Mesoporous Composite Networks of Linked MnFe2O4 and ZnFe2O4 Nanoparticles as Efficient Photocatalysts for the Reduction of Cr(VI)
Abstract
1. Introduction
2. Results and Discussion
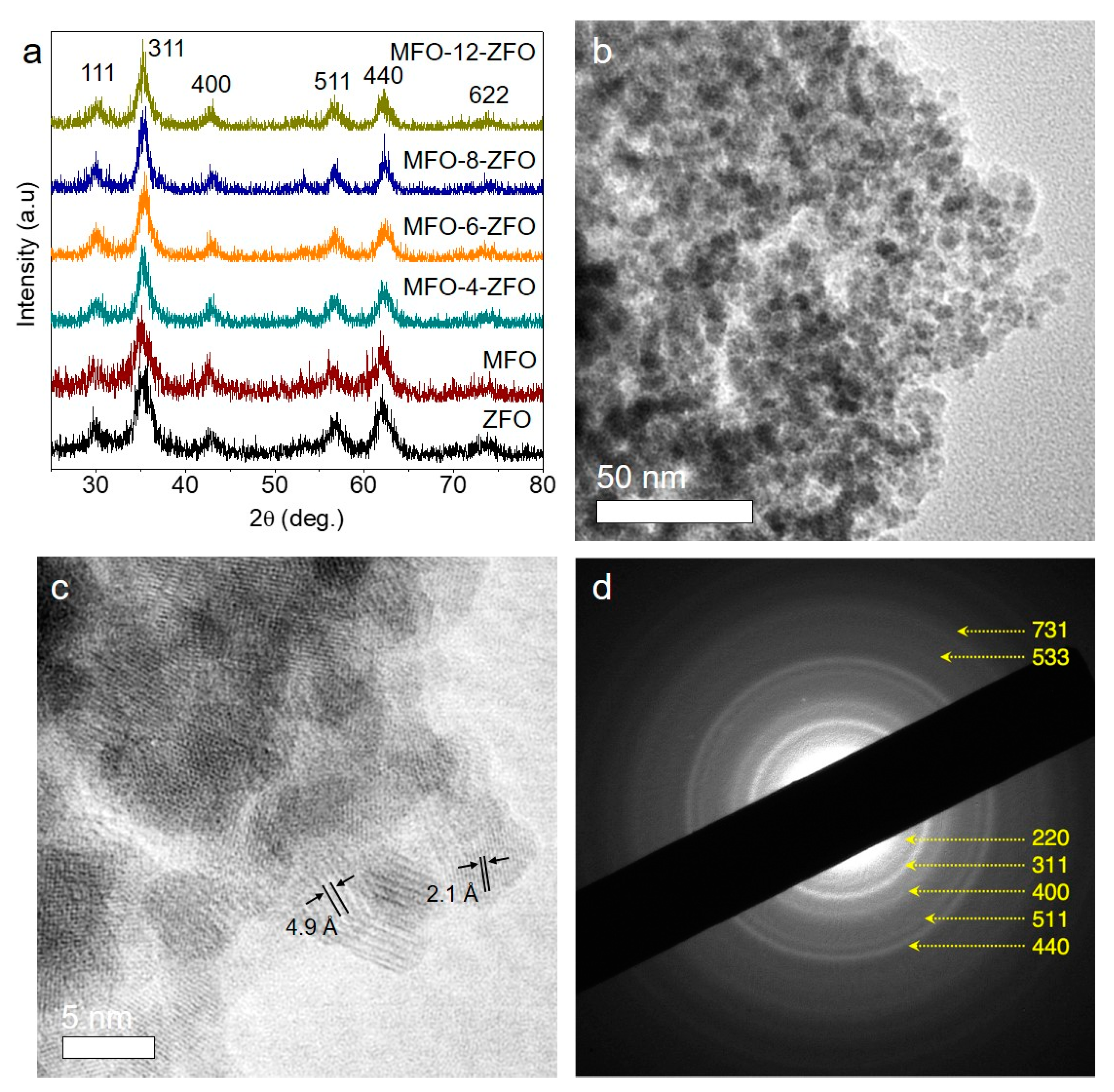
2.1. Structure and Morphology of MFO-ZFO MNAs
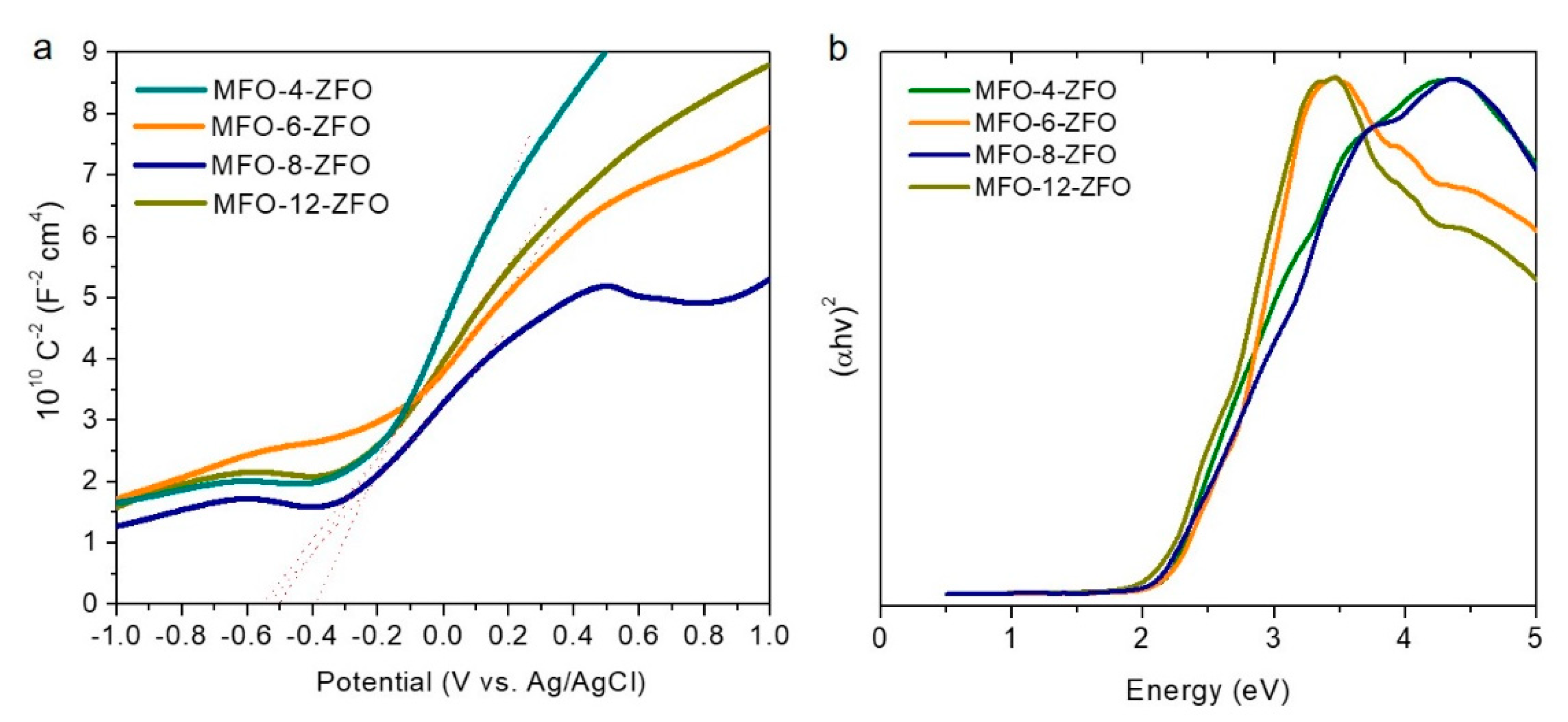
2.2. Photocatalytic Study of MFO-ZFO MNAs
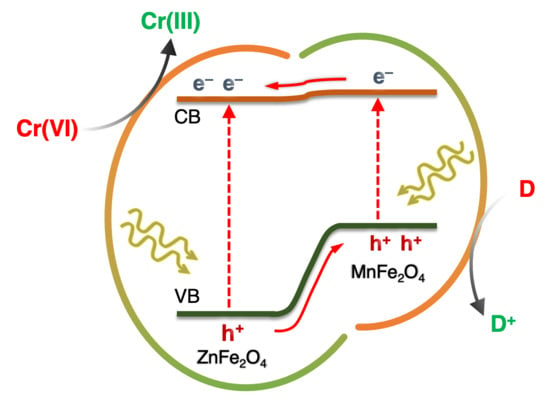
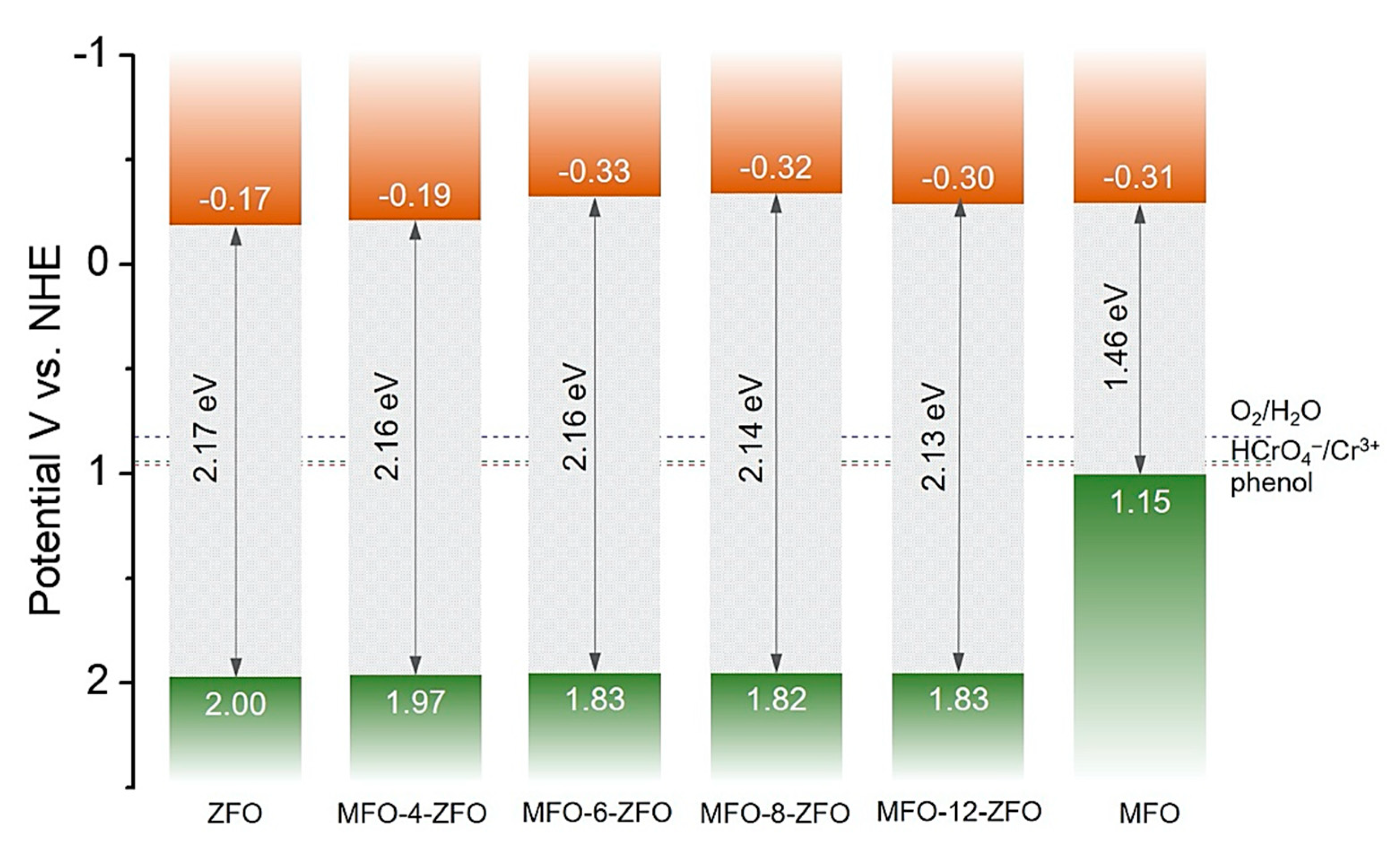
2.3. Mechanism of Photocatalytic Cr(VI) Reduction
3. Materials and Methods
3.1. Materials
3.2. Preparetion of Spinel Ferrite NPs
3.3. Preparation of Colloidal Metal Ferrite NPs
3.4. Preparetion of Mesoporous MFO-ZFO NP Assemblies
3.5. Photocatalytic Experiments
3.6. Physical Characterization
3.7. Electrochemical Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Tchounwou, P.B.; Yedjou, C.G.; Patlolla, A.K.; Sutton, D.J. Heavy metal toxicity and the environment. Exp. Suppl. 2012, 101, 133–164. [Google Scholar] [CrossRef] [PubMed]
- Bakshi, A.; Panigrahi, A.K. A comprehensive review on chromium induced alterations in fresh water fishes. Toxicol. Rep. 2018, 5, 440–447. [Google Scholar] [CrossRef] [PubMed]
- Joutey, N.T.; Sayel, H.; Bahafid, W.; Ghachtouli, N.E. Mechanisms of hexavalent chromium resistance and removal by microorganisms. Rev. Environ. Contam. Toxicol. 2015, 233, 45–69. [Google Scholar] [CrossRef] [PubMed]
- Laxmi, V.; Kaushik, G. Toxicity of hexavalent chromium in environment, health threats, and its bioremediation and detoxification from tannery wastewater for environmental safety. In Bioremediation of Industrial Waste for Environmental Safety: Volume I: Industrial Waste and Its Management; Saxena, G., Bharagava, R.N., Eds.; Springer: Singapore, 2020; pp. 223–243. [Google Scholar]
