A Hybrid Microbial–Enzymatic Fuel Cell Cathode Overcomes Enzyme Inactivation Limits in Biological Fuel Cells
Abstract
:1. Introduction
2. Results
2.1. Assessment of Laccase Secretion
Inducing Laccase Secretion with a Biocompatible Inducer
2.2. Trametes Versicolor Secretome Analysis
2.3. Comparison of Electrode Coatings
2.4. Comparison of Electrochemical Properties with and without Enzyme Regeneration
3. Discussion
4. Materials and Methods
5. Conclusions
Supplementary Materials
Supplementary File 1Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Spiegel, C. Designing and Building Fuel Cells; Mcgraw-Hill: New York, NY, USA, 2007; Volume 87. [Google Scholar]
- Vielstich, W.; Lamm, A.; Gasteiger, H.A. Handbook of Fuel Cells: Fundamentals Technology and Applications; Wiley: New York, NY, USA, 2003; Volume 2. [Google Scholar]
- Minteer, S.D.; Liaw, B.Y.; Cooney, M.J. Enzyme-based biofuel cells. Curr. Opin. Biotechnol. 2007, 18, 228–234. [Google Scholar] [CrossRef]
- Moehlenbrock, M.J.; Minteer, S.D. Extended lifetime biofuel cells. Chem. Soc. Rev. 2008, 37, 1188–1196. [Google Scholar] [CrossRef]
- Bhatt, M.D.; Lee, J.Y. Advancement of Platinum (Pt)-Free (Non-Pt Precious Metals) and/or Metal-Free (Non-Precious Metals) Electrocatalysts in Energy Applications: A Review and Perspectives. Energy Fuels 2020, 34, 6634–6695. [Google Scholar] [CrossRef]
- Kim, J.; Jia, H.; Wang, P. Challenges in biocatalysis for enzyme-based biofuel cells. Biotechnol. Adv. 2006, 24, 296–308. [Google Scholar] [CrossRef] [PubMed]
- Togo, M.; Oike, M.; Kaji, H.; Abe, T.; Nishizawa, M. Stepwise electric power generation for prolonging lifetime of miniaturized biofuel cell. In Proceedings of the 214th Meeting of the Electrochemical Society, Sendai, Japan, 17–19 November 2008; Volume 1422. [Google Scholar]
- Yeager, E. Electrocatalysts for O2 reduction. Electrochim. Acta 1984, 29, 1527–1537. [Google Scholar] [CrossRef]
- Kakarla, R.; Min, B. Evaluation of microbial fuel cell operation using algae as an oxygen supplier: Carbon paper cathode vs. carbon brush cathode. Bioprocess Biosyst. Eng. 2014, 37, 2453–2461. [Google Scholar] [CrossRef] [PubMed]
- Larminie, J.; Dicks, A.; McDonald, M.S. Fuel Cell Systems Explained; J. Wiley: Chichester, UK, 2003; Volume 2. [Google Scholar]
- Stauffer, D.B.; Hirschenhofer, J.H.; Klett, M.G.; Engleman, R.R. Fuel Cell Handbook; (No. DOE/FETC-99/1076); Federal Energy Technology Center (FETC): Morgantown, WV, USA; Pittsburgh, PA, USA, 1998. [Google Scholar]
- Minteer, S.D.; Atanassov, P.; Luckarift, H.R.; Johnson, G.R. New materials for biological fuel cells. Mater. Today 2012, 1, 166–173. [Google Scholar] [CrossRef]
- Horozova, E.; Dimcheva, N. Kinetic study of catalase adsorption on disperse carbonaceous matrices. Cent. Eur. J. Chem. 2004, 2, 627–637. [Google Scholar] [CrossRef]
- Ye, J.S.; Wen, Y.; De Zhang, W.; Cui, H.F.; Xu, G.Q.; Sheu, F.S. Electrochemical biosensing platforms using phthalocyanine-functionalized carbon nanotube electrode. Electroanal. Int. J. Devoted Fundam. Pract. Asp. Electroanal. 2005, 17, 89–96. [Google Scholar] [CrossRef]
- Ramasamy, R.P.; Luckarift, H.R.; Ivnitski, D.M.; Atanassov, P.B.; Johnson, G.R. High electrocatalytic activity of tethered multicopper oxidase–carbon nanotube conjugates. Chem. Commun. 2010, 46, 6045–6047. [Google Scholar] [CrossRef] [PubMed]
- Coll, P.M.; Perez, P.; Villar, E.; Shnyrov, V.L. Domain structure of laccase I from the lignin-degrading basidiomycete PM1 revealed by differential scanning calorimetry. Biochem. Mol. Biol. Int. 1994, 34, 1091–1098. [Google Scholar]
- Parimi, N.S.; Umasankar, Y.; Atanassov, P.; Ramasamy, R.P. Kinetic and mechanistic parameters of laccase catalyzed direct electrochemical oxygen reduction reaction. ACS Catal. 2012, 2, 38–44. [Google Scholar] [CrossRef]
- Kataoka, K.; Sugiyama, R.; Hirota, S.; Inoue, M.; Urata, K.; Minagawa, Y.; Sakurai, T. Four-electron reduction of dioxygen by a multicopper oxidase, CueO, and roles of Asp112 and Glu506 located adjacent to the trinuclear copper center. J. Biol. Chem. 2009, 284, 14405–14413. [Google Scholar] [CrossRef] [Green Version]
- Palmore, G.T.R.; Kim, H.H. Electro-enzymatic reduction of dioxygen to water in the cathode compartment of a biofuel cell. J. Electroanal. Chem. 1999, 464, 110–117. [Google Scholar] [CrossRef]
