Investigation of Co3O4 and LaCoO3 Interaction by Performing N2O Decomposition Tests under Co3O4-CoO Transition Temperature
Abstract
1. Introduction
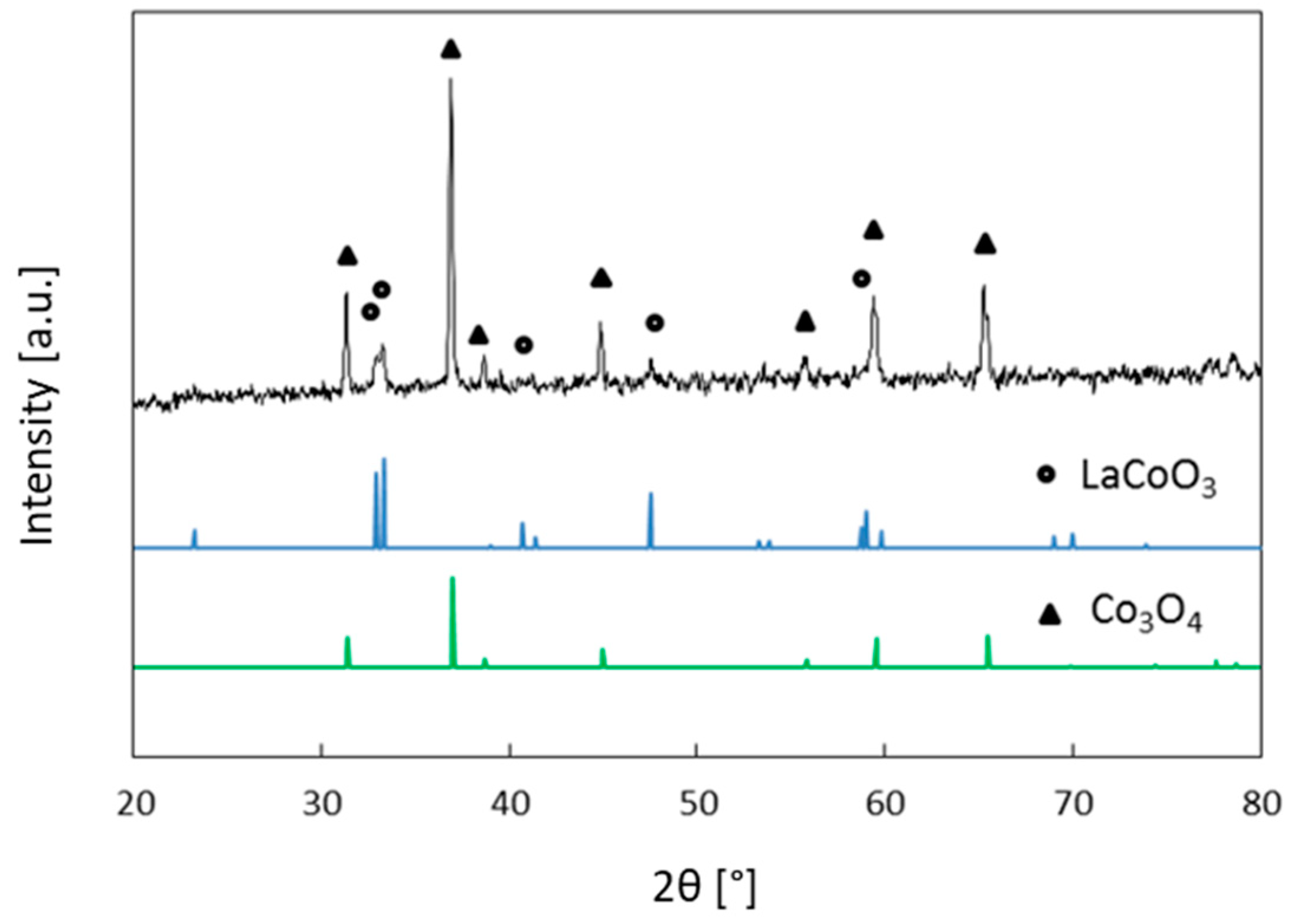
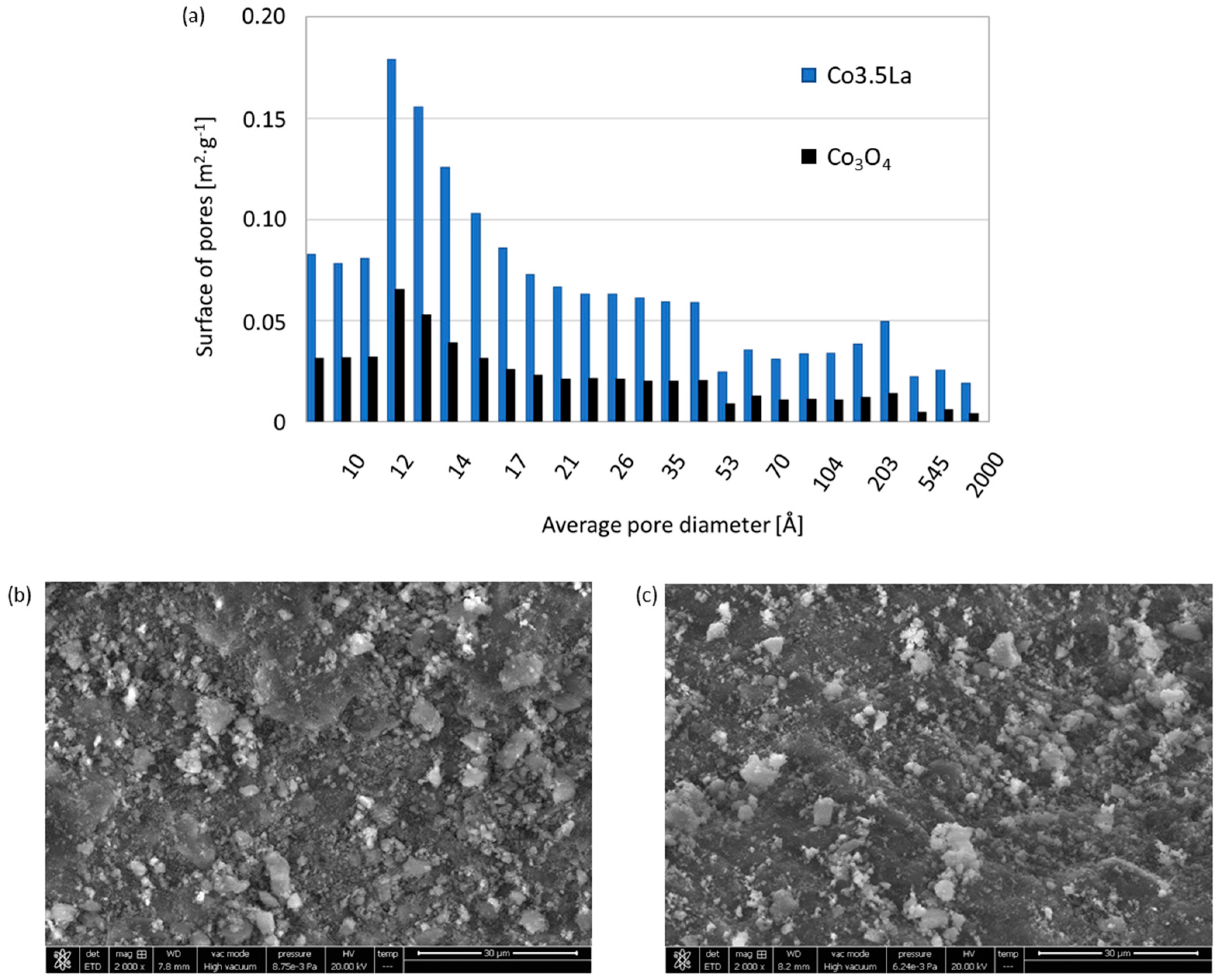
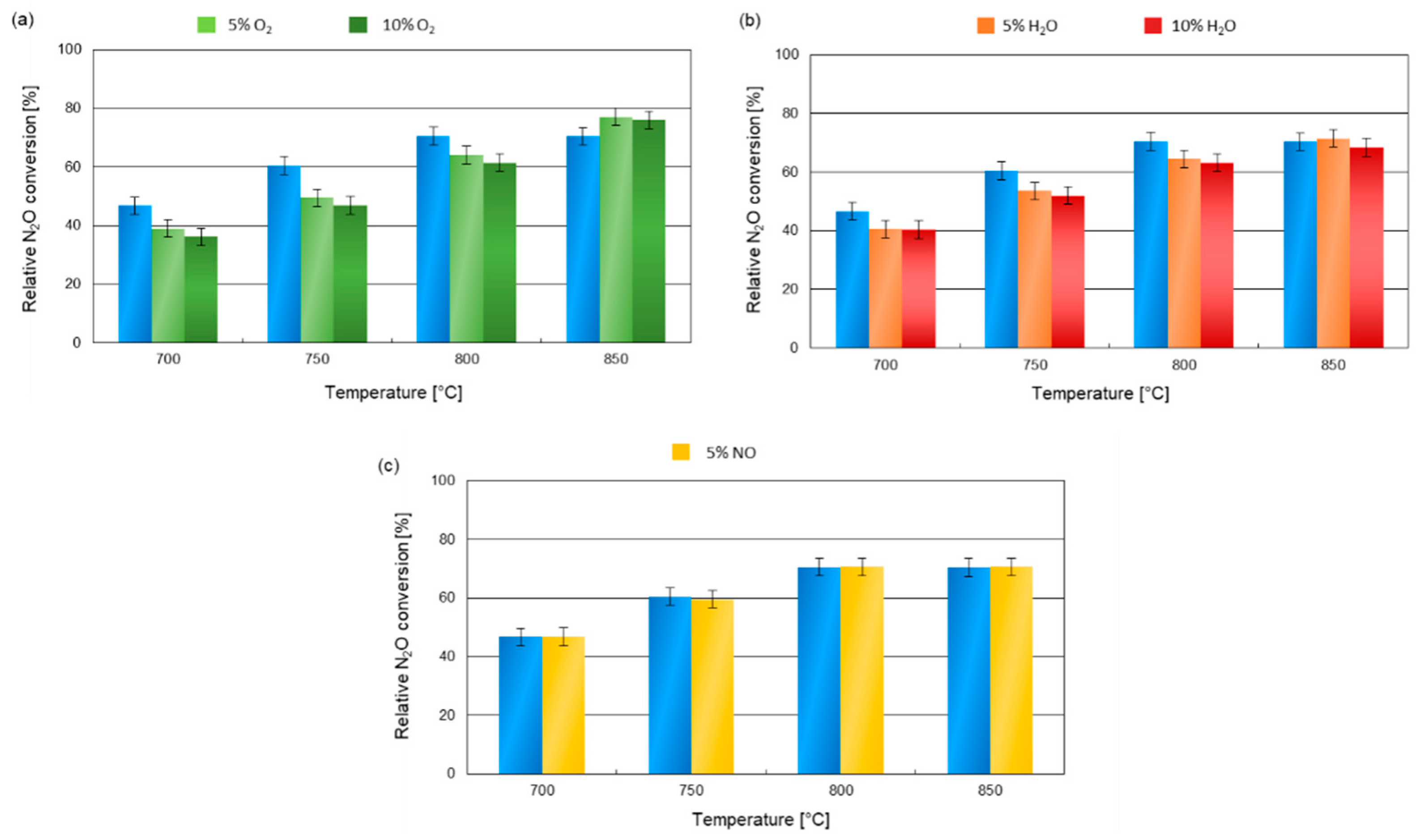
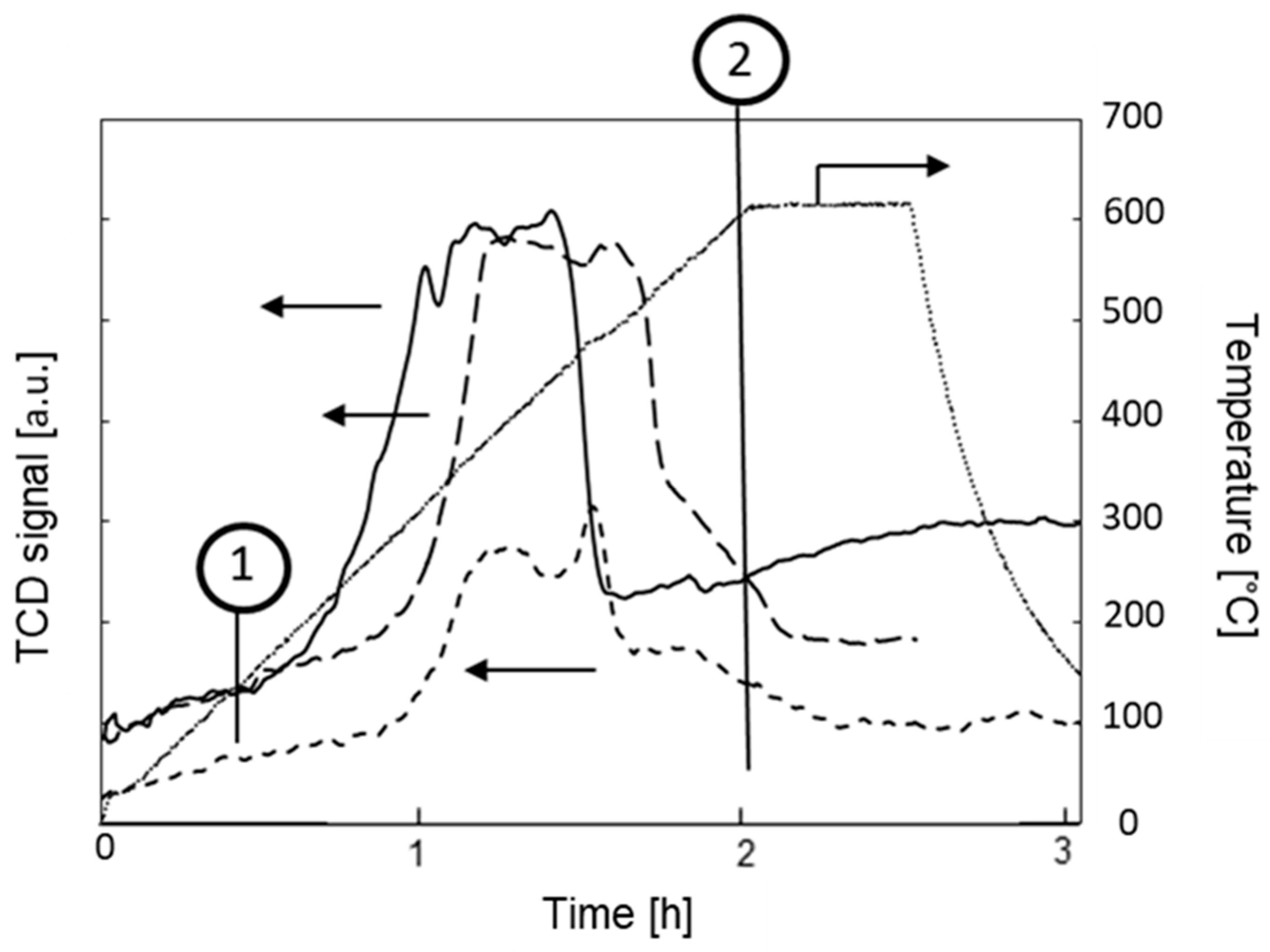
2. Results and Discussion
- (1)
- adsorption of an oxygen molecule on the surface of the catalyst (filling two anion vacancies) and adsorption of two CO molecules by the oxygen atom,
- (2)
- reaction of each CO molecule with an oxygen anion,
- (3)
- desorption of two molecules of CO2 with the regeneration of the oxygen ion vacancies on the surface.
- (1)
- adsorption of a N2O molecule by the oxygen atom on the catalyst surface,
- (2)
- cleavage of the N-O bond and departure of N2, leaving an oxygen anion on the surface of the catalyst,
- (3)
- adsorption of another N2O molecule directly onto a surface oxygen anion to form N2 and O2 (Eley–Rideal mechanism), or onto another spot, cleavage of the N-O bond and subsequent migration of oxygen anions to form an O2 molecule (Langmuir–Hinshelwood mechanism)
- (4)
- desorption of oxygen from the surface of the catalyst (rate determining step [6]).
3. Materials and Methods
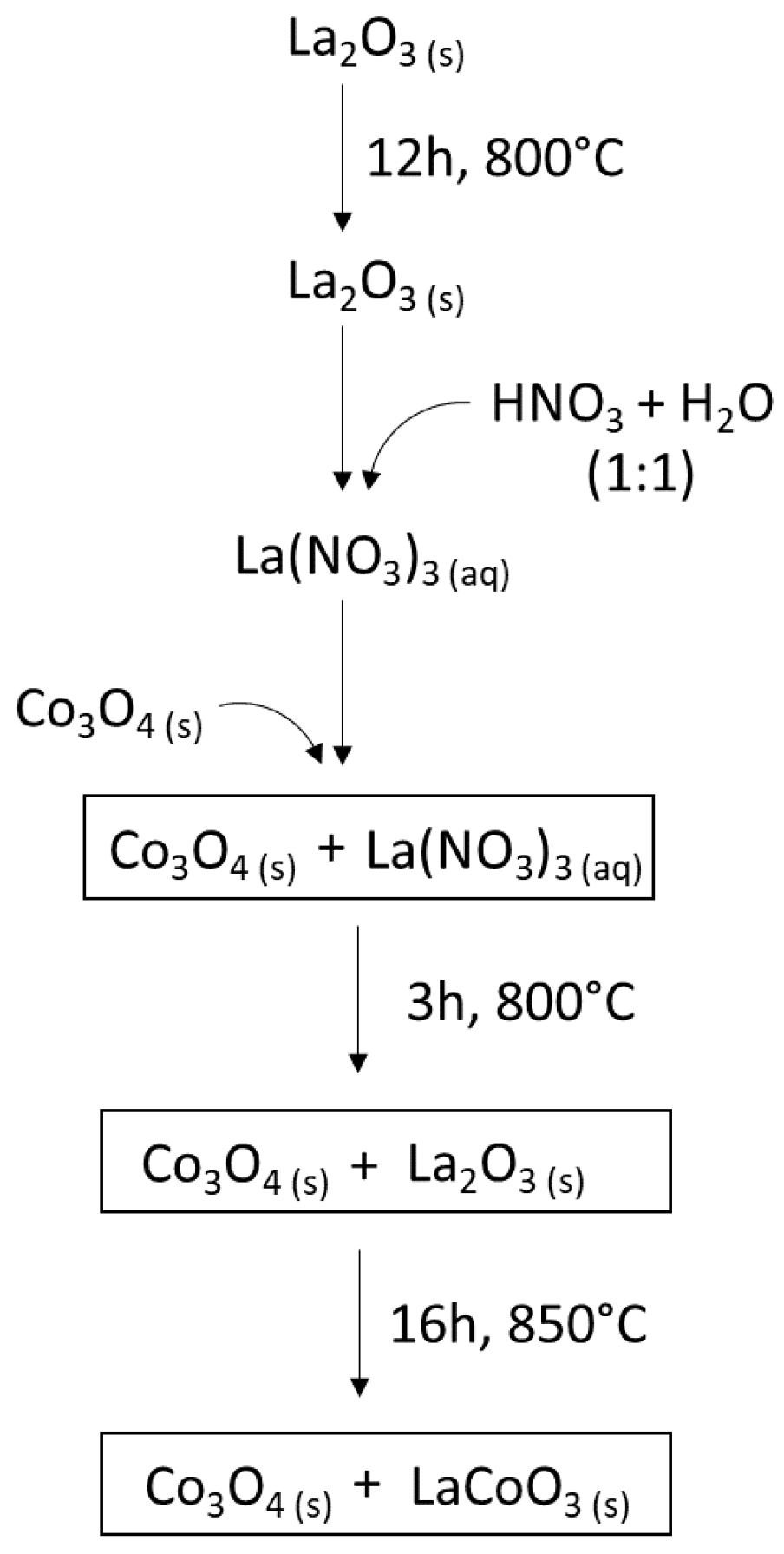
3.1. Catalyst Preparation
3.2. Activity Measurements and Characterization Studies
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cole, K.M.; Kirk, D.W.; Thorpe, S.J. Co3O4 nanoparticles characterized by XPS and UPS. Surf. Sci. Spectra 2021, 28, 014001. [Google Scholar] [CrossRef]
