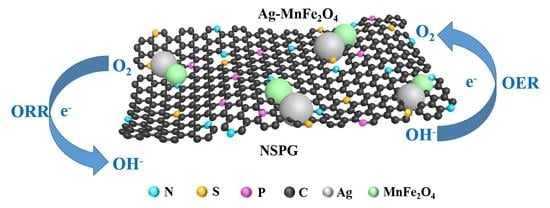
N, S, P-Codoped Graphene-Supported Ag-MnFe2O4 Heterojunction Nanoparticles as Bifunctional Oxygen Electrocatalyst with High Efficiency
Abstract
:1. Introduction
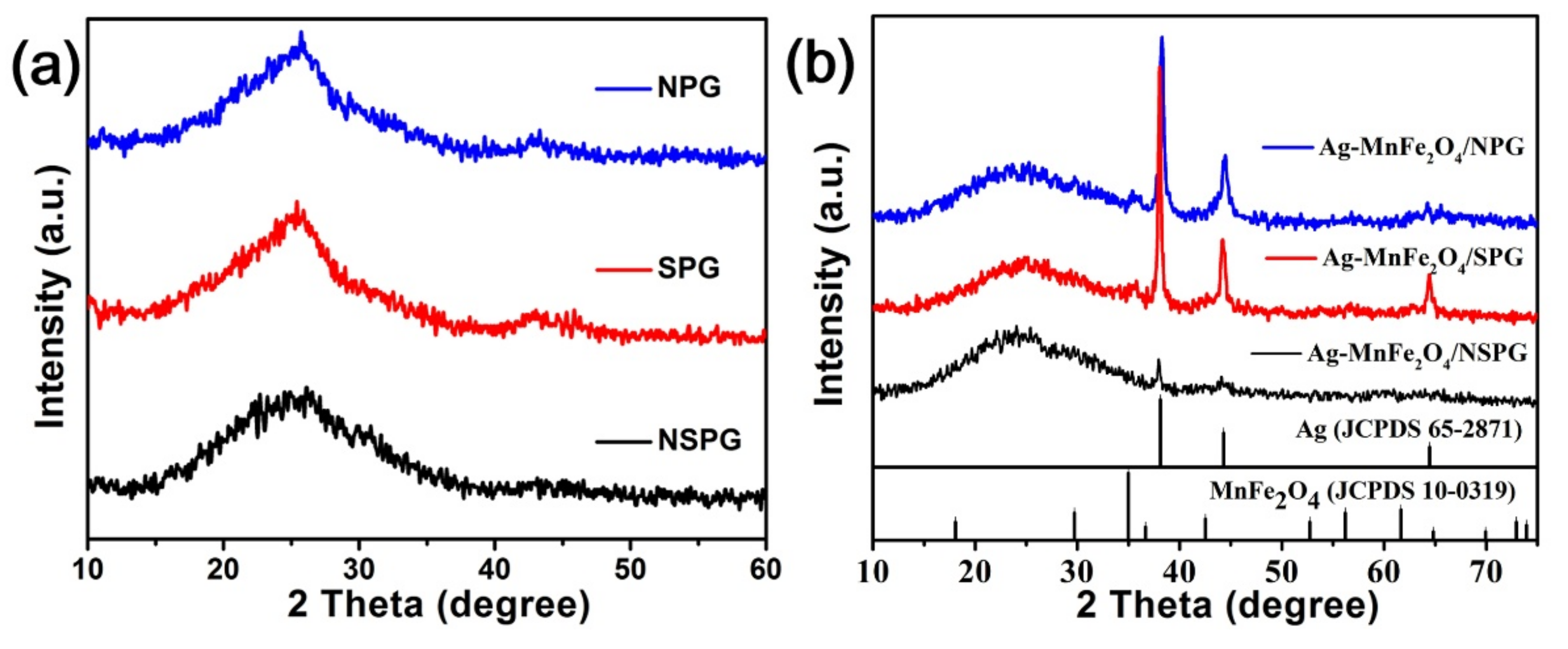
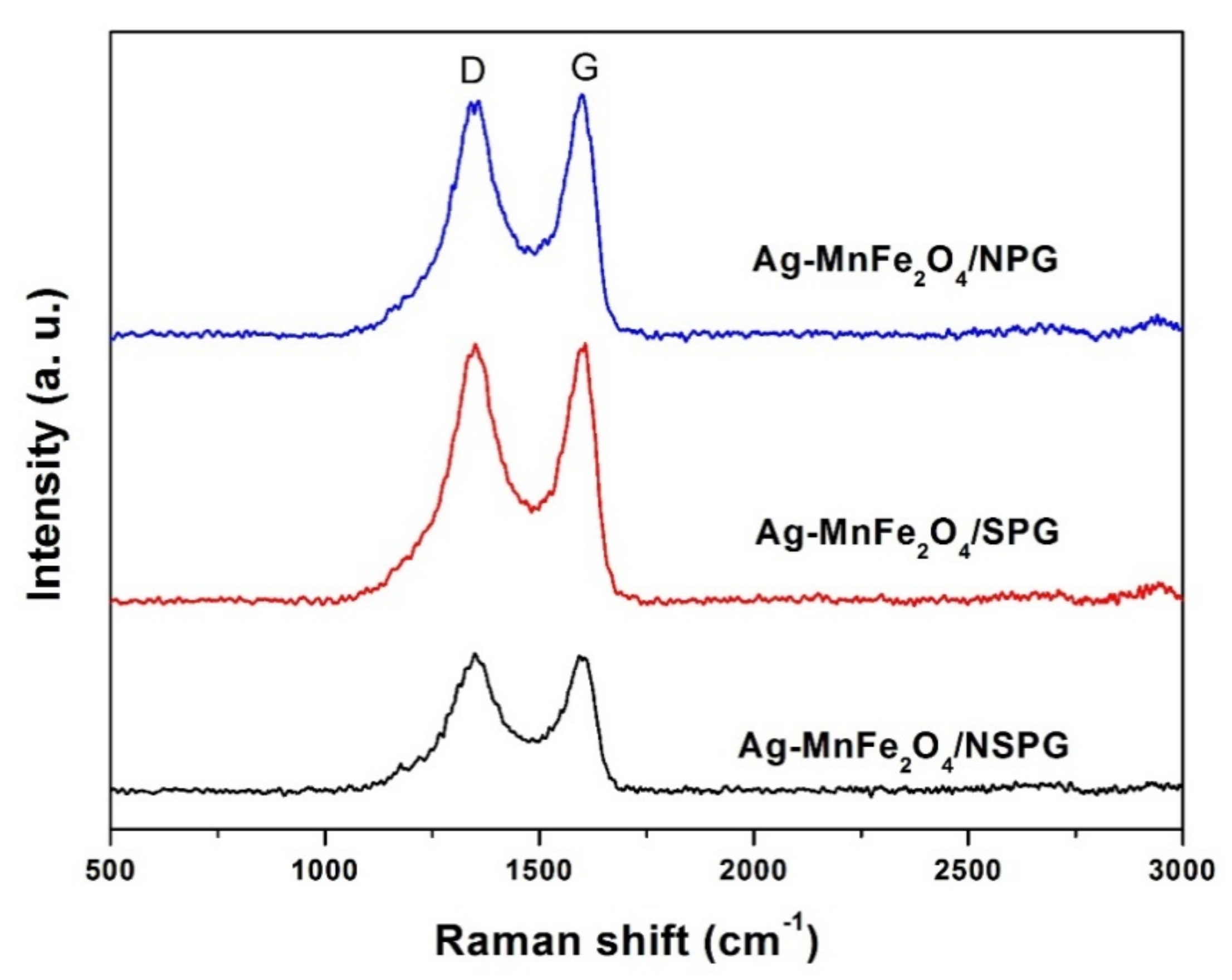
