Effects of Supports BET Surface Areas on Membrane Electrode Assembly Performance at High Current Loads
Abstract
1. Introduction
2. Results
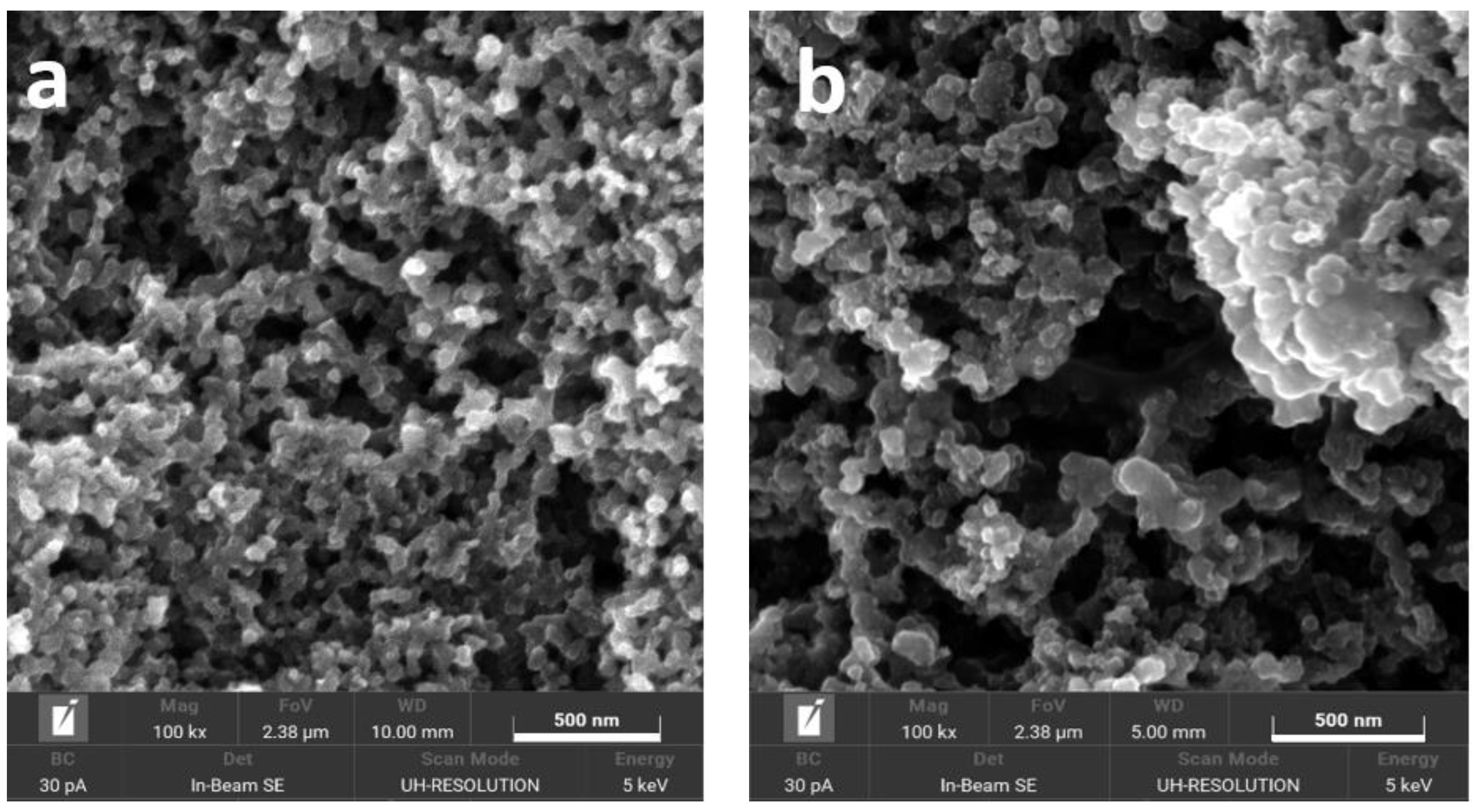
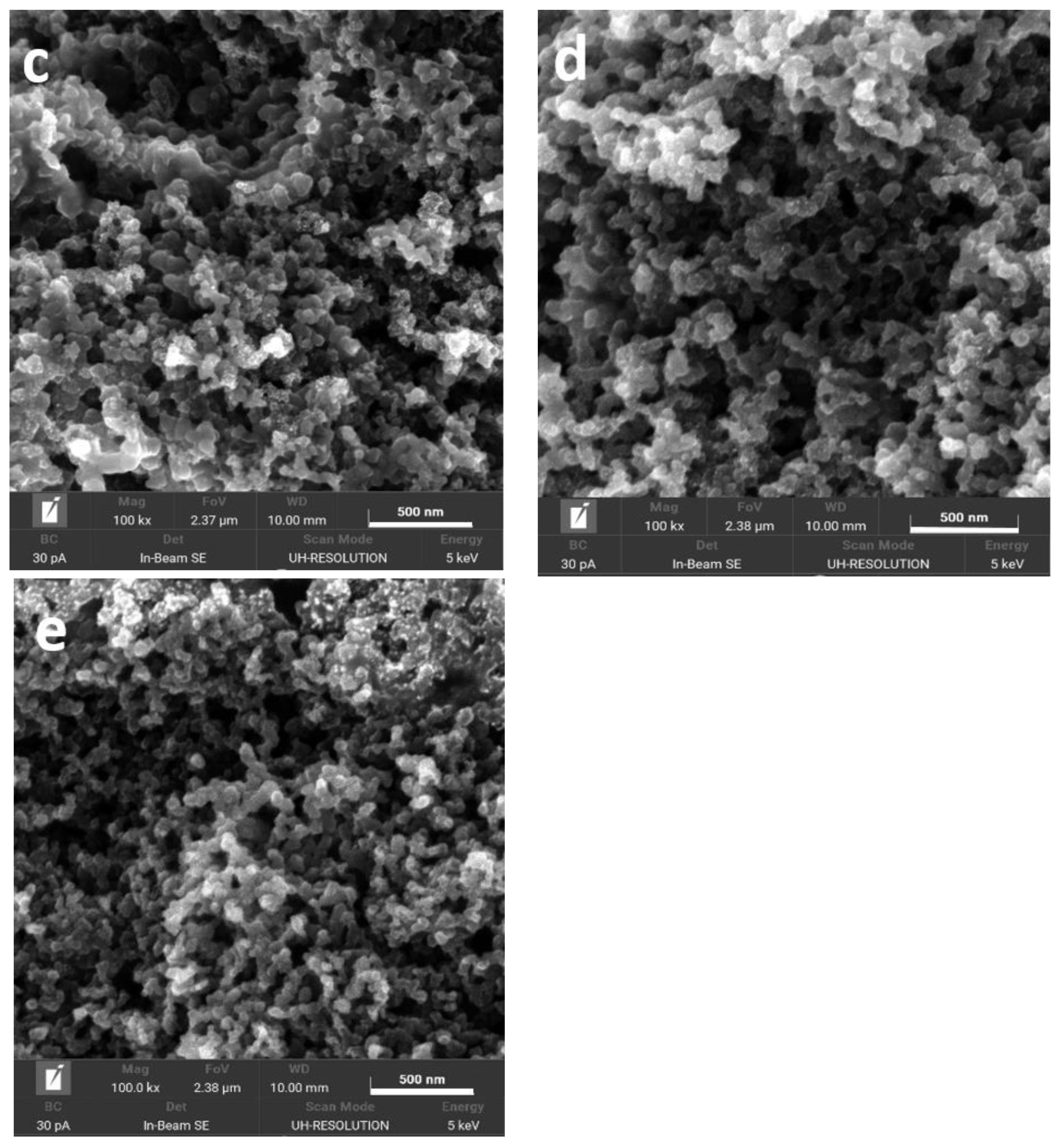
2.1. Physical Characterization of the Supports and the Catalysts
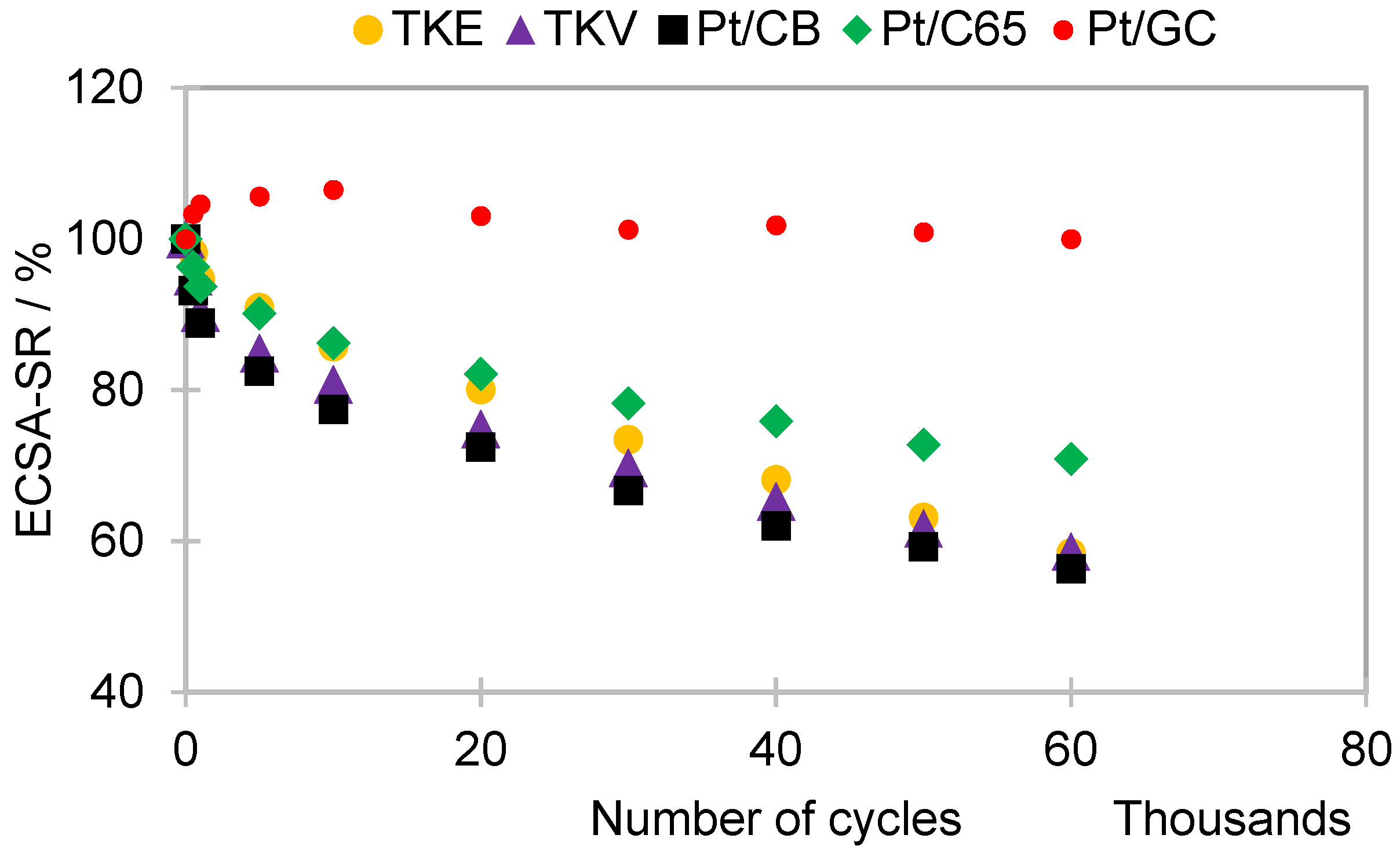
2.2. Supports Corrosion
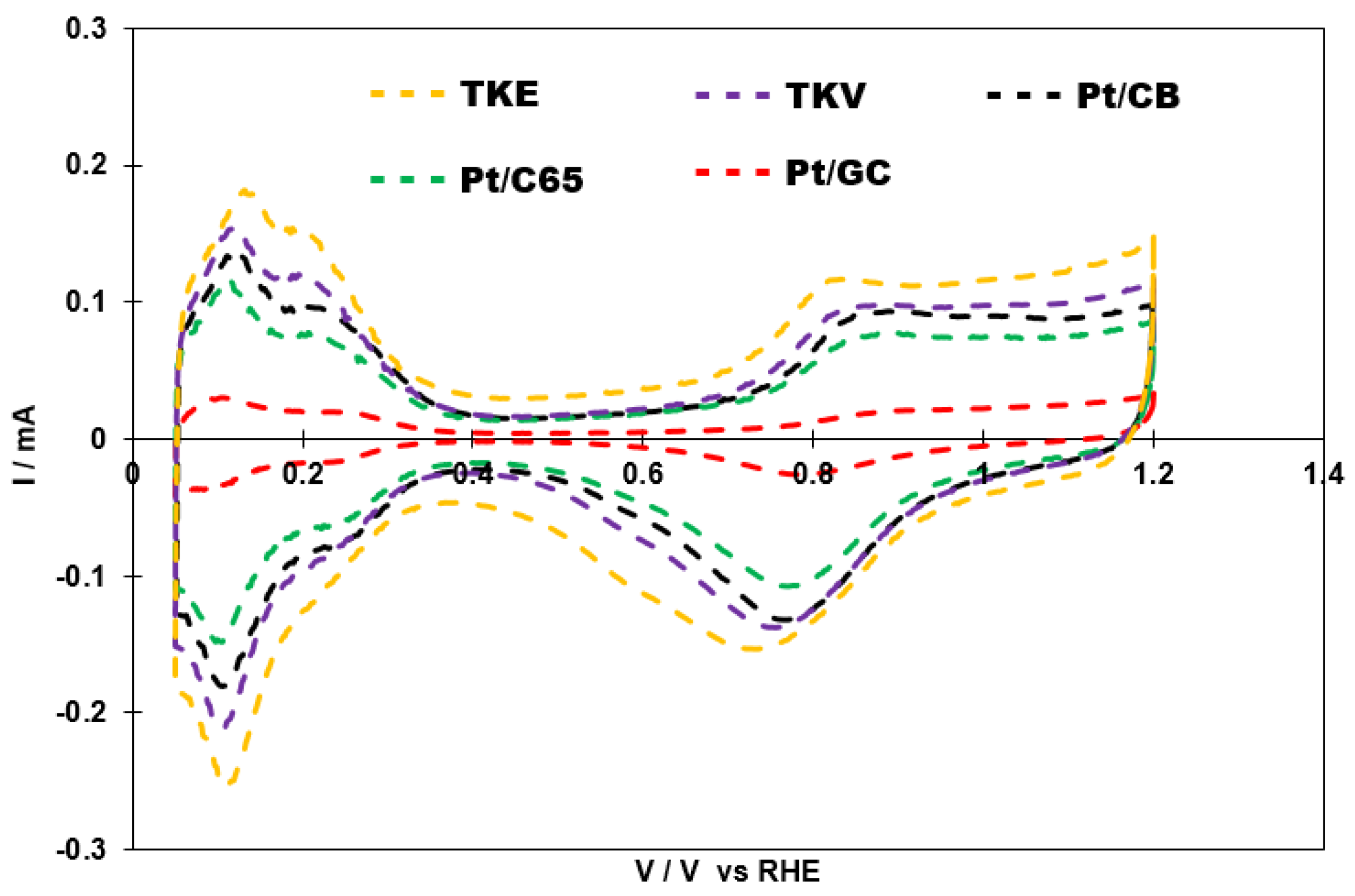
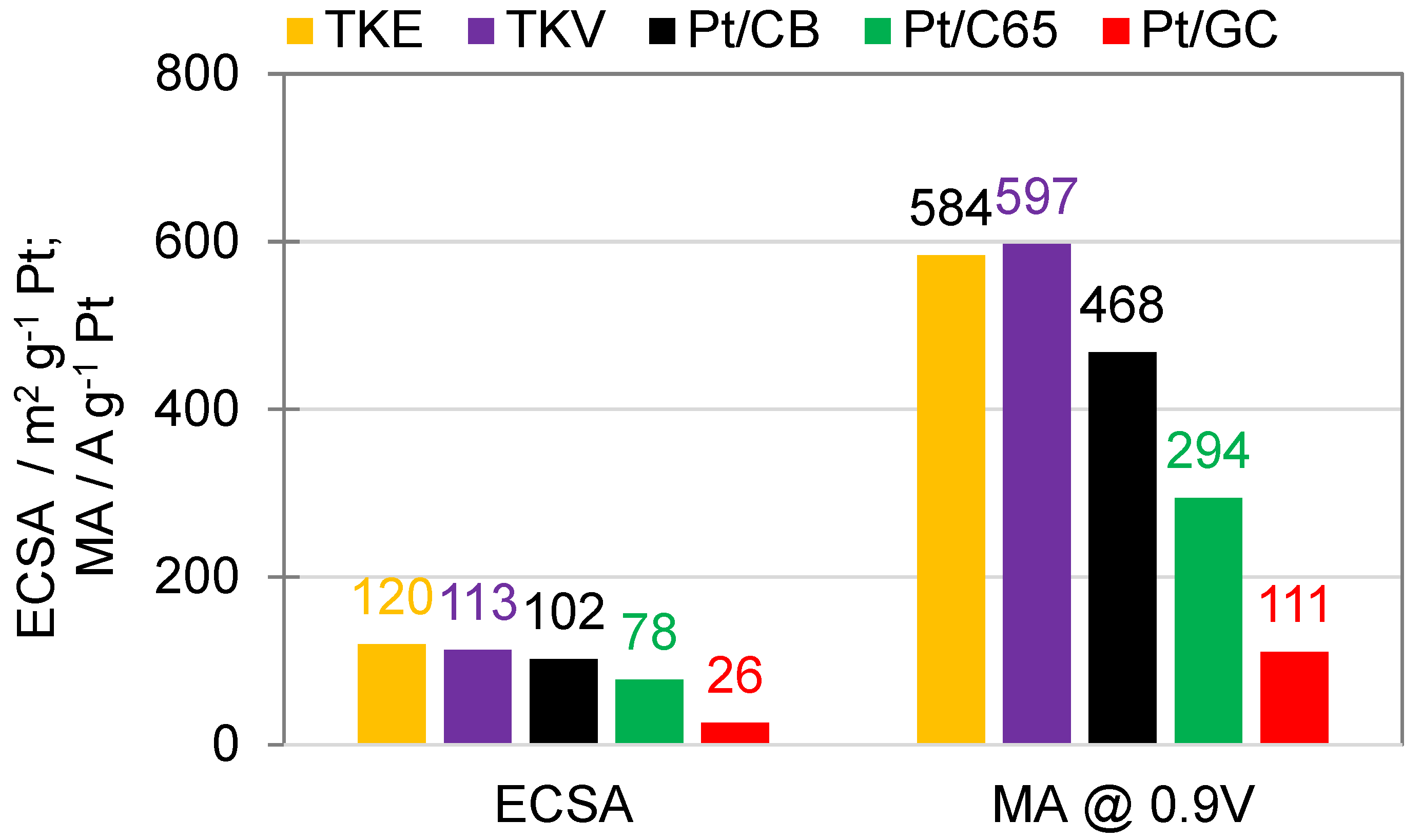
2.3. ECSA and ORR Activities of the Catalysts
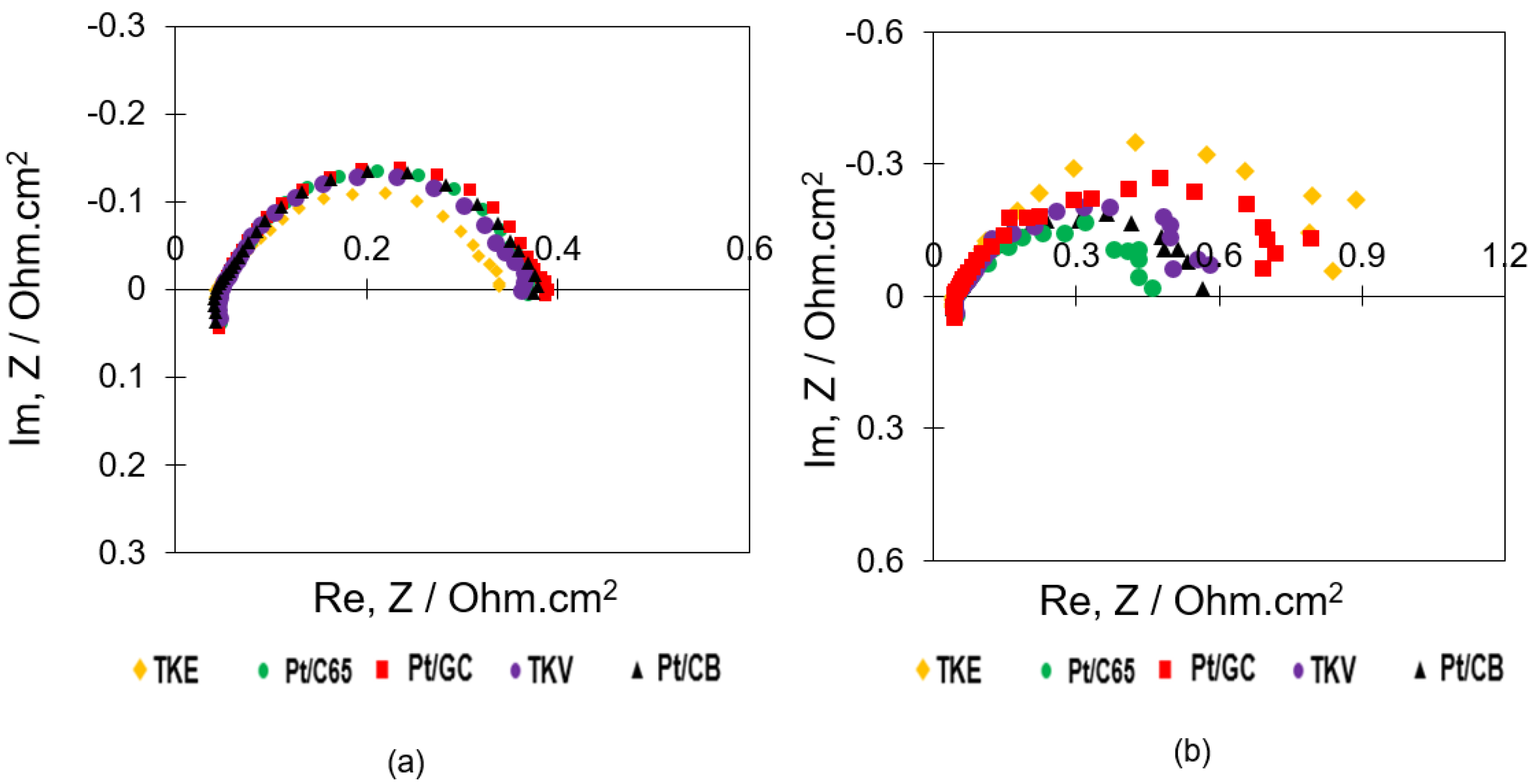
2.4. MEA Performance and Diagnostics
3. Summary and Conclusions
4. Materials and Methods
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Roen, L.M.; Paik, C.H.; Jarvi, T.D. Electrocatalytic Corrosion of Carbon Support in PEMFC Cathodes. Electrochem. Solid State Lett. 2004, 7, A19–A22. [Google Scholar] [CrossRef]
- Kreitmeier, S.; Wokaun, A.; Büchi, F.N. Local Catalyst Support Degradation during Polymer Electrolyte Fuel Cell Start-Up and Shutdown. J. Electrochem. Soc. 2012, 159, F787–F793. [Google Scholar] [CrossRef]
- Selvaganesh, S.V.; Sridhar, P.; Pitchumani, S.; Shukla, A.K. A Durable Graphitic-Carbon Support for Pt and Pt3Co Cathode Catalysts in Polymer Electrolyte Fuel Cells. J. Electrochem. Soc. 2012, 160, F49–F59. [Google Scholar] [CrossRef]
- Takei, C.; Kakinuma, K.; Kawashima, K.; Tashiro, K.; Watanabe, M.; Uchida, M. Load cycle durability of a graphitized carbon black-supported platinum catalyst in polymer electrolyte fuel cell cathodes. J. Power Sources 2016, 324, 729–737. [Google Scholar] [CrossRef]
