2.1. Catalyst Characterization
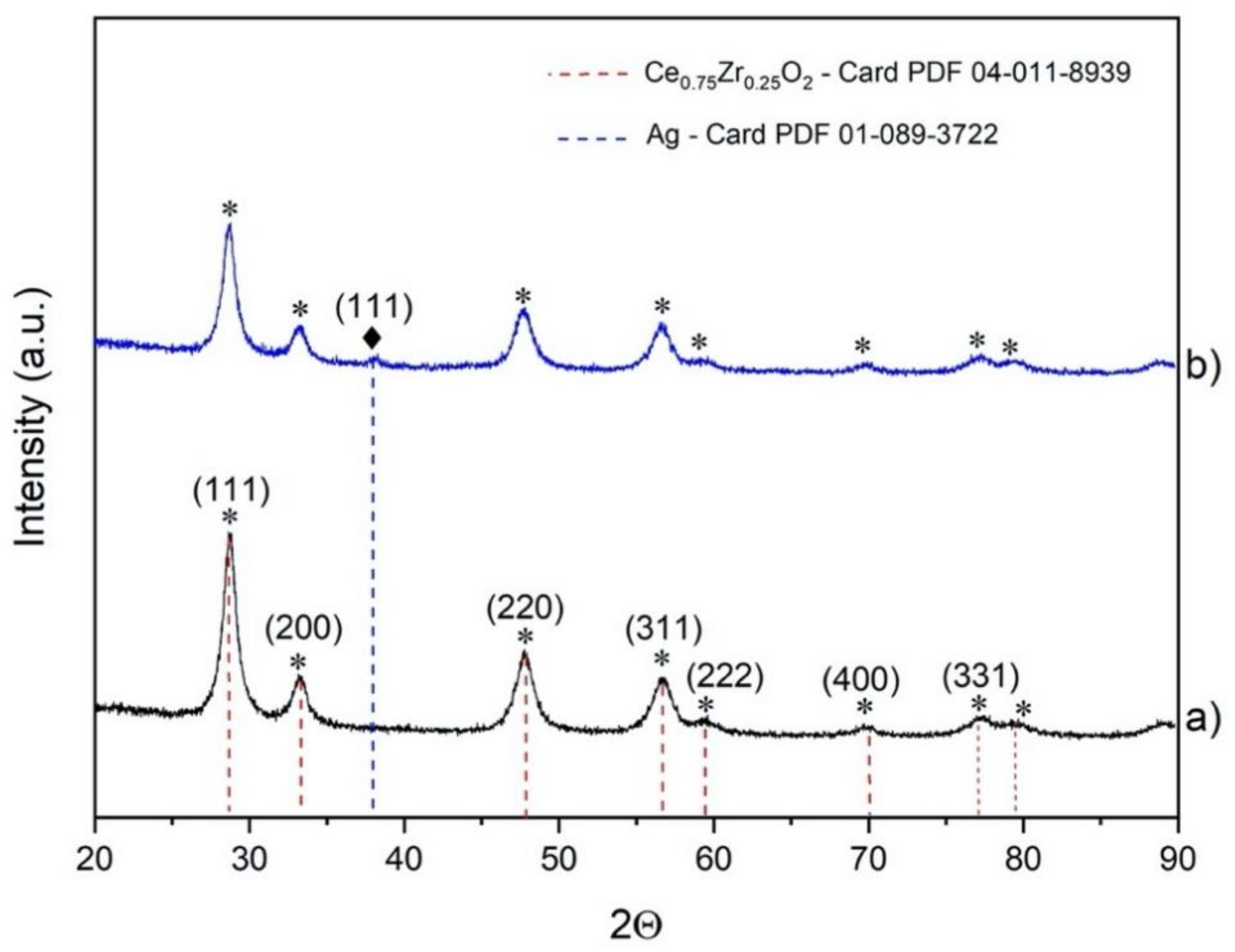
The diffractograms of the Ce
0.75Zr
0.25O
2 support and the 5% Ag/Ce
0.75Zr
0.25O
2 catalyst are shown in
Figure 1. The characteristic peaks of the cubic Ce
0.75Zr
0.25O
2 phase were detected in both cases. The highest peak was observed at 2
Θ = 28.66°, which corresponded to the (111) plane. We also identified the main characteristic peaks of the (200) at 2
Θ = 33.19°, (220) at 2
Θ = 47.74°, (311) at 2
Θ = 56.68°, (222) at 2
Θ = 59.44°, (400) at 2
Θ = 70.06°, and (331) planes at 2
Θ = 76.99°, which suggested the presence of a pure cubic phase (JCPDS file: 04-011-8939) [
24]. The size of the support crystallites was estimated from the peak at 2
Θ = 28.6°, obtaining a value of 5.8 nm. The characteristic peaks of the precursors were not found, which suggested a rather high purity of the obtained support. After silver deposition, the same peaks were still observed, indicating that the support structure was preserved upon deposition. A small peak located at 2
Θ = 38°, corresponding to the (111) plane of the metallic silver phase, was then detected (JCPDS file: 01-089-3722). No peaks due to impurities or silver precursors were observed.
Figure 2 shows the nitrogen adsorption/desorption profiles and the pore volume distribution of the support before and after silver deposition. The obtained adsorption isotherms (
Figure 2a,b) were type IV corresponding to mesoporous solids. It can also be seen that after silver deposition, the textural properties of the support were preserved.
Table 1 shows the specific surface area values, the pore diameter, and the pore volume determined by the BJH method for the support before and after silver loading. Upon silver loading, the specific surface area decreased slightly from 97 m
2/g to 86 m
2/g. On the other hand, pore size and pore volume did not show significant change, meaning that silver was well dispersed on the surface of the support and did not cause blockage of the pores.
