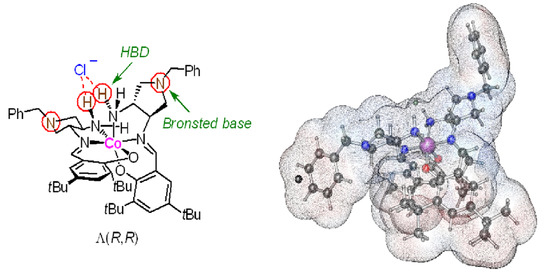
Expanding the Family of Octahedral Chiral-at-Metal Cobalt(III) Catalysts by Introducing Tertiary Amine Moiety into the Ligand
Abstract
1. Introduction
2. Results and Discussion
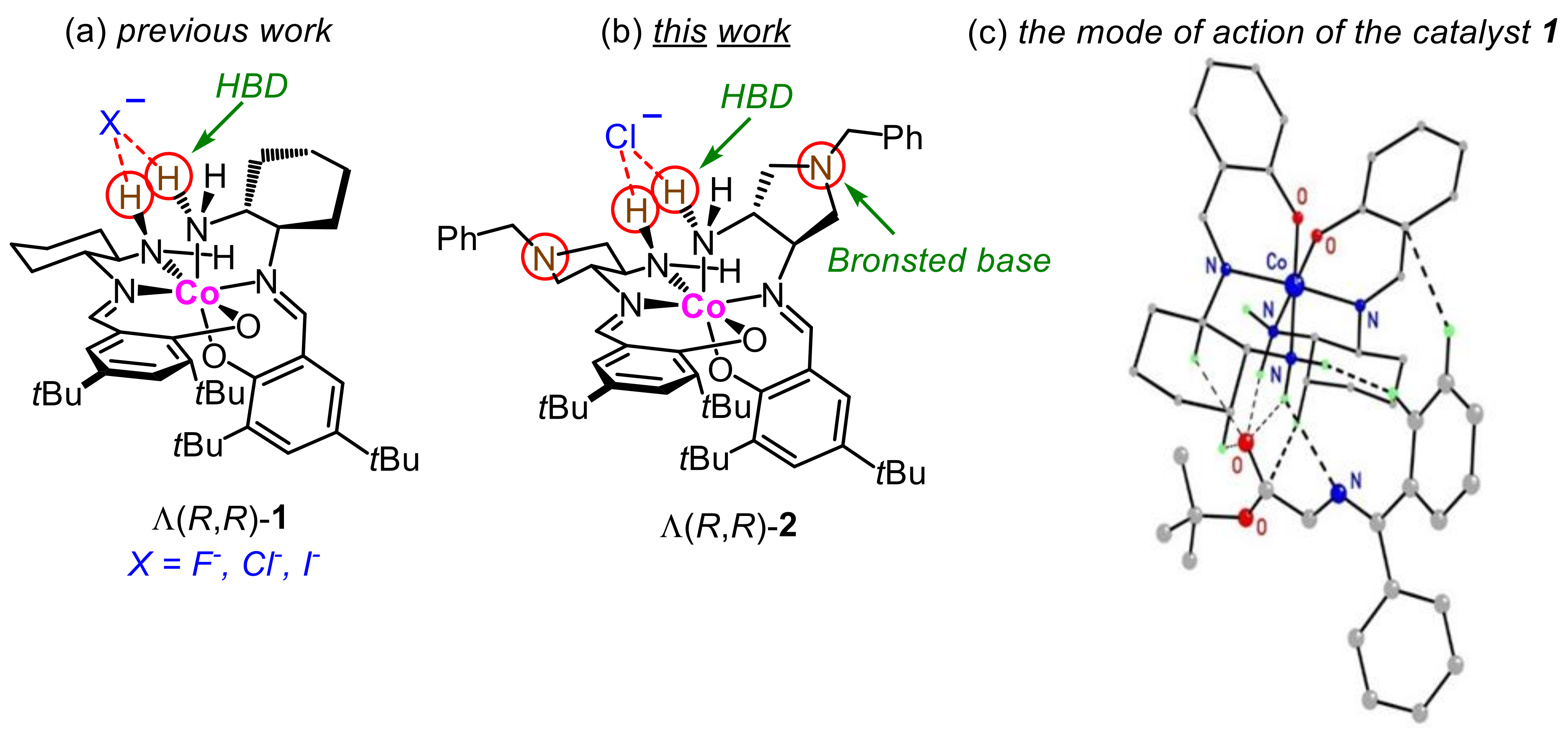
2.1. Synthesis of the Complexes 2
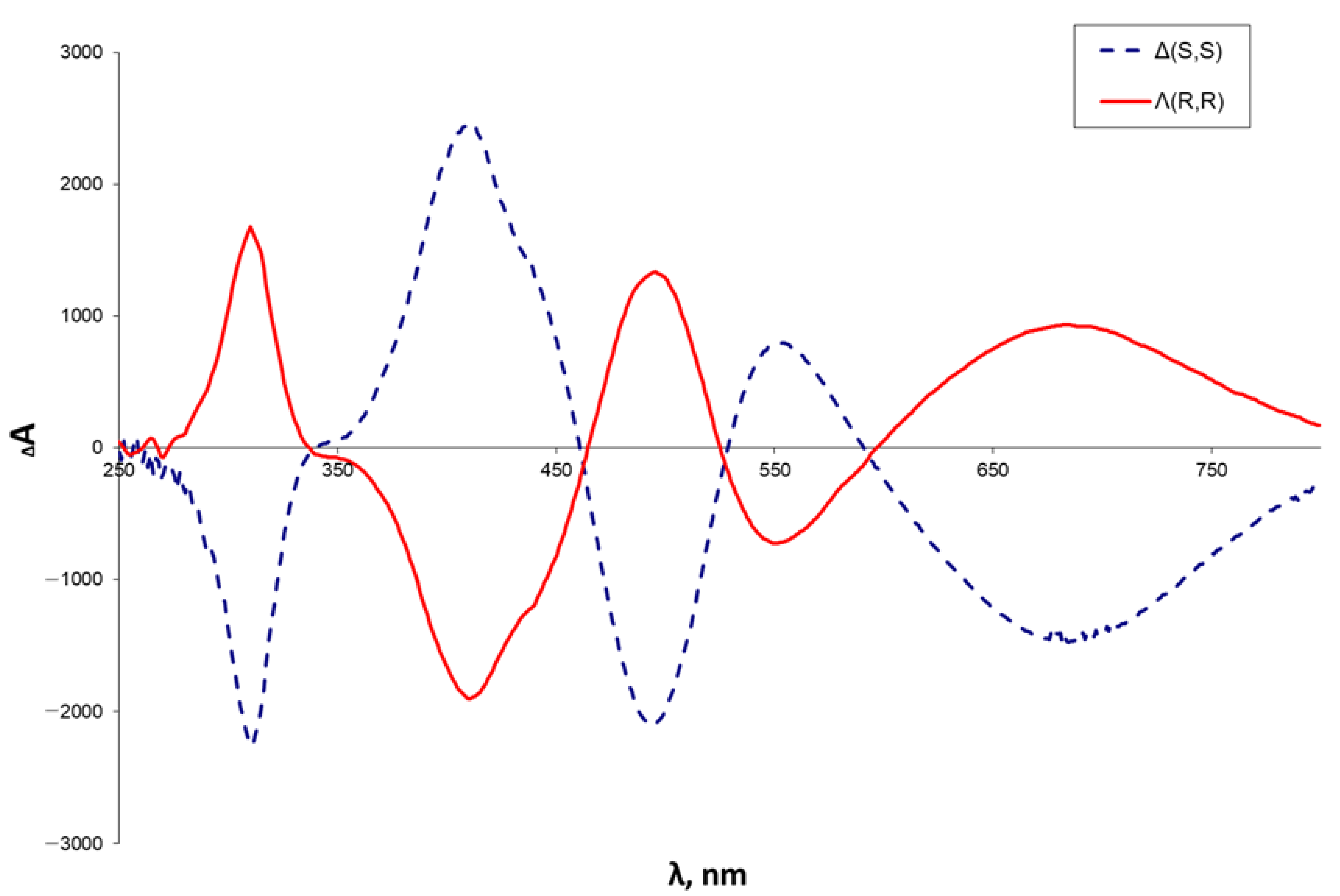
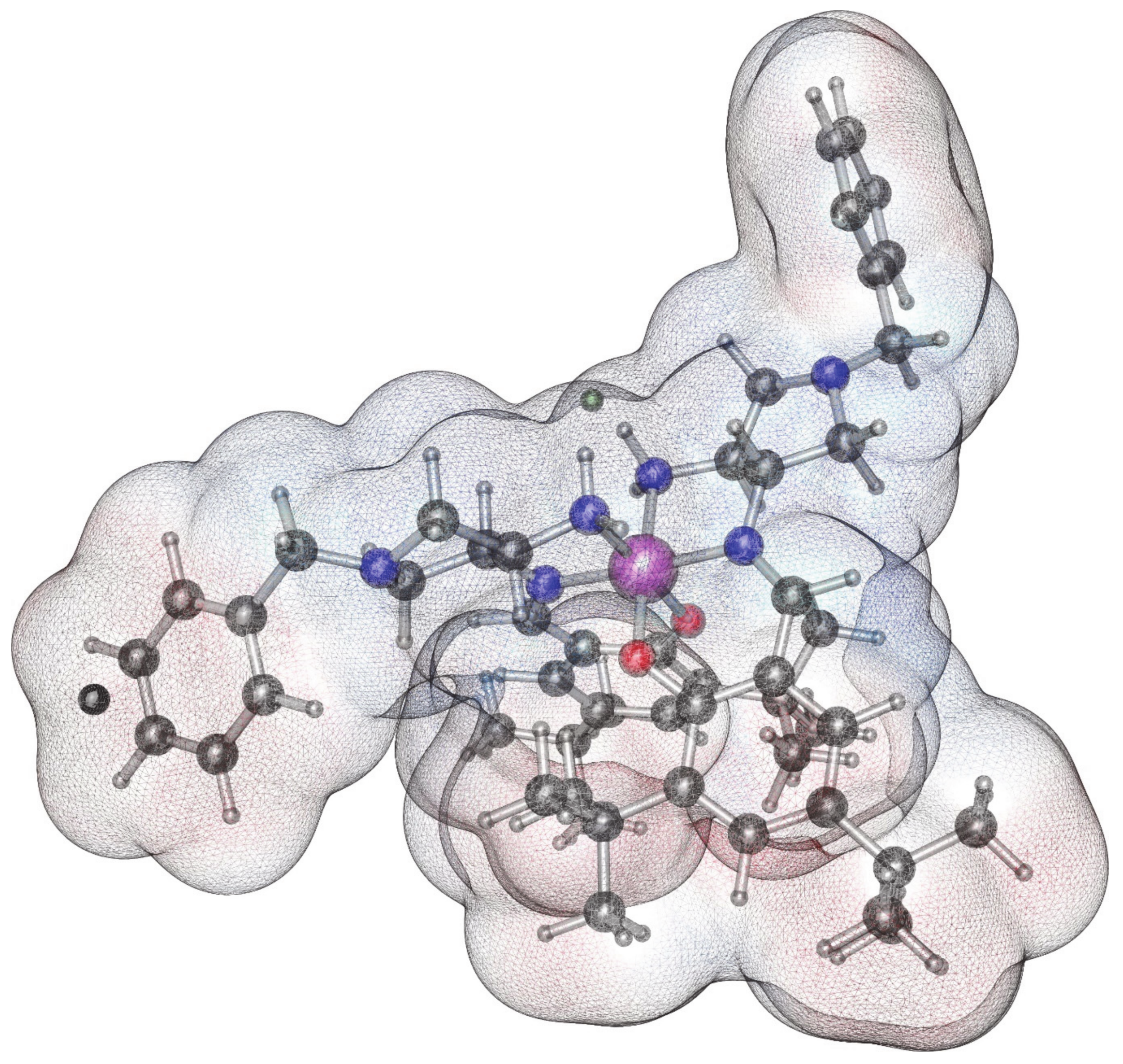
2.2. Characterization of the Complexes 2
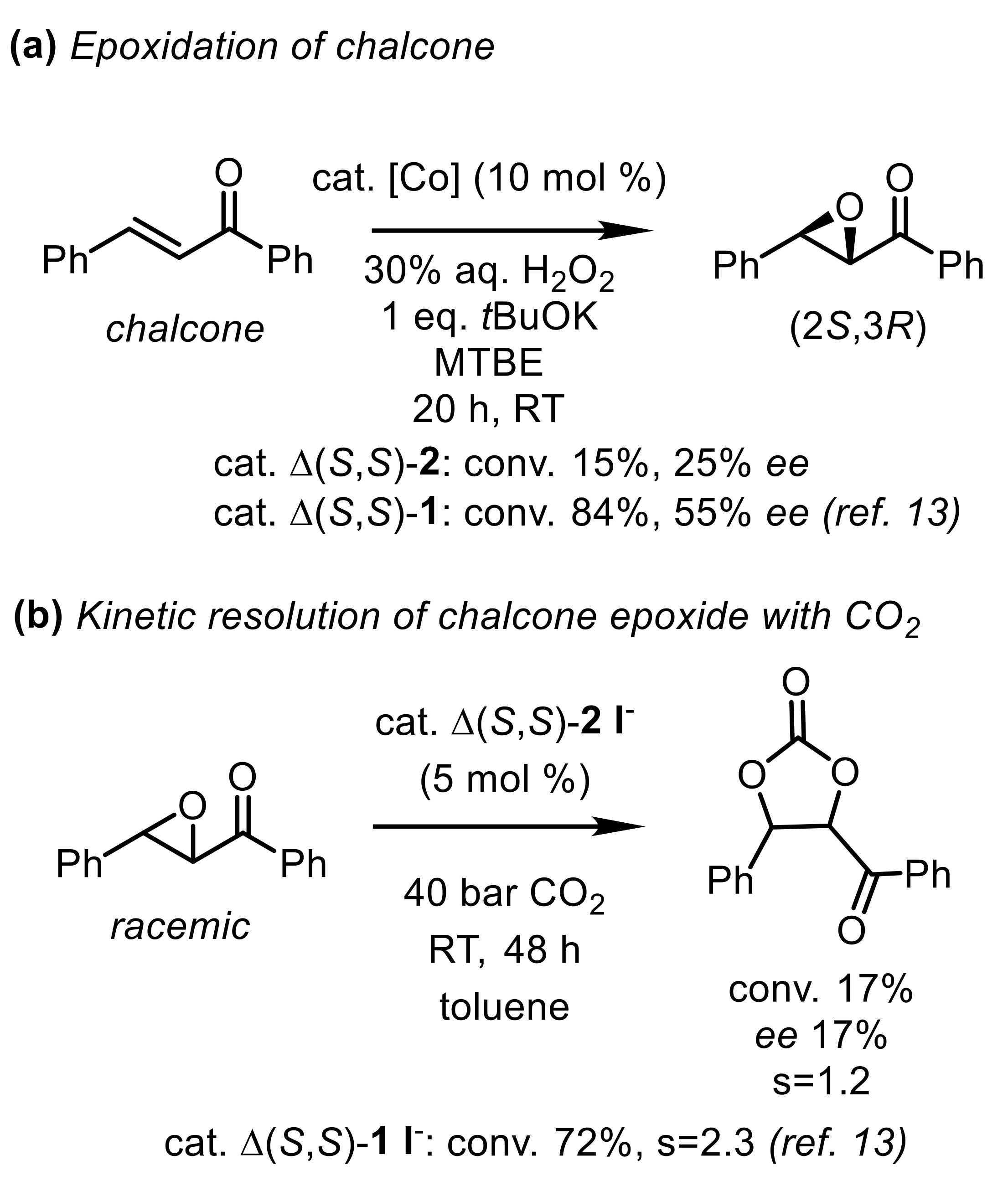
2.3. Catalytic Activity of the Complex 2
2.4. DFT Calculations of Charges and Molecular Electrostatic Potential of the Complex 2
3. Materials and Methods
3.1. Synthesis of the Complexes 2
3.2. X-ray Diffraction Study
3.3. DFT Calculations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gong, L.; Chen, L.-A.; Meggers, E. Asymmetric catalysis Mmediated by the ligand sphere of octahedral chiral-at-metal complexes. Angew. Chem. Int. Ed. 2014, 53, 10868–10874. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.K.; Ehnbom, A.; Lewis, K.G.; Gladysz, J.A. Hydrogen bonding motifs in structurally characterized salts of the tris(ethylenediamine) cobalt trication, [Co(en)3]3+; An interpretive review, including implications for catalysis. Coord. Chem. Rev. 2017, 350, 30–48. [Google Scholar] [CrossRef]
- Cruchter, T.; Larionov, V.A. Asymmetric catalysis with octahedral stereogenic-at-metal complexes featuring chiral ligands. Coord. Chem. Rev. 2018, 376, 95–113. [Google Scholar] [CrossRef]
- Wegener, A.R.; Kabes, C.Q.; Gladysz, J.A. Launching Werner complexes into the modern era of catalytic enantioselective organic synthesis. Acc. Chem. Res. 2020, 53, 2299–2313. [Google Scholar] [CrossRef]
- Ganzmann, C.; Gladysz, J.A. Phase transfer of enantiopure Werner cations into organic solvents: An overlooked family of chiral hydrogen bond donors for enantioselective catalysis. Chem. Eur. J. 2008, 14, 5397–5400. [Google Scholar] [CrossRef]
- Belokon, Y.N.; Larionov, V.A.; Mkrtchyan, A.F.; Khrustalev, V.N.; Nijland, A.; Saghiyan, A.S.; Godovikov, I.A.; Peregudov, A.S.; Babievsky, K.K.; Ikonnikov, N.S.; et al. A novel type of catalysts for the asymmetric C-C bond formation based on chiral stereochemically inert cationic CoIII complexes. Russ. Chem. Bull. 2012, 61, 2252–2260. [Google Scholar] [CrossRef]
