Recent Developments for the Application of 3D Structured Material Nickel Foam and Graphene Foam in Direct Liquid Fuel Cells and Electrolyzers
Abstract
1. Introduction
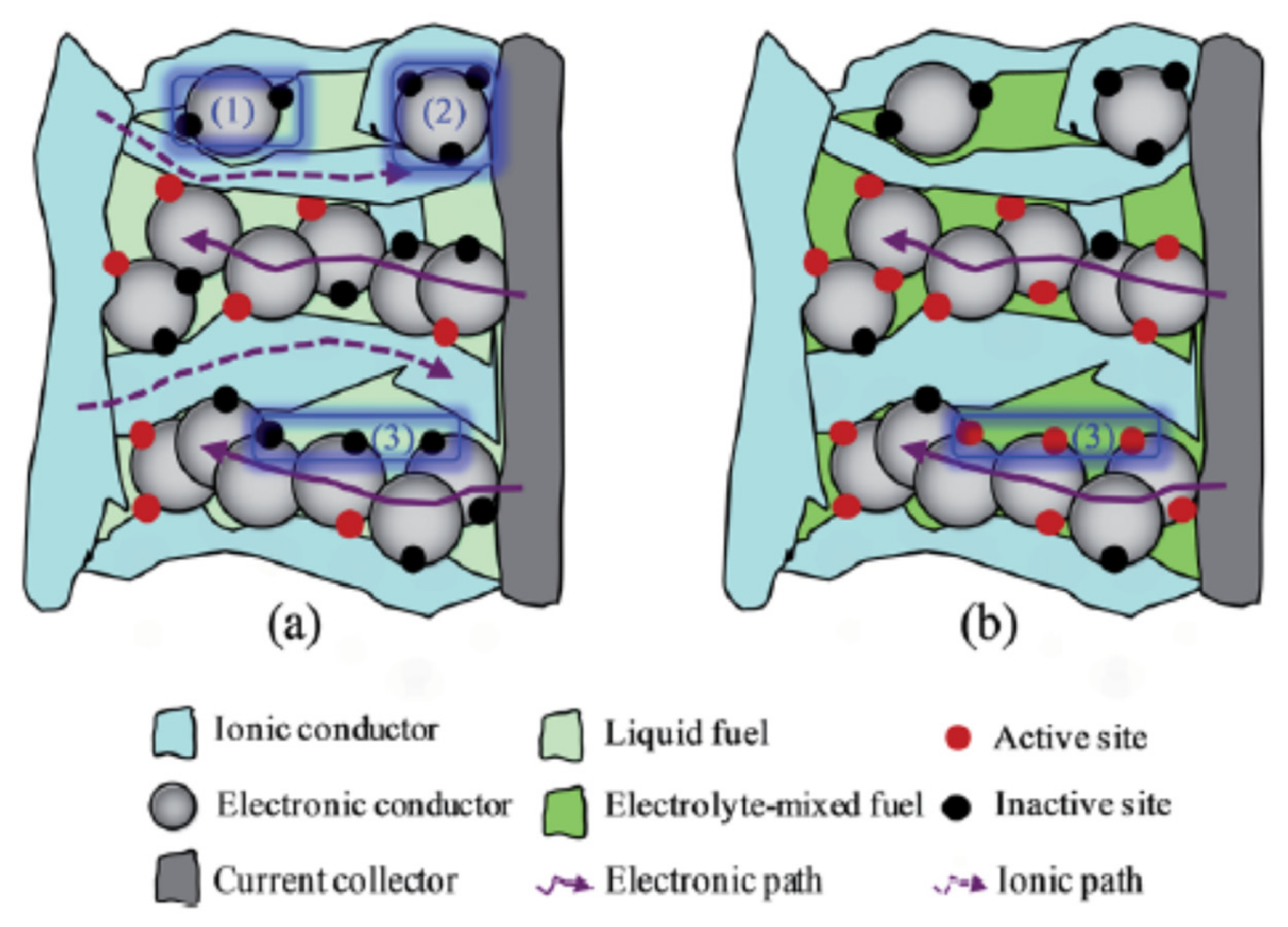
2. Challenges in Conventional Catalyst Support and Future Perspective Using 3D Structured Material Foam in DLFC
3. Current Achievement and Development of the Application of Nickel Foam and Graphene Foam in Direct Liquid Fuel Cells
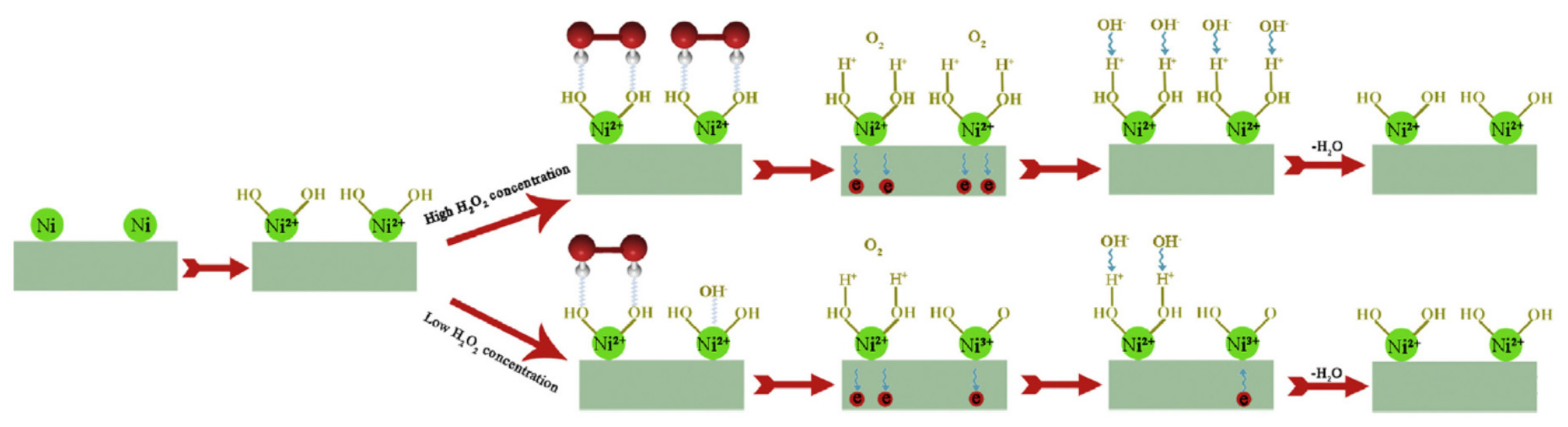
3.1. Methanol Electro-Oxidation Reaction
3.2. Ethanol Electro-Xxidation Reaction
3.3. Borohydride Electro-Oxidation Reaction
3.4. Urea Electro-Oxidation Reaction
3.5. Glucose Electro-Oxidation Reaction
3.6. Hydrogen Peroxide Electro-Oxidation and Electro-Reduction Reaction
3.7. Other Electro-Oxidation Reaction in DLFC
3.8. Selected Application of Foam in the Electrolyzers
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
Acronyms
| AQ | Anthraquinone |
| BOR | Borohydride Electro-Oxidation Reaction |
| CO | Carbon Monoxide |
| CoNPs | Co nanoparticles |
| CVD | Chemical Vapor Deposition |
| DA | 1,5-Dichloro-Anhraquinone |
| DAFC | Direct Ammonia Fuel Cells |
| DBFC | Direct Borohydride Fuel Cell |
| DEFC | Direct Ethanol Fuel Cell |
| DEGFC | Direct Ethylene Glycol Fuel Cell |
| DGFC | Direct Glycerol Fuel Cell |
| DGluFC | Direct Glucose Fuel Cell |
| DHFC | Direct Hydrazine Fuel Cell |
| DLFC | Direct Liquid Fuel Cell |
| DMFC | Direct Methanol Fuel Cell |
| DUPFC | Direct Hydrogen Peroxide Fuel Cell |
| ECSA | Electrochemical Surface Area |
| EOR | Ethanol Oxidation Reaction |
| GA | Graphene Aerogel |
| GDL | Gas Diffusion Layer |
| GF | Graphene Foam |
| GluOR | Glucose Electro-Oxidation Reaction |
| H2O2OR | Hydrogen Peroxide Electro-Oxidation |
| H2O2RR | Hydrogen Peroxide Electro-Reduction Reaction |
| if/ib | Forward Scan/Backward Scan |
| ITO | Indium Tin Oxide |
| LDH | Layered Double Hydroxides |
| MB | Methylene Blue |
| MEA | Membrane Electrolyte Assembly |
| MOR | Methanol Electro-Oxidation Reaction |
| MV | Methyl Viologen |
| NF | Nickel Foam |
| NQ | 2-Hydroxy-1,4-Naphthoquinone |
| NR | Neutral Red |
| Pd | Palladium |
| PEMFC | Polymer Electrolyte Membrane Fuel Cells |
| Pt | Platinum |
| rGO | Reduced Graphene Oxide |
| SSFF | Stainless Steel Fiber Felt |
| UER | Urea Electro-Oxidation Reaction |
| ZIF-67 | Zeolitic Imidazolate Framework-67 |
References
- Chen, X.; Ge, F.; Lai, N. Cobalt-Based coordination polymer as high activity electrocatalyst for oxygen reduction reaction: Catalysis by novel active site CoO4N2. Int. J. Energy Res. 2020, 44, 2164–2172. [Google Scholar] [CrossRef]
- Deokate, R.J.; Mujawar, S.H.; Chavan, H.S.; Mali, S.S.; Hong, C.K.; Im, H.; Inamdar, A.I. Chalcogenide nanocomposite elec-trodes grown by chemical etching of Ni-foam as electrocatalyst for efficient oxygen evolution reaction. Int. J. Energy Res. 2020, 44, 1233–1243. [Google Scholar] [CrossRef]
- Karim, N.; Rubinsin, N.; Burukan, M.; Kamarudin, S. Sustainable route of synthesis platinum nanoparticles using orange peel extract. Int. J. Green Energy 2019, 16, 1518–1526. [Google Scholar] [CrossRef]
- Hanapi, I.; Kamarudin, S.; Zainoodin, A.; Hasran, U. Membrane-Less micro fuel cell system design and performance: An overview. Int. J. Energy Res. 2019, 43, 8956–8972. [Google Scholar] [CrossRef]
- Rajeshkhanna, G.; Rao, G.R. Micro and nano-architectures of Co3O4 on Ni foam for electro-oxidation of methanol. Int. J. Hydrog. Energy 2018, 43, 4706–4715. [Google Scholar] [CrossRef]
- Yu, Y.; Cheng, Y.; Guo, M.; Li, C.; Hu, J. Ag nanoparticles supported on nickel foam: A flexible 3D electrode for methanol electrocatalytic oxidation. RSC Adv. 2017, 7, 39539–39545. [Google Scholar] [CrossRef]
- Ince, A.C.; Karaoglan, M.U.; Glüsen, A.; Colpan, C.O.; Müller, M.; Stolten, D. Semiempirical thermodynamic modeling of a direct methanol fuel cell system. Int. J. Energy Res. 2019, 43, 3601–3615. [Google Scholar] [CrossRef]
- Ong, B.; Kamarudin, S.; Basri, S. Direct liquid fuel cells: A review. Int. J. Hydrog. Energy 2017, 42, 10142–10157. [Google Scholar] [CrossRef]
- Yu, X.; Manthiram, A. Scalable membraneless direct liquid fuel cells based on a catalyst-selective strategy. Energy Environ. Mater. 2018, 1, 13–19. [Google Scholar] [CrossRef]
- Velisala, V.; Srinivasulu, G.N.; Reddy, B.S.; Rao, K.V.K. Review on challenges of direct liquid fuel cells for portable application. World J. Eng. 2015, 12, 591–606. [Google Scholar] [CrossRef]
- Fadzillah, D.; Kamarudin, S.; Zainoodin, M.; Masdar, M. Critical challenges in the system development of direct alcohol fuel cells as portable power supplies: An overview. Int. J. Hydrog. Energy 2019, 44, 3031–3054. [Google Scholar] [CrossRef]
- Cui, X.; Li, Y.; Zhao, M.; Xu, Y.; Chen, L.; Yang, S.; Wang, Y. Facile growth of ultra-small Pd nanoparticles on zeolite-templated mesocellular graphene foam for enhanced alcohol electrooxidation. Nano Res. 2019, 12, 351–356. [Google Scholar] [CrossRef]
- Shakibi Nia, N.; Guillén-Villafuerte, O.; Griesser, C.; Manning, G.; Kunze-Liebhäuser, J.; Arévalo, C.; Pastor, E.; García, G. W2C-Supported PtAuSn—A catalyst with the earliest ethanol oxidation onset potential and the highest ethanol conversion efficiency to CO2 known till date. ACS Catal. 2020, 10, 1113–1122. [Google Scholar] [CrossRef]
- Yang, M.; Shang, C.; Li, F.; Liu, C.; Wang, Z.; Gu, S.; Liu, D.; Cao, L.; Zhang, J.; Lu, Z.; et al. Synergistic electronic and mor-phological modulation on ternary Co1−xVxP nanoneedle arrays for hydrogen evolution reaction with large current density. Sci. China Mater. 2020. [Google Scholar] [CrossRef]
- Lin, Y.; Zhang, M.; Zhao, L.; Wang, L.; Cao, D.; Gong, Y. Ru doped bimetallic phosphide derived from 2D metal organic framework as active and robust electrocatalyst for water splitting. Appl. Surf. Sci. 2021, 536, 147952. [Google Scholar] [CrossRef]
- Gamea, O.E.; Ookawara, S.; Mori, S.; Ahmed, M. Performance enhancement of direct methanol fuel cell using multi-zone narrow flow fields. Int. J. Energy Res. 2019, 43, 8257–8274. [Google Scholar]
- Shrivastava, N.K.; Thombre, S.B.; Chadge, R.B. Liquid feed passive direct methanol fuel cell: Challenges and recent advances. Ionics 2015, 22, 1–23. [Google Scholar] [CrossRef]
- Ning, X.; Zhou, X.; Luo, J.; Ma, L.; Xu, X.; Zhan, L. Glycerol and formic acid electro-oxidation over Pt on S-Doped carbon nanotubes: Effect of carbon support and synthesis method on the metal-support interaction. Electrochim. Acta 2019, 319, 129–137. [Google Scholar] [CrossRef]
- Yahya, N.; Kamarudin, S.; Karim, N.; Masdar, M.; Loh, K.; Lim, K. Durability and performance of direct glycerol fuel cell with palladium-aurum/vapor grown carbon nanofiber support. Energy Convers. Manag. 2019, 188, 120–130. [Google Scholar] [CrossRef]
- Eisa, T.; Mohamed, H.O.; Choi, Y.-J.; Park, S.-G.; Ali, R.; Abdelkareem, M.A.; Oh, S.-E.; Chae, K.-J. Nickel nanorods over nickel foam as standalone anode for direct alkaline methanol and ethanol fuel cell. Int. J. Hydrog. Energy 2020, 45, 5948–5959. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, Z.; Shen, J.; Hu, Z. Micro direct methanol fuel cell: Functional components, supplies management, packaging technology and application. Int. J. Energy Res. 2016, 41, 613–627. [Google Scholar] [CrossRef]