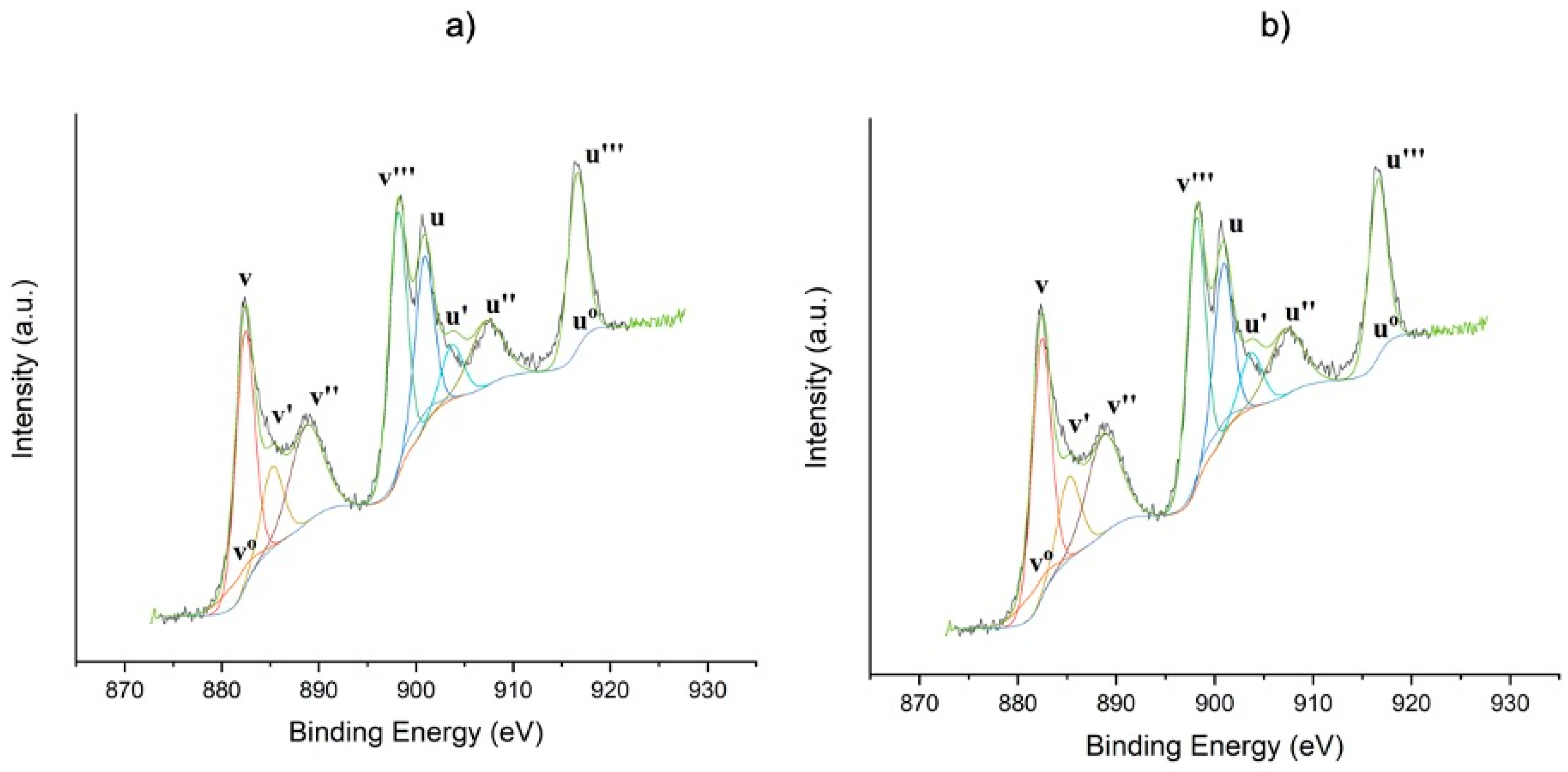
The XPS spectra for cerium (Ce 3d) are shown in
Figure 3. The obtained peaks were decomposed in eight peaks corresponding to the spin–orbit doublets: 3d 5/2 and 3d 3/2 (
Figure 3). The peaks v′, u′, v
0, and u
0 correspond to Ce
3+ species, while the peaks v, u, v‴, u‴, v″, and u″ correspond to Ce
4+ species. Likewise, the proportion of Ce
3+ and Ce
4+ with respect to total cerium was calculated (
Table 2), finding that for the Ce
0.75Zr
0.25O
2 support there was 83.2% of Ce
4+, and for 5%Ag/Ce
0.75Zr
0.25O there was 82%. This may suggest oxygen vacancies or O atoms relaxations resulting from silver deposition [
25,
26].
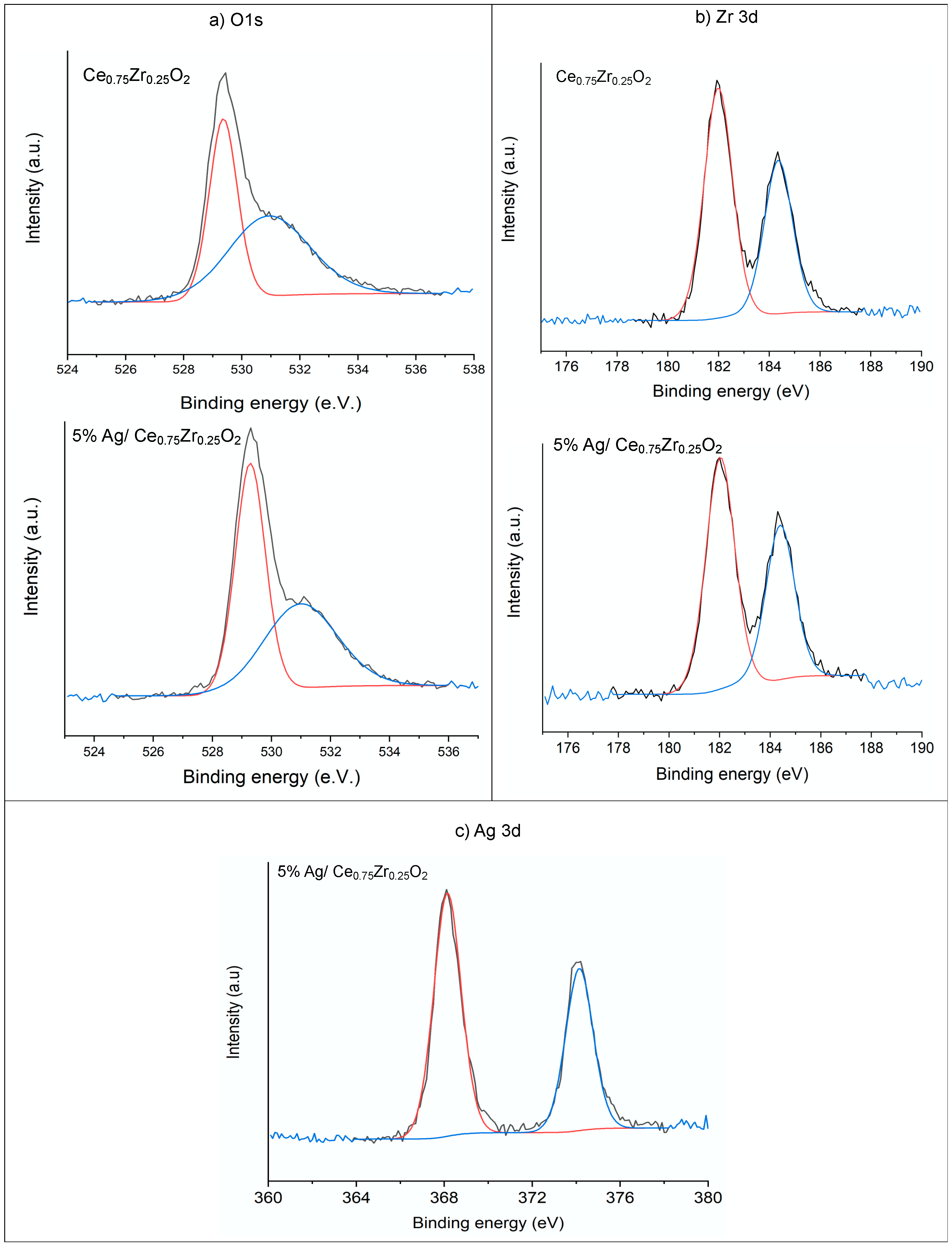
Figure 4 shows the XPS spectra of Ce
0.75Zr
0.25O
2 support and 5%Ag/Ce
0.75Zr
0.25O
2 catalyst for O1s, Zr 3d, and Ag 3d peaks. The obtained peak positions were similar to those reported in the literature. For silver (Ag 3d), the peaks were located at binding energies (BEs) equal to 368.1 eV (Ag 3d 5/2) and 374.1 eV (Ag 3d 3/2), which corresponded to metallic silver [
13]. For zirconium (Zr 3d), the peaks were at Bes = 182 and 184.4 eV, corresponding to ZrO