- Chen, L.-A.; Xu, W.; Huang, B.; Ma, J.; Wang, L.; Xi, J.; Harms, K.; Gong, L.; Meggers, E. Asymmetric catalysis with an inert chiral-at-metal iridium complex. J. Am. Chem. Soc. 2013, 135, 10598–10601. [Google Scholar] [CrossRef]
- Belokon, Y.N.; Maleev, V.I.; North, M.; Larionov, V.A.; Savel’Yeva, T.F.; Nijland, A.; Nelyubina, Y.V. Chiral octahedral complexes of CoIII as a family of asymmetric catalysts operating under phase transfer conditions. ACS Catal. 2013, 3, 1951–1955. [Google Scholar] [CrossRef]
- Chen, L.-A.; Tang, X.; Xi, J.; Xu, W.; Gong, L.; Meggers, E. Chiral-at-metal octahedral iridium catalyst for the asymmetric construction of an all-carbon quaternary stereocenter. Angew. Chem. Int. Ed. 2013, 52, 14021–14025. [Google Scholar] [CrossRef]
- Maleev, V.I.; North, M.; Larionov, V.A.; Fedyanin, I.V.; Savel’yeva, T.F.; Moscalenko, M.A.; Smolyakov, A.F.; Belokon, Y.N. Chiral octahedral complexes of cobalt(III) as “organic catalysts in disguise” for the asymmetric addition of a glycine Schiff base ester to activated olefins. Adv. Synth. Catal. 2014, 356, 1803–1810. [Google Scholar] [CrossRef]
- Ma, J.; Ding, X.; Hu, Y.; Huang, Y.; Gong, L.; Meggers, E. Metal-templated chiral Brønsted base organocatalysis. Nat. Commun. 2014, 5, 4531. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, T.; Ganzmann, C.; Bhuvanesh, N.; Gladysz, J.A. Syntheses of enantiopure bifunctional 2-guanidinobenzimidazole cyclopentadienyl ruthenium complexes: Highly enantioselective organometallic hydrogen bond donor catalysts for carbon–carbon bond forming reactions. Organometallics 2014, 33, 6723–6737. [Google Scholar] [CrossRef]
- Larionov, V.A.; Markelova, E.P.; Smol’Yakov, A.F.; Savel’yeva, T.F.; Maleev, V.I.; Belokon, Y.N. Chiral octahedral complexes of Co(III) as catalysts for asymmetric epoxidation of chalcones under phase transfer conditions. RSC Adv. 2015, 5, 72764–72771. [Google Scholar] [CrossRef]
- Ding, X.; Lin, H.; Gong, L.; Meggers, E. Enantioselective sulfa-Michael addition to α,β-unsaturated γ-oxoesters catalyzed by a metal-templated chiral Brønsted base. Asian J. Org. Chem. 2015, 4, 434–437. [Google Scholar] [CrossRef]
