Advances in Oxidative Desulfurization of Fuel Oils over MOFs-Based Heterogeneous Catalysts
Abstract
:1. Introduction
2. MOFs as ODS Catalysts
2.1. Ti-MOFs as ODS Catalysts
2.2. Zr-MOFs as ODS Catalysts
| Catalyst | Substrate (S Content) | Oxidant | O/S Ratio | Temp. (°C) | Time (min) | Sulfur Removal (%) | Activity (mmol·g−1·h−1) | Ref. |
|---|---|---|---|---|---|---|---|---|
| UiO-66(Zr) UiO-66(Zr)mod UiO-66(Zr)HCl,mod UiO-66(Zr)HCl | DBT, 4-MDBT and 4,6-DMDBT (500 ppm for each) | H2O2 | 12 | 50 | 30 | 99.6 71.2 59.9 36.9 | - | [30] |
| UiO-66(Zr)-1h | DBT (1000 ppm) | H2O2 | ≈11 | 60 | 60 | 97 | 8.5 | [31] |
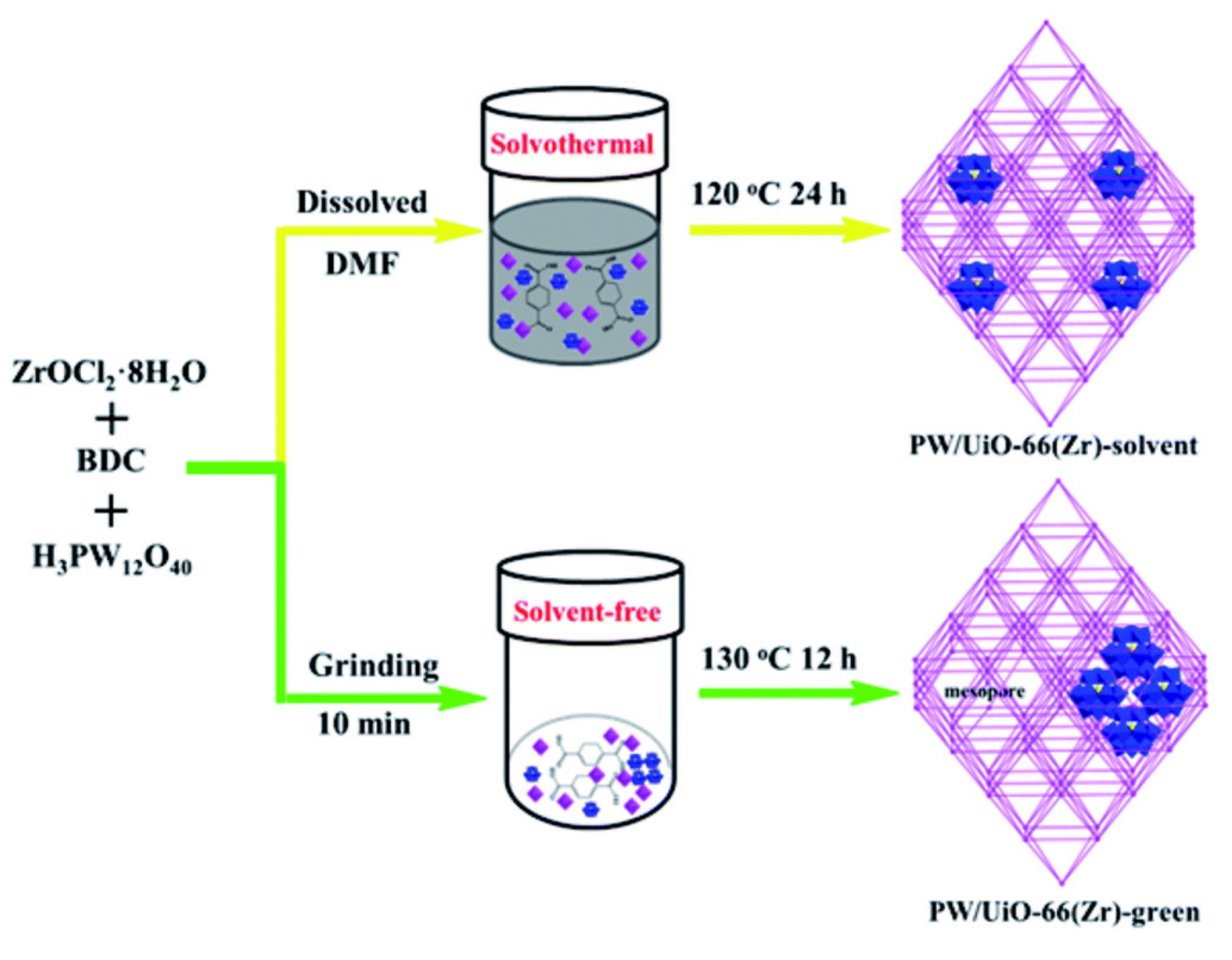
| UiO-66(Zr)-solvent UiO-66(Zr)-free | DBT (1000 ppm) 4,6-DMDBT (500 ppm) DBT (1000 ppm) 4,6-DMDBT (500 ppm) | H2O2 H2O2 | 6 6 | 60 60 | 120 120 | 80.5 52.5 99.6 98.1 | 2.5 0.82 3.1 1.5 | [34] |
| UiO-66(Zr) | DBT (500 ppm) | H2O2 | 12 | 60 | 150 | 100 | - | [36] |
| UiO-66(Zr)-NO2-green UiO-66(Zr)-NH2-green UiO-66(Zr) –green | DBT (1000 ppm) 4,6-DMDBT (500 ppm) DBT (1000 ppm) 4,6-DMDBT (500 ppm) DBT (1000 ppm) 4,6-DMDBT (500 ppm) | H2O2 H2O2 H2O2 | 6 6 6 | 60 60 60 | 30 30 30 | 99.6 99.6 62.6 45.6 90.3 94.2 | - | [17] |
| UiO-66(Zr)-S1 UiO-66(Zr)-MW2 | 1-BT, DBT, 4-MDBT and 4,6-DMDBT (500 ppm for each) | H2O2 | 13 | 50 | 180 | 99.5 96 | - | [39] |
| UiO-66(Zr)-ZrCl4 UiO-66(Zr) | BT, DBT, 4-MDBT and 4,6-DMDBT (500 ppm for each) | H2O2 | 13 | 50 | 60 | 97 62 | - | [40] |
| HP-UiO-66(Zr) | DBT (1000 ppm) 4,6-DMDBT (1000 ppm) | H2O2 | 4 | 30 | 30 60 | 94.3 99.3 | 19.6 10.3 | [42] |
| Ti-UiO-66-D UiO-66-D Ti-UiO-66-H UiO-66-H | DBT (1000 ppm) DBT (1000 ppm) | H2O2 H2O2 | 6 6 | 60 60 | 120 120 | 91.7 50.7 66.3 5.6 | 2.9 1.6 2.1 0.17 | [43] |
| UiO-66(0.13Hf-Zr) | DBT (1000 ppm) BT (1000 ppm) 4,6-DMDBT (1000 ppm) | H2O2 | 4 | 30 | 15 | 99.8 70.8 100 | 17.5 - - | [45] |
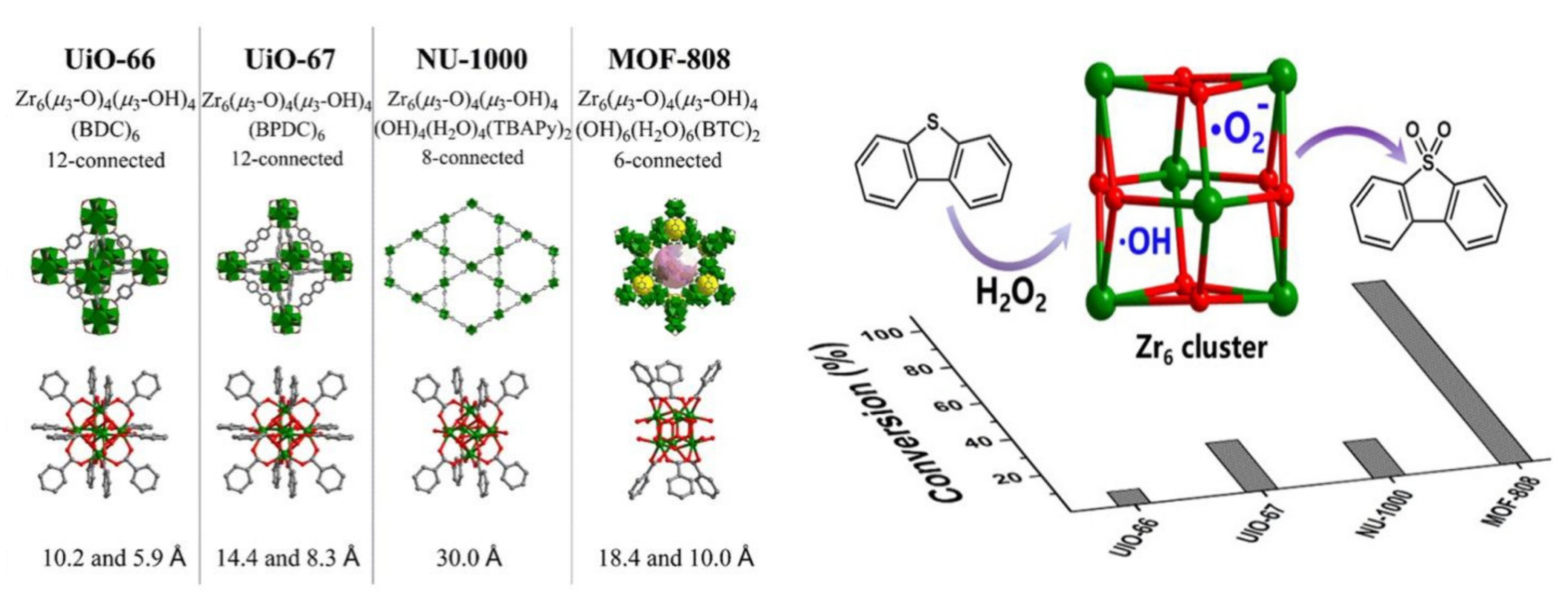
| UiO-66(Zr) UiO-67(Zr) NU-1000(Zr) MOF-808(Zr) | DBT (1000 ppm) DBT (1000 ppm) DBT (1000 ppm) DBT (1000 ppm) | H2O2 H2O2 H2O2 H2O2 | 5 5 5 5 | 50 50 50 50 | 5 5 5 5 | 8.8 20.8 11.1 100 | - | [48] |
| NU-1000(Zr) | DBT (1000 ppm) BT (500 ppm) 3-MDBT (500 ppm) 4,6-DMDBT (500 ppm) | H2O2 | 6 | 60 | 180 | 100 ≈62 ≈81 67.6 | 2.6 - - - | [50] |
| UMCM-309(Zr) MOF-808(Zr)-M | DBT (4319 ppm) BT (4378 ppm) 4,6-DMDBT (4303 ppm) BT, DBT and 4,6-DMDBT (500 ppm for each) | TBHP TBHP | 2.5 2.5 | 60 60 | 480 60 | 96 49 30 68 | 2.5 1.3 0.79 9.2 | [46] |
| MOF-808(Zr)-H | DBT (1000 ppm) BT (1000 ppm) 4,6-DMDBT (500 ppm) | CHP | 3 | 50 | 20 | 93 ≈34 ≈17 | 17.4 - - | [52] |
2.3. Other Metal-Centered MOFs as ODS Catalysts
| Catalyst | Substrate (S Content) | Oxidant | O/S Ratio | Temp. (°C) | Time (min) | Sulfur Removal (%) | Activity (mmol·g−1·h−1) | Ref. |
|---|---|---|---|---|---|---|---|---|
| MIL-47(V) | DBT (15691 ppm) T (7165 ppm) BT (11428 ppm) | TBHP | 2.15 | 80 | - | - | (29) a (1.3) a (9.9) a | [53] |
| TMU-10(Co) TMU-12(Co) | DBT (500 ppm) DBT (500 ppm) | TBHP TBHP | 3 3 | 60 60 | 360 360 | 40.5 75.2 | 0.17 0.32 | [58] |
| NH2–TMU(Co)-53 | DBT (500 ppm) | H2O2 | 3 | 60 | 120 | 79.4 | (10.6) a | [59] |
| MIL-101(Cr) | DBT (1534 ppm) | O2 | - | 120 | 1260 | 99.6 | - | [60] |
| MIL-101(Cr)-NO2 | DBT (200 ppm) | O2 | - | 140 | ≈270 | ≈100 | - | [61] |
| MFM-300(V) | DBT (200 ppm) BT (200 ppm) 4,6-DMDBT (200 ppm) | Air | - | 120 | 300 | 99.6 18.0 98.1 | 0.62 - - | [56] |
3. Composites of MOFs and Additional Active Phases as ODS Catalysts