- Peck, T.C.; Roberts, C.A.; Reddy, G.K. Contrasting Effects of Potassium Addition on M3O4 (M = Co, Fe, and Mn) Oxides during Direct NO Decomposition Catalysis. Catalysts 2020, 10, 561. [Google Scholar] [CrossRef]
- Wójcik, S.; Grzybek, G.; Stelmachowski, P.; Sojka, Z.; Kotarba, A. Bulk, Surface and Interface Promotion of Co3O4 for the Low-Temperature N2O Decomposition Catalysis. Catalysts 2020, 10, 41. [Google Scholar] [CrossRef]
- Liotta, L.F.; Ousmane, M.; Di Carlo, G.; Pantaleo, G.; Deganello, G.; Marcic, G.; Retailleau, L.; Giroir-Fendler, A. Total oxidation of propene at low temperature over Co3O4-CeO2 mixed oxides: Role of surface oxygen vacancies and bulk oxygen mobility in the catalytic activity. Appl. Catal. A 2008, 34, 781–788. [Google Scholar] [CrossRef]
- Iwanek, E.; Krawczyk, K.; Petryk, J.; Sobczak, J.W.; Kaszkur, Z. Direct nitrous oxide decomposition with CoOx-CeO2 catalysts. Appl. Catal. B 2011, 106, 416–422. [Google Scholar] [CrossRef]
- Kapteijn, F.; Rodriguez-Mirasol, J.; Moulijn, J.A. Heterogeneous catalytic decomposition of nitrous oxide. Appl. Catal. B 1996, 9, 25–64. [Google Scholar] [CrossRef]
- Darda, S.; Pachatouridou, E.; Lappas, A.; Iliopoulou, E. Effect of Preparation Method of Co-Ce Catalysts on CH4 Combustion. Catalysts 2019, 9, 219. [Google Scholar] [CrossRef]
- Liotta, L.; Di Carlo, G.; Pantaleo, G.; Venezia, A.; Deganello, G. Co3O4/CeO2 composite oxides for methane emissions abatement: Relationship between Co3O4–CeO2 interaction and catalytic activity. Appl. Catal. B Environ. 2006, 66, 217–227. [Google Scholar] [CrossRef]
- Konsolakis, M.; Sgourakis, M.; Carabineiro, S.A. Surface and redox properties of cobalt–ceria binary oxides: On the effect of Co content and pretreatment conditions. Appl. Surf. Sci. 2015, 341, 48–54. [Google Scholar] [CrossRef]