- Zhang, Y.; Lv, Z.; Zhou, J.; Xin, F.; Ma, J.; Wu, H.; Dong, W. Application of eukaryotic and prokaryotic laccases in biosensor and biofuel cells: Recent advances and electrochemical aspects. Appl. Microbiol. Biotechnol. 2018, 102, 10409–10423. [Google Scholar] [CrossRef] [PubMed]
- Rubenwolf, S.; Strohmeier, O.; Kloke, A.; Kerzenmacher, S.; Zengerle, R.; von Stetten, F. Carbon electrodes for direct electron transfer type laccase cathodes investigated by current density–cathode potential behavior. Biosens. Bioelectron. 2010, 26, 841–845. [Google Scholar] [CrossRef] [PubMed]
- Rubenwolf, S.; Kestel, J.; Kerzenmacher, S.; Zengerle, R.; von Stetten, F. Enhancing the lifetime of laccase-based biofuel cell cathodes by sequential renewal of enzyme. In Proceedings of the 60th Annual Meeting of the International Society of Electrochemistry, Beijing, China, 16–21 August 2009. [Google Scholar]
- Sané, S.; Jolivalt, C.; Mittler, G.; Nielsen, P.J.; Rubenwolf, S.; Zengerle, R.; Kerzenmacher, S. Overcoming bottlenecks of enzymatic biofuel cell cathodes: Crude fungal culture supernatant can help to extend lifetime and reduce cost. ChemSusChem 2013, 6, 1209–1215. [Google Scholar] [CrossRef]
- Stanzione, I.; Pezzella, C.; Giardina, P.; Sannia, G.; Piscitelli, A. Beyond natural laccases: Extension of their potential applications by protein engineering. Appl. Microbiol. Biotechnol. 2020, 104, 915–924. [Google Scholar] [CrossRef]
- Mate, D.M.; Alcalde, M. Laccase engineering: From rational design to directed evolution. Biotechnol. Adv. 2015, 33, 25–40. [Google Scholar] [CrossRef] [Green Version]
- Maté, D.; García-Burgos, C.; García-Ruiz, E.; Ballesteros, A.O.; Camarero, S.; Alcalde, M. Laboratory evolution of high-redox potential laccases. Chem. Biol. 2010, 17, 1030–1041. [Google Scholar] [CrossRef] [PubMed]
- Mateljak, I.; Monza, E.; Lucas, M.F.; Guallar, V.; Aleksejeva, O.; Ludwig, R.; Leech, D.; Shleev, S.; Alcalde, M. Increasing redox potential, redox mediator activity, and stability in a fungal laccase by computer-guided mutagenesis and directed evolution. ACS Catal. 2019, 9, 4561–4572. [Google Scholar] [CrossRef] [Green Version]
- Mateljak, I.; Rice, A.; Yang, K.; Tron, T.; Alcalde, M. The generation of thermostable fungal laccase chimeras by SCHEMA-RASPP structure-guided recombination in vivo. ACS Synth. Biol. 2019, 8, 833–843. [Google Scholar] [CrossRef] [Green Version]
- Furukawa, T.; Sawaguchi, C.; Watanabe, A.; Takahashi, M.; Nigorikawa, M.; Furukawa, K.; Iimura, Y.; Kajita, S.; Oguchi, T.; Ito, Y.; et al. Application of fungal laccase fused with cellulose-binding domain to develop low- lignin rice plants. J. Biosci. Bioeng. 2013, 116, 616–619. [Google Scholar] [CrossRef]
- Galli, C.; Gentili, P.; Jolivalt, C.; Madzak, C.; Vadalà, R. How is the reactivity of laccase affected by single-point mutations? Engineering laccase for improved activity towards sterically demanding substrates. Appl. Microbiol. Biotechnol. 2011, 91, 123–131. [Google Scholar] [CrossRef]
- Zhu, Z.; Momeu, C.; Zakhartsev, M.; Schwaneberg, U. Making glucose oxidase fit for biofuel cell applications by directed protein evolution. Biosens. Bioelectron. 2006, 21, 2046–2051. [Google Scholar] [CrossRef]
- Güven, G.; Prodanovic, R.; Schwaneberg, U. Protein engineering—An option for enzymatic biofuel cell design. Electroanal. Int. J. Devoted Fundam. Pract. Asp. Electroanal. 2010, 22, 765–775. [Google Scholar] [CrossRef]
- Yamaguchi, M.; Tahara, Y.; Nakano, A.; Taniyama, T. Secretory and continuous expression of Aspergillus niger glucose oxidase gene in Pichia pastoris. Protein Expr. Purif. 2007, 55, 273–278. [Google Scholar] [CrossRef] [PubMed]
- Christenson, A.; Dimcheva, N.; Ferapontova, E.E.; Gorton, L.; Ruzgas, T.; Stoica, L.; Shleev, S.; Yaropolov, A.I.; Haltrich, D.; Thorneley, R.N.; et al. Direct electron transfer between ligninolytic redox enzymes and electrodes. Electroanal. Int. J. Devoted Fundam. Pract. Asp. Electroanal. 2004, 16, 1074–1092. [Google Scholar] [CrossRef]
- Canero, D.C.; Roncero, M.I.G. Functional analyses of laccase genes from Fusarium oxysporum. Phytopathology 2008, 98, 509–518. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thangadurai, D.; Sangeetha, J.; David, M. Fundamentals of Molecular Mycology; CRC Press: Boca Raton, FL, USA, 2016. [Google Scholar]
- Patel, H.; Gupte, S.; Gahlout, M.; Gupte, A. Purification and characterization of an extracellular laccase from solid-state culture of Pleurotus ostreatus HP-1. 3 Biotech 2014, 4, 77–84. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baldrian, P. Fungal laccases–occurrence and properties. FEMS Microbiol. Rev. 2006, 30, 215–242. [Google Scholar] [CrossRef] [Green Version]
- Stamets, P.E. Notes on nutritional properties of culinary-medicinal mushrooms. Int. J. Med. Mushrooms 2005, 7, 103–110. [Google Scholar] [CrossRef]
- Nagygyörgy, E.; Kovács, B.; Leiter, É.; Miskei, M.; Pócsi, I.; Hornok, L.; Ádám, A. Toxicity of abiotic stressors to Fusarium species: Differences in hydrogen peroxide and fungicide tolerance. Acta Microbiol. Immunol. Hung. 2014, 61, 189–208. [Google Scholar] [CrossRef] [PubMed]
- Finnegan, M.; Linley, E.; Denyer, S.P.; McDonnell, G.; Simons, C.; Maillard, J.Y. Mode of action of hydrogen peroxide and other oxidizing agents: Differences between liquid and gas forms. J. Antimicrob. Chemother. 2010, 65, 2108–2115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Müntz, K. Protein dynamics and proteolysis in plant vacuoles. J. Exp. Bot. 2007, 58, 2391–2407. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rubenwolf, S.; Sané, S.; Hussein, L.; Kestel, J.; von Stetten, F.; Urban, G.; Kerzenmacher, S. Prolongation of electrode lifetime in biofuel cells by periodic enzyme renewal. Appl. Microbiol. Biotechnol. 2012, 96, 841–849. [Google Scholar] [CrossRef] [PubMed]
- Makhdoomi, H.; Moghadam, H.M.; Zabihi, O. Effect of different conditions on the size and quality of titanium dioxide nanoparticles synthesized by a reflux process. Res. Chem. Intermed. 2015, 41, 1777–1788. [Google Scholar] [CrossRef]
- Kim, J.H.; Hwang, J.Y.; Hwang, H.R.; Kim, H.S.; Lee, J.H.; Seo, J.W.; Shin, U.S.; Lee, S.-H. Simple and cost-effective method of highly conductive and elastic carbon nanotube/polydimethylsiloxane composite for wearable electronics. Sci. Rep. 2018, 8, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tarasevich, M.R.; Bogdanovskaya, V.A.; Kuznetsova, L.N. Bioelectrocatalytic reduction of oxygen in the presence of laccase adsorbed on carbon electrodes. Russ. J. Electrochem. 2001, 37, 833–837. [Google Scholar] [CrossRef]
- Choi, H.N.; Han, J.H.; Park, J.A.; Lee, J.M.; Lee, W.Y. Amperometric glucose biosensor based on glucose oxidase encapsulated in carbon nanotube–titania–Nafion composite film on platinized glassy carbon electrode. Electroanal. Int. J. Devoted Fundam. Pract. Asp. Electroanal. 2007, 19, 1757–1763. [Google Scholar] [CrossRef]
- Koh, B.; Cheng, W. Mechanisms of carbon nanotube aggregation and the reversion of carbon nanotube aggregates in aqueous medium. Langmuir 2014, 30, 10899–10909. [Google Scholar] [CrossRef]
- Yang, Q.S.; He, X.Q.; Liu, X.; Leng, F.F.; Mai, Y.W. The effective properties and local aggregation effect of CNT/SMP composites. Compos. Part B Eng. 2012, 43, 33–38. [Google Scholar] [CrossRef]
- Mathieu, B.; Anthony, C.; Arnaud, A.; Lionel, F. CNT aggregation mechanisms probed by electrical and dielectric measurements. J. Mater. Chem. C 2015, 3, 5769–5774. [Google Scholar] [CrossRef]
- Mynttinen, E.; Wester, N.; Lilius, T.; Kalso, E.; Mikladal, B.; Varjos, I.; Sainio, S.; Jiang, H.; Kauppinen, E.I.; Koskinen, J.; et al. Electrochemical detection of oxycodone and its main metabolites with Nafion-coated single-walled carbon nanotube electrodes. Anal. Chem. 2020, 92, 8218–8227. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, A.; Zheng, W.; Soutschek, K.; Roy, J.; Yurkov, A.M.; Rillig, M.C. Tradeoffs in hyphal traits determine mycelium architecture in saprobic fungi. Sci. Rep. 2019, 9, 1–9. [Google Scholar]
- Rittstieg, K.; Suurnäkki, A.; Suortti, T.; Kruus, K.; Guebitz, G.M.; Buchert, J. Polymerization of Guaiacol and a Phenolic β-O-4-Substructure by Trameteshirsuta Laccase in the Presence of ABTS. Biotechnol. Prog. 2003, 19, 1505–1509. [Google Scholar] [CrossRef]
- McFedries, A.; Schwaid, A.; Saghatelian, A. Methods for the elucidation of protein-small molecule interactions. Chem. Biol. 2013, 20, 667–673. [Google Scholar] [CrossRef] [Green Version]
