Status Update on Bioelectrochemical Systems: Prospects for Carbon Electrode Design and Scale-Up
Abstract
1. Current Developments
2. Recent Highlights in Carbon Bioelectrodes
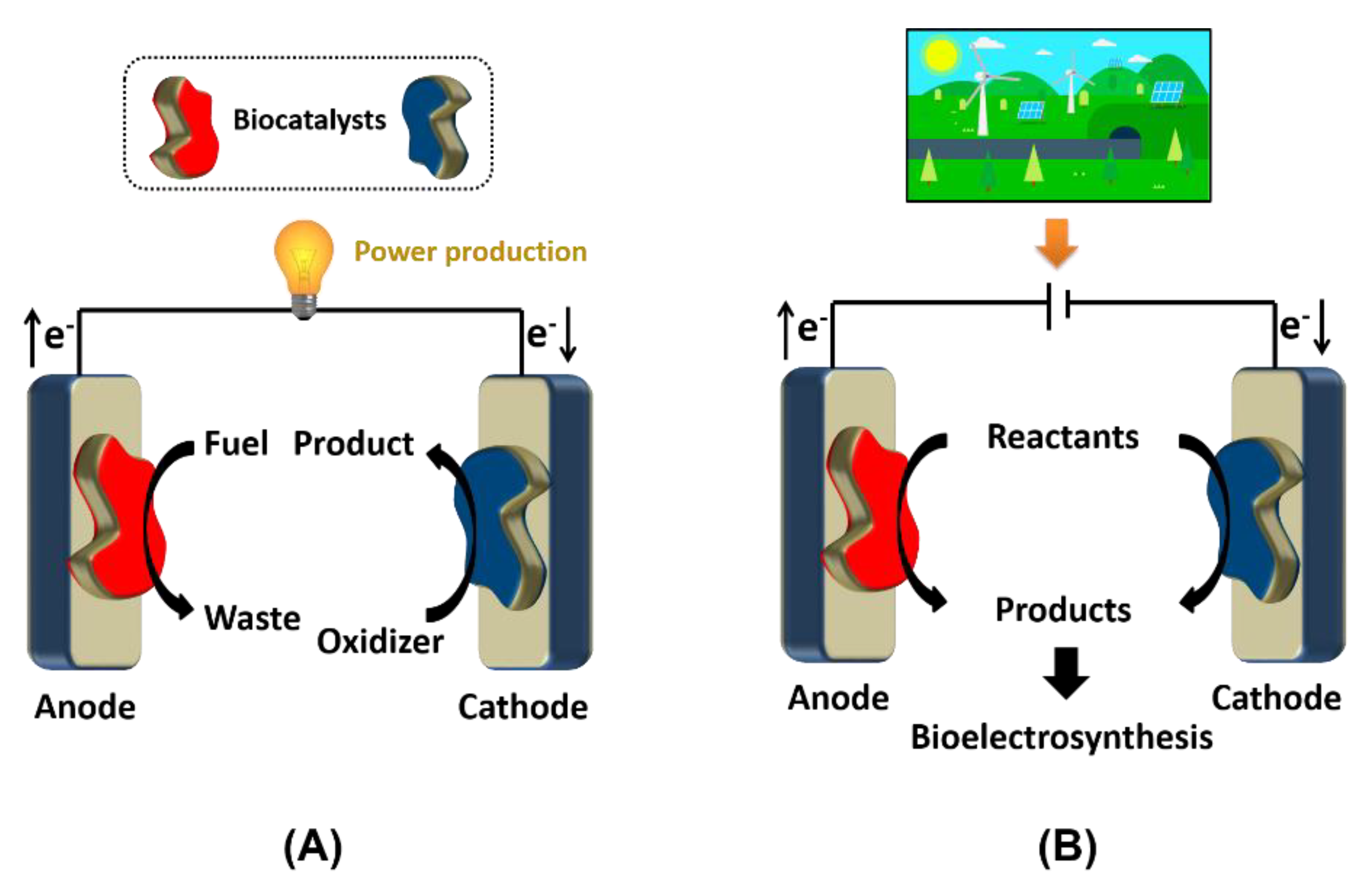
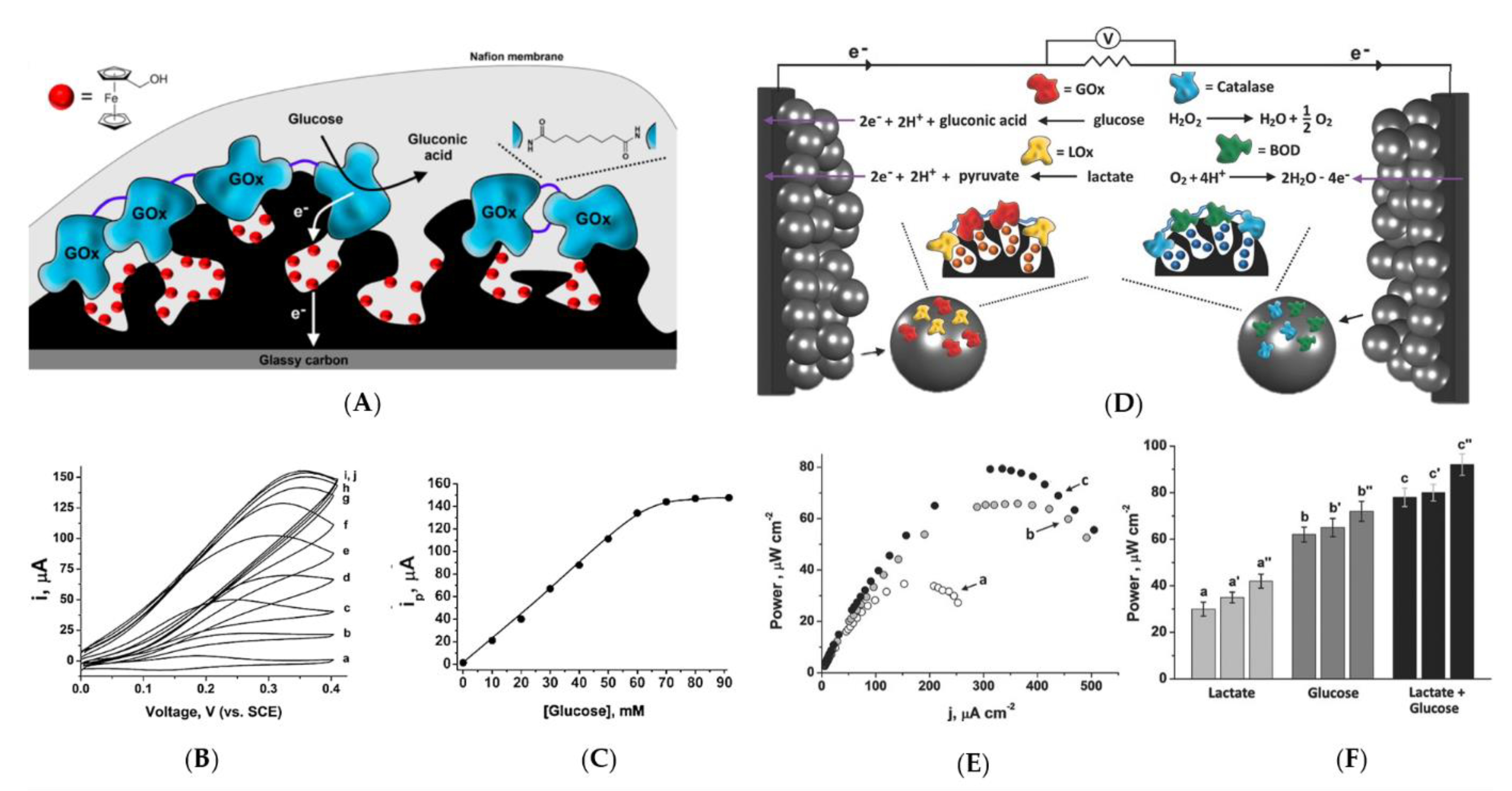
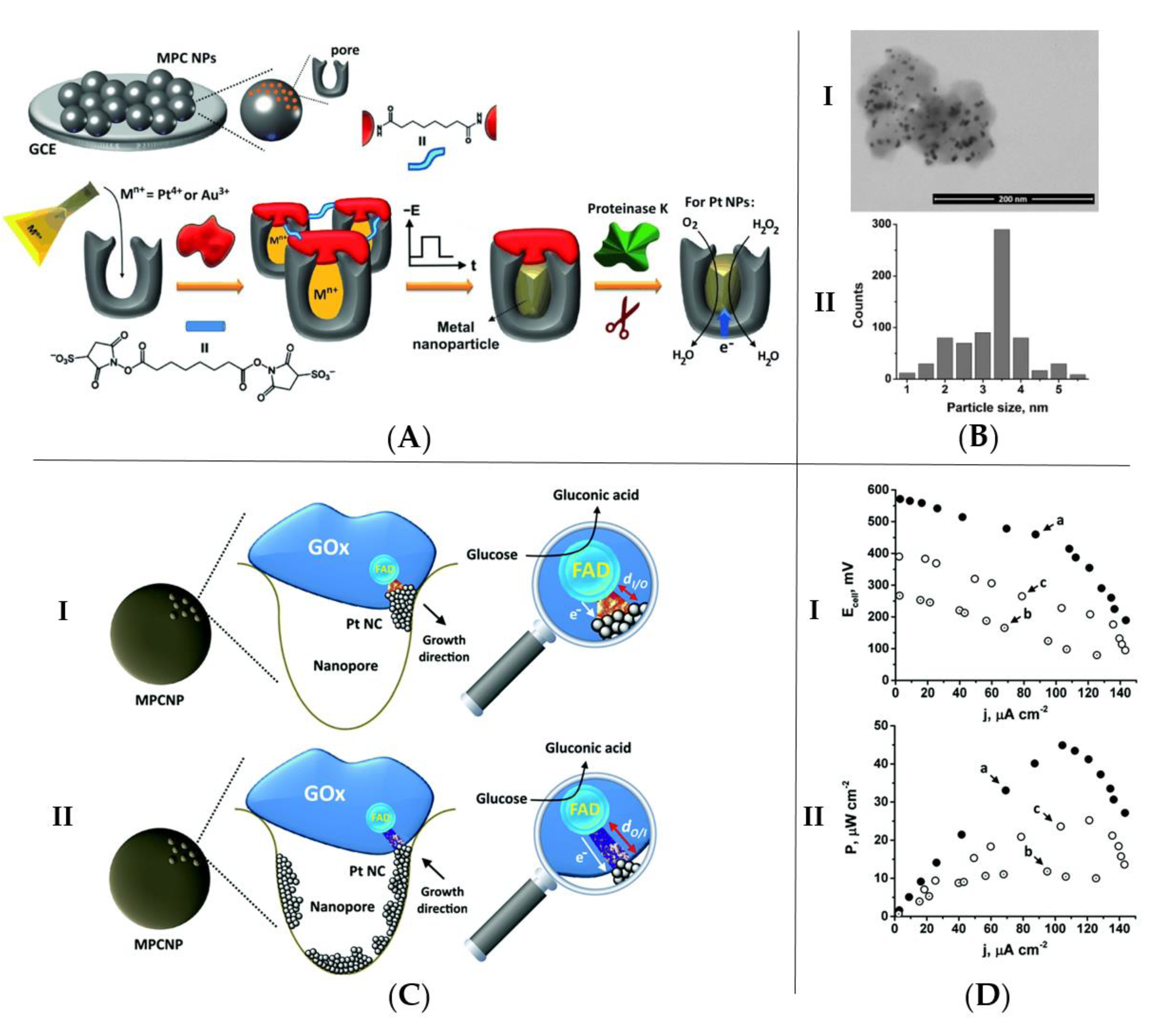
2.1. Enzymatic Electrode Designs
2.2. Microbial Electrode Designs
2.3. Applicability
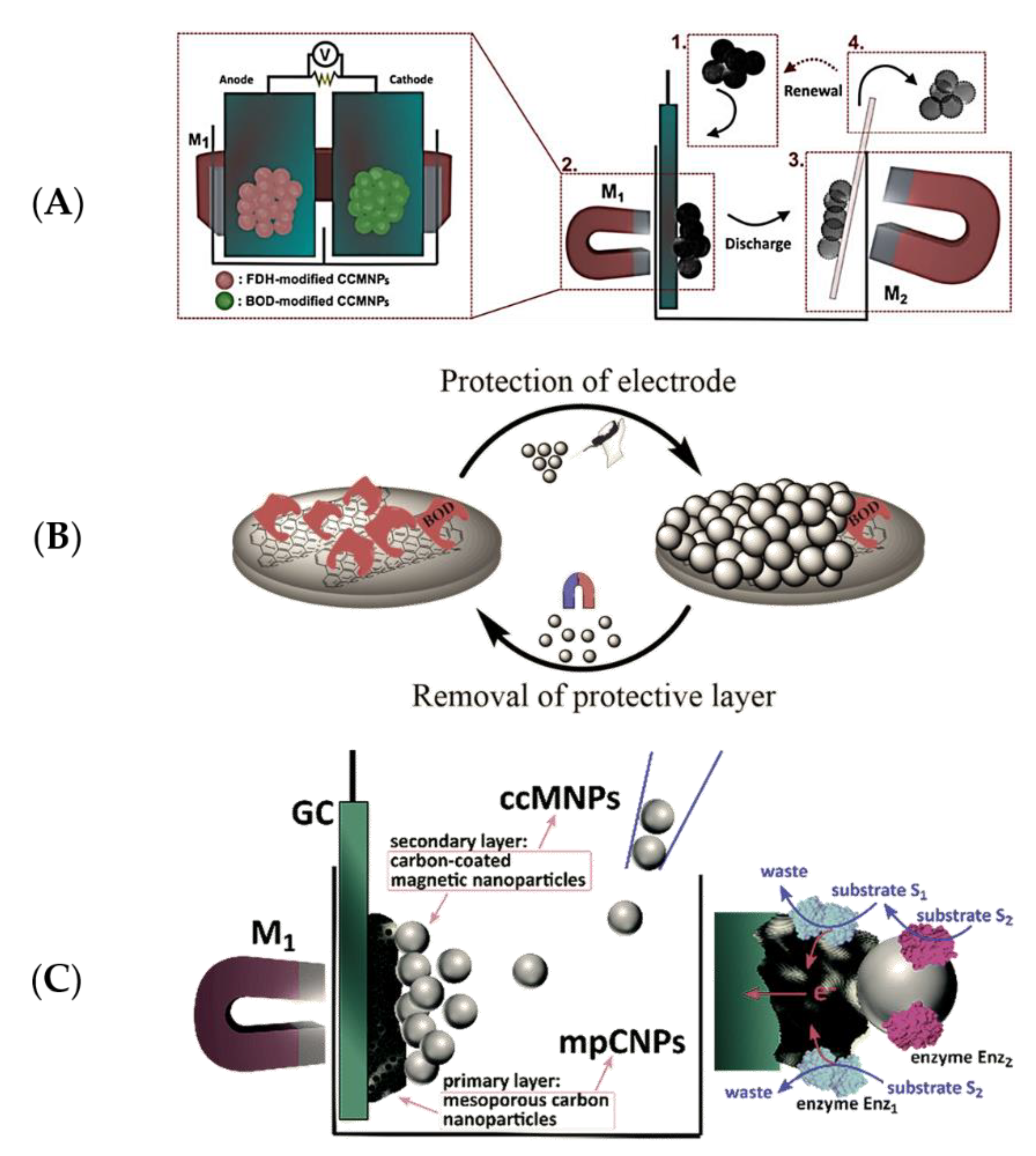
2.3.1. Stability
2.3.2. Substrate Flexibility
3. Future Synergies
Funding
Conflicts of Interest
References
- Chen, H.; Simoska, O.; Lim, K.; Grattieri, M.; Yuan, M.; Dong, F.; Lee, Y.S.; Beaver, K.; Weliwatte, S.; Gaffney, E.M.; et al. Fundamentals, applications, and future directions of bioelectrocatalysis. Chem. Rev. 2020, 120, 12903–12993. [Google Scholar] [CrossRef] [PubMed]
- Ganiyu, S.O.; Martínez-Huitle, C.A.; Rodrigo, M.A. Renewable energies driven electrochemical wastewater/soil decontamination technologies: A critical review of fundamental concepts and applications. Appl. Catal. B Environ. 2020, 270, 118857. [Google Scholar] [CrossRef]
- Miehe, R.; Full, J.; Scholz, P.; Demmer, A.; Bauernhansl, T.; Sauer, A.; Schuh, G. The biological transformation of industrial manufacturing-Future fields of action in bioinspired and bio-based production technologies and organization. Procedia Manuf. 2019, 39, 737–744. [Google Scholar] [CrossRef]
- Rovira-Alsina, L.; Balaguer, M.D.; Puig, S. Thermophilic bio-electro carbon dioxide recycling harnessing renewable energy surplus. Bioresour. Technol. 2021, 321, 124423. [Google Scholar] [CrossRef] [PubMed]
- Frijns, J.; Hofman, J.; Nederlof, M. The potential of (waste)water as energy carrier. Energy Convers. Manag. 2013, 65, 357–363. [Google Scholar] [CrossRef]
- Ghangrekar, M.M.; Das, S.; Tiwari, B.R. Integration of bioelectrochemical systems with other existing wastewater treatment processes. In Integrated Microbial Fuel Cells for Wastewater Treatment; Elsevier: Amsterdam, The Netherlands, 2020; pp. 229–248. [Google Scholar]
- Kim, B.; Jang, N.; Lee, M.; Jang, J.K.; Chang, I.S. Microbial fuel cell driven mineral rich wastewater treatment process for circular economy by creating virtuous cycles. Bioresour. Technol. 2021, 320, 124254. [Google Scholar] [CrossRef]
- Gude, V.G.; Kokabian, B.; Gadhamshetty, V. Beneficial bioelectrochemical systems for energy, water, and biomass production. J. Microb. Biochem. Technol. 2013, 5, 1–14. [Google Scholar] [CrossRef]
- Berger, M. Generating energy becomes personal. In Nanotechnology: The Future is Tiny; Royal Society of Chemistry: London, UK, 2016; pp. 1–27. [Google Scholar]
- Stegmann, P.; Londo, M.; Junginger, M. The circular bioeconomy: Its elements and role in European bioeconomy clusters. Resour. Conserv. Recycl. X 2020, 6, 100029. [Google Scholar] [CrossRef]
- Geppert, F.; Liu, D.; van Eerten-Jansen, M.; Weidner, E.; Buisman, C.; ter Heijne, A. Bioelectrochemical power-to-gas: State of the art and future perspectives. Trends Biotechnol. 2016, 34, 879–894. [Google Scholar] [CrossRef]
- Kano, K.; Shirai, O.; Kitazumi, Y.; Sakai, K.; Xia, H.-Q.; Kano, K.; Shirai, O.; Kitazumi, Y.; Sakai, K.; Xia, H.-Q. Applications to biofuel cells and bioreactors. In Enzymatic Bioelectrocatalysis; Springer: Singapore, 2021; pp. 115–131. [Google Scholar]
- Costa De Oliveira, M.A.; D’Epifanio, A.; Ohnuki, H.; Mecheri, B. Platinum group metal-free catalysts for oxygen reduction reaction: Applications in microbial fuel cells. Catalysts 2020, 10, 475. [Google Scholar] [CrossRef]
- Hernández-Flores, G.; Poggi-Varaldo, H.M.; Solorza-Feria, O. Comparison of alternative membranes to replace high cost Nafion ones in microbial fuel cells. Int. J. Hydrog. Energy 2016, 41, 23354–23362. [Google Scholar] [CrossRef]
- Mohan, S.V.; Nikhil, G.N.; Chiranjeevi, P.; Reddy, C.N.; Rohit, M.V.; Kumar, A.N.; Sarkar, O. Waste biorefinery models towards sustainable circular bioeconomy: Critical review and future perspectives. Bioresour. Technol. 2016, 215, 2–12. [Google Scholar] [CrossRef] [PubMed]
- Tominaga, M.; Kuwahara, K.; Tsushida, M.; Shida, K. Cellulose nanofiber-based electrode as a component of an enzyme-catalyzed biofuel cell. RSC Adv. 2020, 10, 22120–22125. [Google Scholar] [CrossRef]
- González-Pabón, M.J.; Cardeña, R.; Cortón, E.; Buitrón, G. Hydrogen production in two-chamber MEC using a low-cost and biodegradable poly(vinyl) alcohol/chitosan membrane. Bioresour. Technol. 2021, 319, 124168. [Google Scholar] [CrossRef]
- Winfield, J.; Chambers, L.D.; Rossiter, J.; Stinchcombe, A.; Walter, X.A.; Greenman, J.; Ieropoulos, I. Fade to green: A biodegradable stack of microbial fuel cells. ChemSusChem 2015, 8, 2705–2712. [Google Scholar] [CrossRef]
- Chen, W.; Feng, H.; Shen, D.; Jia, Y.; Li, N.; Ying, X.; Chen, T.; Zhou, Y.; Guo, J.; Zhou, M. Carbon materials derived from waste tires as high-performance anodes in microbial fuel cells. Sci. Total Environ. 2018, 618, 804–809. [Google Scholar] [CrossRef]