- Hu, Y.; Zhou, Z.; Gong, L.; Meggers, E. Asymmetric aza-Henry reaction to provide oxindoles with quaternary carbon stereocenter catalyzed by a metal-templated chiral Brønsted base. Org. Chem. Front. 2015, 2, 968–972. [Google Scholar] [CrossRef]
- Liu, J.; Gong, L.; Meggers, E. Asymmetric Friedel–Crafts alkylation of indoles with 2-nitro-3-arylacrylates catalyzed by a metal-templated hydrogen bonding catalyst. Tetrahedron Lett. 2015, 56, 4653–4656. [Google Scholar] [CrossRef]
- Lewis, K.G.; Ghosh, S.K.; Bhuvanesh, N.; Gladysz, J.A. Cobalt(III) Werner complexes with 1,2-diphenylethylenediamine ligands: Readily available, inexpensive, and modular chiral hydrogen bond donor catalysts for enantioselective organic synthesis. ACS Cent. Sci. 2015, 1, 50–56. [Google Scholar] [CrossRef]
- Rulev, Y.A.; Larionov, V.A.; Lokutova, A.V.; Moskalenko, M.A.; Lependina, O.L.; Maleev, V.I.; North, M.; Belokon, Y.N. Chiral cobalt(III) complexes as bifunctional Brønsted acid-Lewis base catalysts for the preparation of cyclic organic carbonates. ChemSusChem 2015, 9, 216–222. [Google Scholar] [CrossRef]
- Xu, W.; Arieno, M.; Löw, H.; Huang, K.; Xie, X.; Cruchter, T.; Ma, Q.; Xi, J.; Huang, B.; Wiest, O.; et al. Metal-templated design: Enantioselective hydrogen-bond-driven catalysis requiring only parts-per-million catalyst loading. J. Am. Chem. Soc. 2016, 138, 8774–8780. [Google Scholar] [CrossRef]
- Huang, K.; Ma, Q.; Shen, X.; Gong, L.; Meggers, E. Metal-templated asymmetric Catalysis: (Z)-1-bromo-1-nitrostyrenes as versatile substrates for Friedel-Crafts alkylation of indoles. Asian J. Org. Chem. 2016, 5, 1198–1203. [Google Scholar] [CrossRef]
- Ma, Q.; Gong, L.; Meggers, E. Enantioselective β-alkylation of pyrroles with the formation of an all-carbon quaternary stereocenter. Org. Chem. Front. 2016, 3, 1319–1325. [Google Scholar] [CrossRef]
- Xu, W.; Shen, X.; Ma, Q.; Gong, L.; Meggers, E. Restricted conformation of a hydrogen bond mediated catalyst enables the highly efficient enantioselective construction of an all-carbon quaternary stereocenter. ACS Catal. 2016, 6, 7641–7646. [Google Scholar] [CrossRef]
- Ding, X.; Tian, C.; Hu, Y.; Gong, L.; Meggers, E. Tuning the basicity of a metal-templated Brønsted base to facilitate the enantioselective sulfa-Michael addition of aliphatic thiols to α,β-unsaturated N-acylpyrazoles. Eur. J. Org. Chem. 2016, 887–890. [Google Scholar] [CrossRef]