- Simonot, L.; Garin, F.; Maire, G. A comparative study of LaCoO3, Co3O4 and LaCoO3—Co3O4: I. Preparation, characterisation and catalytic properties for the oxidation of CO. Appl. Catal. B Environ. 1997, 11, 167–179. [Google Scholar] [CrossRef]
- Liotta, L.; Di Carlo, G.; Longo, A.; Pantaleo, G.; Deganello, G.; Marci, G.; Martorana, A. Structural and morphological properties of Co–La catalysts supported on alumina/lanthana for hydrocarbon oxidation. J. Non-Cryst. Solids 2004, 345, 620–623. [Google Scholar] [CrossRef]
- Liu, S.; Zhang, W.; Deng, T.; Wang, D.; Wang, X.; Zhang, X.; Zhang, C.; Zheng, W. Mechanistic Origin of Enhanced CO Catalytic Oxidation over Co3O4/LaCoO3 at Lower Temperature. ChemCatChem 2017, 9, 3102–3106. [Google Scholar] [CrossRef]
- Abadian, L.; Malekzadeh, A.; Khodadadi, A.A.; Mortazavi, Y. Effects of Excess Cobalt Oxide Nanocrystallites on LaCoO3 Catalyst on Lowering the Light off Temperature of CO and Hydrocarbons Oxidation. Iran. J. Chem. Chem. Eng. 2008, 27, 71–77. [Google Scholar]
- Atkinson, A. Chemically-induced stresses in gadolinium-doped ceria solid oxide fuel cell electrolytes. Solid State Ionics 1997, 95, 249–258. [Google Scholar] [CrossRef]
- Lee, J.G.; Park, J.H.; Shul, Y.G. Tailoring gadolinium-doped ceria-based solid oxide fuel cells to achieve 2 W cm−2 at 550 °C. Nat. Commun. 2014, 5, 4045. [Google Scholar] [CrossRef] [PubMed]
- Fuentes, R.; Baker, R. Synthesis and properties of Gadolinium-doped ceria solid solutions for IT-SOFC electrolytes. Int. J. Hydrog. Energy 2008, 33, 3480–3484. [Google Scholar] [CrossRef]