- Kim, J.Y.; Lee, S.; Kim, T.-Y.; Pak, C.; Kim, H.-T. Highly durable electrocatalyst with graphitized carbon supports modified by diazonium reaction for polymer electrolyte membrane fuel cell. Carbon 2014, 77, 525–537. [Google Scholar] [CrossRef]
- Mohanta, P.K.; Regnet, F.; Jörissen, L. Graphitized Carbon: A Promising Stable Cathode Catalyst Support Material for Long Term PEMFC Applications. Materials 2018, 11, 907. [Google Scholar] [CrossRef]
- Shao-Horn, Y.; Sheng, W.C.; Chen, S.; Ferreira, P.J.; Holby, E.F.; Morgan, D. Instability of supported Pt nanoperticles in low temperature fuel cells. Top. Catal. 2007, 46, 285–305. [Google Scholar] [CrossRef]
- Wang, X.X.; Tan, Z.H.; Zeng, M.; Wanga, J.N. Carbon nanocages: A new support material for Pt catalyst with remarka-bly high durability. Sci. Rep. 2014, 4, 4437. [Google Scholar] [CrossRef]
- Peera, S.G.; Arunchander, A.; Sahu, A. Platinum nanoparticles supported on nitrogen and fluorine co-doped graphite nanofibers as an excellent and durable oxygen reduction catalyst for polymer electrolyte fuel cells. Carbon 2016, 107, 667–679. [Google Scholar] [CrossRef]
- Golovin, V.A.; Maltsev, N.V.; Gribov, E.N.; Okunev, A.G. New nitrogen-containing carbon supports with improved cor-rosion resistance for proton exchange membrane fuel cells. Int. J. Hydrog. Energy 2017, 42, 11159–11165. [Google Scholar] [CrossRef]