- Miran, W.; Nawaz, M.; Jang, J.; Lee, D.S. Sustainable electricity generation by biodegradation of low-cost lemon peel biomass in a dual chamber microbial fuel cell. Int. Biodeterior. Biodegrad. 2016, 106, 75–79. [Google Scholar] [CrossRef]
- Jiang, Y.; May, H.D.; Lu, L.; Liang, P.; Huang, X.; Ren, Z.J. Carbon dioxide and organic waste valorization by microbial electrosynthesis and electro-fermentation. Water Res. 2019, 149, 42–55. [Google Scholar] [CrossRef]
- Jafary, T.; Ghasemi, M.; Alam, J.; Aljlil, S.A.; Yusup, S. Carbon-based polymer nanocomposites as electrodes for microbial fuel cells. In Carbon-Based Polymer Nanocomposites for Environmental and Energy Applications; Elsevier Inc.: Amsterdam, The Netherlands, 2018; pp. 361–390. [Google Scholar]
- Roy, S.; Schievano, A.; Pant, D. Electro-stimulated microbial factory for value added product synthesis. Bioresour. Technol. 2015, 213, 129–139. [Google Scholar] [CrossRef]
- Mohanakrishna, G.; Kondaveeti, S.; Desale, P.; El Mekawy, A.; Abu-Reesh, I.M. Enzymatic electrosynthesis toward value addition. In Biomass, Biofuels, Biochemicals: Microbial Electrochemical Technology: Sustainable Platform for Fuels, Chemicals and Remediation; Elsevier: Amsterdam, The Netherlands, 2018; pp. 955–973. [Google Scholar]
- Srikanth, S.; Maesen, M.; Dominguez-Benetton, X.; Vanbroekhoven, K.; Pant, D. Enzymatic electrosynthesis of formate through CO2 sequestration/reduction in a bioelectrochemical system (BES). Bioresour. Technol. 2014, 165, 350–354. [Google Scholar] [CrossRef]
- Wu, R.; Ma, C.; Zhu, Z. Enzymatic electrosynthesis as an emerging electrochemical synthesis platform. Curr. Opin. Electrochem. 2020, 19, 1–7. [Google Scholar] [CrossRef]
- Logan, B.E.; Rossi, R.; Ragab, A.; Saikaly, P.E. Electroactive microorganisms in bioelectrochemical systems. Nat. Rev. Microbiol. 2019, 17, 307–319. [Google Scholar] [CrossRef] [PubMed]
- Dmitrieva, M.V.; Zolotukhina, E.V. Data describing the cofactor additives effect on bioelectrocatalytic activity of «crude» extracts. Data BR 2020, 30, 105513. [Google Scholar] [CrossRef]
- Pant, D.; Singh, A.; Van Bogaert, G.; Irving Olsen, S.; Singh Nigam, P.; Diels, L.; Vanbroekhoven, K. Bioelectrochemical systems (BES) for sustainable energy production and product recovery from organic wastes and industrial wastewaters. RSC Adv. 2012, 2, 1248–1263. [Google Scholar] [CrossRef]
- Lapinsonnière, L.; Picot, M.; Barrière, F. Enzymatic versus Microbial Bio-Catalyzed Electrodes in Bio-Electrochemical Systems. ChemSusChem 2012, 5, 995–1005. [Google Scholar] [CrossRef]
- Freguia, S.; Virdis, B.; Harnisch, F.; Keller, J. Bioelectrochemical systems: Microbial versus enzymatic catalysis. Electrochim. Acta 2012, 82, 165–174. [Google Scholar] [CrossRef]
- Liu, L.; Choi, S. Miniature microbial solar cells to power wireless sensor networks. Biosens. Bioelectron. 2021, 177, 112970. [Google Scholar] [CrossRef]
- Guang, L.; Koomson, D.A.; Jingyu, H.; Ewusi-Mensah, D.; Miwornunyuie, N. Performance of exoelectrogenic bacteria used in microbial desalination cell technology. Int. J. Environ. Res. Public Health 2020, 17, 1121. [Google Scholar] [CrossRef]
- Al-Mamun, A.; Ahmad, W.; Baawain, M.S.; Khadem, M.; Dhar, B.R. A review of microbial desalination cell technology: Configurations, optimization and applications. J. Clean. Prod. 2018, 183, 458–480. [Google Scholar] [CrossRef]
- Babadi, A.A.; Bagheri, S.; Hamid, S.B.A. Progress on implantable biofuel cell: Nano-carbon functionalization for enzyme immobilization enhancement. Biosens. Bioelectron. 2016, 79, 850–860. [Google Scholar] [CrossRef]
- Mazurenko, I.; de Poulpiquet, A.; Lojou, E. Recent developments in high surface area bioelectrodes for enzymatic fuel cells. Curr. Opin. Electrochem. 2017, 5, 74–84. [Google Scholar] [CrossRef]
- Palanisamy, G.; Jung, H.Y.; Sadhasivam, T.; Kurkuri, M.D.; Kim, S.C.; Roh, S.H. A comprehensive review on microbial fuel cell technologies: Processes, utilization, and advanced developments in electrodes and membranes. J. Clean. Prod. 2019, 221, 598–621. [Google Scholar] [CrossRef]
- Guo, K.; Prévoteau, A.; Patil, S.A.; Rabaey, K. Engineering electrodes for microbial electrocatalysis. Curr. Opin. Biotechnol. 2015, 33, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Kumar, G.G.; Sarathi, V.G.S.; Nahm, K.S. Recent advances and challenges in the anode architecture and their modifications for the applications of microbial fuel cells. Biosens. Bioelectron. 2013, 43, 461–475. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Fernández, F.J.; Pérez De Los Ríos, A.; Salar-García, M.J.; Ortiz-Martínez, V.M.; Lozano-Blanco, L.J.; Godínez, C.; Tomás-Alonso, F.; Quesada-Medina, J. Recent progress and perspectives in microbial fuel cells for bioenergy generation and wastewater treatment. Fuel Process. Technol. 2015, 138, 284–297. [Google Scholar] [CrossRef]
- Sokic-Lazic, D.; Arechederra, R.L.; Treu, B.L.; Minteer, S.D. Oxidation of biofuels: Fuel diversity and effectiveness of fuel oxidation through multiple enzyme cascades. Electroanalysis 2010, 22, 757–764. [Google Scholar] [CrossRef]
