Active Site Engineering on Two-Dimensional-Layered Transition Metal Dichalcogenides for Electrochemical Energy Applications: A Mini-Review
Abstract
1. Introduction
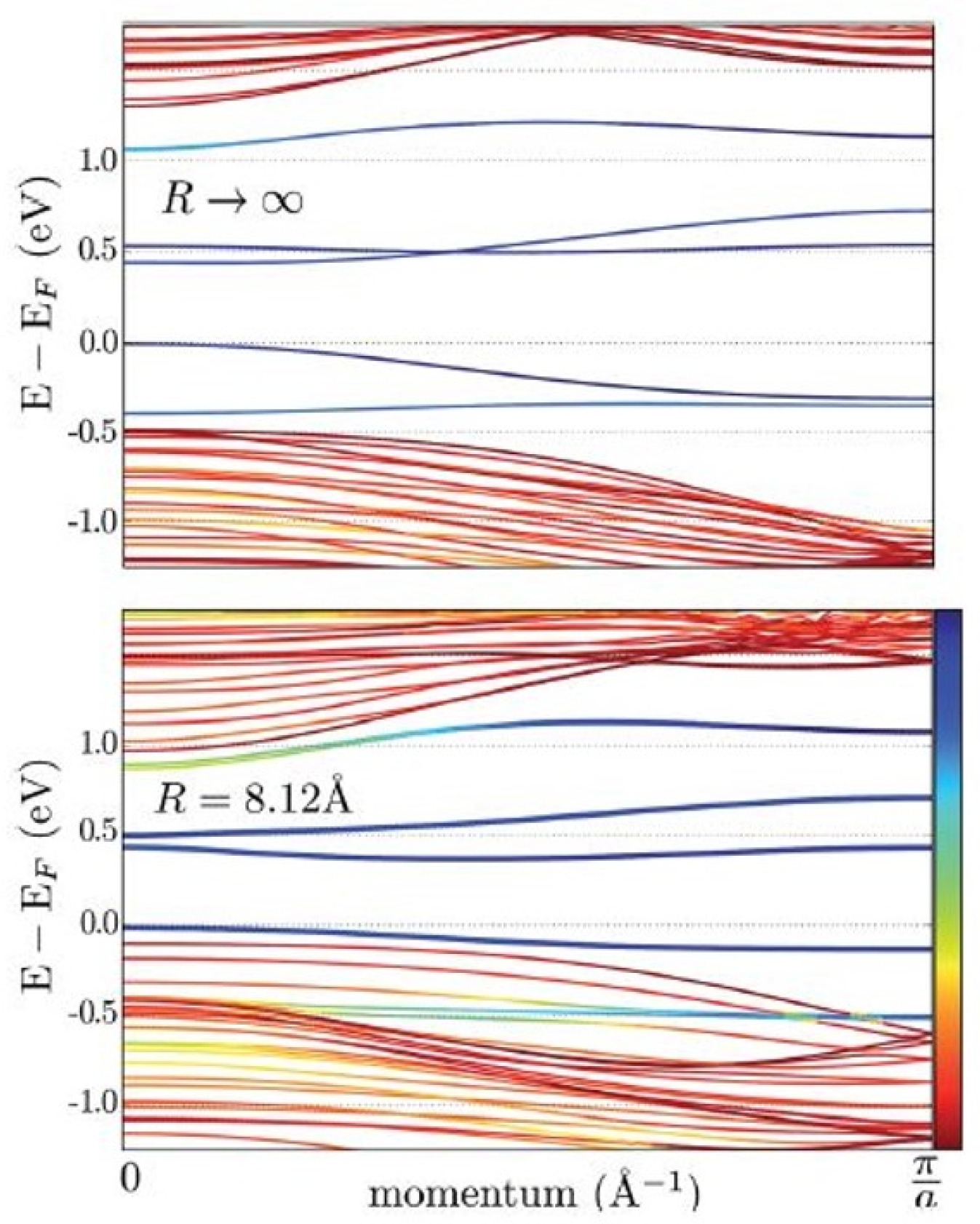
2. Curvature Engineering on 2D-Layered TMDs
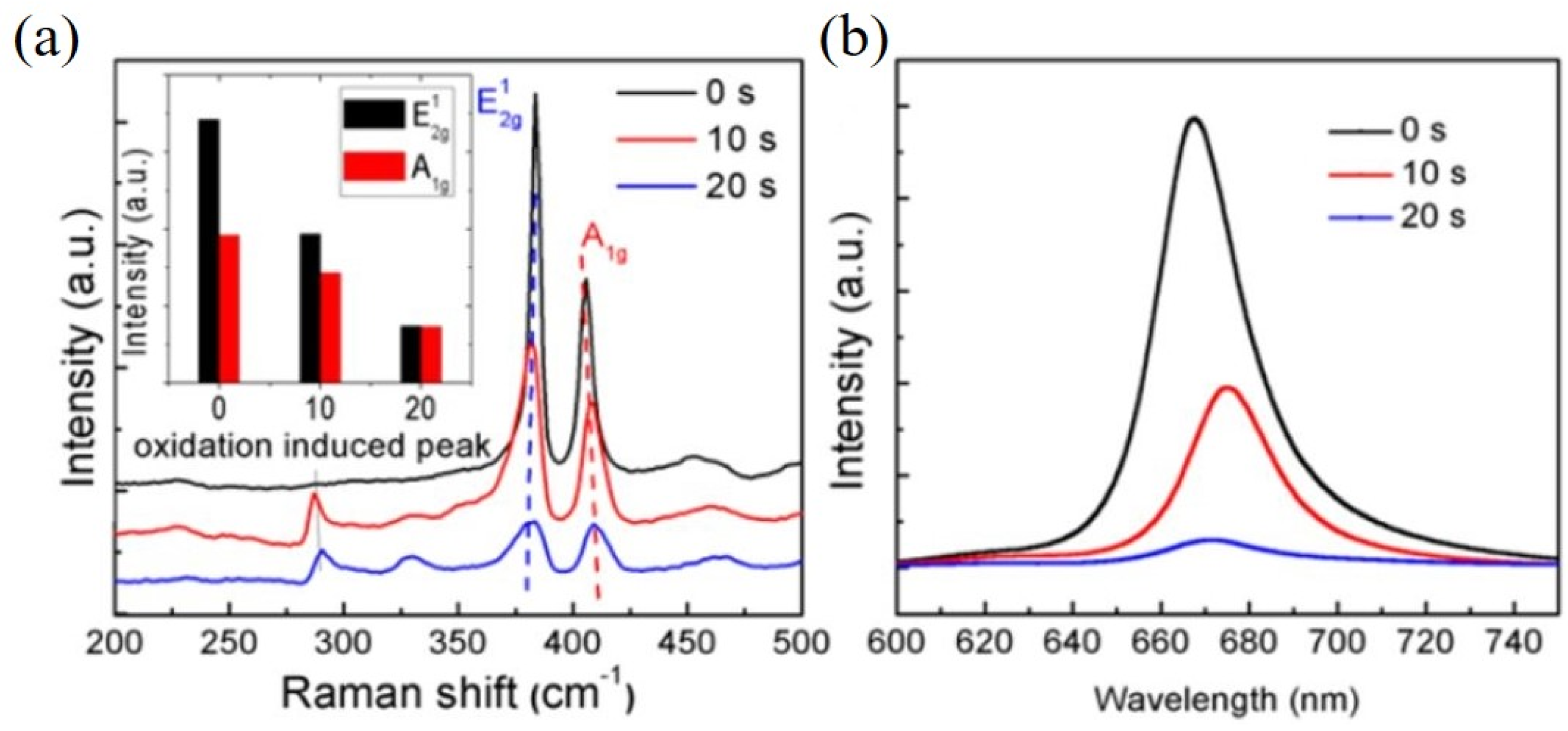
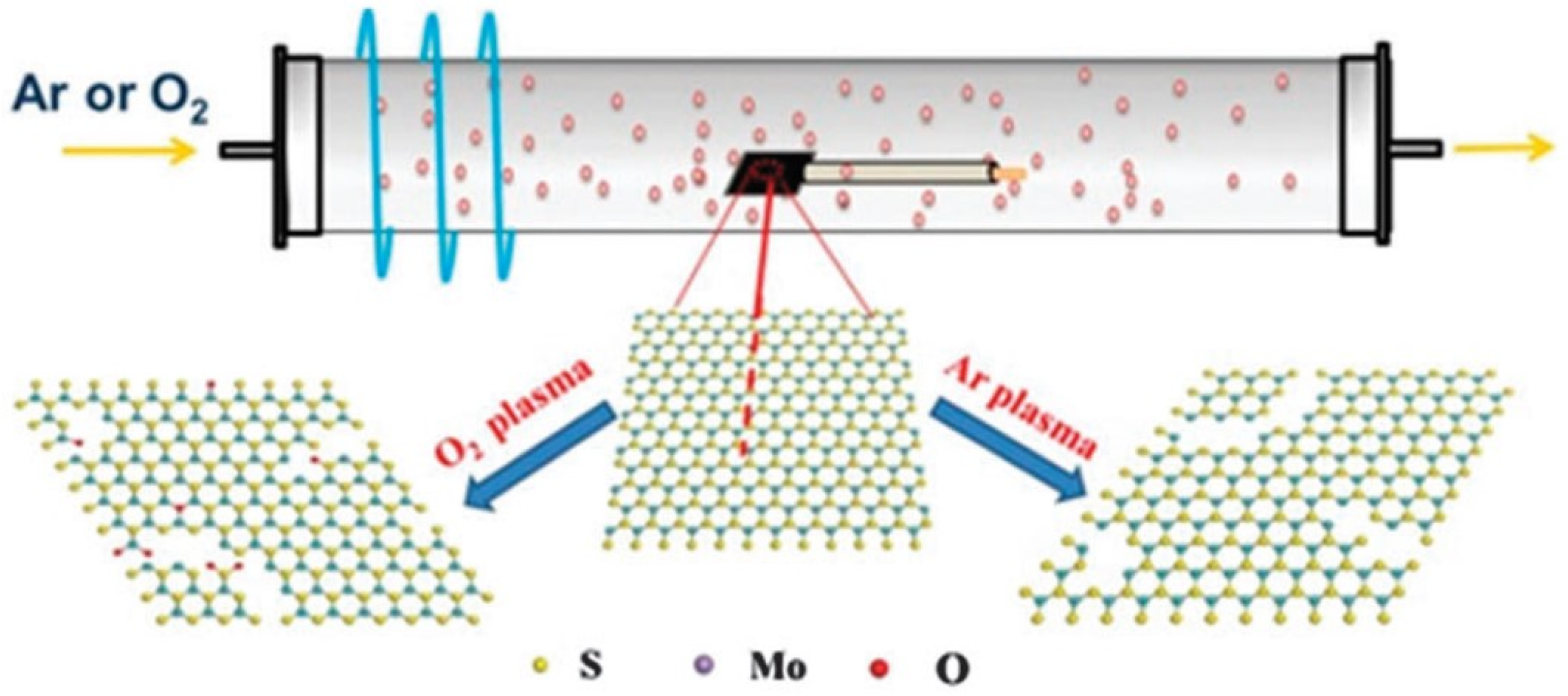
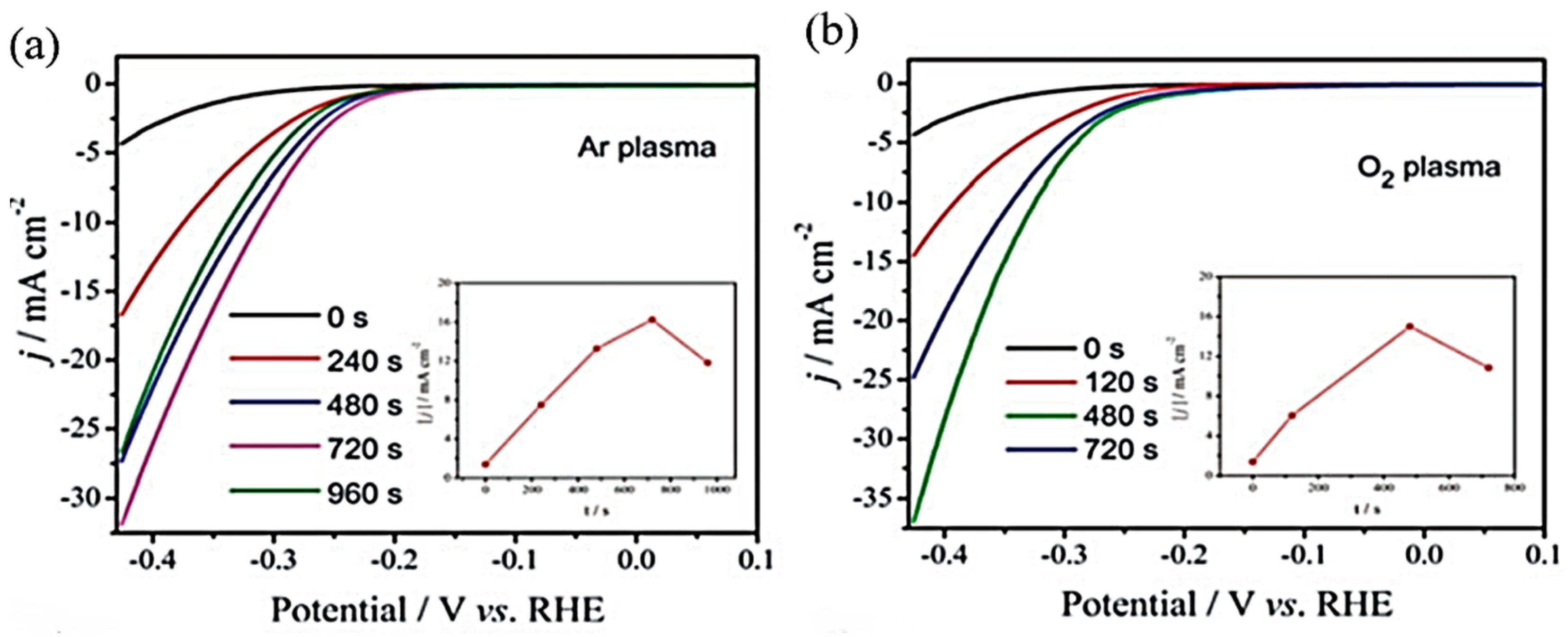
3. Plasma Treatment on 2D-Layered TMDs
4. Heteroatom-Doping on 2D-Layered TMDs
5. Surface Modification on 2D-Layered TMDs
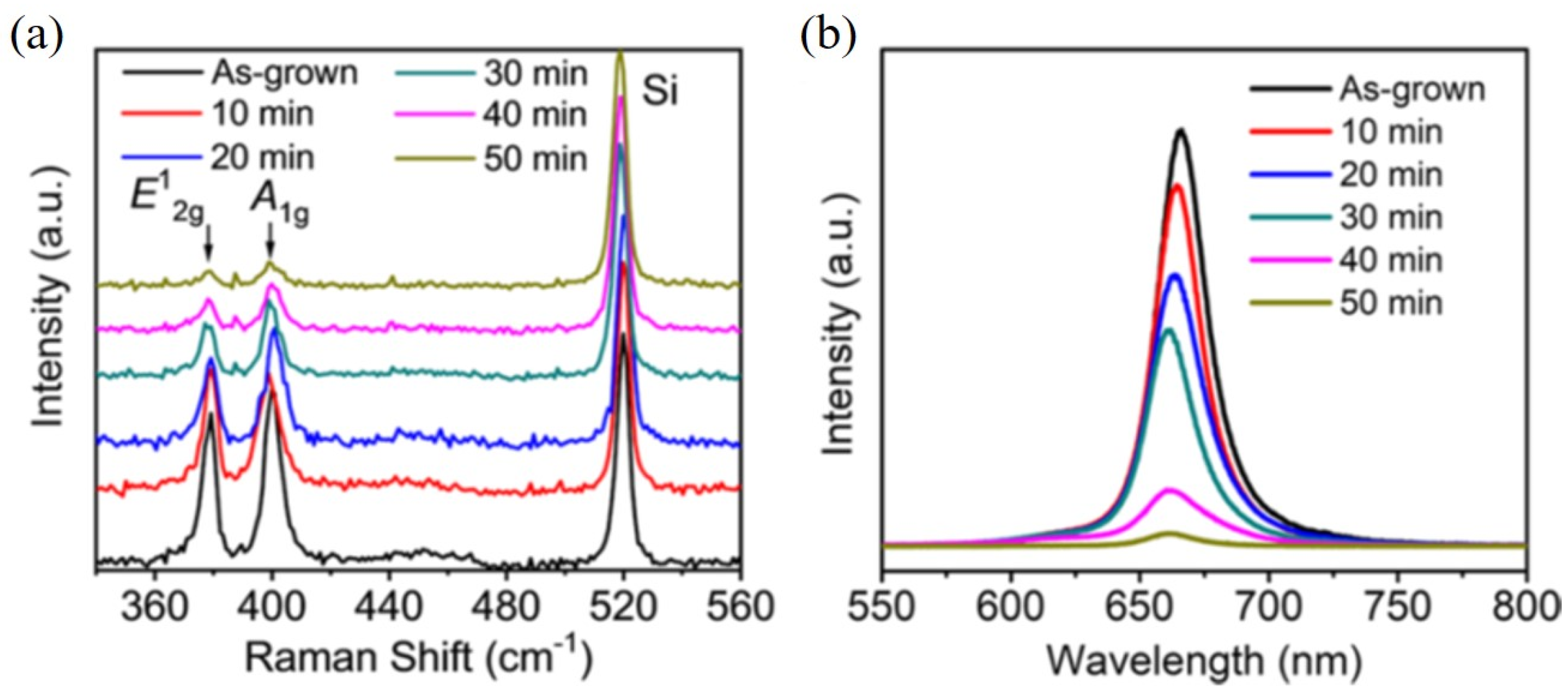
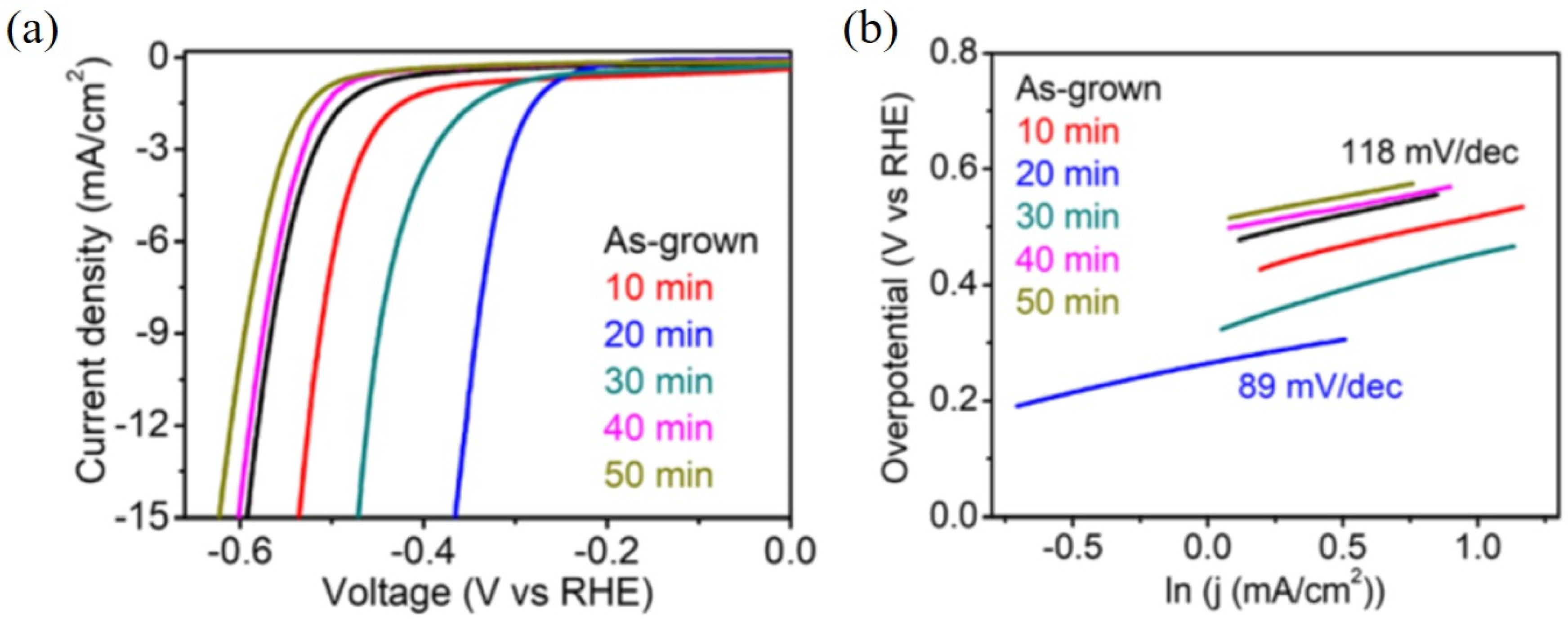
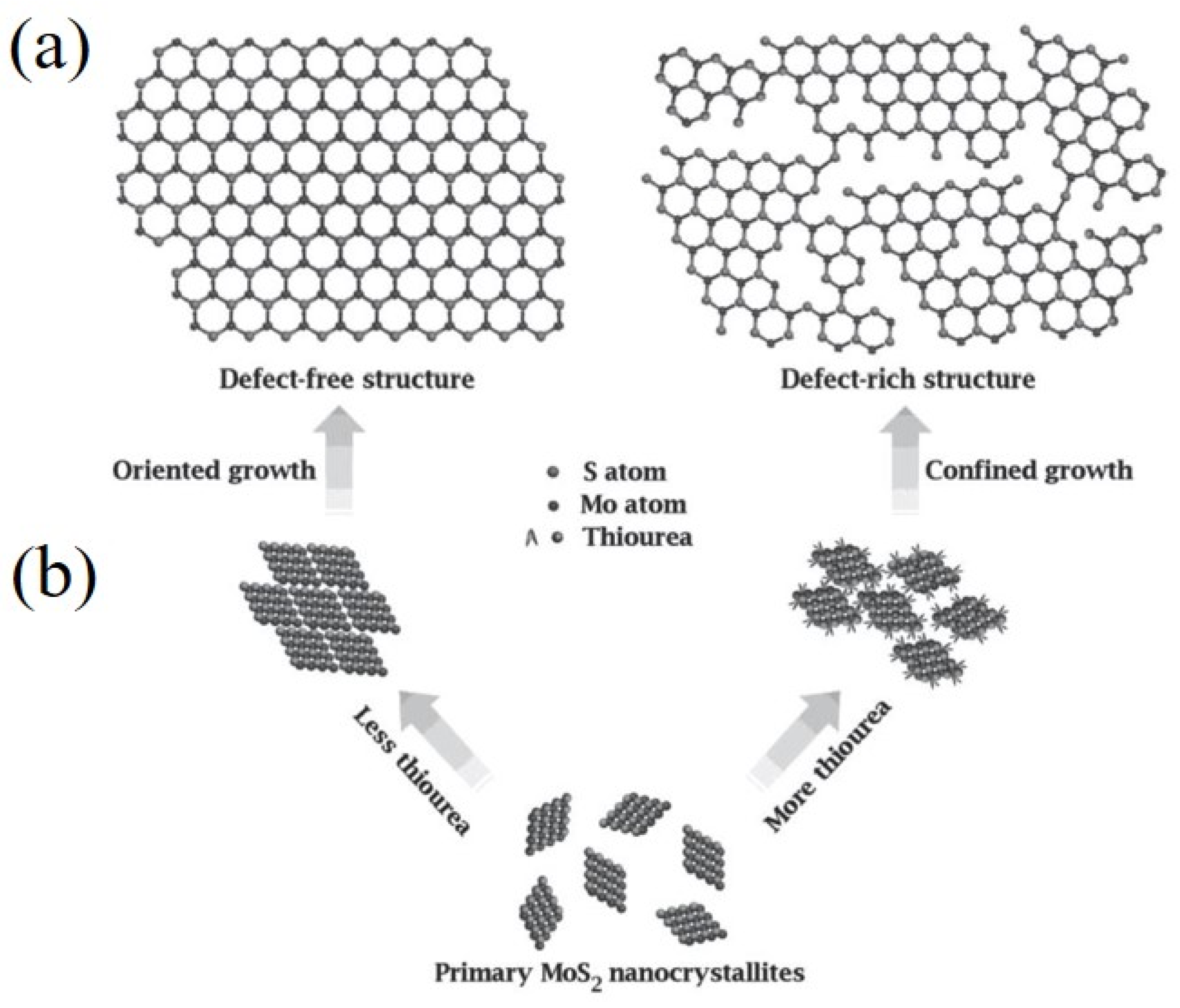
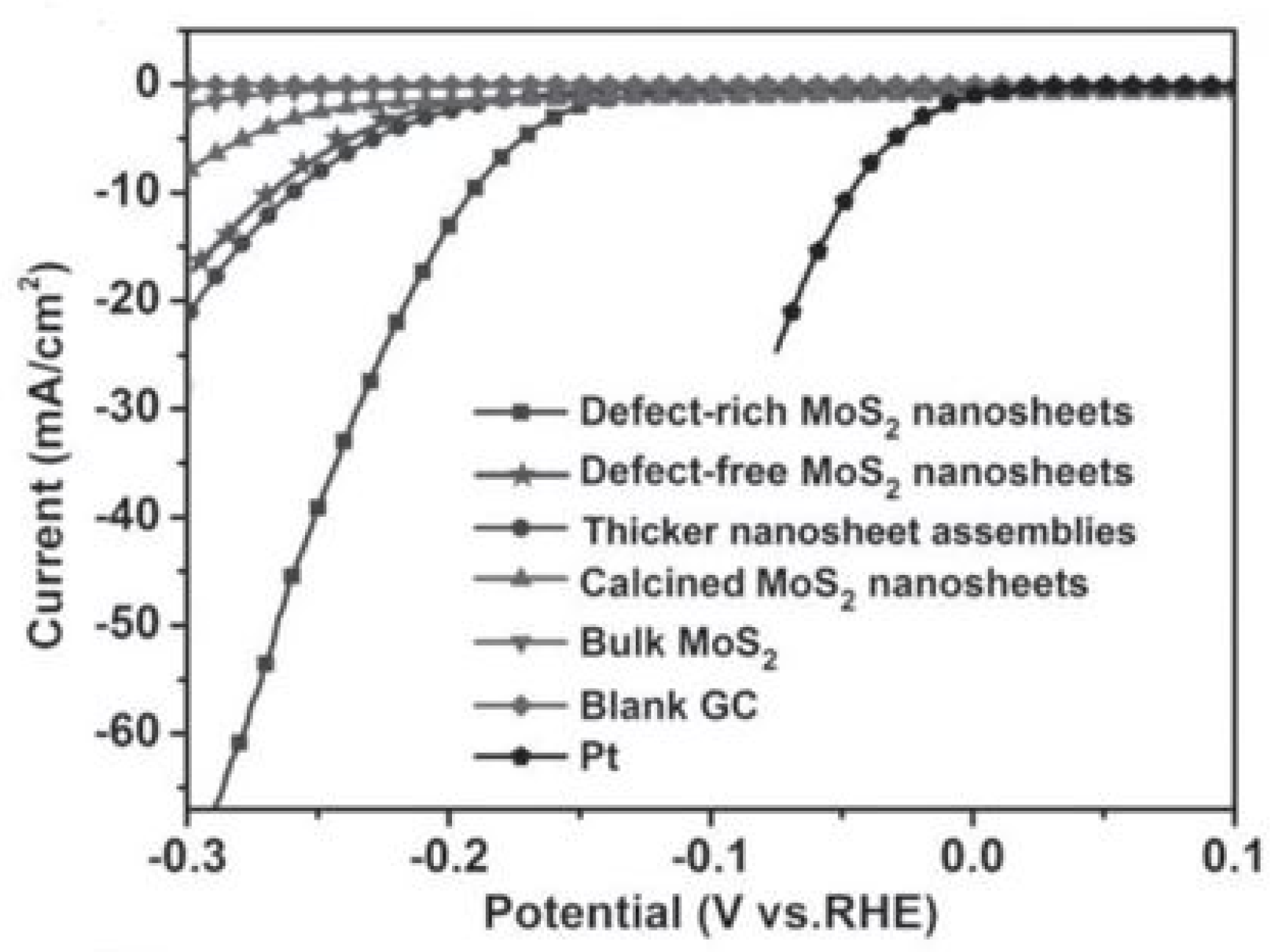
6. Edge Site Formation on 2D-Layered TMDs
7. Conclusions and Outlook
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dean, C.R.; Young, A.F.; Meric, I.; Lee, C.; Wang, L.; Sorgenfrei, S.; Watanabe, K.; Taniguchi, T.; Kim, P.; Shepard, K.L.; et al. Boron nitride substrates for high-quality graphene electronics. Nat. Nanotechnol. 2010, 5, 722–726. [Google Scholar] [CrossRef] [PubMed]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric field effect in atomically thin carbon films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef] [PubMed]
- Tseng, C.-A.; Chen, C.-C.; Ulaganathan, R.K.; Lee, C.-P.; Chiang, H.-C.; Chang, C.-F.; Chen, Y.-T. One-step synthesis of antioxidative graphene-wrapped copper nanoparticles on flexible substrates for electronic and electrocatalytic applications. ACS Appl. Mater. Interfaces 2017, 9, 25067–25072. [Google Scholar] [CrossRef] [PubMed]
- Tseng, C.-A.; Lee, C.-P.; Huang, Y.-J.; Pang, H.-W.; Ho, K.-C.; Chen, Y.-T. One-step synthesis of graphene hollow nanoballs with various nitrogen-doped states for electrocatalysis in dye-sensitized solar cells. Mater. Today Energy 2018, 8, 15–21. [Google Scholar] [CrossRef]
- Tseng, C.-A.; Sahoo, P.K.; Lee, C.-P.; Lin, Y.-T.; Xu, J.-H.; Chen, Y.-T. Synthesis of CoO-decorated graphene hollow nanoballs for high-performance flexible supercapacitors. ACS Appl. Mater. Interfaces 2020, 12, 40426–40432. [Google Scholar] [CrossRef]
- Osada, M.; Sasaki, T. Two-dimensional dielectric nanosheets: Novel nanoelectronics from nanocrystal building blocks. Adv. Mater. 2012, 24, 210–228. [Google Scholar] [CrossRef]