3.1. Composites of MOFs and Polyoxometalates
| Catalyst | Substrate (S Content) | Oxidant | O/S Ratio | Temp. (°C) | Time (min) | Sulfur Removal (%) | Activity (mmol·g−1·h−1) | Ref. |
|---|---|---|---|---|---|---|---|---|
| 50%PTA@MIL-101(Cr) | DBT (≈912 ppm) | H2O2 | 50 | 45 | 180 | 91 | - | [64] |
| Tb(PW11)2@MIL-101(Cr) | 1-BT (500 ppm) DBT (500 ppm) 4,6-DMDBT (500 ppm) | H2O2 | 30 | 50 | 120 | 95 100 96 | 0.064 0.067 0.065 | [66] |
| PW12@MIL-101(Cr) | DBT (500 ppm) | H2O2 | 10 | 50 | 60 | 100 | 0.32 | [65] |
| PW9@MIL-101(Cr) | 1-BT (500 ppm) DBT (500 ppm) 4,6-DMDBT (500 ppm) | H2O2 | ≈89 | 50 | 60 | 98.6 100 98.9 | 0.088 0.089 0.088 | [67] |
| PW11Zn@MIL-101(Cr) | 1-BT (500 ppm) DBT (500 ppm) 4,6-DMDBT (500 ppm) | H2O2 | 8 | 50 | 120 | 100 100 100 | - | [68] |
| LaW10@ MIL-101(Cr) | BT (500 ppm) DBT (500 ppm) 4,6-DMDBT (500 ppm) | H2O2 | 6 | 60 | 180 | 87.4 99.1 94.5 | 0.40 0.45 0.43 | [70] |
| PTA@MIL-101(Cr)-NH2 | BT (950 ppm) DBT (950 ppm) 4,6-DMDBT (950 ppm) | H2O2 | 10 4 10 | 50 | 240 60 240 | 70.5 100 88.2 | (76) a | [71] |
| 2PTA/NH2-MOF(Al) | DBT (500 ppm) | H2O2 | 20 | 65 | 240 | ≈100 | 0.39 | [72] |
| POM/MIL(Cr) POM/MIL(Al) | T, 1-BT, DBT, 4-MDBT and 4,6-DMDBT (500 ppm for each) | H2O2 | 23 | 70 | 120 | 82.0 99.9 | - | [73] |
| PMo12@NH2-MIL-101(Cr) | BT, DBT, 4-MDBT and 4,6-DMDBT (500 ppm for each) | H2O2 | 6 | 50 | 120 | 95 | - | [77] |
| PTA@MIL-101(Cr)@diatomite | DBT (500 ppm) | H2O2 | 5 | 60 | 120 | 98.6 | 0.32 | [78] |
| PMo@HKUST-1(Cu) | T (250 ppm) DBT (500 ppm) | H2O2 | 6 | 65 | 120 | 90 95 | - | [82] |
| PW/UiO-66(Zr)-green | BT (1000 ppm) DBT (1000 ppm) 4,6-DMDBT (500 ppm) | H2O2 | 6 | 25 | 25 | 81 99.7 99.8 | 12.1 14.9 7.5 | [96] |
| [mim(CH2)3COO]3PW @UiO-66(Zr) | DBT (1000 ppm) | H2O2 | 5 | 70 | 60 | 100 | 2.7 | [97] |
| PTA@MIL-100(Fe) PTA@UiO-66(Zr) PTA@ZIF-8(Zn) | BT (950 ppm) DBT (950 ppm) 4,6-DMDBT (950 ppm) BT (950 ppm) DBT (950 ppm) 4,6-DMDBT (950 ppm) BT (950 ppm) DBT (950 ppm) 4,6-DMDBT (950 ppm) | H2O2 H2O2 H2O2 | 4 4 4 | 70 70 70 | 60 60 60 | 61.8 100 92.8 94.8 100 39.1 9.1 28.6 25.7 | - | [92] |
| PW12@UiO-67(Zr) | BT (1000 ppm) DBT (1000 ppm) 4,6-DMDBT (1000 ppm) | H2O2 | 13 | 70 | 60 | 75 99.5 80 | - | [98] |
| 42%PTA@MOF-808(Zr)-A | DBT (1000 ppm) | H2O2 | 5 | 60 | 30 | 100 | 7.1 | [99] |
| PTA@TMU-17(Zn)-NH2 | BT (169 ppm) DBT (124 ppm) 4,6-DMDBT (107 ppm) | H2O2 | 2 | - | 15 | 71 98 87 | 2.6 2.7 2.0 | [79] |
| PMo6W6O40@MOF-199(Cu) @MCM-41 PMo6W6O40@MOF-199(Cu) | DBT (2000 ppm) | O2 | - | 85 | 180 | 98.5 61.4 | - | [83] |
| Cs-POM@MOF-199(Cu) @MCM -41 | DBT (2000 ppm) | O2 | - | 80 | 180 | 99.6 | (104.5) a | [84] |
| C-POM@MOF-199(Cu) @MCM-41 | DBT (2000 ppm) | O2 | - | 50 | 90 | 100 | 29.2 | [85] |
| SRL-POM@MOF-199(Cu) @MCM-41 | DBT (2000 ppm) | O2 | 60 | 150 | 100 | - | [86] | |
| POM@MOF-199(Cu) @ZSM-5 POM@MOF-199(Cu) @MCM-41 | DBT (2000 ppm) | O2 | - | - | - | 91.2 83.5 | - | [88] |
| POM@MOF-199@LZSM-5 | DBT (2000 ppm) | O2 | - | 60 | 120 | 100 | (44.6) a | [89] |
| 1.5HPA@MOF-199(Cu) @CA-4 | T (1000 ppm) | O2 | - | 40 | 180 | 99.23 | (27.6) a | [87] |
| CNTs@MOF-199(Cu)-Mo16V2 | DBT (2000 ppm) | O2 | - | 80 | 180 | 98.3 | (21.0) a | [90] |
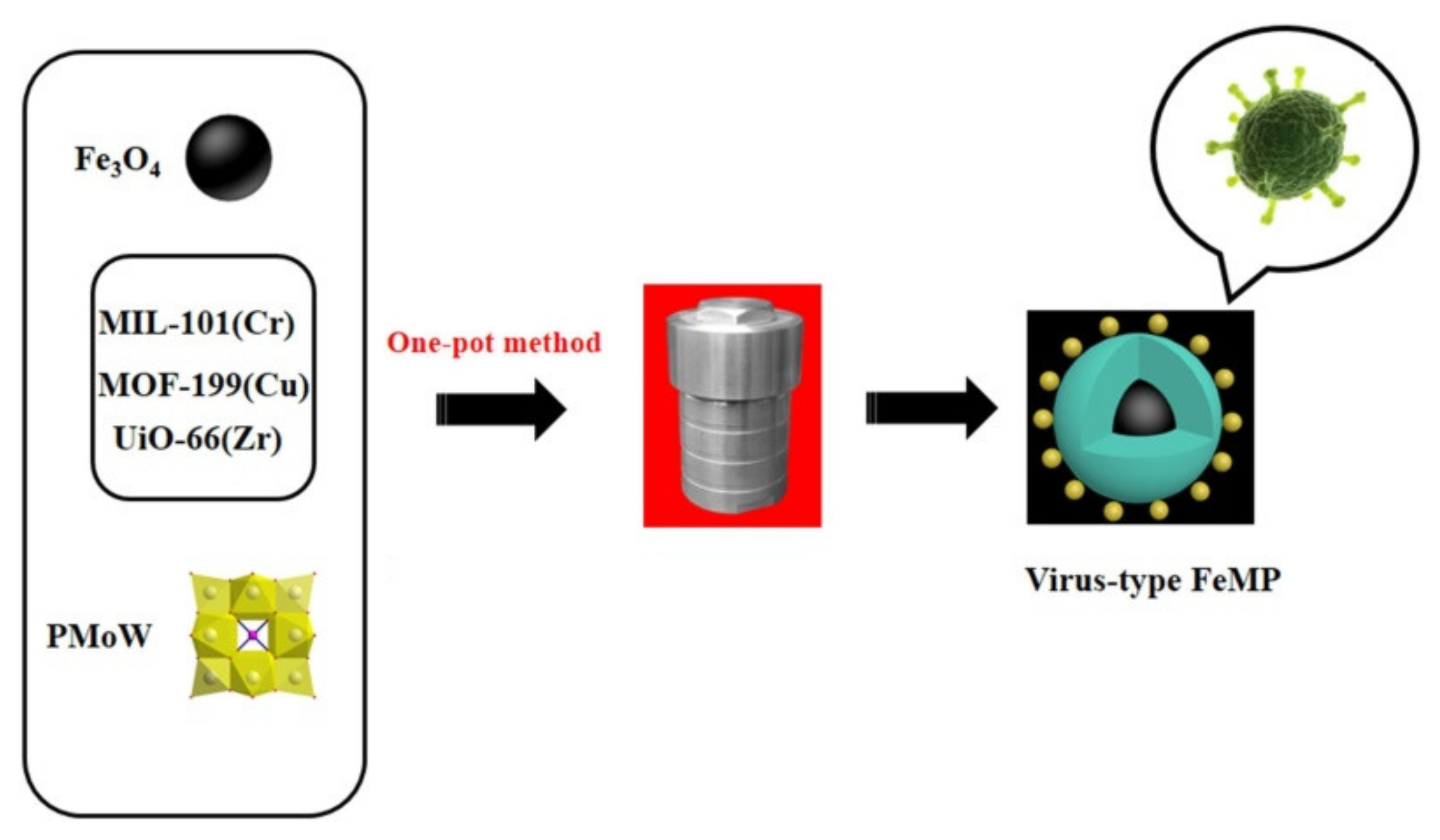
| Fe3O4@MIL-101(Cr)-PMoW Fe3O4@MOF-199(Cu)-PMoW Fe3O4@UiO-66(Zr)-PMoW | DBT (2000 ppm) | Air | - | 40 40 60 | 60 75 75 | 100 97.0 91.6 | 93.8 72.8 68.8 | [19] |
| PMA@UiO-66(Zr) | DBT (500 ppm) | TBHP | 3 | 80 | 55 | 100 | 3.4 | [93] |