2. No significant changes were observed on the zirconium peaks before and after silver deposition.
For the O1s region, no significant changes were found in the shape of the peaks upon silver deposition. Two peaks were evidenced at BE = 529 and 531 eV, which were characteristic of lattice and lattice-defective oxygen vacancies, respectively [
27]. Regarding the atomic concentration of oxygen (
Table 2), a slight decrease after silver deposition was observed, which supported the hypothesis of formation of oxygen vacancies.
Table 2 shows the atomic concentration (%) determined by XPS surface analysis, before and after silver deposition. The XPS analysis evidenced that the surface silver loading (wt.%) was a little bit lower (4.64 wt.%) than the theoretical one of 5 wt.%.
2.2. Experimental Tests and Optimization
A preliminary benchmark test was carried out with the initial conditions that corresponded to those evaluated in previous investigations carried out in our laboratory [
5]. These conditions corresponded to a 0.3 M glycerol solution, a reaction temperature of 60 °C, 5 bar of oxygen, 0.5 g of catalyst, and a NaOH/glycerol molar ratio of 4. These tests were performed in triplicate to prove reproducibility of the system. The results obtained at these initial operating conditions are shown in
Figure 5. The carbon balance was around 100%. After two hours of reaction, we observed almost full conversion (>98%) with a high selectivity to GLYA (69%). The GLYA yield (i.e., 68%) obtained with the 5%Ag/Ce
0.75Zr
0.25O
2 catalyst was the highest one reported in the literature so far, to the best of our knowledge. [
3,
13].
The other products formed in lower selectivity were GA (10%), which is also a high-added-value product [
16,
28], and formic acid (FA, 20%), which resulted from C–C bond cleavage during the GLYA production. A yield of 63.7% of GLYA was thus obtained under these conditions (bottom of
Figure 5).
Experimental tests to optimize the glycolic acid were carried out in two stages. In the first stage, we determined the most sensitive experimental parameters. For this, a second-degree central composite design (CCD) was developed, which was elaborated using XLSTAT [
29].
In the first CCD, named CCD 1, the studied parameters (variables) were glycerol concentration, NaOH/glycerol molar ratio, reaction temperature, and glycerol/catalyst ratio. The response variable was the yield of GLYA. In this experimental design, twenty-five observations were proposed. The levels of each variable are shown in
Table 3. Such levels were defined according to previous results obtained by our laboratory and according to bibliographical data.
The experimental test results were analyzed by an ANOVA analysis, in which the most influential variables and their interactions were found.
In order to obtain the maximum yield of GLYA by increasing the glycerol concentration and minimizing the amount of NaOH, the experimental tests of the CCD 1 design were carried out for 300 min. It was found for all the tests that once the maximum conversion was reached, the production of glycolic acid remained stable until the end of the 300 min test, like it was observed in the preliminary benchmark test (
Figure 5).
Table 4 presents the operating conditions of each test, as well as the obtained yield of glycolic acid.
Upon calculation of the test statistics, the
p-value was obtained, which is defined as the probability of observing the given value of the test statistic, being a measure of evidence against the null hypothesis [
30,
31]. The value to reject the null hypothesis was 0.05, which means that no significant difference existed.
Table 5 shows the results of the statistical analysis of variance (ANOVA) for GLYA with four variables and their interactions. In this case, two effects had
p-values less than 0.05, indicating that they were significantly different from zero at the 95.0% confidence level.
The R-squared statistic indicated that the model as fitted explains ca. 82% of the variability in GLYA yield. Since the p-value was larger than 5.0%, there was no indication of serial autocorrelation in the residuals at the 5.0% significance level. Those variables with a p-value less than 0.05 indicated a significant correlation, being the variables with the largest influence on the yield of GLYA. The most influential variables were glycerol concentration and the interaction (NaOH/Glycerol)2, with p-values of 0.0003 and 0.0421, respectively.
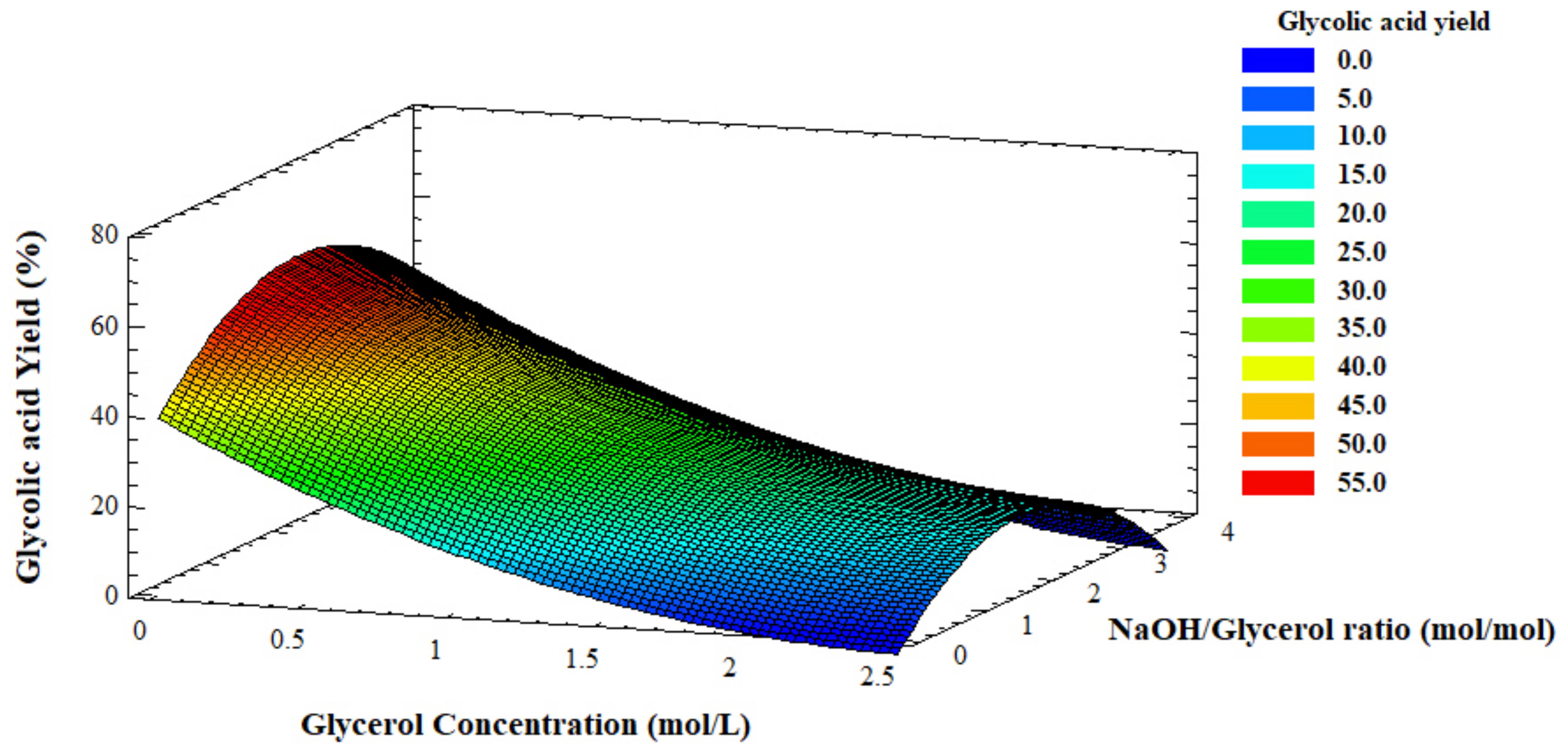
Figure 6 shows the combination of factor levels, which maximize the desirable function (GLYA yield) over the indicated region. It also shows the combination of the factors’ values at which that optimum was achieved. With these results, we could find the optimal influential variables and the corresponding GLYA yield. The optimal as-determined values were 0.51 M for glycerol concentration, 61.4 °C for the temperature, a NaOH/glycerol ratio equal to 2.22, and 0.73 g of catalyst. With these results, compared to the benchmark, we could then decrease the NaOH/glycerol ratio from 4 to 2 and increase the glycerol concentration from 0.3 M to 0.5 M, which corresponded to an overall increase of glycolic acid recovery from 0.27 mol/L g to 0.44 mol/L in the same reaction volume (200 mL).