- World Health Organization. Guidelines for Drinking-Water Quality: Third Edition, Incorporating the First and Second Addenda; World Health Organization: Geneva, Switzerland, 2008; Volume 1, pp. 334–335. [Google Scholar]
- Siboni, M.S.; Samarghandi, M.R.; Azizian, S.; Kim, W.G.; Lee, S.M. The Removal of hexavalent chromium from aqueous solutions using modified holly sawdust: Equilibrium and kinetics studies. Environ. Eng. Res. 2011, 16, 55–60. [Google Scholar] [CrossRef]
- Kongsricharoern, N.; Polprasert, C. Electrochemical precipitation of chromium (Cr6+) from an electroplating wastewater. Water Sci. Technol. 1995, 31, 109–117. [Google Scholar] [CrossRef]
- Etemadi, M.; Samadi, S.; Yazd, S.S.; Jafari, P.; Yousefi, N.; Aliabadi, M. Selective adsorption of Cr(VI) ions from aqueous solutions using Cr6+-imprinted Pebax/chitosan/GO/APTES nanofibrous adsorbent. Int. J. Biol. Macromol. 2017, 95, 725–733. [Google Scholar] [CrossRef]
- Xing, Y.; Chen, X.; Wang, D. Electrically Regenerated ion exchange for removal and recovery of Cr(VI) from wastewater. Environ. Sci. Technol. 2007, 41, 1439–1443. [Google Scholar] [CrossRef]
- Khorramabadi, G.S.; Soltani, R.D.C.; Rezaee, A.; Khataee, A.R.; Jafari, A.J. Utilisation of immobilised activated sludge for the biosorption of chromium (VI). Can. J. Chem. Eng. 2012, 90, 1539–1546. [Google Scholar] [CrossRef]