- Chen, M.; Hwang, S.; Li, J.; Karakalos, S.G.; Chen, K.; He, Y.; Mukherjee, S.; Su, D.; Wu, G. Pt alloy nanoparticles decorated on large-size nitrogen-doped graphene tubes for highly stable oxygen-reduction catalysts. Nanoscale 2018, 10, 17318–17326. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.; Popov, B.N. Development of highly-active and stable Pt/C catalyst for polymer electrolyte membrane fuel cells under simulated start-up/shut-down cycling. Int. J. Hydrogen Energy 2016, 41, 1828–1836. [Google Scholar] [CrossRef]
- Sebastián, D.; Alegre, C.; Gálvez, M.E.; Moliner, R.; Lázaro, M.J.; Aricò, A.S.; Baglio, V. Towards new generation fuel cell electrocatalysts based on xerogel–nanofiber carbon composites. J. Mater. Chem. A 2014, 2, 13713. [Google Scholar] [CrossRef]
- Yli-Rantala, E.; Pasanen, A.; Kauranen, P.; Ruiz, V.; Borghei, M.; Kauppinen, E.I.; Oyarce, A.; Lindbergh, G.; Lagergren, C.; Darab, M.; et al. Graphitised Carbon Nanofibres as Catalyst Support for PEMFC. Fuel Cells 2011, 11, 715–725. [Google Scholar] [CrossRef]
- Wang, Y.; Li, G.; Jin, J.; Yang, S. Hollow porous carbon nanofibers as novel support for platinum-based oxygen reduction reaction electrocatalysts. Int. J. Hydrog. Energy 2017, 42, 5938–5947. [Google Scholar] [CrossRef]
- Sakae, T.; Hiroshi, M.; Hideki, M.; Eishi, T.; Masahiro, K. High durability of carbon nanotube-supported Pt electrocata-lysts covered with silica layers for the cathode in a PEMFC. J. Electrochem. Soc. 2008, 155, B929. [Google Scholar]