- Ghosh, S.K.; Ganzmann, C.; Bhuvanesh, N.; Gladysz, J.A. Werner complexes with ω-dimethylaminoalkyl substituted ethylenediamine ligands: Bifunctional hydrogen-bond-donor catalysts for highly enantioselective Michael additions. Angew. Chem. Int. Ed. 2016, 55, 4356–4360. [Google Scholar] [CrossRef]
- Kumar, A.; Ghosh, S.K.; Gladysz, J.A. Tris(1,2-diphenylethylenediamine)cobalt(III) complexes: Chiral hydrogen bond donor catalysts for enantioselective α-aminations of 1,3-dicarbonyl compounds. Org. Lett. 2016, 18, 760–763. [Google Scholar] [CrossRef]
- Larionov, V.A.; Peregudova, S.M.; Maleev, V.I.; Belokon, Y.N. A novel type of catalysts for asymmetric oxidative coupling of 2-naphthol. Russ. Chem. Bull. 2016, 65, 685–688. [Google Scholar] [CrossRef]
- Joshi, H.; Ghosh, S.K.; Gladysz, J.A. Enantioselective additions of stabilized carbanions to imines generated from α-amido sulfones by using lipophilic salts of chiral tris(1,2-diphenylethylenediamine) cobalt(III) trications as hydrogen bond donor catalysts. Synthesis 2017, 49, 3905–3915. [Google Scholar] [CrossRef]
- Gugkaeva, Z.T.; Larionov, V.A.; Moskalenko, M.A.; Khrustalev, V.N.; Nelyubina, Y.V.; Peregudov, A.S.; Tsaloev, A.T.; Maleev, V.I.; Belokon, Y.N. Economical synthesis of α-amino acids from a novel family of easily available Schiff bases of glycine esters and 2-hydroxybenzophenone. Synthesis 2018, 50, 607–616. [Google Scholar]
- Maximuck, W.J.; Gladysz, J.A. Lipophilic chiral cobalt (III) complexes of hexaamine ligands: Efficacies as enantioselective hydrogen bond donor catalysts. Mol. Catal. 2019, 473, 110360. [Google Scholar] [CrossRef]
- Kabes, C.Q.; Maximuck, W.J.; Ghosh, S.K.; Kumar, A.; Bhuvanesh, N.S.; Gladysz, J.A. Chiral tricationic tris(1,2-diphenylethylenediamine) cobalt(III) hydrogen bond donor catalysts with defined carbon/metal configurations; matched/mismatched effects upon enantioselectivities with enantiomeric chiral counter anions. ACS Catal. 2020, 10, 3249–3263. [Google Scholar] [CrossRef]
- Maximuck, W.J.; Ganzmann, C.; Alvi, S.; Hooda, K.R.; Gladysz, J.A. Rendering classical hydrophilic enantiopure Werner salts [M(en)3]n+nX− lipophilic (M/n = Cr/3, Co/3, Rh/3, Ir/3, Pt/4); new chiral hydrogen bond donor catalysts and enantioselectivities as a function of metal and charge. Dalton Trans. 2020, 49, 3680–3691. [Google Scholar] [CrossRef] [PubMed]