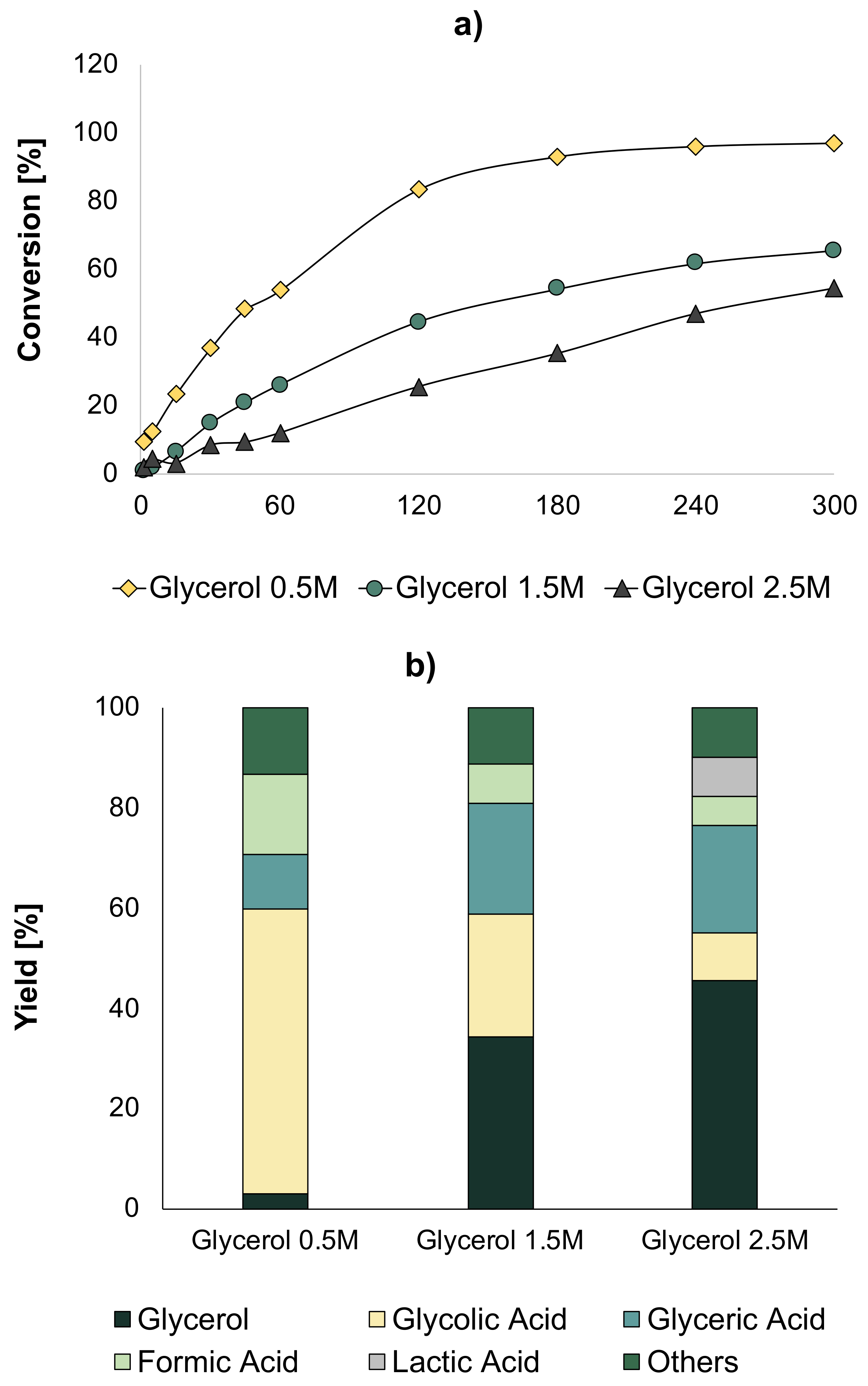
Once the optimal conditions were obtained, we carried out tests increasing the glycerol concentration to 1.5 M and 2.5 M at the optimal conditions obtained in the previous study—temperature (60 °C) and NaOH/Glycerol ratio (2), O
2 pressure of 5 bar, and a quantity of catalyst equal to 0.7 g. The obtained results are shown in
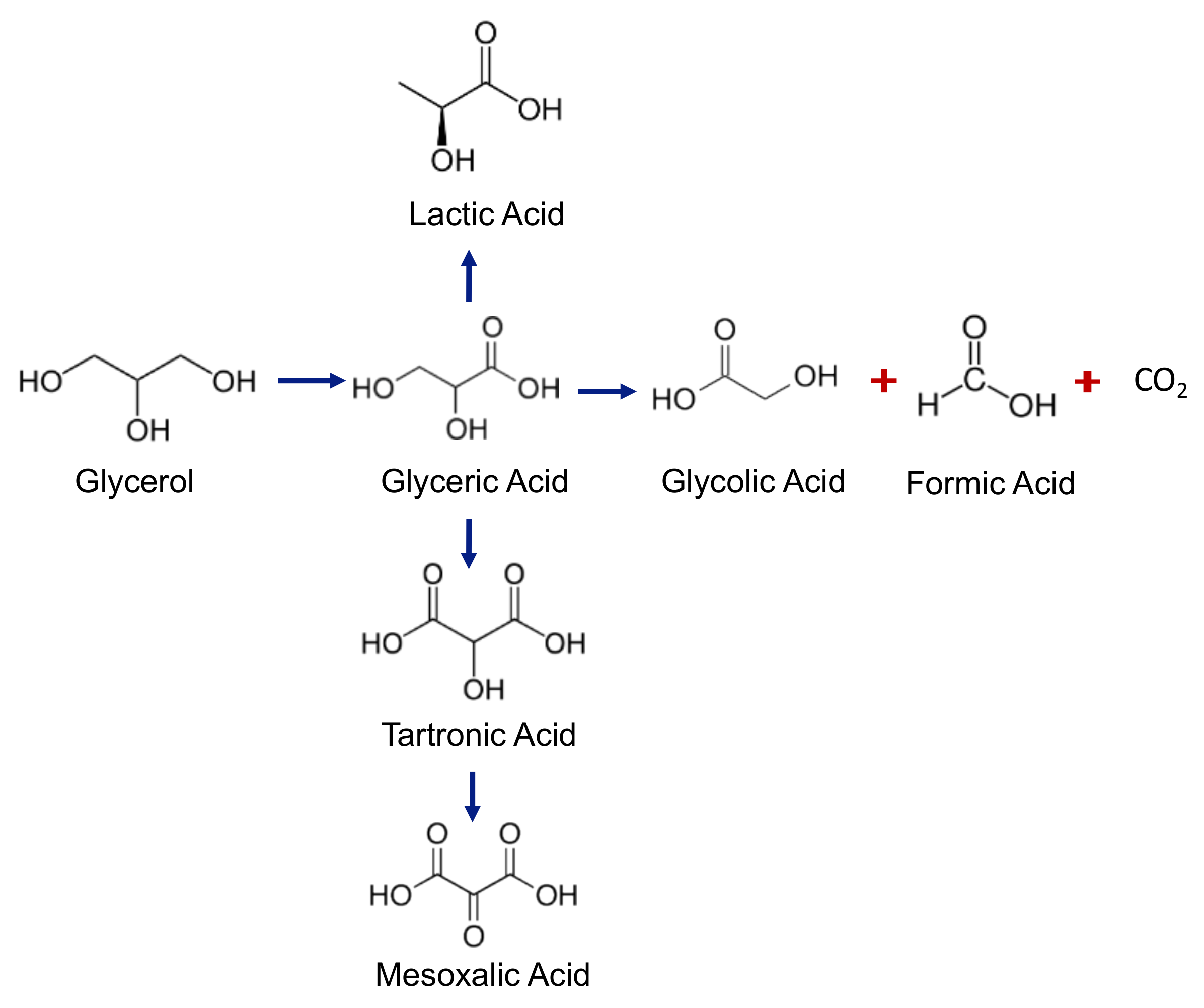
Figure 7. It was found that when the concentration of glycerol was increased, the conversion decreased considerably, leading to a lower GLYA yield. In addition, it could be found that at higher glycerol concentrations, lactic acid (LA) was detected, which was produced due to an oxygen deficiency in the medium (