- Gilmartin, M.A.T.; Hart, J.P. Sensing with chemically and biologically modified carbon electrodes: A review. Analyst 1995, 120, 1029–1045. [Google Scholar] [CrossRef] [PubMed]
- Gong, K.; Yan, Y.; Zhang, M.; Su, L.; Xiong, S.; Mao, L. Electrochemistry and electroanalytical applications of carbon nanotubes: A review. Anal. Sci. 2005, 21, 1383–1393. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ravi, S.; Vadukumpully, S. Sustainable carbon nanomaterials: Recent advances and its applications in energy and environmental remediation. J. Environ. Chem. Eng. 2016, 4, 835–856. [Google Scholar] [CrossRef]
- Jung, S.; Myung, Y.; Das, G.S.; Bhatnagar, A.; Park, J.W.; Tripathi, K.M.; Kim, T. Carbon nano-onions from waste oil for application in energy storage devices. New J. Chem. 2020, 44, 7369–7375. [Google Scholar] [CrossRef]
- Titirici, M.M.; White, R.J.; Brun, N.; Budarin, V.L.; Su, D.S.; Del Monte, F.; Clark, J.H.; MacLachlan, M.J. Sustainable carbon materials. Chem. Soc. Rev. 2015, 44, 250–290. [Google Scholar] [CrossRef] [PubMed]
- Panda, A.; Arumugasamy, S.K.; Lee, J.; Son, Y.; Yun, K.; Venkateswarlu, S.; Yoon, M. Chemical-free sustainable carbon nano-onion as a dual-mode sensor platform for noxious volatile organic compounds. Appl. Surf. Sci. 2021, 537, 147872. [Google Scholar] [CrossRef]
- Licht, S.; Douglas, A.; Ren, J.; Carter, R.; Lefler, M.; Pint, C.L. Carbon nanotubes produced from ambient carbon dioxide for environmentally sustainable lithium-ion and sodium-ion battery anodes. ACS Cent. Sci. 2016, 2, 162–168. [Google Scholar] [CrossRef] [PubMed]
- Kuzmenko, V.; Naboka, O.; Haque, M.; Staaf, H.; Göransson, G.; Gatenholm, P.; Enoksson, P. Sustainable carbon nanofibers/nanotubes composites from cellulose as electrodes for supercapacitors. Energy 2015, 90, 1490–1496. [Google Scholar] [CrossRef]
- Lim, J.; Jin, X.; Hwang, S.J.; Scheu, C. Structural changes of 2D FexMn1−xO2 nanosheets for low-temperature growth of carbon nanotubes. Adv. Funct. Mater. 2020, 30, 2003849. [Google Scholar] [CrossRef]
- Trifonov, A.; Stemmer, A.; Tel-Vered, R. Enzymatic self-wiring in nanopores and its application in direct electron transfer biofuel cells. Nanoscale Adv. 2019, 1, 347–356. [Google Scholar] [CrossRef]
- Herkendell, K.; Stemmer, A.; Tel-Vered, R. Extending the operational lifetimes of all-direct electron transfer enzymatic biofuel cells by magnetically assembling and exchanging the active biocatalyst layers on stationary electrodes. Nano Res. 2019, 12, 767–775. [Google Scholar] [CrossRef]
- Xia, H.; Zeng, J. Rational surface modification of carbon nanomaterials for improved direct electron transfer-type bioelectrocatalysis of redox enzymes. Catalysts 2020, 10, 1447. [Google Scholar] [CrossRef]
- Kim, J.H.; Lee, D.; Bae, T.S.; Lee, Y.S. The electrochemical enzymatic glucose biosensor based on mesoporous carbon fibers activated by potassium carbonate. J. Ind. Eng. Chem. 2015, 25, 192–198. [Google Scholar] [CrossRef]
- Fu, C.; Yi, D.; Deng, C.; Wang, X.; Zhang, W.; Tang, Y.; Caruso, F.; Wang, Y. A partially graphitic mesoporous carbon membrane with three-dimensionally networked nanotunnels for ultrasensitive electrochemical detection. Chem. Mater. 2017, 29, 5286–5293. [Google Scholar] [CrossRef]
- Zhou, M.; Shang, L.; Li, B.; Huang, L.; Dong, S. The characteristics of highly ordered mesoporous carbons as electrode material for electrochemical sensing as compared with carbon nanotubes. Electrochem. Commun. 2008, 10, 859–863. [Google Scholar] [CrossRef]
- Ghasemi, E.; Shams, E.; Nejad, F.N. Covalent modification of ordered mesoporous carbon with glucose oxidase for fabrication of glucose biosensor. J. Electroanal. Chem. 2015, 752, 60–67. [Google Scholar] [CrossRef]
- Tsujimura, S.; Murata, K.; Akatsuka, W. Exceptionally high glucose current on a hierarchically structured porous carbon electrode with “wired” flavin adenine dinucleotide-dependent glucose dehydrogenase. J. Am. Chem. Soc. 2014, 136, 14432–14437. [Google Scholar] [CrossRef]
- Tao, Y.; Kanoh, H.; Abrams, L.; Kaneko, K. Mesopore-modified zeolites: Preparation, characterization, and applications. Chem. Rev. 2006, 106, 896–910. [Google Scholar] [CrossRef] [PubMed]
- Marthala, V.R.R.; Friedrich, M.; Zhou, Z.; Distaso, M.; Reuss, S.; Al-Thabaiti, S.A.; Peukert, W.; Schwieger, W.; Hartmann, M. Zeolite-coated porous arrays: A novel strategy for enzyme encapsulation. Adv. Funct. Mater. 2015, 25, 1832–1836. [Google Scholar] [CrossRef]
- Hartmann, M.; Kostrov, X. Immobilization of enzymes on porous silicas—Benefits and challenges. Chem. Soc. Rev. 2013, 42, 6277–6289. [Google Scholar] [CrossRef]
- Caldas, E.M.; Novatzky, D.; Deon, M.; de Menezes, E.W.; Hertz, P.F.; Costa, T.M.H.; Arenas, L.T.; Benvenutti, E.V. Pore size effect in the amount of immobilized enzyme for manufacturing carbon ceramic biosensor. Microporous Mesoporous Mater. 2017, 247, 95–102. [Google Scholar] [CrossRef]