- Pacilé, D.; Meyer, J.C.; Girit, Ç.Ö.; Zettl, A. The two-dimensional phase of boron nitride: Few-atomic-layer sheets and suspended membranes. Appl. Phys. Lett. 2008, 92, 133107. [Google Scholar] [CrossRef]
- Mishra, A.K.; Lakshmi, K.V.; Huang, L. Eco-friendly synthesis of metal dichalcogenides nanosheets and their environmental remediation potential driven by visible light. Sci. Rep. 2015, 5, 15718. [Google Scholar] [CrossRef]
- Ding, Y.; Wang, Y.; Ni, J.; Shi, L.; Shi, S.; Tang, W. First principles study of structural, vibrational and electronic properties of graphene-like MX2 (M=Mo, Nb, W, Ta; X=S, Se, Te) monolayers. Phys. B Condens. Matter 2011, 406, 2254–2260. [Google Scholar] [CrossRef]
- Zhang, X.; Qiao, X.-F.; Shi, W.; Wu, J.-B.; Jiang, D.-S.; Tan, P.-H. Phonon and Raman scattering of two-dimensional transition metal dichalcogenides from monolayer, multilayer to bulk material. Chem. Soc. Rev. 2015, 44, 2757–2785. [Google Scholar] [CrossRef]
- Ayari, A.; Cobas, E.; Ogundadegbe, O.; Fuhrer, M.S. Realization and electrical characterization of ultrathin crystals of layered transition-metal dichalcogenides. J. Appl. Phys. 2007, 101, 014507. [Google Scholar] [CrossRef]
- Tran, P.D.; Wong, L.H.; Barber, J.; Loo, J.S.C. Recent advances in hybrid photocatalysts for solar fuel production. Energy Environ. Sci. 2012, 5, 5902. [Google Scholar] [CrossRef]
- Han, S.A.; Bhatia, R.; Kim, S.-W. Synthesis, properties and potential applications of two-dimensional transition metal dichalcogenides. Nano Converg. 2015, 2, 17. [Google Scholar] [CrossRef]
- Zhang, C.; Gong, C.; Nie, Y.; Min, K.-A.; Liang, C.; Oh, Y.J.; Zhang, H.; Wang, W.; Hong, S.; Colombo, L.; et al. Systematic study of electronic structure and band alignment of monolayer transition metal dichalcogenides in Van der Waals heterostructures. 2D Mater. 2016, 4, 015026. [Google Scholar] [CrossRef]
- Chhowalla, M.; Shin, H.S.; Eda, G.; Li, L.-J.; Loh, K.P.; Zhang, H. The chemistry of two-dimensional layered transition metal dichalcogenide nanosheets. Nat. Chem. 2013, 5, 263–275. [Google Scholar] [CrossRef] [PubMed]
- Pan, H. Metal dichalcogenides monolayers: Novel catalysts for electrochemical hydrogen production. Sci. Rep. 2014, 4, 5348. [Google Scholar] [CrossRef] [PubMed]