- Shirzad-Siboni, M.; Farrokhi, M.; Soltani, R.D.C.; Khataee, A.; Tajassosi, S. Photocatalytic reduction of hexavalent chromium over ZnO nanorods immobilized on kaolin. Ind. Eng. Chem. Res. 2014, 53, 1079–1087. [Google Scholar] [CrossRef]
- Sun, B.; Reddy, E.P.; Smirniotis, P.G. Visible light Cr(VI) reduction and organic chemical oxidation by TiO2 photocatalysis. Environ. Sci. Technol. 2005, 39, 6251–6259. [Google Scholar] [CrossRef]
- Mondal, C.; Ganguly, M.; Pal, J.; Roy, A.; Jana, J.; Pal, T. Morphology controlled synthesis of SnS2 nanomaterial for promoting photocatalytic reduction of aqueous Cr(VI) under visible light. Langmuir 2014, 30, 4157–4164. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Zhuang, S.; Xu, X.; Zhu, W.; Feng, B.; Hu, J. Photogenerated electron reservoir in hetero-p–n CuO–ZnO nanocomposite device for visible-light-driven photocatalytic reduction of aqueous Cr(VI). J. Mater. Chem. A 2015, 3, 1199–1207. [Google Scholar] [CrossRef]
- Wang, Q.; Shi, X.; Liu, E.; Crittenden, J.C.; Ma, X.; Zhang, Y.; Cong, Y. Facile synthesis of AgI/BiOI-Bi2O3 multi-heterojunctions with high visible light activity for Cr(VI) reduction. J. Hazard. Mater. 2016, 317, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Velegraki, G.; Vamvasakis, I.; Papadas, I.T.; Tsatsos, S.; Pournara, A.; Manos, M.J.; Choulis, S.A.; Kennou, S.; Kopidakis, G.; Armatas, G.S. Boosting photochemical activity by Ni doping of mesoporous CoO nanoparticle assemblies. Inorg. Chem. Front. 2019, 6, 765–774. [Google Scholar] [CrossRef]
