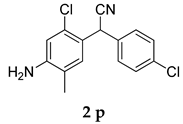
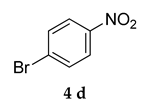
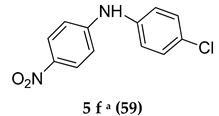
Abstract
We herein report the preparation and characterization of an inexpensive polymer supported 1,3-bis(benzimidazolyl)benzeneCo(II) complex [PS-Co(BBZN)Cl2] as a catalyst by using the polymer (divinylbenzene cross-linked chloromethylated polystyrene), on which 1,3-bis(benzimidazolyl)benzeneCo(II) complex (PS-Co(BBZN)Cl2) has been immobilized. This catalyst was employed to develop arylamination reaction and robustness of the same reaction was demonstrated by synthesizing various bioactive adamantanyl-tethered-biphenylamines. Our synthetic methodology was much improved than reported methods due to the use of an inexpensive and recyclable catalyst.
1. Introduction
Transition metal-catalyzed cross-coupling reactions between aryl halides and primary/secondary amines to obtain aminated aryl compounds has been an area of interest due to the wide applications of arylamines in the synthetics and pharmaceutical industries [1,2,3,4,5]. In this direction, the Buchwald–Hartwig cross-coupling reaction was performed by using transition metal catalysts, ligands and bases with substrates to obtain the desired arylamine products [6,7,8]. The disadvantage of this reaction is the use of expensive catalysts, which offers the chemist the opportunity to discover cheaper, reusable catalysts to drive the arylamination reactions. Inspired by major developments in cobalt-catalyzed arylamination reactions, we developed a complementary method to perform an arylamination reaction using cobalt as a metal catalyst [9,10,11]
In addition, benzimidazole ligand coordinated metal complexes are widely used as catalysts in arylamination reactions [12]. Since these catalysts were found to be less hydrophobic, immobilization of such metal complexes with polymer support was observed to be stable, selective, and recyclable, attributed to the steric, electrostatic, hydrophobic and conformational effects of the polymer support [13]. Hence, several reports pertaining to the synthesis of arylamines using polymer-supported transition metal complexes are found [14,15,16]. Specifically, chloromethylated polystyrene cross-linked with divinyl-benzene was employed as a macromolecular support to perform the arylamination reactions [17,18,19,20,21,22].
In medicinal chemistry, an adamantane-coupled bicyclical core structure was used as an important pharmacophore, which was inserted in many drugs [23]. Hence, the adamantane structure was recognized as a readily available “liphophilic bullet” for providing critical liphophilicity to known pharmacophoric units. Given the remarkable importance of adamantane chemistry, we recently reported the synthesis and biology of adamantyl-tethered biphenylic compounds as potent anticancer agents [24]. In our continued efforts to synthesize newer bioactive agents [25,26,27,28,29,30,31], we herein report a practical, economically feasible and efficient arylamination reaction using polymer-supported 1,3-bis(benzimidazolyl)benzeneCo(II) complex (PS-Co(BBZN)Cl2) as a catalyst. Interestingly, the recovered (PS-Co(BBZN)Cl2) could be reused three times without a significant loss of activity.
2. Results
2.1. Chemistry of Catalyst Design and Method Development
We initially synthesized polymer-supported 1,3-bis(benzimidazolyl)benzeneCo(II) complex [PS-Co(BBZN)Cl2] as shown in Scheme 1.
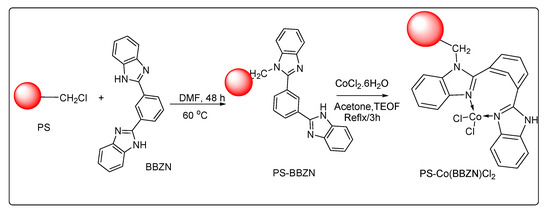
Scheme 1.
Schematic representation to show synthesis of PS-Co(BBZN)Cl2.
For this, 1, 3-bis(benzimidazolyl)benzene was treated with chloromethylated polystyrene divinylbenzene and followed by the addition of cobalt chloride. The obtained PS-Co (BBZN)Cl2 was characterized by analytical techniques including CHNS, UV-Vis, FT-IR, SEM-EDX and TGA as presented in supporting information (Figure 1, Supplementary SI-02). Based on N% and Co% obtained through elemental and metal ion analysis, the complex formed on the polymer support was about 0.0053 moles per 1 g of the polymer support which corresponded to 7.16% of Co intake. This further confirmed the formation of the complex on the polymer support.
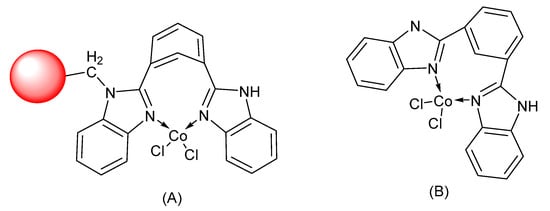
Figure 1.
Structure of (A) PS-Co (BBZN)Cl2 and (B) unbound Co(BBZN)Cl2.
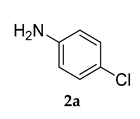
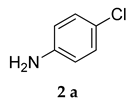
Motivated by the increased understanding of the Co-catalyzed amination reaction, we next investigated the applicability of (PS-Co (BBZN)Cl2) in the arylamination reaction. To examine this hypothesis, 1-(5-bromo-2-methoxyphenyl)adamantine (1a) and 4-chloro aniline (2a) were selected as model substrates and reagents for the reaction in 1,4-dioxane media and Cs2CO3 as a base (Scheme 2).
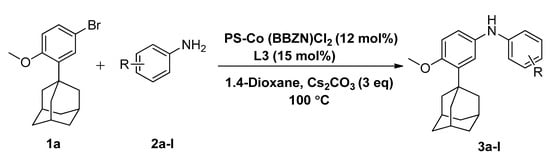
Scheme 2.
General scheme of arylamination reaction between adamantane bromide and various amines using PS-Co(BBZN)Cl2 as a catalyst.