- Zhang, Y.; Shao, Z.; Shen, Q.; Li, M.; Xu, L.; Luo, Z. Aqueous preparation of platinum nanoflowers on three-dimensional graphene for efficient methanol oxidation. Catalysts 2018, 8, 519. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, J.; Jiang, Y.; Zhou, F.; Zhong, J.; Wang, G.; Kiani, M.; Wang, R. Facile synthesis of flower-like platinum nanostructures as an efficient electrocatalyst for methanol electro-oxidation. J. Colloid Interface Sci. 2016, 479, 64–70. [Google Scholar] [CrossRef]
- Kamyabi, M.A.; Mohammadian, H.; Jadali, S.; Moharramnezhad, M. Hydrothermal syntheses of NiO-GO nanocomposite on 3D nickel foam as a support for Pt nanoparticles and its superior electrocatalytic activity towards methanol oxidation. Electroanalysis 2019, 31, 1484–1493. [Google Scholar] [CrossRef]
- Zhang, Y.-L.; Li, J.-L.; Zhao, L.; Sui, X.-L.; Zhou, Q.-Y.; Gong, X.-F.; Cai, J.-J.; Gu, D.-M.; Wang, Z.-B. Nitrogen doped carbon coated Mo modified TiO2 nanowires (NC@MTNWs-FI) with functionalized interfacial as advanced PtRu catalyst support for methanol electrooxidation. Electrochim. Acta 2020, 331, 135410. [Google Scholar] [CrossRef]
- Li, P.-W.; Li, Y.-H.; Ma, Y.-M.; Li, Q.-X. Various morphology of WO₃ modified activated carbon supported Pd catalysts with enhanced formic acid electrooxidation. J. Nanosci. Nanotechnol. 2019, 19, 7777–7784. [Google Scholar] [CrossRef]
- Li, J.-L.; Zhao, L.; Li, X.-F.; Hao, S.-E.; Wang, Z.-B. Carbon-Coated and interfacial-functionalized mixed-phase MoxTi1−xO2-δ nanotubes as highly active and durable PtRu catalyst support for methanol electrooxidation. Chem. Asian J. 2019, 14, 1549–1556. [Google Scholar] [CrossRef]
- Pham, H.Q.; Huynh, T.T.; Mai, A.T.N.; Ngo, T.M.; Bach, L.G.; Ho, V.T.T. Wire-Like Pt on mesoporous Ti0.7W0.3O2 nanomaterial with compelling electro-activity for effective alcohol electro-oxidation. Sci. Rep. 2019, 9, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.-K.; Yeh, T.-K.; Tsai, M.-C.; Chou, H.-Y.; Wu, H.-C.; Hsieh, C.-K. The hybrid nanostructure of vertically aligned cobalt sulfide nanoneedles on three-dimensional graphene decorated nickel foam for high performance methanol oxidation. Surf. Coat. Technol. 2017, 320, 536–541. [Google Scholar] [CrossRef]
- Zhang, F.; Wang, Z.; Xu, K.Q.; Xia, J.; Liu, Q.; Wang, Z. Highly dispersed ultrafine Pt nanoparticles on nickel-cobalt layered double hydroxide nanoarray for enhanced electrocatalytic methanol oxidation. Int. J. Hydrog. Energy 2018, 43, 16302–16310. [Google Scholar] [CrossRef]
- Song, C.; Wang, G.; Li, B.; Miao, C.; Ma, K.; Zhu, K.; Cheng, K.; Ye, K.; Yan, J.; Cao, D.; et al. A novel electrode of ternary CuNiPd nanoneedles decorated Ni foam and its catalytic activity toward NaBH4 electrooxidation. Electrochim. Acta 2019, 299, 395–404. [Google Scholar] [CrossRef]
- Tang, B.; Lv, Y.; Du, J.; Dai, Y.; Pan, S.; Xie, Y.; Zou, J. MoS2-Coated Ni3S2 nanorods with exposed {110} high-index facets as excellent CO-tolerant cocatalysts for Pt: Ultradurable catalytic activity for methanol oxidation. ACS Sustain. Chem. Eng. 2019, 7, 11101–11109. [Google Scholar] [CrossRef]
- Jiang, Y.; Zhou, F.; Chen, J.; Zhang, J.; Li, A.; Zeng, Y.; Wang, G. Ultralow loading palladium nanocatalysts prepared by atomic layer deposition on three-dimensional graphite-coated nickel foam to enhance the ethanol electro-oxidation reaction. RSC Adv. 2016, 6, 13207–13216. [Google Scholar] [CrossRef]
- Yi, F.; Gao, Y.; Li, H.; Yi, L.; Chen, D.; Lu, S. Nitrogen- and oxygen-codoped porous carbonaceous foam templated from high internal emulsion as PtRu catalyst support for direct methanol fuel cell. Electrochim. Acta 2016, 211, 768–776. [Google Scholar] [CrossRef]
- Kung, C.-C.; Lin, P.-Y.; Xue, Y.; Akolkar, R.; Dai, L.; Yu, X.; Liu, C.-C. Three dimensional graphene foam supported platinum–ruthenium bimetallic nanocatalysts for direct methanol and direct ethanol fuel cell applications. J. Power Sources 2014, 256, 329–335. [Google Scholar] [CrossRef]
- Li, J.; Luo, F.; Zhao, Q.; Xiao, L.; Yang, J.; Liu, W.; Xiao, D. Crystalline nickel boride nanoparticle agglomerates for enhanced electrocatalytic methanol oxidation. Int. J. Hydrog. Energy 2019, 44, 23074–23080. [Google Scholar] [CrossRef]
- Mikolajczyk, T.; Turemko, M.; Pierozynski, B. Ethanol oxidation reaction at Pd-Modified nickel foam obtained by PVD method in alkaline solution. J. Electroanal. Chem. 2014, 735, 32–35. [Google Scholar] [CrossRef]
- Zhang, C.; Lee, B.-J.; Li, H.; Samdani, J.; Kang, T.-H.; Yu, J.-S. Catalytic mechanism of graphene-nickel interface dipole layer for binder free electrochemical sensor applications. Commun. Chem. 2018, 1, 94. [Google Scholar] [CrossRef]
- Sim, Y.; Kwak, J.; Kim, S.-Y.; Jo, Y.; Kim, S.; Kim, S.Y.; Kim, J.H.; Lee, C.-S.; Jo, J.H.; Kwon, S.-Y. Formation of 3D Graphene-Ni foam heterostructures with enhanced performance and durability for bipolar plates in a polymer electrolyte membrane fuel cell. J. Mater. Chem. A 2017, 6, 1504–1512. [Google Scholar] [CrossRef]
- Pierozynski, B.; Mikolajczyk, T.; Turemko, M. On the temperature performance of ethanol oxidation reaction at palladium-activated nickel foam. Electrocatalysis 2015, 6, 173–178. [Google Scholar] [CrossRef]
- Roy, A.; Jadhav, H.S.; Cho, M.; Gil Seo, J. Electrochemical deposition of self-supported bifunctional copper oxide electrocatalyst for methanol oxidation and oxygen evolution reaction. J. Ind. Eng. Chem. 2019, 76, 515–523. [Google Scholar] [CrossRef]
- Cheng, Y.; Guo, M.; Yu, Y.; Zhai, M.; Guo, R.; Hu, J. Fabrication of coral-like Pd based Porous MnO2 nanosheet arrays on nickel foam for methanol electrooxidation. Ind. Eng. Chem. Res. 2018, 57, 10893–10904. [Google Scholar] [CrossRef]
- Jadhav, H.S.; Roy, A.; Thorat, G.M.; Gil Seo, J. Facile and cost-effective growth of a highly efficient MgCo2O4 electrocatalyst for methanol oxidation. Inorg. Chem. Front. 2018, 5, 1115–1120. [Google Scholar] [CrossRef]
- Qian, L.; Luo, S.; Wu, L.; Hu, X.; Chen, W.; Wang, X. In situ growth of metal organic frameworks derived hierarchical hollow porous Co3O4/NiCo2O4 nanocomposites on nickel foam as self-supported flexible electrode for methanol electrocatalytic oxida-tion. Appl. Surf. Sci. 2020, 503, 144306. [Google Scholar] [CrossRef]
- Wang, X.; Li, C.; Shi, G. A high-performance platinum electrocatalyst loaded on a graphene hydrogel for high-rate methanol oxidation. Phys. Chem. Chem. Phys. 2014, 16, 10142. [Google Scholar] [CrossRef]
- Sreekanth, T.; Ramaraghavulu, R.; Vattikuti, S.P.; Shim, J.; Yoo, K. Microwave synthesis: ZnCo2O4 NPs as an efficient electrocatalyst in the methanol oxidation reaction. Mater. Lett. 2019, 253, 450–453. [Google Scholar] [CrossRef]
- Shi, R.; Zhang, Y.; Wang, Z. Facile synthesis of a ZnCo2O4 electrocatalyst with three-dimensional architecture for methanol oxidation. J. Alloys Compd. 2019, 810, 151879. [Google Scholar] [CrossRef]
