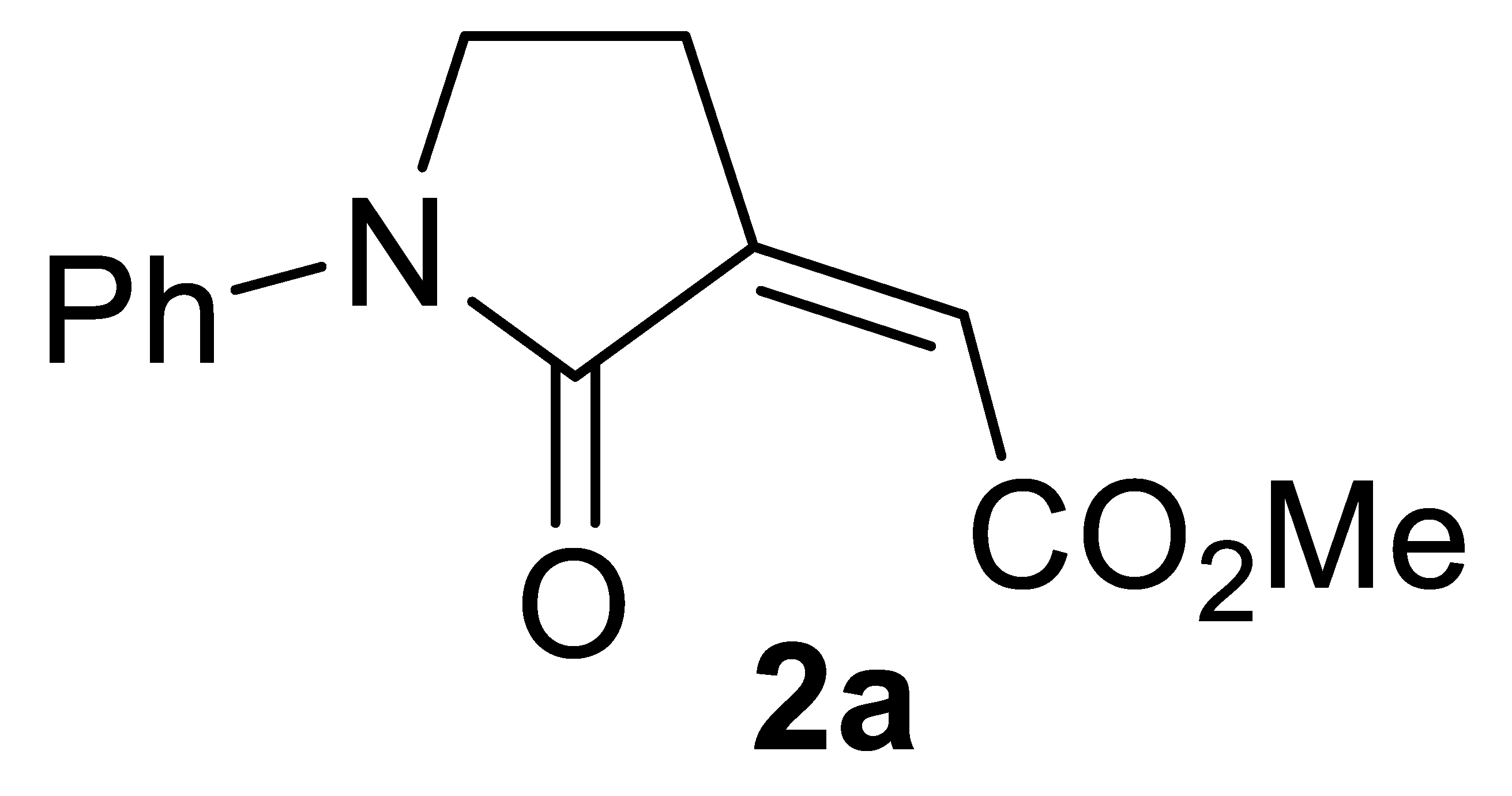
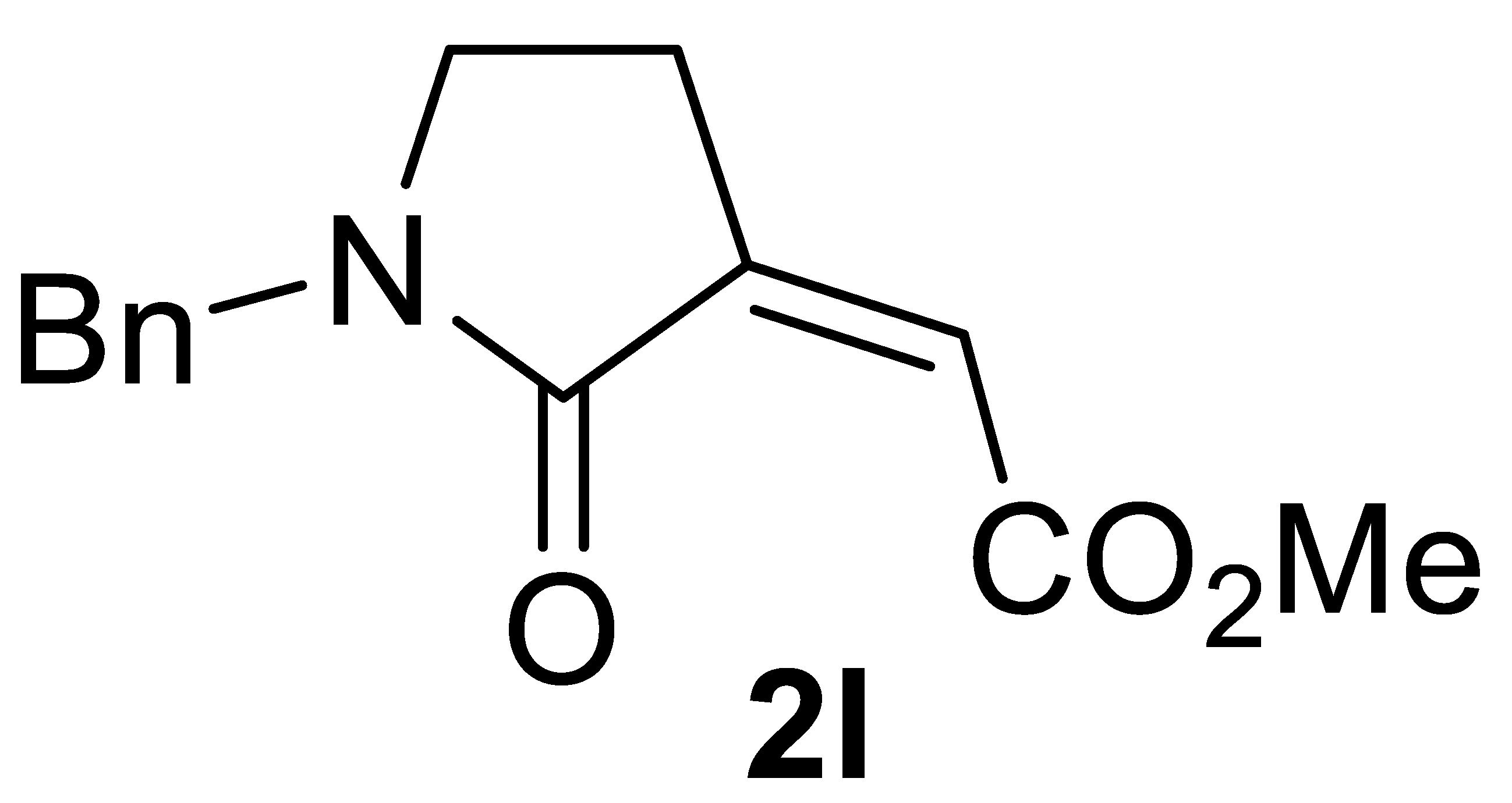
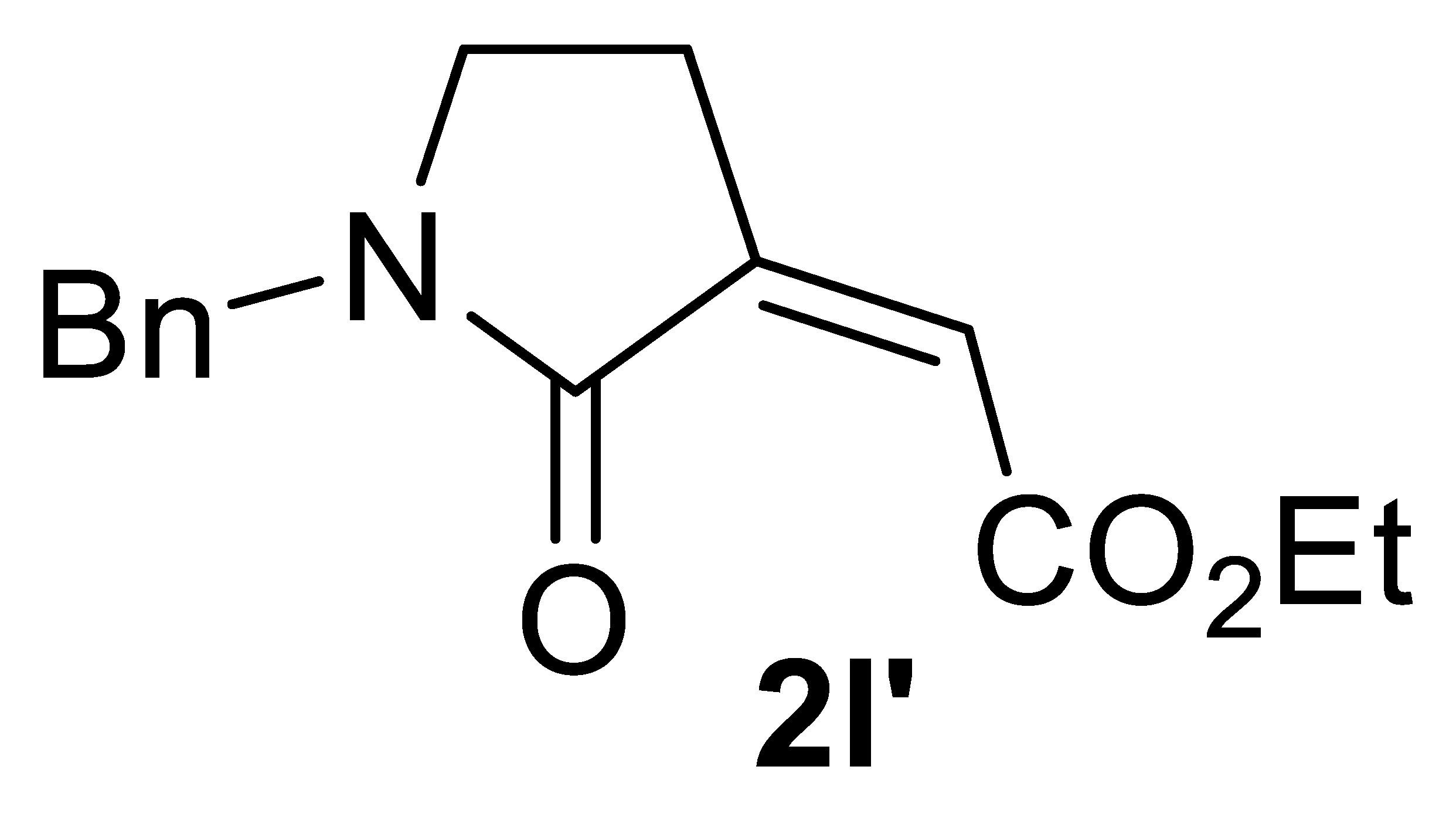
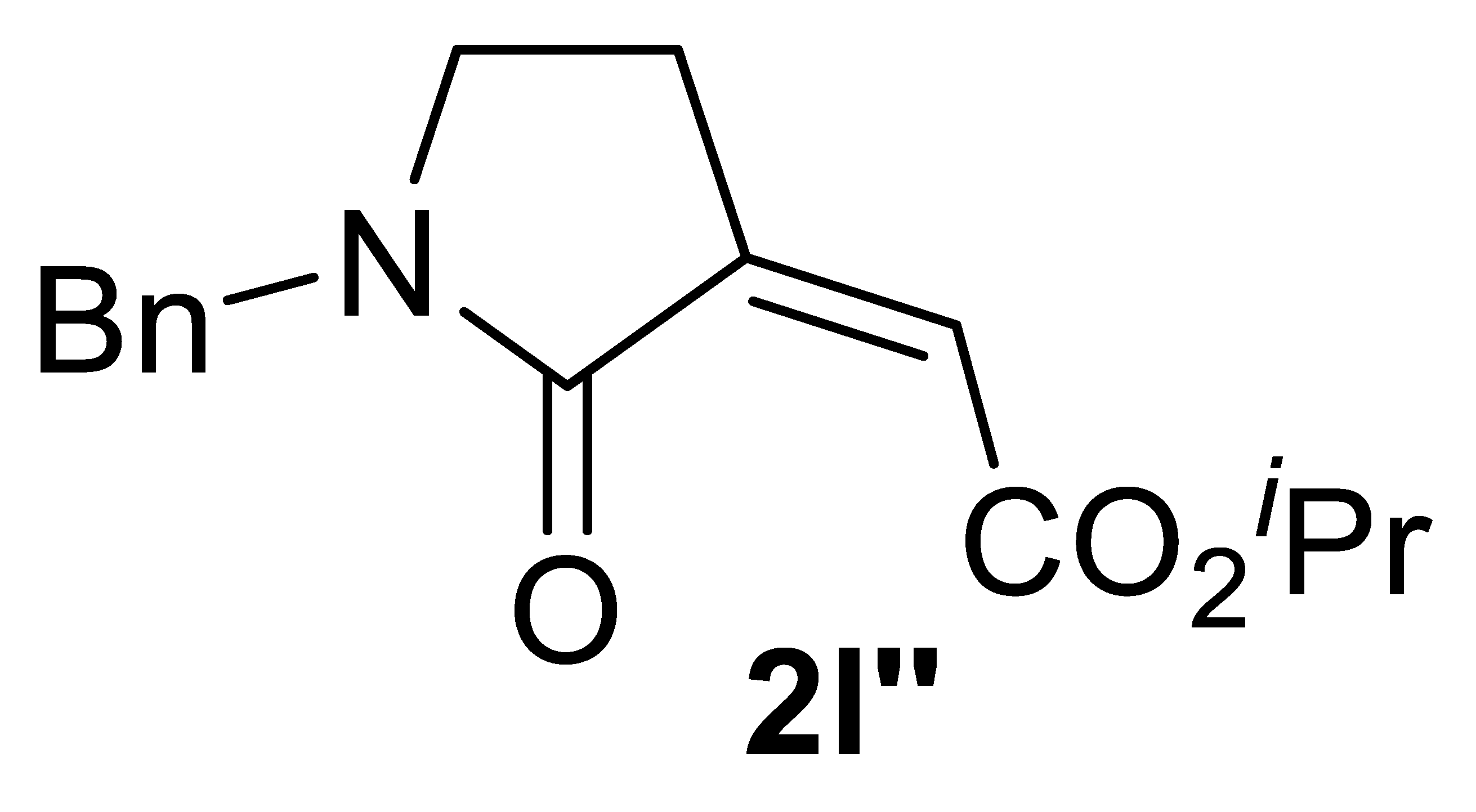
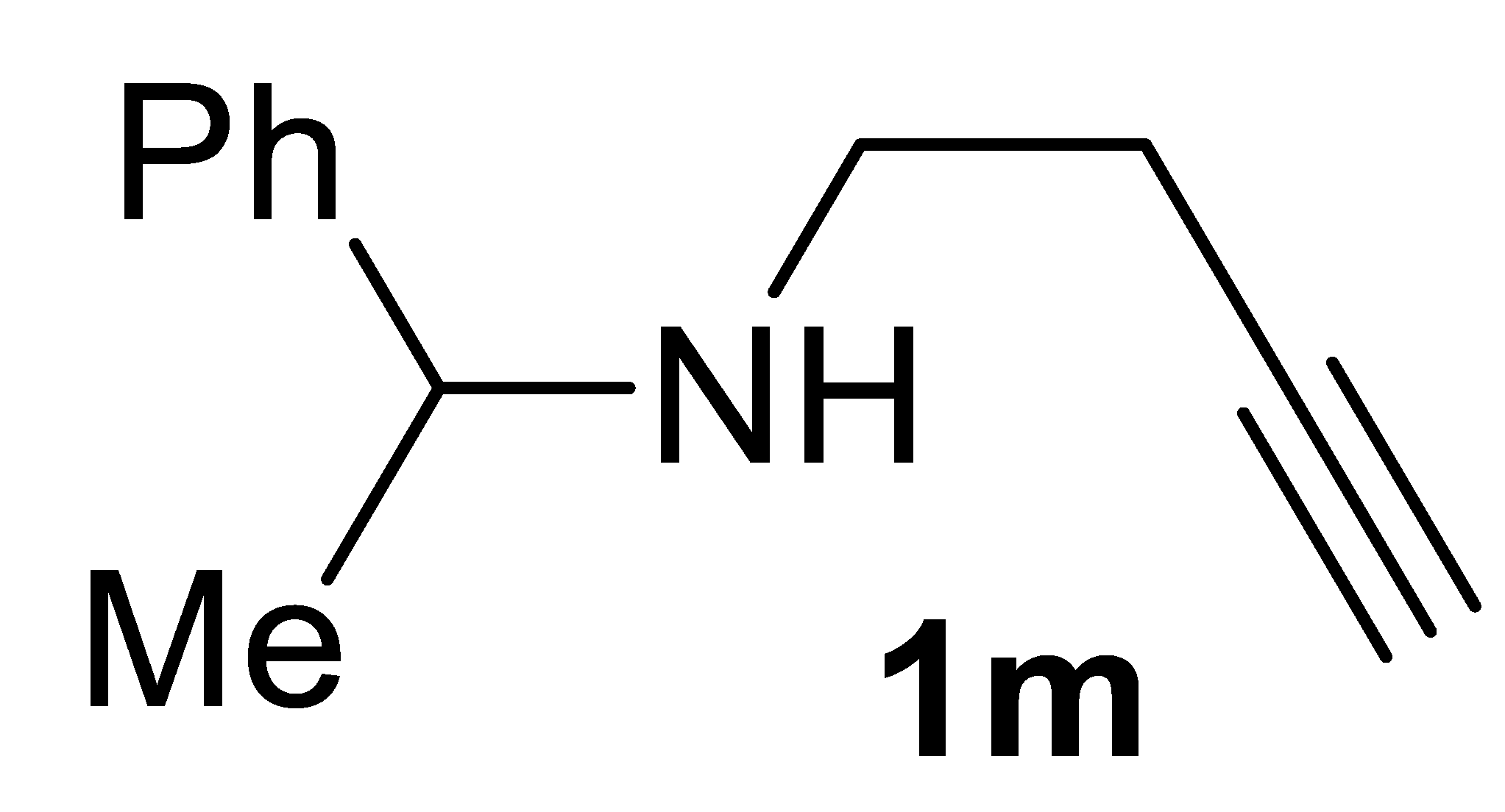
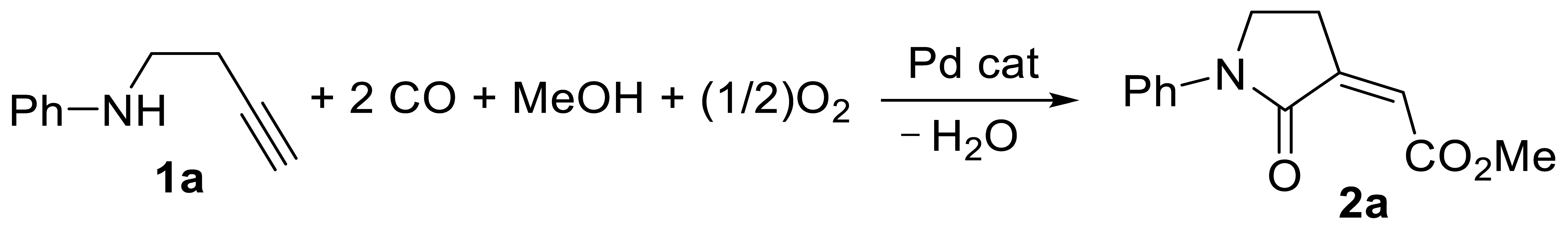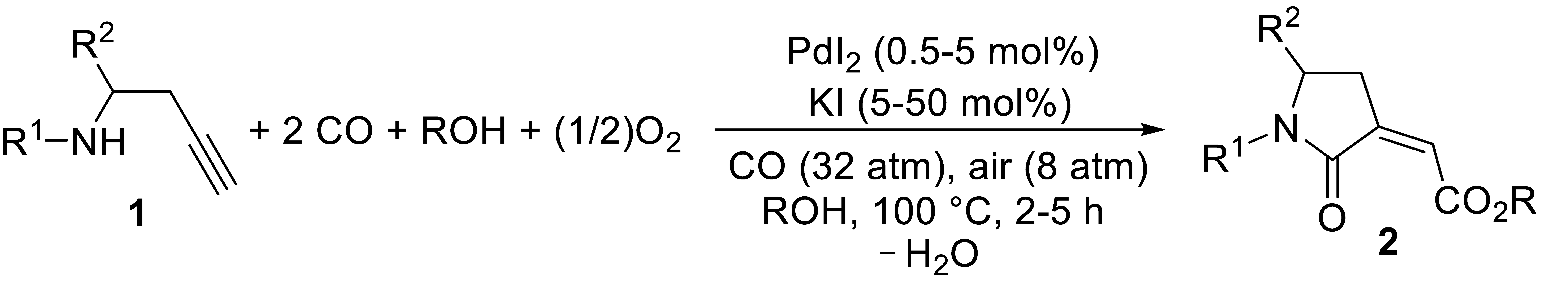Abstract
We report a stereoselective, multicomponent catalytic carbonylative approach to a new class of α,β-unsaturated γ-lactam derivatives with potential biological activity, that are, alkyl (Z)-2-(2-oxopyrrolidin-3-ylidene)acetates. Our method is based on the catalytic assembly of readily available building blocks, namely, homopropargylic amines, carbon monoxide, an alcohol, and oxygen (from air). These simple substrates are efficiently activated in ordered sequence under the action of a very simple catalytic system, consisting of PdI2 in conjunction with KI to give the γ-lactam products in 47–85% yields. Carbonylation reactions are carried out at 100 °C for 2–5 h under 40 atm of a 4:1 mixture of CO‒air, with 0.5–5 mol% of PdI2 and 5–50 mol% of KI.
1. Introduction
Carbonylation reactions represent an excellent approach for the direct synthesis of carbonyl compounds using the simplest and readily available C-1 source, that is, carbon monoxide [1,2,3,4,5]. CO is produced industrially starting from petroleum hydrocarbons, natural gas, and coal, and, in the future, a growing amount of carbon monoxide is also expected to be available from renewable feedstocks, such as biowastes and CO2 [6].
In this work, a stereoselective, multicomponent catalytic carbonylative approach to a new class of α,β-unsaturated γ-lactam derivatives, that are, alkyl (Z)-2-(2-oxopyrrolidin-3-ylidene)acetates 2, is presented. The synthetic approach is based on the use of a particularly simple catalytic system, consisting of PdI2 in conjunction with KI [7,8], for the catalytic activation of very simple starting materials (homopropargylic amines 1, carbon monoxide, an alcohol, and oxygen; Scheme 1).
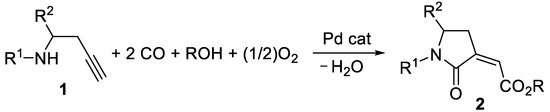
Scheme 1.
Stereoselective multicomponent catalytic carbonylative synthesis of alkyl (Z)-2-(2-oxopyrrolidin-3-ylidene)acetates 2 from homopropargylic amines 1.