- Hussain, S.; Yangping, L. Review of solid oxide fuel cell materials: Cathode, anode, and electrolyte. Energy Transit. 2020, 4, 113–126. [Google Scholar] [CrossRef]
- Rehman, S.U.; Song, R.-H.; Lim, T.-H.; Park, S.-J.; Hong, J.-E.; Lee, J.-W.; Lee, S.-B. High-performance nanofibrous LaCoO3 perovskite cathode for solid oxide fuel cells fabricated via chemically assisted electrodeposition. J. Mater. Chem. A 2018, 6, 6987–6996. [Google Scholar] [CrossRef]
- Ivers-Tiffée, E.L.; Weber, A.; Herbstritt, D. Materials and technologies for SOFC-components. J. Eur. Ceram. Soc. 2001, 21, 1805–1811. [Google Scholar] [CrossRef]
- Mihara, S.; Kobayashi, K.; Akashi, T.; Sakka, Y. Chemical Reactivity and Cathode Properties of LaCoO3 on Lanthanum Silicate Oxyapatite Electrolyte. Key Eng. Mater. 2014, 616, 120–128. [Google Scholar] [CrossRef]
- Khalil, M.S. Synthesis, X-ray, infrared spectra and electrical conductivity of La/Ba–CoO3 systems. Mater. Sci. Eng. A 2003, 352, 64–70. [Google Scholar] [CrossRef]
- Wang, C.; Xiao, M.; Hu, J.; Ling, C.; Zhang, C.; Lan, J.; Hui, Y.; Liu, D. Conductivity and Infrared Absorption of La1−xBaxCoO3 Conductive Ceramics. J. Solid State Chem. 1998, 137, 211–213. [Google Scholar] [CrossRef]
- Shuk, P.; Charton, V.; Samochval, V. Mixed Conductors on the Lanthanid Cobaltites Basis. Mater. Sci. Forum 1991, 76, 161–164. [Google Scholar] [CrossRef]
- Tišler, Z.; Klegová, A.; Svobodová, E.; Šafář, J.; Strejcová, K.; Kohout, J.; Šlang, S.; Pacultová, K.; Rodríguez-Padrón, D.; Bulánek, R. Cobalt Based Catalysts on Alkali-Activated Zeolite Foams for N2O Decomposition. Catalysts 2020, 10, 1398. [Google Scholar] [CrossRef]