- Funabashi, H.; Takeuchi, S.; Tsujimura, S. Hierarchical meso/macro-porous carbon fabricated from dual MgO templates for direct electron transfer enzymatic electrodes. Sci. Rep. 2017, 7, 1–9. [Google Scholar] [CrossRef] [PubMed]
- You, C.; Xu, X.; Tian, B.; Kong, J.; Zhao, D.; Liu, B. Electrochemistry and biosensing of glucose oxidase based on mesoporous carbons with different spatially ordered dimensions. Talanta 2009, 78, 705–710. [Google Scholar] [CrossRef]
- Bobrowski, T.; Conzuelo, F.; Ruff, A.; Hartmann, V.; Frank, A.; Erichsen, T.; Nowaczyk, M.M.; Schuhmann, W. Scalable fabrication of biophotoelectrodes by means of automated airbrush spray-coating. ChemPlusChem 2020, 85, 1396–1400. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Tian, C.; Yang, D.; Jiang, X.; Yang, R. Bioanalytical application of the ordered mesoporous carbon modified electrodes. Electroanalysis 2008, 20, 2518–2525. [Google Scholar] [CrossRef]
- Zhou, Y.; Tang, L.; Zeng, G.; Chen, J.; Wang, J.; Fan, C.; Yang, G.; Zhang, Y.; Xie, X. Amplified and selective detection of manganese peroxidase genes based on enzyme-scaffolded-gold nanoclusters and mesoporous carbon nitride. Biosens. Bioelectron. 2015, 65, 382–389. [Google Scholar] [CrossRef] [PubMed]
- Olyveira, G.M.; Kim, J.H.; Martins, M.V.A.; Iost, R.M.; Chaudhari, K.N.; Yu, J.S.; Crespilho, F.N. Flexible carbon cloth electrode modified by hollow core-mesoporous shell carbon as a novel efficient bio-anode for biofuel cell. J. Nanosci. Nanotechnol. 2012, 12, 356–360. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.; Zhou, Y.; Zeng, G.; Li, Z.; Liu, Y.; Zhang, Y.; Chen, G.; Yang, G.; Lei, X.; Wu, M. A tyrosinase biosensor based on ordered mesoporous carbon-Au/l-lysine/Au nanoparticles for simultaneous determination of hydroquinone and catechol. Analyst 2013, 138, 3552–3560. [Google Scholar] [CrossRef]
- Dai, M.; Haselwood, B.; Vogt, B.D.; La Belle, J.T. Amperometric sensing of norepinephrine at picomolar concentrations using screen printed, high surface area mesoporous carbon. Anal. Chim. Acta 2013, 788, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Zhao, H.; Deng, G.; Ran, X.; Li, Y.; Xie, X.; Li, C.P. Immunosensor for prostate-specific antigen using Au/Pd@flower-like SnO2 as platform and Au@mesoporous carbon as signal amplification. RSC Adv. 2015, 5, 74046–74053. [Google Scholar] [CrossRef]
- Zhang, Q.; Xu, G.; Gong, L.; Dai, H.; Zhang, S.; Li, Y.; Lin, Y. An enzyme-assisted electrochemiluminescent biosensor developed on order mesoporous carbons substrate for ultrasensitive glyphosate sensing. Electrochim. Acta 2015, 186, 624–630. [Google Scholar] [CrossRef]
- Justino, C.I.L.; Duarte, A.C.; Rocha-Santos, T.A.P. Recent progress in biosensors for environmental monitoring: A review. Sensors 2017, 17, 2918. [Google Scholar] [CrossRef]
- Wang, X.; Lu, X.; Wu, L.; Chen, J. Direct electrochemical tyrosinase biosensor based on mesoporous carbon and Co3O4 nanorods for the rapid detection of phenolic pollutants. ChemElectroChem 2014, 1, 808–816. [Google Scholar] [CrossRef]
- Lee, J.H.; Park, J.Y.; Min, K.; Cha, H.J.; Choi, S.S.; Yoo, Y.J. A novel organophosphorus hydrolase-based biosensor using mesoporous carbons and carbon black for the detection of organophosphate nerve agents. Biosens. Bioelectron. 2010, 25, 1566–1570. [Google Scholar] [CrossRef]
- Zhou, Y.; Tang, L.; Zeng, G.; Chen, J.; Cai, Y.; Zhang, Y.; Yang, G.; Liu, Y.; Zhang, C.; Tang, W. Mesoporous carbon nitride based biosensor for highly sensitive and selective analysis of phenol and catechol in compost bioremediation. Biosens. Bioelectron. 2014, 61, 519–525. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Campbell, A.S.; de Ávila, B.E.F.; Wang, J. Wearable biosensors for healthcare monitoring. Nat. Biotechnol. 2019, 37, 389–406. [Google Scholar] [CrossRef]
- Zeng, Y.; Zhu, Z.; Du, D.; Lin, Y. Nanomaterial-based electrochemical biosensors for food safety. J. Electroanal. Chem. 2016, 781, 147–154. [Google Scholar] [CrossRef]
- Mello, L.D.; Kubota, L.T. Review of the use of biosensors as analytical tools in the food and drink industries. Food Chem. 2002, 77, 237–256. [Google Scholar] [CrossRef]
- Kwon, K.Y.; Youn, J.; Kim, J.H.; Park, Y.; Jeon, C.; Kim, B.C.; Kwon, Y.; Zhao, X.; Wang, P.; Sang, B.I.; et al. Nanoscale enzyme reactors in mesoporous carbon for improved performance and lifetime of biosensors and biofuel cells. Biosens. Bioelectron. 2010, 26, 655–660. [Google Scholar] [CrossRef] [PubMed]
- Itoh, T.; Shibuya, Y.; Yamaguchi, A.; Hoshikawa, Y.; Tanaike, O.; Tsunoda, T.; Hanaoka, T.A.; Hamakawa, S.; Mizukami, F.; Hayashi, A.; et al. High-performance bioelectrocatalysts created by immobilization of an enzyme into carbon-coated composite membranes with nano-tailored structures. J. Mater. Chem. A 2017, 5, 20244–20251. [Google Scholar] [CrossRef]