- Zhang, D.; Zhang, J.; Wang, H.; Cui, C.; Jiao, W.; Gao, J.; Liu, Y. Novel Ni foam based nickel oxalate derived porous NiO nanostructures as highly efficient electrodes for the electrooxidation of methanol/ethanol and urea. J. Alloys Compd. 2019, 806, 1419–1429. [Google Scholar] [CrossRef]
- Sun, X.; Li, Y.; Li, M.-J. Highly dispersed palladium nanoparticles on carbon-decorated porous nickel electrode: An effective strategy to boost direct ethanol fuel cell up to 202 mW cm–2. ACS Sustain. Chem. Eng. 2019, 7, 11186–11193. [Google Scholar] [CrossRef]
- Yang, F.; Cheng, K.; Wang, G.; Cao, D. Preparation of Au nanosheets supported on Ni foam and its electrocatalytic performance towards NaBH4 oxidation. Electrochim. Acta 2015, 159, 111–115. [Google Scholar] [CrossRef]
- Song, C.; Zhang, D.; Wang, B.; Cai, Z.; Yan, P.; Sun, Y.; Ye, K.; Cao, D.; Cheng, K.; Wang, G. Uniformly grown PtCo-Modified Co3O4 nanosheets as a highly efficient catalyst for sodium borohydride electrooxidation. Nano Res. 2016, 9, 3322–3333. [Google Scholar] [CrossRef]
- Ye, K.; Zhang, D.; Zhang, H.; Cheng, K.; Wang, G.; Cao, D. Platinum-Modified cobalt nanosheets supported on three-dimensional carbon sponge as a high-performance catalyst for hydrogen peroxide electroreduction. Electrochim. Acta 2015, 178, 270–279. [Google Scholar] [CrossRef]
- Li, Y.; He, Y. Layer reduction method for fabricating Pd-Coated Ni foams as high-performance ethanol electrode for anion-exchange membrane fuel cells. RSC Adv. 2014, 4, 16879–16884. [Google Scholar] [CrossRef]
- Verlato, E.; Cattarin, S.; Comisso, N.; Gambirasi, A.; Musiani, M.; Vázquez-Gómez, L. Preparation of Pd-Modified Ni foam electrodes and their use as anodes for the oxidation of alcohols in basic media. Electrocatalysis 2011, 3, 48–58. [Google Scholar] [CrossRef]
- Gamil, S.; El Rouby, W.M.A.; Antuch, M.; Zedan, I.T. Nanohybrid layered double hydroxide materials as efficient catalysts for methanol electrooxidation. RSC Adv. 2019, 9, 13503–13514. [Google Scholar] [CrossRef]
- Yuan, G.; Wang, L.; Zhang, X.; Wang, Q. Self-Supported Pt nanoflakes-doped amorphous Ni(OH)2 on Ni foam composite electrode for efficient and stable methanol oxidation. J. Colloid Interface Sci. 2019, 536, 189–195. [Google Scholar] [CrossRef]
- Du, J.; You, S.; Li, X.; Tang, B.; Jiang, B.; Yu, Y.; Cai, Z.; Ren, N.; Zou, J. In situ crystallization of active NiOOH/CoOOH hetero-structures with hydroxide ion adsorption sites on velutipes-like CoSe/NiSe nanorods as catalysts for oxygen evolution and Co-Catalysts for methanol oxidation. ACS Appl. Mater. Interfaces 2020, 12, 686–697. [Google Scholar] [CrossRef] [PubMed]
- Niu, X.; Zhao, H.; Lan, M. Palladium deposits spontaneously grown on nickel foam for electro-catalyzing methanol oxidation: Effect of precursors. J. Power Sources 2016, 306, 361–368. [Google Scholar] [CrossRef]
- Niu, X.; Xiong, Q.; Li, X.; Zhang, W.; He, Y.; Pan, J.; Qiu, F.; Yan, Y. Incorporating Ag into Pd/Ni Foam via cascade galvanic replacement to promote the methanol electro-oxidation reaction. J. Electrochem. Soc. 2017, 164, F651–F657. [Google Scholar] [CrossRef]
- Guo, M.; Yu, Y.; Hu, J. Enhanced electrooxidation of methanol via ion implantation of Co nanoparticles onto 3D Ni foam templates. J. Electrochem. Soc. 2017, 164, H198–H202. [Google Scholar] [CrossRef]
- Jadhav, H.S.; Roy, A.; Chung, W.-J.; Gil Seo, J. Free standing growth of MnCo2O4 nanoflakes as an electrocatalyst for methanol electro-oxidation. New J. Chem. 2017, 41, 15058–15063. [Google Scholar] [CrossRef]
- Jadhav, H.S.; Roy, A.; Chung, W.-J.; Gil Seo, J. Growth of urchin-like ZnCo2O4 microspheres on nickel foam as a binder-free electrode for high-performance supercapacitor and methanol electro-oxidation. Electrochim. Acta 2017, 246, 941–950. [Google Scholar] [CrossRef]
- Zhong, C.; Hu, W.B.; Cheng, Y.F. Recent advances in electrocatalysts for electro-oxidation of ammonia. J. Mater. Chem. A 2013, 1, 3216–3238. [Google Scholar] [CrossRef]
- Wang, Y.; Zou, S.; Cai, W.-B. Recent advances on electro-oxidation of ethanol on Pt-And Pd-Based catalysts: From reaction mechanisms to catalytic materials. Catalysts 2015, 5, 1507–1534. [Google Scholar] [CrossRef]
- Gu, L.; Qian, L.; Lei, Y.; Wang, Y.; Li, J.; Yuan, H.; Xiao, D. Microwave-Assisted synthesis of nanosphere-like NiCo2O4 consisting of porous nanosheets and its application in electro-catalytic oxidation of methanol. J. Power Sources 2014, 261, 317–323. [Google Scholar] [CrossRef]
- Wang, W.; Chu, Q.; Zhang, Y.; Zhu, W.; Wang, X.; Liu, X. Nickel foam supported mesoporous NiCo2O4 arrays with excellent methanol electro-oxidation performance. New J. Chem. 2015, 39, 6491–6497. [Google Scholar] [CrossRef]
- Tomboc, G.M.; Abebe, M.W.; Baye, A.F.; Kim, H. Utilization of the superior properties of highly mesoporous PVP modified NiCo2O4 with accessible 3D nanostructure and flower-like morphology towards electrochemical methanol oxidation reaction. J. Energy Chem. 2019, 29, 136–146. [Google Scholar] [CrossRef]
- Hassan, D.K.; El-Safty, S.A.; Khalil, K.A.; Dewidar, M.; Abu El-Maged, G. Mesoporous Carbon/Co3O4 hybrid as efficient electrode for methanol electrooxidation in alkaline conditions. Int. J. Electrochem. Sci. 2016, 11, 8374–8390. [Google Scholar] [CrossRef]
- Roy, A.; Jadhav, H.S.; Thorat, G.M.; Seo, J.G. Electrochemical growth of Co(OH)2 nanoflakes on Ni foam for methanol electrooxidation. New J. Chem. 2017, 41, 9546–9553. [Google Scholar] [CrossRef]
- Yuan, G.; Niu, X.; Chen, Z.; Wang, L.; Zhang, X.; Wang, Q. Self-Supported hierarchical shell@core Ni3 S2 @Ni foam composite electrocatalyst with high efficiency and long-term stability for methanol oxidation. ChemElectroChem 2018, 5, 2376–2382. [Google Scholar] [CrossRef]
- Moura, A.S.; Fajín, J.L.C.; Mandado, M.; Cordeiro, M.N.D.S. Ruthenium-Platinum catalysts and direct methanol fuel cells (DMFC): A review of theoretical and experimental breakthroughs. Catalysts 2017, 7, 47. [Google Scholar] [CrossRef]
- Xiao, J.; Zhang, X.; Gao, T.; Zhou, C.; Xiao, D. Electrochemical formation of multilayered NiO film/Ni foam as a high-efficient anode for methanol electrolysis. J. Solid State Electrochem. 2017, 21, 2301–2311. [Google Scholar] [CrossRef]
- Luo, Q.; Peng, M.; Sun, X.; Asiri, A.M. Hierarchical nickel oxide nanosheet@nanowire arrays on nickel foam: An efficient 3D electrode for methanol electro-oxidation. Catal. Sci. Technol. 2015, 6, 1157–1161. [Google Scholar] [CrossRef]
- Cheng, Y.; Zhai, M.; Hu, J. The fabrication of NiCu2S2 from NiCu film on Nickel foam for methanol electrooxidation and supercapacitors. Appl. Surf. Sci. 2019, 480, 505–513. [Google Scholar] [CrossRef]
- Hong, F.; Wang, M.; Ni, Y. NiO-CoO hybrid nanostructures: Preparation, characterization and application in methanol electrooxidation. J. Clust. Sci. 2018, 29, 663–672. [Google Scholar] [CrossRef]
- Gao, S.; Yang, X.; Wei, M.-J.; Liang, S.; Zang, H.-Y.; Tan, H.-Q.; Wang, Y.-H.; Li, Y.-G. One-Step synthesis of Pt based electrocatalysts encapsulated by polyoxometalate for methanol oxidation. New J. Chem. 2018, 42, 198–203. [Google Scholar] [CrossRef]