| [Bmim]3PMo12O40/UiO-66(Zr) PMA/Thiourea/UiO-66(Zr) | DBT (500 ppm) | TBHP | 3 | 80 | 50 30 | 100 100 | 3.7 6.2 | [94] |
| TiF4-PU-200 | DBT (500 ppm) | TBHP | 3 | 80 | 30 | ≈100 | - | [95] |
3.2. Composites of MOFs with Other Active Species
| Catalyst | Substrate (S Content) | Oxidant | O/S Ratio | Temp. (°C) | Time (min) | Sulfur Removal (%) | Activity (mmol·g−1·h−1) | Ref. |
|---|---|---|---|---|---|---|---|---|
| 5 wt.% MoO3-TMU-5(Zn) | DBT (521 ppm) | Air | - | 20 | 60 | 95.6 | 10.9 | [100] |
| Fe3O4@MoO3@MOF-199(Cu) | DBT (2000 ppm) | Air | - | 50 | 45 | 100 | 125.1 | [102] |
| Fe3O4@W-MoO3@MOF-199(Cu) | DBT (2000 ppm) | Air | - | 40 | 60 | 100 | - | [103] |
| MnO2/UiO-66(Zr) | DBT (347 ppm) 4,6-DMDBT (347 ppm) | NaClO | 4 | 25 | 5 | 100 94 | - | [104] |
| MoUiO-66(Zr) | DBT (500 ppm) | H2O2 | 2 | 25 | 50 | 95 | 0.89 | [105] |
| W/UiO-66(Zr)-0.12 | DBT (1000 ppm) BT (500 ppm) 4,6-DMDBT (500 ppm) | H2O2 | 4 | 30 30 20 | 30 | 99.9 65.3 100 | 14.6 4.8 11.0 | [106] |
| PSMIMHSO4@UiO-66(Zr) | BT (2000 ppm) | H2O2 | 7 | 30 | 20 | 94.6 | 3.0 | [107] |
| 1.1-ILs@MIL-100(Fe) | DBT (50 ppm) | H2O2 | 25 | 60 | 180 | 99.31 | 0.073 | [108] |
| [mim(CH2)3COO]FeCl4@UiO-66(Zr) | DBT (1000 ppm) | H2O2 | 5 | 40 | 120 | 99.1 | 1.4 | [109] |
| MOF-76(Tb)@LDH | DBT (500 ppm) | H2O2 | 3 | 60 | 25 | 100 | 13.2 | [111] |
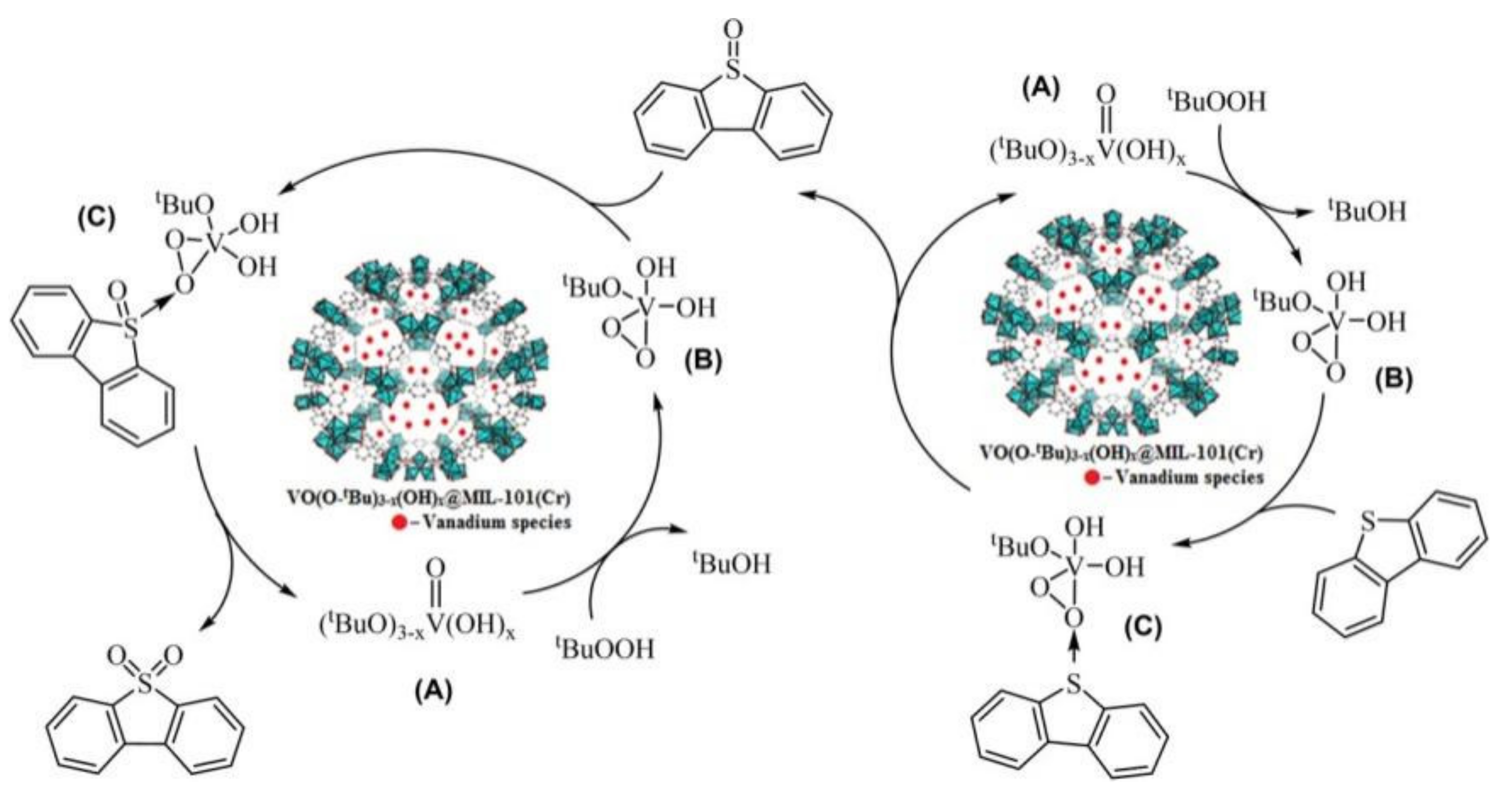
| OV(OtBu)3−x(OH)x@MIL-101(Cr) OV(OtBu)3−x(OH)x@A520(Al) | DBT (500 ppm) | TBHP | 5 | 60 | 60 80 | 98 98 | 10.5 7.9 | [110] |
4. MOFs-Derived Materials as ODS Catalysts
| Catalyst | Precursor MOF | Substrate (S Content) | Oxidant | O/S Ratio | Temp. (°C) | Time (min) | Sulfur Removal (%) | Activity (mmol·g−1·h−1) | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| Ti/NC | IRMOF-3(Zn)/Ti | DBT (4896 ppm) | TBHP | 2.14 | 100 | 35 | ≈25 | (3.4) a | [112] |
| meso-1000 | meso-MIL-125(Ti) | DBT (500 ppm) | TBHP | 10 | 80 | 3 | - | - | [113] |
| C1000 | MIL-47(V) | DBT (4870 ppm) | TBHP | 2.15 | 104 | 14 | ≈57 | (61.9) a | [114] |
| 50%Ti-MIL-101-550 | 50%Ti-MIL-101(Cr) | DBT (1000 ppm) | CHP | - | 60 | 30 | 90 | 11.3 | [116] |
| TiO2@M-6 TiO2@M-74 | TiCl4@MAF-6(Zn) Ti(SO4)2@MOF-74(Zn) | DBT (1000 ppm) | H2O2 | 15 | 80 | 60 | 97.4 84.1 | (50) a (32) a | [115] |
| MDC-C | ZIF-8@MIL-125(Ti) -NH2 | DBT (1000 ppm) | H2O2 | 15 | 80 | 120 | 99.5 | 5.5 | [117] |
| MDNM(75Zn25Mn) | MOF-74(75Zn25Mn) | DBT (1000 ppm) | H2O2 | 20 | 80 | 120 | ≈95 | (58) a | [118] |
| MDC-6(75Zn25Co)-900 | MDC-6(75Zn25Co) | DBT (≈248 ppm) | H2O2 | 15 | 70 | 120 | 93.6 | (48) a | [119] |
| Mo2N@C-3 | PMA(3)@MAF-6(Zn) | DBT (1000 ppm) | H2O2 | 10 | 60 | 20 | 100 | (190) a | [122] |
| Mela(10)PWA(15)@C | Mela(10)PWA(15)@ MAF-6(Zn) | DBT (2000 ppm) | H2O2 | 7.5 | 60 | 15 | 98 | 20.8 | [123] |
5. Summary and Outlook
- As for MOFs as ODS catalysts, most of pure MOFs exhibited poor catalytic ODS performance due to the lack of active sites. However, it has been demonstrated that MOFs with defect sites may improve their performance, especially Zr-based and Ti-based MOFs. Therefore, the design and synthesis of novel Zr-MOFs and Ti-MOFs with abundant defect sites is still challenging.
- As for composites of MOFs and additional active phases as ODS catalysts, most research works have been focused on the combination of POMs and MOFs. Because the type of POMs is varied, it is undoubted that such combination could supply more chances to develop new catalysts for catalytic ODS of fuel oils, particularly for aerobic ODS.