- Habibi, B.; Jahanbakhshi, M. Direct electrochemistry of hemoglobin in a renewable mesoporous carbon ceramic electrode: A new kind of hydrogen peroxide biosensor. Microchim. Acta 2015, 182, 957–963. [Google Scholar] [CrossRef]
- Lu, X.; Xiao, Y.; Lei, Z.; Chen, J.; Zhang, H.; Ni, Y.; Zhang, Q. A promising electrochemical biosensing platform based on graphitized ordered mesoporous carbon. J. Mater. Chem. 2009, 19, 4707–4714. [Google Scholar] [CrossRef]
- Wu, L.; Lu, X.; Zhang, H.; Chen, J. Amino acid ionic liquid modified mesoporous carbon: A tailor-made nanostructure biosensing platform. ChemSusChem 2012, 5, 1918–1925. [Google Scholar] [CrossRef]
- Ania, C.O.; Gomis-Berenguer, A.; Dentzer, J.; Vix-Guterl, C. Nanoconfinement of glucose oxidase on mesoporous carbon electrodes with tunable pore sizes. J. Electroanal. Chem. 2018, 808, 372–379. [Google Scholar] [CrossRef]
- Trifonov, A.; Herkendell, K.; Tel-Vered, R.; Yehezkeli, O.; Woerner, M.; Willner, I. Enzyme-capped relay-functionalized mesoporous carbon nanoparticles: Effective bioelectrocatalytic matrices for sensing and biofuel cell applications. ACS Nano 2013, 7, 11358–11368. [Google Scholar] [CrossRef] [PubMed]
- Trifonov, A.; Tel-Vered, R.; Fadeev, M.; Willner, I. Electrically contacted bienzyme-functionalized mesoporous carbon nanoparticle electrodes: Applications for the development of dual amperometric biosensors and multifuel-driven biofuel cells. Adv. Energy Mater. 2015, 5, 1401853. [Google Scholar] [CrossRef]
- Herkendell, K.; Tel-Vered, R.; Stemmer, A. Switchable aerobic/anaerobic multi-substrate biofuel cell operating on anodic and cathodic enzymatic cascade assemblies. Nanoscale 2017, 9, 14118–14126. [Google Scholar] [CrossRef]
- Guterl, J.-K.; Sieber, V. Biosynthesis “debugged”: Novel bioproduction strategies. Eng. Life Sci. 2013, 13, 4–18. [Google Scholar] [CrossRef]
- Moehlenbrock, M.J.; Toby, T.K.; Pelster, L.N.; Minteer, S.D. Metabolon catalysts: An efficient model for multi-enzyme cascades at electrode surfaces. ChemCatChem 2011, 3, 561–570. [Google Scholar] [CrossRef]
- Mondal, K.; Ali, M.A.; Singh, C.; Sumana, G.; Malhotra, B.D.; Sharma, A. Highly sensitive porous carbon and metal/carbon conducting nanofiber based enzymatic biosensors for triglyceride detection. Sens. Actuators B 2017, 246, 202–214. [Google Scholar] [CrossRef]
- Sakai, K.; Kitazumi, Y.; Shirai, O.; Takagi, K.; Kano, K. Direct electron transfer-type four-way bioelectrocatalysis of CO2/formate and NAD+/NADH redox couples by tungsten-containing formate dehydrogenase adsorbed on gold nanoparticle-embedded mesoporous carbon electrodes modified with 4-mercaptopyridine. Electrochem. Commun. 2017, 84, 75–79. [Google Scholar] [CrossRef]
- Korani, A.; Salimi, A.; Karimi, B. Guanine/ionic liquid derived ordered mesoporous carbon decorated with AuNPs as efficient NADH biosensor and suitable platform for enzymes immobilization and biofuel cell design. Electroanalysis 2017, 29, 2646–2655. [Google Scholar] [CrossRef]
- Liu, Z.; Xu, Q.; Fu, J.; Shi, Z.; Yang, Q.; Guo, Y.; Zhang, Y.; Sun, X.; Wang, Z. Dual-signal amplification strategy aptasensor based on exonuclease III and ordered mesoporous carbon-gold nanocomposites for tetracycline detection in milk. Int. J. Electrochem. Sci. 2018, 13, 8260–8274. [Google Scholar] [CrossRef]
- Wang, L.; Bai, J.; Bo, X.; Zhang, X.; Guo, L. A novel glucose sensor based on ordered mesoporous carbon-Au nanoparticles nanocomposites. Talanta 2011, 83, 1386–1391. [Google Scholar] [CrossRef] [PubMed]
- Trifonov, A.; Tel-Vered, R.; Fadeev, M.; Cecconello, A.; Willner, I. Metal nanoparticle-loaded mesoporous carbon nanoparticles: Electrical contacting of redox proteins and electrochemical sensing applications. Electroanalysis 2015, 27, 2150–2157. [Google Scholar] [CrossRef]
- You, C.; Li, X.; Zhang, S.; Kong, J.; Zhao, D.; Liu, B. Electrochemistry and biosensing of glucose oxidase immobilized on Pt-dispersed mesoporous carbon. Microchim. Acta 2009, 167, 109–116. [Google Scholar] [CrossRef]
- Yu, J.; Yu, D.; Zhao, T.; Zeng, B. Development of amperometric glucose biosensor through immobilizing enzyme in a Pt nanoparticles/mesoporous carbon matrix. Talanta 2008, 74, 1586–1591. [Google Scholar] [CrossRef]
- Haghighi, B.; Karimi, B.; Tavahodi, M.; Behzadneia, H. Electrochemical behavior of glucose oxidase immobilized on Pd-nanoparticles decorated ionic liquid derived fibrillated mesoporous carbon. Electroanalysis 2014, 26, 2010–2016. [Google Scholar] [CrossRef]
- Sakai, K.; Sugimoto, Y.; Kitazumi, Y.; Shirai, O.; Takagi, K.; Kano, K. Direct electron transfer-type bioelectrocatalytic interconversion of carbon dioxide/formate and NAD+/NADH redox couples with tungsten-containing formate dehydrogenase. Electrochim. Acta 2017, 228, 537–544. [Google Scholar] [CrossRef]
- Yehezkeli, O.; Ovits, O.; Tel-Vered, R.; Raichlin, S.; Willner, I. Reconstituted enzymes on electropolymerizable FAD-modified metallic nanoparticles: Functional units for the assembly of effectively “wired” enzyme electrodes. Electroanalysis 2010, 22, 1817–1823. [Google Scholar] [CrossRef]