- Yu, M.; Chen, J.; Liu, J.; Li, S.; Ma, Y.; Zhang, J.; An, J. Mesoporous NiCo2O4 nanoneedles grown on 3D graphene-nickel foam for supercapacitor and methanol electro-oxidation. Electrochim. Acta 2015, 151, 99–108. [Google Scholar] [CrossRef]
- Thoufeeq, S.; Rastogi, P.K.; Sreekanth, N.; Anantharaman, M.M.R.I.; Narayanan, T.N. Nickel-Reduced graphene oxide composite foams for electrochemical oxidation processes: Towards biomolecule sensing. MRS Commun. 2018, 8, 695–702. [Google Scholar] [CrossRef]
- Sesu, D.C.; Patil, I.M.; Lokanathan, M.; Parse, H.B.; Marbaniang, P.; Kakade, B.A. Low density three-dimensional metal foams as significant electrocatalysts toward methanol oxidation reaction. ACS Sustain. Chem. Eng. 2018, 6, 2062–2068. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhang, X.; Li, J.; Qi, G. Three-Dimensional graphene as gas diffusion layer for micro direct methanol fuel cell. Int. J. Mod. Phys. B 2018, 32, 1850145. [Google Scholar] [CrossRef]
- Li, Y.; Lv, J.; He, Y. A monolithic carbon foam-supported Pd-Based catalyst towards ethanol electro-oxidation in alkaline media. J. Electrochem. Soc. 2016, 163, F424–F427. [Google Scholar] [CrossRef]
- Wang, Y.-L.; Zhao, Y.-Q.; Xu, C.-L.; Zhao, D.-D.; Xu, M.-W.; Su, Z.-X.; Li, H.-L. Improved performance of Pd electrocatalyst supported on three-dimensional nickel foam for direct ethanol fuel cells. J. Power Sources 2010, 195, 6496–6499. [Google Scholar] [CrossRef]
- Li, C.; Wen, H.; Tang, P.-P.; Wen, X.-P.; Wu, L.-S.; Dai, H.-B.; Wang, P. Effects of Ni(OH)2 morphology on the catalytic performance of Pd/Ni(OH)2/Ni foam hybrid catalyst toward ethanol electrooxidation. ACS Appl. Energy Mater. 2018, 1, 6040–6046. [Google Scholar] [CrossRef]
- Pierozynski, B.; Mikolajczyk, T. Platinum dissolution and ethanol oxidation reaction on Pt-Activated nickel foam in sodium hydroxide solution. Pol. J. Chem. Technol. 2017, 19, 41–43. [Google Scholar] [CrossRef]
- Pierozynski, B.; Mikolajczyk, T. Enhancement of ethanol oxidation reaction on Pt (PtSn)-Activated nickel foam through in situ formation of nickel oxyhydroxide layer. Electrocatalysis 2017, 8, 252–260. [Google Scholar] [CrossRef]
- Hatamie, A.; Rezvani, E.; Rasouli, A.S.; Simchi, A. Electrocatalytic oxidation of ethanol on flexible three-dimensional interconnected Nickel/Gold composite foams in alkaline media. Electroanalysis 2019, 31, 504–511. [Google Scholar] [CrossRef]
- Xu, H.-T.; Qiu, H.-J.; Fang, L.; Mu, Y.; Wang, Y. A novel monolithic three-dimensional graphene-based composite with enhanced electrochemical performance. J. Mater. Chem. 2015, 3, 14887–14893. [Google Scholar] [CrossRef]
- Liu, M.; He, S.; Chen, W. Free-Standing 3D hierarchical carbon foam-supported PtCo nanowires with “Pt Skin” as advanced electrocatalysts. Electrochim. Acta 2016, 199, 218–226. [Google Scholar] [CrossRef]
- Zhang, Z.; Dong, Y.; Wang, L.; Wang, S. Scalable synthesis of a Pd nanoparticle loaded hierarchically porous graphene network through multiple synergistic interactions. Chem. Commun. 2015, 51, 8357–8360. [Google Scholar] [CrossRef] [PubMed]
- Tsang, C.-H.A.; Hui, K.N.; Ren, L. Deposition of Pd/Graphene aerogel on nickel foam as a binder-free electrode for direct electro-oxidation of methanol and ethanol. J. Mater. Chem. A 2014, 2, 17986–17993. [Google Scholar] [CrossRef]
- Tsang, C.H.A.; Leung, D. Use of Pd-Pt loaded graphene aerogel on nickel foam in direct ethanol fuel cell. Solid State Sci. 2018, 75, 21–26. [Google Scholar] [CrossRef]
- Tsang, C.H.A.; Hui, K.N. Influence of Pd1Ptx alloy NPs on graphene aerogel/nickel foam as binder-free anodic electrode for electrocatalytic ethanol oxidation reaction. J. Power Sources 2019, 413, 98–106. [Google Scholar] [CrossRef]
- Doğan, H.Ö. Ethanol electro-oxidation in alkaline media on Pd/Electrodeposited reduced graphene oxide nanocomposite modified nickel foam electrode. Solid State Sci. 2019, 98, 106029. [Google Scholar] [CrossRef]
- Ma, J.; Gao, X.; Li, J.; Li, H. Promoting effect of tin on binder-free CoSnx-B/Ni-foam catalysts for fuel conversion efficiency in direct borohydride fuel cell. Fuel Cells 2019, 19, 609–615. [Google Scholar] [CrossRef]
- Guo, M.; Cheng, Y.; Yu, Y.; Hu, J. Ni-Co nanoparticles immobilized on a 3D Ni foam template as a highly efficient catalyst for borohydride electrooxidation in alkaline medium. Appl. Surf. Sci. 2017, 416, 439–445. [Google Scholar] [CrossRef]
- Santos, D.M.F.; Eugénio, S.; Cardoso, D.S.P.; Šljukić, B.; Montemor, M.F. Three-Dimensional nanostructured Ni–Cu foams for borohydride oxidation. Russ. J. Phys. Chem. A 2015, 89, 2449–2454. [Google Scholar] [CrossRef]
- Gouveia, W.; Bello, M.; Balčiūnaitė, A.; Eugénio, S.; Santos, D.M.F. 3d metallic foams as catalysts for hydrolysis and electrooxidation of sodium borohydride. ECS Trans. 2018, 86, 603–612. [Google Scholar] [CrossRef]
- Li, B.; Song, C.; Huang, X.; Ye, K.; Cheng, K.; Zhu, K.; Yan, J.; Cao, D.; Wang, G. A novel anode for direct borohydride-hydrogen peroxide fuel cell: Au nanoparticles decorated 3d self-supported reduced graphene oxide foam. ACS Sustain. Chem. Eng. 2019, 7, 11129–11137. [Google Scholar] [CrossRef]
- Li, B.; Song, C.; Zhang, D.; Ye, K.; Cheng, K.; Zhu, K.; Yan, J.; Cao, D.; Wang, G. Novel self-supported reduced graphene oxide foam-based CoAu electrode: An original anode catalyst for electrooxidation of borohydride in borohydride fuel cell. Carbon 2019, 152, 77–88. [Google Scholar] [CrossRef]
- Li, B.; Yan, Q.; Song, C.; Yan, P.; Ye, K.; Cheng, K.; Zhu, K.; Yan, J.; Cao, D.; Wang, G. Reduced graphene oxide foam supported CoNi nanosheets as an efficient anode catalyst for direct borohydride hydrogen peroxide fuel cell. Appl. Surf. Sci. 2019, 491, 659–669. [Google Scholar] [CrossRef]
- Guo, F.; Cao, D.; Du, M.; Ye, K.; Wang, G.; Zhang, W.; Gao, Y.; Cheng, K. Enhancement of direct urea-hydrogen peroxide fuel cell performance by three-dimensional porous nickel-cobalt anode. J. Power Sources 2016, 307, 697–704. [Google Scholar] [CrossRef]
- Ye, K.; Zhang, H.; Zhao, L.; Huang, X.; Cheng, K.; Wang, G.; Cao, D. Facile preparation of three-dimensional Ni(OH)2/Ni foam anode with low cost and its application in a direct urea fuel cell. New J. Chem. 2016, 40, 8673–8680. [Google Scholar] [CrossRef]
- Li, J.; Yao, C.; Kong, X.; Li, Z.; Jiang, M.; Zhang, F.; Lei, X. Boosting hydrogen production by electrooxidation of urea over 3D hierarchical Ni4N/Cu3N nanotube arrays. ACS Sustain. Chem. Eng. 2019, 7, 13278–13285. [Google Scholar] [CrossRef]
- Sha, L.; Ye, K.; Wang, G.; Shao, J.; Zhu, K.; Cheng, K.; Yan, J.; Wang, G.; Cao, D. Rational design of NiCo2S4 nanowire arrays on nickle foam as highly efficient and durable electrocatalysts toward urea electrooxidation. Chem. Eng. J. 2019, 359, 1652–1658. [Google Scholar] [CrossRef]
- Tang, C.; Zhao, Z.L.; Chen, J.; Li, B.; Chen, L.; Li, C.M. Se-Ni(OH)2-shelled vertically oriented NiSe nanowires as a superior electrocatalyst toward urea oxidation reaction of fuel cells. Electrochim. Acta 2017, 248, 243–249. [Google Scholar] [CrossRef]