The (Z)-2-(2-oxopyrrolidin-3-ylidene)acetate core has been previously obtained by Rh(I)-catalyzed enyne cycloisomerization [9], intramolecular palladium-catalyzed allylic alkylation of phosphonoacetamides followed by Horner-Wadsworth-Emmons olefination with ethyl glyoxalate [10], condensation of dimethyl itaconate with glycine methyl ester [11], and the reaction of 2-benzyl 1-(2,2,2-trichloroethyl) (Z)-4-((dimethylamino)methylene)-5-oxopyrrolidine-1,2-dicarboxylate with 2,2,2-trichloroethoxycarbonyl chloride [12]. To the best of our knowledge, however, no method has been reported so far to synthesize the γ-lactam derivatives 2 disclosed in this work.
Interestingly, heterocyclic compounds bearing the strictly related 2-(2-oxopyrrolidin-3-yl)acetate moiety (with a saturated exocyclic carbon-carbon bond) have been reported to exhibit different biological activities, including peptidomimetic inhibitory effect [13], and anti-hepatitis B [14], anti-stroke [15], and anti-thrombotic [16] activity. Therefore, the disclosure of an efficient method for the synthesis of 2 can be of interest for the development of novel bioactive small molecules. In particular, the target compounds incorporate into their structure the 2-(2-oxopyrrolidin-3-ylidene)acetate moiety with an exocyclic double bond, which, as a Michael acceptor group, may react with biological thiols (e.g., glutathione, cysteine) [17], thus inducing diverse biological activities. For this purpose, a biochemical and pharmacological investigation on the cytotoxicity and antiproliferative activity of 2 in tumor cell lines is being carried out in our laboratories, and the first noteworthy results will be reported in due course.
2. Results and Discussion
The synthetic method developed in our work for the preparation of new alkyl (Z)-2-(2-oxopyrrolidin-3-ylidene)acetate derivatives 2 is based on the oxidative carbonylation of readily available 3-yn-1-amines (homopropargylic amines) 1 carried out with the very simple PdI2/KI catalytic system (Scheme 1).
Our initial experiments were based on the use of N-(but-3-yn-1-yl)aniline 1a as model substrate to assess the feasibility of our hypothesis and to optimize the carbonylation conditions. When 1a was allowed to react with CO, MeOH, and O2 (from air), in MeOH as the solvent (0.04 mmol of 1a per mL of MeOH) under 40 atm of a 4:1 mixture of CO-air at 100 °C and in the presence of PdI2 (5 mol%) and KI (0.5 equiv), after 2 h the GLC-MS analysis of the reaction mixture evidenced a complete substrate conversion and the formation of a product, whose MS spectrum was compatible with the desired γ-lactam derivative 2a. This compound was then isolated from the mixture (74% based on starting 1a, Table 1, entry 1) and fully characterized by IR, 1HNMR, and 13CNMR spectroscopies and by XRD analysis, which confirmed the structure corresponding to methyl (Z)-2-(2-oxo-1-phenylpyrrolidin-3-ylidene)acetate 2a (Figure 1; see the Supplementary Materials for full XRD data).

Table 1.
Optimization of carbonylation conditions a.
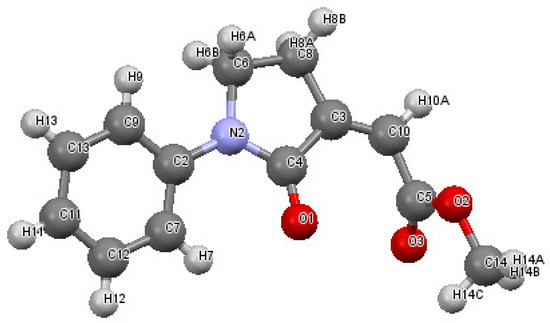
Figure 1.
Molecular structure of methyl (Z)-2-(2-oxo-1-phenylpyrrolidin-3-ylidene)acetate 2a. The figure shows the atom labelling scheme for non-H atoms and displacement ellipsoids at the 50% probability level.
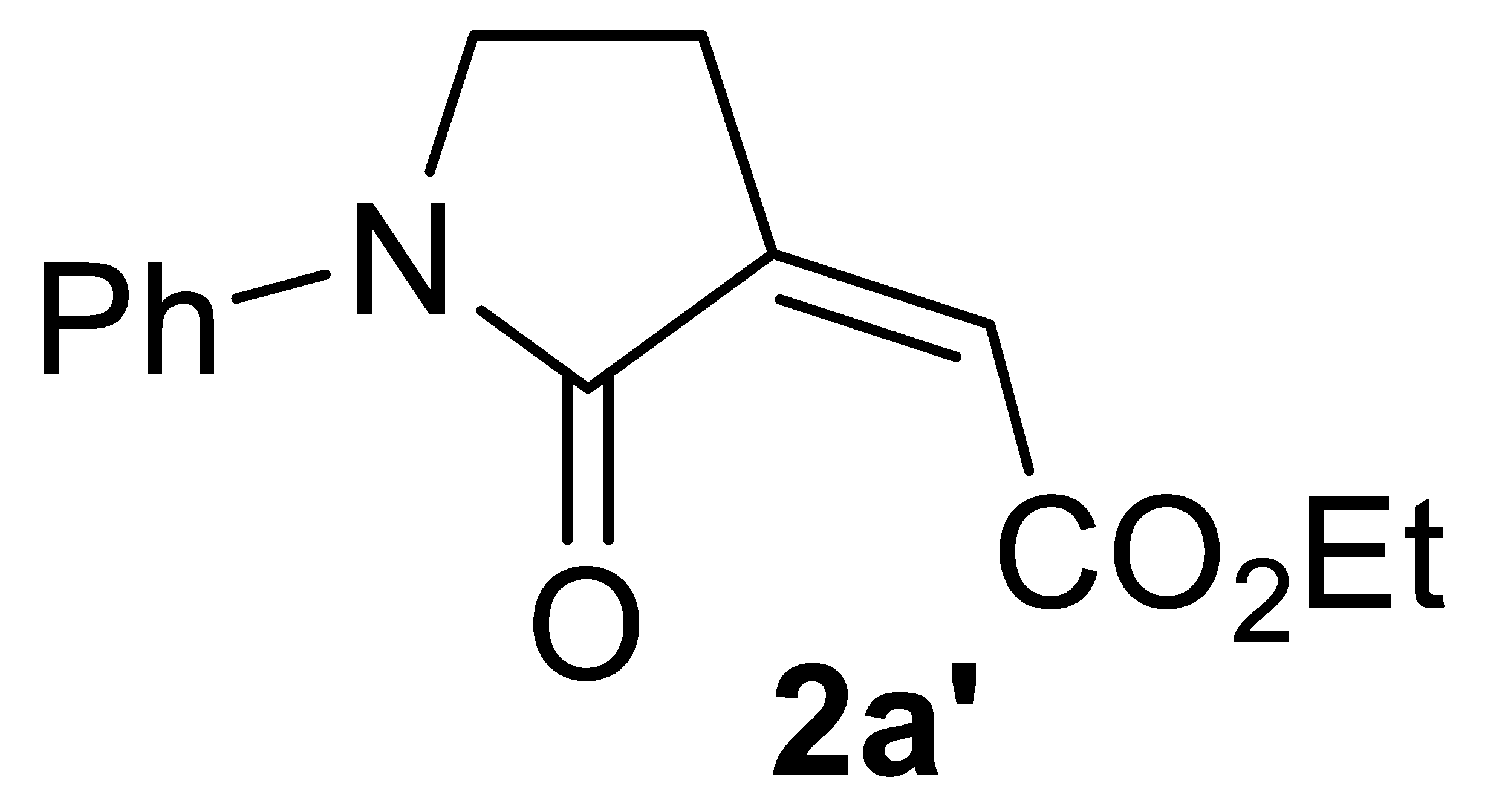
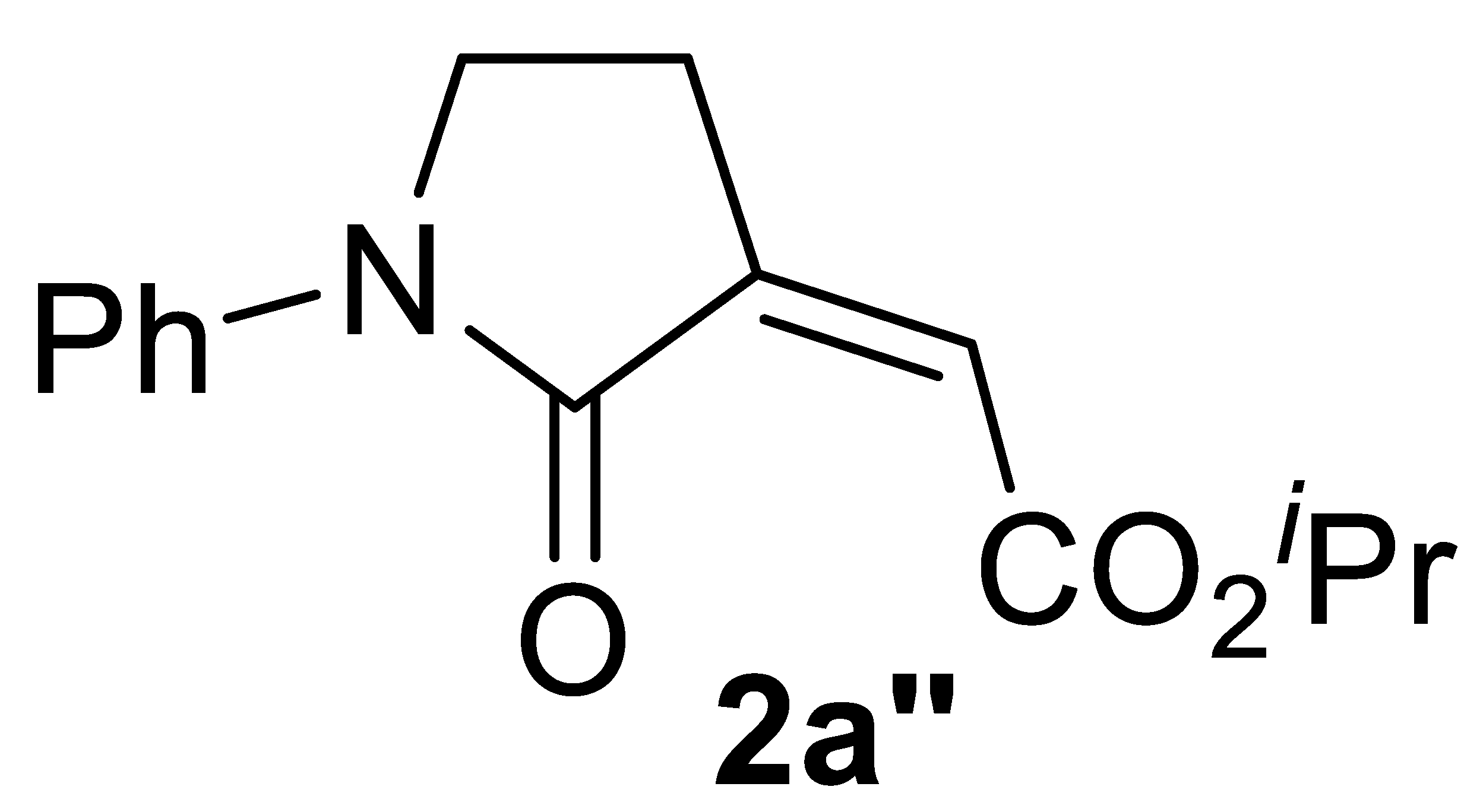
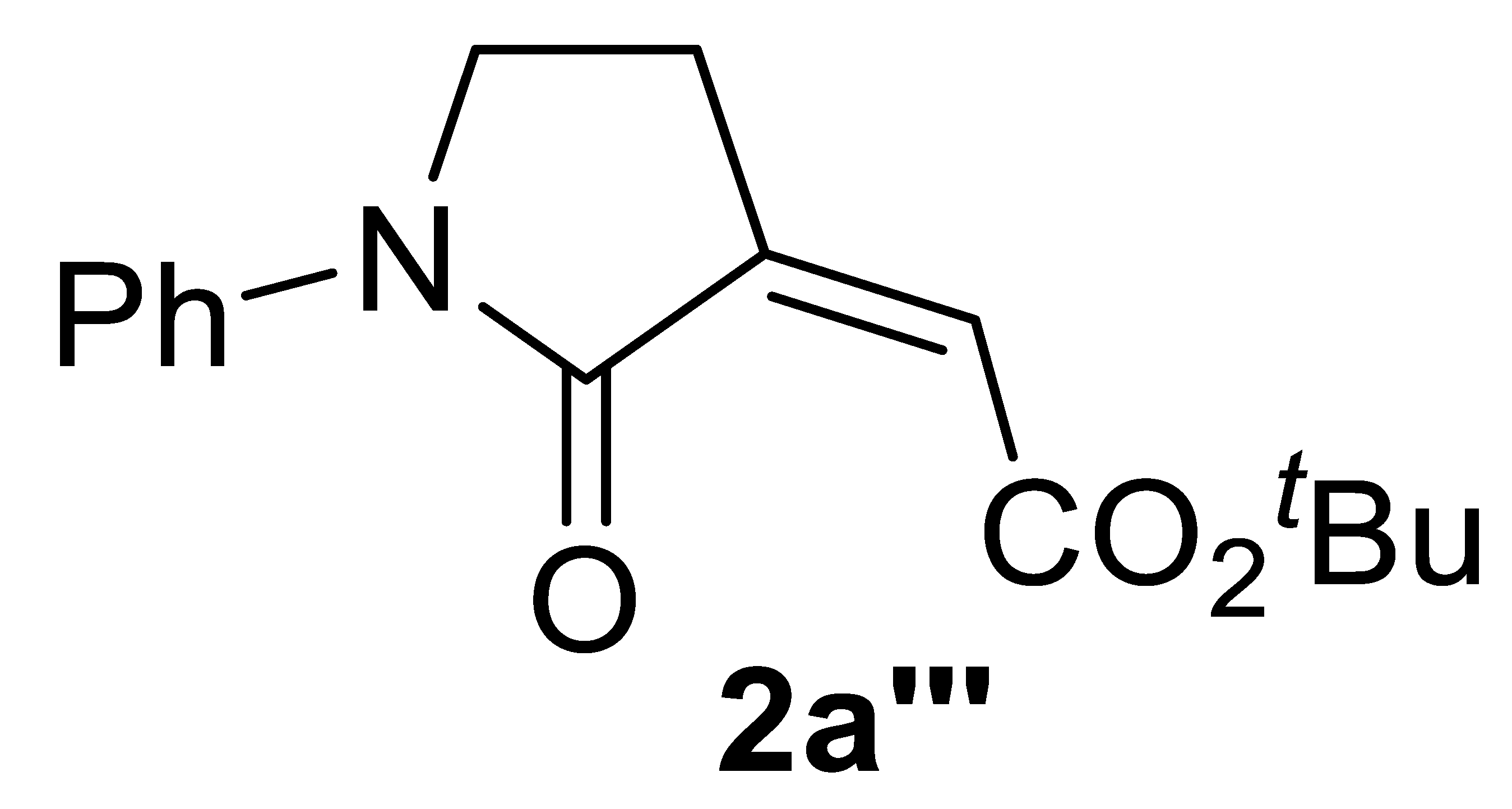
With the aim of obtaining a higher yield of 2a, we then changed the reaction parameters, such as the KI amount, substrate concentration, temperature, and pressure. After this brief optimization study (Table 1, Entries 2–10), 2a was obtained in 85% isolated yield working under the same conditions as those of entry 1, but with a substrate concentration of 0.1 mmol/mL of MeOH (Table 1, entry 7, and Table 2, entry 1). Good yields were still obtained when the process was carried out with lower catalyst loadings (72% yield with 1 mol% of PdI2, and 64% yield with 0.5 mol% of PdI2; Table 2, entries 2 and 3, respectively). The use of EtOH instead of MeOH did not cause a significant change in product yield, the corresponding ethyl ester 2a′ being obtained in 82% yield with 5 mol% PdI2 (Table 2, entry 4). On the other hand, more hindered and less nucleophilic isopropanol led to a lower yield of the corresponding ester 2a″ (72%; Table 2, entry 5). With tert-butanol, the yield of 2a‴ was 33% (Table 2, entry 6), which, however, could be improved to 54% working under more diluted conditions (Table 2, entry 7).

Table 2.
Multicomponent PdI2/KI-catalyzed carbonylative synthesis of alkyl (Z)-2-(2-oxopyrrolidin-3-ylidene)acetates 2 a,b.
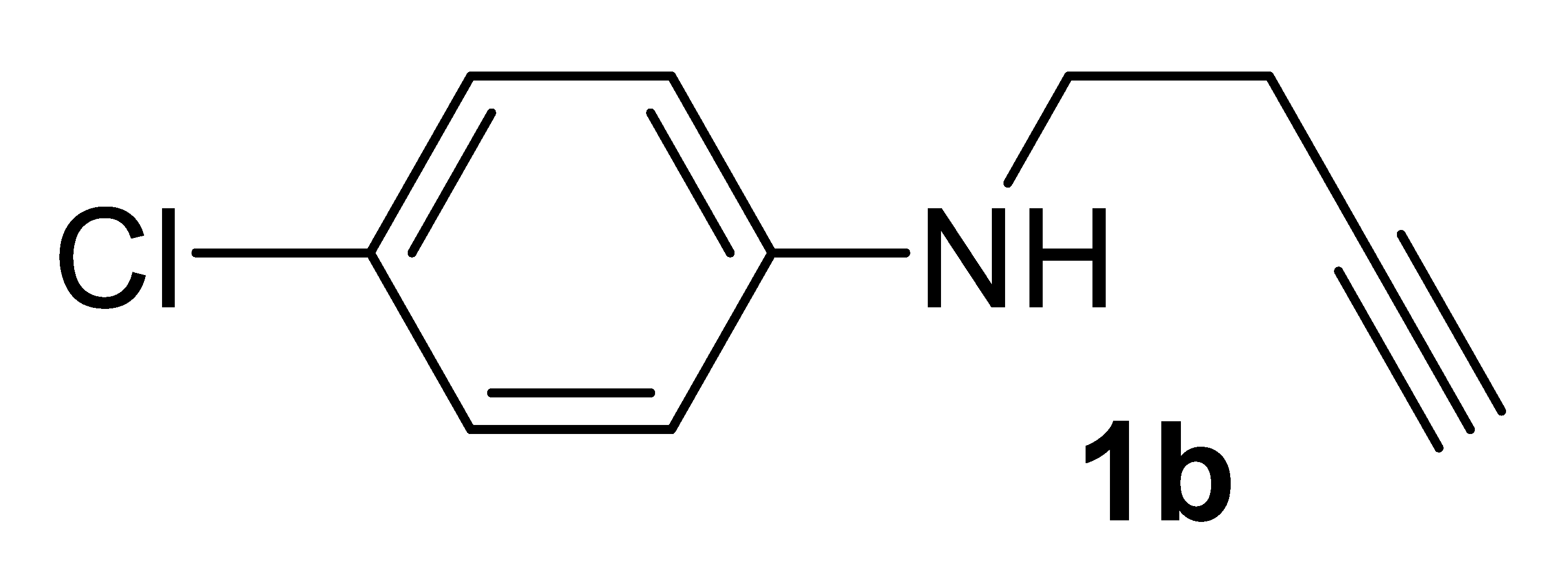
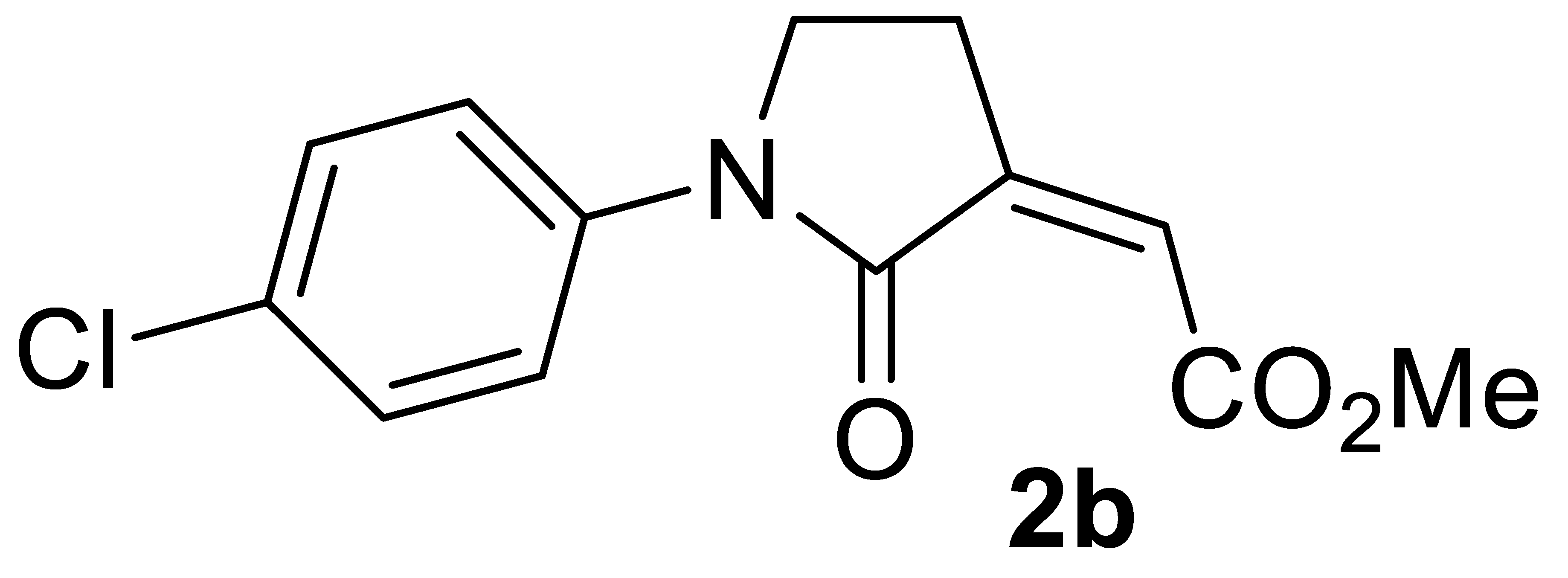
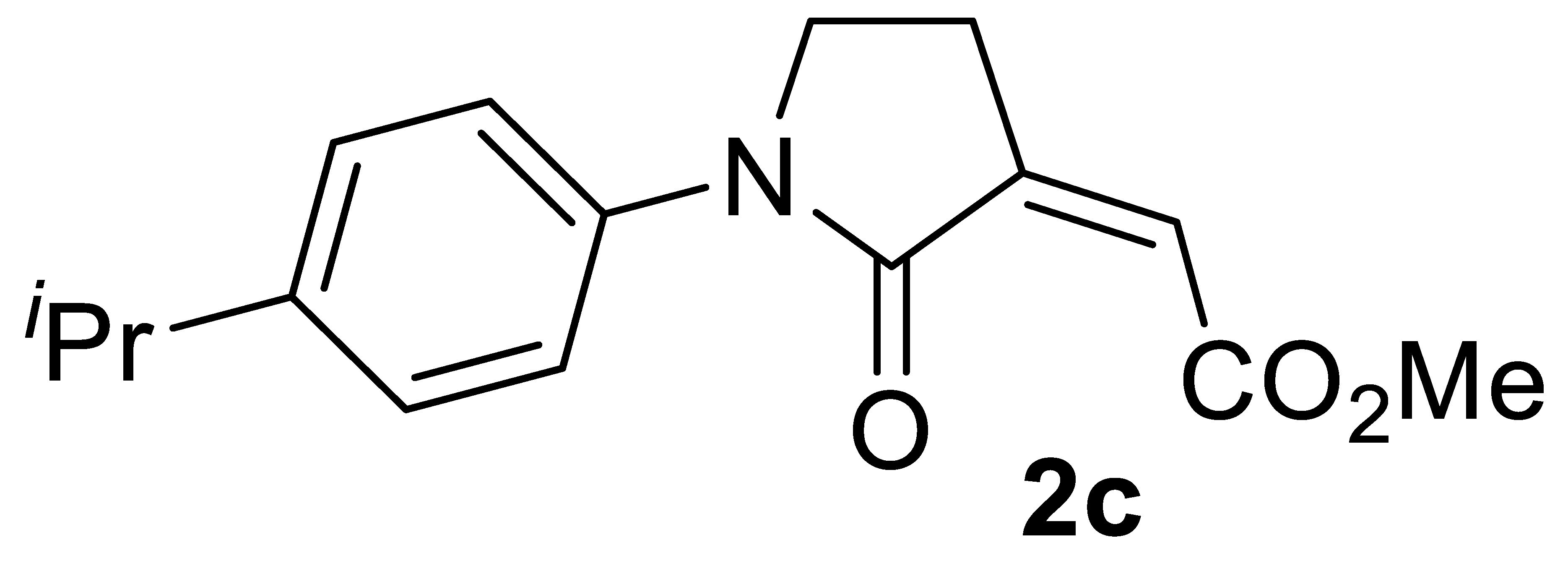
Our next step was to verify the generality of the process using other differently substituted homopropargylic amines 1b–n (Table 2, entries 8–27). Very good results were consistently obtained with all the tested N-aryl-substituted substrates 1b–1k, bearing electron-withdrawing as well as electron-donating substituents (entries 8–20). The structure of another representative product, methyl (Z)-2-(1-(4-chlorophenyl)-2-oxopyrrolidin-3-ylidene)acetate 2b, was confirmed again by XRD analysis (Figure 2; see the Supplementary Materials for full XRD data). We assessed the possibility to work with a lower catalyst loading (1 mol% of PdI2) also for substrates 1b (Table 2, entry 9), 1c (Table 2, entry 11), and 1e (Table 2, entry 14), still with satisfactory results. In fact, the yields of products 2b, 2c, and 2e were only slightly inferior with respect to those obtained with 5 mol% of PdI2 (Table 2; compare entries 9, 11, and 14 with entries 8, 10, and 13, respectively).
