Synthesis of Au or Pt@Perovskite Nanocrystals via Interfacial Photoreduction
Abstract
1. Introduction
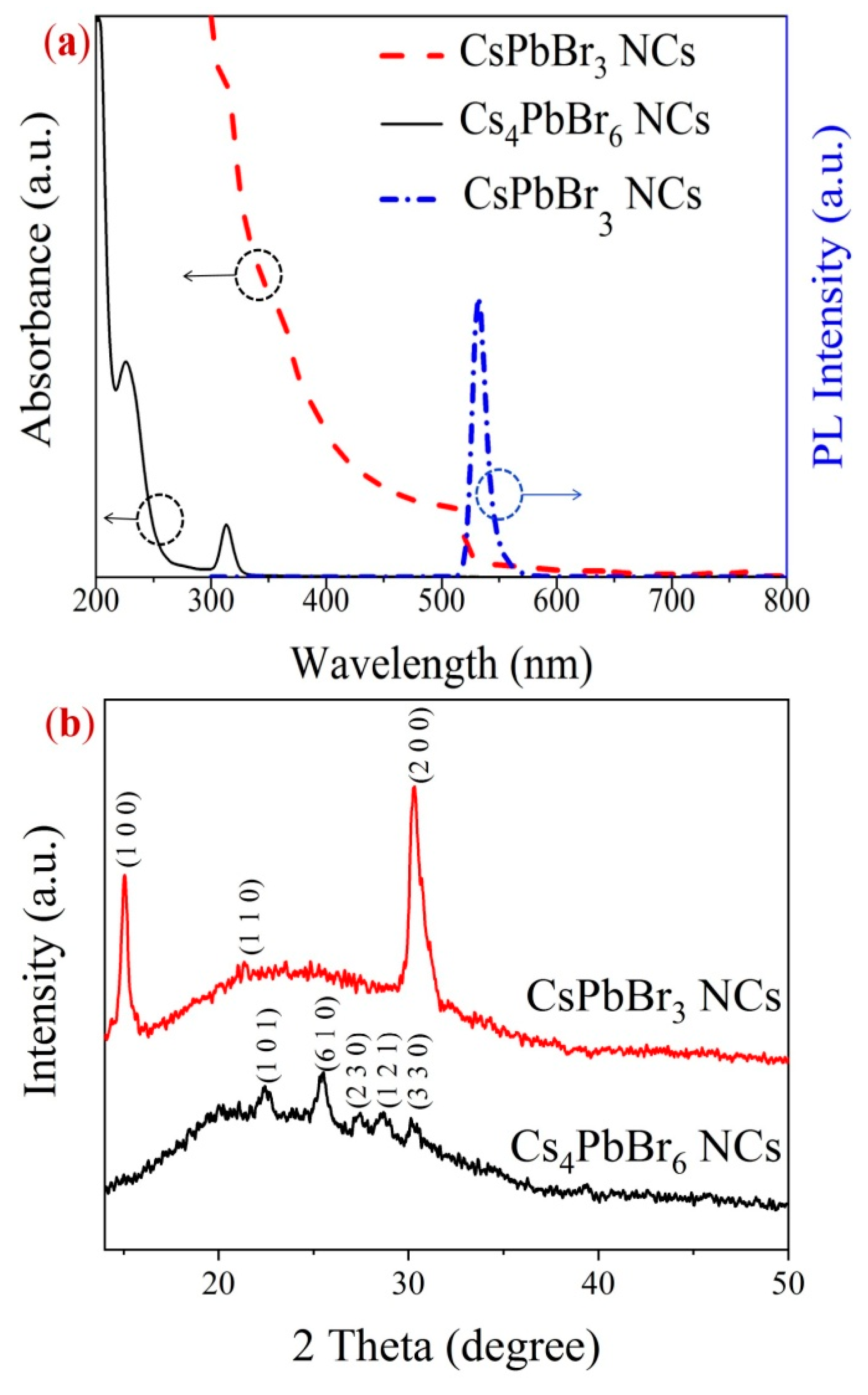
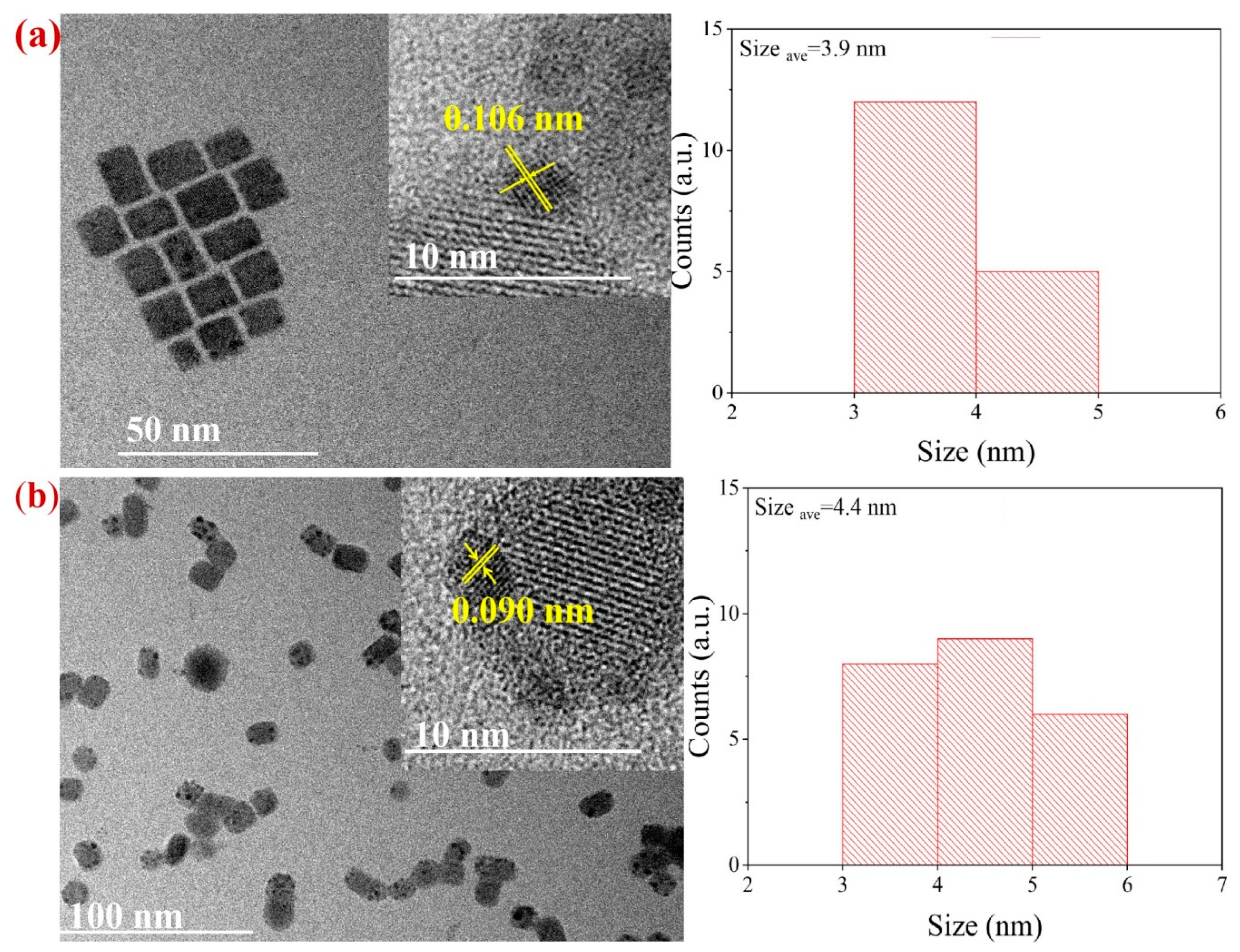
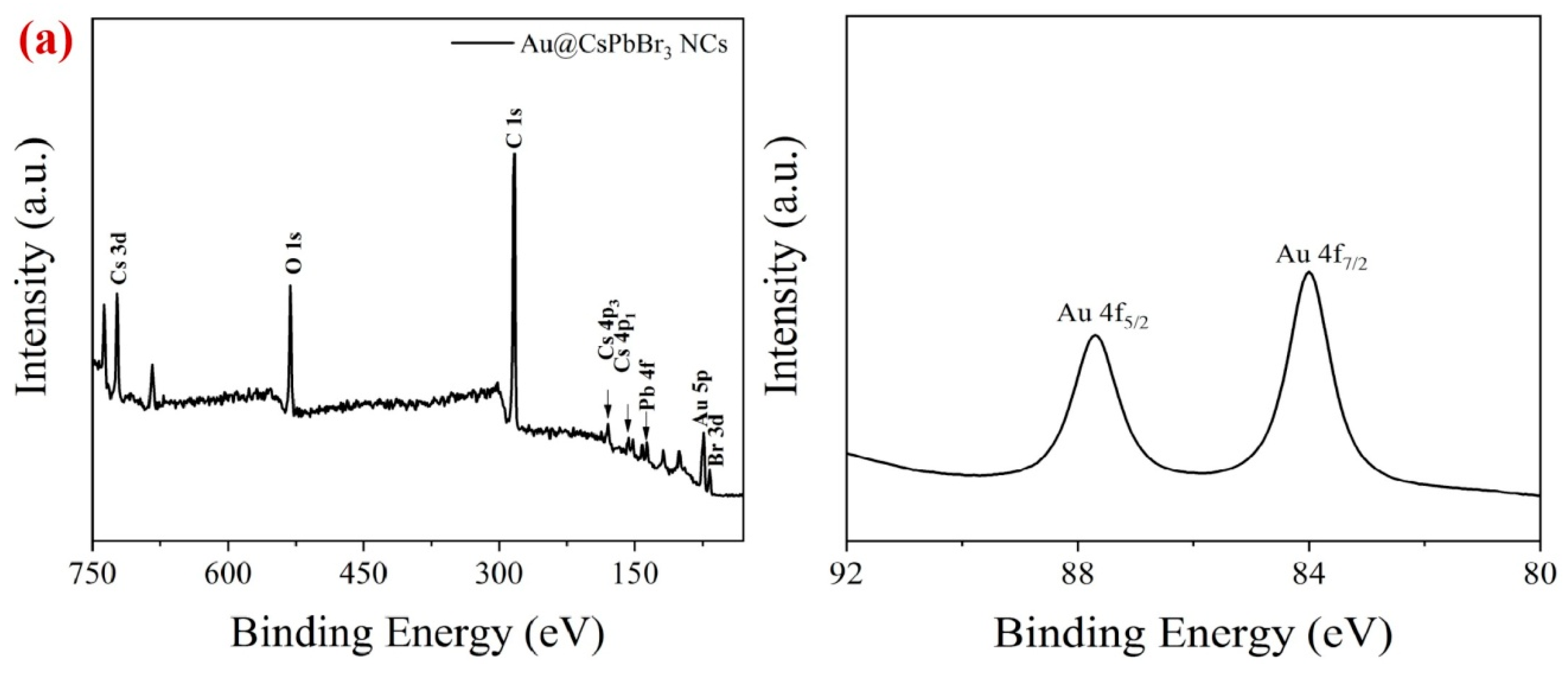
2. Results and Discussion
3. Materials and Methods
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Wiley, A.; Dunlap, S.; Zhou, Y.; Nitin, P.; David, B.M. Synthetic Approaches for Halide Perovskite Thin Films. Chem. Rev. 2019, 119, 3193–3295. [Google Scholar]
- Zhang, S.; Hu, Z.; Zhang, J.; Jia, X.; Jiang, J.; Chen, Y.; Lin, B.; Jiang, H.; Fang, B.; Yuan, N.; et al. Interface engineering via phthalocyanine decoration of perovskite solar cells with high efficiency and stability. J. Power Sources 2019, 438, 226987. [Google Scholar] [CrossRef]
- Zhang, S.; Lu, Y.; Lin, B.; Zhu, Y.; Zhang, K.; Yuan, N.; Ding, J.; Fang, B. PVDF-HFP additive for visible-light-semitransparent perovskite films yielding enhanced photovoltaic performance. Sol. Energy Mater. Sol. Cells 2017, 170, 178–186. [Google Scholar] [CrossRef]
- Kojima, A.; Teshima, K.; Shirai, Y.; Miyasaka, T. Organometal Halide Perovskites as Visible-Light Sensitizers for Photovoltaic Cells. J. Am. Chem. Soc. 2009, 131, 6050–6051. [Google Scholar] [CrossRef]
- Best Research-Cell Efficiency Chart. Available online: https://www.nrel.gov/pv/cell-efficiency.html (accessed on 13 December 2020).