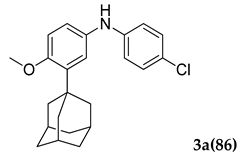
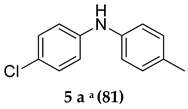
Control experiments established the importance of both PS-Co(BBZN)Cl2 and ligand, as no product was obtained (Table 1, entry 1). Gratifyingly, the substrate was transformed into the desired product 3-(adamantan-1-yl)-N-(4-chlorophenyl)-4-methoxyaniline (3a) with 51% yield in the presence of catalyst (PS-Co(BBZN)Cl2) (10 mol%) and ligand L3 (Table 2, entry 10). Screening of various classes of ligands (Figure 2) to improve the yield revealed that the use of phosphine based ligand BINAP (L3) or Xphose (L4) gave improved yields at different catalyst concentrations (Table 1, entry 10, 11, 14, 15), whereas the other ligands such as bidentate ligands (L1, L2) and N-heterocyclic carbine ligands (L5, L6) yielded no products indicating the high role of selectivity of ligands in the forward reaction. The most robust reaction was achieved by the use of 12 mol% of PS-Co(BBZN)Cl2 in the presence of BINAP with an 86% yield at 10 h reaction condition (Table 1, entry 14). Further investigation revealed that there was no considerable improvement in yield when the catalyst load was increased to 15 mol% (Table 1, entry 18, 19) whereas the yield dropped to 69% when the reaction time was reduced to 6 h with 15 mol% catalyst (Table 1, entry 20). Using the above better protocol, we further synthesized ABTAs by reacting adamantine bromo compounds (1a) and various amines (Table 2). It was observed that all amine partners productively coupled with good yields of around 70–86%.

Table 1.
PS-Co(BBZN)Cl2-catalyzed coupling of 1-(5-bromo-2-methoxyphenyl)adamantane with 4-Chloro aniline a.

Table 2.
PS-Co(BBZN)Cl2 composite-catalyzed coupling of various substituted halo aromatic compounds with various substituted aromatic amines a.
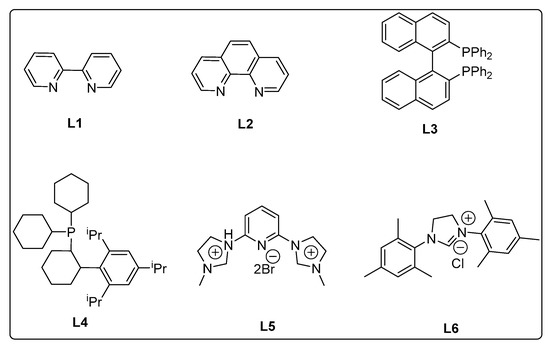
Figure 2.
Various classes of ligands used in this study.
All novel compounds exhibited spectral properties consistent with the assigned structures and were fully characterized by their spectroscopic data (mass, elemental, 1H and 13C NMR analysis).
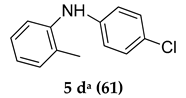
The majority of reactions were done by keeping time point for 16 h and when the concentration of the catalyst was increased to 12%, the reaction was completed in 12 h and in many cases pure product was produced with excellent yield. The above developed method tolerated the presence of substituent in the aromatic amino-compounds. Specifically, we observed that the electron-donating para-substituted aromatic amine partners were well-tolerated to produce corresponding products in good to excellent yields (Table 2, entries 1–12). However, ortho-substituted and electron-withdrawing group bearing compounds were not productive giving lower yields (Table 2, entries 5, 11, 12, 13).
With the reaction conditions established we tried to investigate the scope of the new protocol on different substituted aromatic bromo compounds by treating with various amines (Table 3). We found that electron donating para-substituted on aromatic halo partner was tolerated well to give corresponding products in good to excellent yields (entries 1, 2, 3 and 5), but with ortho-substituted and electron-withdrawing group bearing aromatic bromo compounds observed a loss in yield (entries 4, 6 and 7) with no improvement in the reaction conversion on prolonged reaction.

Table 3.
Various substrates and reagents used to optimization of arylamination reaction.
All novel compounds exhibited spectral properties consistent with the assigned structures and were fully characterized by their spectroscopic data (mass, elemental, 1H and 13C NMR analysis). It was found that the use of a catalyst PS-Co(BBZN)Cl2, in combination with some ligands provided a robust catalytic system. On the basis of previous mechanistic studies in cobalt-catalyzed C−N bond formation reactions, it was possible to propose a mechanism for the conversion of 3-(adamantan-1-yl)-N-(4-chlorophenyl)-4-methoxyaniline (3 a) as shown in Figure 3 [32,33,34].
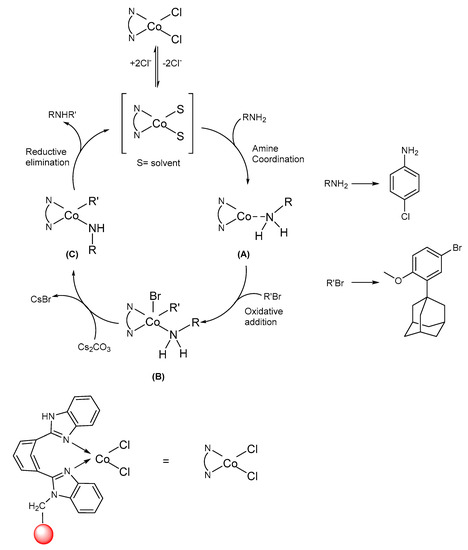
Figure 3.
Plausible mechanism for the generation of arylamines using PS-Co(BBZN)Cl2 as a catalyst.
Initially, the catalyst makes a complex with amine to form a catalyst-amine complex A, which undergoes an oxidative addition reaction with 1-(5-bromo-2-methoxyphenyl)adamantane and complex B formation occurs. Complex B reacts with cesium carbonate base and undergoes metathesis step, which gave complex C. Finally, the reductive elimination reaction complex C takes place and thereby catalyst regeneration and the desired product formation occur in the last step (Figure 3).