- As for MOFs-derived materials as ODS catalysts, more active sites could be created and well dispersed into final materials by this strategy. However, the stability of active sites is still an issue. Meanwhile, high cost resulting from the collapse of MOFs is not beneficial for industrial application. Certainly, such a study is meaningful for the understanding of reaction mechanisms for basic research.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Smith, L.V.; Tarui, N.; Yamagata, T. Assessing the impact of COVID-19 on global fossil fuel consumption and CO2 emissions. Energy Econ. 2021, 97, 105170. [Google Scholar] [CrossRef]
- Poudyal, R.; Loskot, P.; Nepal, R.; Parajuli, R.; Khadka, S.K. Mitigating the current energy crisis in Nepal with renewable energy sources. Renew. Sustain. Energy Rev. 2019, 116, 109388. [Google Scholar] [CrossRef]
- Xu, X.; Nie, S.; Ding, H.; Hou, F.F. Environmental pollution and kidney diseases. Nat. Rev. Nephrol. 2018, 14, 313–324. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Zhao, X.; Xiao, D. Acid rain: An unsuspected factor predisposing Panzhihua airport landslide, China. Environ. Sci. Pollut. Res. Int. 2021, 28, 36753–36764. [Google Scholar] [CrossRef]
- Singh, D.; Chopra, A.; Mahendra, P.K.; Kagdiyal, V.; Saxena, D. Sulfur compounds in the fuel range fractions from different crude oils. Pet. Sci. Technol. 2016, 34, 1248–1254. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, H.; Zhao, J.; Liu, Y.; Liu, C. Ultra-deep desulfurization by reactive adsorption desulfurization on copper-based catalysts. J. Energy Chem. 2019, 29, 8–16. [Google Scholar] [CrossRef]
- Julião, D.; Gomes, A.C.; Pillinger, M.; Valença, R.; Ribeiro, J.C.; Gonçalves, I.S.; Balula, S.S. Desulfurization of liquid fuels by extraction and sulfoxidation using H2O2 and [CpMo(CO)3R] as catalysts. Appl. Catal. B Environ. 2018, 230, 177–183. [Google Scholar] [CrossRef]
- De Rink, R.; Klok, J.B.M.; van Heeringen, G.J.; Sorokin, D.Y.; Ter Heijne, A.; Zeijlmaker, R.; Mos, Y.M.; de Wilde, V.; Keesman, K.J.; Buisman, C.J.N. Increasing the Selectivity for Sulfur Formation in Biological Gas Desulfurization. Environ. Sci. Technol. 2019, 53, 4519–4527. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Liu, G.; Zhang, B.; Zhang, X. Oxidation of refractory sulfur compounds with molecular oxygen over a Ce–Mo–O catalyst. Green Chem. 2016, 18, 5273–5279. [Google Scholar] [CrossRef]
- Wu, P.; Zhu, W.; Chao, Y.; Zhang, J.; Zhang, P.; Zhu, H.; Li, C.; Chen, Z.; Li, H.; Dai, S. A template-free solvent-mediated synthesis of high surface area boron nitride nanosheets for aerobic oxidative desulfurization. Chem. Commun. 2016, 52, 144–147. [Google Scholar] [CrossRef]
- Gu, Q.; Wen, G.; Ding, Y.; Wu, K.-H.; Chen, C.; Su, D. Reduced graphene oxide: A metal-free catalyst for aerobic oxidative desulfurization. Green Chem. 2017, 19, 1175–1181. [Google Scholar] [CrossRef]
- Leng, K.; Sun, Y.; Zhang, X.; Yu, M.; Xu, W. Ti-modified hierarchical mordenite as highly active catalyst for oxidative desulfurization of dibenzothiophene. Fuel 2016, 174, 9–16. [Google Scholar] [CrossRef]
- Leng, K.; Li, X.; Ye, G.; Du, Y.; Sun, Y.; Xu, W. Ti-containing hierarchical Beta with highly active sites for deep desulfurization of fuels under mild conditions. Catal. Sci. Technol. 2016, 6, 7615–7622. [Google Scholar] [CrossRef]
- Piscopo, C.G.; Granadeiro, C.M.; Balula, S.S.; Bošković, D. Metal-Organic Framework-Based Catalysts for Oxidative Desulfurization. ChemCatChem 2020, 12, 4721–4731. [Google Scholar] [CrossRef]
- Bhadra, B.N.; Jhung, S.H. Oxidative desulfurization and denitrogenation of fuels using metal-organic framework-based/-derived catalysts. Appl. Catal. B Environ. 2019, 259, 118021. [Google Scholar] [CrossRef]
- Kirchon, A.; Feng, L.; Drake, H.F.; Joseph, E.A.; Zhou, H.C. From fundamentals to applications: A toolbox for robust and multifunctional MOF materials. Chem. Soc. Rev. 2018, 47, 8611–8638. [Google Scholar] [CrossRef] [PubMed]
- Ye, G.; Qi, H.; Zhou, W.; Xu, W.; Sun, Y. Green and scalable synthesis of nitro- and amino-functionalized UiO-66(Zr) and the effect of functional groups on the oxidative desulfurization performance. Inorg. Chem. Front. 2019, 6, 1267–1274. [Google Scholar] [CrossRef]
- Bueken, B.; Van Velthoven, N.; Krajnc, A.; Smolders, S.; Taulelle, F.; Mellot-Draznieks, C.; Mali, G.; Bennett, T.D.; De Vos, D. Tackling the Defect Conundrum in UiO-66: A Mixed-Linker Approach to Engineering Missing Linker Defects. Chem. Mater. 2017, 29, 10478–10486. [Google Scholar] [CrossRef]
- Li, S.-W.; Zhang, H.-Y.; Han, T.-H.; Wu, W.-Q.; Wang, W.; Zhao, J.-S. A spinosus Fe3O4@MOF-PMoW catalyst for the highly effective oxidative desulfurization under oxygen as oxidant. Sep. Purif. Technol. 2021, 264, 118460. [Google Scholar] [CrossRef]
- Jin, C.; Li, G.; Wang, X.; Zhao, L.; Liu, L.; Liu, H.; Liu, Y.; Zhang, W.; Han, X.; Bao, X. Synthesis, Characterization and Catalytic Performance of Ti-Containing Mesoporous Molecular Sieves Assembled from Titanosilicate Precursors. Chem. Mater. 2007, 19, 1664–1670. [Google Scholar] [CrossRef]
- Kim, T.-W.; Kim, M.-J.; Kleitz, F.; Nair, M.M.; Guillet-Nicolas, R.; Jeong, K.-E.; Chae, H.-J.; Kim, C.-U.; Jeong, S.-Y. Tailor-Made Mesoporous Ti-SBA-15 Catalysts for Oxidative Desulfurization of Refractory Aromatic Sulfur Compounds in Transport Fuel. ChemCatChem 2012, 4, 687–697. [Google Scholar] [CrossRef]
- Dan-Hardi, M.; Serre, C.; Frot, T.; Rozes, L.; Maurin, G.; Sanchez, C.; Ferey, G. A new photoactive crystalline highly porous titanium(IV) dicarboxylate. J. Am. Chem. Soc. 2009, 131, 10857–10859. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-N.; Kim, J.; Kim, H.-Y.; Cho, H.-Y.; Ahn, W.-S. Adsorption/catalytic properties of MIL-125 and NH2-MIL-125. Catal. Today 2013, 204, 85–93. [Google Scholar] [CrossRef]
- McNamara, N.D.; Hicks, J.C. Chelating agent-free, vapor-assisted crystallization method to synthesize hierarchical microporous/mesoporous MIL-125 (Ti). ACS Appl. Mater. Interfaces 2015, 7, 5338–5346. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, G.; Kong, L.; Lu, H. Deep oxidative desulfurization catalyzed by Ti-based metal-organic frameworks. Fuel 2018, 219, 103–110. [Google Scholar] [CrossRef]
- Smolders, S.; Willhammar, T.; Krajnc, A.; Sentosun, K.; Wharmby, M.T.; Lomachenko, K.A.; Bals, S.; Mali, G.; Roeffaers, M.B.J.; De Vos, D.E.; et al. A Titanium(IV)-Based Metal-Organic Framework Featuring Defect-Rich Ti-O Sheets as an Oxidative Desulfurization Catalyst. Angew. Chem. Int. Ed. Engl. 2019, 58, 9160–9165. [Google Scholar] [CrossRef] [PubMed]