- Li, X.; Cao, F.; Yu, D.; Chen, J.; Sun, Z.; Shen, Y.; Zhu, Y.; Wang, L.; Wei, Y.; Wu, Y.; et al. All Inorganic Halide Perovskites Nanosystem: Synthesis, Structural Features, Optical Properties and Optoelectronic Applications. Small 2017, 13, 1603996. [Google Scholar] [CrossRef]
- Huang, J.; Lai, M.; Lin, J.; Yang, P. Rich Chemistry in Inorganic Halide Perovskite Nanostructures. Adv. Mater. 2018, 30, 1802856. [Google Scholar] [CrossRef]
- Wu, L.; Hu, H.; Xu, Y.; Jiang, S.; Chen, M.; Zhong, Q.; Yang, D.; Liu, Q.; Zhao, Y.; Sun, B.; et al. From Nonluminescent Cs4PbX6 (X = Cl, Br, I) Nanocrystals to Highly Luminescent CsPbX3 Nanocrystals: Water-Triggered Transformation through a CsX-Stripping Mechanism. Nano Lett. 2017, 17, 5799–5804. [Google Scholar] [CrossRef]
- Protesescu, L.; Yakunin, S.; Bodnarchuk, M.I.; Krieg, F.; Caputo, R.; Hendon, C.H.; Yang, R.-X.; Walsh, A.; Kovalenko, M.V. Nanocrystals of Cesium Lead Halide Perovskites (CsPbX3, X = Cl, Br, and I): Novel Optoelectronic Materials Showing Bright Emission with Wide Color Gamut. Nano Lett. 2015, 15, 3692–3696. [Google Scholar] [CrossRef]
- Zhang, D.; Yu, Y.; Bekenstein, Y.; Wong, A.-B.; Alivisatos, P.; Yang, P. Ultrathin Colloidal Cesium Lead Halide Perovskite Nanowires. J. Am. Chem. Soc. 2016, 138, 13155–13158. [Google Scholar] [CrossRef]
- Nedelcu, G.; Protesescu, L.; Yakunin, S.; Bodnarchuk, M.I.; Grotevent, M.J.; Kovalenko, M.V. Fast Anion-Exchange in Highly Luminescent Nanocrystals of Cesium Lead Halide Perovskites (CsPbX3, X = Cl, Br, I). Nano Lett. 2015, 15, 5635–5640. [Google Scholar] [CrossRef]
- Chen, M.; Zou, Y.; Wu, L.; Pan, Q.; Yang, D.; Hu, H.; Tan, Y.; Zhong, Q.; Xu, Y.; Liu, H.; et al. Solvothermal Synthesis of High-Quality All-Inorganic Cesium Lead Halide Perovskite Nanocrystals: From Nanocube to Ultrathin Nanowire. Adv. Funct. Mater. 2017, 27, 1701121. [Google Scholar] [CrossRef]
- Tong, Y.; Bladt, E.; Aygüler, M.F.; Manzi, A.; Milowska, K.Z.; Hintermayr, V.A.; Docampo, P.; Bals, S.; Urban, A.S.; Polavarapu, L.; et al. Highly Luminescent Cesium Lead Halide Perovskite Nanocrystals with Tunable Composition and Thickness by Ultrasonication. Angew. Chem. Int. Ed. 2016, 55, 13887–13892. [Google Scholar] [CrossRef]