- Qian, S.; Rao, Z.; Liu, Y.; Yan, J.; Fan, B.; Gui, Y.; Guo, F. Nickel-Rhodium bimetallic dispersions supported on nickel foam as the efficient catalyst for urea electrooxidation in alkaline medium. Electrochim. Acta 2020, 330, 135211. [Google Scholar] [CrossRef]
- Yan, X.; Zhang, W.-D.; Hu, Q.-T.; Liu, J.; Li, T.; Liu, Y.; Gu, Z.-G. Defects-Rich nickel nanoparticles grown on nickel foam as integrated electrodes for electrocatalytic oxidation of urea. Int. J. Hydrog. Energy 2019, 44, 27664–27670. [Google Scholar] [CrossRef]
- Zhan, S.; Zhou, Z.; Liu, M.; Jiao, Y.; Wang, H. 3D NiO nanowalls grown on Ni foam for highly efficient electro-oxidation of urea. Catal. Today 2019, 327, 398–404. [Google Scholar] [CrossRef]
- Sha, L.; Ye, K.; Wang, G.; Shao, J.; Zhu, K.; Cheng, K.; Yan, J.; Wang, G.; Cao, D. Hierarchical NiCo2O4 nanowire array supported on Ni foam for efficient urea electrooxidation in alkaline medium. J. Power Sources 2019, 412, 265–271. [Google Scholar] [CrossRef]
- Liu, X.; Hao, M.; Feng, M.; Zhang, L.; Zhao, Y.; Du, X.; Wang, G. A one-compartment direct glucose alkaline fuel cell with methyl viologen as electron mediator. Appl. Energy 2013, 106, 176–183. [Google Scholar] [CrossRef]
- Yang, Y.-L.; Liu, X.-H.; Hao, M.-Q.; Zhang, P.-P. Performance of a low-cost direct glucose fuel cell with an anion-exchange membrane. Int. J. Hydrog. Energy 2015, 40, 10979–10984. [Google Scholar] [CrossRef]
- Chen, J.; Zhao, C.X.; Zhi, M.M.; Wang, K.; Deng, L.; Xu, G. Alkaline direct oxidation glucose fuel cell system using silver/nickel foams as electrodes. Electrochim. Acta 2012, 66, 133–138. [Google Scholar] [CrossRef]
- Li, Z.; Liu, X.; Liu, P.; Zhang, P. The performance of electron-mediator modified activated carbon as anode for direct glucose alkaline fuel cell. Catalysts 2016, 6, 95. [Google Scholar] [CrossRef]
- Chen, J.; Zheng, H.; Kang, J.; Yang, F.; Cao, Y.; Xiang, M. An alkaline direct oxidation glucose fuel cell using three-dimensional structural Au/Ni-foam as catalytic electrodes. RSC Adv. 2017, 7, 3035–3042. [Google Scholar] [CrossRef]
- Tsang, C.H.A.; Leung, D. Pd-Pt loaded graphene aerogel on nickel foam composite as binder-free anode for a direct glucose fuel cell unit. Solid State Sci. 2017, 71, 123–129. [Google Scholar] [CrossRef]
- Zhao, Y.; Liu, X.; Wang, X.; Zhang, P.; Shi, J. Peony petal-like 3D graphene-nickel oxide nanocomposite decorated nickel foam as high-performance electrocatalyst for direct glucose alkaline fuel cell. Int. J. Hydrog. Energy 2017, 42, 29863–29873. [Google Scholar] [CrossRef]
- Wu, W.; Miao, F.; Tao, B.; Zang, Y.; Zhu, L.; Shi, C.; Chu, P.K. Hybrid ZnO-Graphene electrode with palladium nanoparticles on Ni foam and application to self-powered nonenzymatic glucose sensing. RSC Adv. 2019, 9, 12134–12145. [Google Scholar] [CrossRef]
- Rice, C.A.; Urchaga, P.; Pistono, A.O.; McFerrin, B.W.; McComb, B.T.; Hu, J. Platinum dissolution in fuel cell electrodes: Enhanced degradation from surface area assessment in automotive accelerated stress tests. J. Electrochem. Soc. 2015, 162, 1175–1180. [Google Scholar] [CrossRef]
- El-Nagar, G.A.; Derr, I.; Kottakkat, T.; Roth, C. Auspicious metal-doped-Cu2O/Cu dendrite (M = Ni, Co, Fe) catalysts for direct alkaline fuel cells: Effect of dopants. ECS Trans. 2017, 80, 1013–1022. [Google Scholar] [CrossRef]
- Song, C.; Cao, L.; Li, B.; Huang, X.; Ye, K.; Zhu, K.; Cao, D.; Cheng, K.; Wang, G. Highly efficient palladium nanoparticles decorated reduced graphene oxide sheets supported on nickel foam for hydrogen peroxide electroreduction. Appl. Surf. Sci. 2017, 426, 1046–1054. [Google Scholar] [CrossRef]
- Yang, F.; Cao, B.; Tao, Y.; Cao, D.; Zhang, Y. Nicotinamide-Assisted fabrication of high-stability gold-palladium nanoparticles on carbon fiber cloth for hydrogen peroxide electroreduction. Electrochim. Acta 2016, 210, 199–205. [Google Scholar] [CrossRef]
- Xiao, X.; Yang, F.; Cheng, K.; Wang, X.; Zhang, H.; Ye, K.; Wang, G.; Cao, D. Enhanced performance of direct peroxide/peroxide fuel cell by using ultrafine nickel ferric ferrocyanide nanoparticles as the cathode catalyst. Int. J. Hydrog. Energy 2017, 42, 22856–22865. [Google Scholar] [CrossRef]
- Yang, Y.; Dong, R.; Zhu, Y.; Li, H.; Zhang, H.; Fan, X.; Chang, H. High-Performance direct hydrogen peroxide fuel cells (DHPFCs) with silver nanowire-graphene hybrid aerogel as highly-conductive mesoporous electrodes. Chem. Eng. J. 2020, 381, 122749. [Google Scholar] [CrossRef]
- Sun, L.; He, W.; Li, S.; Shi, L.; Zhang, Y.; Liu, J. The high performance mushroom-like Pd@SnO2/Ni foam electrode for H2O2 reduction in alkaline media. J. Power Sources 2018, 395, 386–394. [Google Scholar] [CrossRef]
- Yang, F.; Cheng, K.; Xue, X.; Yin, J.; Wang, G.; Cao, D. Three-Dimensional porous Ni film electrodeposited on Ni foam: High performance and low-cost catalytic electrode for H2O2 electrooxidation in KOH solution. Electrochim. Acta 2013, 107, 194–199. [Google Scholar] [CrossRef]
- Xiao, X.; Yang, F.; Cheng, K.; Wang, X.; Yin, J.; Ye, K.; Wang, G.; Cao, D. NiCo2O4 nanostructures with various morphologies as the high-performance electrocatalysts for H2O2 electroreduction and electrooxidation. J. Electroanal. Chem. 2014, 729, 103–108. [Google Scholar] [CrossRef]
- Cheng, K.; Cao, D.; Yang, F.; Xu, Y.; Sun, G.; Ye, K.; Yin, J.; Wang, G. Facile synthesis of morphology-controlled Co3O4 nanostructures through solvothermal method with enhanced catalytic activity for H2O2 electroreduction. J. Power Sources 2014, 253, 214–223. [Google Scholar] [CrossRef]
- Shih, Y.-J.; Huang, Y.-H.; Huang, C. In-Situ electrochemical formation of nickel oxyhydroxide (NiOOH) on metallic nickel foam electrode for the direct oxidation of ammonia in aqueous solution. Electrochim. Acta 2018, 281, 410–419. [Google Scholar] [CrossRef]
- Wen, H.; Gan, L.-Y.; Dai, H.-B.; Wen, X.-P.; Wu, L.-S.; Wu, H.; Wang, P. In situ grown Ni phosphide nanowire array on Ni foam as a high-performance catalyst for hydrazine electrooxidation. Appl. Catal. B Environ. 2019, 241, 292–298. [Google Scholar] [CrossRef]
- Feng, Z.; Li, D.; Wang, L.; Sun, Q.; Lu, P.; Xing, P.; An, M. In situ grown nanosheet NiZn alloy on Ni foam for high performance hydrazine electrooxidation. Electrochim. Acta 2019, 304, 275–281. [Google Scholar] [CrossRef]
- Zhao, M.; Li, H.; Li, W.; Li, J.; Yi, L.; Hu, W.; Li, C.M. Ru-Doping enhanced Electrocatalysis of metal-organic framework nanosheets toward overall water splitting. Chem. A Eur. J. 2020, 26, 17091–17096. [Google Scholar] [CrossRef]
- Cheng, X.; Yuan, J.; Cao, J.; Lei, C.; Yang, B.; Li, Z.; Zhang, X.; Yuan, C.; Lei, L.; Hou, Y. Strongly coupling of amor-phous/crystalline reduced FeOOH/α-Ni(OH)2 heterostructure for extremely efficient water oxidation at ultra-high current density. J. Colloid Interface Sci. 2020, 579, 340–346. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.S.; Lee, J.H.; Jang, M.J.; Jeong, J.; Park, S.M.; Choi, W.-S.; Kim, Y.; Yang, J.; Choi, S.M. Co3S4 nanosheets on Ni foam via electrodeposition with sulfurization as highly active electrocatalysts for anion exchange membrane electrolyzer. Int. J. Hydrog. Energy 2020, 45, 36–45. [Google Scholar] [CrossRef]