- Luu, Q.H.; Gladysz, J.A. An air- and water-stable hydrogen-bond-donor catalyst for the enantioselective generation of quaternary carbon stereocenters by additions of substituted cyanoacetate esters to acetylenic esters. Chem. Eur. J. 2020, 26, 10230–10239. [Google Scholar] [CrossRef] [PubMed]
- Wititsuwannakul, T.; Mukherjee, T.; Hall, M.B.; Gladysz, J.A. Computational investigations of enantioselection in carbon–carbon bond forming reactions of ruthenium guanidinobenzimidazole second coordination sphere hydrogen bond donor catalysts. Organometallics 2020, 39, 1149–1162. [Google Scholar] [CrossRef]
- Mukherjee, T.; Ghosh, S.K.; Wititsuwannakul, T.; Bhuvanesh, N.; Gladysz, J.A. Chiral-at-metal ruthenium complexes with guanidinobenzimidazole and pentaphenylcyclopentadienyl ligands: Synthesis, resolution, and preliminary screening as enantioselective second coordination sphere hydrogen bond donor catalysts. Organometallics 2020, 39, 1163–1175. [Google Scholar] [CrossRef]
- Belokon, Y.N.; Bulychev, A.G.; Maleev, V.I.; North, M.; Malfanov, I.L.; Ikonnikov, N.S. Asymmetric synthesis of cyanohydrins catalysed by a potassium Δ-bis[N-salicylidene-(R)-tryptophanato]cobaltate complex. Mendeleev Commun. 2004, 14, 249–250. [Google Scholar] [CrossRef]
- Belokon, Y.N.; Maleev, V.I.; Mal’fanov, I.L.; Savel’eva, T.F.; Ikonnikov, N.S.; Bulychev, A.G.; Usanov, D.L.; Kataev, D.A.; North, M. Anionic chiral cobalt(III) complexes as catalysts of asymmetric synthesis of cyanohydrins. Russ. Chem. Bull. 2006, 115, 821–827. [Google Scholar] [CrossRef]
- Belokon, Y.N.; Maleev, V.I.; Kataev, D.A.; Mal’Fanov, I.L.; Bulychev, A.G.; Moskalenko, M.A.; Saveleva, T.F.; Skrupskaya, T.V.; Lyssenko, K.A.; Godovikov, I.A.; et al. Potassium and silver chiral cobaltate(III) complexes as precatalysts for asymmetric C–C bond formation. Tetrahedron: Asymmetry 2008, 19, 822–831. [Google Scholar] [CrossRef]
- Belokon’, Y.N.; Maleev, V.I.; Kataev, D.A.; Saveleva, T.F.; Skrupskaya, T.V.; Nelyubina, Y.V.; North, M. Chiral ion pairs in catalysis: Lithium salts of chiral metallocomplex anions as catalysts for asymmetric C–C bond formation. Tetrahedron: Asymmetry 2009, 20, 1746–1752. [Google Scholar] [CrossRef]