- Velegraki, G.; Miao, J.; Drivas, C.; Liu, B.; Kennou, S.; Armatas, G.S. Fabrication of 3D mesoporous networks of assembled CoO nanoparticles for efficient photocatalytic reduction of aqueous Cr(VI). Appl. Catal. B Environ. 2018, 221, 635–644. [Google Scholar] [CrossRef]
- Hernández-Gordillo, L.; Tzompantzi, F.J.; Gomez, R. Enhanced photoreduction of Cr(VI) using ZnS(en)0.5 hybrid semiconductor. Catal. Commun. 2012, 19, 51–55. [Google Scholar] [CrossRef]
- Wang, D.; Ye, Y.; Liu, H.; Ma, H.; Zhang, W. Effect of alkaline precipitation on Cr species of Cr(III)-bearing complexes typically used in the tannery industry. Chemosphere 2018, 193, 42–49. [Google Scholar] [CrossRef]
- Srivastava, S.; Ahmad, A.H.; Thakur, I.S. Removal of chromium and pentachlorophenol from tannery effluents. Bioresour. Technol. 2007, 98, 1128–1132. [Google Scholar] [CrossRef]
- Kulkarni, A.M.; Desai, U.V.; Pandit, K.S.; Kulkarni, M.A.; Wadgaonkar, P.P. Nickel ferrite nanoparticles–hydrogen peroxide: A green catalyst-oxidant combination in chemoselective oxidation of thiols to disulfides and sulfides to sulfoxides. RSC Adv. 2014, 4, 36702–36707. [Google Scholar] [CrossRef]
- Guo, X.; Zhu, H.; Li, Q. Visible-light-driven photocatalytic properties of ZnO/ZnFe2O4 core/shell nanocable arrays. Appl. Catal. B Environ. 2014, 160–161, 408–414. [Google Scholar] [CrossRef]
- Hou, Y.; Li, X.; Zhao, Q.; Chen, G. ZnFe2O4 multi-porous microbricks/graphene hybrid photocatalyst: Facile synthesis, improved activity and photocatalytic mechanism. Appl. Catal. B Environ. 2013, 142–143, 80–88. [Google Scholar] [CrossRef]