- Inger, M.; Moszowski, B.; Ruszak, M.; Rajewski, J.; Wilk, M. Two-Stage Catalytic Abatement of N2O Emission in Nitric Acid Plants. Catalysts 2020, 10, 987. [Google Scholar] [CrossRef]
- Karásková, K.; Pacultová, K.; Jirátová, K.; Fridrichová, D.; Koštejn, M.; Obalová, L. K-Modified Co–Mn–Al Mixed Oxide—Effect of Calcination Temperature on N2O Conversion in the Presence of H2O and NOx. Catalysts 2020, 10, 1134. [Google Scholar] [CrossRef]
- Bernauer, M.; Bernauer, B.; Sádovská, G.; Sobalík, Z. High-temperature decomposition of N2O from the HNO3 production: Process feasibility using a structured catalyst. Chem. Eng. Sci. 2020, 220, 115624. [Google Scholar] [CrossRef]
- Pasha, N.; Lingaiah, N.; Babu, N.S.; Reddy, P.S.S.; Prasad, P.S. Studies on cesium doped cobalt oxide catalysts for direct N2O decomposition in the presence of oxygen and steam. Catal. Commun. 2008, 10, 132–136. [Google Scholar] [CrossRef]
- Armor, J.; Braymer, T.; Farris, T.; Li, Y.; Petrocelli, F.; Weist, E.; Kannan, S.; Swamy, C. Calcined hydrotalcites for the catalytic decomposition of N2O in simulated process streams. Appl. Catal. B 1996, 7, 397–406. [Google Scholar] [CrossRef]
- Centi, G.; Cerrato, G.; D’Angelo, S.; Finardi, U.; Giamello, E.; Morterra, M.; Perathoner, S. Reactivity, Stability and Characterization of Cu-ZrO2 Catalysts for the Decomposition of N2O in Industrial Effluents. In Proceedings of the Environmental Catalysis: 1st World Conference, Pisa, Italy, 1–5 May 1995; pp. 175–178. [Google Scholar]
- Centi, G.; Galli, A.; Montanari, B.; Perathoner, S.; Vaccaria, A. Catalytic decomposition of N2O over noble and transition metal containing oxides and zeolites. Role of some variables on reactivity. Catal. Today 1997, 35, 113–120. [Google Scholar] [CrossRef]