- Dong, Y.; Gu, Y.; Zou, Y.; Song, J.; Xu, L.; Li, J.; Xue, J.; Li, X.; Zeng, H. Improving All-Inorganic Perovskite Photodetectors by Preferred Orientation and Plasmonic Effect. Small 2016, 12, 5622–5632. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, Z.; Deschler, F.; Sun, X.; Lu, T.; Wertz, E.A.; Hu, J.; Shi, J. Epitaxial Halide Perovskite Lateral Double Heterostructure. ACS Nano 2017, 11, 3355–3364. [Google Scholar] [CrossRef]
- Su, S.; Shen, J.; Sun, H.; Tao, J.; Xu, D.; Wei, T.; Fan, C.; Wang, Z.; Sun, C.; Bi, W. Shape-controlled synthesis of Ag/Cs4PbBr6 Janus nanoparticles. Nanotechnology 2020, 32, 075601. [Google Scholar] [CrossRef]
- Hu, H.; Wu, L.; Tan, Y.; Zhong, Q.; Chen, M.; Qiu, Y.; Yang, D.; Sun, B.; Zhang, Q.; Yin, Y. Interfacial Synthesis of Highly Stable CsPbX3/Oxide Janus Nanoparticles. J. Am. Chem. Soc. 2018, 140, 406–412. [Google Scholar] [CrossRef]
- Balakrishnan, B.K.; Kamat, P.V. Au-CsPbBr3 Hybrid Architecture: Anchoring Gold Nanoparticles on Cubic Perovskite Nanocrystals. ACS Energy Lett. 2017, 2, 88–93. [Google Scholar] [CrossRef]
- Gong, M.; Alamri, M.; Ewing, D.; Sadeghi, S.M.; Wu, J.Z. Localized Surface Plasmon Resonance Enhanced Light Absorption in AuCu/CsPbCl3 Core/Shell Nanocrystals. Adv. Mater. 2020, 32, 2002163. [Google Scholar] [CrossRef]
- Zhong, Q.-X.; Cao, M.-H.; Hu, H.-C.; Yang, D.; Chen, M.; Li, P.-L.; Wu, L.-Z.; Zhang, Q. One-Pot Synthesis of Highly Stable CsPbBr3@SiO2 Core-Shell Nanoparticles. ACS Nano 2018, 12, 8579–8587. [Google Scholar] [CrossRef]
- Hu, C.; Peng, T.-W.; Hu, X.-X.; Nie, Y.-L.; Zhou, X.-F.; Qu, J.; He, H. Plasmon-Induced Photodegradation of Toxic Pollutants with Ag-AgI/Al2O3 under Visible-Light Irradiation. J. Am. Chem. Soc. 2010, 132, 857–862. [Google Scholar] [CrossRef]
- Zhang, H.-Y.; Yu, A.-C. Photophysics and Photocatalysis of Carbon Nitride Synthesized at Different Temperatures. J. Am. Chem. Soc. 2017, 139, 5216–5224. [Google Scholar] [CrossRef]
- Zhu, M.; Chen, P.; Liu, M. Graphene Oxide Enwrapped Ag/AgX (X = Br, Cl) Nanocomposite as a Highly Efficient Visible-Light Plasmonic Photocatalyst. ACS Nano 2011, 5, 4529–4536. [Google Scholar] [CrossRef] [PubMed]
- Saidaminov, M.I.; Almutlaq, J.; Sarmah, S.; Dursun, I.; Zhumekenov, A.A.; Begum, R.; Pan, J.; Cho, N.; Mohammed, O.F.; BakrPure, O.M. Pure Cs4PbBr6: Highly Luminescent Zero-Dimensional Perovskite Solids. ACS Energy Lett. 2016, 1, 840–845. [Google Scholar] [CrossRef]