- Huo, H.; Fu, C.; Wang, C.; Harms, K.; Meggers, E. Metal-templated enantioselective enamine/H-bonding dual activation catalysis. Chem. Commun. 2014, 50, 10409–10411. [Google Scholar] [CrossRef]
- Yu, J.; Jiang, H.-J.; Zhou, Y.; Luo, S.-W.; Gong, L.-Z. Sodium salts of anionic chiral cobalt(III) complexes as catalysts of the enantioselective Povarov reaction. Angew. Chem. Int. Ed. 2015, 54, 11209–11213. [Google Scholar] [CrossRef]
- Cruchter, T.; Medvedev, M.G.; Shen, X.; Mietke, T.; Harms, K.; Marsch, M.; Meggers, E. Asymmetric nucleophilic catalysis with an octahedral chiral-at-metal iridium(III) complex. ACS Catal. 2017, 7, 5151–5162. [Google Scholar] [CrossRef]
- Jiang, H.-J.; Liu, K.; Yu, J.; Zhang, L.; Gong, L.-Z. Switchable stereoselectivity in bromoaminocyclization of olefins: Using Brønsted acids of anionic chiral cobalt(III) complexes. Angew. Chem. Int. Ed. 2017, 56, 11931–11935. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.-J.; Liu, K.; Wang, J.; Li, N.; Yu, J. Brønsted acids of anionic chiral Co(III) complexes as catalysts for the stereoselective synthesis of cis-4-aminofuranobenzopyrans. Org. Biomol. Chem. 2017, 15, 9077–9080. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Jiang, H.-J.; Li, N.; Li, H.; Wang, J.; Zhang, Z.; Yu, J. Enantioselective bromocyclization of tryptamines induced by chiral Co(III)-complex-templated Brønsted acids under an air atmosphere. J. Org. Chem. 2018, 83, 6815–6823. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Yu, H.; Wang, R.; Shen, J.; Wu, W.-Q.; Liu, K.; Sun, T.-T.; Zhang, Z.; Yao, C.-Z.; Yu, J. Enantioselective intermolecular iodoacetalization of enol ethers catalyzed by chiral Co(III)-complex-templated Brønsted acids. Tetrahedron Lett. 2018, 59, 3605–3608. [Google Scholar] [CrossRef]
- Wang, R.; Wu, W.-Q.; Li, N.; Shen, J.; Liu, K.; Yu, J. Brønsted acids of anionic chiral cobalt(III) complexes as catalysts for the iodoglycosylation or iodocarboxylation of glycals. Synlett 2019, 30, 1077–1084. [Google Scholar] [CrossRef]
- Skubi, K.L.; Kidd, J.B.; Jung, H.; Guzei, I.A.; Baik, M.-H.; Yoon, T.P. Enantioselective excited-state photoreactions controlled by a chiral hydrogen-bonding iridium sensitizer. J. Am. Chem. Soc. 2017, 139, 17186–17192. [Google Scholar] [CrossRef]