- Koroglu, E.O.; Yoruklu, H.C.; Demir, A.; Ozkaya, B. Scale-up and commercialization issues of the MFCs: Challenges and implications. In Microbial Electrochemical Technology; Elsevier: Amsterdam, The Netherlands, 2019; pp. 565–583. [Google Scholar]
- Krieg, T.; Wood, J.A.; Mangold, K.M.; Holtmann, D. Mass transport limitations in microbial fuel cells: Impact of flow configurations. Biochem. Eng. J. 2018, 138, 172–178. [Google Scholar] [CrossRef]
- Binyamin, G.; Chen, T.; Heller, A. Sources of instability of ‘wired’enzyme anodes in serum: Urate and transition metal ions. J. Electroanal. Chem. 2001, 500, 604–611. [Google Scholar] [CrossRef]
- Guggenheim, A.G.; Wright, K.M.; Zwickey, H.L. Immune modulation from five major mushrooms: Application to integrative oncology. Integr. Med. A Clin. J. 2014, 13, 32. [Google Scholar]
- Royse, D.J. A global perspective on the high five: Agaricus, Pleurotus, Lentinula, Auricularia & Flammulina. In Proceedings of the 8th International Conference on Mushroom Biology and Mushroom Products (ICMBMP8), New Delhi, India, 19–22 November 2014; Volume 1, pp. 1–6. [Google Scholar]
- Moore, D.; Chiu, S.W. Fungal Products as Food. In Bio-Exploitation of Filamentous Fungi; Fungal Diversity Press: Hong Kong, China, 2001. [Google Scholar]
- Cardwell, G.; Bornman, J.F.; James, A.P.; Black, L.J. A review of mushrooms as a potential source of dietary vitamin D. Nutrients 2018, 10, 1498. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mleczek, M.; Siwulski, M.; Rzymski, P.; Budzyńska, S.; Gąsecka, M.; Kalač, P.; Niedzielski, P. Cultivation of mushrooms for production of food biofortified with lithium. Eur. Food Res. Technol. 2017, 243, 1097–1104. [Google Scholar] [CrossRef] [Green Version]
- Aguilar-Rivera, N.; de Jesús Debernardi-Vázquez, T. Sustainable Development for Farmers Transforming Agroindustrial Wastes into Profitable Green Products. In Sustainable Development Research and Practice in Mexico and Selected Latin American Countries; Springer: Cham, Swizerland, 2018; pp. 53–75. [Google Scholar]
- Istiyanti, E.; Fivintari, F.R.; Syaftiana, M. Potential development of oyster mushrooms in the lowlands of Bantul Regency, Special Region of Yogyakarta, Indonesia. In IOP Conference Series: Earth and Environmental Science; IOP Publishing: Bristol, UK, 2020; Volume 423, p. 012037. [Google Scholar]
- Mazlan, S.Z.; Hanifah, S.A. Effects of temperature and pH on immobilized laccase activity in conjugated methacrylate-acrylate microspheres. Int. J. Polym. Sci. 2017, 2017, 5657271. [Google Scholar] [CrossRef] [Green Version]
- Kim, K.; Kim, E.; Lee, S.J. New enzymatic time–temperature integrator (TTI) that uses laccase. J. Food Eng. 2012, 113, 118–123. [Google Scholar] [CrossRef]
- Ghosh, P.; Ghosh, U. Statistical optimization of laccase production by Aspergillus flavus PUF5 through submerged fermentation using agro-waste as cheap substrate. Acta Biol. Szeged. 2017, 61, 25–33. [Google Scholar]
- Zhang, R.; Wang, L.; Han, J.; Wu, J.; Li, C.; Ni, L.; Wang, Y. Improving laccase activity and stability by HKUST-1 with cofactor via one-pot encapsulation and its application for degradation of bisphenol A. J. Hazard. Mater. 2020, 383, 121130. [Google Scholar] [CrossRef] [PubMed]
- You, S.; Zhao, Q.; Jiang, J.; Zhang, J. Treatment of domesticwastewaterwith simultaneous electricity generation in microbial fuel cell under continuous operation. Chem. Biochem. Eng. Q. 2006, 20, 407–412. [Google Scholar]
- Igiri, B.E.; Okoduwa, S.I.; Idoko, G.O.; Akabuogu, E.P.; Adeyi, A.O.; Ejiogu, I.K. Toxicity and bioremediation of heavy metals contaminated ecosystem from tannery wastewater: A review. J. Toxicol. 2018, 2018, 2568038. [Google Scholar] [CrossRef]
- Pardo, I.; Chanagá, X.; Vicente, A.I.; Alcalde, M.; Camarero, S. New colorimetric screening assays for the directed evolution of fungal laccases to improve the conversion of plant biomass. BMC Biotechnol. 2013, 13, 90. [Google Scholar] [CrossRef] [Green Version]
- Kruger, N.J. The Bradford method for protein quantitation. In The Protein Protocols Handbook; Springer: Berlin/Heidelberg, Germany, 2009; pp. 17–24. [Google Scholar]
- Motulsky, H.J. GraphPad Statistics Guide—Options for Multiple t Tests. 2016. Available online: http://www.graphpad.com/guides/prism/7/statistics/index.htm (accessed on 11 February 2021).