- Mohanta, P.K.; Regnet, F.; Jörissen, L. Impact of Highly Stable Catalyst Support Materials on Polymer Electrolyte Membrane Fuel Cell Performance. Energy Technol. 2020, 8, 2000081. [Google Scholar] [CrossRef]
- Huang, S.; Ganesan, P.; Park, S.; Popov, B.N. Development of a Titanium Dioxide-Supported Platinum Catalyst with Ul-trahigh Stability for Polymer Electrolyte Membrane Fuel Cell Applications. J. Am. Chem. Soc. 2009, 131, 13898–13899. [Google Scholar] [CrossRef]
- Zhang, P.; Huang, S.; Popov, B.N. Mesoporous Tin Oxide as an Oxidation-Resistant Catalyst Support for Proton Ex-change Membrane Fuel Cells. J. Electrochem. Soc. 2010, 157, B1163–B1172. [Google Scholar] [CrossRef]
- Ioroi, T.; Akita, T.; Yamazaki, S.; Siroma, Z.; Fujiwara, N.; Yasuda, K. Corrosion-Resistant PEMFC Cathode Catalysts Based on a Magnéli-Phase Titanium Oxide Support Synthesized by Pulsed UV Laser Irradiation. J. Electrochem. Soc. 2011, 158, C329–C334. [Google Scholar] [CrossRef]
- Masao, S.; Noda, F.; Takasaki, K.; Ito, K.; Sasaki, K. Carbon-Free Pt Electrocatalysts Supported on SnO2 for Polymer Elec-trolyte Fuel Cells. Electrochem. Solid State Lett. 2009, 12, B119–B122. [Google Scholar] [CrossRef]
- Mohanta, P.K.; Glökler, C.; Arenas, A.O.; Jörissen, L. Sb doped SnO2 as a stable cathode catalyst support for low temper-ature polymer electrolyte membrane fuel cell. Int. J. Hydrog. Energy 2017, 42, 27950–27961. [Google Scholar] [CrossRef]
- Cavaliere, S.; Jiménez-Morales, I.; Ercolano, G.; Savych, I.; Jones, D.J.; Rozière, J. Highly Stable PEMFC Electrodes Based on Electrospun Antimony-Doped SnO2. ChemElectroChem 2015, 2, 1966–1973. [Google Scholar] [CrossRef]