- Zheng, J.; Swords, W.B.; Jung, H.; Skubi, K.L.; Kidd, J.B.; Meyer, G.J.; Baik, M.-H.; Yoon, T.P. Enantioselective intermolecular excited-state photoreactions using a chiral Ir triplet sensitizer: Separating association from energy transfer in asymmetric photocatalysis. J. Am. Chem. Soc. 2019, 141, 13625–13634. [Google Scholar] [CrossRef]
- Brunner, H. Optically active organometallic compounds of transition elements with chiral metal atoms. Angew. Chem. Int. Ed. 1999, 38, 1194–1208. [Google Scholar] [CrossRef]
- Fontecave, M.; Hamelin, O.; Ménage, S. Chiral-at-metal complexes as asymmetric Catalysts. Chiral Diazaligands Asymmetric Synth. 2005, 15, 271–288. [Google Scholar] [CrossRef]
- Zhang, L.; Meggers, E. Stereogenic-only-at-metal asymmetric catalysts. Chem. Asian J. 2017, 12, 2335–2342. [Google Scholar] [CrossRef]
- Emelyanov, M.A.; Stoletova, N.V.; Lisov, A.A.; Medvedev, M.G.; Smol’yakov, A.F.; Maleev, V.I.; Larionov, V.A. Fixation of CO2 with epoxides at ambient conditions catalyzed by a sustainable bifunctional metal-templated hydrogen bond donor catalyst. J. CO2 Utiliz. 2021. (under review). [Google Scholar]
- Reed, A.E.; Weinstock, R.B.; Weinhold, F. Natural population analysis. J. Chem. Phys. 1985, 83, 735–746. [Google Scholar] [CrossRef]
- Breneman, C.M.; Wiberg, K.B. Determining atom-centered monopoles from molecular electrostatic potentials. The need for high sampling density in formamide conformational analysis. J. Comput. Chem. 1990, 11, 361–373. [Google Scholar] [CrossRef]
- Kano, T.; Hato, Y.; Maruoka, K. Design of a C2-symmetric chiral pyrrolidine-based amino sulfonamide: Application to anti-selective direct asymmetric Mannich reactions. Tetrahedron Lett. 2006, 47, 8467–8469. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXT – Integrated space-group and crystal-structure determination. Acta Cryst. 2015, A71, 3–8. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Cryst. 2015, C71, 3–8. [Google Scholar] [CrossRef]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Neese, F. The ORCA program system. Wiley Interdiscip. Rev. Comput. Mol. Sci. 2012, 2, 73–78. [Google Scholar] [CrossRef]