- Ye, G.; Sun, Y.; Zhang, D.; Zhou, W.; Lancelot, C.; Rives, A.; Lamonier, C.; Xu, W. Hierarchical porous titanium terephthalate based material with highly active sites for deep oxidative desulfurization. Microporous Mesoporous Mater. 2018, 270, 241–247. [Google Scholar] [CrossRef]
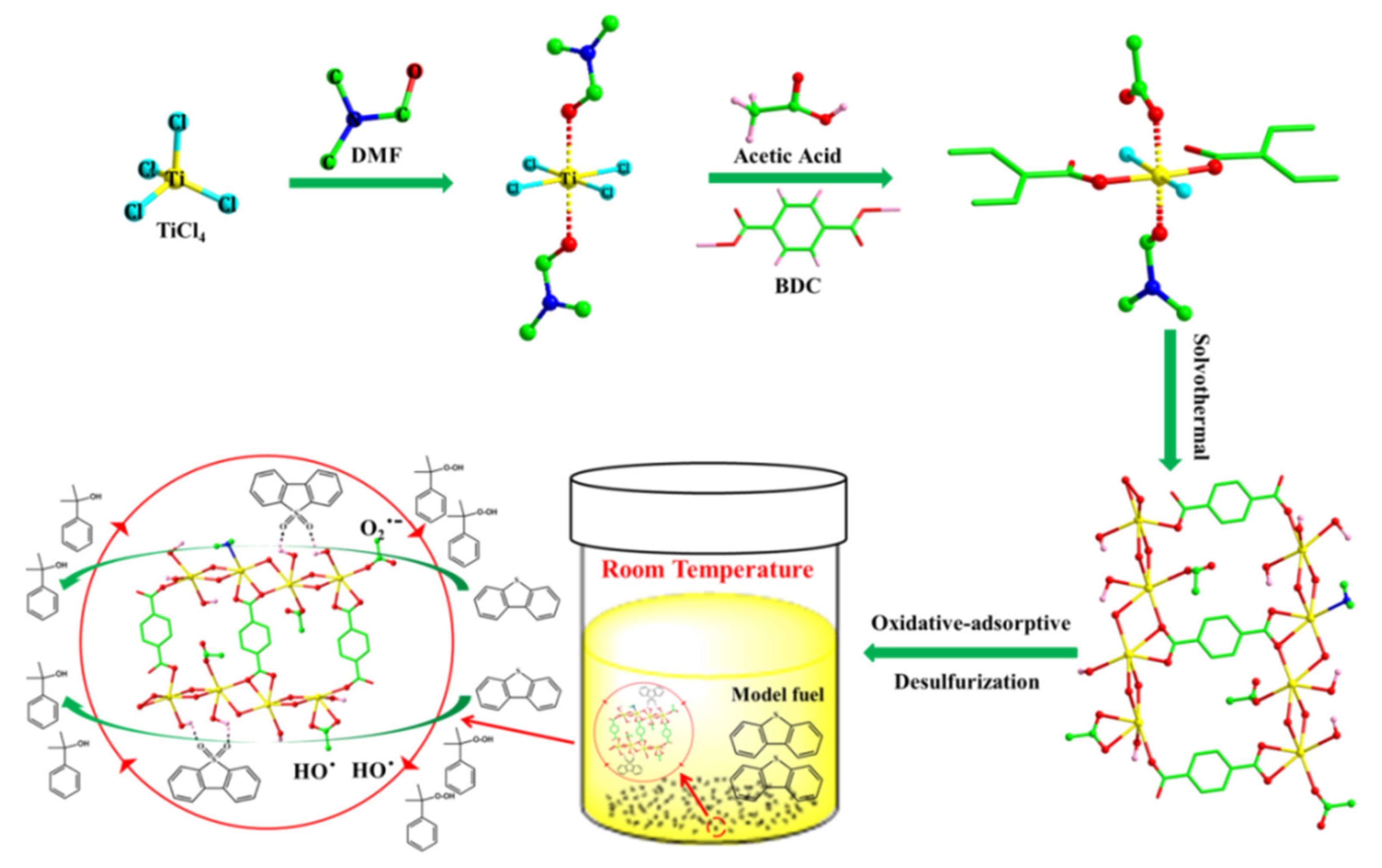
- Ye, G.; Gu, Y.; Zhou, W.; Xu, W.; Sun, Y. Synthesis of Defect-Rich Titanium Terephthalate with the Assistance of Acetic Acid for Room-Temperature Oxidative Desulfurization of Fuel Oil. ACS Catal. 2020, 10, 2384–2394. [Google Scholar] [CrossRef]
- Cavka, J.H.; Jakobsen, S.; Olsbye, U.; Guillou, N.; Lamberti, C.; Bordiga, S.; Lillerud, K.P. A new zirconium inorganic building brick forming metal organic frameworks with exceptional stability. J. Am. Chem. Soc. 2008, 130, 13850–13851. [Google Scholar] [CrossRef]
- Granadeiro, C.M.; Ribeiro, S.O.; Karmaoui, M.; Valenca, R.; Ribeiro, J.C.; de Castro, B.; Cunha-Silva, L.; Balula, S.S. Production of ultra-deep sulfur-free diesels using a sustainable catalytic system based on UiO-66(Zr). Chem. Commun. 2015, 51, 13818–13821. [Google Scholar] [CrossRef]
- Xiao, W.; Dong, Q.; Wang, Y.; Li, Y.; Deng, S.; Zhang, N. Time modulation of defects in UiO-66 and application in oxidative desulfurization. CrystEngComm 2018, 20, 5658–5662. [Google Scholar] [CrossRef]
- Zhuang, S.; Cheng, R.; Wang, J. Adsorption of diclofenac from aqueous solution using UiO-66-type metal-organic frameworks. Chem. Eng. J. 2019, 359, 354–362. [Google Scholar] [CrossRef]
- Dhakshinamoorthy, A.; Santiago-Portillo, A.; Asiri, A.M.; Garcia, H. Engineering UiO-66 Metal Organic Framework for Heterogeneous Catalysis. ChemCatChem 2019, 11, 899–923. [Google Scholar] [CrossRef]
- Ye, G.; Zhang, D.; Li, X.; Leng, K.; Zhang, W.; Ma, J.; Sun, Y.; Xu, W.; Ma, S. Boosting Catalytic Performance of Metal-Organic Framework by Increasing the Defects via a Facile and Green Approach. ACS Appl. Mater. Interfaces 2017, 9, 34937–34943. [Google Scholar] [CrossRef]
- Vermoortele, F.; Ameloot, R.; Vimont, A.; Serre, C.; De Vos, D. An amino-modified Zr-terephthalate metal-organic framework as an acid-base catalyst for cross-aldol condensation. Chem. Commun. 2011, 47, 1521–1523. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, X.; Huang, P.; Liu, A.; Zhu, M. A metal–organic framework for oxidative desulfurization: UIO-66(Zr) as a catalyst. Fuel 2017, 209, 417–423. [Google Scholar] [CrossRef]
- Vermoortele, F.; Vandichel, M.; Van de Voorde, B.; Ameloot, R.; Waroquier, M.; Van Speybroeck, V.; De Vos, D.E. Electronic effects of linker substitution on Lewis acid catalysis with metal-organic frameworks. Angew. Chem. Int. Ed. Engl. 2012, 51, 4887–4890. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Huang, H.; Zhang, Y.; Kang, C.; Chen, S.; Song, L.; Liu, D.; Zhong, C. A versatile MOF-based trap for heavy metal ion capture and dispersion. Nat. Commun. 2018, 9, 187. [Google Scholar] [CrossRef] [Green Version]
- Viana, A.M.; Ribeiro, S.O.; Castro, B.; Balula, S.S.; Cunha-Silva, L. Influence of UiO-66(Zr) Preparation Strategies in Its Catalytic Efficiency for Desulfurization Process. Materials 2019, 12, 3009. [Google Scholar] [CrossRef] [Green Version]
- Viana, A.M.; Julião, D.; Mirante, F.; Faria, R.G.; de Castro, B.; Balula, S.S.; Cunha-Silva, L. Straightforward activation of metal-organic framework UiO-66 for oxidative desulfurization processes. Catal. Today 2021, 362, 28–34. [Google Scholar] [CrossRef]
- Yang, D.; Gates, B.C. Catalysis by Metal Organic Frameworks: Perspective and Suggestions for Future Research. ACS Catal. 2019, 9, 1779–1798. [Google Scholar] [CrossRef]
- Hao, L.; Stoian, S.A.; Weddle, L.R.; Zhang, Q. Zr-Based MOFs for oxidative desulfurization: What matters? Green Chem. 2020, 22, 6351–6356. [Google Scholar] [CrossRef]
- Ye, G.; Qi, H.; Li, X.; Leng, K.; Sun, Y.; Xu, W. Enhancement of Oxidative Desulfurization Performance over UiO-66(Zr) by Titanium Ion Exchange. Chemphyschem 2017, 18, 1903–1908. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Wu, Y.; Chen, T.; Weng, W.; Wan, H. Low-temperature catalytic performance for oxidative dehydrogenation of propane on nanosized Ti(Zr)–Ni–O prepared by modified sol–gel method. Catal. Commun. 2006, 7, 268–271. [Google Scholar] [CrossRef]
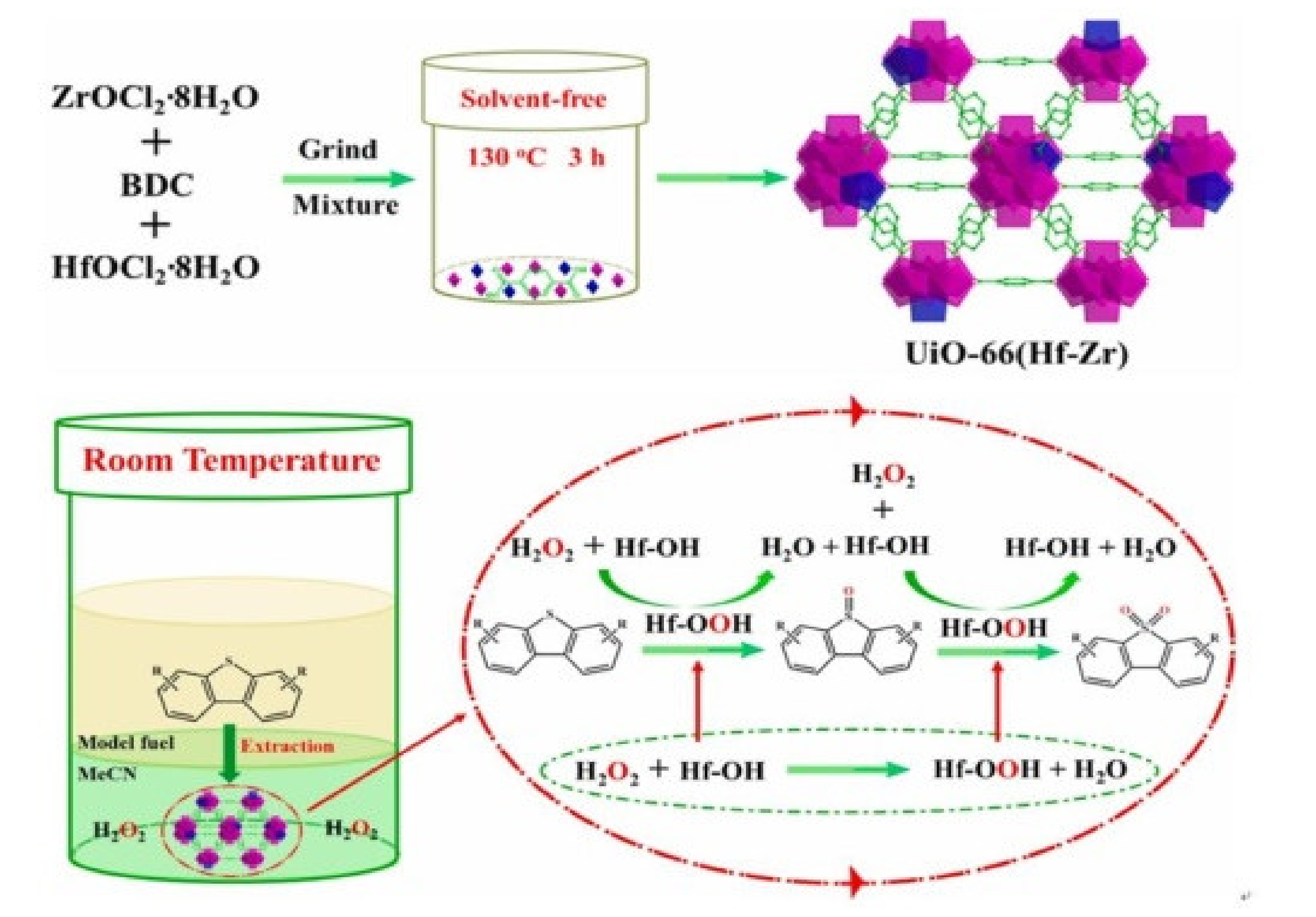
- Ye, G.; Wang, H.; Zeng, X.; Wang, L.; Wang, J. Defect-rich bimetallic UiO-66(Hf-Zr): Solvent-free rapid synthesis and robust ambient-temperature oxidative desulfurization performance. Appl. Catal. B Environ. 2021, 299, 120659. [Google Scholar] [CrossRef]
- Fu, G.; Bueken, B.; De Vos, D. Zr-Metal-Organic Framework Catalysts for Oxidative Desulfurization and Their Improvement by Postsynthetic Ligand Exchange. Small Methods 2018, 2, 1800203. [Google Scholar] [CrossRef]
- Wang, J.C.; Ding, F.W.; Ma, J.P.; Liu, Q.K.; Cheng, J.Y.; Dong, Y.B. Co(II)-MOF: A Highly Efficient Organic Oxidation Catalyst with Open Metal Sites. Inorg. Chem. 2015, 54, 10865–10872. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.Q.; Zeng, Y.N.; Chen, J.; Lin, R.G.; Zhuang, W.E.; Cao, R.; Lin, Z.J. Zr-Based Metal-Organic Frameworks with Intrinsic Peroxidase-Like Activity for Ultradeep Oxidative Desulfurization: Mechanism of H2O2 Decomposition. Inorg. Chem. 2019, 58, 6983–6992. [Google Scholar] [CrossRef] [PubMed]
- Ji, P.; Drake, T.; Murakami, A.; Oliveres, P.; Skone, J.H.; Lin, W. Tuning Lewis Acidity of Metal-Organic Frameworks via Perfluorination of Bridging Ligands: Spectroscopic, Theoretical, and Catalytic Studies. J. Am. Chem. Soc. 2018, 140, 10553–10561. [Google Scholar] [CrossRef]
- Hao, L.; Hurlock, M.J.; Li, X.; Ding, G.; Kriegsman, K.W.; Guo, X.; Zhang, Q. Efficient oxidative desulfurization using a mesoporous Zr-based MOF. Catal. Today 2020, 350, 64–70. [Google Scholar] [CrossRef]
- Mondloch, J.E.; Bury, W.; Fairen-Jimenez, D.; Kwon, S.; DeMarco, E.J.; Weston, M.H.; Sarjeant, A.A.; Nguyen, S.T.; Stair, P.C.; Snurr, R.Q.; et al. Vapor-phase metalation by atomic layer deposition in a metal-organic framework. J. Am. Chem. Soc. 2013, 135, 10294–10297. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; Ye, G.; Xu, W.; Zhou, W.; Sun, Y. Creation of Active Sites in MOF-808(Zr) by a Facile Route for Oxidative Desulfurization of Model Diesel Oil. ChemistrySelect 2020, 5, 244–251. [Google Scholar] [CrossRef]
- McNamara, N.D.; Neumann, G.T.; Masko, E.T.; Urban, J.A.; Hicks, J.C. Catalytic performance and stability of (V) MIL-47 and (Ti) MIL-125 in the oxidative desulfurization of heterocyclic aromatic sulfur compounds. J. Catal. 2013, 305, 217–226. [Google Scholar] [CrossRef]