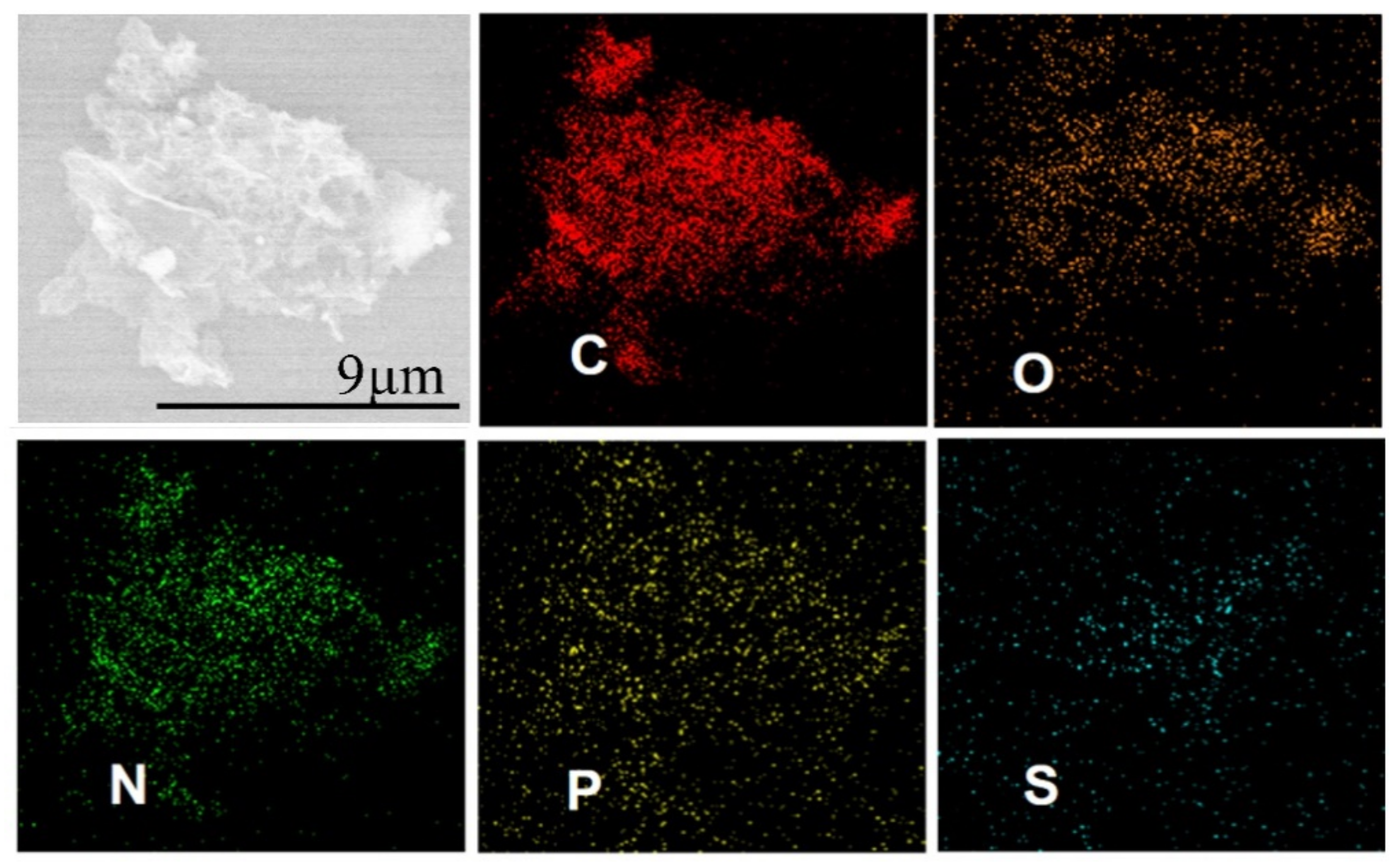
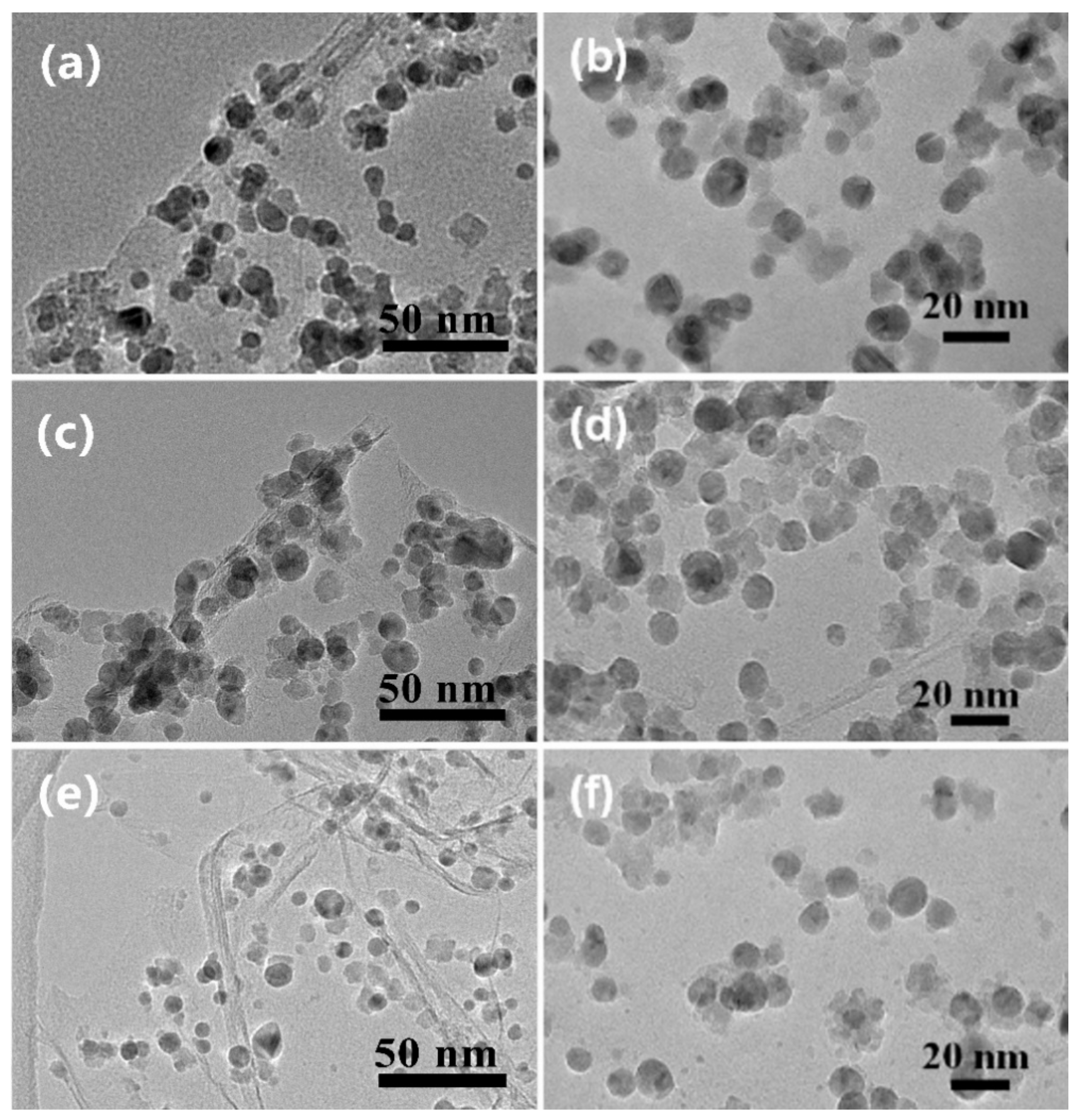
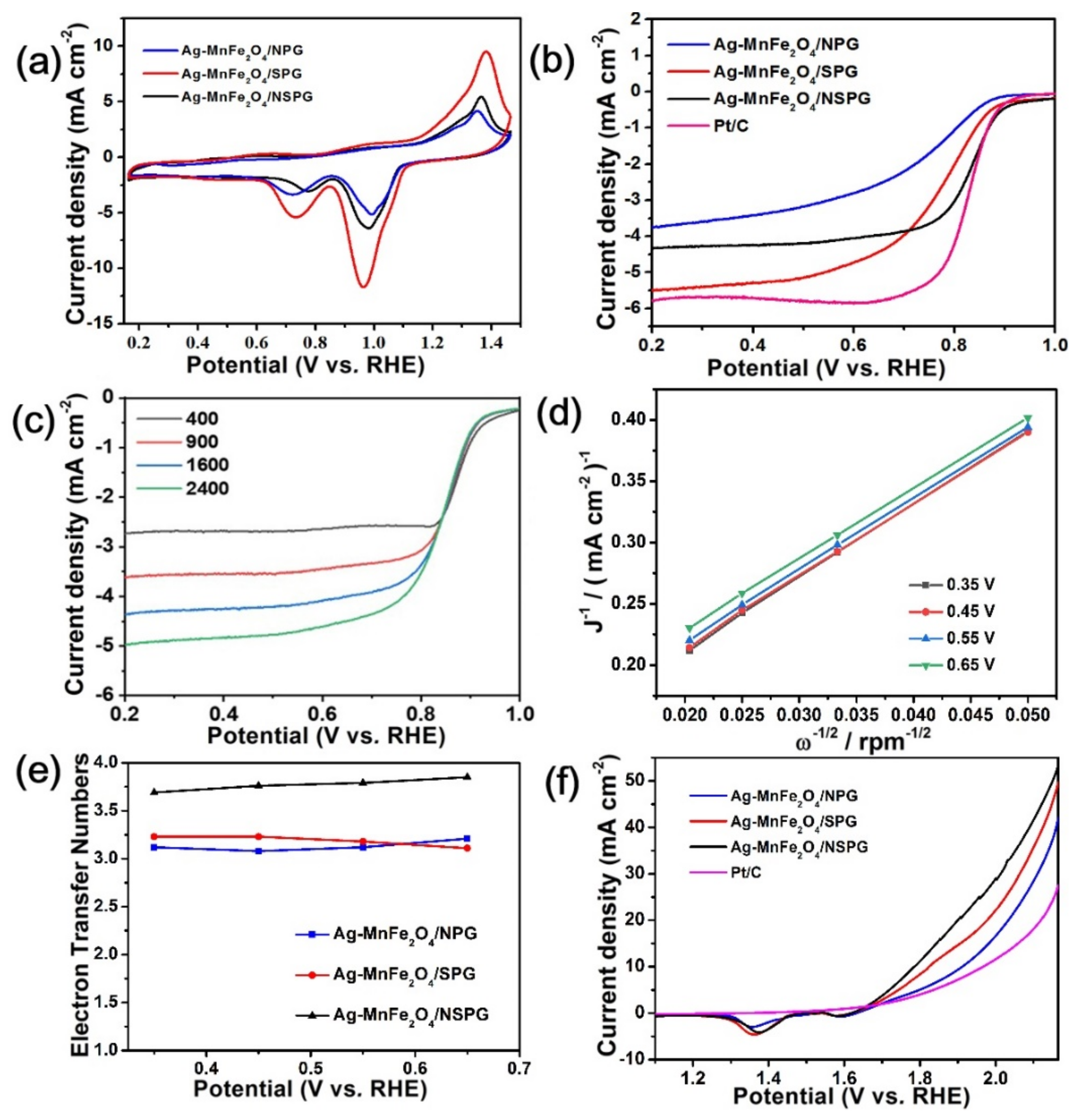
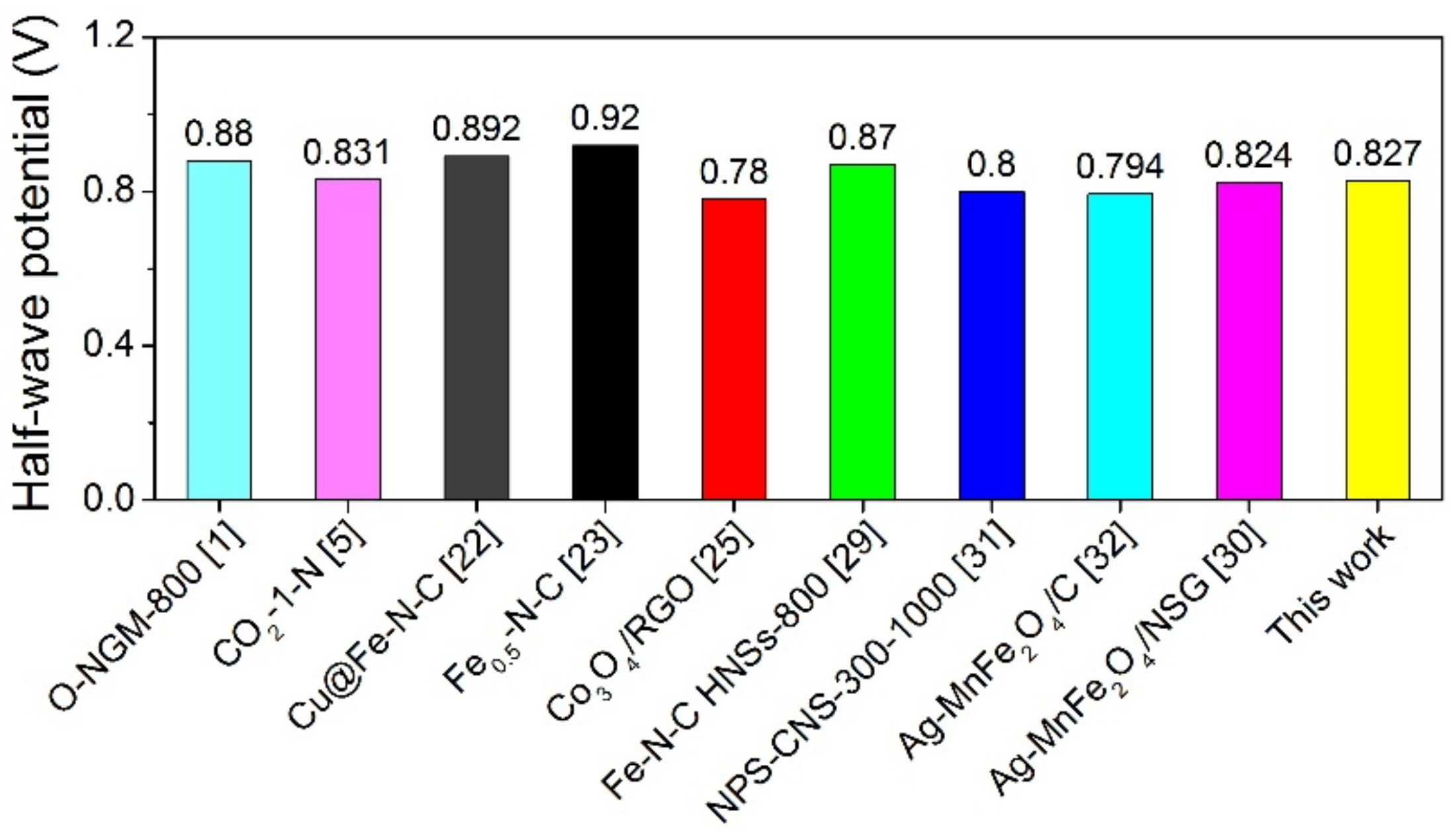
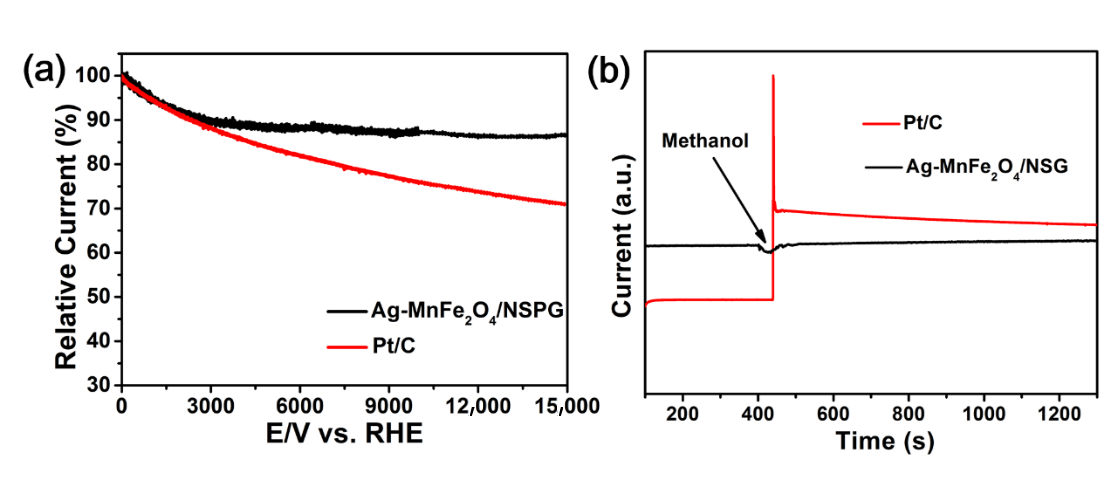
2. Results and Discussion
3. Experimental Section
3.1. Chemical Reagents and Materials
3.2. Material Synthesis