- Sonawane, J.M.; Yadav, A.; Ghosh, P.C.; Adeloju, S.B. Recent advances in the development and utilization of modern anode materials for high performance microbial fuel cells. Biosens. Bioelectron. 2017, 90, 558–576. [Google Scholar] [CrossRef]
- Zhang, T.; Nie, H.; Bain, T.S.; Lu, H.; Cui, M.; Snoeyenbos-West, O.L.; Franks, A.E.; Nevin, K.P.; Russell, T.P.; Lovley, D.R. Improved cathode materials for microbial electrosynthesis. Energy Environ. Sci. 2013, 6, 217–224. [Google Scholar] [CrossRef]
- Jafary, T.; Daud, W.R.W.; Ghasemi, M.; Kim, B.H.; Jahim, J.M.; Ismail, M.; Lim, S.S. Biocathode in microbial electrolysis cell; Present status and future prospects. Renew. Sustain. Energy Rev. 2015, 47, 23–33. [Google Scholar] [CrossRef]
- Alatraktchi, F.A.A.; Zhang, Y.; Noori, J.S.; Angelidaki, I. Surface area expansion of electrodes with grass-like nanostructures and gold nanoparticles to enhance electricity generation in microbial fuel cells. Bioresour. Technol. 2012, 123, 177–183. [Google Scholar] [CrossRef] [PubMed]
- Abada, B.; Boumerfeg, S.; Haddad, A.; Etienne, M. Electrochemical investigation of thiobacillus denitrificans in a bacterial composite. J. Electrochem. Soc. 2020, 167, 135502. [Google Scholar] [CrossRef]
- Li, S.; Cheng, C.; Thomas, A. Carbon-based microbial-fuel-cell electrodes: From conductive supports to active catalysts. Adv. Mater. 2017, 29, 1602547. [Google Scholar] [CrossRef]
- Ghasemi, M.; Daud, W.R.W.; Hassan, S.H.A.; Oh, S.E.; Ismail, M.; Rahimnejad, M.; Jahim, J.M. Nano-structured carbon as electrode material in microbial fuel cells: A comprehensive review. J. Alloys Compd. 2013, 580, 245–255. [Google Scholar] [CrossRef]
- Lamberg, P.; Bren, K.L. Extracellular electron transfer on sticky paper electrodes: Carbon paste paper anode for microbial fuel cells. ACS Energy Lett. 2016, 1, 895–898. [Google Scholar] [CrossRef]
- Logan, B.; Cheng, S.; Watson, V.; Estadt, G. Graphite fiber brush anodes for increased power production in air-cathode microbial fuel cells. Environ. Sci. Technol. 2007, 41, 3341–3346. [Google Scholar] [CrossRef]
- Santoro, C.; Guilizzoni, M.; Correa Baena, J.P.; Pasaogullari, U.; Casalegno, A.; Li, B.; Babanova, S.; Artyushkova, K.; Atanassov, P. The effects of carbon electrode surface properties on bacteria attachment and start up time of microbial fuel cells. Carbon 2014, 67, 128–139. [Google Scholar] [CrossRef]
- Penteado, E.D.; Fernandez-Marchante, C.M.; Zaiat, M.; Gonzalez, E.R.; Rodrigo, M.A. Influence of carbon electrode material on energy recovery from winery wastewater using a dual-chamber microbial fuel cell. Environ. Technol. 2017, 38, 1333–1341. [Google Scholar] [CrossRef]
- Kipf, E.; Koch, J.; Geiger, B.; Erben, J.; Richter, K.; Gescher, J.; Zengerle, R.; Kerzenmacher, S. Systematic screening of carbon-based anode materials for microbial fuel cells with Shewanella oneidensis MR-1. Bioresour. Technol. 2013, 146, 386–392. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Chi, M.; Luo, J.; He, H.; Jin, T. An overview of electrode materials in microbial fuel cells. J. Power Sources 2011, 196, 4427–4435. [Google Scholar] [CrossRef]
- Guo, K.; Hidalgo, D.; Tommasi, T.; Rabaey, K. Pyrolytic carbon-coated stainless steel felt as a high-performance anode for bioelectrochemical systems. Bioresour. Technol. 2016, 211, 664–668. [Google Scholar] [CrossRef]
- Kerzenmacher, S. Engineering of microbial electrodes. In Advances in Biochemical Engineering/Biotechnology; Springer: Berlin/Heidelberg, Germany, 2019; Volume 167, pp. 135–180. [Google Scholar]
- Krieg, T.; Sydow, A.; Schröder, U.; Schrader, J.; Holtmann, D. Reactor concepts for bioelectrochemical syntheses and energy conversion. Trends Biotechnol. 2014, 32, 645–655. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.F.; Du, L.; Guo, P.B.; Zhu, B.; Luong, J.H.T. Controlled modification of carbon nanotubes and polyaniline on macroporous graphite felt for high-performance microbial fuel cell anode. J. Power Sources 2015, 283, 46–53. [Google Scholar] [CrossRef]
- Chen, X.; Cui, D.; Wang, X.; Wang, X.; Li, W. Porous carbon with defined pore size as anode of microbial fuel cell. Biosens. Bioelectron. 2015, 69, 135–141. [Google Scholar] [CrossRef]
- Flexer, V.; Jourdin, L. Purposely designed hierarchical porous electrodes for high rate microbial electrosynthesis of acetate from carbon dioxide. Acc. Chem. Res. 2020, 53, 311–321. [Google Scholar] [CrossRef]
- Flexer, V.; Chen, J.; Donose, B.C.; Sherrell, P.; Wallace, G.G.; Keller, J. The nanostructure of three-dimensional scaffolds enhances the current density of microbial bioelectrochemical systems. Energy Environ. Sci. 2013, 6, 1291–1298. [Google Scholar] [CrossRef]
- Nie, H.; Zhang, T.; Cui, M.; Lu, H.; Lovley, D.R.; Russell, T.P. Improved cathode for high efficient microbial-catalyzed reduction in microbial electrosynthesis cells. Phys. Chem. Chem. Phys. 2013, 15, 14290–14294. [Google Scholar] [CrossRef] [PubMed]