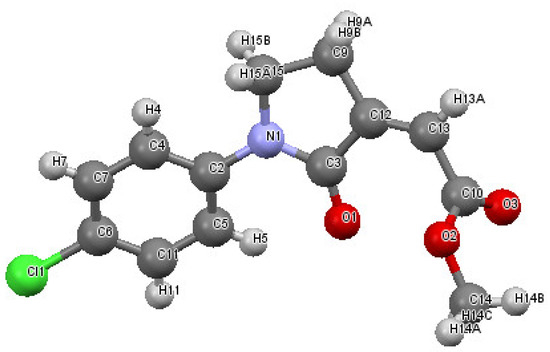
Figure 2.
Molecular structure of methyl (Z)-2-(1-(4-chlorophenyl)-2-oxopyrrolidin-3-ylidene)acetate 2b. The figure shows the atom labelling scheme for non-H atoms and displacement ellipsoids at the 50% probability level.
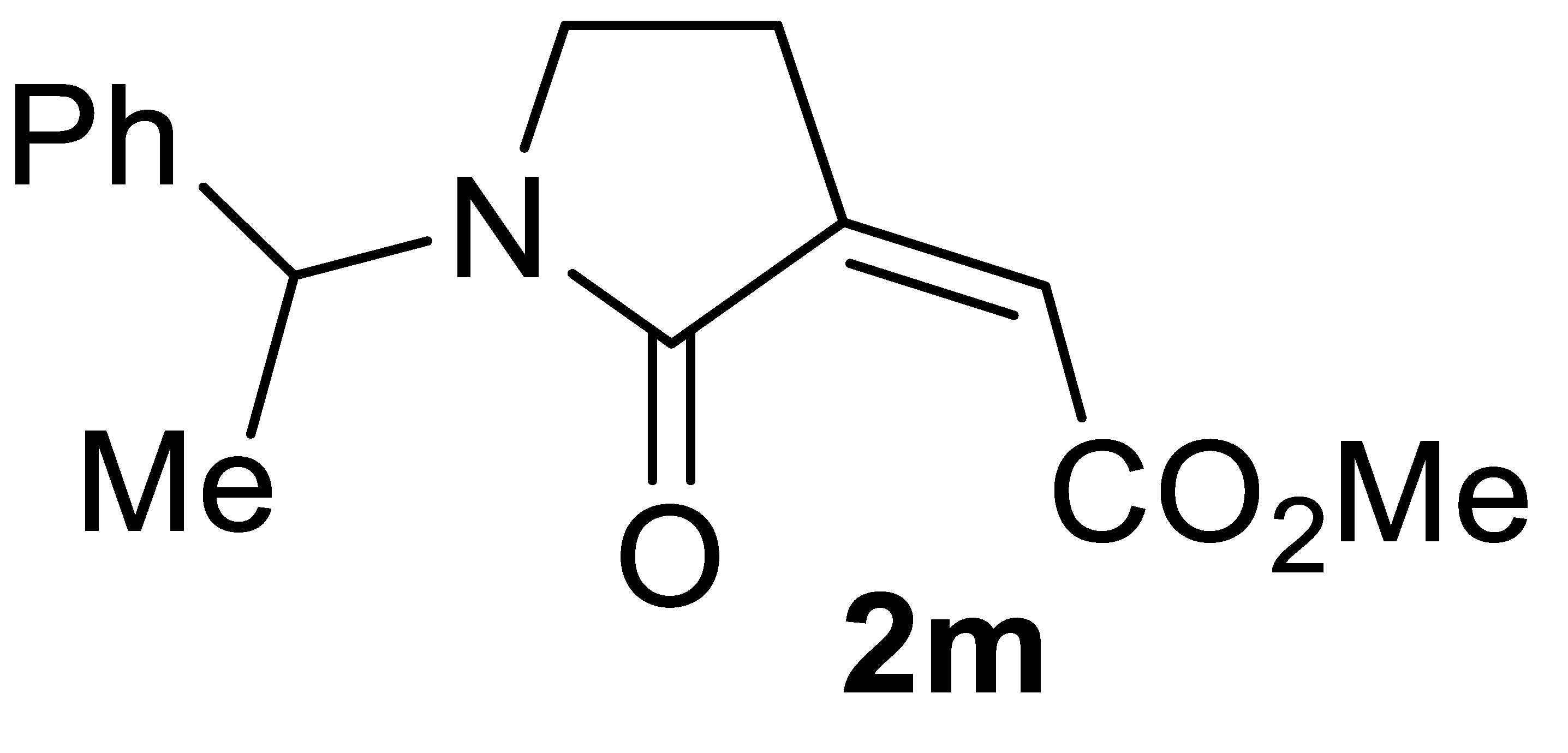
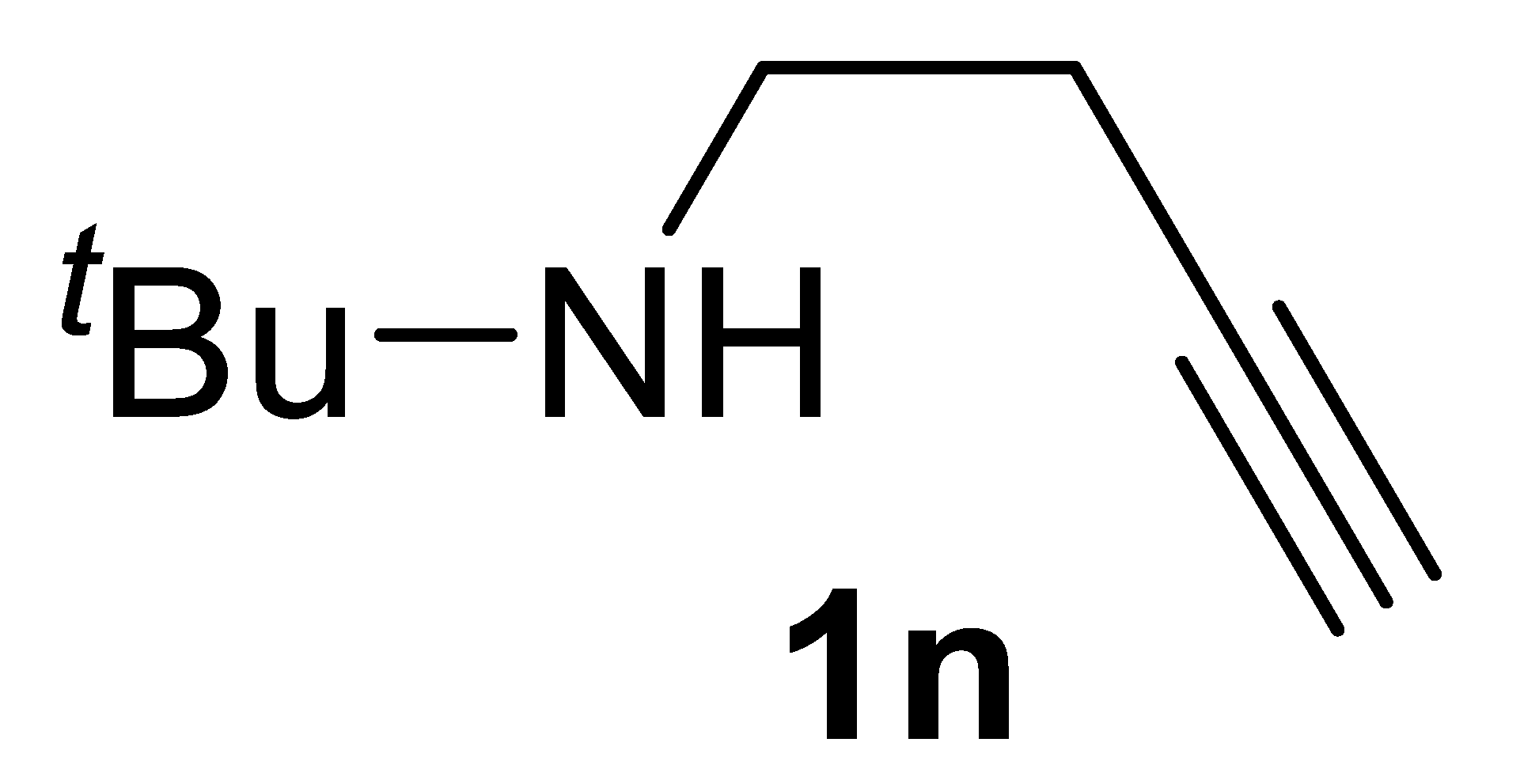
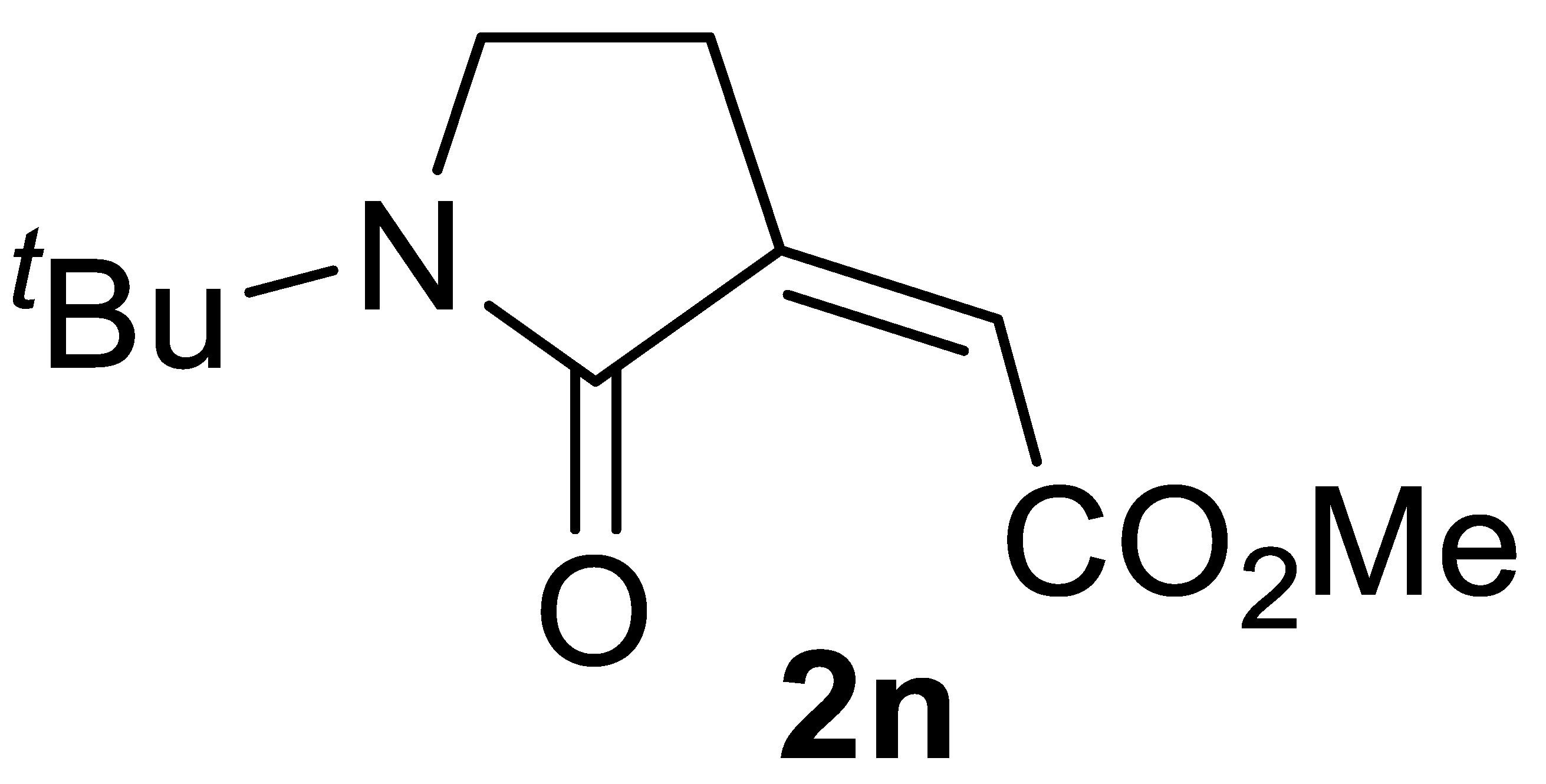
The carbonylation protocol led to lower γ-lactam yields when starting from N-alkyl substituted substrates, such as N-benzylbut-3-yn-1-amine 1l and N-(1-phenylethyl)but-3-yn-1-amine 1m, as shown in entries 21-23 and 25, respectively. Interestingly, however, the yields of products 2l and 2m could be significantly improved by carrying out the catalytic process under more diluted conditions (entries 24 and 26, respectively). This effect of substrate concentration on the reaction outcome is difficult to rationalize, and we refrain from proposing speculative interpretations. These conditions permitted to achieve an acceptable product yield even when starting from substrate 1n, bearing a highly bulky alkyl group on nitrogen (yield of methyl (Z)-2-(1-(tert-butyl)-2-oxopyrrolidin-3-ylidene)acetate 2n was 47%; Table 2, entry 27).
Based on the existing knowledge on carbonylations [1,2,3,4,5] and on our expertise in PdI2/KI-catalyzed carbonylation reactions [7,8], we can propose the mechanistic pathway shown in Scheme 2 for the formation of (Z)-2-(2-oxopyrrolidin-3-ylidene)acetates 2. Palladation of the nitrogen of 1 initially leads to alkynylaminopalladium intermediate I, stabilized by the intramolecular triple bond coordination (anionic iodide ligands are omitted for clarity). Coordination of CO followed by migratory insertion then affords the carbamoylpalladium complex II, which undergoes intramolecular syn triple bond insertion to yield III, followed by a second insertion of carbon monoxide to give IV. Nucleophilic displacement by ROH on IV finally yields 2 and Pd(0), which is readily oxidized back to catalytically active PdI2 by the action of O2.
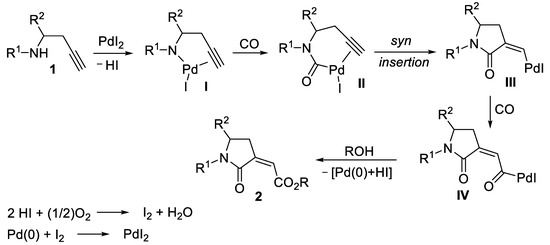
Scheme 2.
Proposed mechanism for the stereoselective catalytic carbonylative synthesis of alkyl (Z)-2-(2-oxopyrrolidin-3-ylidene)acetates 2 from homopropargyic amines 1.
3. Materials and Methods
3.1. General Experimental Methods
All reactions were analyzed by TLC on silica gel 60 F254 or on neutral alumina and by GLC-MS using a Shimadzu QP-2010 GC–MS apparatus (Shimadzu Italia s.r.l., Milano, Italy) and capillary columns with polymethylsilicone + 5% phenylsilicone as the stationary phase. Column chromatography was performed on silica gel 60 (Merck, 70–230 mesh; Merck Life Science s.r.l., Milano, Italy). Evaporation refers to the removal of solvent under reduced pressure.
Melting points are uncorrected. 1H NMR and 13C NMR spectra were recorded at 25 °C in CDCl3 at 300 MHz and 75 MHz, respectively, with Me4Si as internal standard, using a Bruker DPX Avance 300 NMR Spectrometer (Bruker Italia s.r.l., Milano, Italy); chemical shifts (δ) and coupling constants (J) are given in ppm and in Hz, respectively. IR spectra were taken with a JASCO FT-IR 4200 spectrometer (Jasco Europe s.r.l., Cremella, Lecco, Italy). Mass spectra were obtained using a Shimadzu QP-2010 GC–MS apparatus (Shimadzu Italia s.r.l., Milano, Italy) at 70 eV ionization voltage (normal resolution) and by electrospray ionization mass spectrometry (ESI-MS) (high resolution) with an Agilent 1260 Infinity UHD accurate-mass Q-TOF spectrometer (Agilent Technologies Italia s.p.a., Cernusco Sul Naviglio, Milano, Italy), equipped with a Dual AJS ESI source working in positive mode, and were recorded in the 150–1000 m/z range. The LC-MS experimental conditions were as follows: The flow-rate was 0.4 mL/min and the column temperature was set to 30 °C. The eluents were formic acid–water (0.1:99.9, v/v) (phase A) and formic acid–acetonitrile (0.1:99.9, v/v) (phase B). The following gradient was employed: 0–10 min, linear gradient from 5% to 95% B; 10–15 min, washing and reconditioning of the column to 5% B. Injection volume was 10 uL. N2 was employed as desolvation gas at 300 °C and a flow rate of 8 L/min. The nebulizer was set to 45 psig. The Sheat gas temperature was set at 400 °C and a flow of 12 L/min. A potential of 3.5 kV was used on the capillary for positive ion mode. The fragmentor was set to 175 V.
3.2. Preparation of Substrates 1a–g and 1l–n
N-Substituted 3-yn-1-amines 1a–g and 1l–n were prepared by the reaction of 3-yn-1-yl tosylates with a primary amine as described below. All starting materials were commercially available (Merck Life Science s.r.l., Milano, Italy) and were used without further purification.
A mixture of the 3-yn-1-yl tosylate (10 mmol; but-3-yn-1-yl tosylate, 2.24 g; pent-4-yn-2-yl tosylate, 2.38 g; hex-5-yn-3-yl tosylate, 2.52 g) and the primary amine (30 mmol; aniline, 2.79 g; 4-chloroaniline, 3.83 g; 4-isopropylaniline, 4.06 g; 4-(tert-butyl)aniline, 4.48; 3-bromo-5-methylaniline, 5.58 g; benzylamine, 3.21 g; α-methylbenzylamine, 3.62 g; tert-butylamine, 2.20 g) in MeCN (10 mL) was stirred for 3–24 h (3 h for the synthesis of 1n; 4 h for 1l; 5 h for 1m; 24 h for 1a–1g) under reflux under nitrogen. After cooling to room temperature, saturated Na2CO3 aqueous solution and AcOEt were added to the mixture. The layers were separated and the aqueous layer was extracted with AcOEt (for 1a–g, 1l–m) or Et2O (for 1n) (3 × 15 mL). The combined organic layers were washed with brine, dried over K2CO3, filtered, and concentrated under vacuum (30 °C and 100 mbar for 1a–g, 1l–m or 30 °C and 850 mbar for 1n). The crude products were purified by column chromatography on silica gel using as eluent hexane−AcOEt from 100:0 to 95:5 (1a–g) or 95:5 CH2Cl2-MeOH (1l–1m). In the case of 1n, column chromatography with Et2O was followed by fractional distillation to remove the solvent first at 760 mmHg and the under vacuum to collect the pure product.
3.2.1. N-(But-3-yn-1-yl)aniline 1a
Yield: 755.0 mg, 52% based on but-3-yn-1-yl tosylate. Yellow oil. IR (film): ν = 3403 (m, br), 3292 (s, br), 2116 (w), 1604 (s), 1505 (s), 1317 (m), 1262 (m), 751 (m), 693 (m) cm−1; 1H NMR (CDCl3, 300 MHz): δ = 7.25–7.02 (m, 2 H, aromatic), 6.72 (t, J = 7.3, 1H, aromatic), 6.63 (d, J = 8.1, 2H, aromatic), 3.92 (s, br, 1H, NH), 3.31 (s, br, 2H, NCH2CH2), 2.49 (td, J = 6.5, 2.5, 2H, NCH2CH2), 2.03 (t, J = 2.5, 1H, ≡CH); 13C{1H} NMR (CDCl3, 75 MHz): δ = 147.6, 129.3 (2C), 117.9, 113.1 (2C), 81.8, 70.0, 42.4, 19.1; GC-MS (EI, 70 eV): m/z = 145 (M+, 16), 106 (100); HRMS (ESI-TOF) m/z: [(M + H)+] cald for C10H12N+: 146.0964; found, 146.0975. The spectroscopic data agreed with those reported [18].
3.2.2. N-(But-3-yn-1-yl)-4-Chloroaniline 1b
Yield: 988.0 mg, 55% based on but-3-yn-1-yl tosylate. Yellow oil. IR (film): ν = 3406 (m, br), 3296 (s), 2117 (w), 1601 (s), 1504 (s), 1293 (m), 1262 (m), 1089 (m), 817 (s) cm−1; 1H NMR (CDCl3, 300 MHz): δ = 7.16–7.08 (m, 2H, aromatic), 6.58–6.49 (m, 2H, aromatic), 3.95 (s, br, 1H, NH), 3.27 (t, J = 6.6, NCH2CH2), 2.48 (td, J = 6.6, 2.6, 2H, NCH2CH2), 2.05 (t, J = 2.6, 1H, ≡CH); 13C{1H} NMR (CDCl3, 75 MHz): δ = 146.1, 129.1 (2C), 122.3, 114.2 (2C), 81.5, 70.3, 42.4, 19.0; GC-MS (EI, 70 eV): m/z = 179 (M+, 16), 142 (34), 140 (100); HRMS (ESI-TOF) m/z: [(M + H)+] cald for C10H11ClN+: 180.0575; found, 180.0578.