- Wang, W.; Yang, Z.; Jiao, F.; Gong, Y. (P, W)-Codoped MoO2 nanoflowers on nickel foam as an efficient bifunctional electro-catalyst for overall water splitting. Appl. Surf. Sci. 2020, 529, 146987. [Google Scholar] [CrossRef]
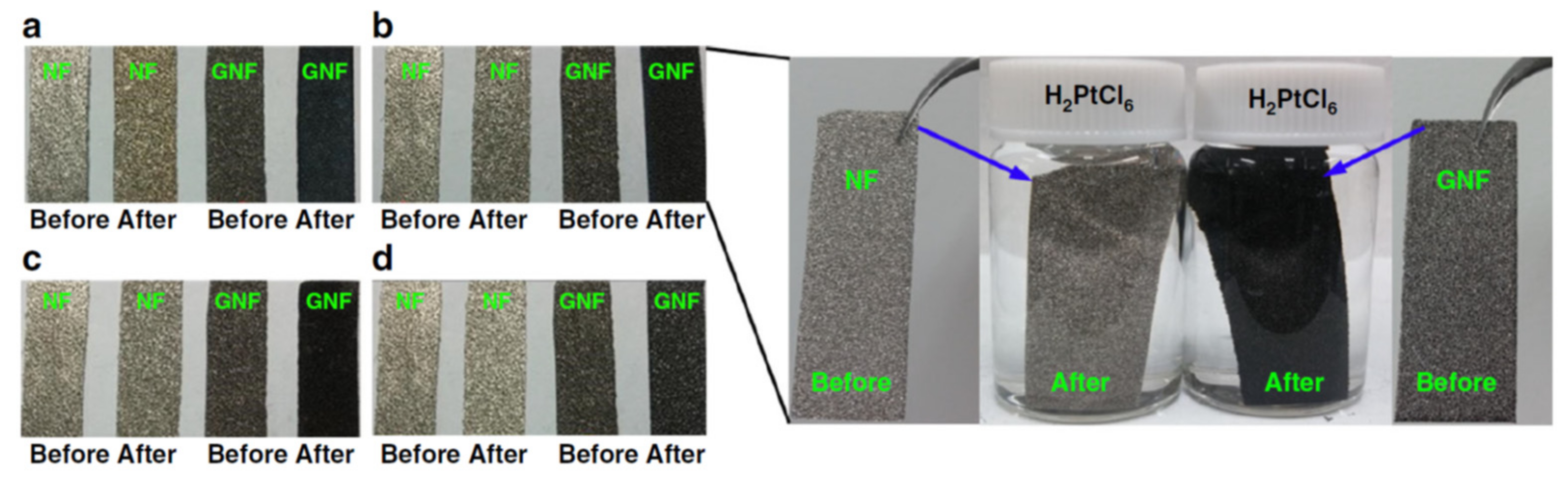

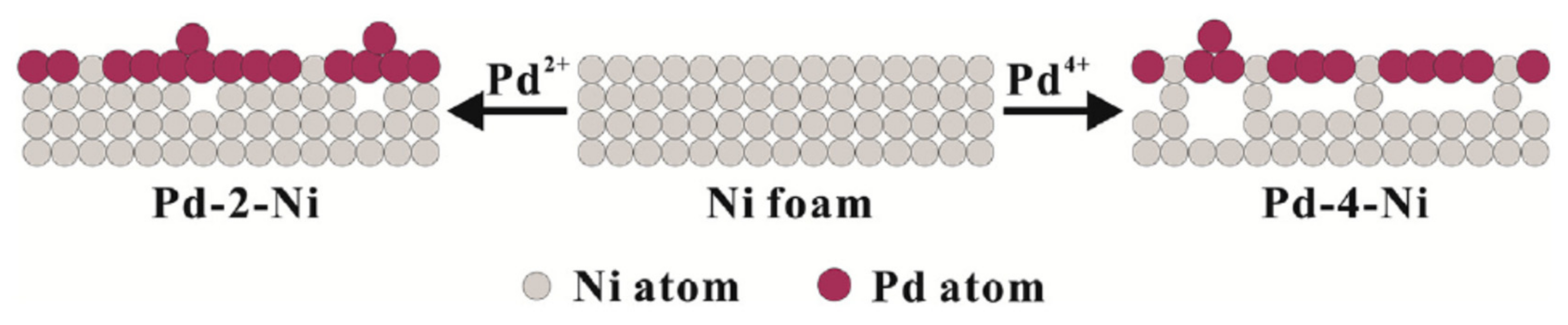

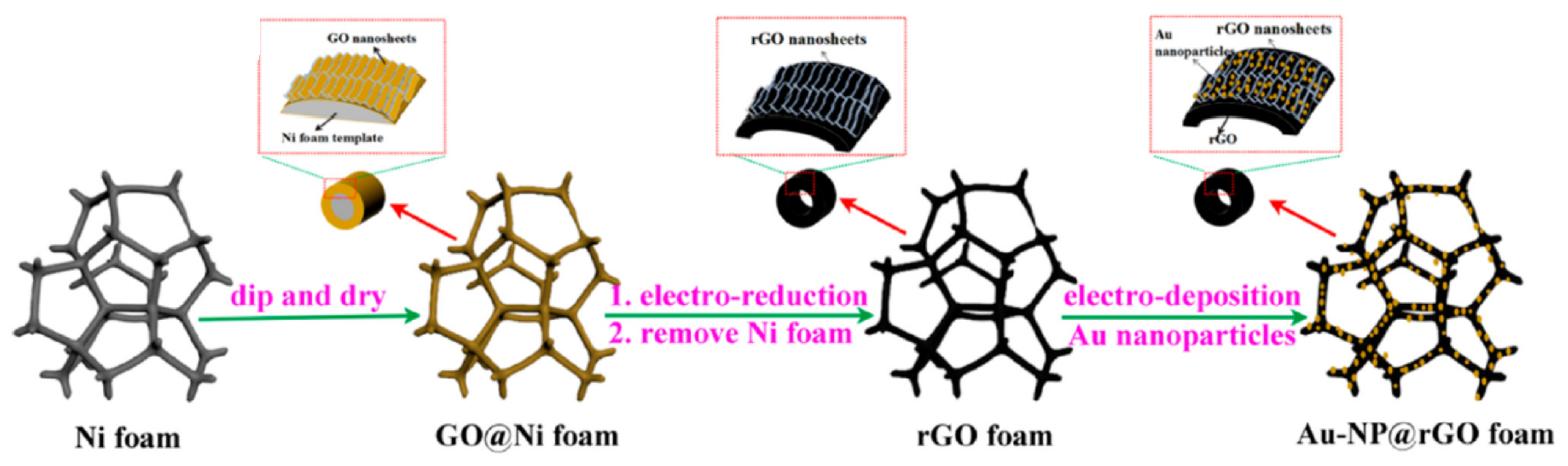
| Foam | Catalyst | Fuel Concentration (M) | Medium | Onset Potential, Oxidation Potential, Current Density | If/Ib, Mass Activity | ECSA/Surface Area, Charge Transfer Resistance (Rct), Electron Transfer | Activation Energy, Power Density | Stability, Durability and Other Information | Ref. |
| NF | Pd | 0.5 M Methanol | 1.0 M KOH | - | Mass activity = 650 A/g | - | - | - | [54] |
| NF | Pd | 1.0 M Methanol | 1.0 M NaOH | Onset potential = −0.562 V (vs. Ag/AgCl); Oxidation potential = −0.047 V (vs. Ag/AgCl) | Mass activity = 180.8 mA/mg | Electron transfer = 6 | - | The catalyst still maintain 86% of the activity after 200 cycles | [58] |
| NF | Co | 50 mM Methanol | 1.0 M NaOH | Oxidation potential = 0.8 V (vs. Ag/AgCl); Current density = 56.39 mA/cm2 | - | - | - | - | [60] |
| NF | Ag | 0.1 M Methanol | 0.1 M NaOH | Oxidation potential = 0.440 V (vs. Ag/AgCl) | - | - | - | The current density of the N-AgNPs/NF electrode reached a steady state until 5000 s. | [6] |
| NF | AgPd | 1.5 M Methanol | 1.0 M NaOH | Onset potential = −0.36 V (vs. Ag/AgCl); Oxidation potential = −0.12 V (vs. Ag/AgCl); Current density = 7.673 mA/cm2 | Mass activity = 1.887 A/mgPd | ECSA/surface area = 24.5 m2/g | - | The decrease in forward current has only a ∼9% after 50 cycles | [59] |
| NF | Pt/CoSe/NiSe | 1.0 M Methanol | 1.0 M KOH | - | If/Ib = 5.02; Mass activity = 1437.1 mA | ECSA/surface area = 85 m2/gPt; Rct = 9.6 Ω | - | The decay corresponding to the ratio of current at 3600 s to initial current (I3600/Iinitial) is approximately 0.72 | [57] |
| NF | Pt-CoNi-LDH | 0.5 M Methanol | 1.0 M NaOH | - | If/Ib = 2.06 | ECSA/surface area = 131.86 m2/g | - | High current than Pt/C/NF for 6000 s at 0.10 V | [30] |
| NF | NiO | 0.3 M Methanol | 1.0 M KOH | Oxidation potential = 0.6 V (Hg/HgO); Current density = 161.5 mA/cm2 | - | Rct = 0.4 Ω | - | The electrodes current reveal no obvious decay within 3600 s and the current retention is >92% after 500 cycles | [72] |
| NF | NiO | 0.4 M Methanol | 1.0 M NaOH | Onset potential = 0.2–0.4 V; Oxidation potential = 0.6 V; Current density = 257 mA/cm2 | - | ECSA/surface area = 4.56 m2/g | - | The CAs reached stability in a few seconds and the electrooxidation current density increases with the increase of applied potential. | [48] |
| NF | NiO | 1.0 M Methanol | 1.0 M KOH | Onset potential = 0.2–0.4 V (vs. Hg/HgO); Oxidation potential = 0.54 V (vs. Hg/HgO); Current density = 479 mA/cm2 | If/Ib > 1 | ECSA/surface area = 11.9 m2/g | - | After 3 h of CA testing, the shape was clearly maintained without any change and stable for 60 min. | [20] |
| NF | NiO nanosheet @ nanowires | 0.5 M Methanol | 1.0 M KOH | Oxidation potential = 1.62 V (vs. RHE); Current density = 89 mA/cm2 | - | Rct = 1.1 Ω | - | All electrodes show no decay in 8000 s and retains 81% of the anodic current density at 1.62 V after 1000 cycles | [73] |
| NF | NiO-CoO | 0.5 M Methanol | 1.0 M KOH | Onset potential = 0.35 V (vs. Hg/HgO); Current density = 175 µA/cm2 at 0.6 V | - | - | - | The current density of the NiO-CoO/NF electrode gradually decreased from 110 to 85 A/cm2 within the initial 2.5 h | [75] |
| NF | C/Co3O4 | 0.5 M Methanol | 0.5 M KOH | Onset potential = 0.39 V (vs. Hg/HgO) | - | ECSA/surface area = 85.7 m2/g; Rct = 5.21 Ω | - | MOR at 0.6 V (vs. Hg/HgO) exhibits about 80% retention of the original value after 600 scans. | [68] |
| NF | Co(OH)2 | 0.5 M Methanol | 0.5 M KOH | Onset potential = 0.27 V (vs. SCE) | Mass activity = 150 A/g | Rct = 1.4 Ω | - | CA test showed the 84% retention based on the initial. CV curves of Co(OH)2 catalyst are quite stable and the current density exhibits 82% retention after 500 cycles. | [69] |
| NF | Co3O4 | 0.5 M Methanol | 1.0 M KOH | Onset potential = 0.32–0.34 V (vs. Hg/HgO) | Mass activity = 28–36.2 A/g at 0.6 V (vs. Hg/HgO) | ECSA/surface area = 53–100 m2/g | - | The current retentions of nanograss Co3O4, microsphere- Co3O4 and microflower- Co3O4 materials in methanol solution after 1000 cycles at a potential of 0.6 V are found to be 66, 96 and 32%, respectively. | [5] |
| NF | NiCo2O4 | 0.5 M Methanol | 1.0 M KOH | - | Mass Activity = 40.9 A/g | ECSA/surface area = 146.5 m2/g | - | The current density performs 89% retention after 500 cycles. The current density can be returned to 97% of the original value by replacing the new solution. | [65] |
| NF | NiCo2O4 | 0.5 M Methanol | 1.0 M KOH | Onset potential = 0.16 V (vs. SCE); Current density = 134 mA/cm2 | - | Rct = 0.86 Ω cm2 | - | CA tests are performed at 0.6 V for 1000 s showed stability 88%. | [77] |
| NF | ZnCo2O4 | 0.5 M Methanol | 1.0 M KOH | Onset potential = 0.50 V (vs. Ag/AgCl) | Mass activity = 110 A/g | ECSA/surface area = 66 m2/g | - | 65% of the current density retention observed after 1000 CV cycles. | [62] |
| NF | MgCo2O4 | 0.5 M Methanol | 1.0 M KOH | Onset potential = 0.32 V (vs. Ag/AgCl) | Mass activity = 98 A/g | - | - | The current density retained was 73% after 1000 cycles. But the current density can be returned to 94% of the original value by replacing with new solution | [62] |
| GF | Pt | 1.0 MMethanol | 0.5 M H2SO4 | Onset potential = 0–0.1 V (vs. Ag/AgCl); Oxidation potential = 0.6–0.8 V (vs. Ag/AgCl) | If/Ib = 1.3; Mass activity = 113.8 mA/mgPt | ECSA/surface area = 67.5 cm2/g | - | - | [22] |
| GF | Pd | 1.0 M Methanol | 1.0 M KOH | Onset potential = −0.64 V (vs. SCE) | Mass activity = 0.835 A/mgPd | ECSA/surface area = 59 m2/g | - | Pd/GF well reserved the much higher current density after 10,000 s for the MOR reactions as compared to the Pd/C | [12] |
| GF | PtRu | 1.0 M Methanol | 0.5 M H2SO4 | Oxidation potential = 0.8–1.0 V (vs. SCE); Current density = 109.3 mA/cm2 | If/Ib = 1.14 | ECSA/surface area = 186.2 m2/g | - | The current density of PtRu/C, PtRu/Graphene, PtRu/GF were reduced by 78.8%, 54.6%, and 0.7% of their initial current density for MOR after 900 cycles, respectively | [35] |
| GA-NF | Pd | 1.0 M Methanol | 1.0 M KOH | Onset potential = 0.18 V (vs. SCE) | If/Ib = 3.11; Mass activity = 798.8 A/g | - | - | - | [89] |
| G-NF | Pt | 1.0 M Methanol | 0.5 M KOH | Onset potential = 0.42–0.45 V; Oxidation potential = 0.04 V; Current density = 139.0 mA/cm2 | If/Ib = 18.2 | ECSA/surface area = 150.3 m2/g; Rct = 4.9 Ω | - | The current density of Pt/G-Gel/NF-4 became stable to 3600 s | [45] |
| GO-NF | Pt-polyoxometalate | 1.0 M Methanol | 0.1 M KOH | Current density = 61.9 mA/cm2 | If/Ib = 5.1; Mass activity = 250.6 mA/mg | ECSA/surface area = 69.3 m2/g | - | Showed the highest mass activity until 7200 s. | [76] |