- Cui, M.; Nie, H.; Zhang, T.; Lovley, D.; Russell, T.P. Three-dimensional hierarchical metal oxide-carbon electrode materials for highly efficient microbial electrosynthesis. Sustain. Energy Fuels 2017, 1, 1171–1176. [Google Scholar] [CrossRef]
- Kim, K.R.; Kang, J.; Chae, K.J. Improvement in methanogenesis by incorporating transition metal nanoparticles and granular activated carbon composites in microbial electrolysis cells. Int. J. Hydrog. Energy 2017, 42, 27623–27629. [Google Scholar] [CrossRef]
- Champigneux, P.; Delia, M.L.; Bergel, A. Impact of electrode micro- and nano-scale topography on the formation and performance of microbial electrodes. Biosens. Bioelectron. 2018, 118, 231–246. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Zhou, J.; Wang, Y.; Wang, Y. Nano on micro: Tuning microbial metabolisms by nano-based artificial mediators to enhance and expand production of biochemicals. Curr. Opin. Biotechnol. 2020, 64, 161–168. [Google Scholar] [CrossRef]
- Kaur, R.; Marwaha, A.; Chhabra, V.A.; Kim, K.H.; Tripathi, S.K. Recent developments on functional nanomaterial-based electrodes for microbial fuel cells. Renew. Sustain. Energy Rev. 2020, 119, 109551. [Google Scholar] [CrossRef]
- Wang, W.; You, S.; Gong, X.; Qi, D.; Chandran, B.K.; Bi, L.; Cui, F.; Chen, X. Bioinspired nanosucker array for enhancing bioelectricity generation in microbial fuel cells. Adv. Mater. 2016, 28, 270–275. [Google Scholar] [CrossRef]
- Hindatu, Y.; Annuar, M.S.M.; Gumel, A.M. Mini-review: Anode modification for improved performance of microbial fuel cell. Renew. Sustain. Energy Rev. 2017, 73, 236–248. [Google Scholar] [CrossRef]
- Pinck, S.; Jorand, F.P.A.; Xu, M.; Etienne, M. Protamine promotes direct electron transfer between shewanella oneidensis cells and carbon nanomaterials in bacterial biocomposites. ChemElectroChem 2019, 6, 2398–2406. [Google Scholar] [CrossRef]
- Jeon, M.S.; Jeon, Y.; Hwang, J.H.; Heu, C.S.; Jin, S.; Shin, J.; Song, Y.; Chang Kim, S.; Cho, B.K.; Lee, J.K.; et al. Fabrication of three-dimensional porous carbon scaffolds with tunable pore sizes for effective cell confinement. Carbon 2018, 130, 814–821. [Google Scholar] [CrossRef]
- Bi, L.; Ci, S.; Cai, P.; Li, H.; Wen, Z. One-step pyrolysis route to three dimensional nitrogen-doped porous carbon as anode materials for microbial fuel cells. Appl. Surf. Sci. 2018, 427, 10–16. [Google Scholar] [CrossRef]
- Hung, Y.H.; Liu, T.Y.; Chen, H.Y. Renewable coffee waste-derived porous carbons as anode materials for high-performance sustainable microbial fuel cells. ACS Sustain. Chem. Eng. 2019, 7, 16991–16999. [Google Scholar] [CrossRef]
- Rethinasabapathy, M.; Lee, J.H.; Roh, K.C.; Kang, S.M.; Oh, S.Y.; Park, B.; Lee, G.W.; Cha, Y.L.; Huh, Y.S. Silver grass-derived activated carbon with coexisting micro-, meso- and macropores as excellent bioanodes for microbial colonization and power generation in sustainable microbial fuel cells. Bioresour. Technol. 2020, 300, 122646. [Google Scholar] [CrossRef]
- Logan, B.E. Scaling up microbial fuel cells and other bioelectrochemical systems. Appl. Microbiol. Biotechnol. 2010, 85, 1665–1671. [Google Scholar] [CrossRef]
- Blasco-Gómez, R.; Batlle-Vilanova, P.; Villano, M.; Balaguer, M.D.; Colprim, J.; Puig, S. On the edge of research and technological application: A critical review of electromethanogenesis. Int. J. Mol. Sci. 2017, 18, 874. [Google Scholar] [CrossRef]
- Neubert, M.; Hauser, A.; Pourhossein, B.; Dillig, M.; Karl, J. Experimental evaluation of a heat pipe cooled structured reactor as part of a two-stage catalytic methanation process in power-to-gas applications. Appl. Energy 2018, 229, 289–298. [Google Scholar] [CrossRef]
- Christodoulou, X.; Okoroafor, T.; Parry, S.; Velasquez-Orta, S.B. The use of carbon dioxide in microbial electrosynthesis: Advancements, sustainability and economic feasibility. J. CO2 Util. 2017, 18, 390–399. [Google Scholar] [CrossRef]
- Bian, B.; Bajracharya, S.; Xu, J.; Pant, D.; Saikaly, P.E. Microbial electrosynthesis from CO2: Challenges, opportunities and perspectives in the context of circular bioeconomy. Bioresour. Technol. 2020, 302, 122863. [Google Scholar] [CrossRef] [PubMed]
- Mateos, R.; Escapa, A.; San-Martín, M.I.; De Wever, H.; Sotres, A.; Pant, D. Long-term open circuit microbial electrosynthesis system promotes methanogenesis. J. Energy Chem. 2020, 41, 3–6. [Google Scholar] [CrossRef]
- Enzmann, F.; Mayer, F.; Stöckl, M.; Mangold, K.M.; Hommel, R.; Holtmann, D. Transferring bioelectrochemical processes from H-cells to a scalable bubble column reactor. Chem. Eng. Sci. 2019, 193, 133–143. [Google Scholar] [CrossRef]
- Miyake, T.; Oike, M.; Yoshino, S.; Yatagawa, Y.; Haneda, K.; Nishizawa, M. Automatic, sequential power generation for prolonging the net lifetime of a miniature biofuel cell stack. Lab Chip 2010, 10, 2574–2578. [Google Scholar] [CrossRef]
- Kipf, E.; Sané, S.; Morse, D.; Messinger, T.; Zengerle, R.; Kerzenmacher, S. An air-breathing enzymatic cathode with extended lifetime by continuous laccase supply. Bioresour. Technol. 2018, 264, 306–310. [Google Scholar] [CrossRef]