3.3. Material Characterizations
3.4. Electrochemical Measurements
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Wang, M.; Wang, W.; Qian, T.; Liu, S.; Li, Y.; Hou, Z.; Goodenough, J.B.; Ajayan, P.M.; Yan, C. Oxidizing vacancies in nitrogen-doped carbon enhance air-cathode activity. Adv. Mater. 2019, 31, 1803339. [Google Scholar] [CrossRef] [PubMed]
- Shi, F.; He, J.; Zhang, B.; Peng, J.; Ma, Y.; Chen, W.; Li, F.; Qin, Y.; Liu, Y.; Shang, W.; et al. Plasmonic-enhanced oxygen reduction reaction of silver/graphene electrocatalysts. Nano Lett. 2019, 19, 1371–1378. [Google Scholar] [CrossRef] [PubMed]
- Thaheem, I.; Kim, K.J.; Lee, J.J.; Joh, D.W.; Jeong, I.; Lee, K.T. High performance Mn1.3Co1.3Cu0.4O4 spinel based composite cathodes for intermediate temperature solid oxide fuel cells. J. Mater. Chem. A 2019, 7, 19696–19703. [Google Scholar] [CrossRef]
- Santori, P.G.; Speck, F.D.; Cherevko, S.; Firouzjaie, H.A.; Peng, X.; Mustain, W.E.; Jaouen, F. High performance FeNC and Mn-oxide/FeNC layers for AEMFC cathodes. J. Electr. Soc. 2020, 167, 134505. [Google Scholar] [CrossRef]
- Ratso, S.; Walke, P.R.; Mikli, V.; Ločs, J.; Šmits, K.; Vītola, V.; Šutka, A.; Kruusenberg, I. CO2 turned into a nitrogen doped carbon catalyst for fuel cells and metal–air battery applications. Green Chem. 2021, 23, 4435–4445. [Google Scholar] [CrossRef]
- Kaare, K.; Yu, E.; Käämbre, T.; Volperts, A.; Dobele, G.; Zhurinsh, A.; Niaura, G.; Tamasauskaite Tamasiunaite, L.; