Abstract
In this study, a highly efficient carbon-supported Pd catalyst for the direct ethanol fuel cell was developed by electrodepositing nanostructured Pd on oxygen plasma-treated carbon fiber paper (Pd/pCFP). The oxygen plasma treatment has been shown to effectively remove the surface organic contaminants and add oxygen species onto the CFP to facilitate the deposition of nano-structured Pd on the surface of carbon fibers. Under the optimized and controllable electrodeposition method, nanostructured Pd of ~10 nm can be easily and evenly deposited onto the CFP. The prepared Pd/pCFP electrode exhibited an extraordinarily high electrocatalytic activity towards ethanol oxidation, with a current density of 222.8 mA mg−1 Pd. Interestingly, the electrode also exhibited a high tolerance to poisoning species and long-term stability, with a high ratio of the forward anodic peak current density to the backward anodic peak current density. These results suggest that the Pd/pCFP catalyst may be a promising anodic material for the development of highly efficient direct alcohol fuel cells.
1. Introduction
Energy supply by consumption of fossil fuels, such as petroleum, coal and natural gas, raises severe problems for the environment and public health, including depletion of natural resources, air pollution and global warming [1,2]. Hence, finding clean and reliable power sources for various mechanical, electrical, and medical devices and vehicles without increasing stress on the environment and public health is a top priority worldwide. Compared to conventional power generators, fuel cells convert chemical energy directly to electrical energy by electrochemical reactions and operate with high efficiency, low emissions and clean processes [3,4,5,6]. Among various fuel cells, direct liquid fuel cells have attracted much attention because they exhibit high energy density and high efficiency and can be operated under ambient conditions [7,8]. In addition, liquid fuels, such as ethanol, methanol and formic acid, exhibit many advantages, such as safe, easy handling and storage and convenient refill. Among commonly used liquid fuels, ethanol is particularly attractive due to its high specific energy (8.0 kWh/kg), low toxicity, renewability and low crossover problems [9,10,11]. Moreover, the large-scale production of ethanol is readily available [12,13]. Hence, the direct ethanol fuel cell (DEFC) is recognized as one of the most efficient power systems for powering portable and mobile devices [14,15,16]. DEFC works by electrochemical oxidation of ethanol at the anode, while at cathode oxygen is reduced. Ideally, ethanol can be completely oxidized on the anode of DEFC to H2O and CO2 with sequential transfer of 12 electrons, and it delivers high energy density [17,18]. However, high overpotential, intermediate to high temperatures and a highly active catalyst are usually required for the complete oxidation of ethanol.
Two basic types of DEFC were developed on the basis of the usage of a permeable membrane, i.e., proton-exchange membrane (PEM) and anion-exchange membrane (AEM), that separates the anode and cathode. The PEM-based DEFC works well under the acidic condition, with platinum (Pt) and various Pt-based bimetallic materials having long been used as highly efficient catalysts for the ethanol oxidation reaction (EOR) [19,20,21]. As a catalyst, Pt exhibits several appealing properties, such as low operating temperatures, high power densities, high stability and relative ease of scale-up. However, the requirement of a relatively large amount of scarce and expensive Pt, and low poisoning tolerance to the CO-like species generated during EOR [22,23], are major disadvantages of this type of DEFC. Alloying Pt with less expensive metals, such as ruthenium (Ru), tungsten (W), tin (Sn), gold (Au) and 3d transition metals, helps to reduce the CO intolerance and improve the catalytic activity of Pt [19,20,22,24,25]. The second metal in the alloy may enhance the electro-oxidation of ethanol by activating water molecules and providing preferential sites for–OH adsorption (–OHads) at lower potentials than Pt. The –OHads species are required to completely convert the poisoning intermediates to CO2. However, the dissolution of these metals under the acidic condition remains the major reason for the severe degradation of the catalytic performance of these alloyed catalysts [25,26]. In comparison, the AEM-based DEFC works well under the alkaline condition and exhibits some advantages over the PEM-based process by showing faster kinetics in both ethanol oxidation and oxygen reduction reactions. In addition, palladium (Pd) can be adopted as the catalyst for the oxidation of ethanol. Pd is a good alternative for the development of an active catalyst for DEFC because it is more abundant, cost-effective and has relatively high catalytic activity in alkaline media and tolerance to CO-like species poisoning [27,28,29,30].
Although Pd is a good electrocatalyst for EOR [31], its performance can be further enhanced by changing its size, morphology and the supporting substrate [31,32,33,34]. Carbon materials have long been used to support metal catalysts because of their inertness to chemical reactions, resistance to corrosion, large surface area and cost-effectiveness [35,36,37]. Various carbon-based nano- or micro-materials, such as carbon nanotube (CNT) [29,38], graphene or graphene oxide [39,40,41,42,43], carbon microspheres [44] and helical carbon nanofibers [45], have been used as Pd-based catalyst supports to increase its catalytic activity and efficiency. However, these carbon-based materials are typically produced by methods with harsh synthetic conditions, complicated synthetic procedures and low production yields, and their economical and mass production are limited [46,47,48]. In addition, the presence of organic contaminants and/or the surface hyper-hydrophobicity of these carbon supports may cause weak interaction with noble metal nanoparticles and eventually lead to their detachment and uneven distribution [49,50].
Carbon fiber paper (CFP) is a thin sheet of laminated short carbon fibers with high electrical conductivity [51]. CFP has been used for the development of various fuel cells or biofuel cells due to its high surface area, high porosity, strong mechanical property, good electrical and thermal conductivity and excellent chemical stability [52,53,54,55,56,57]. CFP has also been adopted for the development of a Pd-supported anode (Pd/CFP) in DEFCs by directly electrodepositing Pd onto the CFP without any treatment [43]. Unfortunately, its performance, in terms of its poisoning tolerance to intermediate species, was poor [43]. Although the true reason for the poor performance of Pd/CFP is unknown, the inefficient deposition, loose attachment and non-uniform distribution of nano-scaled Pd on CFP have been postulated. Recently, plasma was shown to improve the surface chemical and electrical properties of carbon-based electrodes such as the screen-printed carbon paste electrode, graphite nanofiber electrode, graphite felt electrode, glassy carbon electrode and CFP [49,50,58,59,60,61,62,63]. The great improvement in electrical properties of carbon-based electrodes, such as electrochemical reactivity and capacitance, by plasma treatment may be due to the removal of the organic contaminants and the increase in oxygen species and other functional groups on the surface of electrodes [49,50,58,59,60,61,62,63]. The attachment of electrochemical active microorganisms on the glassy carbon electrode and carbon/graphite felt electrode can also be enhanced after the pre-treatment of electrodes by oxygen plasma [60,61]. Interestingly, the removal of the organic contaminants may increase hydrophilicity of the electrode surface and allow the easy access of metal ions. Adding oxygen species to the surface of carbon-based electrodes can further enhance the surface adsorption of metal ions [35,36], leading to the enhancement of chemical deposition and loading of Pt-Ru on the graphite nanofibers [63], and the improvement of electrodeposition and even distribution of gold nanoparticles on CFP [64]. Based on these observations, the development of a highly efficient electrode for DEFC by direct electrodeposition of Pd on the oxygen plasma-treated CFP was proposed.
In this study, we demonstrated that Pd could be effectively electrodeposited onto the surface of the oxygen plasma-treated CFP (pCFP). The deposition of Pd onto pCFP was analyzed by a scanning electron microscope (SEM), energy-dispersive X-ray spectroscopy (EDS) and thermogravimetric analysis (TGA). The generated Pd-deposited pCFP (Pd/pCFP) exhibited great catalytic activity towards ethanol oxidation in alkaline media, with high specific current density and good tolerance to poisoning intermediates.
2. Results and Discussion
2.1. Effect of Plasma Treatment in the Electrodeposition of Pd on CFP
Oxygen plasma treatment was previously shown to improve the electrodeposition of Au nanoparticles onto CFP [64]. In this study, oxygen plasma treatment was proposed to improve the electrodeposition of Pd onto CFP, as well as the catalytic activity of the electrodeposited Pd catalyst towards ethanol oxidation. As shown in Figure S1, without oxygen plasma treatment, the Pd nanoparticles (PdNP) could barely be electrodeposited onto the CFP by CV in 100 mM phosphate buffer, pH 7.0, containing 2 mM K2PdCl4 in a potential range from −0.6 to +1.0 V (vs. Ag/AgCl) with a scan rate of 50 mV s−1 for 20 cycles (panels A and B). In contrast, after oxygen plasma treatment, the PdNPs could be electrodeposited onto the CFP under the same conditions with a size distribution between 5 and 90 nm (panels C and D). The Pd signals on the Energy Dispersive X-ray Spectrometry of the PdNP/pCFP further demonstrated the electrodeposition of Pd onto the surface of CFP (Figure S2). These results indicate that oxygen plasma treatment can improve the deposition of the Pd onto CFP. However, the low deposition rate of Pd onto pCFP suggests that optimization for Pd electrodeposition is required.
2.2. Preparation of Pd/pCFP Electrode for Ethanol Oxidation
The effect of pH on the electrodeposition of Pd on pCFP was first investigated by the catalytic activity of electrodes in H2O2 oxidation. The electrodeposition of Pd onto pCFP was performed by CV in 2 mM K2PdCl4 at various pH values (pH 2.9, 5.0, 5.8, 7.0 and 8.0) in a potential range from −0.5 V to +0.9 V for 10 cycles. The electrochemical responses of the Pd/pCFP electrodes towards H2O2 oxidation showed that the electrode prepared at pH 5.8 exhibited the highest peak response (Figure S3). The electrochemical responses of Pd/pCFP electrodes to H2O2 were moderately decreased when they were prepared at the pHs of 2.9 and 5.0, while the electrochemical responses of the electrodes significantly decreased when they were prepared at higher pH values, i.e., pH 7.0 and 8.0. The SEM images showed that the surface of the Pd/pCFP electrode prepared at pH 5.8 was covered by a layer of Pd nanoparticles, with an average size of about 10 nm (Figure 1A,B). This result suggests that the electrodeposition of Pd onto pCFP at pH 5.8 is optimal and can be used for the future preparation of Pd/pCFP electrodes.
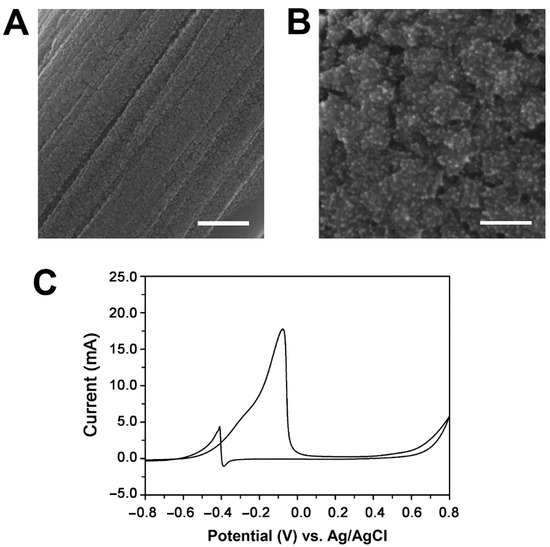
Figure 1.
The effect of plasma treatment on the preparation of Pd/CFP electrodes. The Pd/pCFP was prepared by CV in 0.1 M phosphate buffer, pH 5.8, containing 2 mM K2PdCl4 in a potential range between −0.5 V and +0.9 V (vs. Ag/AgCl) with a scan rate of 50 mV s−1 for 10 cycles. SEM images of Pd electrodeposited onto pCFP at low (20,000×) (A) and high magnifications (200,000×) (B). The scale bars in panels (A) and (B) are 1 μm and 100 nm, respectively. (C) The cyclic voltammogram of 1 M ethanol oxidation on the Pd/pCFP electrode in 1 M KOH in a potential range of −0.8 V to +0.6 V at scan rate of 50 mV s−1.
The catalytic activity of the Pd/pCFP electrode prepared at pH 5.8 to 1 M ethanol under the alkaline condition (1 M KOH) was further analyzed by CV in a potential range from −0.8 V to +0.6 V. As shown in Figure 1C, the prepared Pd/pCFP electrode exhibited a broad oxidation peak corresponding to ethanol oxidation in a forward scan starting from −0.6 V to the onset potential peak at −0.08 V (vs. Ag/AgCl). A small oxidative peak corresponding to the depletion of the adsorbed poisonous species appeared at −0.41 V in the reverse scan with forward and backward peak current densities of 118.67 mA cm−2 and 29.20 mA cm−2, respectively. The ratio of the forward anodic peak current (If) to the backward anodic peak current (Ib) was calculated as If/Ib = 4.02. This redox profile is similar to that reported previously [65]. Although the electrocatalytic activity (118.67 mA) of the prepared Pd/pCFP towards ethanol oxidation is lower than that of Pd/CFP reported previously (413 mA mg−1, Pd corresponding to 165.2 mA cm−2) [43], the prepared Pd/pCFP electrode exhibited a higher tolerance to the poisonous intermediates during the ethanol oxidation [38,66] than that of Pd/CFP, with an If/Ib ratio of 2.14 [43].
To generate the electrode with both high catalytic activity to ethanol and high tolerance to poisonous intermediates from ethanol oxidation, the preparation of Pd/pCFP was further optimized by using different potential cycling numbers (10, 20, 30 and 40 cycles) and scan rates (100, 50, 40, 30, 20, 10 mV s−1). As shown in Figure 2A, the If of Pd/pCFP prepared at pH 5.8 increased in response to the decrease in scan rates, with the highest response appearing at the scan rate of 10 mV s−1 with a current density of 224.3 ± 28.1 mA cm−2 and an If/Ib ratio of 1.77. Next, the effect of different potential cycling numbers on the preparation of Pd/pCFP was studied. The electrodes were prepared using 2 mM K2PdCl4, pH 5.8, with a scan rate of 10 mV s−1 for 10, 20, 30 and 40 cycles. As shown in Figure 2B, the Pd/pCFP prepared at a scan rate of 10 mV s−1 for 40 cycles reached the highest response to ethanol oxidation with a current density of 477.9 ± 29.6 mA cm−2 and If/Ib ratio of 3.37. These observations suggest that anodes for future experiments could be prepared on pCFP by CV in 100 mM phosphate, pH 5.8, containing 2 mM K2PdCl4 in a potential range of −0.5 V to +0.9 V (vs. Ag/AgCl) at a scan rate of 10 mV s−1 for 40 cycles.
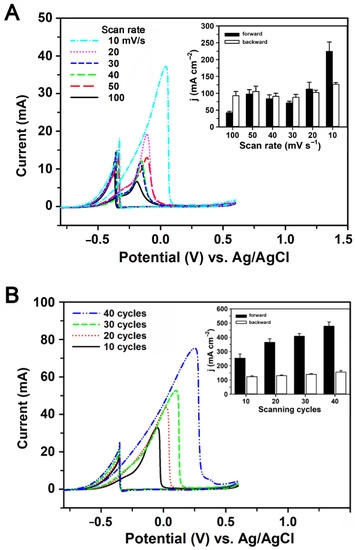
Figure 2.
The cyclic voltammograms of ethanol oxidation onto Pd/pCFP electrodes prepared under different conditions. The effect of the cycling numbers and scan rates of the CV on the preparation of Pd/pCFP was studied. The performance of the prepared electrodes was analyzed by the electrochemical oxidation of 1 M ethanol in 1 M KOH. (A) The preparation of Pd/pCFP was carried out by CV in 100 mM phosphate buffer, pH 5.8, containing 2 mM K2PdCl4 in a potential range of −0.5 V to +0.9 V at different scan rates (100, 50, 40, 30, 20, 10 mV s−1) for 10 cycles. (inset) The average maximum forward (closed bars) and backward (open bars) current densities of Pd/pCFPs to 1 M ethanol were obtained from three independent experiments and presented as mean ± S.D. (B) The Pd/pCFP electrodes were prepared as described in (A) except that different potential cycling numbers (i.e., 10, 20, 30 and 40 cycles) were adopted. (inset) The average maximum forward (closed bars) and backward (open bars) current densities of Pd/pCFPs to 1 M ethanol were obtained from three independent experiments and presented as mean ± S.D.
2.3. Characterization of Pd/pCFP Electrode
The surface characteristics of the prepared Pd/pCFP under the optimized condition was monitored under the SEM. It was found that Pd formed a uniform layer with numerous nano-granules on the surface of pCFP (Figure 3A,B). The EDS analysis of the surface of Pd/pCFP exhibited a characteristic energy peak of Pd and some minor peaks representing the C and O elements, demonstrating the deposition of Pd on the CFP (Figure 3B).
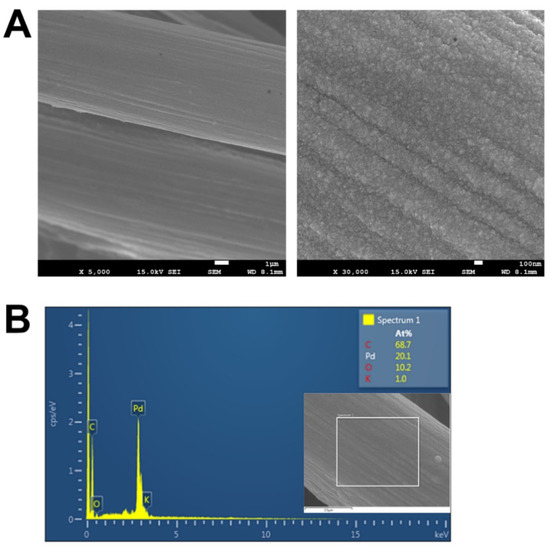
Figure 3.
Scanning electron microscopic (SEM) images and energy dispersive X-ray spectrometry of Pd/pCFP electrodes. The Pd/pCFP electrodes were prepared by CV in 2 mM K2PdCl4/100 mM phosphate buffer, pH 5.8, in a potential range of −0.5 V to +0.9 V at a scan rate of 10 mV s−1 for 40 cycles. The SEM images of Pd/pCFP under magnification powers of 5000× (A) and 30,000× (B) were recorded. The scale bar in (A) and (B) is 1 μm and 200 nm, respectively. (C) The energy dispersive X-ray spectroscopy was carried out in the region on the Pd/pCFP (inset).
The loading content of Pd onto pCFP was determined by thermogravimetric analysis. In this study, the prepared Pd/pCFP electrode was thermally decomposed in air at a heating rate of 10 °C min−1. The Pd/pCFP exhibited almost no weight loss until about 600 °C (Figure 4). A major weight loss occurred between 615 °C and 850 °C, which was due to the decomposition of carbon materials. The remaining residue after thermal decomposition represents the loading of Pd onto pCFP, which was estimated to be 2.43 mg/cm2.
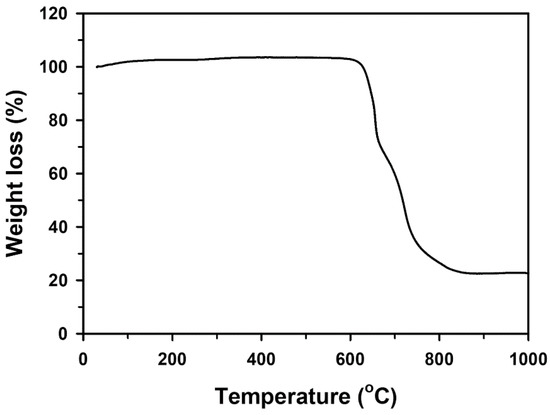
Figure 4.
Thermogravimetric analysis of Pd/pCFP electrodes in air. The Pd/pCFP was prepared as described in Methodology and Figure 3. The thermal decomposition of Pd/pCFP was conducted in air heated from 30 °C to 1000 °C at a rate of 10 °C min−1.
2.4. Electro-Oxidation of Ethanol on Pd/pCFP Electrodes
The prepared Pd/pCFP electrode was designed for the ethanol oxidation under the alkaline condition. Therefore, CV of the prepared electrode in 1 M KOH was first performed to determine the basal responses of the Pd/pCFP without ethanol (Figure S4). The electrochemical response of the Pd/pCFP electrode to 1 M KOH was performed at a scan rate of 50 mV s−1 in the potential range of −1.0 V to +1.0 V. The prepared Pd/pCFP exhibited similar electrochemical behavior as that of the reported carbon-based Pd catalysts. Three responsive peaks were observed in the cyclic voltammogram. Pd has been known to absorb hydrogen at room temperature and atmospheric pressure [67]. Hence, the anodic peak A in the potential range between −0.65 V and −0.45 V represents the oxidation of the absorbed hydrogen on the surface of active Pd [65,68,69]. Peak B emerged from about −0.1 V to +0.7 V and was due to the formation of Pd(II) oxide on the surface of the catalyst [68,69,70]. The potential and current increased successively over time until reaching a plateau. The process beyond the potential range could be attributed to the oxygen evolution reaction [71]. In the reverse scan, a cathodic peak C, situated at −0.6 V, was attributed to the reduction of Pd(II) oxide on the surface of the Pd nanoparticles [65,72].
The electrochemical responses of ethanol oxidation on the prepared Pd/pCFP were further investigated by CV in different potential ranges (i.e., −0.8 V to +0.6 V, −0.8 V to +0.8 V, −0.8 V to +1.0 V, and −0.8 V to +1.2 V) to find the optimal operational condition (Figure 5). Interestingly, the oxidative current of ethanol on Pd/pCFP reached a maximum in the potential range of −0.8 V to +0.8 V (dotted curve), with the peak potential at +0.29 V (vs. Ag/AgCl) and a current density of 222.8 mA mg−1 Pd or 541.3 mA cm−2, whereas an anodic peak in the backward scan appeared at −0.4 V, with a current density of 40.6 mA mg−1 Pd or 98.7 mA cm−2. The If/Ib ratio for the prepared Pd/pCFP was about 5.48. In comparison, the ethanol oxidation responses across different potential ranges (Figure 5) were lower with the current densities of 168.2 mA mg−1 Pd, 187.7 mA mg−1 Pd, and 146.0 mA mg−1 Pd for the potential ranges of −0.8 V to +0.6 V (solid line), −0.8 V to +1.0 V (dash line), and −0.8 V to +1.2 V (dash-dotted line), respectively. Interestingly, the If/Ib ratios of ethanol oxidation in the potential ranges of −0.8 V to +1.0 V (If/Ib = 7.24) and −0.8 V to +1.2 V (If/Ib = 8.04) were much higher than that in the potential range of −0.8 V to +0.8 V. This may be due to the decomposition of the poisoning species at high anodic potentials. Considering the possible occurrence of unnecessary electrochemical reactions at high potentials, the potential range of −0.8 V to +0.8 V was suggested to be the optimal condition for the ethanol oxidation reaction.
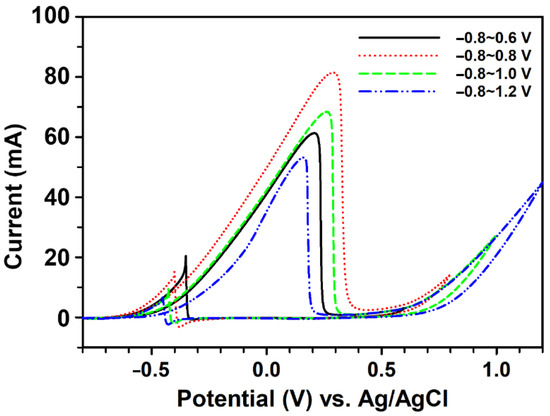
Figure 5.
The electrochemical responses of ethanol oxidation across different potential ranges. The electrochemical responses of 1 M ethanol oxidation in 1 M KOH on the Pd/pCFP electrode were performed by CV at a scan rate of 50 mV s−1 in the potential ranges of −0.8~0.6 V (solid line), −0.8~0.8 V (dotted line), −0.8~1.0 V (dash line), and −0.8~1.2 V (dash-dotted line).
The presence of poisonous intermediates during ethanol oxidation may block the electrode surface, poison the catalyst, and reduce the oxidation of ethanol. The long-term electrochemical stability and poisoning resistance capability of Pd/pCFP catalyst was further evaluated by chronoamperometry (CA) [73]. In this study, the electrochemical responses of 1 M ethanol in an alkaline condition were monitored at different potentials (i.e., −0.10, −0.15, −0.20, −0.25 and −0.30 V) for 3000 s. As shown in Figure 6, the Pd/pCFP electrode exhibited a slow current decay at the potential of −0.15 V (dotted line), with a current density of 30.1 mA cm−2 at the end of 3000 s. In comparison, the initial current of ethanol oxidation decayed quickly in chronoamperometry analysis at the rest of the potentials tested, i.e., −0.10 V (solid line), −0.20 V (short dash line), −0.25 V (dash-dotted line) and −0.30 V (long dash line). These results suggest that at the potential of −0.15 V, the Pd/pCFP electrode exhibited better catalytic activity and resistance against poisoning intermediates during ethanol oxidation.
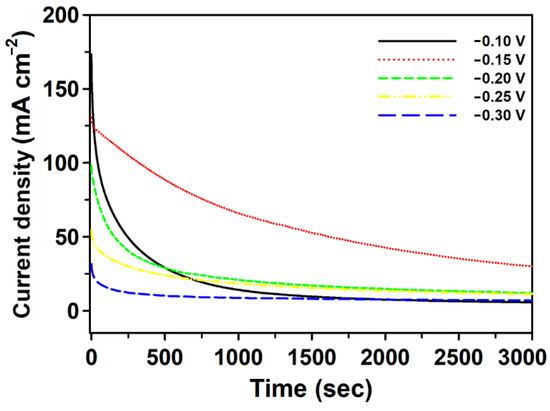
Figure 6.
Electrochemical characterization of Pd/pCFP. Chronoamperometric curves of 1 M ethanol was carried out in 1 M KOH at various fixed potentials (−0.10, −0.15, −0.20, −0.25 and −0.30 V) (vs. Ag/AgCl) for 3000 s.
The poisoning effect of intermediates, such as acetaldehyde, from the incomplete oxidation of ethanol was demonstrated by the electrochemical oxidation of 1 M ethanol without (solid line) and with 0.5 M acetaldehyde (dash line) (Figure 7). The anodic peak potential blue-shifted from 0.49 V to 0.26 V, and the peak current density (j) reduced about 33% from 205.5 mA mg−1 Pd to 138.3 mA mg−1 Pd.
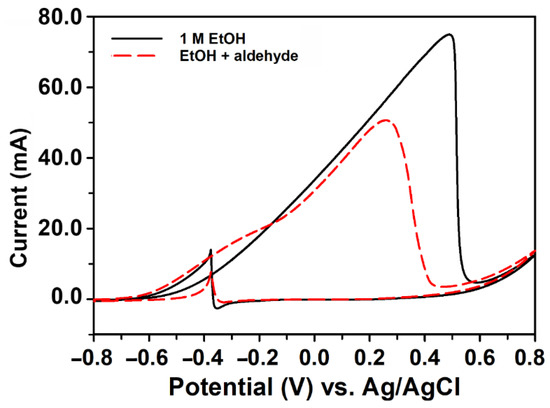
Figure 7.
The influence of acetaldehyde on ethanol oxidation. The CV of 1 M ethanol on the Pd/pCFP electrode was carried out in 1 KOH in the potential range of −0.8 V to +0.8 V with (dash line) or without (solid line) 0.5 M acetaldehyde. The scan rate was 50 mV s−1.
3. Materials and Methods
3.1. Materials
Carbon fiber paper (TGP-H-060) was purchased from Toray Inc. (Tokyo, Japan). Copper foil, potassium tetrachloropalladate (II) (K2PdCl4), ethanol (99.8%) and acetaldehyde were obtained from Sigma-Aldrich (St. Louis, MO, USA). Potassium dihydrogen phosphate, potassium hydrogen phosphate, sodium chloride, potassium chloride and potassium hydroxide were obtained from Showa. Acetic acid and 2-propanol were obtained from J.T. Baker (Randor, PA, USA). The rest of the reagents were analytical grade.
3.2. Apparatus
The electrochemical measurements were carried out on a CHI6116E (CH Instruments, Austin, TX, USA) by using the standard three-electrode system with an Ag/AgCl electrode, platinum wire and Pd/pCFP as the reference electrode, counter electrode and working electrode, respectively. Oxygen plasma treatment was performed in the plasma cleaner (Zepto, Diener electronic, Ebhausen, Germany). Scanning electron microscopy (SEM) and EDS were carried out using a high-resolution thermal field emission scanning electron microscope, JSM-7610F (JEOL Ltd., Tokyo, Japan), of the Instrumentation Center at National Tsing Hua University. Thermal Gravimetric Analysis (TGA) was performed using 2-HT (Mettler-Toledo, Greifensee, Switzerland) of the Instrumentation Center at National Tsing Hua University and was conducted in air heated from 30 °C to 1000 °C at a rate of 10 °C min−1.
3.3. Preparation of Working Electrode for Ethanol Oxidation
The working electrodes were prepared from pCFP (working area of 3 × 5 mm2) as described previously with some modification [50]. The plasma treatment of CFP prior to the Pd electrodeposition was carried out in the chamber of the plasma cleaner at an air pressure of 0.4 bar, power of 75 W, and oxygen flow rate of 40 N h−1 for 15 s. Electrodeposition of Pd was performed in 0.1 M phosphate buffer, pH 5.8, containing 2 mM K2PdCl4 at a scan rate of 10 mV s−1. The potential cycling between −0.5 V and +0.9 V was performed at room temperature for 40 cycles.
3.4. Electrochemical Analysis
Unless defined, the electrochemical measurements of the oxidation of 1 M ethanol were performed by cyclic voltammetry (CV) in 1 M KOH between the potential range of −0.8 V and +0.8 V with a scan rate of 50 mV s−1. The electrolyte was deaerated with N2 gas at ambient temperature for 10 min before measurements were obtained. The chronoamperometric measurement (CA) of ethanol oxidation on the Pd/pCFP electrode was performed in 1 M KOH containing 1 M ethanol at the overpotentials of −0.10, −0.15, −0.20, −0.25 and −0.30 V for 3000 s.
4. Conclusions
In this study, a highly efficient Pd-based electrode for ethanol oxidation was simply fabricated by electrodepositing Pd on the oxygen plasma-treated CFP. The direct deposition of Pd catalyst on the plasma-treated CFP exhibits several advantages, including easy preparation, inexpensiveness and high catalytic activity. The condition for the preparation of Pd/pCFP was also optimized by changing pH values, scanning rates and the cycling numbers for CV. The electrochemical response of Pd/pCFP, prepared under optimized conditions for ethanol oxidation, was 222.6 mAmg−1 Pd loaded, which was about 54% of the reported Pd/CFP [41]. However, the If/Ib ratio of 5.49, an indicator of the capability of a catalyst to tolerate the poisonous intermediate species during reactions, was much higher than that of Pd/CFP (If/Ib ratio = 2.14). In addition, the Pd/pCFP showed a long stability. In conclusion, a highly efficient Pd-supported carbon-based electrode with high catalytic activity towards ethanol and high tolerance to poisonous species was developed and demonstrated in this study. These excellent characteristics made Pd/pCFP a more favorable anodic electrode for the development of DEFC.
Supplementary Materials
The following are available online at https://www.mdpi.com/2073-4344/11/2/248/s1, Figure S1: Scanning electron microscopic images of palladium-electrodeposited CFP electrodes, Figure S2: Energy dispersive X-ray spectrometry of the Pd/pCFP. Figure S3: Effect of pH value on the electrodeposition of Pd on pCFP, Figure S4: Electrochemical behavior of Pd/pCFP electrode to 1 M KOH.
Author Contributions
Conceptualization, C.-J.Y.; Methodology, H.-Y.P. and C.-H.L.; Investigation, H.-Y.P. and C.-H.L.; Resources, C.-J.Y.; Data curation: H.-Y.P. and C.-H.L.; Supervision, C.-J.Y.; Formal analysis, C.-J.Y. and W.L.; Writing—original draft, H.-Y.P.; Writing—review and editing, C.-J.Y.; Funding acquisition, C.-J.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This research was financially supported by the research grants MOST 107-2314-B-009-002 and MOST 109-2314-B-009-002 from the Ministry of Science and Technology, Taiwan (ROC).
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
The authors would like to thank the Ministry of Science and Technology (MOST), Taiwan (ROC) for financially supporting this research and the Instrumentation Center at National Tsing Hua University for helping to perform the SEM and chronoamperometric analysis.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Perera, F. Pollution from Fossil-Fuel Combustion is the Leading Environmental Threat to Global Pediatric Health and Equity: Solutions Exist. Int. J. Environ. Res. Public Health 2018, 15, 16. [Google Scholar] [CrossRef]
- Martins, F.; Felgueiras, C.; Smitkova, M.; Caetano, N. Analysis of Fossil Fuel Energy Consumption and Environmental Impacts in European Countries. Energies 2019, 12, 964. [Google Scholar] [CrossRef]
- Arshad, A.; Ali, H.M.; Habib, A.; Bashir, M.A.; Jabbal, M.; Yan, Y. Energy and exergy analysis of fuel cells: A review. Therm. Sci. Eng. Progress 2019, 9, 308–321. [Google Scholar] [CrossRef]
- Kirubakaran, A.; Jain, S.; Nema, R.K. A review on fuel cell technologies and power electronic interface. Renew. Sustain. Energy Rev. 2009, 13, 2430–2440. [Google Scholar] [CrossRef]
- Yan, F.; Xu, L.; Wang, Y. Application of hydrogen enriched natural gas in spark ignition IC engines: From fundamental fuel properties to engine performances and emissions. Renew. Sustain. Energy Rev. 2018, 82, 1457–1488. [Google Scholar] [CrossRef]
- Sharaf, O.Z.; Orhan, M.F. An overview of fuel cell technology: Fundamentals and applications. Renew. Sustain. Energy Rev. 2014, 32, 810–853. [Google Scholar] [CrossRef]
- Ong, B.C.; Kamarudin, S.K.; Basri, S. Direct liquid fuel cells: A review. Intel. J. Hydrog. Energy 2017, 42, 10142–10157. [Google Scholar] [CrossRef]
- Velisala, V.; Srinivasulu, G.N.; Reddy, B.S.; Rao, K.V.K. Review on challenges of direct liquid fuel cells for portable application. World J. Eng. 2015, 12, 591–606. [Google Scholar] [CrossRef]
- Yüksel, F.; Yüksel, B. The use of ethanol–gasoline blend as a fuel in an SI engine. Renew. Energy 2004, 29, 1181–1191. [Google Scholar] [CrossRef]
- Kwanchareon, P.; Luengnaruemitchai, A.; Jai-In, S. Solubility of a diesel–biodiesel–ethanol blend, its fuel properties, and its emission characteristics from diesel engine. Fuel 2007, 86, 1053–1061. [Google Scholar] [CrossRef]
- Papagiannakis, R.; Hountalas, D. Combustion and exhaust emission characteristics of a dual fuel compression ignition engine operated with pilot diesel fuel and natural gas. Energy Convers. Manag. 2004, 45, 2971–2987. [Google Scholar] [CrossRef]
- Manochioa, C.; Andradea, B.R.; Rodrigueza, R.P.; Moraesb, B.S. Ethanol from biomass: A comparative overview. Renew. Sustain. Energy Rev. 2017, 80, 743–755. [Google Scholar] [CrossRef]
- Demirbas, A. Political, economic and environmental impacts of biofuels: A review. Appl. Energy 2009, 86, S108–S117. [Google Scholar] [CrossRef]
- Cermenek, B.; Ranninger, J.; Hacker, V. Alkaline Direct Ethanol Fuel Cell. In Ethanol: Science and Engineering; Basile, A., Iulianelli, A., Dalena, F., Veziroğlu, T.N., Eds.; Elsevier Inc.: Amsterdam, The Netherlands, 2019; pp. 383–905. [Google Scholar]
- Kamarudin, M.Z.F.; Kamarudin, S.K.; Masdar, M.S.; Daud, W.R.W. Direct ethanol fuel cells. Int. J. Hydrog. Energy 2013, 38, 9438–9458. [Google Scholar] [CrossRef]
- Lamy, C.; Belgsir, E.M.; Leger, J.-M. Electrocatalytic oxidation of aliphatic alcohols: Application to the direct alcohol fuel cell (DAFC). J. Appl. Electrochem. 2001, 31, 799–809. [Google Scholar] [CrossRef]
- Almeida, T.S.; Andrade, A.R. New trends in direct ethanol fuel cells. In New and Future Developments in Catalysis: Batteries, Hydrogen Storage and Fuel Cells; Suib, S.L., Ed.; Elsevier: Amsterdam, The Netherlands, 2013; pp. 429–452. [Google Scholar]
- Niwa, K.; Murata, R.; Arai, K.; Ikuma, Y. Intermediate oxidation in direct ethanol fuel cells. Trans. Mat. Res. Soc. 2014, 39, 43–46. [Google Scholar] [CrossRef][Green Version]
- Wang, X.-H.; Yuan, S.-M.; Zhu, Y.; Ni, H.-J. Preparation and performance research of PtSn catalyst supported on carbon fiber for direct ethanol fuel cells. J. Fuel Chem. Technol. 2012, 40, 1454–1458. [Google Scholar] [CrossRef]
- Zhou, W.; Zhou, Z.; Song, S.; Li, W.; Sun, G.; Tsiakaras, P.; Xin, Q. Pt based anode catalysts for direct ethanol fuel cells. Appl. Catal. B 2003, 46, 273–285. [Google Scholar] [CrossRef]
- Seselj, N.; Engelbrekt, C.; Zhang, J. Graphene-supported platinum catalysts for fuel cells. Sci. Bull. 2015, 60, 864–876. [Google Scholar] [CrossRef]
- Zhou, W.J.; Song, S.Q.; Li, W.Z.; Zhou, Z.H.; Sun, G.Q.; Xin, Q.; Douvartzides, S.; Tsiakaras, P. Direct Ethanol Fuel Cells Based on PtSn Anodes: The Effect of Sn Content on the Fuel Cell Performance. J. Power Sources 2005, 140, 50–58. [Google Scholar] [CrossRef]
- Tilquin, J.Y.; Côté, R.; Guay, D.; Dodelet, J.P.; Denès, G. Carbon monoxide poisoning of platinum-graphite catalysts for polymer electrolyte fuel cells: Comparison between platinum-supported on graphite and intercalated in graphite. J. Power Sources 1996, 61, 193–200. [Google Scholar] [CrossRef]
- Cai, B.; Wen, D.; Liu, W.; Herrmann, A.K.; Benad, A.; Eychmüller, A. Function-led design of aerogels: Self-assembly of alloyed PdNi hollow nanospheres for efficient electrocatalysis. Angew. Chem. Int. Ed. 2015, 54, 13101–13105. [Google Scholar] [CrossRef] [PubMed]
- Hoshi, Y.; Yoshida, T.; Atsushi Nishikata, A.; Tsuru, T. Dissolution of Pt–M (M: Cu, Co, Ni, Fe) binary alloys in sulfuric acid solution. Electrochimica Acta 2011, 56, 5302–5309. [Google Scholar] [CrossRef]