3.2.3. N-(But-3-yn-1-yl)-4-Isopropylaniline 1c
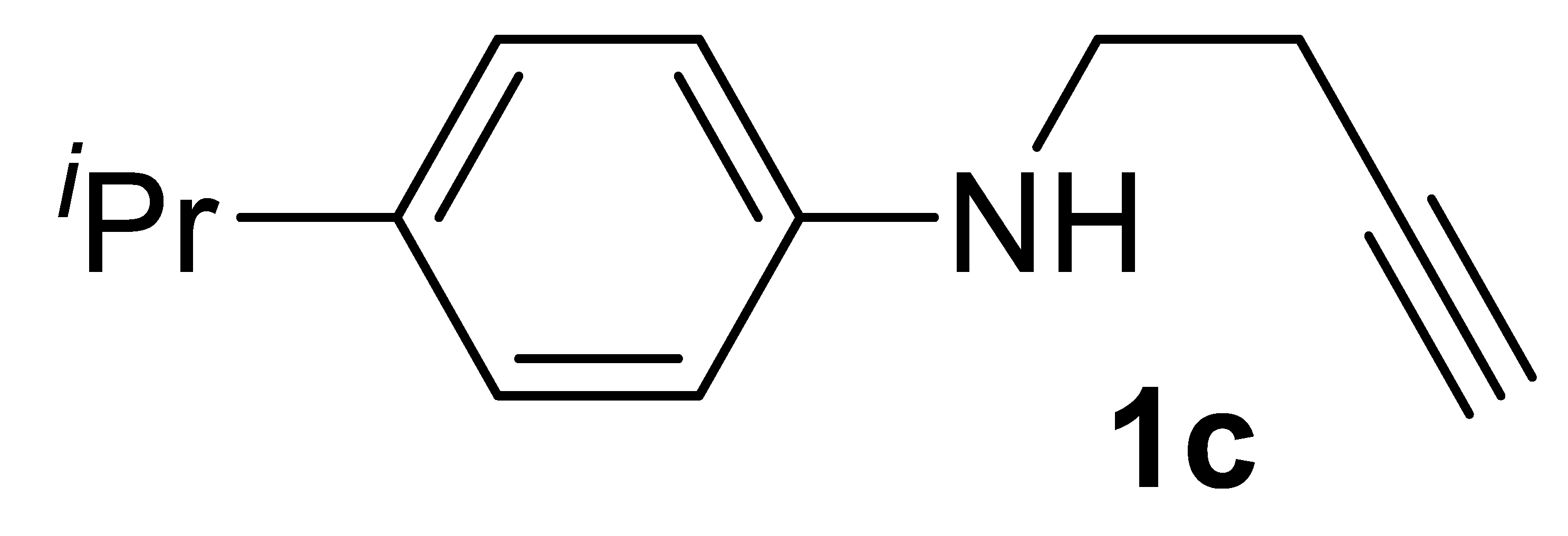
Yield: 1.07 g, 57% based on but-3-yn-1-yl tosylate. Yellow oil. IR (film): ν = 3397 (m, br), 3298 (m, br), 2118 (w), 1616 (s), 1519 (s), 1288 (m), 1259 (m), 1186 (m), 1051 (w), 822 (m) cm−1; 1H NMR (CDCl3, 300 MHz): δ = 7.09–7.01 (m, 2 H, aromatic), 6.62–6.54 (m, 2 H, aromatic), 3.81 (s, br, 1H, NH), 3.29 (t, J = 6.7, NCH2CH2), 2.80 (heptuplet, 1H, J = 6.9, CH3CHCH3), 2.48 (td, J = 6.7, 2.6, 2H, NCH2CH2), 2.02 (t, J = 2.6, 1H, ≡CH), 1.20 (d, J = 6.9, 6H, (CH3)2CH); 13C{1H} NMR (CDCl3, 75 MHz): δ = 145.6, 138.4, 127.2 (2C), 113.2 (2C), 81.9, 77.2, 70.0, 42.8, 33.1, 24.2, 19.2; GC-MS (EI, 70 eV): m/z = 187 (M+, 18), 148 (100); HRMS (ESI-TOF) m/z: [(M + H)+] cald for C13H18N+: 188.1434; found, 188.1444.
3.2.4. N-(But-3-yn-1-yl)-4-(tert-Butyl)aniline 1d
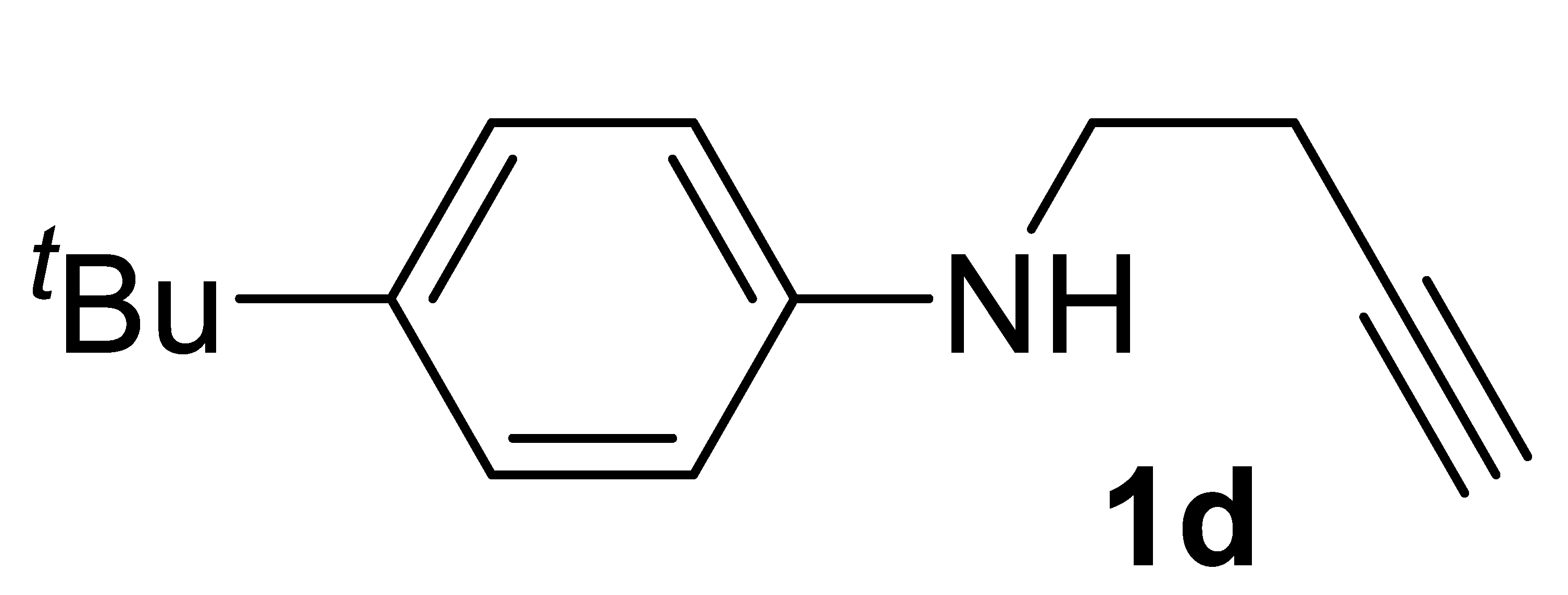
Yield: 1.13 g, 56% based on but-3-yn-1-yl tosylate. Yellow oil. IR (film): ν = 3402 (w, br), 3294 (m, br), 2118 (vw), 1616 (m), 1520 (s), 1261 (m), 1192 (m), 822 (m) cm−1; 1H NMR (CDCl3, 300 MHz): δ = 7.26–7.16 (m, 2 H, aromatic), 6.63–6.53 (d, J = 8.6, 2 H, aromatic), 3.83 (s, br, 1H, NH), 3.28 (t, J = 6.5, 2 H, NCH2CH2), 2.46 (td, J = 6.5, 2.5, 2 H, NCH2CH2), 2.01 (t, J = 2.5, 1 H, ≡CH), 1.27(s, 9H, t-Bu); 13C{1H} NMR (CDCl3, 75 MHz): δ = 145.2, 140.6, 126.0 (2C), 112.9 (2C), 81.9, 70.0, 42.7, 33.8, 31.5 (3C), 19.2; GC-MS (EI, 70 eV): m/z = 201 (M+, 20), 186 (22), 162 (100), 147 (29); HRMS (ESI-TOF) m/z: [(M + H)+] cald for C14H20N+: 202.1590; found, 202.1599.
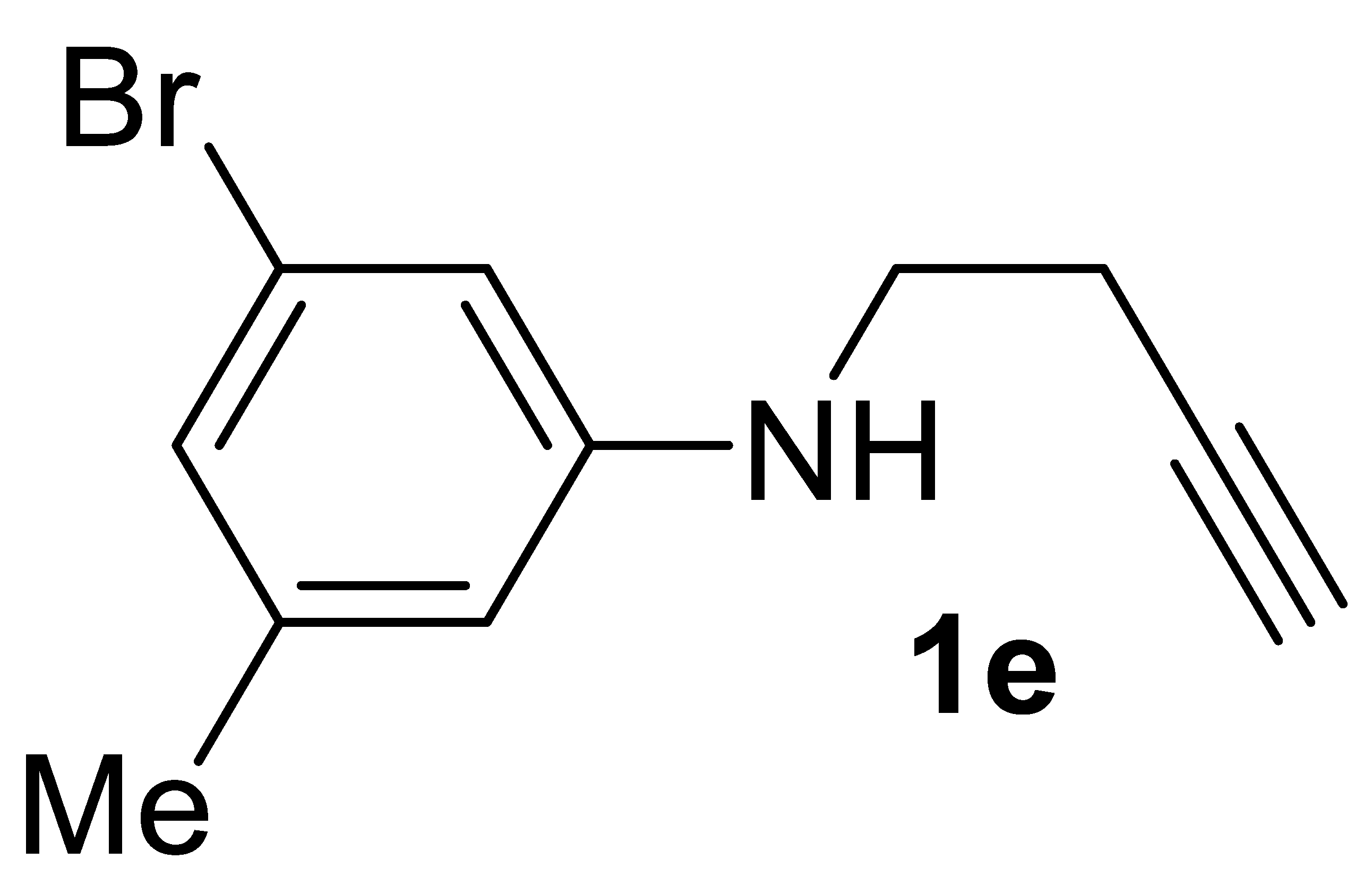
3.2.5. 3-Bromo-N-(but-3-yn-1-yl)-5-Methylaniline 1e
Yield: 998.0 mg, 42% based on but-3-yn-1-yl tosylate. Yellow oil. IR (film): ν = 3394 (w, br), 3299 (m), 2119 (vw), 1517 (s), 1316 (m), 1100 (w), 1037 (w), 803 (m) cm−1; 1H NMR (CDCl3, 300 MHz): δ = 7.29–7.24 (m, 1 H, aromatic), 7.01–6.94 (m, 1 H, aromatic), 6.55 (d, J = 8.2, 1 H, aromatic), 4.45 (s, br, 1H, NH), 3.34 (q, J = 6.6, NCH2CH2), 2.51 (td, J = 6.6, 2.6, 2H, NCH2CH2), 2.22 (s, 3H, Me), 2.06 (t, J = 2.6, 1 H, ≡CH); 13C{1H} NMR (CDCl3, 75 MHz): δ = 142.1, 132.9, 129.0, 127.8, 111.4, 110.0, 81.4, 70.3, 42.6, 20.0, 19.0; GC-MS (EI, 70 eV): m/z = 239 [(M + 2)+, 20], 237 (M+, 21), 200 (96), 198 (100), 119 (45); HRMS (ESI-TOF) m/z: [(M + H)+] cald for C11H13BrN+: 238.0226; found, 238.0235.
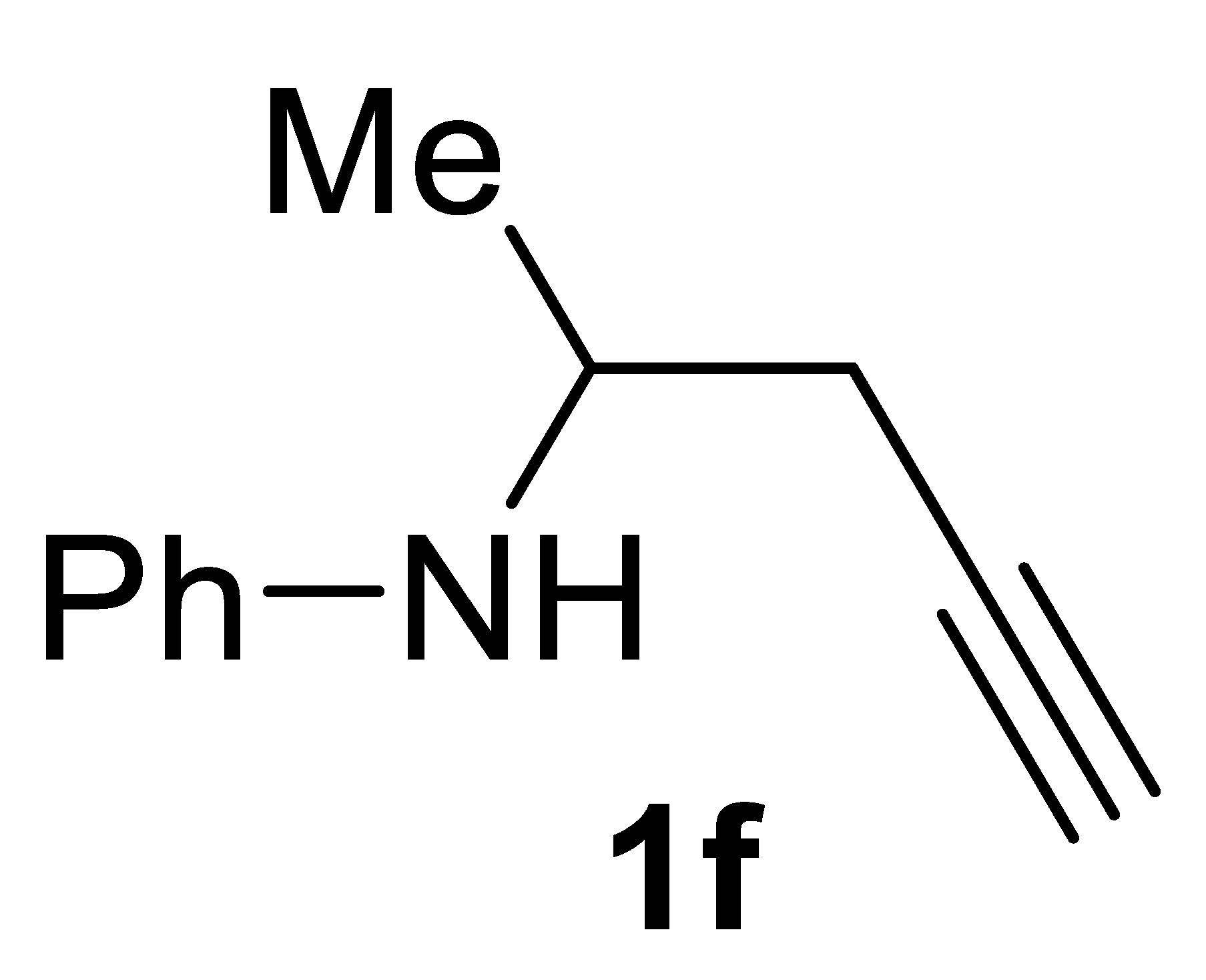
3.2.6. N-(Pent-4-yn-2-yl)aniline 1f
Yield: 891.7 mg, 56% based on pent-4-yn-2-yl tosylate. Yellow oil. IR (film): ν = 3403 (m), 3294 (m), 2116 (w), 1603 (s), 1506 (s), 1316 (s), 1255 (w), 1154 (w), 750 (s) cm−1; 1H NMR (CDCl3, 300 MHz): δ = 7.22–7.12 (m, 2 H, aromatic), 6.75–6.66 (m, 1 H, aromatic), 6.64–6.55 (m, 2 H, aromatic, 3.77–3.61 (m, 2 H, NH+ CHCH3), 2.53–2.31 (m, 2H, CH2C≡), 2.03 (t, J = 2.6, 1H, ≡CH), 1.31 (d, J = 6.4, 3H, Me); 13C{1H} NMR (CDCl3, 75 MHz): δ = 146.8, 129.4 (2C), 117.6, 113.6 (2C), 80.9, 70.7, 47.0, 25.5, 20.0; GC-MS (EI, 70 eV): m/z = 159 (M+, 13), 120 (100); HRMS (ESI-TOF) m/z: [(M + H)+] cald for C11H14N+: 160.1121; found, 160.1131.
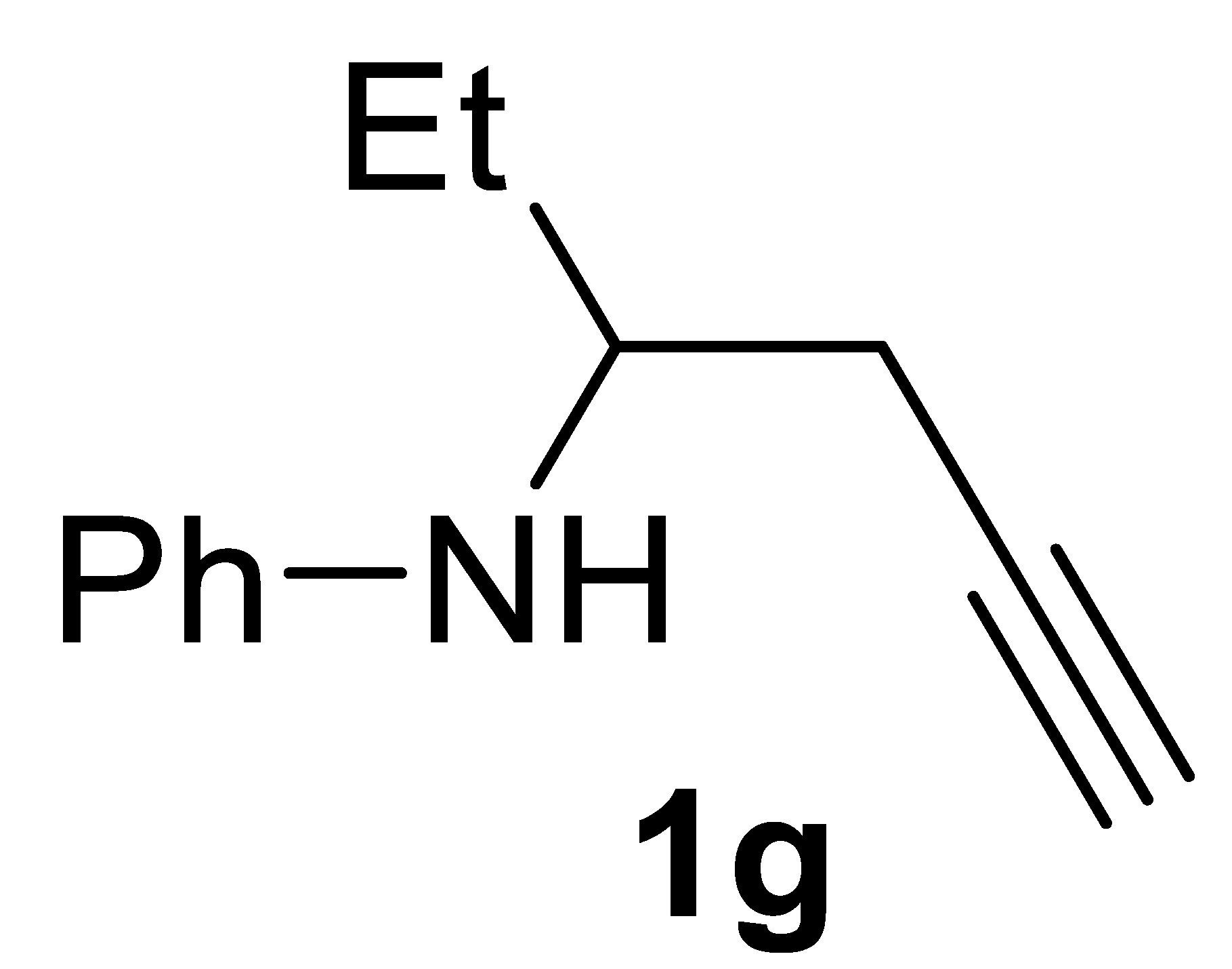
3.2.7. N-(Hex-5-yn-3-yl)aniline 1g
Yield: 762.3 mg, 44% based on hex-5-yn-3-yl tosylate. Yellow oil. IR (film): ν = 3399 (m), 3294 (m), 2114 (w), 1601 (s), 1504 (s), 1315 (m), 1281 (m), 1153 (w), 748 (m) cm−1; 1H NMR (CDCl3, 300 MHz): δ = 7.23–7.09 (m, 2 H, aromatic), 6.75–6.65 (m, 1 H), 6.65–6.55 (m, 2 H, aromatic), 3.69 (s, br, NH), 3.52–3.33 (m, 1H, CHCH3), 2.51–2.36 (m, 2H, NCH2CH2), 2.00 (t, J = 2.6, 1H, ≡CH), 1.84–1.52 (m, 2 H, CH2CH3), 0.99 (t, 3H, CH3); 13C{1H} NMR (CDCl3, 75 MHz): δ = 147.2, 129.4 (2C), 117.5, 113.5 (2C), 80.9, 70.5, 52.8, 26.8, 23.2, 10.7; GC-MS (EI, 70 eV): m/z = 173 (M+, 11), 134 (100); HRMS (ESI-TOF) m/z: [(M + H)+] cald for C12H16N+: 174.1277; found, 174.1300.
