Liquids as a Halogen Sources in Cytostatic Drugs Removal. J. Photochem. Photobiol. A Chem. 2019, 382, 111932. [Google Scholar] [CrossRef]
- Hao, H.-Y.; Xu, Y.-Y.; Liu, P.; Zhang, G.-Y. BiOCl Nanostructures with Different Morphologies: Tunable Synthesis and Visible-Light-Driven Photocatalytic Properties. Chin. Chem. Lett. 2015, 26, 133–136. [Google Scholar] [CrossRef]
- Li, X.; Zhu, C.; Song, Y.; Du, D.; Lin, Y. Solvent Co-Mediated Synthesis of Ultrathin BiOCl Nanosheets with Highly Efficient Visible-Light Photocatalytic Activity. RSC Adv. 2017, 7, 10235–10241. [Google Scholar] [CrossRef]
- Li, L.; Ai, L.; Zhang, C.; Jiang, J. Hierarchical {001}-Faceted BiOBr Microspheres as a Novel Biomimetic Catalyst: Dark Catalysis towards Colorimetric Biosensing and Pollutant Degradation. Nanoscale 2014, 6, 4627. [Google Scholar] [CrossRef]
- Xing, H.; Ma, H.; Fu, Y.; Zhang, X.; Dong, X.; Zhang, X. Preparation of BiOBr by Solvothermal Routes with Different Solvents and Their Photocatalytic Activity. J. Renew. Sustain. Energy 2015, 7, 063120. [Google Scholar] [CrossRef]
- Liu, Z.; Wu, B.; Xiang, D.; Zhu, Y. Effect of Solvents on Morphology and Photocatalytic Activity of BiOBr Synthesized by Solvothermal Method. Mater. Res. Bull. 2012, 47, 3753–3757. [Google Scholar] [CrossRef]
- Li, R.; Ren, H.; Ma, W.; Hong, S.; Wu, L.; Huang, Y. Synthesis of BiOBr Microspheres with Ethanol as Self-Template and Solvent with Controllable Morphology and Photocatalytic Activity. Catal. Commun. 2018, 106, 1–5. [Google Scholar] [CrossRef]
- Li, J.; Sun, S.; Qian, C.; He, L.; Chen, K.K.; Zhang, T.; Chen, Z.; Ye, M. The Role of Adsorption in Photocatalytic Degradation of Ibuprofen under Visible Light Irradiation by BiOBr Microspheres. Chem. Eng. J. 2016, 297, 139–147. [Google Scholar] [CrossRef]
- Fang, Y.; Hua, T.; Feng, W.; Johnson, D.M.; Huang, Y. Mannitol Ligand-Assisted Assembly of BiOBr Photocatalyst in the Cationic Micelles of Cetylpyridinium Bromide. Catal. Commun. 2016, 80, 15–19. [Google Scholar] [CrossRef]
- Hu, J.; Weng, S.; Zheng, Z.; Pei, Z.; Huang, M.; Liu, P. Solvents Mediated-Synthesis of BiOI Photocatalysts with Tunable Morphologies and Their Visible-Light Driven Photocatalytic Performances in Removing of Arsenic from Water. J. Hazard. Mater. 2014, 264, 293–302. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, F.; Li, D.; Liu, R.; Liu, S. Facile Synthesis of Flower-like BiOI Hierarchical Spheres at Room Temperature with High Visible-Light Photocatalytic Activity. Mater. Sci. Eng. B 2015, 193, 112–120. [Google Scholar] [CrossRef]
- Gnayem, H.; Sasson, Y. Hierarchical Nanostructured 3D Flowerlike BiOClx Br1−x Semiconductors with Exceptional Visible Light Photocatalytic Activity. ACS Catal. 2013, 3, 186–191. [Google Scholar] [CrossRef]
- Shi, X.; Chen, X.; Chen, X.; Zhou, S.; Lou, S.; Wang, Y.; Yuan, L. PVP Assisted Hydrothermal Synthesis of BiOBr Hierarchical Nanostructures and High Photocatalytic Capacity. Chem. Eng. J. 2013, 222, 120–127. [Google Scholar] [CrossRef]
- Liu, J.; Hu, J.; Ruan, L.; Wu, Y. Facile and Environment Friendly Synthesis of Hierarchical BiOCl Flowery Microspheres with Remarkable Photocatalytic Properties. Chin. Sci. Bull. 2014, 59, 802–809. [Google Scholar] [CrossRef]
- Zhao, Y.; Tan, X.; Yu, T.; Wang, S. SDS-Assisted Solvothermal Synthesis of BiOBr Microspheres with Highly Visible-Light Photocatalytic Activity. Mater. Lett. 2016, 164, 243–247. [Google Scholar] [CrossRef]
- Wang, X.; Chen, H.; Li, H.; Mailhot, G.; Dong, W. Preparation and Formation Mechanism of BiOCl0.75I0.25 Nanospheres by Precipitation Method in Alcohol–Water Mixed Solvents. J. Colloid Interface Sci. 2016, 478, 1–10. [Google Scholar] [CrossRef]
- Gao, X.; Zhang, X.; Wang, Y.; Peng, S.; Yue, B.; Fan, C. Rapid Synthesis of Hierarchical BiOCl Microspheres for Efficient Photocatalytic Degradation of Carbamazepine under Simulated Solar Irradiation. Chem. Eng. J. 2015, 263, 419–426. [Google Scholar] [CrossRef]
- Yang, J.; Xie, T.; Zhu, Q.; Wang, J.; Xu, L.; Liu, C. Boosting the Photocatalytic Activity of BiOX under Solar Light via Selective Crystal Facet Growth. J. Mater. Chem. C 2020, 8, 2579–2588. [Google Scholar] [CrossRef]
- Zhang, H.; Yang, Y.; Zhou, Z.; Zhao, Y.; Liu, L. Enhanced Photocatalytic Properties in BiOBr Nanosheets with Dominantly Exposed (102) Facets. J. Phys. Chem. C 2014, 118, 14662–14669. [Google Scholar] [CrossRef]
- Dai, B.; Zhang, A.; Liu, Z.; Wang, T.; Li, C.; Zhang, C.; Li, H.; Liu, Z.; Zhang, X. Facile Synthesis of Metallic Bi Deposited BiOI Composites with the Aid of EDTA-2Na for Highly Efficient Hg0 Removal. Catal. Commun. 2019, 121, 53–56. [Google Scholar] [CrossRef]
- Chang, C.; Zhu, L.; Fu, Y.; Chu, X. Highly Active Bi/BiOI Composite Synthesized by One-Step Reaction and Its Capacity to Degrade Bisphenol A under Simulated Solar Light Irradiation. Chem. Eng. J. 2013, 233, 305–314. [Google Scholar] [CrossRef]
- Montoya-Zamora, J.M.; Martínez-de la Cruz, A.; López Cuéllar, E. Enhanced Photocatalytic Activity of BiOI Synthesized in Presence of EDTA. J. Taiwan Inst. Chem. Eng. 2017, 75, 307–316. [Google Scholar] [CrossRef]
- Bárdos, E.; Márta, V.; Baia, L.; Todea, M.; Kovács, G.; Baán, K.; Garg, S.; Pap, Z.; Hernadi, K. Hydrothermal Crystallization of Bismuth Oxybromide (BiOBr) in the Presence of Different Shape Controlling Agents. Appl. Surf. Sci. 2020, 518, 146184. [Google Scholar] [CrossRef]
- Hu, T.; Li, H.; Zhang, R.; Du, N.; Hou, W. Thickness-Determined Photocatalytic Performance of Bismuth Tungstate Nanosheets. RSC Adv. 2016, 6, 31744–31750. [Google Scholar] [CrossRef]
- Zhang, G.; Cai, L.; Zhang, Y.; Wei, Y. Bi5+, Bi(3−x)+, and Oxygen Vacancy Induced BiOClx I1−x Solid Solution toward Promoting Visible-Light Driven Photocatalytic Activity. Chem. Eur. J. 2018, 24, 7434–7444. [Google Scholar] [CrossRef]
- Heidari, S.Z.; Haghighi, M.; Shabani, M. Sunlight-activated BiOCl/BiOBr–Bi24O31Br10 photocatalyst for the removal of pharmaceutical compounds. J. Clean. Prod. 2020, 259, 120679. [Google Scholar] [CrossRef]
- Lin, Z.; Zhe, F.; Wang, Y.; Zhang, Q.; Zhao, X.; Hu, X.; Wu, Y.; He, Y. Preparation of interstitial carbon doped BiOI for enhanced performance in photocatalytic nitrogen fixation and methyl orange degradation. J. Colloid Interface Sci. 2019, 539, 563–574. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, L.; Zhou, Z. First-Principles Studies on Facet-Dependent Photocatalytic Properties of Bismuth Oxyhalides (BiOXs). RSC Adv. 2012, 2, 9224. [Google Scholar] [CrossRef]
- Weng, S.; Fang, Z.; Wang, Z.; Zheng, Z.; Feng, W.; Liu, P. Construction of Teethlike Homojunction BiOCl (001) Nanosheets by Selective Etching and Its High Photocatalytic Activity. ACS Appl. Mater. Interfaces 2014, 6, 18423–18428. [Google Scholar] [CrossRef] [PubMed]
- Gao, M.; Zhang, D.; Pu, X.; Li, H.; Li, J.; Shao, X.; Ding, K. BiOBr Photocatalysts with Tunable Exposing Proportion of {001} Facets: Combustion Synthesis, Characterization, and High Visible-Light Photocatalytic Properties. Mater. Lett. 2015, 140, 31–34. [Google Scholar] [CrossRef]
- Xu, J.; Meng, W.; Zhang, Y.; Li, L.; Guo, C. Photocatalytic Degradation of Tetrabromobisphenol A by Mesoporous BiOBr: Efficacy, Products and Pathway. Appl. Catal. B Environ. 2011, 107, 355–362. [Google Scholar] [CrossRef]
- Zhang, L.; Cao, X.-F.; Chen, X.-T.; Xue, Z.-L. BiOBr Hierarchical Microspheres: Microwave-Assisted Solvothermal Synthesis, Strong Adsorption and Excellent Photocatalytic Properties. J. Colloid Interface Sci. 2011, 354, 630–636. [Google Scholar] [CrossRef]
- Arumugam, M.; Choi, M.Y. Recent Progress on Bismuth Oxyiodide (BiOI) Photocatalyst for Environmental Remediation. J. Ind. Eng. Chem. 2020, 81, 237–268. [Google Scholar] [CrossRef]
- Xiao, X.; Xing, C.; He, G.; Zuo, X.; Nan, J.; Wang, L. Solvothermal Synthesis of Novel Hierarchical Bi4O5I2 Nanoflakes with Highly Visible Light Photocatalytic Performance for the Degradation of 4-Tert-Butylphenol. Appl. Catal. B Environ. 2014, 148, 154–163. [Google Scholar] [CrossRef]
- Fan, Z.; Zhao, Y.; Zhai, W.; Qiu, L.; Li, H.; Hoffmann, M.R. Facet-Dependent Performance of BiOBr for Photocatalytic Reduction of Cr(VI). RSC Adv. 2016, 6, 2028–2031. [Google Scholar] [CrossRef]
- Hussain, M.B.; Khan, M.S.; Loussala, H.M.; Bashir, M.S. The Synthesis of a BiOCl x Br 1−x Nanostructure Photocatalyst with High Surface Area for the Enhanced Visible-Light Photocatalytic Reduction of Cr(VI). RSC Adv. 2020, 10, 4763–4771. [Google Scholar] [CrossRef]
- Li, H.; Deng, F.; Zheng, Y.; Hua, L.; Qu, C.; Luo, X. Visible-Light-Driven Z-Scheme RGO/Bi2S3 –BiOBr Heterojunctions with Tunable Exposed BiOBr (102) Facets for Efficient Synchronous Photocatalytic Degradation of 2-Nitrophenol and Cr(VI) Reduction. Environ. Sci. Nano 2019, 6, 3670–3683. [Google Scholar] [CrossRef]


| Photocatalyst | Particle Size | Morphology | Eg (eV) | MVB (eV) | MCB (eV) | I110/I102 |
|---|---|---|---|---|---|---|
| BiOCl_0M | 0.48–1.9 μm | microplate | 3.3 | 3.51 | 0.21 | 0.65 |
| BiOCl_0.1M | 41.3–66.6 nm | nanoplate | 3.2 | 3.46 | 0.26 | 1.03 |
| BiOCl_0.5M | 13.4–25.4 nm | nanoplate | 3.05 | 3.38 | 0.33 | 1.14 |
| BiOCl_1M | 15–20 nm | nanoparticle | 3.0 | 3.36 | 0.36 | 1.13 |
| BiOBr_0M | 1.45–5.12 μm | microplate | 2.58 | 2.99 | 0.41 | 0.25 |
| BiOBr_0.1M | 40.8–81.0 nm | nanoplate | 2.65 | 3.03 | 0.38 | 0.76 |
| BiOBr_0.5M | 58.5–72.7 nm | nanoplate | 2.65 | 3.03 | 0.38 | 1.08 |
| BiOBr_1M | 530–734 nm | microstructure flower-like | 2.56 | 2.98 | 0.42 | 1.40 |
| BiOI_0M | 4.13–5.65 μm | microplate | 1.65 | 2.26 | 0.61 | 0.46 |
| BiOI_0.1M | 0.74–1.21 μm | microstructure rose-like | 1.65 | 2.26 | 0.61 | 0.75 |
| BiOI_0.5M | 0.74–1.21 μm | microplate | 1.58 | 2.23 | 0.65 | 0.72 |
| BiOI_1M | 183–361 nm | nanoplate | 1.50 | 2.19 | 0.69 | 0.63 |
| Type of Solution | Density [g cm−3] | Viscosity [cP] |
|---|---|---|
| deionized water | 0.99823 | 1.005 |
| 0.1 M mannitol | 1.00383 | 1.087 |
| 0.5 M mannitol | 1.03534 | 1.269 |
| 1 M mannitol | 1.06681 | 1.763 |
| Sample | BE Bi 4f 7/2 (eV) | BE Bi 4f 5/2 (eV) | ||||||
|---|---|---|---|---|---|---|---|---|
| Bi(3−x)+ | Bi3+ | Bi4+ | Bi5+ | Bi(3−x)+ | Bi3+ | Bi4+ | Bi5+ | |
| BiOCl_0M | 158.8 | 160.1 | - | - | 163.7 | 165.4 | 166.6 | - |
| BiOCl_0.1M | 158.7 | 159.9 | 161.1 | - | 163.6 | 165.2 | 166.4 | - |
| BiOCl_0.5M | 158.4 | 159.8 | 161.1 | - | 163.4 | 165.0 | 166.3 | - |
| BiOCl_1M | 158.6 | 160.0 | 161.1 | - | 163.6 | 165.3 | 166.5 | - |
| BiOBr_0M | 158.0 | 160.1 | - | - | 163.3 | 165.4 | - | - |
| BiOBr_0.1M | 158.2 | 159.9 | 160.9 | - | 163.2 | 165.2 | 166.1 | - |
| BiOBr_0.5M | 158.3 | 159.8 | 161.0 | - | 163.1 | 165.1 | 166.3 | - |
| BiOBr_1M | 158.3 | 159.9 | 160.9 | - | 163.6 | 165.2 | 166.2 | - |
| BiOI_0M | - | 159.4 | 161.5 | - | - | 165.0 | 166.6 | - |
| BiOI_0.1M | - | 159.6 | 161.3 | 162.7 | - | 164.8 | 166.6 | 167.7 |
| BiOI_0.5M | - | 159.9 | 161.1 | 162.5 | - | 165.2 | 166.4 | 167.8 |
| BiOI_1M | - | 159.9 | 161.0 | 162.5 | - | 165.2 | 166.3 | 167.7 |
| Sample Label | Rh B | Cr6+ | 5-FU | |||
|---|---|---|---|---|---|---|
| kapp [min−1] | R2 | kapp [min−1] | R2 | kapp [min−1] | R2 | |
| BiOCl_0M | 0.152 | 0.9736 | 0.003 | 0.9627 | 0.076 | 0.9972 |
| BiOCl_0.1M | 0.045 | 0.9743 | 0.006 | 0.9703 | 0.008 | 0.9569 |
| BiOCl_0.5M | 0.084 | 0.9761 | 0.021 | 0.9715 | 0.022 | 0.9799 |
| BiOCl_1M | 0.070 | 0.9746 | 0.017 | 0.968 | 0.093 | 0.9989 |
| BiOBr_0M | 0.046 | 0.9667 | 0.001 | 0.9554 | 0.007 | 0.9909 |
| BiOBr_0.1M | 0.068 | 0.9822 | 0.010 | 0.9635 | 0.097 | 0.9521 |
| BiOBr_0.5M | 0.115 | 0.9977 | 0.022 | 0.9613 | 0.021 | 0.9902 |
| BiOBr_1M | 0.092 | 0.9986 | 0.025 | 0.9813 | 0.017 | 0.9796 |
| BiOI_0M | 0.008 | 0.9791 | 0.003 | 0.9782 | inactive | |
| BiOI_0.1M | 0.005 | 0.9387 | 0.004 | 0.9667 | 0.095 | 0.9886 |
| BiOI_0.5M | 0.021 | 0.9756 | 0.029 | 0.9938 | inactive | |
| BiOI_1M | 0.021 | 0.9700 | 0.012 | 0.9913 | inactive | |
| No. | Sample Label | BiOX Precursors | Solvent |
|---|---|---|---|
| Bismuth oxychloride | |||
| 1. | BiOCl_0M | 2 mmol KCl, 2 mmol Bi(NO3)3·5H2O | deionized water |
| 2. | BiOCl_0.1M | 0.1 M mannitol | |
| 3. | BiOCl_0.5M | 0.5 M mannitol | |
| 4. | BiOCl_1M | 1 M mannitol | |
| Bismuth oxybromide | |||
| 5. | BiOBr_0M | 2 mmol KBr, 2 mmol Bi(NO3)3·5H2O | deionized water |
| 6. | BiOBr_0.1M | 0.1 M mannitol | |
| 7. | BiOBr_0.5M | 0.5 M mannitol | |
| 8. | BiOBr_1M | 1 M mannitol | |
| Bismuth oxyiodide | |||
| 9. | BiOI_0M | 2 mmol KI, 2 mmol Bi(NO3)3·5H2O | deionized water |
| 10. | BiOI_0.1M | 0.1 M mannitol | |
| 11. | BiOI_0.5M | 0.5 M mannitol | |
| 12. | BiOI_1M | 1 M mannitol | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wilczewska, P.; Bielicka-Giełdoń, A.; Szczodrowski, K.; Malankowska, A.; Ryl, J.; Tabaka, K.; Siedlecka, E.M. Morphology Regulation Mechanism and Enhancement of Photocatalytic Performance of BiOX (X = Cl, Br, I) via Mannitol-Assisted Synthesis. Catalysts 2021, 11, 312. https://doi.org/10.3390/catal11030312
Wilczewska P, Bielicka-Giełdoń A, Szczodrowski K, Malankowska A, Ryl J, Tabaka K, Siedlecka EM. Morphology Regulation Mechanism and Enhancement of Photocatalytic Performance of BiOX (X = Cl, Br, I) via Mannitol-Assisted Synthesis. Catalysts. 2021; 11(3):312. https://doi.org/10.3390/catal11030312
Chicago/Turabian StyleWilczewska, Patrycja, Aleksandra Bielicka-Giełdoń, Karol Szczodrowski, Anna Malankowska, Jacek Ryl, Karol Tabaka, and Ewa Maria Siedlecka. 2021. "Morphology Regulation Mechanism and Enhancement of Photocatalytic Performance of BiOX (X = Cl, Br, I) via Mannitol-Assisted Synthesis" Catalysts 11, no. 3: 312. https://doi.org/10.3390/catal11030312
APA StyleWilczewska, P., Bielicka-Giełdoń, A., Szczodrowski, K., Malankowska, A., Ryl, J., Tabaka, K., & Siedlecka, E. M. (2021). Morphology Regulation Mechanism and Enhancement of Photocatalytic Performance of BiOX (X = Cl, Br, I) via Mannitol-Assisted Synthesis. Catalysts, 11(3), 312. https://doi.org/10.3390/catal11030312