- Yu, J.; Tu, J.; Zhao, F.; Zeng, B. Direct electrochemistry and biocatalysis of glucose oxidase immobilized on magnetic mesoporous carbon. J. Solid State Electrochem. 2010, 14, 1595–1600. [Google Scholar] [CrossRef]
- Zheng, J.; Xu, J.L.; Jin, T.B.H.; Wang, J.L.; Zhang, W.Q.; Hu, Y.X.; He, P.G.; Fang, Y.Z. Preparation of magnetic ordered mesoporous carbon composite and its application in direct electrochemistry of horseradish peroxidase. Electroanalysis 2013, 25, 2159–2165. [Google Scholar] [CrossRef]
- Trifonov, A.; Stemmer, A.; Tel-Vered, R. Carbon-coated magnetic nanoparticles as a removable protection layer extending the operation lifetime of bilirubin oxidase-based bioelectrode. Bioelectrochemistry 2021, 137, 107640. [Google Scholar] [CrossRef] [PubMed]
- Diender, M.; Uhl, P.S.; Bitter, J.H.; Stams, A.J.M.; Sousa, D.Z. High rate biomethanation of carbon monoxide-rich gases via a thermophilic synthetic coculture. ACS Sustain. Chem. Eng. 2018, 6, 2169–2176. [Google Scholar] [CrossRef] [PubMed]
- Thema, M.; Weidlich, T.; Hörl, M.; Bellack, A.; Mörs, F.; Hackl, F.; Kohlmayer, M.; Gleich, J.; Stabenau, C.; Trabold, T.; et al. Biological CO2-methanation: An approach to standardization. Energies 2019, 12, 1670. [Google Scholar] [CrossRef]
- Wang, H.; Ren, Z.J. A comprehensive review of microbial electrochemical systems as a platform technology. Biotechnol. Adv. 2013, 31, 1796–1807. [Google Scholar] [CrossRef]
- Herkendell, K.; Stemmer, A.; Tel-Vered, R. Magnetically induced enzymatic cascades-advancing towards multi-fuel direct/mediated bioelectrocatalysis. Nanoscale Adv. 2019, 1, 1686–1692. [Google Scholar] [CrossRef]
- Chiranjeevi, P.; Bulut, M.; Breugelmans, T.; Patil, S.A.; Pant, D. Current trends in enzymatic electrosynthesis for CO2 reduction. Curr. Opin. Green Sustain. Chem. 2019, 16, 65–70. [Google Scholar] [CrossRef]
- Dubrawski, K.L.; Shao, X.; Milton, R.D.; Deutzmann, J.S.; Spormann, A.M.; Criddle, C.S. Microbial battery powered enzymatic electrosynthesis for carbon capture and generation of hydrogen and formate from dilute organics. ACS Energy Lett. 2019, 4, 2929–2936. [Google Scholar] [CrossRef]
- Bhattacharjee, U.; Pandey, L.M. Novel nanoengineered materials-based catalysts for various bioelectrochemical systems. ACS Symp. Ser. 2020, 1342, 45–71. [Google Scholar] [CrossRef]
- Zhai, T.; Wang, C.; Gu, F.; Meng, Z.H.; Liu, W.; Wang, Y. Dopamine/Polyethylenimine-Modified Silica for Enzyme Immobilization and Strengthening of Enzymatic CO2 Conversion. ACS Sustain. Chem. Eng. 2020, 8, 15250–15257. [Google Scholar] [CrossRef]
- Wu, R.; Zhu, Z. Self-powered enzymatic electrosynthesis of l -3,4-dihydroxyphenylalanine in a hybrid bioelectrochemical system. ACS Sustain. Chem. Eng. 2018, 6, 12593–12597. [Google Scholar] [CrossRef]
- Sahoo, P.C.; Pant, D.; Kumar, M.; Puri, S.K.; Ramakumar, S.S.V. Material–microbe interfaces for solar-driven CO2 bioelectrosynthesis. Trends Biotechnol. 2020, 38, 1245–1261. [Google Scholar] [CrossRef]
- Mazurenko, I.; Etienne, M.; Kohring, G.W.; Lapicque, F.; Walcarius, A. Enzymatic bioreactor for simultaneous electrosynthesis and energy production. Electrochim. Acta 2016, 199, 342–348. [Google Scholar] [CrossRef]
- Hoa, L.Q.; Vestergaard, M.C.; Tamiya, E. Carbon-based nanomaterials in biomass-based fuel-fed fuel cells. Sensors 2017, 17, 2587. [Google Scholar] [CrossRef] [PubMed]
- Sharma, M.; Sarma, P.M. Microbially mediated electrosynthesis processes. In Microbial Fuel Cell: A Bioelectrochemical System that Converts Waste to Watts; Springer: Berlin/Heidelberg, Germany, 2017; pp. 421–442. ISBN 9783319667935. [Google Scholar]
- Foley, J.M.; Rozendal, R.A.; Hertle, C.K.; Lant, P.A.; Rabaey, K. Life cycle assessment of high-rate anaerobic treatment, microbial fuel cells, and microbial electrolysis cells. Environ. Sci. Technol. 2010, 44, 3629–3637. [Google Scholar] [CrossRef] [PubMed]
- Rosenbaum, M.A.; Franks, A.E. Microbial catalysis in bioelectrochemical technologies: Status quo, challenges and perspectives. Appl. Microbiol. Biotechnol. 2014, 98, 509–518. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Herkendell, K. Status Update on Bioelectrochemical Systems: Prospects for Carbon Electrode Design and Scale-Up. Catalysts 2021, 11, 278. https://doi.org/10.3390/catal11020278
Herkendell K. Status Update on Bioelectrochemical Systems: Prospects for Carbon Electrode Design and Scale-Up. Catalysts. 2021; 11(2):278. https://doi.org/10.3390/catal11020278
Chicago/Turabian StyleHerkendell, Katharina. 2021. "Status Update on Bioelectrochemical Systems: Prospects for Carbon Electrode Design and Scale-Up" Catalysts 11, no. 2: 278. https://doi.org/10.3390/catal11020278
APA StyleHerkendell, K. (2021). Status Update on Bioelectrochemical Systems: Prospects for Carbon Electrode Design and Scale-Up. Catalysts, 11(2), 278. https://doi.org/10.3390/catal11020278