- Marnellos, G.E.; Efthimiadis, E.A.; Vasalos, I.A. Effect of SO2 and H2O on the N2O decomposition in the presence of O2 over Ru/Al2O3. Appl. Catal. B 2003, 46, 523–539. [Google Scholar] [CrossRef]
- Oi, J.; Obuchi, A.; Bamwenda, G.R.; Ogata, A.; Yagita, H.; Kushiyama, S.; Mizuno, K. Decomposition of nitrous oxide over supported rhodium catalysts and dependency on feed gas composition. Appl. Catal. B 1997, 12, 277–286. [Google Scholar] [CrossRef]
- Kapteijn, F.; Marbán, G.; Rodríguez-Mirasol, J.; Moulijn, J.A. Kinetic Analysis of the Decomposition of Nitrous Oxide over ZSM-5 Catalysts. J. Catal. 1997, 167, 256–265. [Google Scholar] [CrossRef]
- Kögel, M.; Abu-Zied, B.M.; Schwefer, M.; Turek, T. The effect of NOx on the catalytic decomposition of nitrous oxide over Fe-MFI zeolites. Catal. Commun. 2001, 2, 273–276. [Google Scholar] [CrossRef]
- Tejuca, L.G.; Fierro, J.L.G.; Tascón, J.M. Structure and Reactivity of Perovskite-Type Oxides. Adv. Catal. 1989, 36, 237–328. [Google Scholar] [CrossRef]
- Wilczkowska, E.; Krawczyk, K.; Petryk, J.; Sobczak, J.W.; Kaszkur, Z. Direct nitrous oxide decomposition with a cobalt oxide catalyst. Appl. Catal. A 2010, 389, 165–172. [Google Scholar] [CrossRef]
- Giecko, G.; Borowiecki, T.; Gac, W.; Kruk, J. Fe2O3/Al2O3 catalysts for the N2O decomposition in the nitric acid industry. Catal. Today 2008, 137, 403–409. [Google Scholar] [CrossRef]
- Shen, Q.; Li, L.; Li, J.; Tian, H.; Hao, Z. A study on N2O catalytic decomposition over Co/MgO catalysts. J. Hazard. Mater. 2009, 163, 1332–1337. [Google Scholar] [CrossRef]