- Chen, H.-S.; Benedetti, T.M.; Gonçales, V.R.; Bedford, N.M.; Scott, R.W.J.; Webster, R.F.; Cheong, S.; Gooding, J.J.; Tilley, R.D. Preserving the Exposed Facets of Pt3Sn Intermetallic Nanocubes During an Order to Disorder Transition Allows the Elucidation of the Effect of the Degree of Alloy Ordering on Electrocatalysis. J. Am. Chem. Soc. 2020, 142, 3231–3239. [Google Scholar] [CrossRef] [PubMed]
- Chen, A.; Ostrom, C. Palladium-based nanomaterials: Synthesis and electrochemical applications. Chem. Rev. 2015, 115, 11999–12044. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Zhou, D.; Zhou, M. Ethanol electrooxidation on Pd/C nanoparticles in alkaline media. J. Energy Chem. 2016, 25, 71–76. [Google Scholar] [CrossRef]
- Bianchini, C.; Shen, P.K. Palladium-based electrocatalysts for alcohol oxidation in half cells and in direct alcohol fuel cells. Chem. Rev. 2009, 109, 4183–4206. [Google Scholar] [CrossRef]
- Ma, L.; Chu, D.; Chen, R. Comparison of ethanol electro-oxidation on Pt/C and Pd/C catalysts in alkaline media. Int. J. Hydrog. Energy 2012, 37, 11185–11194. [Google Scholar] [CrossRef]
- Yarulin, A.E.; Crespo-Quesada, R.M.; Egorova, E.V.; Kiwi-Minsker, L. L Structure sensitivity of selective acetylene hydrogenation over the catalysts with shape-controlled palladium nanoparticles. Kinet. Catal. 2012, 53, 253–261. [Google Scholar] [CrossRef]
- Sawangphruk, M.; Krittayavathananon, A.; Chinwipas, N.; Srimuk, P.; Vatanatham, T.; Limtrakul, S.; Foord, J.S. Ultraporous Palladium Supported on Graphene-Coated Carbon Fiber Paper as a Highly Active Catalyst Electrode for the Oxidation of Methanol. Fuel Cells 2013, 13, 881–888. [Google Scholar] [CrossRef]
- Ghosh, S.; Remita, H.; Kar, P.; Choudhury, S.; Sardar, S.; Beaunier, P.; Roy, P.S.; Bhattacharya, S.K.; Pal, S.K. Facile synthesis of Pd nanostructures in hexagonal mesophases as a promising electrocatalyst for ethanol oxidation. J. Mater. Chem. A 2015, 3, 9517–9527. [Google Scholar] [CrossRef]
- Cerritos, R.C.; Guerra-Balcázar, M.; Ramírez, R.F.; Ledesma-García, J.; Arriaga, L.G. Morphological effect of Pd catalyst on ethanol electro-oxidation reaction. Materials 2012, 5, 1686–1697. [Google Scholar] [CrossRef]
- Kang, M.; Song, M.W.; Kim, K.L. Palladium catalysts supported on activated carbon with different textural and surface chemical properties. React. Kinet. Catal. Lett. 2002, 76, 207–212. [Google Scholar] [CrossRef]
- Rodriguez-Reinoso, F. The role of carbon materials in heterogeneous catalysis. Carbon 1998, 36, 159–175. [Google Scholar] [CrossRef]
- Hu, C.; Xin, W. Highly dispersed palladium nanoparticles on commercial carbon black with significantly high electro-catalytic activity for methanol and ethanol oxidation. Int. J. Hydrog. Energy 2015, 40, 12382–12391. [Google Scholar] [CrossRef]
- Yang, G.; Zhou, Y.; Pan, H.-B.; Zhu, C.; Fu, S.; Wai, C.M.; Du, D.; Zhu, J.-J.; Lin, Y. Ultrasonic-assisted synthesis of Pd–Pt/carbon nanotubes nanocomposites for enhanced electro-oxidation of ethanol and methanol in alkaline medium. Ultrason. Sonochem. 2016, 28, 192–198. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Ma, J.; Huo, H.; Jin, J.; Ma, J.; Yang, H. Ionic liquids-noncovalently functionalized multi-walled carbon nanotubes decorated with palladium nanoparticles: A promising electrocatalyst for ethanol electrooxidation. Int. J. Hydrog. Energy 2016, 41, 12358–12368. [Google Scholar] [CrossRef]
- Serov, A.; Andersen, N.I.; Kabir, S.A.; Roy, A.; Asset, T.; Chatenet, M.; Maillard, F.; Atanassov, P. Palladium Supported on 3D Graphene as an Active Catalyst for Alcohols Electrooxidation. J. Electrochem. Soc. 2015, 162, F1305–F1309. [Google Scholar] [CrossRef][Green Version]
- Höltig, M.; Ruhmlieb, C.; Kipp, T.; Mews, A. Highly efficient fuel cell electrodes from few-layer graphene sheets and electrochemically deposited palladium nanoparticles. J. Phys. Chem. C 2016, 120, 7476–7481. [Google Scholar] [CrossRef]
- Yazdan-Abad, M.Z.; Noroozifar, M.; Nafiseh, A.; Modarresi-Alam, R.A.; Saravani, H. Pd nanonetwork decorated on the rGO as a high-performance electrocatalyst for ethanol oxidation. Appl. Surf. Sci. 2018, 462, 112–117. [Google Scholar] [CrossRef]
- Sawangphruk, M.; Krittayavathananon, A.; Chinwipasa, N. Ultraporous palladium on flexible graphene-coated carbon fiber paper as high-performance electro-catalysts for the electro-oxidation of ethanol. J. Mater. Chem. A 2013, 1, 1030–1034. [Google Scholar] [CrossRef]
- Xu, C.; Cheng, L.; Shen, P.; Liu, Y. Methanol and ethanol electrooxidation on Pt and Pd supported on carbon microsphere in alkaline media. Electrochem. Commun. 2007, 9, 997–1001. [Google Scholar] [CrossRef]
- Hu, G.; Nitze, F.; Sharifi, T.; Barzegar, H.R.; Wagberg, T. Self-assembled palladium on helical carbon nanofibers as enhanced electrocatalysts for electro-oxidation of small molecules. J. Mater. Chem. 2012, 22, 8541–8548. [Google Scholar] [CrossRef]
- Ebbesen, T.W.; Ajayan, P.M. Large-Scale Synthesis of Carbon Nanotubes. Nature 1992, 358, 220–221. [Google Scholar] [CrossRef]
- Thess, A.; Lee, R.; Nikolaev, P.; Dai, H.; Petit, P.; Robert, J.; Xu, C.; Lee, Y.H.; Kim, S.G.; Rinzler, A.G.; et al. Crystalline Ropes of Metallic Carbon Nanotubes. Science 1996, 273, 483–487. [Google Scholar] [CrossRef]
- Fan, S.; Chapline, M.C.; Frankin, N.R.; Tombler, T.W.; Cassell, A.M.; Dai, H. Self-Oriented Regular Arrays of Carbon Nanotubes and Their Field Emission Properties. Science 1999, 283, 512–514. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.C.; Chang, K.S.; Yuan, C.-J. Enhancement of electrochemical properties of screen-printed carbon electrodes by oxygen plasma treatment. Electrochim. Acta 2009, 54, 4937–4949. [Google Scholar] [CrossRef]