Figure 8). The optimal conditions obtained in the previous optimization could not be directly transposed to higher glycerol concentrations.
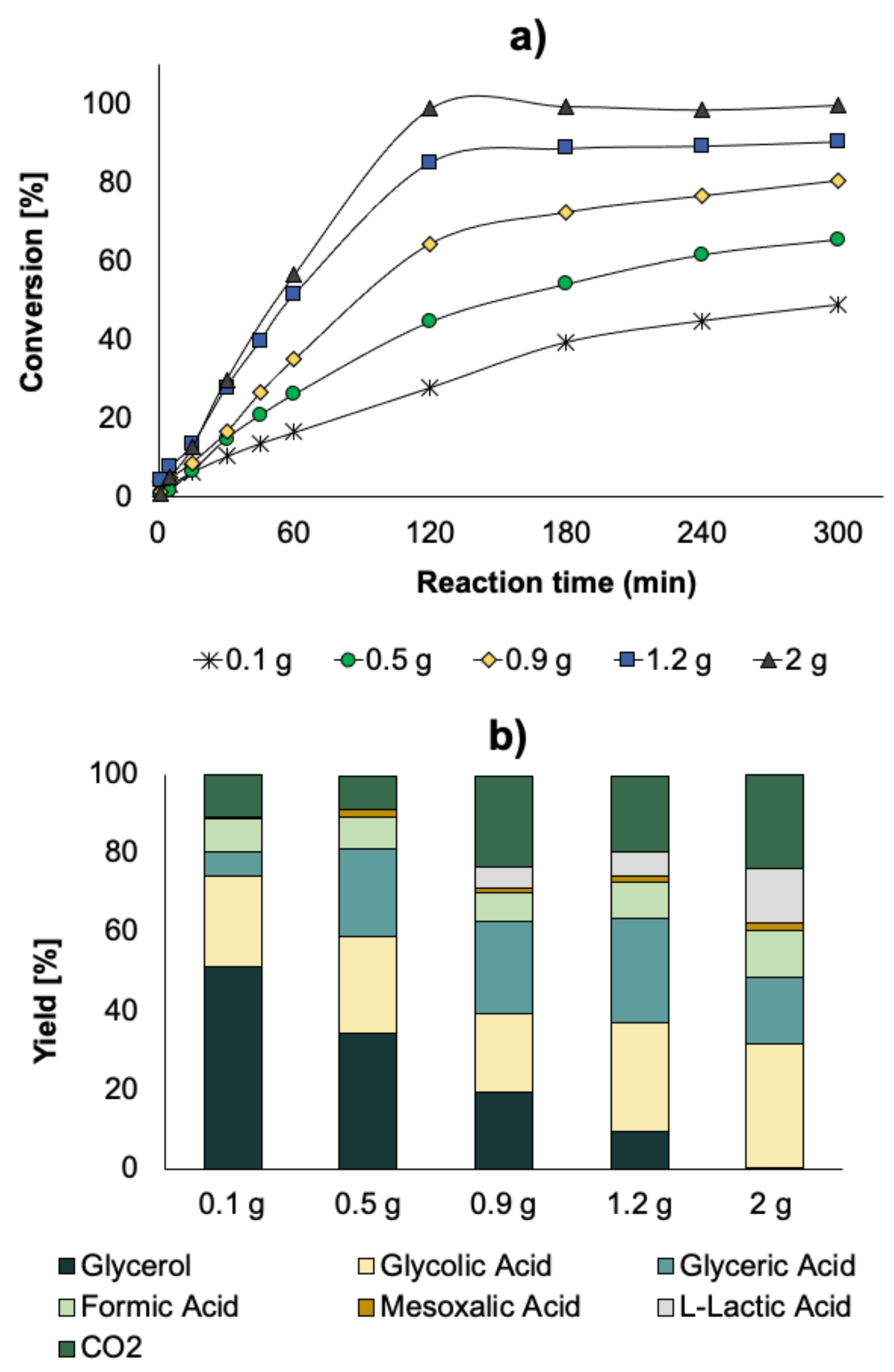
In order to increase the conversion, tests were performed with 1.5 M glycerol, while increasing the amount of catalyst. Catalyst amounts were chosen from 0.1 to 2 g. Increasing the catalyst amount led to a drastic increase in glycerol conversion, with a value of almost 100% when using 2 g of catalyst. This full conversion was linked with a change in terms of selectivity, as depicted in
Figure 9b. With the lowest quantity of catalyst, the main products were FA, GLYA, and GA, while when the quantity of catalyst was larger than 0.9 g, new products appeared, such as mesoxalic acid (MA) and LA, while the quantity of CO
2 (total oxidation) drastically increased (
Figure 8).