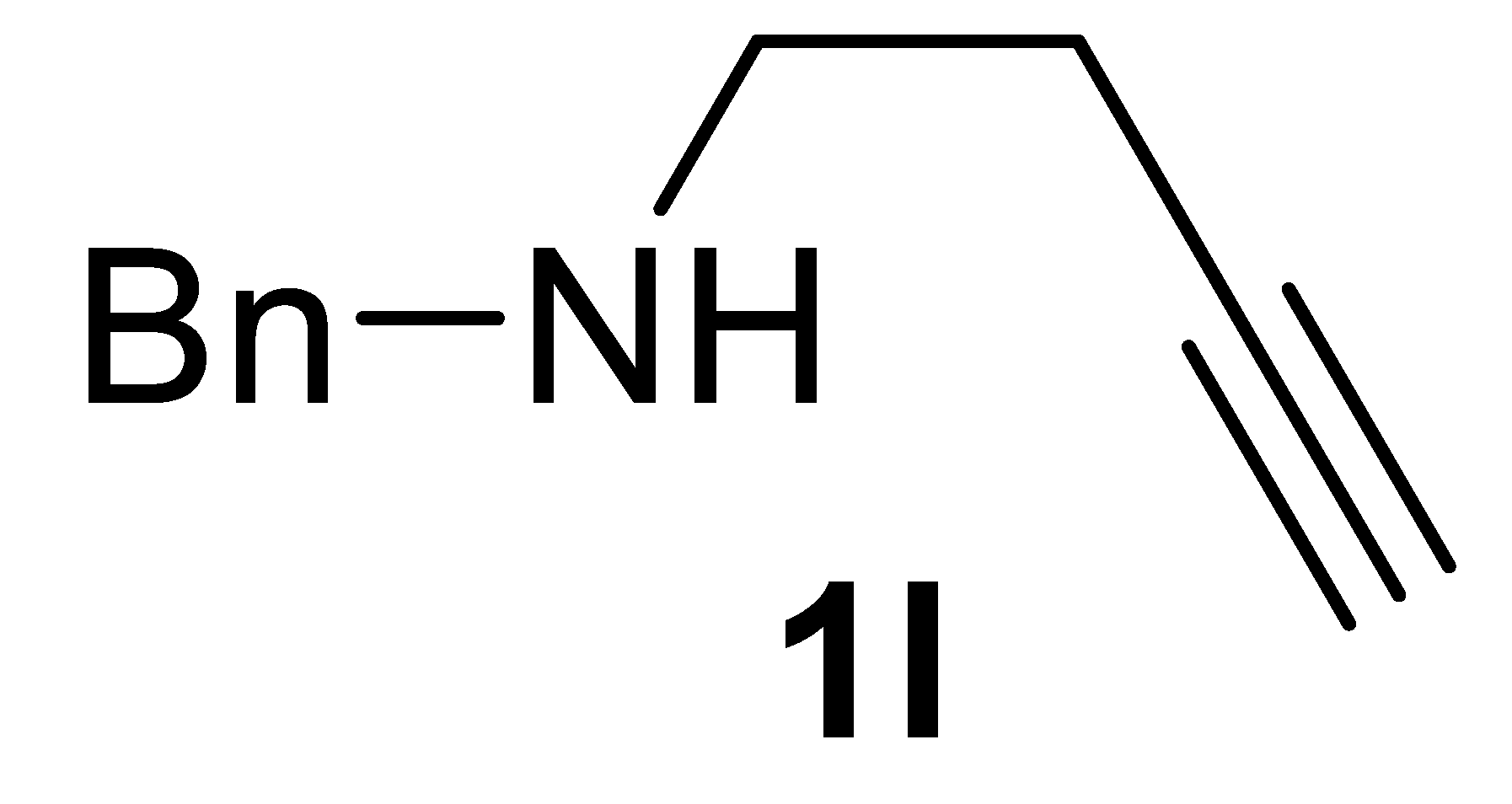
3.2.8. N-Benzylbut-3-yn-1-Amine 1l
Yield: 1.2 g, 75% based on but-3-yn-1-yl tosylate. Yellow oil. IR (film): ν = 3296 (s), 2915 (m), 2836 (m), 2116 (w), 1454 (m), 1120 (m), 738 (s), 699 (m), 640 (s) cm−1; 1H NMR (CDCl3, 300 MHz): δ = 7.37–7.22 (m, 5H, Ph), 3.82 (s, 2 H, CH2Ph), 2.80 (t, J = 6.5, 2H, NCH2CH2), 2.42 (td, J = 6.5, 2.6, 2H, NCH2CH2), 2.00 (t, J = 2.6, 1H, ≡CH), 1.74 (s, br, 1H, NH); 13C{1H} NMR (CDCl3, 75 MHz): δ = 140.1, 128.4 (2C), 128.1 (2C), 127.0, 82.5, 69.6, 53.4, 47.3, 19.5; GC-MS (EI, 70 eV): m/z = 159 (M+, 1), 120 (58), 91 (100). HRMS (ESI-TOF) m/z: [(M + H)+] cald for C11H14N+: 160.1121; found, 160.1130. The spectroscopic data agreed with those reported [19].
3.2.9. N-(1-Phenylethyl)but-3-yn-1-Amine 1m
Yield: 1.1 g, 63% based on but-3-yn-1-yl tosylate. Yellow oil. IR (film): 3302 (s), 2968 (s), 2927 (m), 2117 (w), 1452 (m), 1130 (m), 762 (m), 701 (s) cm−1; 1H NMR (CDCl3, 300 MHz): δ = 7.35–7.10 (m, 5H, Ph), 3.80 (q, J = 6.6, 1H, CHCH3), 2.72–2.53 (m, 2H, NCH2), 2.40–2.27 (m, 2 H, NCH2CH2), 1.98 (s, br, 1 H, ≡CH), 1.78 (s, br, 1H, NH), 1.36 (d, J = 6.6, 3H, Me); 13C{1H} NMR (CDCl3, 75 MHz): δ = 145.4, 128.5 (2C), 127.0, 126.6 (2C), 82.6, 69.5, 57.7, 45.7, 24.4, 19.6; HRMS (ESI-TOF) m/z: [(M + H)+] cald for C12H16N+: 174.1277; found, 174.1301. The spectroscopic data agreed with those reported [20].
3.2.10. N-tert-Butylbut-3-yn-1-Amine 1n
Yield: 689 mg, 55% based on but-3-yn-1-yl tosylate. Colorless oil. IR (film): ν = 3310 (m), 2967 (s), 2866 (s), 2118 (w), 1450 (m), 1365 (m), 1060 (w), 633 (m) cm−1; 1H NMR (CDCl3, 300 MHz): δ = 2.73 (t, J = 6.7, 2H, NCH2), 2.37 (td, J = 6.7, 2.6, 2H, CH2C≡), 2.06–1.97 (m, 2 H, ≡CH + NH), 1.12 (s, 9H, t-Bu); 13C{1H} NMR (CDCl3, 75 MHz): δ = 82.7, 69.4, 50.3, 41.2, 29.0 (3C), 20.5; HRMS (ESI-TOF) m/z: [(M + H)+] cald for C8H16N+: 126.1277; found, 126.1288. The spectroscopic data agreed with those reported [21].
3.3. Preparation of Substrates 1h–k
N-Substituted but-3-yn-1-amines 1h–k were prepared by propargylation of the corresponding imines as described below. All starting materials were commercially available (Merck Life Science s.r.l., Milano, Italy) and were used without further purification.
N-Benzylideneaniline, N-(4-bromobenzylidene)aniline, N-(4-methoxybenzylidene)aniline, 4-chloro-N-(4-methoxybenzylidene)aniline were prepared by mixing the corresponding aldehydes (10 mmol; benzaldehyde, 1.05 g; 4-bromobenzaldehyde, 1.85 g; 4-methoxybenzaldehyde, 1.35 g) with anilines (10 mmol; aniline, 925 mg; 4-chloroaniline, 1.28 g) and Na2SO4 (5 g) in toluene (40 mL). The mixture was refluxed for 24 h. Solvent was removed in vacuo to afford the crude imine which was used directly in the next step. Zinc powder (2 g, 30 mmol) was washed with 2 N HCl (5 mL), H2O (until neutral), MeOH (25 mL), and tetrahydrofuran (THF) (25 mL) in this sequence, before being suspended in anhydrous THF (25 mL) under nitrogen. A THF solution (75 mL) of the crude imine (obtained as above) was added, followed by 3-bromopropyne (30 mmol, 3.5 g; 4.4 mL of an 80 wt % solution in toluene), which was added dropwise at 0 °C followed by stirring for 30 min. The mixture was allowed to warm up to room temperature and stirred for additional 24 h. Water (100 mL) and Et2O (100 mL) were added, and the reaction mixture was filtered through Celite. The aqueous phase was extracted with AcOEt (3 × 100 mL) and the combined organic layers were washed with brine (100 mL), dried over K2CO3, and concentrated under vacuum. The crude products were purified by column chromatography on silica gel using as eluent hexane−AcOEt from 100:0 to 95:5.
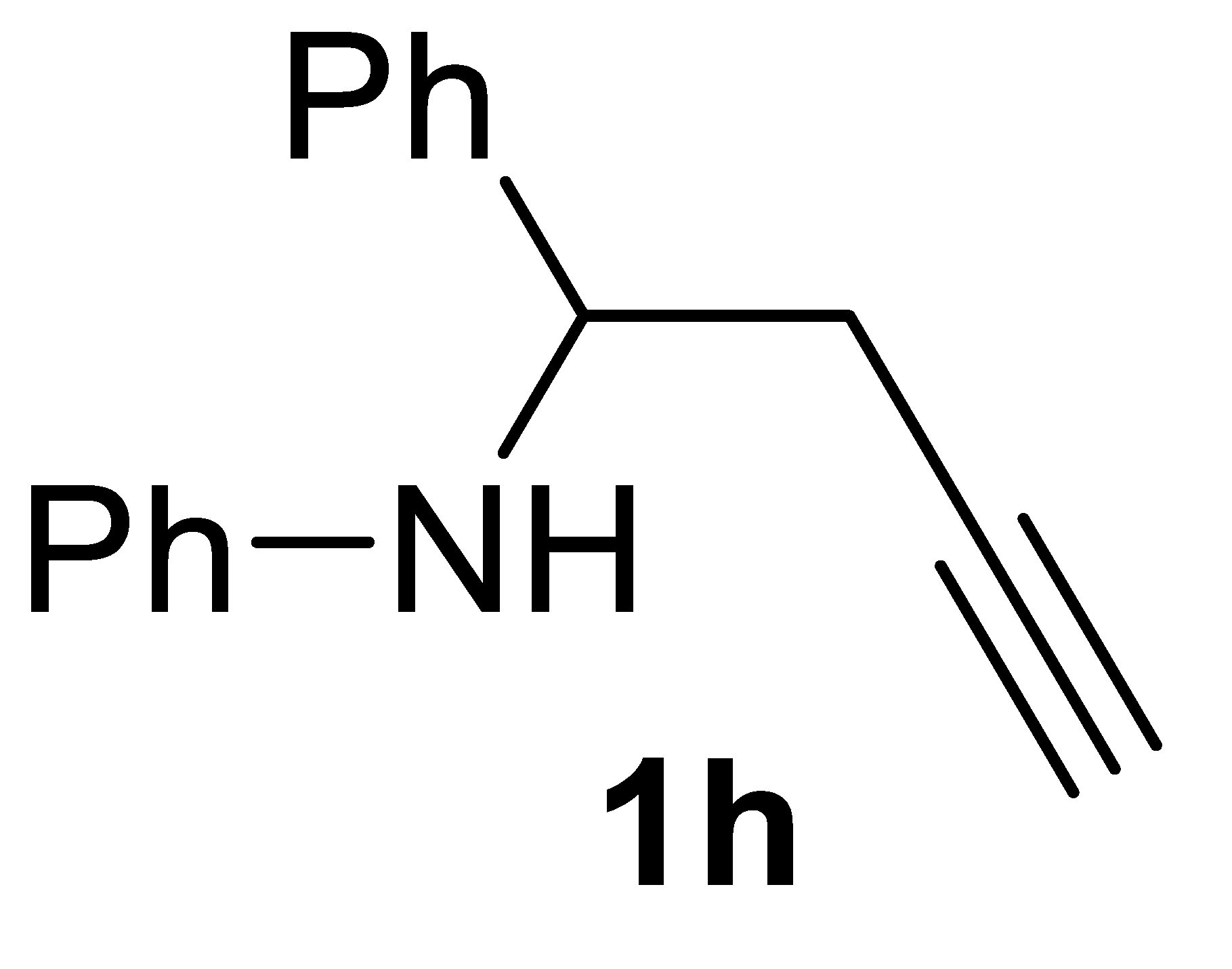
3.3.1. N-(1-Phenylbut-3-yn-1-yl)aniline 1h
Yield: 1.16 g, 52% based on starting N-benzylideneaniline. Yellow solid, mp 67.2–68.2. IR (KBr): ν = 3410 (m), 3256 (m), 2114 (w), 1605 (s), 1512 (s), 1327 (m), 1265 (w), 756 (s) cm−1; 1H NMR (CDCl3, 300 MHz): δ = 7.45–7.20 (m, 5 H, aromatic), 7.16–7.04 (m, 2H, aromatic), 6.72–6.63 (m, 1H, aromatic), 6.60–6.49 (m, 2H, aromatic), 4.54 (t, J = 6.0, 1H, CHPh), 4.41 (s, 1H, NH), 2.85–2.56 (m, 2H, CH2C≡), 2.06 (t, J = 2.6, 1H, ≡CH); 13C{1H} NMR (CDCl3, 75 MHz): δ = 147.0, 142.0, 129.1 (2C), 128.7 (2C), 127.5, 126.4 (2C), 117.8, 113.7 (2C), 80.3, 71.4, 56.4, 28.1; GC-MS (EI, 70 eV): m/z = 221 (M+, 7), 182 (100), 104 (45); HRMS (ESI-TOF) m/z: [(M + H)+] cald for C16H16N+: 222.1277; found, 222.1298. The spectroscopic data agreed with those reported [22].
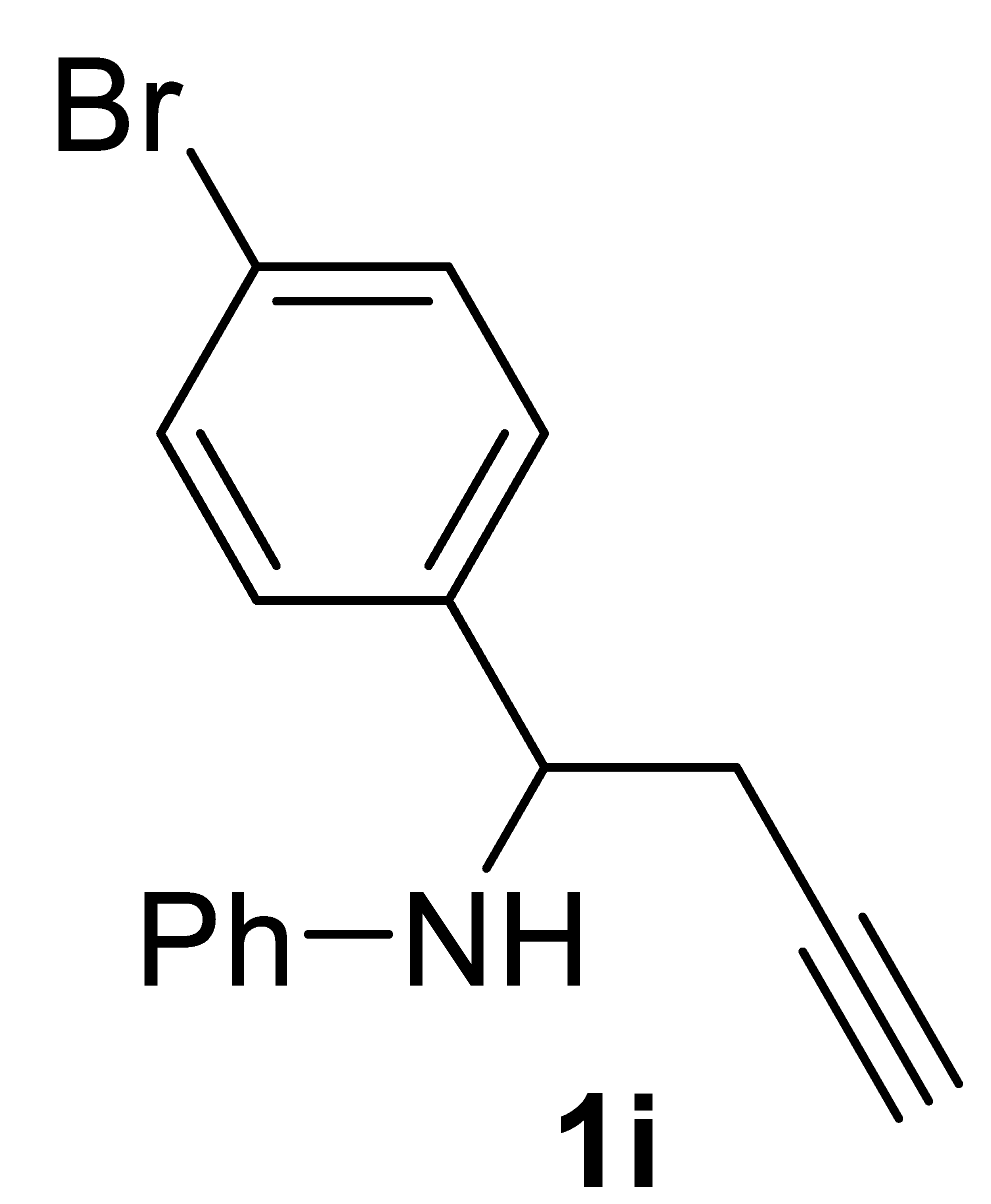
3.3.2. N-(1-(4-Bromophenyl)but-3-yn-1-yl)aniline 1i
Yield: 1.59 g, 53% based on starting N-(4-bromobenzylidene)aniline. Yellow oil. IR (film): ν = 3402 (w), 3294 (m), 3048 (w), 2114 (w), 1605 (s), 1505 (s), 1427 (w), 1312 (m), 1265 (m), 1072 (m), 1011 (m), 818 (m), 748 (s), 694 (m), 648 (m, br) cm−1; 1H NMR (CDCl3, 300 MHz): δ = 7.45 (distorted d, J = 8.3, 2H, aromatic), 7.28 (distorted d, J = 8.3, 2H, aromatic), 7.17–7.05 (m, 2H, aromatic), 6.74–6.64 (m, 1H, aromatic), 6.56–6.45 (m, 2H, aromatic), 4.48 (s, br, 1H, NH or CHPh), 4.39 (s, br, 1H, CHPh or NH), 2.82–2.55 (m, 2H, CH2C≡), 2.08 (t, J = 2.5, 1 H, ≡CH); 13C{1H} NMR (CDCl3, 75 MHz): δ = 146.6, 141.1, 131.8 (2C), 129.2 (2C), 128.2 (2C), 121.3, 118.1, 113.7 (2C), 79.8, 71.8, 55.8, 28.0; GC-MS (EI, 70 eV): m/z = 301 [(M + 2)+, 9], 299 (M+, 10), 262 (96), 260 (100), 104 (57); HRMS (ESI-TOF) m/z: [(M + H)+] cald for C16H15BrN+: 300.0382; found, 300.0420.
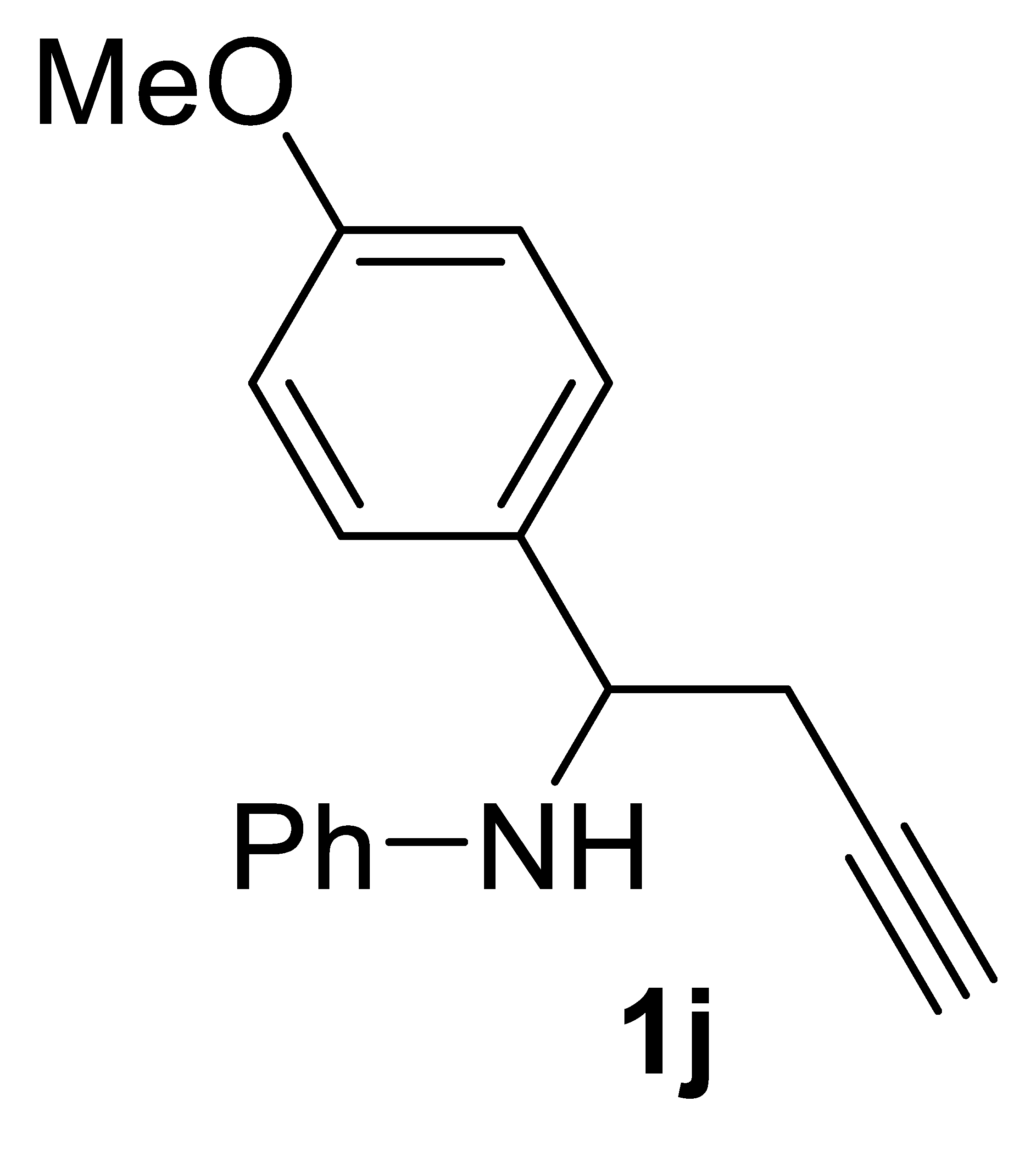
3.3.3. N-(1-(4-Bromophenyl)but-3-yn-1-yl)aniline 1j
Yield: 1.45 g, 57% based on starting N-(4-methoxybenzylidene)aniline. Yellow oil. IR (film): ν = 3402 (m), 3287 (m), 2118 (vw), 1605 (s), 1505 (s), 1173 (m), 1099 (w), 1034 (w), 752 (m) cm−1; 1H NMR (CDCl3, 300 MHz): δ = 7.30 (distorted d, J = 8.3, 2H, aromatic), 7.15–7.04 (m, 2H, aromatic), 6.85 (distorted d, J = 8.3, 2 H, aromatic), 6.71–6.62 (m, 1H, aromatic), 6.57–6.50 (m, 2H, aromatic), 4.48 (t, J = 6.0, 1 H, CHPh), 4.35 (s, br, 1H, NH), 3.75 (s, 3H, OMe), 2.80–2.52 (m, 2 H, CH2C≡), 2.05 (s, br, 1H, ≡CH); 13C{1H} NMR (CDCl3, 75 MHz): δ = 158.9, 147.0, 134.1, 129.1 (2C), 127.4 (2C), 117.7, 114.0 (2C), 113.7 (2C), 80.5, 71.3, 55.8, 55.2, 28.1; GC-MS (EI, 70 eV): m/z = 251 (M+, 9), 212 (100); HRMS (ESI-TOF) m/z: [(M + H)+] cald for C17H18NO+: 252.1383; found, 252.1423.