- Cai, C.; Zhang, Z.; Liu, J.; Shan, N.; Zhang, H.; Dionysiou, D.D. Visible light-assisted heterogeneous Fenton with ZnFe2O4 for the degradation of Orange II in water. Appl. Catal. B Environ. 2016, 182, 456–468. [Google Scholar] [CrossRef]
- Kong, L.; Jiang, Z.; Xiao, T.; Lu, L.; Jones, M.O.; Edwards, P.P. Exceptional visible-light-driven photocatalytic activity over BiOBr–ZnFe2O4 heterojunctions. Chem. Commun. 2011, 47, 5512–5514. [Google Scholar] [CrossRef]
- Lee, K.-T.; Chuah, X.-F.; Cheng, Y.-C.; Lu, S.-Y. Pt coupled ZnFe2O4 nanocrystals as a breakthrough photocatalyst for Fenton-like processes—Photodegradation treatments from hours to seconds. J. Mater. Chem. A 2015, 3, 18578–18585. [Google Scholar] [CrossRef]
- Yu, T.-H.; Cheng, W.-Y.; Chao, K.-J.; Lu, S.-Y. ZnFe2O4 decorated CdS nanorods as a highly efficient, visible light responsive, photochemically stable, magnetically recyclable photocatalyst for hydrogen generation. Nanoscale 2013, 5, 7356–7360. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Jang, Y.J.; Kim, J.H.; Jang, J.-W.; Choi, S.H.; Lee, J.S. Defective ZnFe2O4 nanorods with oxygen vacancy for photoelectrochemical water splitting. Nanoscale 2015, 7, 19144–19151. [Google Scholar] [CrossRef]
- Ren, Y.; Li, N.; Feng, J.; Luan, T.; Wen, Q.; Li, Z.; Zhang, M. Adsorption of Pb(II) and Cu(II) from aqueous solution on magnetic porous ferrospinel MnFe2O4. J. Colloid Interface Sci. 2012, 367, 415–421. [Google Scholar] [CrossRef]
- Skliri, E.; Miao, J.; Xie, J.; Liu, G.; Salim, T.; Liu, B.; Zhang, Q.; Armatas, G.S. Assembly and photochemical properties of mesoporous networks of spinel ferrite nanoparticles for environmental photocatalytic remediation. Appl. Catal. B Environ. 2018, 227, 330–339. [Google Scholar] [CrossRef]