Taking into account that LA was not targeted in this reaction and also because it is quite difficult to separate it from GLYA, reducing the amount of formed LA is important. For doing this, a series of subsequent catalytic tests were performed by increasing the O
2 partial pressure (higher amount of available oxygen in the liquid phase). The reaction parameters were 60 °C, NaOH/Glycerol ratio = 2, and 2 g of catalyst.
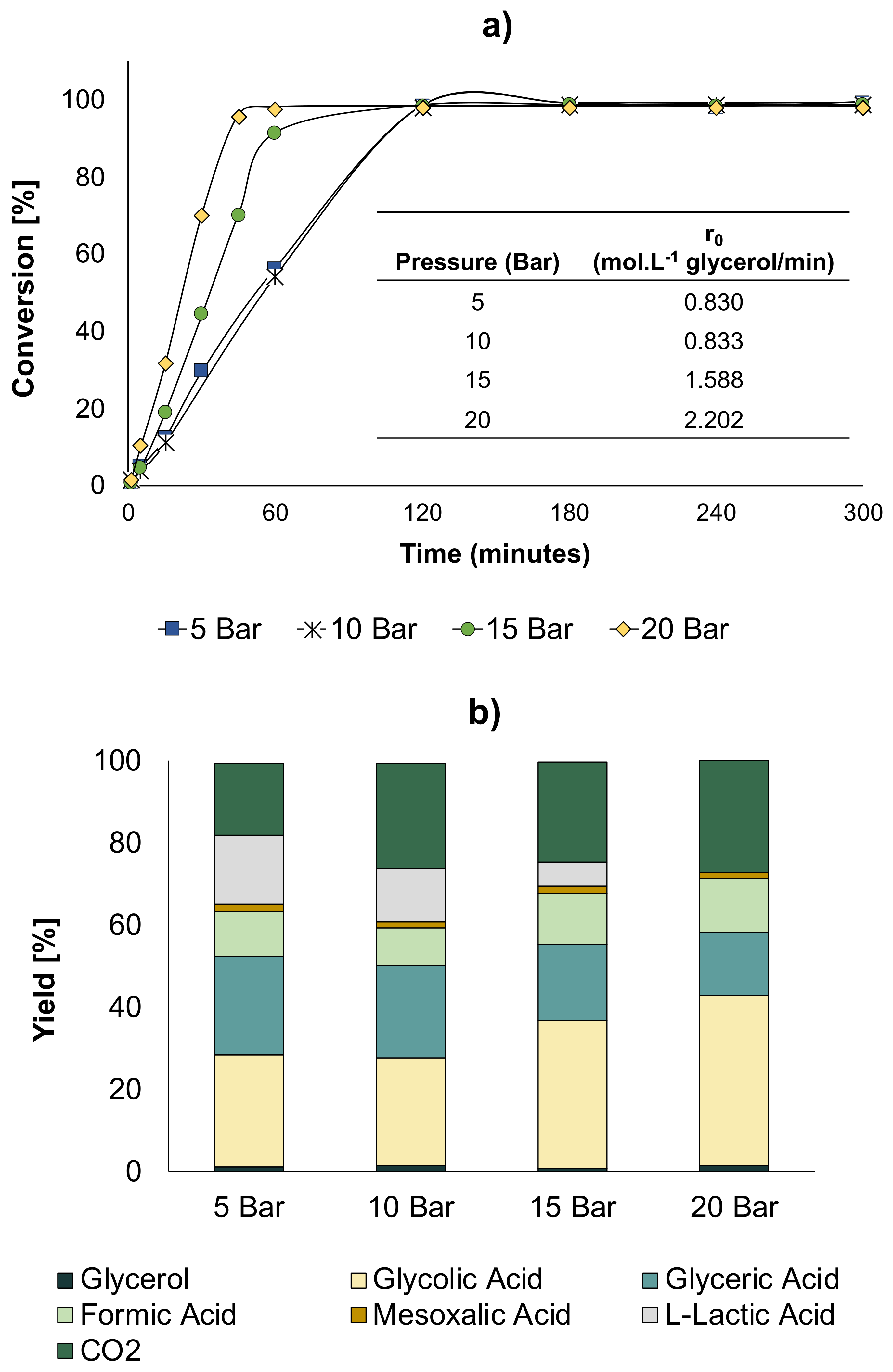
Figure 10 shows the results of these tests.
The results showed that while an increase in O
2 pressure to 10 bar did not yield any significant change in conversion and yields, at 15 bar, a higher reaction rate was actually observed together with an increase in GLYA and a decrease in LA selectivities (
Figure 10a,b). When the pressure was further increased to 20 bar, the reaction rate was even higher than that observed at 15 bar. Further, at this pressure, the GLYA yield increased, while LA was not produced anymore. Furthermore, it was evidenced that as the pressure of O
2 increased, the initial reaction rate proportionally increased. Thus, at a pressure of 20 bar, in addition to favoring the production of GLYA, complete conversion was obtained at a shorter reaction time.
As the availability of oxygen and the amount of catalyst was identified as an important feature, we decided to carry out the evaluation on the catalytic performances of two more variables: the O
2/glycerol ratio and the catalyst/glycerol mass ratio. The O
2/glycerol ratio is indicative of the oxygen availability, and was measured in terms of pressure in the sky of the reactor. We thus carried out CCD 2. In this design, ten observations were proposed. The levels of each variable are shown in
Table 6. These levels were defined according to previous experiences in our laboratory, conditions and technical limitations of the set-up, and according to bibliography. For the O
2 pressure/glycerol ratio, ratios from 0.01 to 0.47 were evaluated, corresponding to pressures from 1 to 40 bar. For the catalyst/glycerol ratio (
g/
g), ratios from 0.03 to 0.13 were evaluated, which corresponded to catalyst quantities from 0.9 g to 3.6 g. These tests were performed with a glycerol solution of 1.5 M and at NaOH/glycerol molar ratio of 2 (optimal condition found in the first stage). The experimental tests results were analyzed by an ANOVA analysis, in which the most influential variables and their interaction were found.