- Dou, M.; Hou, M.; Wang, F.; Liang, D.; Zhao, Q.; Shao, Z.; Yi, B. Sb-Doped SnO2Supported Platinum Catalyst with High Stability for Proton Exchange Membrane Fuel Cells. J. Electrochem. Soc. 2014, 161, F1231–F1236. [Google Scholar] [CrossRef]
- Yin, M.; Xu, J.; Li, Q.; Jensen, J.O.; Huang, Y.; Cleemann, L.N.; Bjerrum, N.J.; Xing, W. Highly active and stable Pt elec-trocatalysts promoted by antimony-doped SnO2 supports for oxygen reduction reaction. Appl. Catal. B Environ. 2014, 144, 112–120. [Google Scholar] [CrossRef]
- Gurrola, M.; Guerra-Balcázar, M.; Álvarez-Contreras, L.; Nava, R.; Ledesma-García, J.; Arriaga, L.G. High surface electrochemical support based on Sb-doped SnO 2. J. Power Sources 2013, 243, 826–830. [Google Scholar] [CrossRef]
- Sun, S.; Zhang, G.; Sun, X.; Cai, M.; Ruthkosky, M. Highly Stable and Active Pt/Nb-TiO2 Carbon-Free Electrocatalyst for Proton Exchange Membrane Fuel Cells. J. Nanotechnol. 2012, 2012, 389505. [Google Scholar] [CrossRef]
- Park, I.-S.; Lee, E.; Manthiram, A. Electrocatalytic Properties of Indium Tin Oxide-Supported Pt Nanoparticles for Methanol Electro-oxidation. J. Electrochem. Soc. 2010, 157, B251–B255. [Google Scholar] [CrossRef]
- Huang, K.; Sasaki, K.; Adzic, R.R.; Xing, Y. Increasing Pt oxygen reduction reaction activity and durability with a car-bon-doped TiO2 nanocoating catalyst support. J. Mater. Chem. 2012, 22, 16824–16832. [Google Scholar] [CrossRef]