Further, we performed density-functional theory calculations using dispersion corrected CAM-B3 LYP functional and 6–31+G method [35]. All electron basis set as implemented in the Gaussian 09 package [36]. The minima nature of the structures has been confirmed based on computed real harmonic vibrational analysis at the same level of theory. Gibbs free energy calculations for four intermediate cobalt complexes were chosen for our mechanistic elucidation. Initially CoCl2 makes the coordination complex with the ligand and reacts with aromatic amine and forms Co-NH bond quickly [intermediate (a); ΔE = −6.03 kcal/mole], which in turn gets stabilized by releasing HCl and attains a lower energy intermediate with a ΔE of −9.62 kcal/mole. Alkyl bromide adds to the intermediate (b) quickly and attains still lower energy of ΔE of −17.18 kcal/mole where the bindentate ligand detachment takes place and immediate loss of HCl takes place and again attains lowest energy intermediate (d) of ΔE = −19.62 kcal/mole, which gives the product immediately. The optimized geometries and the energy profile diagram of intermediates (a–d) are shown in Figure 4 and Figure 5, respectively. On the basis of lower Gibbs free energy of intermediates across (a) to (d), we can conclude that the reaction occurs naturally upon cobalt chloride coordination complex formation occurring with the bidentate ligands.
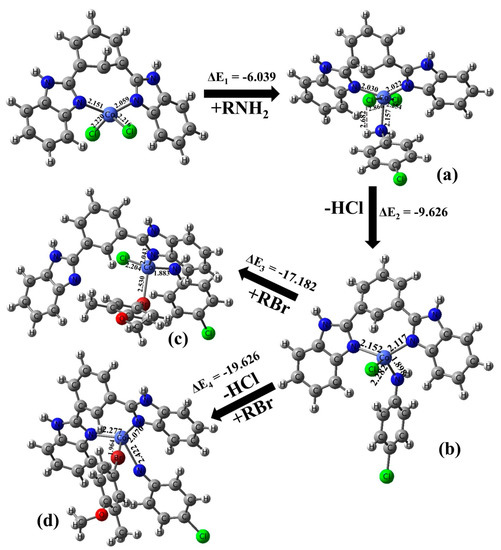
Figure 4.
Computed intermediate structures (a–d) and reaction path. Energy difference ΔE are given in kcal/mole.
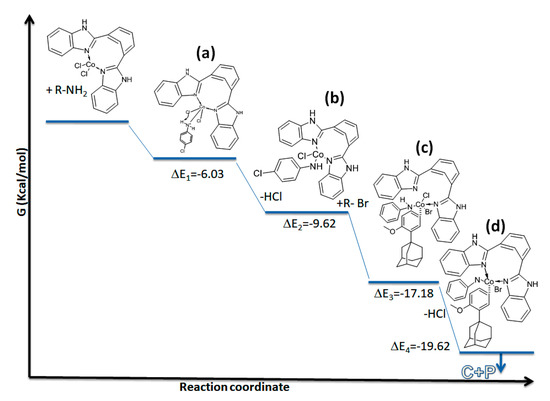
Figure 5.
Energy profile diagram or arylamination reaction. C= catalyst; P = product.
2.2. Recyclability of the Catalyst
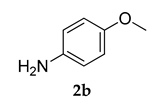
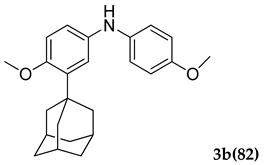
Further, the superiority of PS-Co(BBZN)Cl2 catalyst was its recyclability, which was investigated by using the compound 1 a and 2 b as a model reaction. After each run, the catalyst was filtered off and washed with water followed by methanol, it was then dried in an oven at 120 °C for 15 min and used directly for the next reaction. The results were summarized (Table 4). We recorded that the catalyst could be used thrice and isolated yields achieved were above 70%.

Table 4.
The recycling of the catalyst a.
3. Materials and Methods
3.1. Procedure for the Synthesis of PS-Co(BBZN)Cl2 Complex
3.1.1. Preparation of BBZN Functionalized Polymer Support
The chloromethylated polystyrene beads cross-linked with 6.5% divinylbenzene were first washed with a mixture of THF and water in the ratio 4:1 using Soxhlet extractor for 48 h. The beads were then vacuum dried. The chloromethylated polystyrene beads (3 g) were allowed to swell in DMF solution of BBZN ligand (5.2 g) was added to the above suspension followed by the addition of triethylamine (12 mL) in ethylacetate (105 mL) and was heated at 60 °C for 45 h in a water bath. It was cooled to room temperature, filtered, and washed with DMF. The beads were then Soxhlet extracted with ethanol to remove any unreacted BBZN and dried in an oven at 60 °C overnight.
3.1.2. Preparation of PS-Co(BBZN)Cl2 Complex
The functionalized beads (1.0 g) were allowed to swell in 50 mL acetonitrile and toluene mixture in the ratio 1:1 for 1 h. Then the solvent was decanted. To this, 1.426 g of CoCl2.6 H2 O dissolved in methanol (100 mL) was added at intervals (4 times) and heated at 60 °C for 48 h. It was filtered, washed with alcohol and Soxhlet extracted to remove any unreacted CoCl2.6 H2 O. It was filtered and dried in an oven at 60 °C for 10 h and vacuum dried.
3.2. General Procedure for (PS-Co(BBZN)Cl2) Complex Catalyzed C−N Bond-Formation Reaction
A dried Schlenk tube was charged with substrate 1 a (320 mg, 1 mmol), 2 a (127.6 mg, 1 mmol), BINAP (48 mg, 15 mol%), (PS-Co(BBZN)Cl2](38 mg, 12 mol%). The tube was evacuated and backfilled with N2, and Cs2 CO3 (975 mg, 3 mmol) followed by reagent grade 1, 4-dioxane (5 mL). The reaction mixture was heated to 100 °C for 10 h. After completion of reaction the mass was cooled to room temperature, filtered off the catalyst, the solvent quenched with water and diluted with ethyl acetate (10 mL). The layers were separated, and the aqueous layer was extracted with (5 mL) ethyl acetate. The combined organic layer was washed with water (10 mL), dried over anhydrous sodium sulphate and the solvent was removed in vacuum. The crude product was purified using silica gel column chromatography.