| rGO-NF | Ni | 10 μM to 4.5 mM Methanol | 0.1 M NaOH | Oxidation potential = 1.65 V (vs. RHE); Current density = 4.81 mA/cm2 | - | Rct = 95 Ω | - | - | [78] |
| G-NF | NiCo2O4 | 0.5 M Methanol | 1.0 M KOH | Onset potential = 0.4 V (vs. Hg/HgO) | Mass activity = 93.3 A/g at 0.65 V (vs. Hg/HgO) | Rct = 1.52 Ω | - | The current density has 93.4% retention of the first cycle after 500 cycles. | [77] |
| NF | Pd | 0.5 M Ethanol | 1.0 M KOH | - | Mass activity = 635 A/g | - | - | [54] | |
| NF | Pd | 0.25 M Ethanol | 0.1 M NaOH | Onset potential = 0.5 V (vs. RHE); Oxidation potential = 1.15 V (vs. RHE) | - | Rct = 0.175 Ω | Activation energy = 17 kJ/mol | - | [37] |
| NF | Pd | 1.0 M Ethanol | 1.0 M KOH | Onset potential = 0.27 V (vs. RHE); Current density = 0.2 A | - | ECSA/surface area = 63 m2/g | Power density = 164 mW/cm2 | - | [53] |
| NF | Pd | 0.25 M Ethanol | 0.1 M NaOH | Oxidation potential = 1.4 –1.5 V (vs. RHE) | - | ECSA/surface area = 13,190 cm2/g | Activation energy = 12 kJ/mol | - | [40] |
| NF | Pd | 0.25 M Ethanol | 0.1 M NaOH | Onset potential = 0.45 V (vs. RHE); Oxidation potential = 0.8 V (vs. RHE) | - | - | Activation energy = 73.8 kJ/mol | - | [37] |
| NF | Pt | 0.5 M Ethanol | 0.1 M NaOH | Oxidation potential = 1.0–1.3 V (vs. RHE) | - | - | - | - | [84] |
| NF | PtSn | 0.5 M Ethanol | 0.1 M NaOH | Oxidation potential = 1.5–2.0 V (vs. RHE) | Mass activity = 1.75 A/g | - | - | - | [85] |
| NF | Pd-Ni(OH)2 | 1.0 M Ethanol | 1.0 M NaOH | Oxidation potential = 0.8 V (vs. RHE) | Mass activity = 1295 mA/mg | ECSA/surface area = 24.61 m2/gPd; Rct = 0.49 Ω | - | Demonstrated excellent cycling stability and retained 89.6% of its initial activity after 2000 CV cycles | [83] |
| NF | Au | 1.0 M Ethanol | 0.5 M NaOH | Onset potential = 0.63 V (vs. Ag/AgCl); Oxidation potential = 0.15 V (vs. Ag/AgCl) | - | Rct = 62.5 Ω | - | ~18.8% decline in the current density is observed after 900 s. | [86] |
| NF | NiO | 0.05 M Ethanol | 1.0 M NaOH | Onset potential = 0.2–0.4 V; Current density = 87 mA/cm2 at 0.6 V | - | ECSA/surface area = 4.59 cm2/g | - | - | [48] |
| NF | NiO | 2 M Ethanol | 1.0 M KOH | Onset potential = 0.2–0.4 V (vs. Hg/HgO); Current density = 543 mA/cm2 | - | ECSA/surface area = 11.9 m2/g | - | The current density is stable for 60 min. | [20] |
| GF | Pd | 1.0 M Ethanol | 1.0 M KOH | Onset potential = −0.5 V (vs. Hg/HgO) | - | - | - | The peak current density exhibits negligible change after the 100th cycle | [89] |
| GF | PdCo | 1.0 M Ethanol | 1.0 M KOH | Oxidation potential = −0.2 V (vs. SCE) | - | - | - | The EOR is relatively stable during the 2500 s testing time | [87] |
| GF | PdNi | 0.1 M Ethanol | 0.1 M KOH | Onset potential = −0.58 V (vs. MMO) | Mass activity = 0.372 A/mg | ECSA/surface area = 24.5 m2/g | - | The Pd1Ni2/CF yields a higher current than the Pd/CF within 10,000 s. | [81] |
| GF | PtRu | 1.0 M Ethanol | 0.5 M H2SO4 | Oxidation potential = 0.8−1.0 V (vs. SCE); Current density = 78.6 mA/cm2 | If/Ib = 1 | ECSA/surface area = 186.2 m2/g | - | The current density of PtRu/C, PtRu/Graphene, PtRu/GF were decreased by 98.1%, 92.3%, and 67.5% of their initial current density for EOR after 900 cycles. | [35] |
| GF | PtCo-Pt skin | 0.5 M Ethanol | 0.1 M KOH | Onset potential = −0.55 V (vs. Ag/AgCl) | If/Ib = 1.51; Mass activity = 5.11 A/mgPt | - | - | - | [88] |
| GA-NF | Pd | 1.0 M Ethanol | 1.0 M KOH | Onset potential = 0.564 V (vs. SCE) | If/Ib = 2.72; Mass activity = 874 A/g | - | - | The 7.65 wt% Pd/GA/NF electrode achieved better overall performance and stability in EOR compared to MOR. | [90] |
| Graphite coated NF | Pd | 1.0 M Ethanol | 1.0 M KOH | Onset potential = −601 mV (vs. Hg/HgO); Oxidation potential = −193 mV (vs. Hg/HgO); Current density = 39.97 mA/cm2 | - | ECSA/surface area = 58.84 m2/g | - | The peaking current density of the as-prepared catalysts was about 2.64 times as high as that of commercial Pd/C to EOR. | [33] |
| rGO-NF | Pd | 1.0 M Ethanol | 0.5 M NaOH | Oxidation potential = 0.02 V (vs. Ag/AgCl); Current density = ~130 mA/cm2 | If/Ib = 0.87 | - | - | Current density on the Pd/ERGO was significantly slower to decay for 3200 s. | [93] |
| Carbon-NF | Pd | 1.0 M Ethanol | 1.0 M KOH | Onset potential = 0.3 V (vs. RHE); Current density = 0.16 A/cm2 | - | ECSA/surface area = 121.8 m2/g | Power density = 202 mW/cm2 | Stable in 16 h discharge at 100 mA/cm2 | [49] |
| GA-NF | PdPt | 4.0 M Ethanol | 5.0 M KOH | - | - | - | Power density = 3.6 mW/cm2 | - | [91] |
| GA-NF | PdPt | 1.0 M Ethanol | 1.0 M KOH | Oxidation potential = 0.245 V (vs. SCE) | If/Ib = 1.24; Mass activity = 3408.7 A/g | - | - | The Pd1Pt1.03/GA-NF electrode exhibits high activity and stability in EOR under a long operation (1000 cycles) | [92] |
| NF | Au | 0.05–0.2 M NaBH4 | 2.0 M NaOH | Oxidation potential = −0.7 V (vs. Ag/AgCl); Current density = 827 mA/cm2 | - | - | - | After 1200 s test, the oxidation current densities of 170 mA/cm2, 35 mA/cm2 and 4 mA/cm2 can be obtained at −0.4 V, −0.6 V and −0.8 V, respectively | [50] |
| NF | NiCo | 0.1 M NaBH4 | 1.0 M NaOH | Onset potential = −1.0 V (vs. Ag/AgCl) | - | - | - | The current density reached steady state at all applied potentials after a rapid decrease | [95] |
| NF | CuNiPd | 0.3 M NaBH4 | 2.0 M NaOH | Current density = 710 mA/cm2 at 0 V | - | Electron transfer = 4.9 | Activation energy = 18.42 kJ/mol | The current density is maintain after change several potential. | [31] |
| NF | PtCo-Co3O4 | 0.2 M NaBH4 | 2.0 M NaOH | Current density = 850 mA/cm2 at −0.4 V | - | - | - | the current densities become stable after only dozens of seconds when the applied potentials are fixed at −0.6, −0.8, and −1.0 V | [51] |
| NF | CoSn0.33-B | 0.2 M KBH4 | 1.0 M KOH | - | - | - | Power density = 158 mW/cm2 | Specific capacity or fuel conversion efficiency decrease with the catalytic activity increases. | [94] |
| rGOF | Au | 0.4 M NaBH4 | 2.0 M NaOH | Current density = 661 mA/cm2 | - | Electron transfer = 7.2 | Power density = 50 mW/cm2 | Open-circuit voltage (OCV) of 1.60 V | [98] |
| rGOF | CoAu | 0.3 M NaBH4 | 2.0 M NaOH | Current density = 1.35 A/cm2 at 0 V | - | ECSA/surface area = 390 m2/g; Electron transfer = 6.9 | Power density = 80.5 mW/cm2 at 85 mA/cm2 | 55.4% utilization efficiency of NaBH4 | [99] |
| rGOF | CoNi | 0.5 M NaBH4 | 4.0 M NaOH | Current density = 1.54 A/cm2 | - | Rct = 0.286 Ω cm2; Electron transfer = 6.7 | Activation energy = 8.29 kJ/mol; Power density = 140 mW/cm2 | - | [100] |
| NF | NiCo | 0.33 M Urea | 5.0 M KOH | - | - | - | Power density = 17.4 mW/cm2 and 31.5 mW/cm2 at 20 °C and 70 °C, respectively. | Open circuit voltage of 0.83 V | [101] |
| NF | NiRh | 0.05 M Urea | 1.0 M KOH | Onset potential = 0.33 V; Current density = 131.9 mA/cm2 | - | - | - | The retention current is 17.4% in comparison between 50 s and 1800 s | [106] |
| NF | NiO | 0.1 M Urea | 1.0 M NaOH | Onset potential = 0.2–0.4 V; Current density = 155 mA/cm2 at 0.6 V | - | ECSA/surface area = 4.59 cm2/g | - | The potential of 0.30 V is close to the onset oxidation potential and the stable current density is only around 2 mA/cm2 at this potential. | [48] |