- Liu, X.; Chen, J.; Liu, G.; Zhang, L.; Zhang, H.; Yi, B. Enhanced long-term durability of proton exchange membrane fuel cell cathode by employing Pt/TiO2/C catalysts. J. Power Sources 2010, 195, 4098–4103. [Google Scholar] [CrossRef]
- Chiwata, M.; Kakinuma, K.; Wakisaka, M.; Uchida, M.; Deki, S.; Watanabe, M.; Uchida, H. Oxygen Reduction Reaction Activity and Durability of Pt Catalysts Supported on Titanium Carbide. Catalysts 2015, 5, 966–980. [Google Scholar] [CrossRef]
- Zheng, W.; Wang, L.; Deng, F.; Giles, S.A.; Prasad, A.K.; Advani, S.G.; Yan, Y.; Vlachos, D.G. Durable and self-hydrating tungsten carbide-based composite polymer electrolyte membrane fuel cells. Nat. Commun. 2017, 8, 418. [Google Scholar] [CrossRef] [PubMed]
- Roth, C.; Bleith, P.; Schwöbel, C.A.; Kaserer, S.; Eichler, J. Importance of Fuel Cell Tests for Stability Assess-ment—Suitability of Titanium Diboride as an Alternative Support Material. Energies 2014, 7, 3642–3652. [Google Scholar] [CrossRef]
- Bezerra, C.W.; Zhang, L.; Liu, H.; Lee, K.; Marques, A.L.; Marques, E.P.; Wang, H.; Zhang, J. A review of heat-treatment effects on activity and stability of PEM fuel cell catalysts for oxygen reduction reaction. J. Power Sources 2007, 173, 891–908. [Google Scholar] [CrossRef]
- Han, K.S.; Moon, Y.–S.; Han, O.H.; Hwang, K.J.; Kim, I.; Kim, H. Heat treatment and potential cycling effects on surface morphology, particle size, and catalytic activity of Pt/C catalysts studied by 13C NMR, TEM, XRD and CV, Electro-chemistry. Communications 2007, 9, 317–324. [Google Scholar] [CrossRef]