3.2.1. 3-(Adamantan-1-yl)-N-(4-chlorophenyl)-4-methoxyaniline (3 a)
Pale Yellow colored solid; mp 140–142 °C: 1H NMR (400 MHz,CDCl3) 7.14–7.12 (d, J = 8.0 Hz, 2 H), 6.95–6.91 (m, 2 H), 6.81–6.78 (m, 3 H), 5.46 (s, 1 H), 3.80 (s, 3 H), 2.05 (m, 9 H), 1.74 (m, 6 H); 13C NMR (100 MHz,CDCl3) 155.0, 144.2, 139.9, 134.8, 129.2, 123.6, 120.6, 119.1, 116.4, 112.6, 55.4, 40.6, 37.1, 29.1; LCMS (MM:ES + APCI) 368.4 (M + H)+; Anal.Calcd for C23 H26 ClNO: C, 75.08; H, 7.12; N, 3.81. Found: C, 75.01; H, 7.15; N, 3.88.
3.2.2. 3-(Adamantan-1-yl)-4-methoxy-N-(4-methoxyphenyl)aniline (3 b)
Brown colored solid; mp 117–119 °C: 1H NMR (400 MHz,CDCl3) 7.48–7.46 (d, J = 8.0 Hz, 2 H), 7.24 (m, 1 H), 6.88–6.86 (d, J = 8.0 Hz, 2 H), 6.72–6.70 (d, J = 8.0 Hz, 2 H), 5.39 (s, 1 H), 3.89 (s, 3 H), 3.83 (s, 3 H), 2.06–2.03 (m, 9 H), 1.75 (m, 6 H); 13C NMR (100 MHz, CDCl3) 153.8, 142.2, 138.9, 130.4, 128.2, 122.5, 118.1, 115.5, 111.8, 55.0, 53.7, 40.3, 36.96, 28.9; LCMS (MM:ES + APCI) 364.4 (M + H)+; Anal.Calcd for C24 H29 NO2: C, 79.30; H, 8.04; N, 3.85. Found: C, 79.26; H, 8.11; N, 3.79.
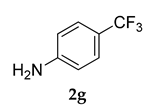
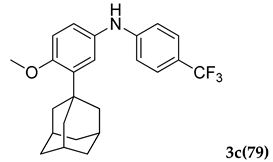
3.2.3. 3-(Adamantan-1-yl)-4-methoxy-N-(4-(trifluoromethyl)phenyl)aniline (3 c)
Off-white colored solid; mp 124–126 °C: 1H NMR (400 MHz,CDCl3) 7.64–7.61 (m, 2 H), 7.45–7.39 (m, 3 H), 7.24 (s, 1 H), 6.96–6.94 (d, J = 8.0 Hz, 1 H), 5.36 (s, 1 H), 3.87 (s, 3 H), 2.13–2.04 (m, 9 H), 1.77 (m, 6 H); 13C NMR (100 MHz,CDCl3) 159.1, 145.1, 139.1, 131.8, 127.0, 125.7 (JCF = 25.7 Hz), 112.1, 55.2, 40.6, 37.2 (JCF = 7.6 Hz), 29.7, 29.1; LCMS (MM : ES + APCI) 402.2 (M + H)+; Anal.Calcd for C24 H26 F3 NO: C, 71.80; H, 6.53; N, 3.49. Found: C, 71.76; H, 6.59; N, 3.41.
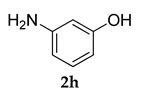
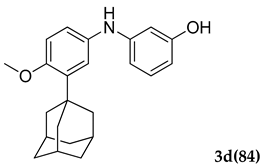
3.2.4. 3-((3-(Adamantan-1-yl)-4-methoxyphenyl)amino)phenol (3 d)
Off-white colored solid; mp 98–100 °C; 1H NMR (400 MHz,CDCl3) 7.43 (s, 1 H), 7.08 (s, 1 H), 6.99–6.96 (m, 4 H), 6.84–6.82 (d, J = 8.0 Hz, 1 H), 5.62 (s, 1 H), 4.80(s, 1 H), 3.82 (s, 3 H), 2.05 (m, 9 H), 1.74 (m, 6 H); 13C NMR (100 MHz,CDCl3) 159.2, 154.3, 146.2, 139.9, 129.7, 121.3, 119.9, 117.7, 115.3, 112.6, 111.2, 55.4, 40.6, 37.1, 29.1; HRMS Calcd 372.1934 Found: 372.1938 (M + H)+; Anal.Calcd for C24 H29 NO2: C, 79.30; H, 8.04; N, 3.85. Found: C, 79.26; H, 8.11; N, 3.79.
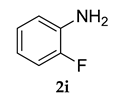
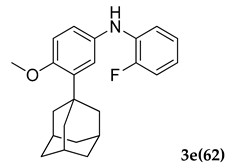
3.2.5. 3-(Adamantan-1-yl)-N-(2-fluorophenyl)-4-methoxyaniline (3 e)
Yellow colored solid; mp 106–108 °C: 1H NMR (400 MHz, CDCl3) 7.10–7.06 (m, 1 H), 6.98–6.96 (dd, J1 = 2.7 Hz, J2 = 2.2 Hz, 2 H), 6.84–6.83 (d, J = 4.0 Hz, 1 H), 6.81 (s, 1 H), 5.50 (s, 1 H), 3.81 (s, 3 H), 2.05 (m, 9 H), 1.75 (m, 6 H); 13C NMR (100 MHz,CDCl3) 158.4, 155.4, 147.1, 139.9, 134.1, 130.3, 121.5, 120.0, 118.8, 114.6, 112.8 (JCF = 53.4 Hz), 55.4, 40.5, 37.2, 29.0; LCMS (MM : ES + APCI) 352.4 (M + H)+; Anal.Calcd for C23 H26 FNO: C, 78.60; H, 7.46; N, 3.99. Found: C, 78.71; H, 7.39; N, 3.91.
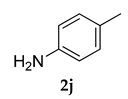
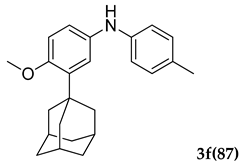
3.2.6. 3-(Adamantan-1-yl)-4-methoxy-N-(p-tolyl)aniline (3 f)
Off-white colored solid; mp 108–110 °C: 1H NMR (400 MHz,CDCl3) 7.03–7.01 (d, J = 8.0 Hz, 2 H), 6.95–6.89 (dd, J1 = 4.0 Hz, J2 = 4.0 Hz, 2 H), 6.85–6.83 (d, J = 8.0 Hz, 2 H), 6.80–6.78(d, J = 8.0 Hz, 1 H), 5.39 (s, 1 H), 3.80 (s, 3 H), 2.26 (s, 3 H), 2.05 (m, 9 H), 1.74 (m, 6 H); 13C NMR (100 MHz,CDCl3) 154.2, 142.6, 139.7, 129.8, 128.9, 119.4, 117.6, 116.3, 112.7, 55.5, 40.6, 37.1, 37.0, 29.1, 20.6; LCMS (MM : ES + APCI) 348.4 (M + H)+; Anal. Calcd for C24 H29 NO: C, 82.95; H, 8.41; N, 4.03. Found: C, 82.90; H, 8.46; N, 3.99.