- Rakočević, L.; Štrbac, S.; Srejić, I. Hydrogen evolution on Au/GC and PdAu/GC nanostructures in acid solution: AFM, XPS, and electrochemical study. Int. J. Hydrogen Energy 2021. [Google Scholar] [CrossRef]
- Zhang, X.; Huang, Q.; Li, Z.; Ma, A.; He, X.; Lin, S. Effects of silicotungstic acid on the physical stability and electrocatalytic activity of platinum nanoparticles assembled on graphene. Mater. Res. Bull. 2014, 60, 57–63. [Google Scholar] [CrossRef]
- Yang, S.; Dai, J.; Yu, Z.; Shao, Y.; Zhou, Y.; Xiao, X.; Zeng, X.; Huang, J. Tailoring Passivation Molecular Structures for Extremely Small Open-Circuit Voltage Loss in Perovskite Solar Cells. J. Am. Chem. Soc. 2019, 141, 5781–5787. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, P.; Wang, Z.; Cai, B.; Zheng, X.; Chen, Y.; Yuan, N.; Ding, J.; Zhang, W. Heterojunction Engineering for High Efficiency Cesium Formamidinium Double-Cation Lead Halide Perovskite Solar Cells. ChemSusChem 2018, 11, 808. [Google Scholar] [CrossRef]



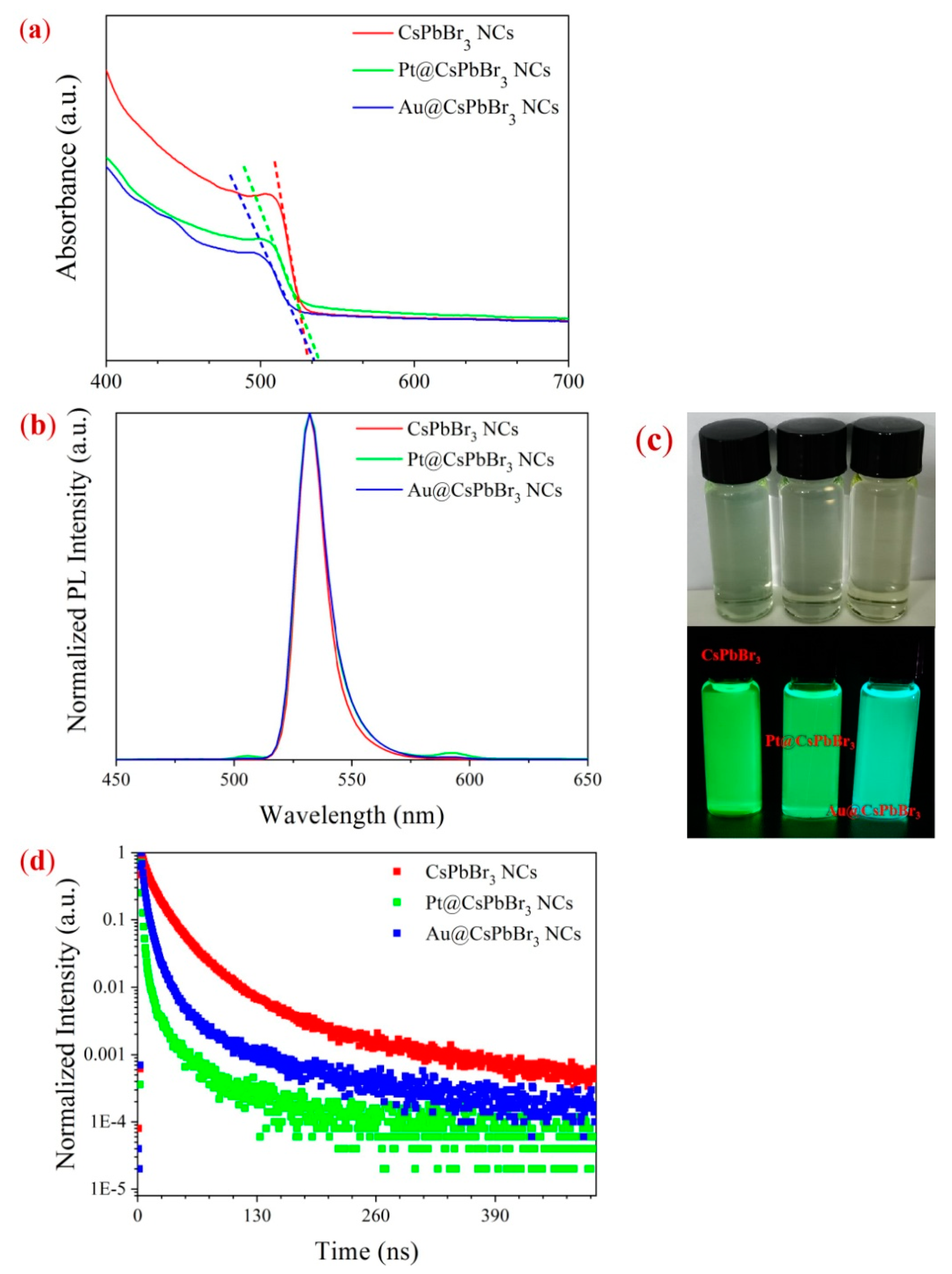
| τ1 (ns) | A1 | τ2 (ns) | A2 | |
|---|---|---|---|---|
| CsPbBr3 | 14.8 | 0.94 | 59.4 | 0.06 |
| Au@CsPbBr3 NCs | 7.03 | 0.95 | 28.2 | 0.05 |
| Pt@CsPbBr3 NCs | 2.49 | 0.96 | 9.97 | 0.04 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Wang, L.; Liu, W.; Cao, M.; Zhang, J.; Yuan, N.; Zhang, S.; Gu, Z. Synthesis of Au or Pt@Perovskite Nanocrystals via Interfacial Photoreduction. Catalysts 2021, 11, 174. https://doi.org/10.3390/catal11020174
Zhang J, Wang L, Liu W, Cao M, Zhang J, Yuan N, Zhang S, Gu Z. Synthesis of Au or Pt@Perovskite Nanocrystals via Interfacial Photoreduction. Catalysts. 2021; 11(2):174. https://doi.org/10.3390/catal11020174
Chicago/Turabian StyleZhang, Jing, Li Wang, Wenwen Liu, Mengsha Cao, Jing Zhang, Ningyi Yuan, Shuai Zhang, and Zhongze Gu. 2021. "Synthesis of Au or Pt@Perovskite Nanocrystals via Interfacial Photoreduction" Catalysts 11, no. 2: 174. https://doi.org/10.3390/catal11020174
APA StyleZhang, J., Wang, L., Liu, W., Cao, M., Zhang, J., Yuan, N., Zhang, S., & Gu, Z. (2021). Synthesis of Au or Pt@Perovskite Nanocrystals via Interfacial Photoreduction. Catalysts, 11(2), 174. https://doi.org/10.3390/catal11020174