- Zhang, L.; Lee, K.; Zhang, J. The effect of heat treatment on nanoparticle size and ORR activity for carbon-supported Pd–Co alloy electrocatalysts. Electrochim. Acta 2007, 52, 3088–3094. [Google Scholar] [CrossRef]
- Viet, N.; Minh, C.; Nogami, M.; Ohtaki, M. Novel Pt and Pd Based Core-Shell Catalysts with Critical New Issues of Heat Treatment, Stability and Durability for Proton Exchange Membrane Fuel Cells and Direct Methanol Fuel Cells. Heat Treat. Conv. Novel Appl. 2012. [Google Scholar] [CrossRef][Green Version]
- Fuller, T.F.; Gray, G. Carbon corrosion induced by partial hydrogen coverage. ECS Trans. 2006, 1, 345–353. [Google Scholar] [CrossRef]
- Mathias, M.F.; Makharia, R.; Gasteiger, H.A.; Conley, J.J.; Fuller, T.J.; Gittleman, C.J.; Kocha, S.S.; Miller, D.P.; Mittelsteadt, C.K.; Xi, T.; et al. Two Fuel Cell Cars in Every Garage? The Electrochemical Society Interface • Fall 2005. Available online: https://www.electrochem.org/dl/interface/fal/fal05/IF8-05_Pg24-35.pdf (accessed on 11 October 2016).
- Reiser, C.A.; Bregolia, L.; Patterson, W.; Yi, J.S.; Yangb, J.D.; Perry, M.L.; Jarvi, T.D. A Reverse-Current De-cay Mechanism for Fuel Cells. Electrochem. Solid State Lett. 2005, 8, A273–A276. [Google Scholar] [CrossRef]
- Testing Oxygen Reduction Reaction Activity with the Rotating Disc Electrode Technique. Available online: https://www.energy.gov/sites/prod/files/2014/03/f12/webinarslides_rde_technique_031213.pdf (accessed on 13 August 2014).