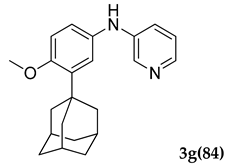
3.2.7. N-(3-Adamantan-1-yl)-4-methoxyphenyl)pyridin-3-amine (3 g)
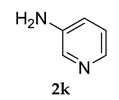
Pale yellow colored solid; mp 103–104 °C; 1H NMR (400 MHz, CDCl3) 8.42–8.41 (d, J = 4.0 Hz, 1 H), 7.47–7.43 (m, 2 H), 7.29–7.27 (m, 2 H), 7.23–7.22 (m, 2 H), 5.58 (s, 1 H), 3.85 (s, 3 H), 2.14–207 (m, 9 H), 1.79–1.73 (m, 6 H); 13C NMR (100 MHz, CDCl3) 161.9, 153.0, 143.7, 136.1, 126.8, 125.9, 124.0, 123.0, 121.9, 117.6, 115.1, 56.1, 40.8, 38.0, 28.6; LCMS (MM : ES + APCI) 335.4 (M + H)+; Anal. Calcd for C22 H26 N2 O: C, 79.00; H, 7.84; N, 8.38. Found: C, 79.08; H, 7.79; N, 8.33.
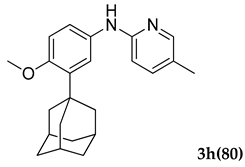
3.2.8. N-(3-Adamantan-1-yl)-4-methoxyphenyl)-5-methylpyridin-2-amine (3 h)
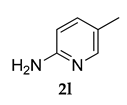
Off-white colored solid; mp 101–102 °C: 1H NMR (400 MHz, CDCl3); 8.42 (s, 1 H), 7.53–7.47 (m, 3 H), 7.03–7.01 (d, J = 8.0 Hz, 2 H), 5.60 (s, 1 H), 3.83 (s, 3 H), 2.30 (s, 3 H), 2.09 (m, 9 H), 1.78 (m, 6 H); 13C NMR (100 MHz, CDCl3) 160.2, 155.2, 145.0, 139.2, 128.9, 126.4, 121.4, 119.5, 117.4, 115.7, 54.8, 39.9, 36.4, 27.5, 27.0, 21.9; HRMS Calcd: 371.2094; Found: 371.2098 (M + H)+; Anal. Calcd for C23 H28 N2 O: C, 79.27; H, 8.10; N, 8.04. Found: C, 79.32; H, 7.99; N, 8.09.
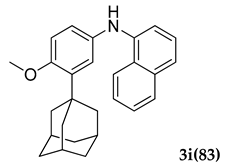
3.2.9. N-(3-Adamantan-1-yl)-4-methoxyphenyl)naphthalen-1-amine (3 i)
Pale yellow colored solid; mp 105–106 °C; 1H NMR (400 MHz, CDCl3) 8.09–8.07 (d, J = 8.0 Hz, 1 H), 8.03–8.01 (d, J = 8.0 Hz, 1 H), 7.96–7.94 (d, J= 8 Hz, 1 H), 7.60–7.28 (m, 6 H), 5.51 (s, 1 H), 3.83 (s, 3 H), 2.13–2.05 (m, 9 H), 1.76–1.73 (m, 6 H); 13C NMR (100 MHz,CDCl3) 153.9, 145.7, 138.3, 133.9, 132.7, 131.9, 128.7, 128.3, 128.2, 127.1, 126.9, 126.3, 125.9, 125.5, 111.4, 55.2, 40.4, 37.2, 29.2; LCMS (MM : ES + APCI) 384.4 (M + H)+; Anal.Calcd for C27 H29 NO: C, 84.55; H, 7.62; N, 3.65. Found: C, 84.61; H, 7.59; N, 3.69.
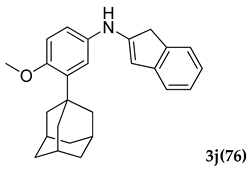
3.2.10. N-(3-Adamantan-1-yl)-4-methoxyphenyl)-1 H-inden-2-amine (3 j)
Pale yellow colored solid; mp 116–118 °C: 1H NMR (400 MHz, CDCl3) 7.64–7.61 (m, 2 H), 7.45–7.39 (m, 3 H), 6.96–7.94 (d, J = 8 Hz, 2 H), 6.19 (s, 1 H), 5.34 (s, 1 H), 3.83 (s, 3 H), 3.29 (s, 2 H), 2.13–2.07 (m, 9 H), 1.77 (m, 6 H); 13C NMR (100 MHz, CDCl3) 154.0, 144.4, 138.9, 132.0, 130.0, 126.0, 125.9, 120.1, 119.9, 115.5, 104.4, 55.4, 44.3, 40.8, 37.3, 29.7, 29.2; LCMS (MM : ES + APCI) 372.2 (M + H)+ Anal.Calcd for C27 H29 NO: C, 84.06; H, 7.87; N, 3.77. Found: C, 84.11; H, 7.94; N, 3.72.