- Velusamy, D.B.; Kim, R.H.; Cha, S.; Huh, J.; Khazaeinezhad, R.; Kassani, S.H.; Song, G.; Cho, S.M.; Cho, S.H.; Hwang, I.; et al. Flexible transition metal dichalcogenide nanosheets for band-selective photodetection. Nat. Commun. 2015, 6, 8063. [Google Scholar] [CrossRef]
- Conley, H.J.; Wang, B.; Ziegler, J.I.; Haglund, R.F., Jr.; Pantelides, S.T.; Bolotin, K.I. Bandgap engineering of strained monolayer and bilayer MoS2. Nano Lett. 2013, 13, 3626–3630. [Google Scholar] [CrossRef]
- Dumcenco, D.; Ovchinnikov, D.; Lopez Sanchez, O.; Gillet, P.; Alexander, D.T.L.; Lazar, S.; Radenovic, A.; Kis, A. Large-area MoS2 grown using H2S as the sulphur source. 2d Mater. 2015, 2, 4. [Google Scholar] [CrossRef]
- Xu, J.; Zhang, J.; Zhang, W.; Lee, C.-S. Interlayer nanoarchitectonics of two-dimensional transition-metal dichalcogenides nanosheets for energy storage and conversion applications. Adv. Energy Mater. 2017, 7, 1700571. [Google Scholar] [CrossRef]
- Voiry, D.; Goswami, A.; Kappera, R.; Silva, C.; Kaplan, D.; Fujita, T.; Chen, M.; Asefa, T.; Chhowalla, M. Covalent functionalization of monolayered transition metal dichalcogenides by phase engineering. Nat. Chem. 2015, 7, 45–49. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.J.; Kim, D.W.; Lim, J.; Cho, S.Y.; Kim, S.O.; Jung, H.T. Large-area buckled MoS2 films on the graphene substrate. ACS Appl. Mater. Interfaces 2016, 8, 13512–13519. [Google Scholar] [CrossRef] [PubMed]
- Bissett, M.A.; Tsuji, M.; Ago, H. Strain engineering the properties of graphene and other two-dimensional crystals. Phys. Chem. Chem. Phys. 2014, 16, 11124–11138. [Google Scholar] [CrossRef]
- Desai, S.B.; Seol, G.; Kang, J.S.; Fang, H.; Battaglia, C.; Kapadia, R.; Ager, J.W.; Guo, J.; Javey, A. Strain-induced indirect to direct bandgap transition in multilayer WSe2. Nano Lett. 2014, 14, 4592–4597. [Google Scholar] [CrossRef] [PubMed]
- Zeng, M.; Liu, J.; Zhou, L.; Mendes, R.G.; Dong, Y.; Zhang, M.-Y.; Cui, Z.-H.; Cai, Z.; Zhang, Z.; Zhu, D.; et al. Bandgap tuning of two-dimensional materials by sphere diameter engineering. Nat. Mater. 2020, 19, 528–533. [Google Scholar] [CrossRef]
- Azcatl, A.; Qin, X.; Prakash, A.; Zhang, C.; Cheng, L.; Wang, Q.; Lu, N.; Kim, M.J.; Kim, J.; Cho, K.; et al. Covalent nitrogen doping and compressive strain in MoS2 by remote N2 plasma exposure. Nano Lett. 2016, 16, 5437–5443. [Google Scholar] [CrossRef]