- Shinozak, K.; Zack, J.W.; Pylypenko, S.; Pivovar, B.S.; Kochaa, S.S. Oxygen Reduction Reaction Measurements on Platinum Electrocatalysts Utilizing Rotating Disk Electrode Technique II. Influence of Ink Formulation, Catalyst Layer Uniformity and Thickness. J. Electrochem. Soc. 2015, 162, F1384–F1396. [Google Scholar] [CrossRef]
- Garsany, Y.; Singer, I.L.; Swider-Lyons, K.E. Impact of film drying procedures on RDE characterization of Pt/VC electrocatalysts. J. Electroanal. Chem. 2011, 662, 396–406. [Google Scholar] [CrossRef]
- Kocha, S.S.; Zack, J.W.; Alia, S.M.; Neyerlin, K.C.; Pivovar, B.S. Influence of Ink Composition on the Electro-chemical Properties of Pt/C Electrocatalysts. ECS Trans. 2013, 50, 1475–1485. [Google Scholar] [CrossRef]
- Mohanta, P.K. Corrosion Resistant Cathode Catalyst Support Materials for Polymer Electrolyte Membrane Fuel Cell. Ph.D. Thesis, Ulm Universität, Ulm, Germany, 2017. [Google Scholar]
- Chen, D.; Kongkanand, A.; Jorne, J. Proton Conduction and Oxygen Diffusion in Ultra-Thin Nafion Films in PEM Fuel Cell: How Thin? J. Electrochem. Soc. 2019, 166, F24–F33. [Google Scholar] [CrossRef]
- Sasikumar, G.; Ihm, J.; Ryu, H. Dependence of optimum Nafion content in catalyst layer on platinum loading. J. Power Sources 2004, 132, 11–17. [Google Scholar] [CrossRef]
- Kongkanand, M.; Mathias, F. The Priority and Challenge of High-Power Performance of Low-Platinum Proton-Exchange Membrane Fuel Cells. J. Phys. Chem. Lett. 2016, 7, 1127–1137. [Google Scholar] [CrossRef]
- Orfanidi, A.; Madkikar, P.; El-Sayed, H.A.; Harzer, G.S.; Kratky, T.; Gasteiger, H.A. The Key to High Performance Low Pt Loaded Electrodes. J. Electrochem. Soc. 2017, 164, F418–F426. [Google Scholar] [CrossRef]
- Mohanta, P.K.; Ripa, M.S.; Regnet, F.; Jörissen, L. Impact of membrane types and catalyst layers composition on PEMFC performance. Chem. Open 2020, 9, 1–10. [Google Scholar]
- Park, Y.-C.; Tokiwa, H.; Kakinuma, K.; Watanabe, M.; Uchida, M. Effects of carbon supports on Pt distribution, ionomer coverage and cathode performance for polymer electrolyte fuel cells. J. Power Sources 2016, 315, 179–191. [Google Scholar] [CrossRef]
- Boehm, H. Chemical Identification of Surface Groups. Adv. Catal. 1966, 16, 179–274. [Google Scholar] [CrossRef]
- Figueiredo, J.; Pereira, M.; Freitas, M.; Órfão, J. Modification of the surface chemistry of activated carbons. Carbon 1999, 37, 1379–1389. [Google Scholar] [CrossRef]
- New Energy and Industrial Technology Development Organization (NEDO). PEFC EvaluationProject, Cell Evaluation and Analysis Protocol Guidline (Electrocatalysts, Supports, Membrane, and MEA); Daido University, Ritsumeikan University, Tokyo Institute of Technology, Japan Automobile Research Institute, PEFC Evaluation Project Promotion Office: Nagoya, Japan, 2014. [Google Scholar]


| Supports | Supports BET, m2 g−1 | Supports El. Conductivity, S cm−1 |
|---|---|---|
| Vulcan XC72 (CB) | 192 (bare) | 2.3 |
| Super C65 (C65) | 174 (oxidized) | 1.0 |
| Timcal RE167 (GC) | 63 (oxidized) | 2.1 |
| Catalyst | Company | Supports | Supports BET, m2 g−1 | Actual Pt, wt% | Pt Crys., nm |
|---|---|---|---|---|---|
| TKE | Tanaka | High surface area CB | 800 * | 28.2 * | 0.7 |
| TKV | Tanaka | Vulcan XC72 | 250 * | 28.9 * | 1.4 |
| Pt/CB | Homemade | Vulcan XC72 | 192 | 28.2 | 2.9 |
| Pt/C65 | Homemade | Super C65 | 174 | 29.1 | 3.3 |
| Pt/GC | Homemade | Timcal RE167 | 63 | 28.7 | 6.8 |
| Catalyst | Supports | Supports BET m2 g−1 | Optimum I/C |
|---|---|---|---|
| TKE | HSCB | 800 | 0.74 |
| TKV | Vulcan XC72 | 250 | 0.72 |
| Pt/CB | Vulcan XC72 | 192 | 0.72 |
| Pt/C65 | Timcal Super C65 | 174 | 0.82 |
| Pt/GC | RE167 | 62 | 0.5 |
| Catalyst | OCP | 0.04 A cm−2 | 0.16 A cm−2 | 1.36 A cm−2 | 2.16 A cm−2 |
|---|---|---|---|---|---|
| TKE | 0.999 | 0.896 | 0.849 | 0.624 | 0.372 |
| TKV | 0.997 | 0.875 | 0.829 | 0.632 | 0.445 |
| Pt/CB | 1.004 | 0.867 | 0.816 | 0.617 | 0.432 |
| Pt/C65 | 1.009 | 0.867 | 0.818 | 0.640 | 0.460 |
| Pt/GC | 0.987 | 0.856 | 0.806 | 0.588 | 0.342 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kumar Mohanta, P.; Ripa, M.S.; Regnet, F.; Jörissen, L. Effects of Supports BET Surface Areas on Membrane Electrode Assembly Performance at High Current Loads. Catalysts 2021, 11, 195. https://doi.org/10.3390/catal11020195
Kumar Mohanta P, Ripa MS, Regnet F, Jörissen L. Effects of Supports BET Surface Areas on Membrane Electrode Assembly Performance at High Current Loads. Catalysts. 2021; 11(2):195. https://doi.org/10.3390/catal11020195
Chicago/Turabian StyleKumar Mohanta, Paritosh, Masuma Sultana Ripa, Fabian Regnet, and Ludwig Jörissen. 2021. "Effects of Supports BET Surface Areas on Membrane Electrode Assembly Performance at High Current Loads" Catalysts 11, no. 2: 195. https://doi.org/10.3390/catal11020195
APA StyleKumar Mohanta, P., Ripa, M. S., Regnet, F., & Jörissen, L. (2021). Effects of Supports BET Surface Areas on Membrane Electrode Assembly Performance at High Current Loads. Catalysts, 11(2), 195. https://doi.org/10.3390/catal11020195