- Xue, L.; Zhang, C.; He, H.; Teraoka, Y. Catalytic decomposition of N2O over CeO2 promoted Co3O4 spinel catalyst. Appl. Catal. B 2007, 75, 167–174. [Google Scholar] [CrossRef]
- Smeets, P.J.; Sels, B.F.; Van Teeffelen, R.M.; Leeman, H.; Hensen, E.J.M.; Schoonheydt, R.A. The catalytic performance of Cu-containing zeolites in N2O decomposition and the influence of O2, NO and H2O on recombination of oxygen. J. Catal. 2008, 256, 183–191. [Google Scholar] [CrossRef]
- Sang, C.; Lund, C.R. Possible role of nitrite/nitrate redox cycles in N2O decomposition and light-off over Fe-ZSM-5. Catal. Lett. 2001, 73, 73–77. [Google Scholar] [CrossRef]
- Mul, G.; Perez-Ramirez, J.; Kapteijn, F.; Moulijn, J.A. NO-Assisted N2O Decomposition over ex-Framework FeZSM-5: Mechanistic Aspects. Catal. Lett. 2001, 77, 7–13. [Google Scholar] [CrossRef]
- Xie, X.; Li, Y.; Liu, Z.-Q.; Haruta, M.; Shen, W. Low-temperature oxidation of CO catalysed by Co3O4 anorods. Nature 2009, 458, 746. [Google Scholar] [CrossRef] [PubMed]
- Asano, K.; Ohnishi, C.; Iwamoto, S.; Shioya, Y.; Inoue, M. Potassium-doped Co3O4 catalyst for direct decomposition of N2O. Appl. Catal. B 2008, 78, 242–249. [Google Scholar] [CrossRef]
- Wilczkowska, E.; Kowalczyk, Z.; Petryk, J.; Raróg-Pilecka, W.; Kaszkur, Z. Catalytic Decomposition of Nitrous Oxide. Pol. J. Chem. 2009, 83, 515–518. [Google Scholar]
- Barreca, D.; Massignam, C. Composition and Microstructure of Cobalt Oxide Thin Films Obtained from a Novel Cobalt(II) Precursor by Chemical Vapor Deposition. Chem. Mater. 2001, 13, 588–593. [Google Scholar] [CrossRef]