- Yuan, C.-J.; Wang, C.-L.; Wu, T.Y.; Hwang, K.-C.; Chao, W.-C. Fabrication of a carbon fiber paper as the electrode and its application toward developing a sensitive unmediated amperometric biosensor. Biosens. Bioelectron. 2011, 26, 2858–2863. [Google Scholar] [CrossRef]
- Mathur, R.; Maheshwari, P.H.; Dhami, T.; Tandon, R. Characteristics of the carbon paper heat-treated to different temperatures and its influence on the performance of PEM fuel cell. Electrochim. Acta 2007, 52, 4809–4817. [Google Scholar] [CrossRef]
- Mathur, R.; Maheshwari, P.H.; Dhami, T.; Sharma, R.; Sharma, C. Processing of carbon composite paper as electrode for fuel cell. J. Power Sources 2006, 161, 790–798. [Google Scholar] [CrossRef]
- Reshetenko, T.V.; Kim, H.T.; Krewer, U.; Kweon, H.J. The Effect of the Anode Loading and Method of MEA Fabrication on DMFC Performance. Fuel Cell 2007, 7, 238–245. [Google Scholar] [CrossRef]
- Gharibi, H.; Mirzaie, R.A. Fabrication of gas-diffusion electrodes at various pressures and investigation of synergetic effects of mixed electrocatalysts on oxygen reduction reaction. J. Power Sources 2003, 115, 194–204. [Google Scholar] [CrossRef]
- Kamitaka, Y.; Tsujimura, S.; Setoyama, N.; Kajino, T.; Kano, K. Fructose/dioxygen biofuel cell based on direct electron transfer-type bioelectrocatalysis. Phys. Chem. Chem. Phys. 2007, 9, 1793–1801. [Google Scholar] [CrossRef]
- Kuwahara, T.; Ohta, H.; Kondo, M.; Shimomura, M. Immobilization of glucose oxidase on carbon paper electrodes modified with conducting polymer and its application to a glucose fuel cell. Bioelectrochemistry 2008, 74, 66–72. [Google Scholar] [CrossRef]
- Tamaki, T.; Ito, T.; Yamaguchi, T. Immobilization of hydroquinone through a spacer to polymer grafted on carbon black for a high-surface-area biofuel cell electrode. J. Phys. Chem. B 2007, 111, 10312–10319. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.-H.; Hsu, C.-L.; Yuan, C.-J.; Tang, S.-F.; Chiang, H.-J.; Jang, H.-D.; Chang, K.-S. Improvement of the inter-electrode reproducibility of screen-printed carbon electrodes by oxygen plasma etching and an image color level method for quality control. Mater. Sci. Eng. C 2011, 31, 1265–1270. [Google Scholar] [CrossRef]
- Tashima, D.; Hirano, M.; Kitazaki, S.; Eguchi, T.; Kumaga, S. Oxygen-Plasma Surface Treatment of an Electrode Sheet Using Carbon from Japanese Distilled Liquor Waste for Double-layer Capacitors. Electrochem 2020, 1, 322–328. [Google Scholar] [CrossRef]
- Flexer, V.; Marque, M.; Donose, B.C.; Virdis, B.; Keller, J. Plasma treatment of electrodes significantly enhances the development of anodic electrochemically active biofilms. Electrochim. Acta 2013, 108, 566–574. [Google Scholar] [CrossRef]
- Epifanio, M.; Inguva, S.; Kitching, M.; Mosnier, J.-P.; Marsili, E. Effects of atmospheric air plasma treatment of graphite and carbon felt electrodes on the anodic current from Shewanella attached cells. Bioelectrochemistry 2015, 106, 186–193. [Google Scholar] [CrossRef]
- Majzlíková, P.; Prášek, J.; Eliáš, M.; Jašek, O.; Pekárek, J.; Hubálek, J.; Zajíčková, L. Comparison of different modifications of screen-printed working electrodes of electrochemical sensors using carbon nanotubes and plasma treatment. Phys. Status Solidi A 2014, 211, 2756. [Google Scholar] [CrossRef]
- Lee, H.; Jung, Y.; Kim, S. Effect of plasma treatments to graphite nanofibers supports on electrochemical behaviors of metal catalyst electrodes. J. Nanosci. Nanotechnol. 2012, 12, 1513–1516. [Google Scholar] [CrossRef]
- Lin, C.-H.; Wei, L.-Y.; Lee, J.-H.; Lien, C.-L.; Lu, C.-H.; Yuan, C.-J. Effect of anions on the oxidation and reduction of hydrogen peroxide on the gold nanoparticle-deposited carbon fiber paper electrode. Electrochim. Acta 2015, 180, 64–70. [Google Scholar] [CrossRef]
- Liang, Z.; Zhao, T.; Xu, J.; Zhu, L. Mechanism study of the ethanol oxidation reaction on palladium in alkaline media. Electrochim. Acta 2009, 54, 2203–2208. [Google Scholar] [CrossRef]
- Bin, D.; Yang, B.; Zhang, K.; Wang, C.; Wang, J.; Zhong, J.; Feng, Y.; Guo, J.; Du, Y. Design of PdAg hollow nanoflowers through galvanic replacement and their application for ethanol electrooxidation. Chem. Eur. J. 2016, 22, 16642–16647. [Google Scholar] [CrossRef]
- Nguyen, H.T.; Kristian, N.; Wang, S.; Chan, S.H.; Wang, X. Enhancement effect of Ag for Pd/C towards the ethanol electro-oxidation in alkaline media. Appl. Catal. B 2009, 91, 507–515. [Google Scholar] [CrossRef]
- Thotiyl, M.O.; Kumar, T.R.; Sampath, S. Pd supported on titanium nitride for efficient ethanol oxidation. J. Phys. Chem. C 2010, 114, 17934–17941. [Google Scholar] [CrossRef]
- Prabhuram, J.; Manoharan, R.; Vasan, H. Effects of incorporation of Cu and Ag in Pd on electrochemical oxidation of methanol in alkaline solution. J. Appl. Electrochem. 1998, 28, 935–941. [Google Scholar] [CrossRef]
- Grdeń, M.; Czerwiński, A. EQCM studies on Pd–Ni alloy oxidation in basic solution. J. Solid State Eelectr. 2008, 12, 375. [Google Scholar] [CrossRef]
- Lee, J.-W.; Pyun, S.-I.; Filipek, S. The kinetics of hydrogen transport through amorphous Pd82− yNiySi18 alloys (y = 0−32) by analysis of anodic current transient. Electrochim. Acta 2003, 48, 1603–1611. [Google Scholar] [CrossRef]
- Jeong, M.C.; Pyun, C.H.; Yeo, I.H. Voltammetric studies on the palladium oxides in alkaline media. J. Electrochem. Soc. 1993, 140, 1986–1989. [Google Scholar] [CrossRef]
- Huang, W.; Ma, X.Y.; Wang, H.; Feng, R.; Zhou, J.; Duchesne, P.N.; Zhang, P.; Chen, F.; Han, N.; Zhao, F. Promoting Effect of Ni(OH)2 on Palladium Nanocrystals Leads to Greatly Improved Operation Durability for Electrocatalytic Ethanol Oxidation in Alkaline Solution. Adv. Mater. 2017, 29, 1703057. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).