- Shi, Y.; Zhou, Y.; Yang, D.-R.; Xu, W.-X.; Wang, C.; Wang, F.-B.; Xu, J.-J.; Xia, X.-H.; Chen, H.-Y. Energy level engineering of MoS2 by transition-metal doping for accelerating hydrogen evolution reaction. J. Am. Chem. Soc. 2017, 139, 15479–15485. [Google Scholar] [CrossRef]
- Kong, D.; Wang, H.; Cha, J.J.; Pasta, M.; Koski, K.J.; Yao, J.; Cui, Y. Synthesis of MoS2 and MoSe2 films with vertically aligned layers. Nano Lett. 2013, 13, 1341–1347. [Google Scholar] [CrossRef]
- Padmajan Sasikala, S.; Singh, Y.; Bing, L.; Yun, T.; Koo, S.H.; Jung, Y.; Kim, S.O. Longitudinal unzipping of 2D transition metal dichalcogenides. Nat. Commun. 2020, 11, 5032. [Google Scholar] [CrossRef]
- Xie, J.; Zhang, H.; Li, S.; Wang, R.; Sun, X.; Zhou, M.; Zhou, J.; Lou, X.; Xie, Y. Defect-rich MoS2 ultrathin nanosheets with additional active edge sites for enhanced electrocatalytic hydrogen evolution. Adv. Mater. 2013, 25. [Google Scholar] [CrossRef]
- Zhang, H.; Yu, L.; Chen, T.; Zhou, W.; Lou, X.W. Surface Modulation of hierarchical MoS2 nanosheets by Ni single atoms for enhanced electrocatalytic hydrogen evolution. Adv. Funct. Mater. 2018, 28. [Google Scholar] [CrossRef]
- He, K.; Poole, C.; Mak, K.F.; Shan, J. Experimental demonstration of continuous electronic structure tuning via strain in atomically thin MoS2. Nano Lett. 2013, 13, 2931–2936. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.R.; Wang, G.; Liu, B.L.; Marie, X.; Qiao, X.F.; Zhang, X.; Wu, X.X.; Fan, H.; Tan, P.H.; Amand, T.; et al. Strain tuning of optical emission energy and polarization in monolayer and bilayer MoS2. Phys. Rev. B 2013, 88, 121301. [Google Scholar] [CrossRef]
- Feng, J.; Qian, X.; Huang, C.-W.; Li, J. Strain-engineered artificial atom as a broad-spectrum solar energy funnel. Nat. Photonics 2012, 6, 866–872. [Google Scholar] [CrossRef]
- González, R.I.; Valencia, F.J.; Rogan, J.; Valdivia, J.A.; Sofo, J.; Kiwi, M.; Munoz, F. Bending energy of 2D materials: Graphene, MoS2 and imogolite. RSC Adv. 2018, 8, 4577–4583. [Google Scholar] [CrossRef]
- Kibsgaard, J.; Chen, Z.; Reinecke, B.N.; Jaramillo, T.F. Engineering the surface structure of MoS2 to preferentially expose active edge sites for electrocatalysis. Nat. Mater. 2012, 11, 963–969. [Google Scholar] [CrossRef]