| NF | NiO | 0.33 M Urea | 1.0 M KOH | Onset potential = 0.35 V (vs. Hg/HgO); Current density = 800 mA/cm2 at 0.7 V | - | - | - | Did not show any morphology change after being used for urea electro-oxidation for 12 h. | [108] |
| NF | Ni(OH)2 | 0.6 M Urea | 5.0 M KOH | Onset potential = 0.21 V (vs. Ag/AgCl); Current density = 559 mA/cm2 at 0.56 V (vs. Ag/AgCl) | - | - | Power density = 19.7 mW/cm2 and 28.8 mW/cm2 at 20 °C and 50 °C, respectively. | Open circuit voltage of 0.86 V | [102] |
| NF | Ni(OH)2 | 0.33 M Urea | 2.0 M KOH | Onset potential = 0.35 V (vs. Hg/HgO) | - | Rct = 0.4 Ω | - | Negligible change in potential over the operation period of 5 h. | [107] |
| NF | Se-Ni(OH)2 shelled-NiSe nanowire | 0.33 M Urea | 1.0 M KOH | Current density = 100 mA/cm2 at 0.366 V (vs. SCE) | - | Rct = 6 Ω | - | The potential remains constant for the rest of 500 s | [105] |
| NF | NiCo2S4 nanowire | 0.33 M Urea | 5.0 M KOH | Onset potential = 0.18 V (vs. Ag/AgCl); Current density = 720 mA/cm2 | - | Rct = 0.12 Ω | - | The current densities at different potentials nearly stable after 7200 s | [104] |
| NF | NiCoO4 nanowire | 0.33 M Urea | 5.0 M KOH | Onset potential = 0.19 V (vs. Ag/AgCl); Current density = 570 mA/cm2 at 0.6 V | - | - | - | The current density remained nearly constant without any reduction after 1800 s | [108] |
| NF | - | 1.0 M Glucose | 3.0 M KOH | Current density = 0.03 A/cm2 | - | Mediator = methyl viologen | Power density = 0.62 mW/cm2 at current density 5.03 mA/cm2 | - | [110] |
| NF | - | 1.0 M Glucose | 3.0 M KOH | - | - | Mediator = methyl viologen | Power density = 5.20 W/m2 at 15 mM methyl viologen | Specific capacity = 153.58 mAh/g | [111] |
| NF | - | 1.0 M Glucose | 3.0 M KOH | - | - | Rct = 0.4522 Ω; Mediator = 4-naphthoquinone (NQ) | Power density = 16.10 W/cm2 at current density 48.09 A/m2 | Open circuit voltage of 0.76 V | [113] |
| NF | Au | 0.5 M Glucose | 6.0 M KOH | - | - | - | Power density = 26.6 W/cm2 at current density 89 mA/cm2 | - | [114] |
| NF | Ag | 0.5 M Glucose | 0.5 M KOH | - | - | - | Power density = 2.03 mW/cm2 at 80 °C | - | [112] |
| GA-NF | PdPt | 0.5 M Glucose | 3.0 M KOH | - | - | - | Power density = 1.25 mW/cm2 | Open circuit voltage (OCV) of the cell at 1.1 V | [115] |
| rGO-NF | NiO | 1.0 M Glucose | 3.0 M KOH | - | - | Rct = 0.1576 Ω | Power density = 13.48 W/m2 | OCV = 0.792 V | [116] |
| rGO-NF | - | 10 µM–4.5 mM Glucose | 0.1 M NaOH | Onset Potential = 1.4 V | - | - | - | - | [78] |
| G-NF | Pd-ZnO | 0.5 M Glucose | 1.0 M KOH | Oxidation potential = 0.742 V; Current density = 222.2 mA/cm2 | If/Ib = 1.96 | - | - | - | [117] |
| Cu/CuO2 Foam | Metal-Doped (M = Ni, Co, Fe) | 2.0 mM Glucose | 0.1 M KOH | Oxidation potential = 0.5 V (vs. SCE); Current density = 30 mA/cm2 | - | - | - | - | [119] |
| NF | Ni | 0.25–2 M H2O2 | 4.0 M KOH | Onset potential = −0.2 V; Current density = 822 mA/cm2 at 0.2 V | - | - | Activation energy = 21.2 kJ/mol; Power density = 19.4 mW/cm2 | Oxidation currents were nearly constant at each potential during the test period 3000 s | [125] |
| NF | Ni | 1 M H2O2 | 4.0 M KOH | - | - | - | Power density = 22.8 mW/cm2 | - | [121] |
| NF | Ni | 1 M H2O2 | 4.0 M KOH | - | - | - | Power density = 36 mW/cm2 at 20 °C | OCP of 1.09 V | [122] |
| NF | NiCoO4 | 0.4 M H2O2 | 3.0 M KOH | Current density = 330 mA/cm2 at 0.6 V | - | - | - | The curves remained smooth without any fluctuation during the 1200 s test period at low reduction potential | [126] |
| NF | Pd | 0.5 M Glycerol | 1.0 M KOH | - | Mass activity = 1470 A/g | - | - | - | [54] |
| GF | Pd | 1.0 M Glycerol | 1.0 M KOH | Onset potential = −0.45 V (vs. SCE) | Mass activity = 2.718 A/mg Pd; ESCA/ surface area = 59 m2/g | - | - | Pd/GF well reserved the much higher current density after 10,000 s for all the EGOR and GOR reactions as compared to the Pd/C | [12] |
| GF | Pd | 1.0 M Ethylene Glycol | 1.0 M KOH | Onset potential = −0.53 V (vs. SCE) | Mass activity = 4.056 A/g Pd; ESCA/ surface area = 59 m2/g | - | - | [12] | |
| NF | Pd | 0.5 M Ethylene Glycol | 1.0 M KOH | - | Mass activity = 2100 A/g | - | - | - | [54] |
| NF | NiZn | 0.1 M N2H4 | 1.0 M NaOH | Onset potential = −0.08 V (vs. RHE); Current density = 370 mA/cm2 at 0.3 V | - | - | - | The catalytic performance of NiZn catalyst maintains 88.7%; however, the NF and Ni film only remain 58.1% and 59.1% of the initial activity after 5000 s, respectively. | [130] |
| NF | Ni(OH)2 / NiOOH | 0.03 M Ammonia | 10 mM Na2SO4 | Onset potential = 0.6 V (vs. Hg/HgO at pH 11) | - | - | - | - | [128] |
| NF | Ni phosphide nanowire | Not mention | 1.0 M NaOH | Onset potential = −0.08 V (vs. RHE); Current density = 580 mA/cm2 at 0.3 V | - | - | - | After 10000 s of constant-potential measurement, the NixP/NF catalyst still retained 80.5% of its initial activity | [129] |
| Foam | Catalyst | Fuel Concentration (M) | Medium | Onset Potential, Reduction Potential, Current Density | If/Ib, Mass Activity | ECSA/Surface Area, Charge Transfer Resistance (Rct), Electron Transfer | Activation Energy, Power Density | Stability, Durability, and Other Information | Ref. |
| rGO-NF | Pd | 0.5 M H2O2 | 2.0 M NaOH | Current density = 450 mA/cm2 at 0.8 V | - | ECSA/ surface area = 67.97 m2/g | Activation energy = 8.202 kJ/mol | All of the curves keep smooth and stable immediately after the start of the test for H2O2 reduction | [120] |
| Carbon Sponge | PtCo | 1.5 M H2O2 | 3.0 M KOH | Reduction potential = −0.30 to −0.6 V; Current density = −1.38 A/cm2.mg at −0.5 V (vs. Ag/AgCl) | - | - | - | Open circuit potentials (OCP) were −0.16 V | [52] |
| NF | Pd@ SnO2 | 0.5 M H2O2 | 3.0 M NaOH | Current density = 320 mA/cm2 at −0.54 V | - | - | - | The catalyst changes barely during 800 potential cycles | [124] |
| NF | Co3O4 | 0.5 M H2O2 | 3.0 M KOH | Current density = 0.214 A/cm2 at 0.4 V (vs. Ag/AgCl) | - | - | - | the open circuit potential (OCP) of the five samples are closed to −0.15 V | [127] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karim, N.A.; Alias, M.S.; Yang, H. Recent Developments for the Application of 3D Structured Material Nickel Foam and Graphene Foam in Direct Liquid Fuel Cells and Electrolyzers. Catalysts 2021, 11, 279. https://doi.org/10.3390/catal11020279
Karim NA, Alias MS, Yang H. Recent Developments for the Application of 3D Structured Material Nickel Foam and Graphene Foam in Direct Liquid Fuel Cells and Electrolyzers. Catalysts. 2021; 11(2):279. https://doi.org/10.3390/catal11020279
Chicago/Turabian StyleKarim, Nabila A., Muhammad Syafiq Alias, and Hsiharng Yang. 2021. "Recent Developments for the Application of 3D Structured Material Nickel Foam and Graphene Foam in Direct Liquid Fuel Cells and Electrolyzers" Catalysts 11, no. 2: 279. https://doi.org/10.3390/catal11020279
APA StyleKarim, N. A., Alias, M. S., & Yang, H. (2021). Recent Developments for the Application of 3D Structured Material Nickel Foam and Graphene Foam in Direct Liquid Fuel Cells and Electrolyzers. Catalysts, 11(2), 279. https://doi.org/10.3390/catal11020279