- Fahraeus, G.; REINHAMMAR, B. Large Scale Production and Purification of Laccase from. Acta Chem. 1967, 21, 2367–2378. [Google Scholar] [CrossRef]
- Gong, K.; Yan, Y.; Zhang, M.; Su, L.; Xiong, S.; Mao, L. Electrochemistry and electroanalytical applications of carbon nanotubes: A review. Anal. Sci. 2005, 21, 1383–1393. [Google Scholar] [CrossRef] [Green Version]
- Hu, C.; Hu, S. Carbon nanotube-based electrochemical sensors: Principles and applications in biomedical systems. J. Sens. 2009, 2009, 187615. [Google Scholar] [CrossRef] [Green Version]
- Bishop, L.; Cena, L.; Orandle, M.; Yanamala, N.; Dahm, M.M.; Birch, M.E.; Erdely, A. In vivo toxicity assessment of occupational components of the carbon nanotube life cycle to provide context to potential health effects. ACS Nano 2017, 11, 8849–8863. [Google Scholar] [CrossRef] [PubMed]
- Hansen, S.F.; Lennquist, A. Carbon nanotubes added to the SIN List as a nanomaterial of Very High Concern. Nat. Nanotechnol. 2020, 15, 3–4. [Google Scholar] [CrossRef] [PubMed]
- Kostarelos, K. The long and short of carbon nanotube toxicity. Nat. Biotechnol. 2008, 26, 774–776. [Google Scholar] [CrossRef] [PubMed]
- Wen, Z.; Ci, S.; Mao, S.; Cui, S.; Lu, G.; Yu, K.; Chen, J. TiO2 nanoparticles-decorated carbon nanotubes for significantly improved bioelectricity generation in microbial fuel cells. J. Power Sources 2013, 234, 100–106. [Google Scholar] [CrossRef]
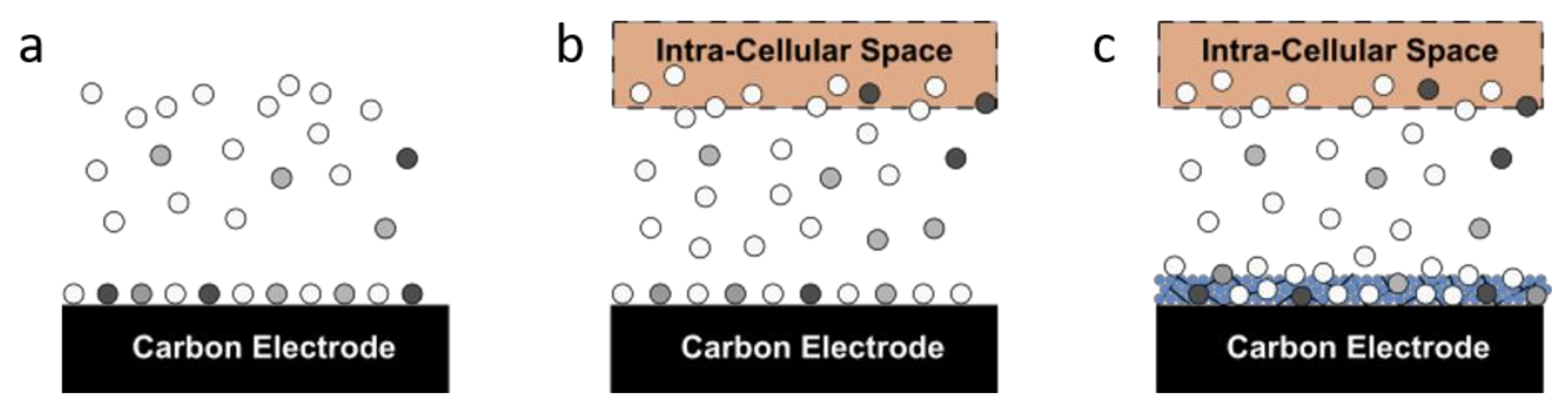