- Wang, D.; Qian, E.W.; Amano, H.; Okata, K.; Ishihara, A.; Kabe, T. Oxidative desulfurization of fuel oil. Appl. Catal. A Gen. 2003, 253, 91–99. [Google Scholar] [CrossRef]
- Otsuki, S.; Nonaka, T.; Takashima, N.; Qian, W.; Ishihara, A.; Imai, T.; Kabe, T. Oxidative Desulfurization of Light Gas Oil and Vacuum Gas Oil by Oxidation and Solvent Extraction. Energy Fuels 2000, 14, 1232–1239. [Google Scholar] [CrossRef]
- Li, X.; Gu, Y.; Chu, H.; Ye, G.; Zhou, W.; Xu, W.; Sun, Y. MFM-300(V) as an active heterogeneous catalyst for deep desulfurization of fuel oil by aerobic oxidation. Appl. Catal. A Gen. 2019, 584, 117152. [Google Scholar] [CrossRef]
- Lu, Z.; Godfrey, H.G.; da Silva, I.; Cheng, Y.; Savage, M.; Tuna, F.; McInnes, E.J.; Teat, S.J.; Gagnon, K.J.; Frogley, M.D.; et al. Modulating supramolecular binding of carbon dioxide in a redox-active porous metal-organic framework. Nat. Commun. 2017, 8, 14212. [Google Scholar] [CrossRef] [Green Version]
- Masoomi, M.Y.; Bagheri, M.; Morsali, A. Application of Two Cobalt-Based Metal-Organic Frameworks as Oxidative Desulfurization Catalysts. Inorg. Chem. 2015, 54, 11269–11275. [Google Scholar] [CrossRef] [PubMed]
- Abazari, R.; Sanati, S.; Morsali, A.; Slawin, A.; Carpenter-Warren, C.L. Dual-Purpose 3D Pillared Metal-Organic Framework with Excellent Properties for Catalysis of Oxidative Desulfurization and Energy Storage in Asymmetric Supercapacitor. ACS Appl. Mater. Interfaces 2019, 11, 14759–14773. [Google Scholar] [CrossRef]
- Gómez-Paricio, A.; Santiago-Portillo, A.; Navalón, S.; Concepción, P.; Alvaro, M.; Garcia, H. MIL-101 promotes the efficient aerobic oxidative desulfurization of dibenzothiophenes. Green Chem. 2016, 18, 508–515. [Google Scholar] [CrossRef]
- Vallés-García, C.; Santiago-Portillo, A.; Álvaro, M.; Navalón, S.; García, H. MIL-101(Cr)-NO2 as efficient catalyst for the aerobic oxidation of thiophenols and the oxidative desulfurization of dibenzothiophenes. Appl. Catal. A Gen. 2020, 590, 117340. [Google Scholar] [CrossRef]
- Zhou, Y.; Guo, Z.; Hou, W.; Wang, Q.; Wang, J. Polyoxometalate-based phase transfer catalysis for liquid–solid organic reactions: A review. Catal. Sci. Technol. 2015, 5, 4324–4335. [Google Scholar] [CrossRef]
- Ahmadian, M.; Anbia, M. Oxidative Desulfurization of Liquid Fuels Using Polyoxometalate-Based Catalysts: A Review. Energy Fuels 2021, 35, 10347–10373. [Google Scholar] [CrossRef]
- Hu, X.; Lu, Y.; Dai, F.; Liu, C.; Liu, Y. Host–guest synthesis and encapsulation of phosphotungstic acid in MIL-101 via “bottle around ship”: An effective catalyst for oxidative desulfurization. Microporous Mesoporous Mater. 2013, 170, 36–44. [Google Scholar] [CrossRef]
- Ribeiro, S.; Barbosa, A.D.S.; Gomes, A.C.; Pillinger, M.; Gonçalves, I.S.; Cunha-Silva, L.; Balula, S.S. Catalytic oxidative desulfurization systems based on Keggin phosphotungstate and metal-organic framework MIL-101. Fuel Process. Technol. 2013, 116, 350–357. [Google Scholar] [CrossRef]
- Ribeiro, S.; Granadeiro, C.M.; Silva, P.; Almeida Paz, F.A.; de Biani, F.F.; Cunha-Silva, L.; Balula, S.S. An efficient oxidative desulfurization process using terbium-polyoxometalate@MIL-101(Cr). Catal. Sci. Technol. 2013, 3, 2404–2414. [Google Scholar] [CrossRef]
- Granadeiro, C.M.; Barbosa, A.D.S.; Ribeiro, S.; Santos, I.C.M.S.; de Castro, B.; Cunha-Silva, L.; Balula, S.S. Oxidative catalytic versatility of a trivacant polyoxotungstate incorporated into MIL-101(Cr). Catal. Sci. Technol. 2014, 4, 1416–1425. [Google Scholar] [CrossRef]
- Julião, D.; Gomes, A.C.; Pillinger, M.; Cunha-Silva, L.; de Castro, B.; Gonçalves, I.S.; Balula, S.S. Desulfurization of model diesel by extraction/oxidation using a zinc-substituted polyoxometalate as catalyst under homogeneous and heterogeneous (MIL-101(Cr) encapsulated) conditions. Fuel Process. Technol. 2015, 131, 78–86. [Google Scholar] [CrossRef]
- Samaniyan, M.; Mirzaei, M.; Khajavian, R.; Eshtiagh-Hosseini, H.; Streb, C. Heterogeneous Catalysis by Polyoxometalates in Metal–Organic Frameworks. ACS Catal. 2019, 9, 10174–10191. [Google Scholar] [CrossRef]
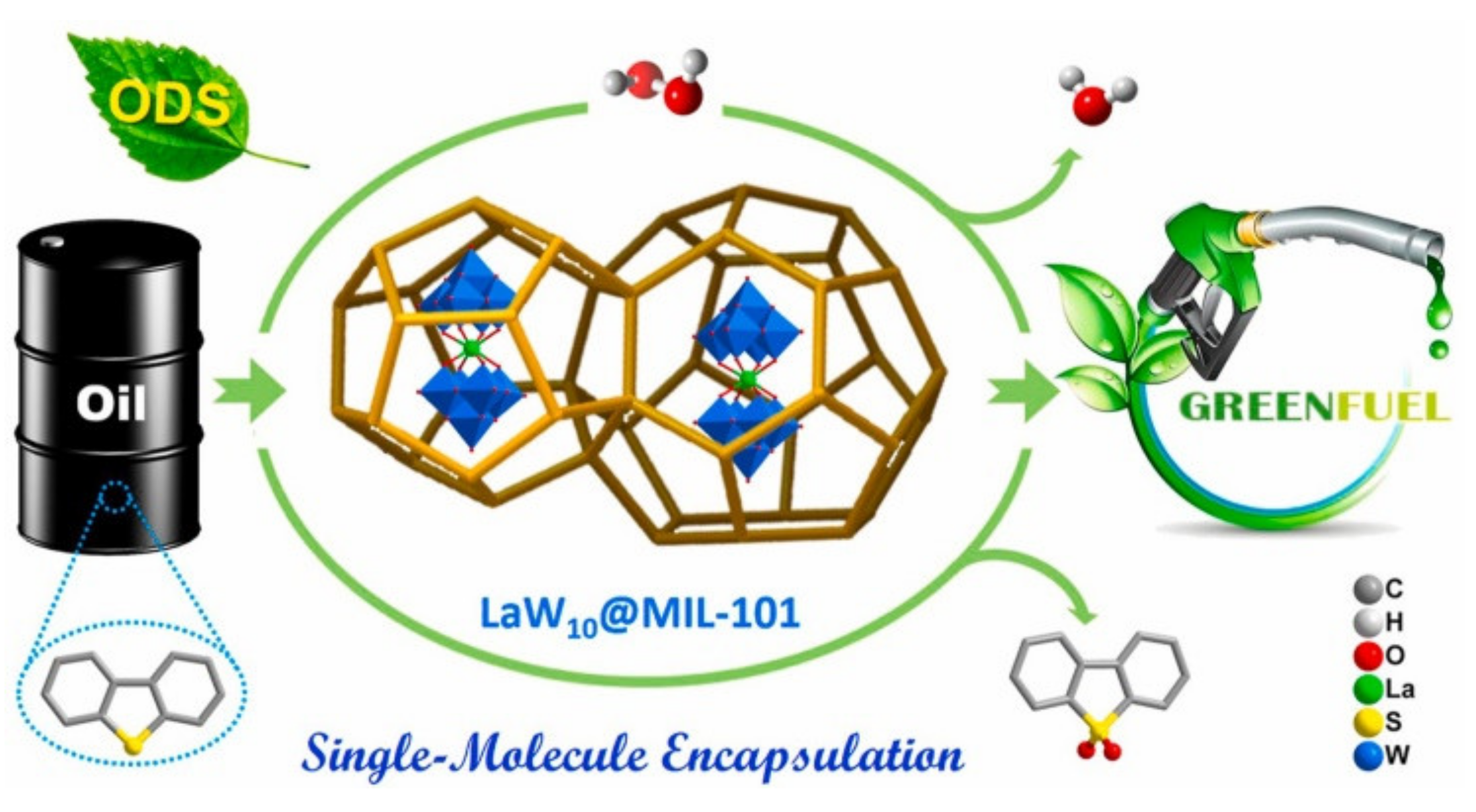
- Lu, Y.; Yue, C.; Liu, B.; Zhang, M.; Li, Y.; Yang, W.; Lin, Y.; Pan, Y.; Sun, D.; Liu, Y. The encapsulation of POM clusters into MIL-101(Cr) at molecular level: LaW10O36@MIL-101(Cr), an efficient catalyst for oxidative desulfurization. Microporous Mesoporous Mater. 2021, 311, 110694. [Google Scholar] [CrossRef]
- Wang, X.S.; Huang, Y.B.; Lin, Z.J.; Cao, R. Phosphotungstic acid encapsulated in the mesocages of amine-functionalized metal-organic frameworks for catalytic oxidative desulfurization. Dalton Trans. 2014, 43, 11950–11958. [Google Scholar] [CrossRef]
- Zhang, L.; Wu, S.; Liu, Y.; Wang, F.; Han, X.; Shang, H. Immobilization of phosphotungstic acid in an amino-containing metal-organic framework for oxidative desulfurization. Appl. Organomet. Chem. 2016, 30, 684–690. [Google Scholar] [CrossRef]
- Granadeiro, C.M.; Nogueira, L.S.; Julião, D.; Mirante, F.; Ananias, D.; Balula, S.S.; Cunha-Silva, L. Influence of a porous MOF support on the catalytic performance of Eu-polyoxometalate based materials: Desulfurization of a model diesel. Catal. Sci. Technol. 2016, 6, 1515–1522. [Google Scholar] [CrossRef]
- Hupp, J.T.; Poeppelmeier, K.R. Chemistry. Better living through nanopore chemistry. Science 2005, 309, 2008–2009. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Zou, X.; Gao, X.; Fan, S.; Sun, F.; Ren, H.; Zhu, G. Hydrogen Selective NH2-MIL-53(Al) MOF Membranes with High Permeability. Adv. Funct. Mater. 2012, 22, 3583–3590. [Google Scholar] [CrossRef]
- Nogueira, L.S.; Ribeiro, S.; Granadeiro, C.M.; Pereira, E.; Feio, G.; Cunha-Silva, L.; Balula, S.S. Novel polyoxometalate silica nano-sized spheres: Efficient catalysts for olefin oxidation and the deep desulfurization process. Dalton Trans. 2014, 43, 9518–9528. [Google Scholar] [CrossRef]
- Granadeiro, C.; Ferreira, P.; Julião, D.; Ribeiro, L.; Valença, R.; Ribeiro, J.; Gonçalves, I.; de Castro, B.; Pillinger, M.; Cunha-Silva, L.; et al. Efficient Oxidative Desulfurization Processes Using Polyoxomolybdate Based Catalysts. Energies 2018, 11, 1696. [Google Scholar] [CrossRef] [Green Version]
- Sun, M.; Chen, W.-C.; Zhao, L.; Wang, X.-L.; Su, Z.-M. A PTA@MIL-101(Cr)-diatomite composite as catalyst for efficient oxidative desulfurization. Inorg. Chem. Commun. 2018, 87, 30–35. [Google Scholar] [CrossRef]