- Ye, G.; Gong, Y.; Lin, J.; Li, B.; He, Y.; Pantelides, S.T.; Zhou, W.; Vajtai, R.; Ajayan, P.M. Defects engineered monolayer MoS2 for improved hydrogen evolution reaction. Nano Lett. 2016, 16, 1097–1103. [Google Scholar] [CrossRef]
- Kang, N.; Paudel, H.P.; Leuenberger, M.N.; Tetard, L.; Khondaker, S.I. Photoluminescence quenching in single-layer MoS2 via oxygen plasma treatment. J. Phys. Chem. C 2014, 118, 21258–21263. [Google Scholar] [CrossRef]
- Nan, H.; Wang, Z.; Wang, W.; Liang, Z.; Lu, Y.; Chen, Q.; He, D.; Tan, P.; Miao, F.; Wang, X.; et al. Strong photoluminescence enhancement of MoS2 through defect engineering and oxygen bonding. ACS Nano 2014, 8, 5738–5745. [Google Scholar] [CrossRef]
- Yang, J.; Kim, S.; Choi, W.; Park, S.H.; Jung, Y.; Cho, M.-H.; Kim, H. Improved growth behavior of atomic-layer-deposited high-k dielectrics on multilayer MoS2 by oxygen plasma pretreatment. ACS Appl. Mater. Interfaces 2013, 5, 4739–4744. [Google Scholar] [CrossRef]
- Islam, M.R.; Kang, N.; Bhanu, U.; Paudel, H.P.; Erementchouk, M.; Tetard, L.; Leuenberger, M.N.; Khondaker, S.I. Tuning the electrical property via defect engineering of single layer MoS2 by oxygen plasma. Nanoscale 2014, 6, 10033–10039. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, A.D.; Nguyen, T.K.; Le, C.T.; Kim, S.; Ullah, F.; Lee, Y.; Lee, S.; Kim, K.; Lee, D.; Park, S.; et al. Nitrogen-Plasma-Treated Continuous Monolayer MoS2 for Improving Hydrogen Evolution Reaction. ACS Omega 2019, 4, 21509–21515. [Google Scholar] [CrossRef] [PubMed]
- Tao, L.; Duan, X.; Wang, C.; Duan, X.; Wang, S. Plasma-engineered MoS2 thin-film as an efficient electrocatalyst for hydrogen evolution reaction. Chem. Commun. 2015, 51, 7470–7473. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.; Li, H.; Xiao, J.; Tu, Y.; Deng, D.; Yang, H.; Tian, H.; Li, J.; Ren, P.; Bao, X. Triggering the electrocatalytic hydrogen evolution activity of the inert two-dimensional MoS2 surface via single-atom metal doping. Energy Environ. Sci. 2015, 8, 1594–1601. [Google Scholar] [CrossRef]
- Wang, H.; Tsai, C.; Kong, D.; Chan, K.; Abild-Pedersen, F.; Nørskov, J.K.; Cui, Y. Transition-metal doped edge sites in vertically aligned MoS2 catalysts for enhanced hydrogen evolution. Nano Res. 2015, 8, 566–575. [Google Scholar] [CrossRef]