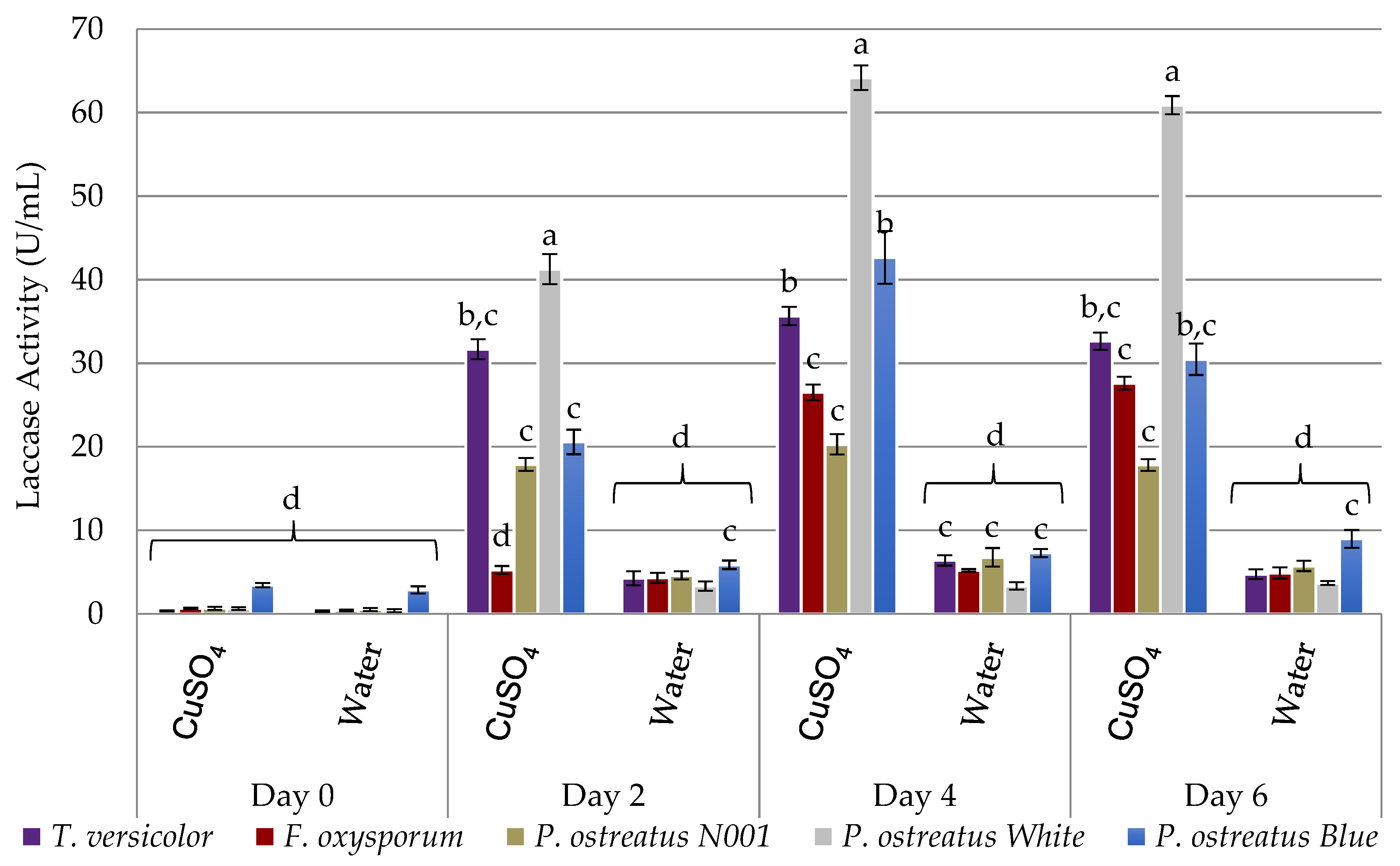
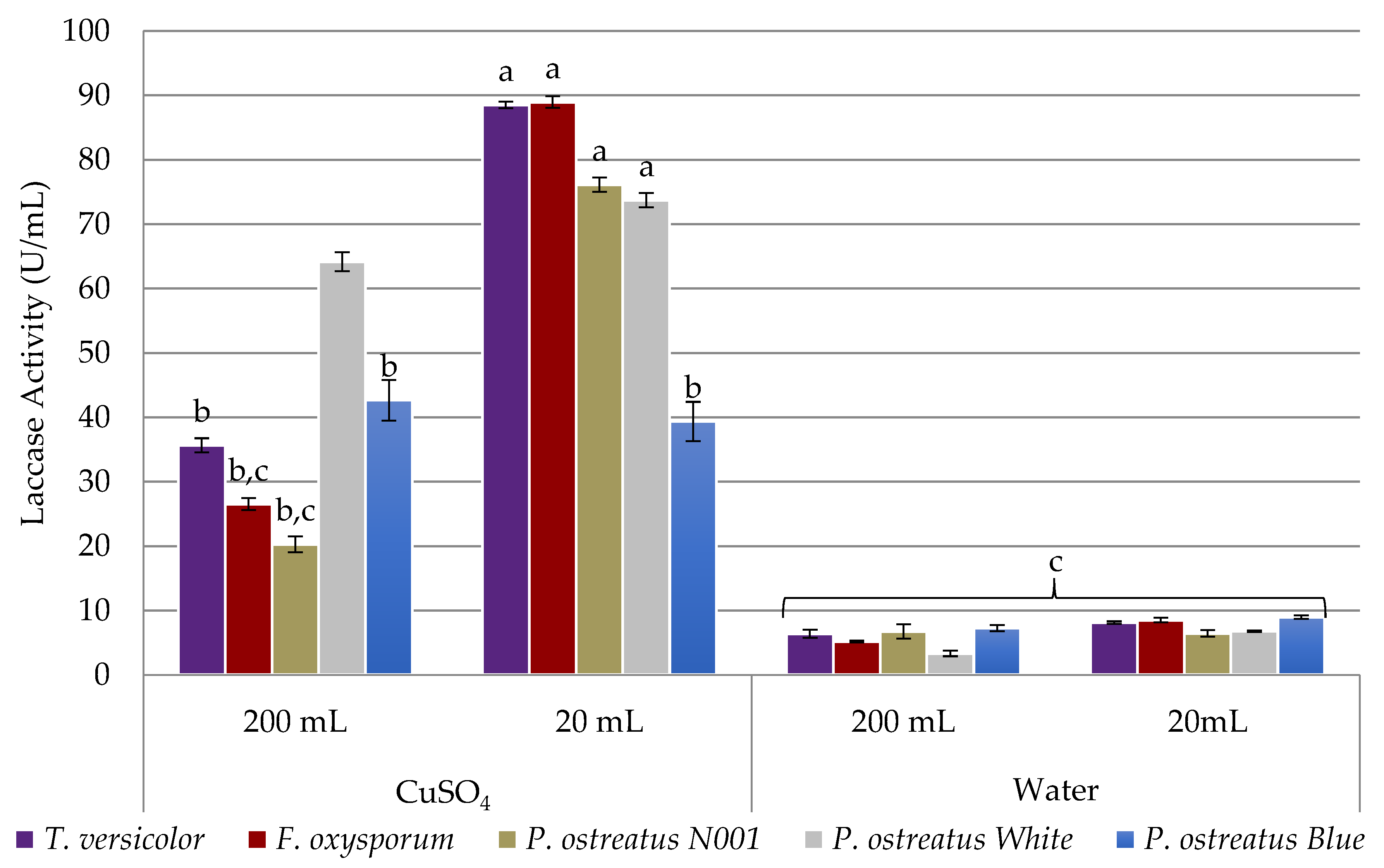

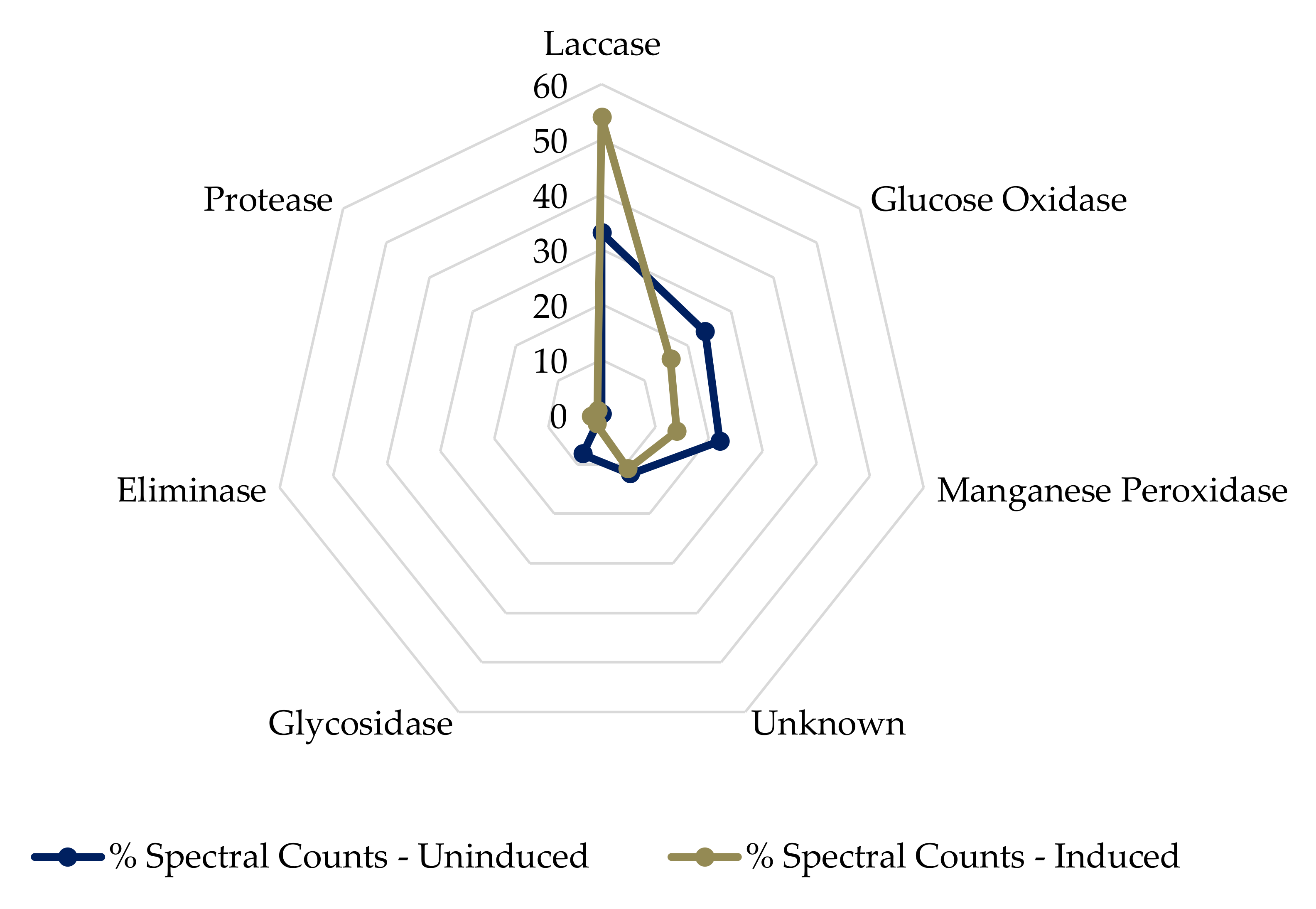
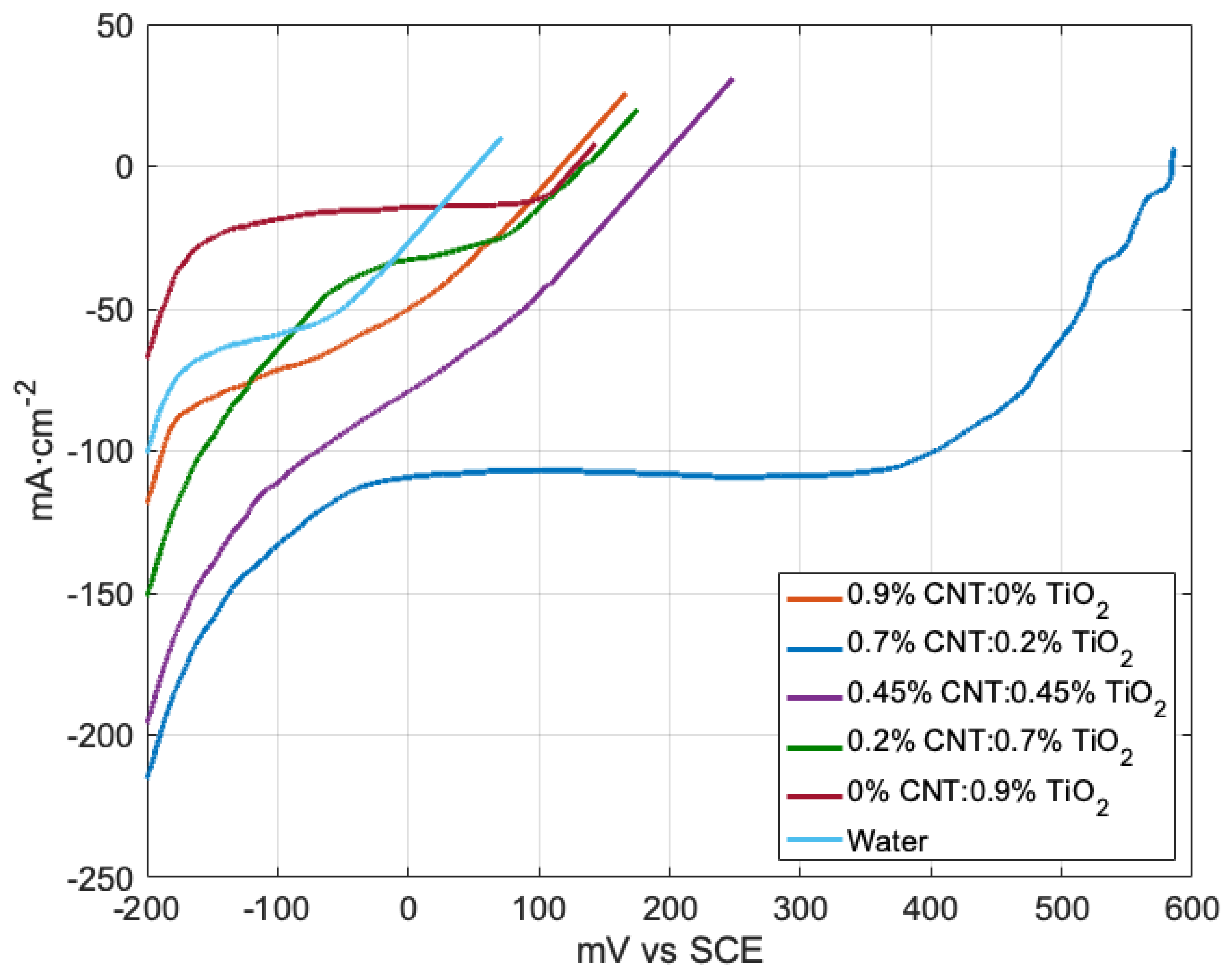


| Coating Composition | Open Circuit Potential (mV vs. SCE) | Current Density at −200 mV vs. SCE |
|---|---|---|
| 0.9% TiO2 | 128.0 | −68 |
| 0.7% TiO2−0.2% CNT | 134 | −152 |
| 0.45% TiO2−0.45% CNT | 189 | −196 |
| 0.2% TiO2−0.7% CNT | 584 | −216 |
| 0.9% CNT | 117 | −119 |
| 18 MΩ H2O | 52 | −101 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Evans, J.P.; Gervasio, D.F.; Pryor, B.M. A Hybrid Microbial–Enzymatic Fuel Cell Cathode Overcomes Enzyme Inactivation Limits in Biological Fuel Cells. Catalysts 2021, 11, 242. https://doi.org/10.3390/catal11020242
Evans JP, Gervasio DF, Pryor BM. A Hybrid Microbial–Enzymatic Fuel Cell Cathode Overcomes Enzyme Inactivation Limits in Biological Fuel Cells. Catalysts. 2021; 11(2):242. https://doi.org/10.3390/catal11020242
Chicago/Turabian StyleEvans, John Parker, Dominic F. Gervasio, and Barry M. Pryor. 2021. "A Hybrid Microbial–Enzymatic Fuel Cell Cathode Overcomes Enzyme Inactivation Limits in Biological Fuel Cells" Catalysts 11, no. 2: 242. https://doi.org/10.3390/catal11020242
APA StyleEvans, J. P., Gervasio, D. F., & Pryor, B. M. (2021). A Hybrid Microbial–Enzymatic Fuel Cell Cathode Overcomes Enzyme Inactivation Limits in Biological Fuel Cells. Catalysts, 11(2), 242. https://doi.org/10.3390/catal11020242