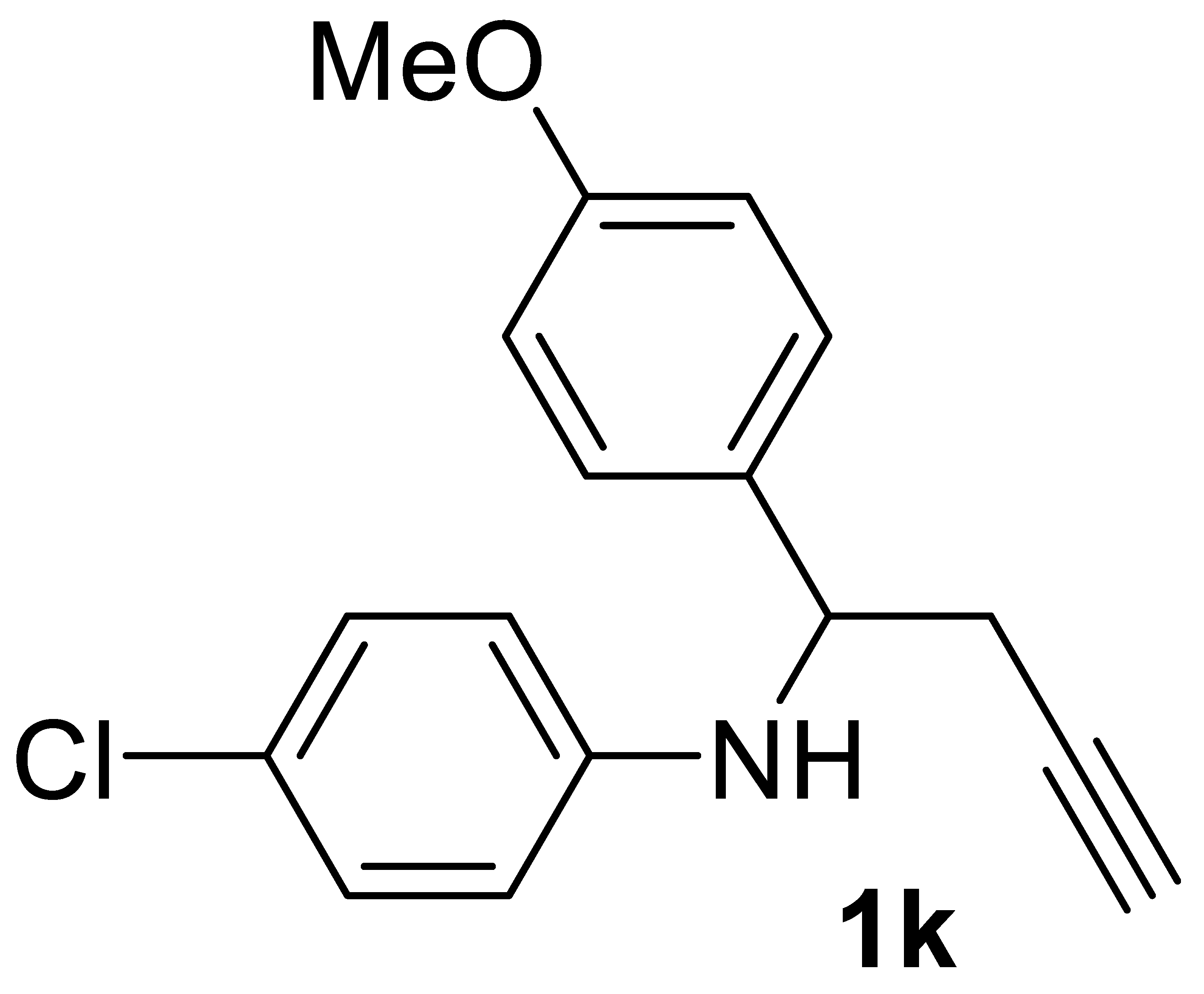
3.3.4. 4-Chloro-N-(1-(4-Methoxyphenyl)but-3-yn-1-yl)aniline 1k
Yield: 1.69 g, 59% based on starting 4-chloro-N-(4-methoxybenzylidene)aniline. Yellow oil. IR (film): ν = 3410 (m), 3294 (m), 2114 (vw), 1605 (s), 1504 (s), 1250 (s), 1173 (m), 1088 (w), 1034 (m), 818 (m) cm−1; 1H NMR (CDCl3, 300 MHz): δ = 7.32–7.20 (m, 2H, aromatic), 7.07–6.97 (m, 2H, aromatic), 6.90–6.80 (m, 2H, aromatic), 6.50–6.40 (m, 2H, aromatic), 4.50–4.30 (m, 2H, CHPh + NH), 3.76 (s, 3H, OMe), 2.80–2.50 (m, 2H, CH2C≡), 2.07 (t, J = 2.5, 1H, ≡CH); 13C{1H} NMR (CDCl3, 75 MHz): δ = 159.0, 145.6, 133.5, 128.9 (2C), 127.4 (2C), 122.3, 114.8 (2C), 114.1 (2C), 80.2, 71.5, 55.9, 55.2, 28.1; GC-MS (EI, 70 eV): m/z = 285 (M+, 9), 245 (100); HRMS (ESI-TOF) m/z: [(M + H)+] cald for C17H17ClNO+: 286,0993; found, 286.0999.
3.4. General Procedure for the Palladium-Catalyzed Oxidative Carbonylation of N-Substituted 3-yn-1-Amines 1a–n in MeOH
A 40 mL stainless steel autoclave was charged in the presence of air with PdI2 (9.0 mg, 2.5 × 10−2 mmol), KI (41.5 mg, 2.5 × 10−1 mmol) and a solution of 1 (0.5 mmol; 1a, 72.6 mg; 1b, 89.8 mg; 1c, 93.6 mg; 1d, 100.7 mg; 1e, 119.1 mg; 1f, 79.6 mg; 1g, 86.6 mg; 1h, 110.7 mg; 1i, 150.1 mg; 1j, 125.7 mg; 1k, 142.9 mg; 1l, 79.6 mg; 1m, 86.6 mg; 1n, 62.6 mg) in MeOH (5 mL for 1a–k and 12.5 mL for 1l–n). The autoclave was sealed and, while the mixture was stirred, the autoclave was pressurized with CO (32 atm) and air (up to 40 atm). After being stirred at 100 °C for the required time (2h for 1a–m or 5h for 1n) the autoclave was cooled, degassed and opened. The solvent was evaporated and the products were purified by column chromatography on silica gel using as eluent hexane/AcOEt 7:3 for 2a, 2b, 2e, 2f, 2l and 2m; hexane/AcOEt 8:2 for 2c, 2d, 2g, 2h, 2i, 2j and 2k; only hexane to hexane/AcOEt 6:4 for 2n.
3.4.1. (Z)-Methyl 2-(2-Oxo-1-Phenylpyrrolidin-3-ylidene)acetate 2a
Yield: 98.3 mg, starting from 72.6 mg of 1a (85%) (Table 2, entry 1). White solid, mp 102-103 °C. IR (KBr): ν = 1733 (s), 1696 (s), 1597 (m), 1496 (m), 1398 (m), 1307 (m), 1238 (m), 762 (m) cm−1; 1H NMR (CDCl3, 300 MHz): δ = 7.72–7.62 (m, 2H, aromatic), 7.42–7.31 (m, 2H, aromatic), 7.21–7.12 (m, 1H, aromatic), 6.17 (t, J = 2.4, 1H, =CH), 3.87 (t, J = 6.6, 2H, NCH2), 3.85 (s, 3H, CO2Me), 2.92 (td, J = 6.6, 2.4, 2H, NCH2CH2); 13C{1H} NMR (CDCl3, 75 MHz): δ = 167.3, 164.4, 139.0, 138.6, 128.9 (2C), 125.2, 122.7, 119.8 (2C), 52.3, 45.3, 24.2; HRMS (ESI-TOF) m/z: [(M + H)+] cald for C13H14NO3+: 232.0968; found, 232.0978.
3.4.2. (Z)-Methyl 2-(1-(4-Chlorophenyl)-2-Oxopyrrolidin-3-Ylidene)acetate 2b
Yield: 102.3 mg, starting from 89.8 mg of 1b (77%) (Table 2, entry 8). White solid, mp 95–96 °C. IR (KBr): ν = 1733 (s), 1696 (s), 1496 (m), 1394 (m), 1241 (m), 1085 (w), 1010 (w), 892 (w), 828 (m) cm−1; 1H NMR (CDCl3, 300 MHz): δ = 7.68–7.60 (m, 2H, aromatic), 7.36–7.28 (m, 2H, aromatic), 6.19 (s, br, 1H, =CH), 3.91–3.78 (m, 2 H, NCH2), 3.85 (s, 3H, CO2Me), 2.93 (td, J = 7.1, 2.3, 2H, NCH2CH2); 13C{1H} NMR (CDCl3, 75 MHz): δ = 167.1, 164.4, 138.2, 137.6, 130.3, 128.9 (2C), 123.1, 120.8 (2C), 52.3, 45.2, 24.0; HRMS (ESI-TOF) m/z: [(M + H)+] cald for C13H13ClNO3+: 266.0578; found, 266.0550.
3.4.3. (Z)-Methyl 2-(1-(4-Isopropylphenyl)-2-Oxopyrrolidin-3-Ylidene)acetate 2c
Yield: 101.1 mg, starting from 93.6 mg of 1c (74%) (Table 2, entry 10). Yellow solid, mp 94–95 °C. IR (KBr): ν = 1734 (s), 1696 (s), 1516 (m), 1398 (m), 1307 (w), 1239 (m), 1017 (m), 835 (m) cm−1; 1H NMR (CDCl3, 300 MHz): δ = 7.62–7.53 (m, 2H, aromatic), 7.26–7.18 (m, 2H, n aromatic), 6.16 (t, J = 2.3, 1H, =CH), 3.86 (t, J = 6.4, 2 H, NCH2), 3.85 (s, 3H, CO2Me), 2.97–2.83 (m, 3H, NCH2CH2 + CHMe2), 1.22 (d, J = 7.0, 6H, CH(CH3)2); 13C{1H} NMR (CDCl3, 75 MHz): δ = 167.4, 164.3, 146.0, 138.7, 136.7, 126.8 (2C), 122.4, 119.9 (2C), 52.3, 45.4, 33.6, 24.1, 23.9 (2C); HRMS (ESI-TOF) m/z: [(M + H)+] cald for C17H20NO3+: 274.1438; found, 274.1434.
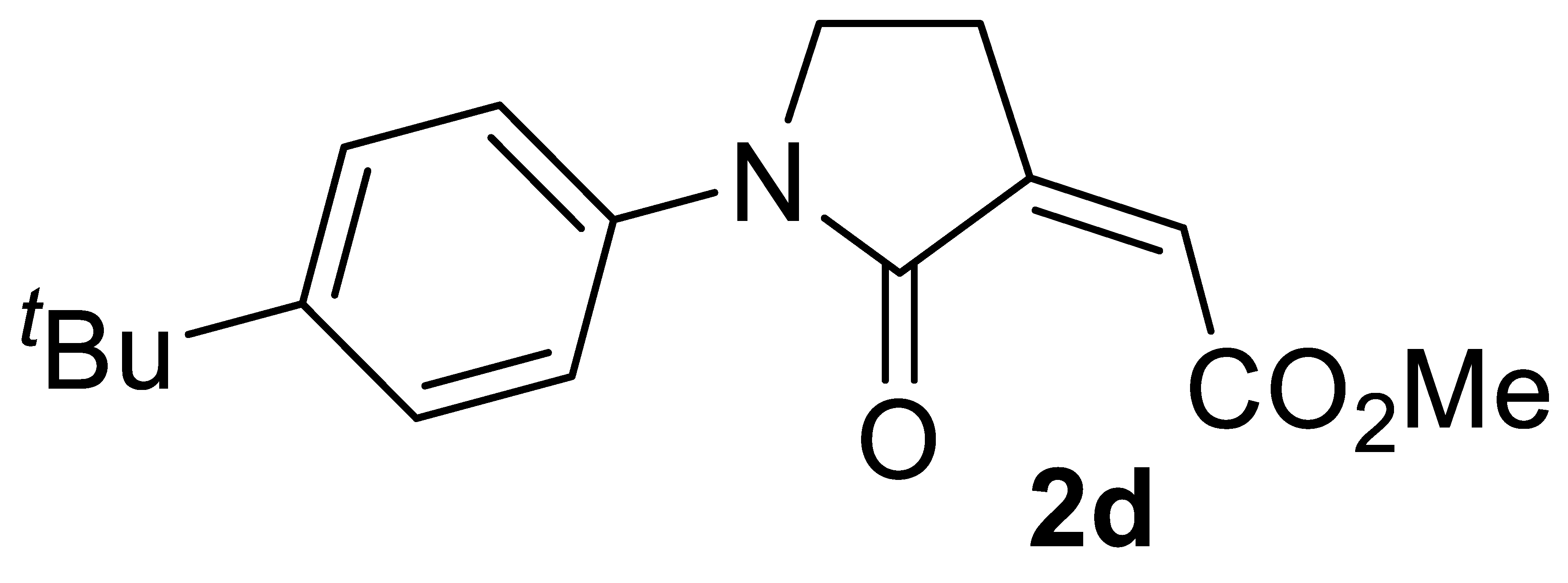
3.4.4. (Z)-Methyl 2-(1-(4-(tert-Butyl)phenyl)-2-Oxopyrrolidin-3-Ylidene)acetate 2d
Yield: 100.6 mg, starting from 100.7 mg of 1d (70%) (Table 2, entry 12). Yellow solid, mp 96–97 °C. IR (KBr): ν = 1736 (s), 1690 (s), 1520 (m), 1396 (m), 1234 (m), 1080 (w), 1049 (m), 895 (m), 833 (s) cm−1; 1H NMR (CDCl3, 300 MHz): δ = 7.62–7.50 (m, 2H, aromatic), 7.43–7.30 (m, 2H, aromatic), 6.15 (s, br, 1H, =CH), 3.92–3.75 (m, 2H, NCH2), 3.84 (s, 3H, CO2Me), 2.95–2.82 (m, 2H, NCH2CH2), 1.30 (s, 9H, t-Bu); 13C{1H} NMR (CDCl3, 75 MHz): δ = 167.3, 164.3, 148.2, 138.8, 136.4, 125.7 (2C), 122.4, 119.6 (2C), 52.3, 45.3, 34.4, 31.3 (3C), 24.2; HRMS (ESI-TOF) m/z: [(M + H)+] cald for C17H22NO3+: 288.1594; found, 288.1589.
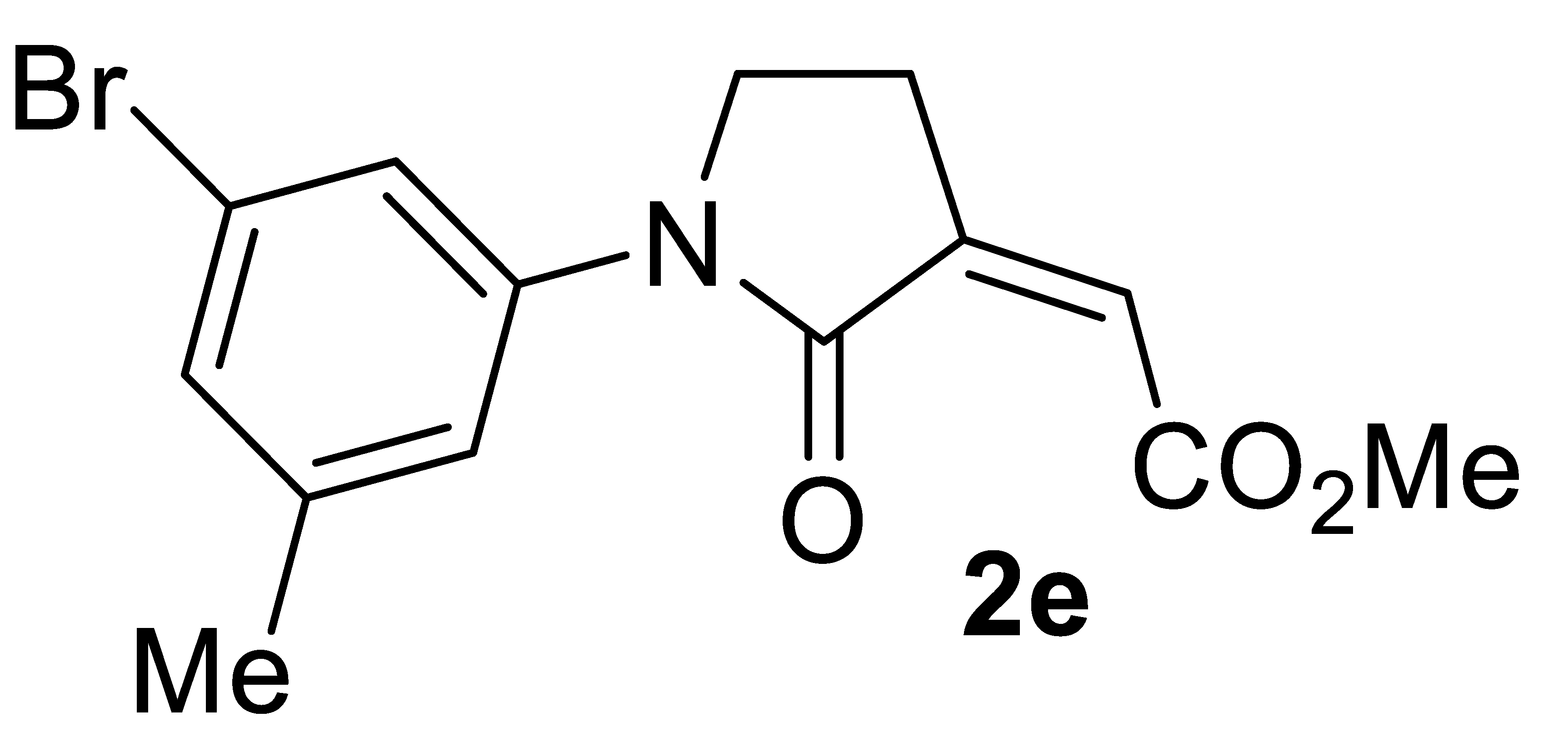
3.4.5. (Z)-Methyl 2-(1-(3-Bromo-5-Methylphenyl)-2-Oxopyrrolidin-3-Ylidene)acetate 2e
Yield: 126.4 mg, starting from 119.1 mg of 1e (78%) (Table 2, entry 13). Yellow solid, mp 89–90 °C. IR (KBr): ν = 1733 (s), 1699 (s), 1496 (m), 1411 (w), 1016 (w) cm−1; 1H NMR (CDCl3, 300 MHz): δ = 7.47 (s, 1 H, aromatic), 7.22–7.11 (m, 2 H, aromatic), 6.20 (t, J = 2.2, 1H, =CH), 3.87–3.75 (m, 2H, NCH2), 3.81 (s, 3H, CO2Me), 3.00 (td, J = 6.5, 2.4, 2H, NCH2CH2), 2.34 (s, 3H, CH3); 13C{1H} NMR (CDCl3, 75 MHz): δ = 167.2, 165.0, 140.3, 137.7, 134.6, 134.0, 129.3, 129.0, 122.8, 121.4, 52.3, 46.8, 25.1, 20.8; HRMS (ESI-TOF) m/z: [(M + H)+] cald for C14H15BrNO3+: 324.0230; found, 324.0212.
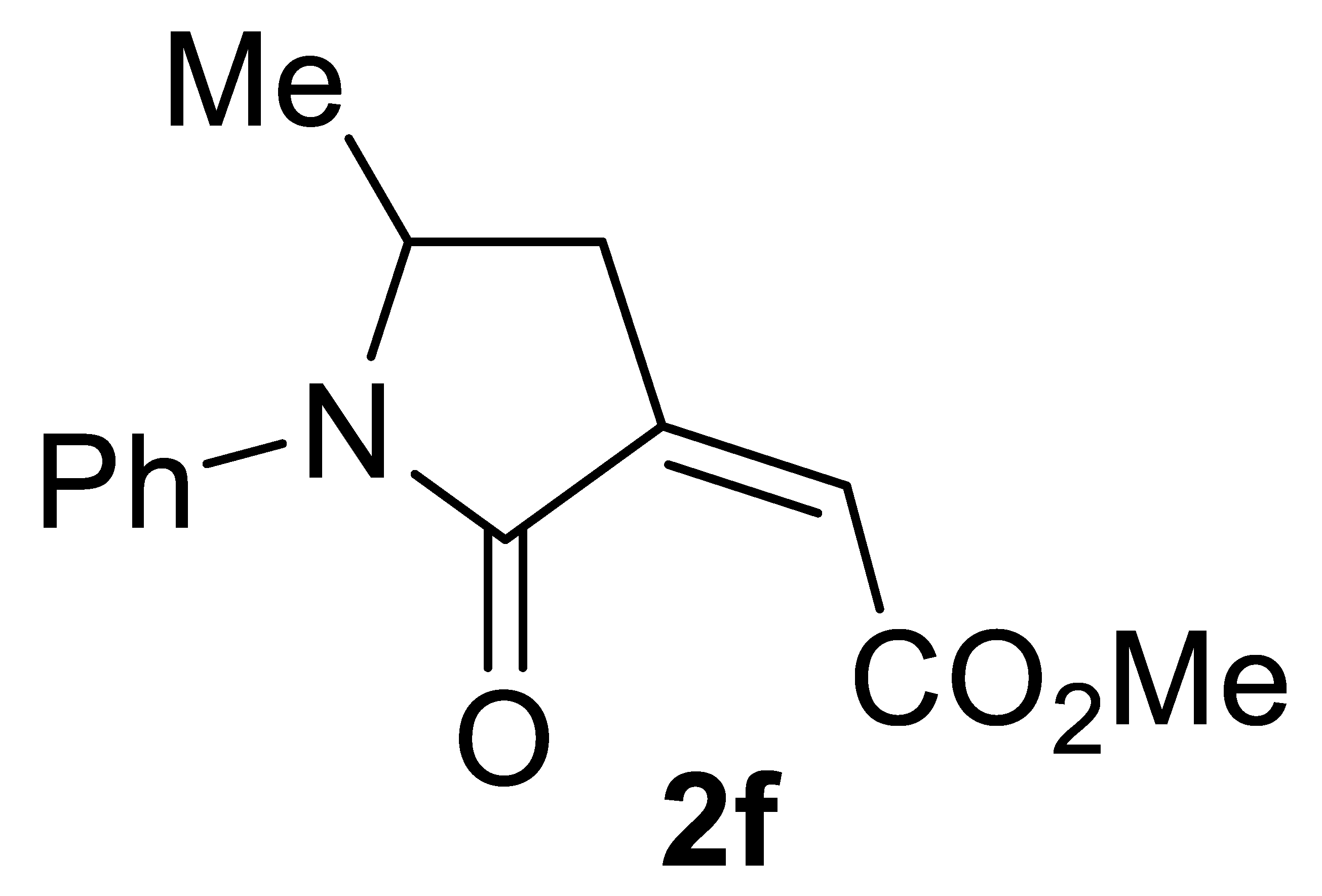
3.4.6. (Z)-Methyl 2-(5-Methyl-2-Oxo-1-Phenylpyrrolidin-3-Ylidene)acetate 2f
Yield: 94.4 mg, starting from 79.6 mg of 1f (77%) (Table 2, entry 15). Yellow solid, mp 36–37 °C. IR (KBr): ν = 1733 (s), 1705 (s), 1596 (m), 1496 (m), 1388 (s), 1239 (m), 1015 (w) cm−1; 1H NMR (CDCl3, 300 MHz): δ = 7.47–7.33 (m, 4H, aromatic), 7.25–7.16 (m, 1H, aromatic), 6.16 (t, J = 2.4, 1H, =CH), 4.43–4.28 (m, 1H, NCHMe), 3.82 (s, 3H, CO2Me), 3.14 (ddd, J = 17.3, 7.7, 2.4, 1H, NCHMeCHH), 2.50 (ddd, J = 17.5, 4.3, 2.4, 1H, NCHMeCHH), 1.23 (d, J = 6.2, 2H, CH3); 13C{1H} NMR (CDCl3, 75 MHz): δ = 167.4, 164.3, 138.1, 136.9, 129.0 (2C), 126.1, 123.5 (2C), 122.7, 52.4, 52.3, 33.1, 20.8; HRMS (ESI-TOF) m/z: [(M + H)+] cald for C14H16NO3+: 246.1125; found, 246.1130.
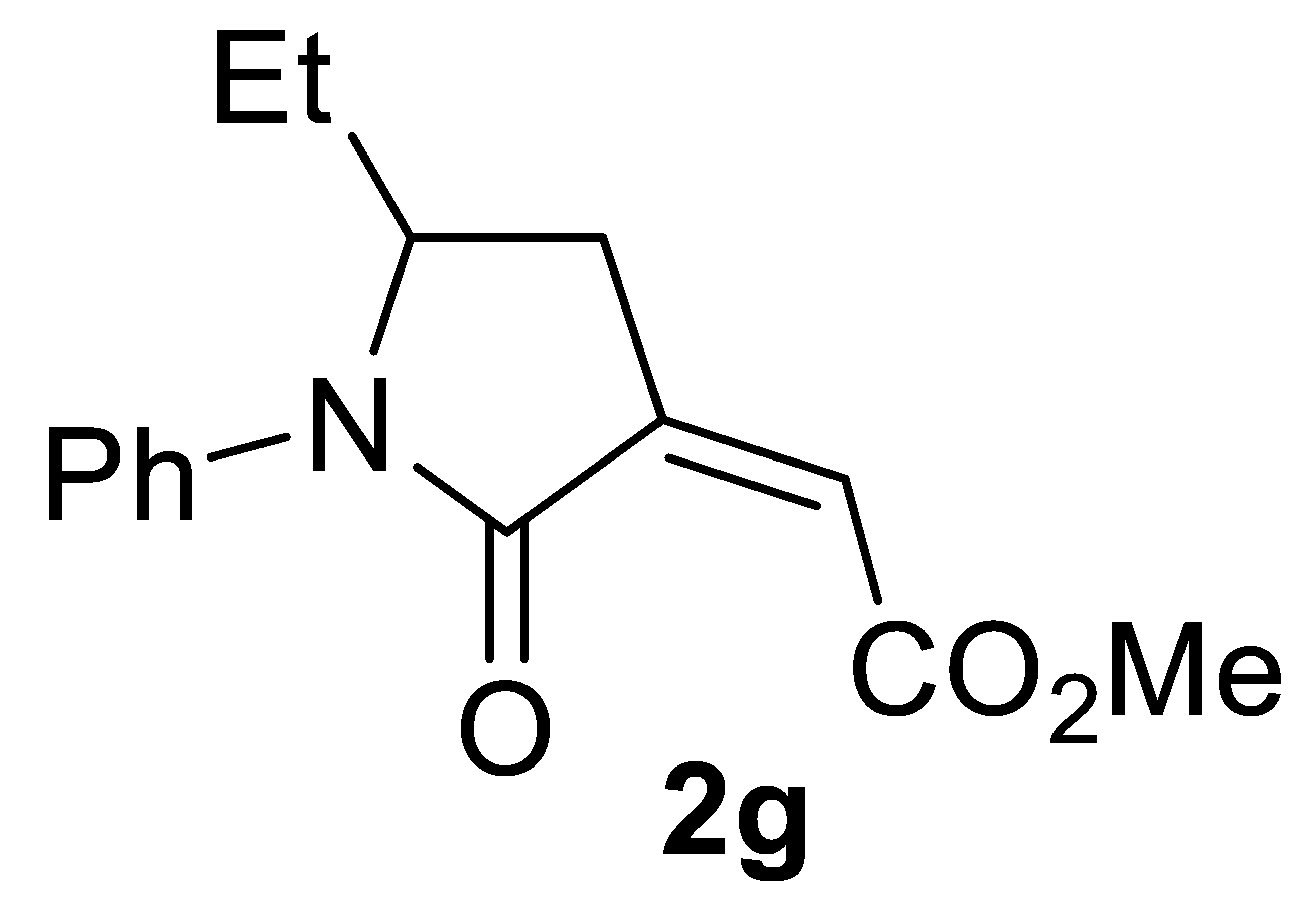
3.4.7. (Z)-Methyl 2-(5-Ethyl-2-Oxo-1-Phenylpyrrolidin-3-Ylidene)acetate 2g
Yield: 101.1 mg, starting from 86.6 mg of 1g (78%) (Table 2, entry 16). Yellow solid, mp 39–40 °C. IR (KBr): ν = 1733 (s), 1671 (s), 1496 (m), 1394 (m), 1240 (m), 1016 (w) cm−1; 1H NMR (CDCl3, 300 MHz): δ = 7.47–7.33 (m, 4H, aromatic), 7.25–7.16 (m, 1H, aromatic), 6.17 (t, J = 2.3, 1H, =CH), 4.31–4.19 (m, 1H, NCHEt), 3.82 (s, 3H, CO2Me), 3.05 (ddd, J = 17.6, 7.9, 2.3, 1H, NCH(Et)CHH), 2.66–2.53 (m, 1H, NCH(Et)CHH), 1.81–1.63 (m, 1H, CHHCH3), 1.52–1.34 (m, 1H, CHHCH3), 0.83 (t, J = 7.4, 3H, CH2CH3); 13C{1H} NMR (CDCl3, 75 MHz): δ = 167.4, 164.5, 138.2, 137.0, 129.0 (2C), 126.2, 123.5 (2C), 122.6, 57.3, 52.3, 29.9, 26.2, 8.2; HRMS (ESI-TOF) m/z: [(M + H)+] cald for C15H18NO3+: 260.1281; found, 260.1309.
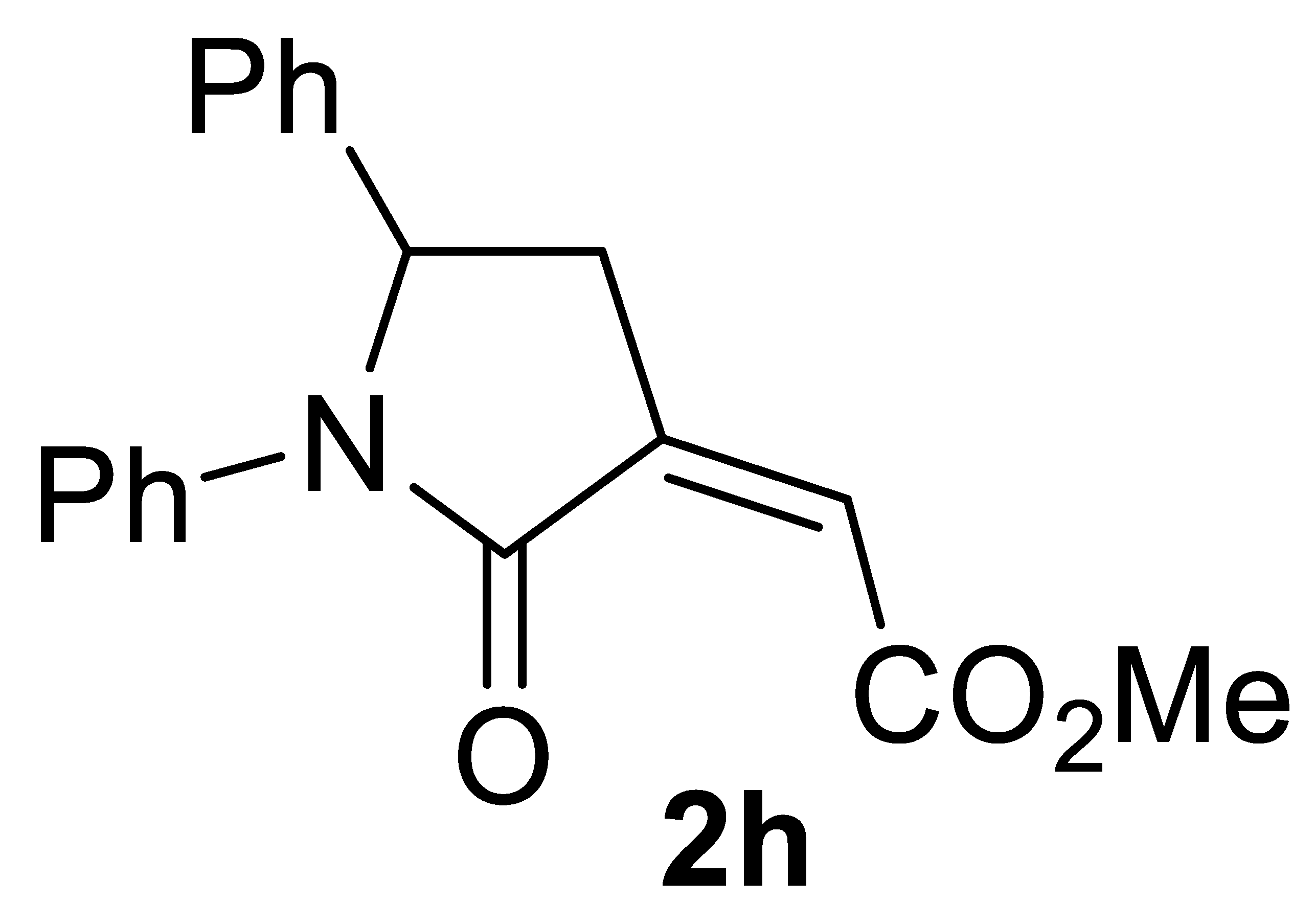
3.4.8. (Z)-Methyl 2-(2-Oxo-1,5-Diphenylpyrrolidin-3-Ylidene)acetate 2h
Yield: 126.0 mg, starting from 110.7 mg of 1h (82%) (Table 2, entry 17). White solid, mp 157–158 °C. IR (KBr): ν = 1732 (s), 1697 (s), 1597 (w), 1492 (m), 1385 (m), 1242 (m), 1011 (m) cm−1; 1H NMR (CDCl3, 300 MHz): δ = 7.48–7.40 (m, 2H, aromatic), 7.33–7.16 (m, 7H, aromatic), 7.10–7.02 (m, 1H, aromatic), 6.17 (t, J = 2.3, 1H, =CH), 5.27 (dd, J = 8.3, 3.6, 1H, CHPh), 3.86 (s, 3H, CO2Me), 3.39 (ddd, J = 17.2, 8.3, 2.3, 1H, NCHPhCHH), 2.73 (ddd, J = 17.2, 3.6, 2.3, 1H, NCH(Ph)CHH); 13C{1H} NMR (CDCl3, 75 MHz): δ = 167.1, 165.0, 140.8, 137.6, 137.5, 129.1 (2C), 128.7 (2C), 128.1, 125.9 (2C), 125.6, 123.2, 122.3 (2C), 60.7, 53.4, 35.6; HRMS (ESI-TOF) m/z: [(M + H)+] cald for C19H18NO3+: 308.1281; found, 308.1286.
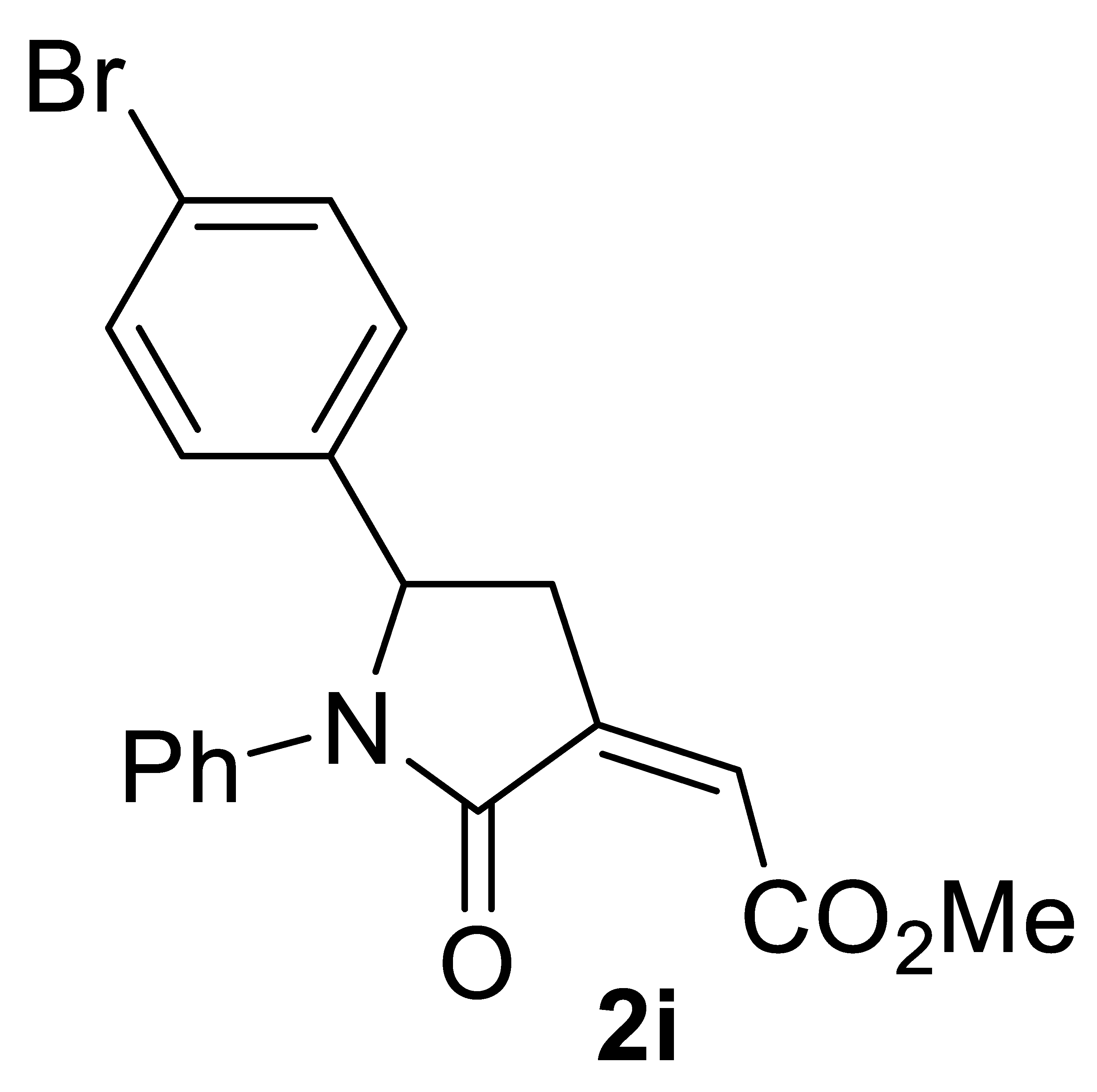
3.4.9. (Z)-Methyl 2-(5-(4-Bromophenyl)-2-Oxo-1-Phenylpyrrolidin-3-Ylidene)acetate 2i
Yield: 135.2 mg, starting from 150.1 mg of 1i (70%) (Table 2, entry 18). White solid, mp 151–152 °C. IR (KBr): ν = 1736 (s), 1697 (s), 1612 (w), 1489 (m), 1389 (m), 1250 (s), 1180 (w), 1026 (w), 833 (m) cm−1; 1H NMR (CDCl3, 300 MHz): δ = 7.45–7.35 (m, 4H, aromatic), 7.28–7.19 (m, 2H, aromatic), 7.13–7.04 (m, 3H, aromatic), 6.19 (t, J = 2.3, 1H, =CH), 5.25 (dd, J = 8.3, 3.8, 1H, CHAr), 3.86 (s, 3H, CO2Me), 3.39 (ddd, J = 17.2, 8.3, 2.3, 1H, CHHC=), 2.69 (ddd, J = 17.2, 3.8, 2.3, 1H, CHHC=); 13C{1H} NMR (CDCl3, 75 MHz): δ = 167.0, 164.9, 139.8, 137.3, 137.0, 132.3 (2C), 128.8 (2C), 127.7 (2C), 125.8, 123.5, 122.3 (2C), 122.0, 60.1, 52.4, 35.4; HRMS (ESI-TOF) m/z: [(M + H)+] cald for C19H17BrNO3+: 386.0386; found, 386.0384.
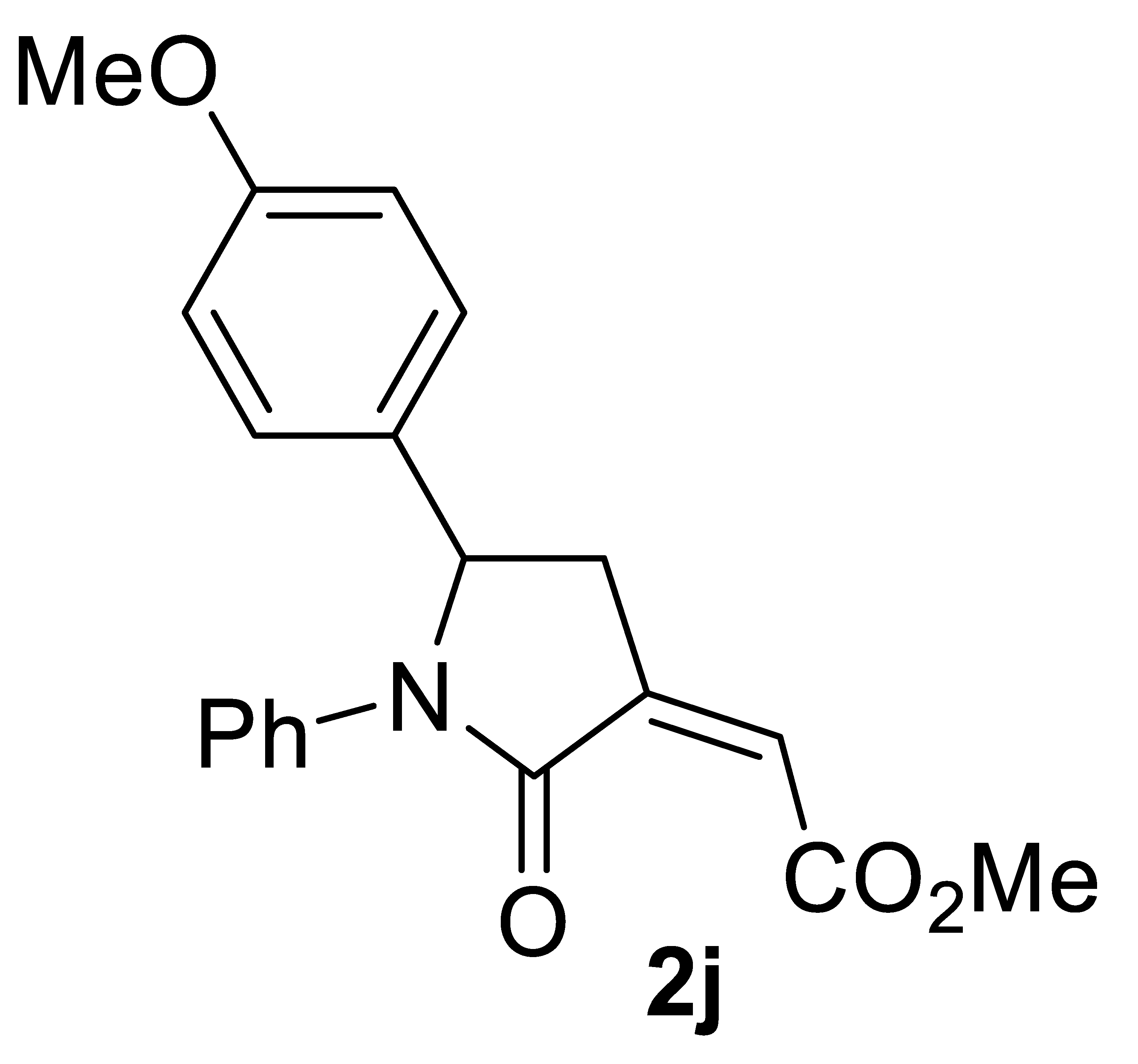
3.4.10. (Z)-Methyl 2-(5-(4-Methoxyphenyl)-2-Oxo-1-Phenylpyrrolidin-3-Ylidene)acetate 2j
Yield: 119.8 mg, starting from 125.7 mg of 1j (71%) (Table 2, entry 19). White solid, mp 95–96 °C. IR (KBr): ν = 1721 (s), 1697 (s), 1597 (m), 1512 (m), 1427 (w), 1373 (m), 1103 (m), 1034 (m), 1003 (m), 895 (m), 841 (m), 772 (m) cm−1; 1H NMR (CDCl3, 300 MHz): δ = 7.50–7.34 (m, 2H, aromatic), 7.31–7.16 (m, 2 H, aromatic), 7.16–6.99 (m, 3H, aromatic), 6.88–6.71 (m, 2H, aromatic), 6.17 (s, br, 1H, =CH), 5.22 (dd, J = 7.8, 3.6, 1H, CHAr), 3.86 (s, 3H, CO2Me), 3.73 (s, 3H, OMe), 3.44–3.25 (m, 1H, CHHC=), 2.81–2.60 (m, 1H, CHHC=); 13C{1H} NMR (CDCl3, 75 MHz): δ = 167.2, 164.9, 159.3, 137.7, 137.6, 132.6, 128.7 (2C), 127.2 (2C), 125.6, 123.0, 122.6 (2C), 114.4 (2C), 60.3, 55.2, 52.3, 35.8; HRMS (ESI-TOF) m/z: [(M + H)+] cald for C20H20NO4+: 338.1387; found, 338.1381.
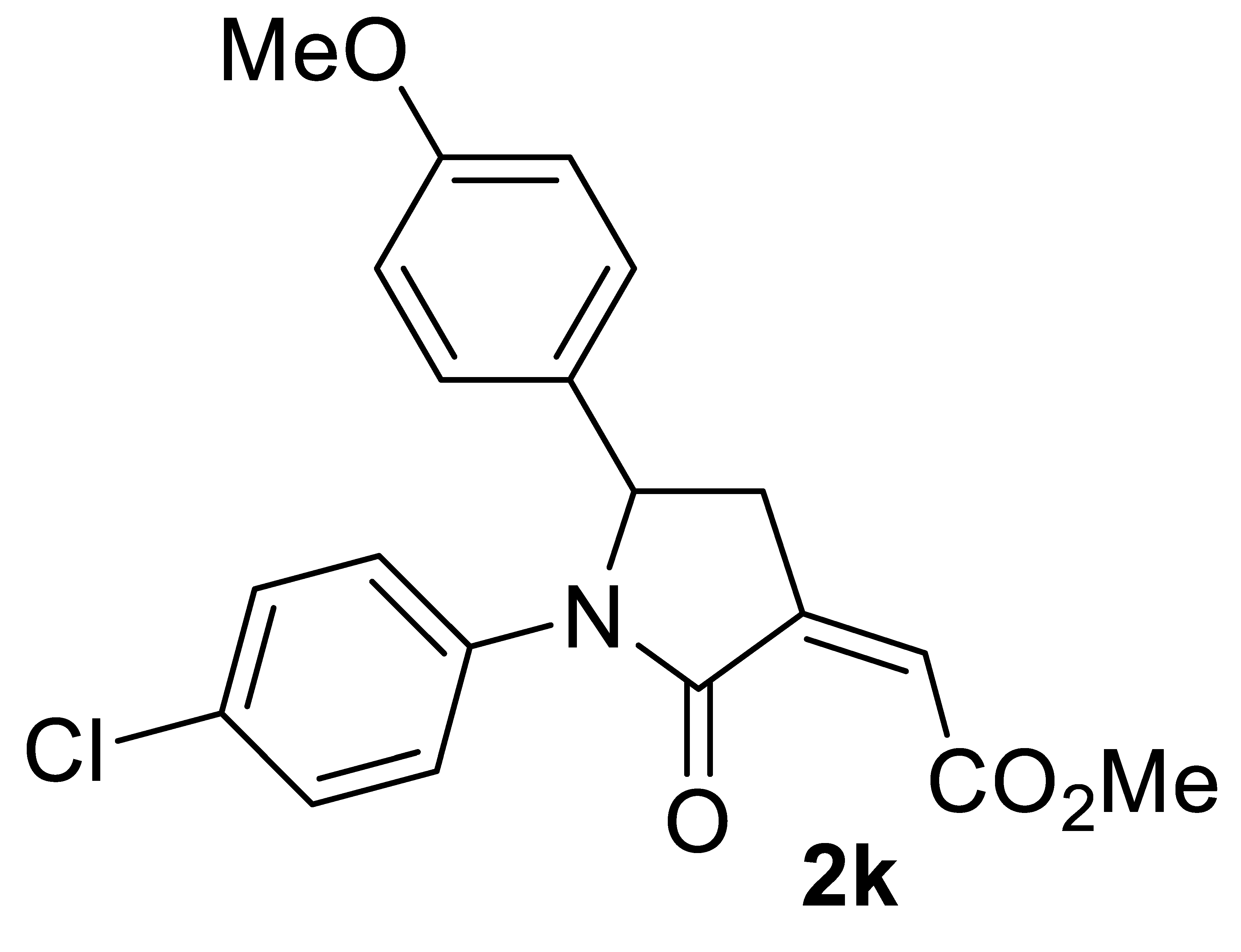
3.4.11. (Z)-Methyl 2-(1-(4-Chlorophenyl)-5-(4-Methoxyphenyl)-2-Oxopyrrolidin-3-Ylidene)acetate 2k
Yield: 130.1 mg, starting from 142.9 mg of 1k (70%) (Table 2, entry 20). Yellow solid, mp 119-120 °C. IR (KBr): ν = 1736 (s), 1695 (s), 1613 (w), 1511 (m), 1690 (m), 1389 (m), 1249 (s), 1180 (m), 1011 (m), 895 (w), 833 (m) cm−1; 1H NMR (CDCl3, 300 MHz): δ = 7.43–7.34 (m, 2H, aromatic), 7.25–7.15 (m, 2H, aromatic), 7.15–7.05 (m, 2H, aromatic), 6.87–6.76 (m, 2 H, aromatic), 6.19 (s, br, 1H, =CH), 5.19 (dd, J = 8.0, 3.6, 1H, CHAr), 3.87 (s, 3H, CO2Me), 3.75 (s, 3H, OMe), 3.37 (ddd, J = 17.1, 8.0, 2.3, 1H, CHHC=), 2.73 (ddd, J = 17.1, 3.6, 2.3, 1H, CHHC=); 13C{1H} NMR (CDCl3, 75 MHz): δ = 167.0, 164.9, 159.4, 137.3, 136.1, 132.2, 130.8, 128.8 (2C), 127.2 (2C), 123.6 (2C), 123.5, 114.6 (2C), 60.3, 55.3, 52.4, 35.7; HRMS (ESI-TOF) m/z: [(M + H)+] cald for C20H19ClNO4+: 372.0997; found, 372.0995.
3.4.12. (Z)-Methyl 2-(1-Benzyl-2-Oxopyrrolidin-3-Ylidene)acetate 2l
Yield: 79.7 mg, starting from 79.6 mg of 1l (65%) (Table 2, entry 24). Yellow oil. IR (film): ν = 1736 (s), 1686 (s), 1497 (w), 1438 (m), 1338 (w), 1245 (m), 702 (m) cm−1; 1H NMR (CDCl3, 300 MHz): δ = 7.37–7.21 (m, 5H, Ph), 6.11 (t, J = 2.4, 1H, =CH), 4.51 (s, 2H, CH2Ph), 3.86 (s, 3H, CO2Me), 3.29 (t, J = 6.5, 2H, NCH2), 2.76 (td, J = 6.5, 2.4, 2H, NCH2CH2); 13C{1H} NMR (CDCl3, 75 MHz): δ = 167.4, 165.2, 138.4, 135.7, 128.8 (2C), 128.4 (2C), 127.8, 121.8, 52.2, 47.2, 43.5, 24.2; HRMS (ESI-TOF) m/z: [(M + H)+] cald for C14H16NO3+: 246.1130; found, 246.1128.
3.4.13. (Z)-Methyl 2-(2-Oxo-1-(1-Phenylethyl)pyrrolidin-3-Ylidene)acetate 2m
Yield: 97.2 mg, starting from 86.6 mg of 1m (75%) (Table 2, entry 26). Yellow oil. IR (film): ν = 1733 (s), 1690 (s), 1423 (m), 1331 (w), 1234 (m), 1017 (w) cm−1; 1H NMR (CDCl3, 300 MHz): δ = 7.43–7.15 (m, 5H, Ph), 6.08 (dist t, J = 2.3, 1H, =CH), 5.56 (q, J = 7.1, 1H, CHMe2), 3.86 (s, 3H, CO2Me), 3.42–3.30 (m, 1H, NCHHCH2), 3.08–2.94 (m, 1H, NCHHCH2), 2.83–2.60 (2H, NCH2CH2), 1.55 (d, J = 7.1, 3H, CH3); 13C{1H} NMR (CDCl3, 75 MHz): δ = 167.4, 164.8, 139.4, 138.9, 128.6 (2C), 127.7, 127.3 (2C), 121.5, 52.3, 49.7, 39.3, 24.3, 16.0; HRMS (ESI-TOF) m/z: [(M + H)+] cald for C15H18NO3+: 260.1281; found, 260.1290.
3.4.14. (Z)-Methyl 2-(1-(tert-Butyl)-2-Oxopyrrolidin-3-Ylidene)acetate 2n
Yield: 49.7 mg, starting from 62.6 mg of 1n (47%) (Table 2, entry 27). Yellow solid, mp 85–86 °C. IR (KBr): ν = 1721 (s), 1667 (s), 1412 (m), 1335 (m), 1211 (m), 1011 (s), 895 (m), 833 (m) cm−1; 1H NMR (CDCl3, 300 MHz): δ = 5.97 (t, J = 2.2, 1H, =CH), 3.84 (s, 3H, CO2Me), 3.50 (t, J = 6.4, 2H, NCH2), 2.72 (td, J = 6.4, 2.2, 2H, NCH2CH2), 1.43 (s, 9H, t-Bu); 13C{1H} NMR (CDCl3, 75 MHz): δ = 167.8, 165.6, 140.4, 120.2, 54.8, 52.2, 42.5, 27.4 (3C), 24.3; HRMS (ESI-TOF) m/z: [(M + H)+] cald for C11H18NO3+: 212.1281; found, 212.1262.
3.5. General Procedure for the Palladium-Catalyzed Oxidative Carbonylation of N-Substituted 3-yn-1-Amines 1a and 1f in Different Alcoholic Solvents
A 40 mL stainless steel autoclave was charged in the presence of air with PdI2 (9.0 mg, 2.5 × 10−2 mmol), KI (41.5 mg, 2.5 × 10−1 mmol) and a solution of 1 (0.5 mmol; 1a, 72.6 mg; 1l, 79.6 mg) in EtOH or isoPrOH (5 mL) or tert-BuOH (12.5 mL only for 1a). The autoclave was sealed and, while the mixture was stirred, the autoclave was pressurized with CO (32 atm) and air (up to 40 atm). After being stirred at 100 °C for the required time (2 h for 1a and 5h for 1l) the autoclave was cooled, degassed and opened. The solvent was evaporated and the products were purified by column chromatography on silica gel using as eluent hexane/AcOEt 7:3 for 2a′, 2a″ and 2a‴; hexane/AcOEt 6:4 for 2l′ and 2l″.
3.5.1. (Z)-Ethyl 2-(2-Oxo-1-Phenylpyrrolidin-3-Ylidene)acetate 2a′
Yield: 100.6 mg, starting from 72.5 mg of 1a (82%) (Table 2, entry 4). White solid, mp 61–62 °C. IR (KBr): ν = 1728 (s), 1690 (s), 1598 (w), 1496 (m), 1398 (m), 1306 (m), 1234 (m), 1095 (w), 1030 (m), 761 (m) cm−1; 1H NMR (CDCl3, 300 MHz): δ = 7.70–7.63 (m, 2H, aromatic), 7.40–7.30 (m, 2H, aromatic), 7.20–7.11 (m, 1H, aromatic), 6.16 (t, J = 2.3, 1H, =CH), 4.33 (q, J = 7.2, 2 H, CO2CH2CH3), 3.85 (t, J = 6.6, 2H, NCH2), 2.90 (td, J = 6.6, 2.2, 2H, NCH2CH2), 7.16 (t, J = 7.2, 3H, CH2CH3); 13C{1H} NMR (CDCl3, 75 MHz): δ = 166.8, 164.4, 139.1, 138.3, 128.9 (2C), 125.1, 123.1, 119.7 (2C), 61.3, 45.2, 24.1, 14.1; HRMS (ESI-TOF) m/z: [(M + H)+] cald for C14H16NO3+: 246.1125; found, 246.1138.
3.5.2. (Z)-Isopropyl 2-(2-Oxo-1-Phenylpyrrolidin-3-Ylidene)acetate 2a″
Yield: 93.4 mg, starting from 72.7 mg of 1a (72%) (Table 2, entry 5). White solid, mp 85–86 °C. IR (KBr): ν = 1723 (s), 1699 (s), 1598 (w), 1496 (m), 1398 (m), 1306 (m), 1241 (m), 1106 (m), 970 (w), 762 (m) cm−1; 1H NMR (CDCl3, 300 MHz): δ = 7.66 (dist d, J = 8.0, 2H, aromatic), 7.34 (t, J = 7.6, 2H, aromatic), 7.14 (dist d, J = 7.1, 1H), 6.14 (s, br, 1H, =CH), 5.22 (quint, J = 6.1, 1H, CHMe2), 3.82 (t, J = 6.5, 2H, NCH2), 2.92–2.80 (m, 2H, NCH2CH2), 1.34 (d, J = 6.1, 6H, CH(CH3)2); 13C{1H} NMR (CDCl3, 75 MHz): δ = 166.2, 164.4, 139.2, 137.9, 128.8 (2C), 125.0, 123.4, 119.7 (2C), 68.9, 45.2, 24.1, 21.7 (2C); HRMS (ESI-TOF) m/z: [(M + H)+] cald for C15H18NO3+: 260.1281; found, 260.1290.
3.5.3. (Z)-tert-Butyl 2-(2-Oxo-1-Phenylpyrrolidin-3-Ylidene)acetate 2a‴
Yield: 73.8 mg, starting from 72.5 mg of 1a (54%) (Table 2, entry 7). Yellow solid, mp 95–96 °C. IR (KBr): ν = 1723 (s), 1699 (s), 1598 (w), 1496 (m), 1400 (m), 1307 (m), 1251 (m), 1161 (m), 759 (w) cm−1; 1H NMR (CDCl3, 300 MHz): δ = 7.67 (dist d, J = 8.1, 2H, aromatic), 7.36 (t, J = 7.7, 2H, aromatic), 7.15 (t, J = 7.2, 1H, aromatic), 6.11 (s, br, 1H, =CH), 3.85 (t, J = 6.4, 2H, NCH2), 2.92-2.80 (m, 2H, NCH2CH2), 1.57 (s, 9H, t-Bu); 13C{1H} NMR (CDCl3, 75 MHz): δ = 165.8, 164.5, 139.3, 136.8, 128.9 (2C), 125.0, 124.4, 119.8 (2C), 82.2, 45.3, 28.1 (3C), 24.2; HRMS (ESI-TOF) m/z: [(M + H)+] cald for C16H20NO3+: 274.1438; found, 274.1444.
3.5.4. (Z)-Ethyl 2-(1-Benzyl-2-Oxopyrrolidin-3-Ylidene)acetate 2l′
Yield: 60.9 mg, starting from 79.7 mg of 1l (47%) (Table 2, entry 22). Yellow solid, mp 54–55 °C. IR (KBr): ν = 1728 (s), 1690 (s), 1668 (s), 1446 (w), 1337 (w), 1230 (m), 1030 (m) cm−1; 1H NMR (CDCl3, 300 MHz): δ = 7.38–7.18 (m, 5H, Ph), 6.11 (t, J = 2.4, 1H, =CH), 4.51 (s, 2H, CH2Ph), 4.34 (q, J = 7.1, 2H, CH2CH3), 3.29 (t, J = 6.4, 2H, NCH2), 2.76 (td, J = 6.4, 2.4, 2H, NCH2CH2), 1.36 (t, J = 7.1, 3H, CH2CH3); 13C{1H} NMR (CDCl3, 75 MHz): δ = 166.8, 165.3, 138.1, 135.8, 128.7 (2C), 128.4 (2C), 127.8, 122.2, 61.3, 47.2, 43.5, 24.3, 14.1; HRMS (ESI-TOF) m/z: [(M + H)+] cald for C15H18NO3+: 260.1281; found, 260.1278.
3.5.5. (Z)-Isopropyl 2-(1-Benzyl-2-Oxopyrrolidin-3-Ylidene)acetate 2l″
Yield: 65.6 mg, starting from 79.5 mg of 1l (48%) (Table 2, entry 23). Yellow solid, mp 51–52 °C. IR (KBr): ν = 1725 (s), 1696 (s), 1496 (w), 1446 (m), 1238 (s), 1108 (m), 970 (w) cm−1; 1H NMR (CDCl3, 300 MHz): δ = 7.37–7.20 (m, 5H, Ph), 6.12–6.06 (m, 1H, =CH), 5.22 (heptuplet, J = 6.3, 1H, CHMe2), 4.51 (s, 2H, CH2Ph), 3.27 (t, J = 6.5, 2H, NCH2), 2.74 (td, J = 6.5, 2.3, 2H, NCH2CH2), 1.36 (d, J = 6.3, 6H, CH(CH3)2); 13C{1H} NMR (CDCl3, 75 MHz): δ = 166.3, 165.2, 137.7, 135.8, 128.7 (2C), 128.4 (2C), 127.8, 122.5, 68.9, 47.2, 43.4, 24.3, 21.8 (2C); HRMS (ESI-TOF) m/z: [(M + H)+] cald for C16H20NO3+: 274.1438; found, 274.1413.
3.6. Palladium-Catalyzed Oxidative Carbonylation of N-(but-3-yn-1-yl)-4-(tert-Butyl)aniline 1d to (Z)-Methyl 2 (1-(4-(tert-Butyl)phenyl)-2-Oxopyrrolidin-3-Ylidene)acetate 2d in Larger Scale
A 300 mL stainless steel autoclave was charged in the presence of air with PdI2 (54.0 mg, 1.5 × 10−1mmol), KI (249.0 mg, 1.5 mmol) and a solution of N-(but-3-yn-1-yl)-4-(tert-butyl)aniline 1d (602.3 mg, 2.99 mmol) in MeOH (30 mL). The autoclave was sealed and, while the mixture was stirred, the autoclave was pressurized with CO (32 atm) and air (up to 40 atm). After being stirred at 100 °C for 2 h, the autoclave was cooled, degassed and opened. The solvent was evaporated and the products were purified by column chromatography on silica gel using as eluent hexane/AcOEt 8:2, to give pure (Z)-methyl 2(1-(4-(tert-butyl)phenyl)-2-oxopyrrolidin-3-ylidene)acetate 2d as a yellow solid (Yield: 620.0 mg, 72% based on starting 1d).
4. Conclusions
In conclusion, we have shown that our simple PdI2-KI catalytic system is able to catalyze the oxidative carbonylation of readily available homopropargylic amines 1 to give (2-(2-oxopyrrolidin-3-ylidene)acetates) 2 under relatively mild conditions (100 °C for 2–5 h under 40 atm of a 4:1 mixture of CO‒air). This stereoselective multicomponent catalytic approach occurs through an ordered sequence of steps starting from simple building blocks, involving nitrogen palladation of the homopropargylic amine followed by CO insertion, intramolecular syn triple bond insertion with 5-exo-dig cyclization, further CO insertion and alcoholysis. The method has been successfully applied to a variety of differently substituted alkyne substrates and different alcohols (including MeOH, primary, secondary, and tertiary alcohols) to give the new α,β-unsaturated γ-lactam derivatives in 47–85% yields.
The biological evaluation of some of the novel α,β-unsaturated γ-lactams synthesized in this work, aimed at assessing their cytotoxicity toward tumor cells and their probable biomolecular targets, is currently underway in our laboratories and the results will be reported elsewhere.
Supplementary Materials
The following are available online at https://www.mdpi.com/2073-4344/11/2/227/s1: X-ray data for compounds 2a and 2b, Figure S1 (Molecular structure of methyl (Z)-2-(2-oxo-1-phenylpyrrolidin-3-ylidene)acetate 2a) and Figure S2 (Molecular structure of methyl (Z)-2-(1-(4-chlorophenyl)-2-oxopyrrolidin-3-ylidene)acetate 2b); Copies of HRMS, 1H NMR, and 13C{1H} NMR spectra for all substrates and products.
Author Contributions
Conceptualization, B.G., R.M., and I.Z.; methodology, R.M., I.Z., M.B., C.D.A., L.F., A.F., N.D.C., A.R.C., B.G.; validation, R.M., I.Z., M.B., C.D.A., L.F., A.F., N.D.C., A.R.C., B.G.; investigation, R.M., I.Z., M.B., C.D.A., L.F., A.F., N.D.C., A.R.C., B.G.; writing, B.G.; supervision, B.G.; funding acquisition, B.G. All authors have read and agreed to the published version of the manuscript.
Funding
Financial support by MIUR PRIN 2017YJMPZN project (Mussel-inspired functional biopolymers for underwater adhesion, surface/interface derivatization and nanostructure/composite self-assembly-MUSSEL) to B.G. is acknowledged.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
We thank Antonio Palumbo Piccionello (University of Palermo, Italy) for HRMS measurements.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Das, D.; Bhanage, B. Double carbonylation reactions: Overview and recent advances. Adv. Synth. Catal. 2020, 362, 3022–3058. [Google Scholar] [CrossRef]
- Peng, J.-B. Recent advances in carbonylative difunctionalization of Alkenes. Adv. Synth. Catal. 2020, 362, 3059–3080. [Google Scholar] [CrossRef]
- Zhang, S.; Neumann, H.; Beller, M. Synthesis of α,β-unsaturated carbonyl compounds by carbonylation reactions. Chem. Soc. Rev. 2020, 49, 3187–3210. [Google Scholar] [CrossRef] [PubMed]
- Yin, Z.; Xu, J.; Wu, X.-F. No making without breaking: Nitrogen-centered carbonylation reactions. ACS Catal. 2020, 10, 6510–6531. [Google Scholar] [CrossRef]
- Peng, J.B.; Geng, H.-Q.; Wu, X.-F. The chemistry of CO: Carbonylation. Chem 2019, 5, 526–552. [Google Scholar] [CrossRef]
- Reimert, R.; Marschner, F.; Renner, H.-J.; Boll, W.; Supp, E.; Brejc, M.; Liebner, W.; Schaub, G. Gas production, 2. In Ullmann’s Encyclopedia of Industrial Chemistry; Baltes, H., Göpel, W., Hesse, J., Eds.; Wiley-VCH: Weinheim, Germany, 2011; pp. 423–479. [Google Scholar]
- Mancuso, R.; Della Ca’, N.; Veltri, L.; Ziccarelli, I.; Gabriele, B. PdI2-based catalysis for carbonylation reactions: A personal account. Catalysts 2019, 9, 610. [Google Scholar] [CrossRef]
- Mancuso, R.; Strangis, R.; Ziccarelli, I.; Della Ca’, N.; Gabriele, B. Palladium catalysis with sulfurated substrates under aerobic conditions: A direct oxidative carbonylation approach to thiophene-3-carboxylic esters. J. Catal. 2021, 393, 335–343. [Google Scholar] [CrossRef]
- Lei, H.; Xin, S.; Qiu, Y.; Zhang, X. Enantioselective total synthesis of (−)-kainic acid and (+)-acromelic acid C via Rh(I)-catalyzed asymmetric enyne cycloisomerization. Chem. Commun. 2018, 54, 727–730. [Google Scholar] [CrossRef] [PubMed]
- Madec, D.; Poli, G.; Thuong, M.; Sottocornola, S.; Prestat, G.; Broggini, G. New access to kainic acid via intramolecular palladium-catalyzed allylic alkylation. Synlett 2007, 2007, 1521–1524. [Google Scholar] [CrossRef]
- Baker, R.; MacLeod, A.M.; Saunders, J.; Merchant, K. Thiadiazoles Useful in the Treatment of Senile Dementia. U.S. Patent 5405853, 11 April 1995. [Google Scholar]
- Danishefsky, S.; Berman, E.; Clizbe, L.A.; Hirama, M. A simple synthesis of L-.gamma.-carboxyglutamate and derivatives thereof. J. Am. Chem. Soc. 1979, 101, 4385–4386. [Google Scholar] [CrossRef]
- De Marco, R.; Mazzotti, G.; Greco, A.; Gentilucci, L. Heterocyclic scaffolds in the design of peptidomimetic integrin ligands: Synthetic strategies, structural aspects, and biological activity. Curr. Top. Med. Chem. 2015, 16, 343–359. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Lindvall, M.; Manning, J.R.; McEnroe, G.; Novartis, A.G. Novel Dihydroisoxazole Compounds and Their Use for the Treatment of Hepatitis B. PCT Patent Application WO2019/97479 A1, 23 May 2019. [Google Scholar]
- Ciccone, A.; Motto, C.; Abraha, I.; Cozzolino, F.; Santilli, I. Glycoprotein IIb-IIIa inhibitors for acute ischaemic stroke. Cochrane Database Syst. Rev. 2014, CD005208. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, T.I.; Kumar, A.; Kumar, K.S.; Dikshit, D.K. Platelets and atherothrombosis: Causes, targets and treatments for thrombosis. Curr. Med. Chem. 2013, 20, 2779–2797. [Google Scholar] [CrossRef] [PubMed]
- Jackson, P.A.; Widen, J.C.; Harki, D.A.; Brummond, K.M. Covalent modifiers: A chemical perspective on the reactivity of α,β-unsaturated carbonyls with thiols via hetero-Michael addition reactions. J. Med. Chem. 2017, 60, 839–885. [Google Scholar] [CrossRef]
- Yuan, B.; Jiang, Y.; Qi, Z.; Guan, X.; Wang, T.; Yan, R. External oxidant-free oxidative tandem cyclization: NaI-catalyzed thiolation for the synthesis of 3-thiosubstituted pyrroles. Adv. Synth. Catal. 2019, 361, 5112–5117. [Google Scholar] [CrossRef]
- Azuma, M.; Yoshikawa, T.; Kogure, N.; Kitajima, M.; Takayama, H. Biogenetically inspired total syntheses of Lycopodium alkaloids, (+)-flabellidine and (−)-lycodine. J. Am. Chem. Soc. 2014, 136, 11618–11621. [Google Scholar] [CrossRef]
- Breuning, M.A.; Harms, K.; Koert, U. The imidato-alkenyllithium route for the synthesis of the isoquinocycline-pyrrolopyrrole substructure. Org. Lett. 2011, 13, 1402–1405. [Google Scholar] [CrossRef] [PubMed]
- Courtois, G.; Mesnard, D.; Dugue, B.; Miginiac, L. Aminomethylation secondaire ou primaire d’organoaluminiques α-insatures a l’aide de gem-aminoethers N-trimethylsilyles: Synthese d’amines secondaires ou primaires, β-ethyleniques, β-acetyleniques ou α-alleniques. Bull. Soc. Chim. Fr. 1987, 124, 93–98. [Google Scholar]
- Tong, S.; Piemontesi, C.; Wang, Q.; Wang, M.-X.; Zhu, J. Silver-catalyzed three-component 1,1-aminoacylation of homopropargylamines: α-additions for both terminal alkynes and isocyanides. Angew. Chem. Int. Ed. 2017, 56, 7958–7962. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).