- Huang, Y.-J.; Fan, M.-S.; Li, C.-T.; Lee, C.-P.; Chen, T.-Y.; Vittal, R.; Ho, K.-C. MoSe2 nanosheet/poly(3,4-ethylenedioxythiophene): Poly(styrenesulfonate) composite film as a Pt-free counter electrode for dye-sensitized solar cells. Electrochim. Acta 2016, 211, 794–803. [Google Scholar] [CrossRef]
- Chen, Z.; Cummins, D.; Reinecke, B.N.; Clark, E.; Sunkara, M.K.; Jaramillo, T.F. Core-shell MoO3-MoS2 nanowires for hydrogen evolution: A functional design for electrocatalytic materials. Nano Lett. 2011, 11, 4168–4175. [Google Scholar] [CrossRef]
- Zhang, Z.; Dong, Y.; Sun, H.; Liu, G.; Liu, S.; Yang, X. Defect-rich 2D reticulated MoS2 monolayers: Facile hydrothermal preparation and marvellous photoelectric properties. J. Taiwan Inst. Chem. Eng. 2019, 101, 221–230. [Google Scholar] [CrossRef]
- Abinaya, R.; Archana, J.; Harish, S.; Navaneethan, M.; Ponnusamy, S.; Muthamizhchelvan, C.; Shimomura, M.; Hayakawa, Y. Ultrathin layered MoS2 nanosheets with rich active sites for enhanced visible light photocatalytic activity. RSC Adv. 2018, 8, 26664–26675. [Google Scholar] [CrossRef]
- Jiang, Y.; Wang, D.; Li, J.; Li, M.; Pan, Z.; Ma, H.; Lv, G.; Qu, W.; Wang, L.; Tian, Z. Designing MoS2 nanocatalysts with increased exposure of active edge sites for anthracene hydrogenation reaction. Catal. Sci. Technol. 2017, 7, 2998–3007. [Google Scholar] [CrossRef]
- Liu, K.-K.; Zhang, W.; Lee, Y.-H.; Lin, Y.-C.; Chang, M.-T.; Su, C.-Y.; Chang, C.-S.; Li, H.; Shi, Y.; Zhang, H.; et al. Growth of large-area and highly crystalline MoS2 thin layers on insulating substrates. Nano Lett. 2012, 12, 1538–1544. [Google Scholar] [CrossRef] [PubMed]
- Gogoi, G.; Arora, S.; Vinothkumar, N.; De, M.; Qureshi, M. Quaternary semiconductor Cu2ZnSnS4 loaded with MoS2 as a co-catalyst for enhanced photo-catalytic activity. RSC Adv. 2015, 5, 40475–40483. [Google Scholar] [CrossRef]
- Jong, A.D.; Borg, H.; Ijzendoorn, L.J.; Soudant, V.G.F.M.; Beer, V.D.; Veen, J.; Niemantsverdriet, J. Sulfidation mechanism of molybdenum catalysts supported on SiO2/Si(100) model support studied by surface spectroscopy. J. Phys. Chem. 1993, 97, 6477–6483. [Google Scholar] [CrossRef]
- Stevens, G.C.; Edmonds, T. Electron spectroscopy for chemical analysis spectra of molybdenum sulfides. J. Catal. 1975, 37, 544–547. [Google Scholar] [CrossRef]