- Afzalinia, A.; Mirzaie, A.; Nikseresht, A.; Musabeygi, T. Ultrasound-assisted oxidative desulfurization process of liquid fuel by phosphotungstic acid encapsulated in a interpenetrating amine-functionalized Zn(II)-based MOF as catalyst. Ultrason. Sonochem 2017, 34, 713–720. [Google Scholar] [CrossRef]
- Chui, S.S.; Lo, S.M.; Charmant, J.P.; Orpen, A.G.; Williams, I.D. A chemically functionalizable nanoporous material. Science 1999, 283, 1148–1150. [Google Scholar] [CrossRef]
- Song, J.; Luo, Z.; Britt, D.K.; Furukawa, H.; Yaghi, O.M.; Hardcastle, K.I.; Hill, C.L. A multiunit catalyst with synergistic stability and reactivity: A polyoxometalate-metal organic framework for aerobic decontamination. J. Am. Chem. Soc. 2011, 133, 16839–16846. [Google Scholar] [CrossRef]
- Rafiee, E.; Nobakht, N. Keggin type heteropoly acid, encapsulated in metal-organic framework: A heterogeneous and recyclable nanocatalyst for selective oxidation of sulfides and deep desulfurization of model fuels. J. Mol. Catal. A Chem. 2015, 398, 17–25. [Google Scholar] [CrossRef]
- Li, S.-W.; Gao, R.-M.; Zhang, R.-L.; Zhao, J.-s. Template method for a hybrid catalyst material POM@MOF-199 anchored on MCM-41: Highly oxidative desulfurization of DBT under molecular oxygen. Fuel 2016, 184, 18–27. [Google Scholar] [CrossRef]
- Li, S.W.; Li, J.R.; Jin, Q.P.; Yang, Z.; Zhang, R.L.; Gao, R.M.; Zhao, J.S. Preparation of mesoporous Cs-POM@MOF-199@MCM-41 under two different synthetic methods for a highly oxidesulfurization of dibenzothiophene. J. Hazard. Mater. 2017, 337, 208–216. [Google Scholar] [CrossRef]
- Li, S.-W.; Gao, R.-M.; Zhao, J.-s. Deep Oxidative Desulfurization of Fuel Catalyzed by Modified Heteropolyacid: The Comparison Performance of Three Kinds of Ionic Liquids. ACS Sustain. Chem. Eng. 2018, 6, 15858–15866. [Google Scholar] [CrossRef]
- Li, S.-W.; Yang, Z.; Gao, R.-M.; Zhang, G.; Zhao, J.-s. Direct synthesis of mesoporous SRL-POM@MOF-199@MCM-41 and its highly catalytic performance for the oxidesulfurization of DBT. Appl. Catal. B Environ. 2018, 221, 574–583. [Google Scholar] [CrossRef]
- Li, J.; Yang, Z.; Hu, G.; Zhao, J. Heteropolyacid supported MOF fibers for oxidative desulfurization of fuel. Chem. Eng. J. 2020, 388, 124325. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, W.; Zhang, J.; Dong, Z.; Zhang, X.; Ding, S. One-pot synthesis of cup-like ZSM-5 zeolite and its excellent oxidative desulfurization performance. RSC Adv. 2018, 8, 31979–31983. [Google Scholar] [CrossRef] [Green Version]
- Li, S.-W.; Gao, R.-M.; Zhang, W.; Zhang, Y.; Zhao, J.-S. Heteropolyacids supported on macroporous materials POM@MOF-199@LZSM-5: Highly catalytic performance in oxidative desulfurization of fuel oil with oxygen. Fuel 2018, 221, 1–11. [Google Scholar] [CrossRef]
- Gao, Y.; Lv, Z.; Gao, R.; Zhang, G.; Zheng, Y.; Zhao, J. Oxidative desulfurization process of model fuel under molecular oxygen by polyoxometalate loaded in hybrid material CNTs@MOF-199 as catalyst. J. Hazard. Mater. 2018, 359, 258–265. [Google Scholar] [CrossRef]
- Gao, Y.; Liu, Z.; Hu, G.; Gao, R.; Zhao, J. Design and synthesis heteropolyacid modified mesoporous hybrid material CNTs@MOF-199 catalyst by different methods for extraction-oxidation desulfurization of model diesel. Microporous Mesoporous Mater. 2020, 291, 109702. [Google Scholar] [CrossRef]
- Wang, X.-S.; Li, L.; Liang, J.; Huang, Y.-B.; Cao, R. Boosting Oxidative Desulfurization of Model and Real Gasoline over Phosphotungstic Acid Encapsulated in Metal-Organic Frameworks: The Window Size Matters. ChemCatChem 2017, 9, 971–979. [Google Scholar] [CrossRef]
- Zhang, X.-m.; Zhang, Z.; Zhang, B.; Yang, X.; Chang, X.; Zhou, Z.; Wang, D.-H.; Zhang, M.-H.; Bu, X.-H. Synergistic effect of Zr-MOF on phosphomolybdic acid promotes efficient oxidative desulfurization. Appl. Catal. B Environ. 2019, 256, 117804. [Google Scholar] [CrossRef]
- Chang, X.; Yang, X.F.; Qiao, Y.; Wang, S.; Zhang, M.H.; Xu, J.; Wang, D.H.; Bu, X.H. Confined Heteropoly Blues in Defected Zr-MOF (Bottle Around Ship) for High-Efficiency Oxidative Desulfurization. Small 2020, 16, e1906432. [Google Scholar] [CrossRef] [PubMed]
- Ding, Z.; Zhang, X.M.; Chang, X.; Wang, S.; Wang, D.H.; Zhang, M.H.; Zhang, T.H. Synergistic Effect between Zr-MOF and Phosphomolybdic Acid with the Promotion of TiF4 Template. Molecules 2020, 25, 4673. [Google Scholar] [CrossRef]
- Ye, G.; Hu, L.; Gu, Y.; Lancelot, C.; Rives, A.; Lamonier, C.; Nuns, N.; Marinova, M.; Xu, W.; Sun, Y. Synthesis of polyoxometalate encapsulated in UiO-66(Zr) with hierarchical porosity and double active sites for oxidation desulfurization of fuel oil at room temperature. J. Mater. Chem. A 2020, 8, 19396–19404. [Google Scholar] [CrossRef]
- Qi, Z.; Huang, Z.; Wang, H.; Li, L.; Ye, C.; Qiu, T. In situ bridging encapsulation of a carboxyl-functionalized phosphotungstic acid ionic liquid in UiO-66: A remarkable catalyst for oxidative desulfurization. Chem. Eng. Sci. 2020, 225, 115818. [Google Scholar] [CrossRef]
- Peng, Y.-L.; Liu, J.; Zhang, H.-F.; Luo, D.; Li, D. A size-matched POM@MOF composite catalyst for highly efficient and recyclable ultra-deep oxidative fuel desulfurization. Inorg. Chem. Front. 2018, 5, 1563–1569. [Google Scholar] [CrossRef]
- Lin, Z.J.; Zheng, H.Q.; Chen, J.; Zhuang, W.E.; Lin, Y.X.; Su, J.W.; Huang, Y.B.; Cao, R. Encapsulation of Phosphotungstic Acid into Metal-Organic Frameworks with Tunable Window Sizes: Screening of PTA@MOF Catalysts for Efficient Oxidative Desulfurization. Inorg. Chem. 2018, 57, 13009–13019. [Google Scholar] [CrossRef]
- Bagheri, M.; Masoomi, M.Y.; Morsali, A. A MoO3–Metal–Organic Framework Composite as a Simultaneous Photocatalyst and Catalyst in the PODS Process of Light Oil. ACS Catal. 2017, 7, 6949–6956. [Google Scholar] [CrossRef]
- Bagheri, M.; Masoomi, M.Y.; Morsali, A.; Schoedel, A. Two Dimensional Host-Guest Metal-Organic Framework Sensor with High Selectivity and Sensitivity to Picric Acid. ACS Appl. Mater. Interfaces 2016, 8, 21472–21479. [Google Scholar] [CrossRef]
- Li, S.-W.; Wang, W.; Zhao, J.-S. Catalytic oxidation of DBT for ultra-deep desulfurization under MoO3 modified magnetic catalyst: The comparison influence on various morphologies of MoO3. Appl. Catal. A Gen. 2020, 602, 117671. [Google Scholar] [CrossRef]
- Li, S.-W.; Wang, W.; Zhao, J.-S. Highly effective oxidative desulfurization with magnetic MOF supported W-MoO3 catalyst under oxygen as oxidant. Appl. Catal. B Environ. 2020, 277, 119224. [Google Scholar] [CrossRef]