- Petitto, S.C.; Marsh, E.M.; Carson, G.A.; Langell, M.A. Cobalt oxide surface chemistry: The interaction of CoO(100), Co3O4(110) and Co3O4(111) with oxygen and water. J. Mol. Catal. A 2008, 281, 49–58. [Google Scholar] [CrossRef]
- Chuang, T.; Brundle, C.; Rice, D. Interpretation of the x-ray photoemission spectra of cobalt oxides and cobalt oxide surfaces. Surf. Sci. 1976, 59, 413–429. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iwanek, E.M.; Liotta, L.F.; Pantaleo, G.; Krawczyk, K.; Gdyra, E.; Petryk, J.; Sobczak, J.W.; Kaszkur, Z. Investigation of Co3O4 and LaCoO3 Interaction by Performing N2O Decomposition Tests under Co3O4-CoO Transition Temperature. Catalysts 2021, 11, 325. https://doi.org/10.3390/catal11030325
Iwanek EM, Liotta LF, Pantaleo G, Krawczyk K, Gdyra E, Petryk J, Sobczak JW, Kaszkur Z. Investigation of Co3O4 and LaCoO3 Interaction by Performing N2O Decomposition Tests under Co3O4-CoO Transition Temperature. Catalysts. 2021; 11(3):325. https://doi.org/10.3390/catal11030325
Chicago/Turabian StyleIwanek (nee Wilczkowska), Ewa M., Leonarda F. Liotta, Giuseppe Pantaleo, Krzysztof Krawczyk, Ewa Gdyra, Jan Petryk, Janusz W. Sobczak, and Zbigniew Kaszkur. 2021. "Investigation of Co3O4 and LaCoO3 Interaction by Performing N2O Decomposition Tests under Co3O4-CoO Transition Temperature" Catalysts 11, no. 3: 325. https://doi.org/10.3390/catal11030325
APA StyleIwanek, E. M., Liotta, L. F., Pantaleo, G., Krawczyk, K., Gdyra, E., Petryk, J., Sobczak, J. W., & Kaszkur, Z. (2021). Investigation of Co3O4 and LaCoO3 Interaction by Performing N2O Decomposition Tests under Co3O4-CoO Transition Temperature. Catalysts, 11(3), 325. https://doi.org/10.3390/catal11030325