- Benoist, L.; Gonbeau, D.; Pfister-Guillouzo, G.; Schmidt, E.; Meunier, G.; Levasseur, A. X-ray photoelectron spectroscopy characterization of amorphous molybdenum oxysulfide thin films. Thin Solid Film. 1995, 258, 110–114. [Google Scholar] [CrossRef]
- Shuxian, Z.; Hall, W.K.; Ertl, G.; Knözinger, H. X-ray photoemission study of oxygen and nitric oxide adsorption on MoS2. J. Catal. 1986, 100, 167–175. [Google Scholar] [CrossRef]
- Wu, H.B.; Xia, B.Y.; Yu, L.; Yu, X.-Y.; Lou, X.W. Porous molybdenum carbide nano-octahedrons synthesized via confined carburization in metal-organic frameworks for efficient hydrogen production. Nat. Commun. 2015, 6, 6512. [Google Scholar] [CrossRef]
- Zeng, M.; Li, Y. Recent advances in heterogeneous electrocatalysts for the hydrogen evolution reaction. J. Mater. Chem. A 2015, 3, 14942–14962. [Google Scholar] [CrossRef]
- Jian, W.; Cheng, X.; Huang, Y.; You, Y.; Zhou, R.; Sun, T.; Xu, J. Arrays of ZnO/MoS2 nanocables and MoS2 nanotubes with phase engineering for bifunctional photoelectrochemical and electrochemical water splitting. Chem. Eng. J. 2017, 328, 474–483. [Google Scholar] [CrossRef]
- Tian, J.; Liu, Q.; Cheng, N.; Asiri, A.M.; Sun, X. Self-supported Cu3P nanowire arrays as an integrated high-performance three-dimensional cathode for generating hydrogen from water. Angew. Chem. (Int. Ed. Engl.) 2014, 53, 9577–9581. [Google Scholar] [CrossRef]
- Hinnemann, B.; Moses, P.G.; Bonde, J.; Jørgensen, K.P.; Nielsen, J.H.; Horch, S.; Chorkendorff, I.; Nørskov, J.K. Biomimetic hydrogen evolution: MoS2 nanoparticles as catalyst for hydrogen evolution. J. Am. Chem. Soc. 2005, 127, 5308–5309. [Google Scholar] [CrossRef] [PubMed]
- Qu, Y.; Ke, Y.; Shao, Y.; Chen, W.; Kwok, C.T.; Shi, X.; Pan, H. Effect of curvature on the hydrogen evolution reaction of graphene. J. Phys. Chem. C 2018, 122, 25331–25338. [Google Scholar] [CrossRef]










| Counter Electrode | η (%) | VOC (V) | jSC (mA cm−2) | FF |
|---|---|---|---|---|
| Pt | 7.81 ± 0.03 | 0.74 ± 0.00 | 16.38 ± 0.03 | 0.65 ± 0.00 |
| Bare PEDOT:PSS | 2.90 ± 0.03 | 0.67 ± 0.00 | 9.32 ± 0.25 | 0.46 ± 0.01 |
| MP-0.25 | 6.28 ± 0.25 | 0.67 ± 0.01 | 15.42 ± 0.55 | 0.61 ± 0.01 |