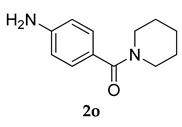
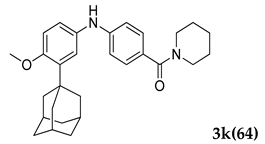
3.2.11. 4.((3-(Adamantan-1-yl)-4-methoxyphenyl)amino)phenyl)(piperidin-1-yl)methanone (3 k)
Off-white colored solid; mp 121–122 °C; 1H NMR (400 MHz, CDCl3) 7.65–7.62 (m, 2 H), 7.45–7.39 (m, 3 H), 6.97–6.95 (d, J = 8.0 Hz, 1 H), 5.37 (s, 1 H), 3.87 (s, 3 H), 3.47–3.39 (m, 4 H), 2.14–2.13 (m, 9 H), 2.08–2.04 (m, 3 H), 1.78 (m, 6 H), 1.45 (m, 6 H); 13CNMR (100 MHz, CDCl3) 170.4, 155.2, 145.6, 138.9, 131.8, 127.0, 125.8, 125.6, 112.5, 55.5, 46.2, 40.5, 37.2, 37.1, 29.7, 29.1, 24.4; LCMS (MM : ES + APCI) 445.2 (M + H)+; Anal.Calcd for C29 H36 N2 O2: C 78.34; H 8.16; N 6.30; Found: C 78.39; H 8.10; N 6.24.
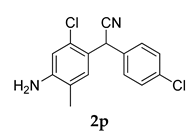
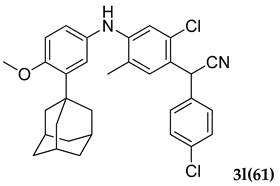
3.2.12. 2.(4-((3-Adamantan-1-yl)-4-methoxyphenyl)amino)-2-chloro-5-methylphenyl)-2-(4-chlorophenyl)acetonitrile (3 l)
Yellow colored solid; mp 129–132 °C; 1H NMR (300 MHz, CDCl3) 7.62–7.51 (m, 3 H), 7.32–7.26 (m, 2 H), 6.84–6.81 (d, J = 12.0 Hz, 2 H), 6.35–6.25 (m, 2 H), 5.66 (s, 1 H), 5.27 (s, 1 H), 3.82 (s, 3 H), 2.16–1.66 (m, 18 H); 13C NMR (75 MHz,CDCl3) 156.0, 1139.8, 136.6, 133.3, 126.8, 125.8, 125.6, 123.3, 121.7, 117.6, 55.3, 42.5, 41.6, 41.1, 40.9, 36.9, 36.5, 36.1, 35.6, 28.5, 27.9, 18.13; HRMS Calcd: 553.1784; Found: 553.1892 (M + H)+; Anal.Calcd for C32 H32 Cl2 N2 O: C 72.31; H 6.07; N 5.27; Found: C 72.39; H 6.01; N 5.24.
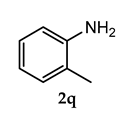
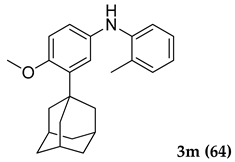
Off-white colored solid; mp 118–120 °C; 3-(adamantan-1-yl)-4-methoxy-N-(o-tolyl)aniline (3 m): 1H NMR (400 MHz, CDCl3) 7.40 (s, 1 H), 7.36 (d, 1 H), 7.17–7.10 (m, 4 H), 6.94 (d, 1 H), 5.37 (s, 1 H), 3.87 (s, 3 H), 2.41 (s, 3 H), 2.14 (m, 6 H), 2.06 (m, 3 H), 1.77 (m, 6 H); HRMS Calcd 370.214. Found: 370.212 (M + H)+.
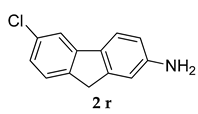
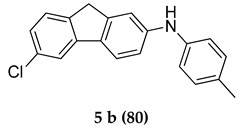
6-Chloro-N-(p-tolyl)-9 H-fluoren-2-amine (5 b)
Yellow colored solid; mp 131–132 °C 1H NMR (400 MHz, DMSO-d6); 8.10 (s, 1 H), 8.05–8.03 (d, J = 8 Hz, 1 H), 7.76 (s, 1 H), 7.53–7.51 (d, J = 8.0 Hz, 1 H), 7.38–7.21 (m, 4 H), 7.16–7.12 (m, 2 H), 5.36 (s, 1 H), 4,37 (s, 2 H), 2.39 (s, 3 H); 13C NMR (100 MHz, DMSO-d6); 140.7, 140.5, 137.8, 135.5, 129.1, 127.8, 127.5, 127.0, 125.4, 123.2, 121.4, 119.9, 112.1, 110.6, 41.20, 23.5; HRMS Calcd 328.0863; Found: 328.0866 (M + Na)+; Anal.Calcd for C20 H16 ClN: C, 78.55; H, 5.27; N, 4.58; Found: C, 78.58; H, 5.21; N, 4.55.
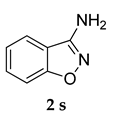
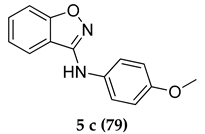
N-(4-Methoxyphenyl)benzo[d]isoxazol-3-amine (5 c)
White colored solid; mp 98–100 °C: 1H NMR (400 MHz, DMSO-d6); 8.44–8.42 (d, J = 8.0 Hz, 1 H), 8.08–8.06 (d, J = 8.0 Hz, 1 H), 8.02–8.00 (d, J = 8.0 Hz, 1 H), 7.95–7.91 (m, J = 8.0 Hz, 2 H), 7.71–7.67 (m, 1 H), 7.26–7.24 (d, J = 8.0 Hz, 2 H), 5.32 (s, 1 H), 3.88 (s, 3 H); 13C NMR (100 MHz, DMSO-d6); 164.1, 159.7, 152.0, 147.4, 132.2, 127.2,125.6, 123.4, 121.7, 118.9, 114.4, 113.8, 55.2; HRMS Calcd 263.0791; Found: 263.0794 (M + Na+); Anal.Calcd for C14 H12 N2 O2: C, 69.99; H, 5.03; N, 11.66; Found: C, 70.05; H, 5.08; N, 11.59.
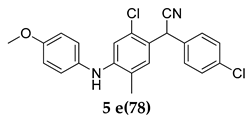
2-(2-Chloro-4-((4-methoxyphenyl)amino)-5-methylphenyl)-2-(4-chlorophenyl)acetonitrile (5 e)
Off-white colored solid; mp 111–112 °C: 1H NMR (400 MHz, DMSO-d6); 7.57–7.53 (m, 3 H), 7.51–7.42 (m, 3 H), 7.35–7.31 (m, 2 H), 7.08–7.02 (m, 2 H), 5.72 (s, 1 H), 5.32 (s, 1 H), 3.83 (s, 3 H), 2.13 (s, 3 H); 13C NMR (100 MHz, DMSO-d6); 152.0, 149.8, 141.1, 140.0, 137.1, 132.4, 132.3, 132.1, 129.1, 128.6, 123.1, 120.8, 116.1, 54.99, 36.6, 17.9; HRMS Calcd 419.0688; Found: 419.0692 (M + Na+); Anal.Calcd for C22 H18 Cl2 N2 O: C, 66.51; H, 4.57; N, 7.05; Found: C, 66.59; H, 4.52; N, 7.11.