- Papadas, I.T.; Vamvasakis, I.; Tamiolakis, I.; Armatas, G.S. Templated self-assembly of colloidal nanocrystals into three-dimensional mesoscopic structures: A perspective on synthesis and catalytic prospects. Chem. Mater. 2016, 28, 2886–2896. [Google Scholar] [CrossRef]
- Kong, X.K.; Zhou, Y.; Xu, T.; Hu, B.; Lei, X.; Chen, H.; Yu, G. A novel technique of COD removal from electroplating wastewater by Fenton-alternating current electrocoagulation. Environ. Sci. Pollut. Res. 2020, 27, 15198–15210. [Google Scholar] [CrossRef]
- Dsikowitzky, L.; Schwarzbauer, J. Organic contaminants from industrial wastewaters: Identification, toxicity and fate in the environment. In Pollutant Diseases, Remediation and Recycling. Environmental Chemistry for a Sustainable World; Lichtfouse, E., Schwarzbauer, J., Robert, D., Eds.; Springer: Cham, Switzerland, 2013; Volume 4, pp. 45–101. [Google Scholar] [CrossRef]
- Hsu, H.T.; Chen, S.S.; Tang, Y.F.; Hsi, H.C. Enhanced photocatalytic activity of chromium(VI) reduction and EDTA oxidization by photoelectrocatalysis combining cationic exchange membrane processes. J. Hazard. Mater. 2013, 248–249, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Yu, Y.; Zhang, L. Highly efficient photocatalytic removal of sodium pentachlorophenate with Bi3O4Br under visible light. Appl. Catal. B Environ. 2013, 136–137, 112–121. [Google Scholar] [CrossRef]