The results obtained with each operating condition with CCD 2 can be seen in
Table 7. The statistical analysis showed the influence of the two variables and the interaction between them.
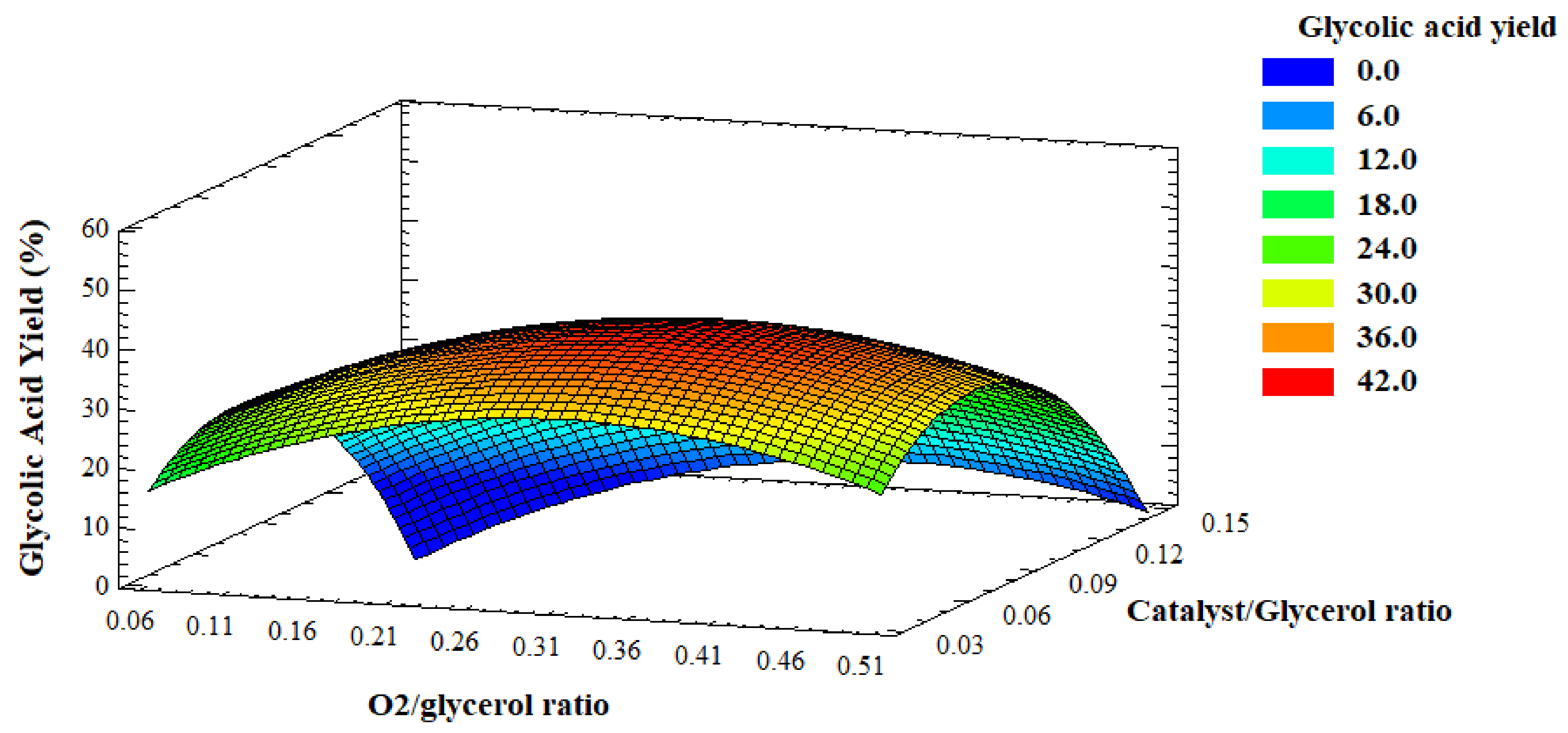
Figure 11 shows the surface response for the GLYA yield evaluated against O
2/glycerol molar ratio and catalyst/glycerol mass ratio. For low catalyst/glycerol and oxygen/glycerol ratios, the GLYA yield was low. This was mainly due to the fact that with less oxygen availability, the selectivity of the reaction turned to other products, such as LA. On the other hand, the maximum GLYA yield was found at intermediate values of catalyst/glycerol (0.07) and also oxygen/glycerol (0.23) ratios. This was due to the fact that when the amount of catalyst is increased, it is necessary to increase in parallel the O
2 pressure to have sufficient oxygen. However, due to technical limitations, the maximum O
2/glycerol ratio studied herein did not allow using sufficient oxygen pressure for the maximum amount of catalyst, and it was not possible to increase the pressure even further. Due to this relative deficiency of oxygen at high catalyst amounts, undesirable products were obtained that affected the GLYA yield.
The optimal values we found were O2/glycerol ratio of 0.23 and catalyst/glycerol ratio of 0.07.
Once the optimal conditions of temperature (60 °C), NaOH/glycerol (2), O2/glycerol ratio (0.23), and catalyst/glycerol ratio (0.07) were determined, the glycerol concentration was increased, and we varied it from 1.5 to 3 M.