- Adamo, C.; Barone, V. Toward reliable density functional methods without adjustable parameters: The PBE0 model. J. Chem. Phys. 1999, 110, 6158–6170. [Google Scholar] [CrossRef]
- Grimme, S.; Ehrlich, S.; Goerigk, L. Effect of the damping function in dispersion corrected density functional theory. J. Comput. Chem. 2011, 32, 1456–1465. [Google Scholar] [CrossRef] [PubMed]
- Weigend, F.; Ahlrichs, R. Balanced basis sets of split valence, triple zeta valence and quadruple zeta valence quality for H to Rn: Design and assessment of accuracy. Phys. Chem. Chem. Phys. 2005, 7, 3297–3305. [Google Scholar] [CrossRef] [PubMed]
- Nikolaienko, T.Y.; Bulavin, L.A.; Hovorun, D.M. JANPA: An open source cross-platform implementation of the Natural Population Analysis on the Java platform. Comput. Theor. Chem. 2014, 1050, 15–22. [Google Scholar] [CrossRef]
- Lu, T.; Chen, F. Multiwfn: A multifunctional wavefunction analyzer. J. Comput. Chem. 2012, 33, 580–592. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Savel’yeva, T.F.; Khromova, O.V.; Larionov, V.A.; Smol’yakov, A.F.; Fedyanin, I.V.; Belokon, Y.N.; Maleev, V.I. Expanding the Family of Octahedral Chiral-at-Metal Cobalt(III) Catalysts by Introducing Tertiary Amine Moiety into the Ligand. Catalysts 2021, 11, 152. https://doi.org/10.3390/catal11020152
Savel’yeva TF, Khromova OV, Larionov VA, Smol’yakov AF, Fedyanin IV, Belokon YN, Maleev VI. Expanding the Family of Octahedral Chiral-at-Metal Cobalt(III) Catalysts by Introducing Tertiary Amine Moiety into the Ligand. Catalysts. 2021; 11(2):152. https://doi.org/10.3390/catal11020152
Chicago/Turabian StyleSavel’yeva, Tat’yana F., Olga V. Khromova, Vladimir A. Larionov, Alexander F. Smol’yakov, Ivan V. Fedyanin, Yuri N. Belokon, and Victor I. Maleev. 2021. "Expanding the Family of Octahedral Chiral-at-Metal Cobalt(III) Catalysts by Introducing Tertiary Amine Moiety into the Ligand" Catalysts 11, no. 2: 152. https://doi.org/10.3390/catal11020152
APA StyleSavel’yeva, T. F., Khromova, O. V., Larionov, V. A., Smol’yakov, A. F., Fedyanin, I. V., Belokon, Y. N., & Maleev, V. I. (2021). Expanding the Family of Octahedral Chiral-at-Metal Cobalt(III) Catalysts by Introducing Tertiary Amine Moiety into the Ligand. Catalysts, 11(2), 152. https://doi.org/10.3390/catal11020152