- Subhan, S.; Yaseen, M.; Ahmad, B.; Tong, Z. Fabrication of MnO2 NPs incorporated UiO-66 for the green and efficient oxidative desulfurization and denitrogenation of fuel oils. J. Environ. Chem. Eng. 2021, 9, 105179. [Google Scholar] [CrossRef]
- Afzali, N.; Kardanpour, R.; Zadehahmadi, F.; Tangestaninejad, S.; Moghadam, M.; Mirkhani, V.; Mechler, A.; Mohammadpoor-Baltork, I.; Bahadori, M. Molybdenum (VI)-functionalized UiO-66 provides an efficient heterogeneous nanocatalyst in oxidation reactions. Appl. Organomet. Chem. 2019, 33, e5225. [Google Scholar] [CrossRef]
- Ye, G.; Wang, H.; Chen, W.; Chu, H.; Wei, J.; Wang, D.; Wang, J.; Li, Y. In Situ Implanting of Single Tungsten Sites into Defective UiO-66(Zr) by Solvent-Free Route for Efficient Oxidative Desulfurization at Room Temperature. Angew. Chem. Int. Ed. Engl. 2021, 60, 20318–20324. [Google Scholar] [CrossRef]
- Wu, J.; Gao, Y.; Zhang, W.; Tan, Y.; Tang, A.; Men, Y.; Tang, B. Deep desulfurization by oxidation using an active ionic liquid-supported Zr metal-organic framework as catalyst. Appl. Organomet. Chem. 2015, 29, 96–100. [Google Scholar] [CrossRef]
- Yang, W.; Guo, G.; Mei, Z.; Yu, Y. Deep oxidative desulfurization of model fuels catalysed by immobilized ionic liquid on MIL-100(Fe). RSC Adv. 2019, 9, 21804–21809. [Google Scholar] [CrossRef] [Green Version]
- Qi, Z.; Qiu, T.; Wang, H.; Ye, C. Synthesis of ionic-liquid-functionalized UiO-66 framework by post-synthetic ligand exchange for the ultra-deep desulfurization. Fuel 2020, 268, 117336. [Google Scholar] [CrossRef]
- Jamali, M.A.; Arvani, A.; Amini, M.M. Vanadium Containing Metal-organic Frameworks as Highly Efficient Catalysts for the Oxidation of Refractory Aromatic Sulfur Compounds. ChemCatChem 2020, 13, 293–303. [Google Scholar] [CrossRef]
- Liu, S.; Li, X.; Zhang, H. Synergistic effects of MOF-76 on layered double hydroxides with superior activity for extractive catalytic oxidative desulfurization. New J. Chem. 2020, 44, 6269–6276. [Google Scholar] [CrossRef]
- Kim, J.; McNamara, N.D.; Her, T.H.; Hicks, J.C. Carbothermal reduction of Ti-modified IRMOF-3: An adaptable synthetic method to support catalytic nanoparticles on carbon. ACS Appl. Mater. Interfaces 2013, 5, 11479–11487. [Google Scholar] [CrossRef] [PubMed]
- McNamara, N.D.; Kim, J.; Hicks, J.C. Controlling the Pyrolysis Conditions of Microporous/Mesoporous MIL-125 To Synthesize Porous, Carbon-Supported Ti Catalysts with Targeted Ti Phases for the Oxidation of Dibenzothiophene. Energy Fuels 2015, 30, 594–602. [Google Scholar] [CrossRef]
- Kim, J.; McNamara, N.D.; Hicks, J.C. Catalytic activity and stability of carbon supported V oxides and carbides synthesized via pyrolysis of MIL-47 (V). Appl. Catal. A Gen. 2016, 517, 141–150. [Google Scholar] [CrossRef] [Green Version]
- Sarker, M.; Bhadra, B.N.; Shin, S.; Jhung, S.H. TiO2-Integrated Carbon Prepared via Pyrolysis of Ti-Loaded Metal–Organic Frameworks for Redox Catalysis. ACS Appl. Nano Mater. 2018, 2, 191–201. [Google Scholar] [CrossRef]
- Li, X.; Zhang, L.; Sun, Y. Titanium-Modified MIL-101(Cr) Derived Titanium-Chromium-Oxide as Highly Efficient Oxidative Desulfurization Catalyst. Catalysts 2020, 10, 1091. [Google Scholar] [CrossRef]
- Bhadra, B.N.; Song, J.Y.; Khan, N.A.; Jhung, S.H. TiO2-Containing Carbon Derived from a Metal-Organic Framework Composite: A Highly Active Catalyst for Oxidative Desulfurization. ACS Appl. Mater. Interfaces 2017, 9, 31192–31202. [Google Scholar] [CrossRef] [PubMed]
- Bhadra, B.N.; Jhung, S.H. Well-dispersed Ni or MnO nanoparticles on mesoporous carbons: Preparation via carbonization of bimetallic MOF-74s for highly reactive redox catalysts. Nanoscale 2018, 10, 15035–15047. [Google Scholar] [CrossRef]
- Bhadra, B.N.; Khan, N.A.; Jhung, S.H. Co supported on N-doped carbon, derived from bimetallic azolate framework-6: A highly effective oxidative desulfurization catalyst. J. Mater. Chem. A 2019, 7, 17823–17833. [Google Scholar] [CrossRef]
- Chen, H.; Shen, K.; Mao, Q.; Chen, J.; Li, Y. Nanoreactor of MOF-Derived Yolk–Shell Co@C–N: Precisely Controllable Structure and Enhanced Catalytic Activity. ACS Catal. 2018, 8, 1417–1426. [Google Scholar] [CrossRef]
- He, L.; Weniger, F.; Neumann, H.; Beller, M. Synthesis, Characterization, and Application of Metal Nanoparticles Supported on Nitrogen-Doped Carbon: Catalysis beyond Electrochemistry. Angew. Chem. Int. Ed. Engl. 2016, 55, 12582–12594. [Google Scholar] [CrossRef] [PubMed]
- Mondol, M.M.H.; Bhadra, B.N.; Jhung, S.H. Molybdenum nitride@porous carbon, derived from phosphomolybdic acid loaded metal-azolate framework-6: A highly effective catalyst for oxidative desulfurization. Appl. Catal. B Environ. 2021, 288, 119988. [Google Scholar] [CrossRef]
- Bhadra, B.N.; Jhung, S.H. Oxidative desulfurization of liquid fuel with tungsten-nitride@porous carbon, derived from MAF-6(Zn) loaded with phosphotungstic acid and melamine. Chem. Eng. J. 2021, 419, 129485. [Google Scholar] [CrossRef]

| Catalyst | Substrate (S Content) | Oxidant | O/S Ratio | Temp. (°C) | Time (min) | Sulfur Removal (%) | Activity (mmol·g−1·h−1) | Ref. |
|---|---|---|---|---|---|---|---|---|
| MIL-125(Ti) | DBT (200 ppm) 4-MDBT (200 ppm) 4,6-DMDBT (200 ppm) | CHP | 15 | 80 | 180 | 36 15 12 | 0.30 0.12 0.10 | [23] |
| meso-MIL-125(Ti) | DBT (500 ppm) | TBHP | 10 | 80 | - | - | (22.9) a | [24] |
| MIL-125(Ti)-L | DBT (240 ppm) | H2O2 TBHP | 8 8 | 60 60 | 30 30 | 95.3 44.9 | 1.0 0.5 | [25] |
| COK-47S(Ti) | DBT (3601 ppm) | TBHP | 2.5 | 60 | 120 | 99 | (41.1) a | [26] |
| Ti-BDC-180 | DBT (1000 ppm) | H2O2 | 6 | 60 | 20 | 99.8 | 18.7 | [27] |
| Ti-BDC-A | DBT (500 ppm) | CHP | 6 | 25 | 10 | 100 | 21.9 | [28] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, H.; Gu, Y.; Liu, D.; Sun, Y. Advances in Oxidative Desulfurization of Fuel Oils over MOFs-Based Heterogeneous Catalysts. Catalysts 2021, 11, 1557. https://doi.org/10.3390/catal11121557
Luo H, Gu Y, Liu D, Sun Y. Advances in Oxidative Desulfurization of Fuel Oils over MOFs-Based Heterogeneous Catalysts. Catalysts. 2021; 11(12):1557. https://doi.org/10.3390/catal11121557
Chicago/Turabian StyleLuo, Hongsi, Yulong Gu, Daqing Liu, and Yinyong Sun. 2021. "Advances in Oxidative Desulfurization of Fuel Oils over MOFs-Based Heterogeneous Catalysts" Catalysts 11, no. 12: 1557. https://doi.org/10.3390/catal11121557
APA StyleLuo, H., Gu, Y., Liu, D., & Sun, Y. (2021). Advances in Oxidative Desulfurization of Fuel Oils over MOFs-Based Heterogeneous Catalysts. Catalysts, 11(12), 1557. https://doi.org/10.3390/catal11121557