| MP-0.50 | 6.67 ± 0.15 | 0.68 ± 0.01 | 15.66 ± 0.29 | 0.61 ± 0.00 |
| MP-1.00 | 7.58 ± 0.05 | 0.70 ± 0.01 | 15.97 ± 0.18 | 0.67 ± 0.01 |
| MP-2.00 | 6.08 ± 0.04 | 0.68 ± 0.01 | 15.81 ± 0.21 | 0.57 ± 0.01 |
| Bare MoSe2 | 2.29 ± 0.04 | 0.66 ± 0.01 | 12.65 ± 0.31 | 0.28 ± 0.01 |
| Materials (a) | Number of Active Sites [10−3 mol g−1] | TOF [s−1] (b) | Tafel Slope [mV dec−1] | Tafel Region [mV] | j0 [µA cm−2] (c) | j [mA cm−2] (d) |
|---|---|---|---|---|---|---|
| Defect-rich ultrathin NSs | 1.785 | 0.725 | 50 | 120–180 | 8.91 | 70.0 |
| Defect-free ultrathin NSs | 0.620 | 0.496 | 87 | 180–240 | 3.16 | 16.8 |
| Calcined NSs | 0.311 | 0.467 | 90 | 230–300 | 5.62 | 8.2 |
| Thicker NSs assembles | 0.582 | 0.653 | 88 | 120–250 | 7.94 | 20.3 |
| Bulk | 0.137 | 0.304 | 81 | 250–350 | 0.32 | 2.3 |
| Elements | Binding Energy/eV | FWHM c/eV | Atomic Ratio/% | Affiliation |
|---|---|---|---|---|
| S (2p) | 161.33/162.51 a | 1.29 | 50.76/25.38 | S in MoS2 |
| 162.75/163.93 a | 1.30 | 15.74/8.12 | Vacant S in MoS2 | |
| Mo (3d) | 228.60/231.74 b | 1.25 | 25.61/16.49 | Mo in MoS2 |
| 229.06/232.39 b | 0.74 | 35.09/22.81 | Vacant Mo in MoS2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, C.-A.; Lee, C.-L.; Yang, P.-K.; Tsai, D.-S.; Lee, C.-P. Active Site Engineering on Two-Dimensional-Layered Transition Metal Dichalcogenides for Electrochemical Energy Applications: A Mini-Review. Catalysts 2021, 11, 151. https://doi.org/10.3390/catal11020151
Chen C-A, Lee C-L, Yang P-K, Tsai D-S, Lee C-P. Active Site Engineering on Two-Dimensional-Layered Transition Metal Dichalcogenides for Electrochemical Energy Applications: A Mini-Review. Catalysts. 2021; 11(2):151. https://doi.org/10.3390/catal11020151
Chicago/Turabian StyleChen, Chueh-An, Chiao-Lin Lee, Po-Kang Yang, Dung-Sheng Tsai, and Chuan-Pei Lee. 2021. "Active Site Engineering on Two-Dimensional-Layered Transition Metal Dichalcogenides for Electrochemical Energy Applications: A Mini-Review" Catalysts 11, no. 2: 151. https://doi.org/10.3390/catal11020151
APA StyleChen, C.-A., Lee, C.-L., Yang, P.-K., Tsai, D.-S., & Lee, C.-P. (2021). Active Site Engineering on Two-Dimensional-Layered Transition Metal Dichalcogenides for Electrochemical Energy Applications: A Mini-Review. Catalysts, 11(2), 151. https://doi.org/10.3390/catal11020151