- Zaban, A.; Greenshtein, M.; Bisquert, J. Determination of the electron lifetime in nanocrystalline dye solar cells by open-circuit voltage decay measurements. ChemPhysChem 2003, 4, 859–864. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Zou, B.; Rondinone, A.J.; Zhang, Z.J. Reverse micelle synthesis and characterization of superparamagnetic MnFe2O4 spinel ferrite nanocrystallites. J. Phys. Chem. B 2000, 104, 1141–1145. [Google Scholar] [CrossRef]
- Brunauer, S.; Deming, L.S.; Deming, W.E.; Teller, E. On a theory of the van der Waals adsorption of gases. J. Am. Chem. Soc. 1940, 62, 1723–1732. [Google Scholar] [CrossRef]
- Ravikovitch, P.I.; Wei, D.; Chueh, W.T.; Haller, G.L.; Neimark, A.V. Evaluation of pore structure parameters of MCM-41 catalyst supports and catalysts by means of nitrogen and argon adsorption. J. Phys. Chem. B 1997, 101, 3671–3679. [Google Scholar] [CrossRef]
- Tauc, J. Optical properties of amorphous semiconductors. In Amorphous and Liquid Semiconductors; Tauc, J., Ed.; Springer: Boston, MA, USA, 1974; pp. 159–220. [Google Scholar]
- Kubelka, P. New contributions to the optics of intensely light-scattering materials. Part I. J. Opt. Soc. Am. 1948, 38, 448–457. [Google Scholar] [CrossRef]
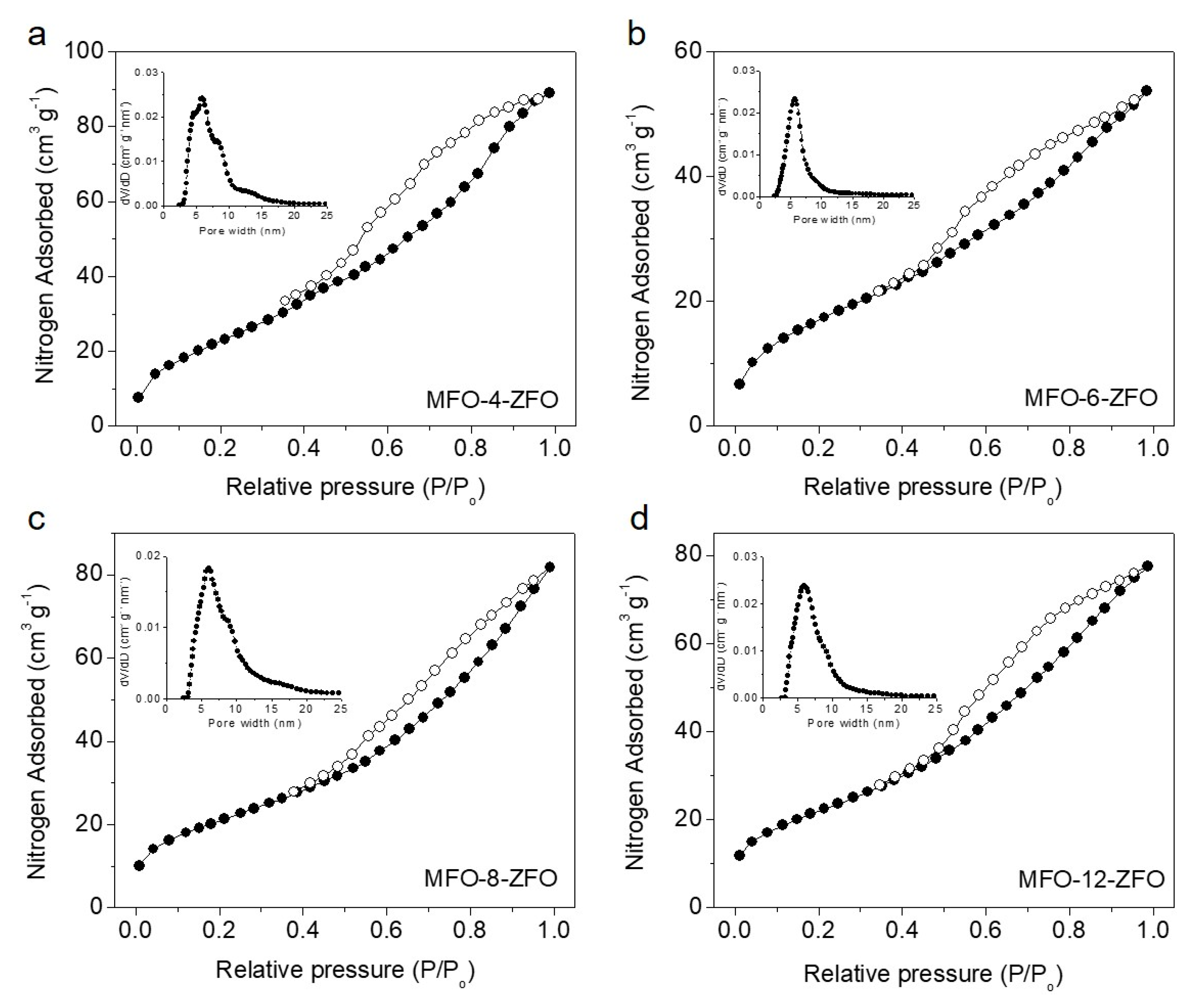



| Sample | Surface Area (m2 g−1) | Pore Volume (cm3 g−1) | Pore Width (nm) | Crystallite Size 1 (nm) |
|---|---|---|---|---|
| ZFO | 105 | 0.15 | 5.8 | 6.2 |
| MFO | 106 | 0.21 | 6.1 | 6.7 |
| MFO-4-ZFO | 91 | 0.14 | 5.9 | 6.5 |
| MFO-6-ZFO | 68 | 0.09 | 5.8 | 6.3 |
| MFO-8-ZFO | 79 | 0.13 | 6.2 | 6.8 |
| MFO-12-ZFO | 82 | 0.12 | 6.0 | 6.4 |