4. Conclusions
In conclusion, we prepared PS-Co (BBZN)Cl2 catalyst and used it for the C−N bond formation reaction. A series of adamantyl-tethered-amino biphenylic compounds were synthesized by new protocol. Our synthetic methodology is much improved compared to existing methodologies as the catalyst is effective, inexpensive and recyclable.
Supplementary Materials
The following are available online at https://www.mdpi.com/2073-4344/10/11/1315/s1, SI-01: Experiment Section, SI-02: Spectral characterization Co(BBZN)Cl2, SI-03 to14: Spectral characterization of compounds 4 a-4 l.
Author Contributions
B.C.P., S.E.R., S.N.D. performed the organic synthesis and material characterization experiments; P.K.M., G.V., B.S.P., V.P., P.E.L. and R.K.S. supported through suggestions and guidance; K.F.-U.-R. and G.P. provided DFT calculations; B. designed the research, provided resources and wrote the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
B. thanks Council of Scientific and Industrial Research (No. 02(0291)17/EMR-II), Department of Biotechnology (BT/PR24978/NER/95/938/2017), Vision Group on Science and Technology, Government of Karnataka for funding. PEL thanks Tsinghua Berkeley Shenzhen Institute Faculty Start-Up Fund, the Shenzhen Development and Reform Commission Subject Construction Project for funding.
Conflicts of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Rasheed, S.; Rao, D.N.; Das, P. Copper-Catalyzed Inter- and Intramolecular C-N Bond Formation: Synthesis of Benzimidazole-Fused Heterocycles. J. Org. Chem. 2015, 80, 9321–9327. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Huang, Q.; Zhou, X.; Yu, L.; Li, Z.; Wu, D. Synthesis of pyrido[1,2-a]benzimidazoles through a copper-catalyzed cascade C-N coupling process. Eur. J. Org. Chem. 2011, 2011, 5242–5245. [Google Scholar] [CrossRef]
- Hesp, K.D.; Bergman, R.G.; Ellman, J.A. Rhodium-catalyzed synthesis of branched amines by direct addition of benzamides to imines. Org. Lett. 2012, 14, 2304–2307. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Ye, M.; Zong, C.; Hu, F.; Feng, L.; Wang, X.; Wang, Y.; Chen, C. Copper-catalyzed intramolecular C-N bond formation: A straightforward synthesis of benzimidazole derivatives in water. J. Org. Chem. 2011, 76, 716–719. [Google Scholar] [CrossRef]
- Brain, C.T.; Steer, J.T. An improved procedure for the synthesis of benzimidazoles, using palladium-catalyzed aryl-amination chemistry. J. Org. Chem. 2003, 68, 6814–6816. [Google Scholar] [CrossRef] [PubMed]
- Wolfe, J.P.; Wagaw, S.; Marcoux, J.; Buchwald, S.L. Rational Development of Practical Catalysts for Aromatic Carbon−nitrogen bond formation. Acc. Chem. Res. 1998, 31, 805–818. [Google Scholar] [CrossRef]
- Tsang, W.C.P.; Zheng, N.; Buchwald, S.L. Combined C-H functionalization/C-N bond formation route to carbazoles. J. Am. Chem. Soc. 2005, 127, 14560–14561. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.G.; Buchwald, S.L. Palladium-catalyzed amination of aryl halides and sulfonates. J. Organomet. Chem. 1999, 576, 125–146. [Google Scholar] [CrossRef]
- Corcoran, E.B.; Pirnot, M.T.; Lin, S.; Dreher, S.D.; Dirocco, D.A.; Davies, I.W.; Buchwald, S.L.; Macmillan, D.W.C. Aryl amination using ligand-free Ni(II) salts and photoredox catalysis. Science 2016, 353, 279–283. [Google Scholar] [CrossRef]
- Toma, G.; Yamaguchi, R. Cobalt-catalyzed C-N bond-forming reaction between chloronitrobenzenes and secondary amines. Eur. J. Org. Chem. 2010, 2010, 6404–6408. [Google Scholar] [CrossRef]
- Ahmad, K.; Chang, C.R.; Li, J. Mechanistic investigations of Co(II)-Catalyzed C-N coupling reactions. J. Organomet. Chem. 2018, 868, 144–153. [Google Scholar] [CrossRef]
- Ruiz-Castillo, P.; Buchwald, S.L. Applications of Palladium-Catalyzed C-N Cross-Coupling Reactions. Chem. Rev. 2016, 116, 12564–12649. [Google Scholar] [CrossRef] [PubMed]
- Cahiez, G.; Moyeux, A. Cobalt-catalyzed cross-coupling reactions. Chem. Rev. 2010, 110, 1435–1462. [Google Scholar] [CrossRef]
- Sherrington, D.C. Polymer-supported metal complex alkene epoxidation catalysts. Catal. Today 2000, 57, 87–104. [Google Scholar] [CrossRef]
- Eshwar Rao, S.; Gayathri, V. Poly(styrene–divinyl benzene)-immobilized Fe(III) complex of 1,3-bis(benzimidazolyl)benzene: Efficient catalyst for the photocatalytic degradation of xylenol orange. J. Appl. Polym. Sci. 2018, 135, 1–13. [Google Scholar] [CrossRef]
- Fan, Q.H.; Ren, C.Y.; Yeung, C.H.; Hu, W.H.; Chan, A.S.C. Highly effective soluble polymer-supported catalysts for asymmetric hydrogenation. J. Am. Chem. Soc. 1999, 121, 7407–7408. [Google Scholar] [CrossRef]