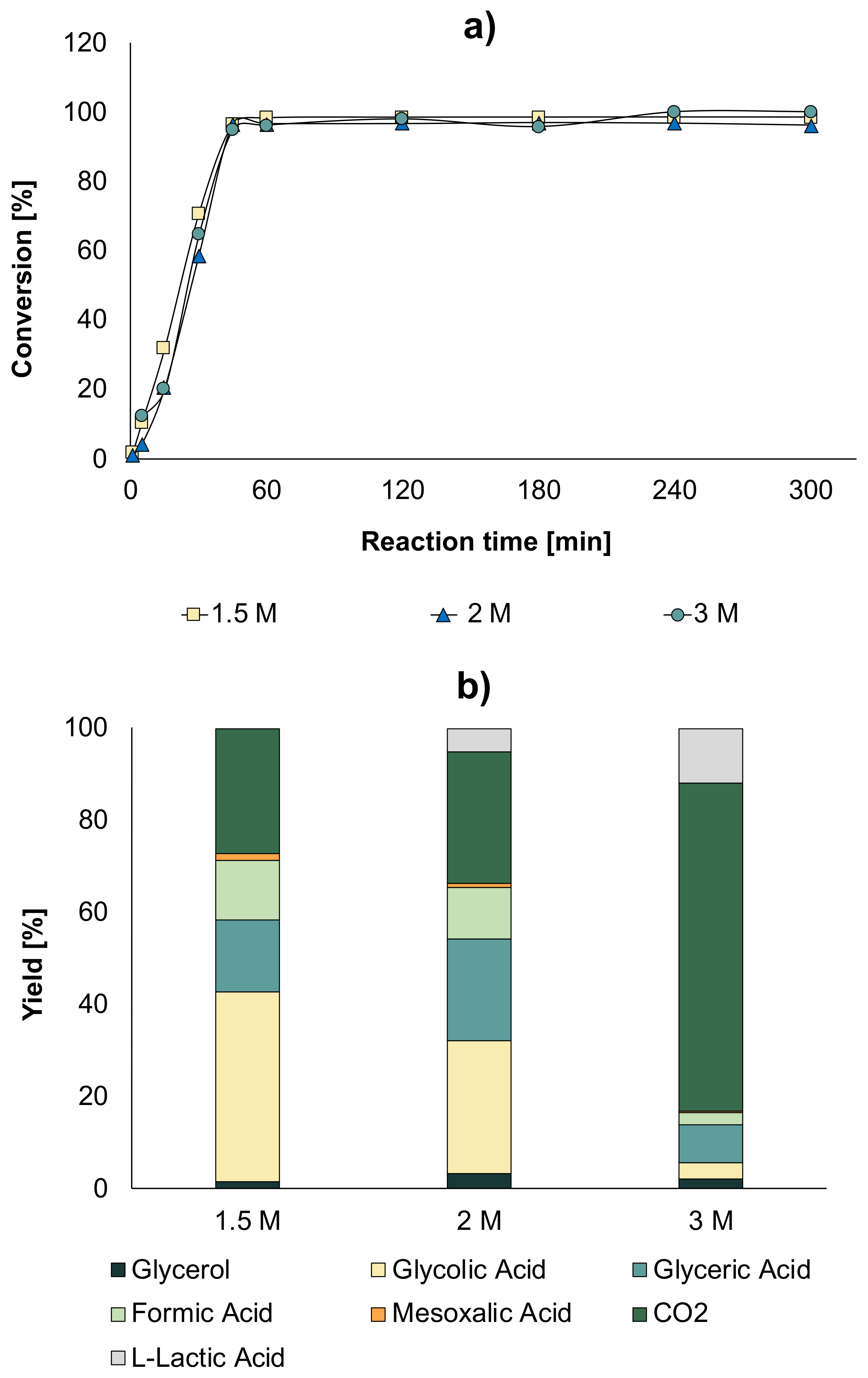
The results showed a significant improvement in the conversion. If we compare the effects of the conditions, for a glycerol concentration of 1.5 M without optimal conditions, a conversion of 60% was obtained at 300 min (
Figure 7a), while with the optimal conditions, a conversion of almost 100% after 45 min was obtained (
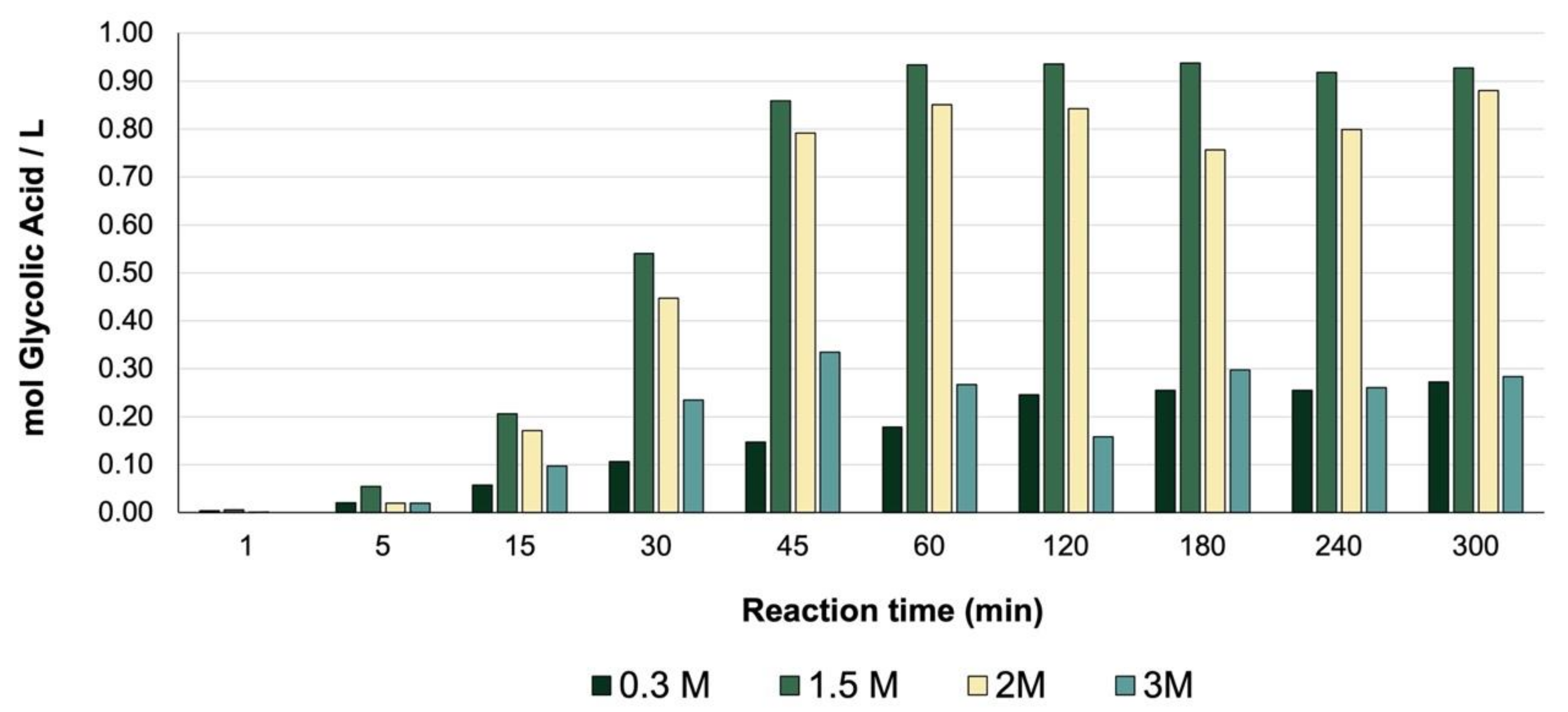
Figure 12a). Comparing the concentration of glycolic acid obtained with an initial solution of 0.3 M glycerol (0.27 mol/L), it was observed that by increasing the initial concentration of glycerol under optimal conditions, a higher concentration of glycolic acid was obtained—0.93 mol/L when a 1.5 M solution was used and 0.88 mol/L when using a 2 M solution.
The distribution of products in the liquid at maximum conversion is given in
Figure 12b. As the glycerol concentration increased, MA appeared as a product that was not observed when a glycerol concentration of 0.3 M (
Figure 5b) and 0.5 M were used (
Figure 7b). In addition, it was found that, as the concentration of glycerol increased, LA appeared in a higher concentration, which was an undesired product in our study.
The quantity (mol) of GLYA produced per liter of solution was evaluated at different concentrations of glycerol under optimal conditions and compared with the initial conditions (
Figure 13). A significant increase in the concentration of GLYA was observed when using a 1.5 M and 2 M solution, leading to the possibility of increasing the glycerol concentration and obtaining a solution rich in the product of interest.