| Catalyst | Band Gap (Eg) (eV) | Flat-Band Potential (EFB) (V vs. NHE) | VB Potential (EVB) 1 (V vs. NHE) | Donor Density (Nd) (cm−3) |
|---|---|---|---|---|
| ZFO | 2.17 | −0.17 | 2.00 | 3.63 × 1016 |
| MFO | 1.46 | −0.31 | 1.15 | 1.10 × 1017 |
| MFO-4-ZFO | 2.16 | −0.19 | 1.97 | 9.71 × 1015 |
| MFO-6-ZFO | 2.16 | −0.33 | 1.83 | 1.79 × 1016 |
| MFO-8-ZFO | 2.14 | −0.32 | 1.82 | 1.88 × 1016 |
| MFO-12-ZFO | 2.13 | −0.30 | 1.83 | 1.46 × 1016 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Skliri, E.; Vamvasakis, I.; Papadas, I.T.; Choulis, S.A.; Armatas, G.S. Mesoporous Composite Networks of Linked MnFe2O4 and ZnFe2O4 Nanoparticles as Efficient Photocatalysts for the Reduction of Cr(VI). Catalysts 2021, 11, 199. https://doi.org/10.3390/catal11020199
Skliri E, Vamvasakis I, Papadas IT, Choulis SA, Armatas GS. Mesoporous Composite Networks of Linked MnFe2O4 and ZnFe2O4 Nanoparticles as Efficient Photocatalysts for the Reduction of Cr(VI). Catalysts. 2021; 11(2):199. https://doi.org/10.3390/catal11020199
Chicago/Turabian StyleSkliri, Euaggelia, Ioannis Vamvasakis, Ioannis T. Papadas, Stelios A. Choulis, and Gerasimos S. Armatas. 2021. "Mesoporous Composite Networks of Linked MnFe2O4 and ZnFe2O4 Nanoparticles as Efficient Photocatalysts for the Reduction of Cr(VI)" Catalysts 11, no. 2: 199. https://doi.org/10.3390/catal11020199
APA StyleSkliri, E., Vamvasakis, I., Papadas, I. T., Choulis, S. A., & Armatas, G. S. (2021). Mesoporous Composite Networks of Linked MnFe2O4 and ZnFe2O4 Nanoparticles as Efficient Photocatalysts for the Reduction of Cr(VI). Catalysts, 11(2), 199. https://doi.org/10.3390/catal11020199