- Howard, I.C.; Hammond, C.; Buchard, A. Polymer-supported metal catalysts for the heterogeneous polymerisation of lactones. Polym. Chem. 2019, 10, 5894–5904. [Google Scholar] [CrossRef]
- Annis, D.A.; Jacobsen, E.N. Polymer-supported chiral Co(salen) complexes: Synthetic applications and mechanistic investigations in the hydrolytic kinetic resolution of terminal epoxides. J. Am. Chem. Soc. 1999, 121, 4147–4154. [Google Scholar] [CrossRef]
- Karjalainen, J.K.; Hormi, O.E.O.; Sherrington, D.C. Efficient Polymer-Supported Sharpless Alkene Epoxidation Catalyst. Molecules 1998, 3, 51–59. [Google Scholar] [CrossRef]
- Canali, L.; Sherrington, D.C. Utilisation of homogeneous and supported chiral metal(salen) complexes in asymmetric catalysis. Chem. Soc. Rev. 1999, 28, 85–93. [Google Scholar] [CrossRef]
- Sherrington, D.C. Polymer-supported metal complex oxidation catalysts. Pure Appl. Chem. 1988, 60, 401–414. [Google Scholar] [CrossRef]
- Conte, V.; Floris, B. Vanadium catalyzed oxidation with hydrogen peroxide. Inorg. Chim. Acta 2010, 363, 1935–1946. [Google Scholar] [CrossRef]
- Farzaneh, S.; Zarghi, A. Estrogen receptor ligands: A review (2013–2015). Sci. Pharm. 2016, 84, 409–427. [Google Scholar] [CrossRef]
- Sebastian, A.; Pandey, V.; Mohan, C.D.; Chia, Y.T.; Rangappa, S.; Mathai, J.; Baburajeev, C.P.; Paricharak, S.; Mervin, L.H.; Bulusu, K.C.; et al. Novel adamantanyl-based thiadiazolyl pyrazoles targeting EGFR in triple-negative breast cancer. ACS Omega 2016, 1, 1412–1424. [Google Scholar] [CrossRef]
- Anusha, S.; Mohan, C.D.; Ananda, H.; Baburajeev, C.P.; Rangappa, S.; Mathai, J.; Fuchs, J.E.; Li, F.; Shanmugam, M.K.; Bender, A.; et al. Adamantyl-tethered-biphenylic compounds induce apoptosis in cancer cells by targeting Bcl homologs. Bioorg. Med. Chem. Lett. 2016, 26, 1056–1060. [Google Scholar] [CrossRef]
- Sadashiva, M.P.; Nanjundaswamy, S.; Li, F.; Manu, K.A.; Sengottuvelan, M.; Prasanna, D.S.; Anilkumar, N.C.; Sethi, G.; Sugahara, K.; Subbegowda, K.; et al. Anti-cancer activity of novel dibenzo[b,f]azepine tethered isoxazoline derivatives. BMC Chem. Biol. 2012, 12. [Google Scholar] [CrossRef]
- Ningegowda, R.; Shivananju, N.S.; Rajendran, P.; Basappa; Rangappa, K.S.; Chinnathambi, A.; Li, F.; Achar, R.R.; Shanmugam, M.K.; Bist, P.; et al. A novel 4,6-disubstituted-1,2,4-triazolo-1,3,4-thiadiazole derivative inhibits tumor cell invasion and potentiates the apoptotic effect of TNFα by abrogating NF-κB activation cascade. Apoptosis 2017, 22, 145–157. [Google Scholar] [CrossRef]
- Priya, B.S.; Nanjunda Swamy, S.; Tejesvi, M.V.; Basappa; Sarala, G.; Gaonkar, S.L.; Naveen, S.; Shashidhara Prasad, J.; Rangappa, K.S. Synthesis, characterization, antimicrobial and single crystal X-ray crystallographic studies of some new sulfonyl, 4-chloro phenoxy benzene and dibenzoazepine substituted benzamides. Eur. J. Med. Chem. 2006, 41, 1262–1270. [Google Scholar] [CrossRef]
- Anusha, S.; Anandakumar, B.S.; Mohan, C.D.; Nagabhushana, G.P.; Priya, B.S.; Rangappa, K.S. Preparation and use of combustion-derived Bi2O3 for the synthesis of heterocycles with anti-cancer properties by Suzuki-coupling reactions. RSC Adv. 2014, 4, 52181–52188. [Google Scholar] [CrossRef]
- Rakesh, K.S.; Jagadish, S.; Vinayaka, A.C.; Hemshekhar, M.; Paul, M.; Thushara, R.M.; Sundaram, M.S.; Swaroop, T.R.; Mohan, C.D.; Sadashiva, M.P.; et al. A new ibuprofen derivative inhibits platelet aggregation and ros mediated platelet apoptosis. PLoS ONE 2014, 9, e2718. [Google Scholar] [CrossRef]
- Nirvanappa, A.C.; Mohan, C.D.; Rangappa, S.; Ananda, H.; Sukhorukov, A.Y.; Shanmugam, M.K.; Sundaram, M.S.; Nayaka, S.C.; Girish, K.S.; Chinnathambi, A.; et al. Novel synthetic oxazines target NF-κB in colon cancer in vitro and inflammatory bowel disease in vivo. PLoS ONE 2016, 11, e0163209. [Google Scholar] [CrossRef] [PubMed]
- Tan, B.Y.H.; Teo, Y.C. Efficient cobalt-catalyzed C-N cross-coupling reaction between benzamide and aryl iodide in water. Org. Biomol. Chem. 2014, 12, 7478–7481. [Google Scholar] [CrossRef] [PubMed]
- Moselage, M.; Li, J.; Ackermann, L. Cobalt-Catalyzed C-H Activation. ACS Catal. 2016, 6, 498–525. [Google Scholar] [CrossRef]
- Ibrahim, H.; Bala, M.D. Air stable pincer (CNC) N-heterocyclic carbene-cobalt complexes and their application as catalysts for C-N coupling reactions. J. Organomet. Chem. 2015, 794, 301–310. [Google Scholar] [CrossRef]
- Yanai, T.; Tew, D.P.; Handy, N.C. A new hybrid exchange-correlation functional using the Coulomb-attenuating method (CAM-B3LYP). Chem. Phys. Lett. 2004, 393, 51–57. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09, Revision D.01; Gaussian Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).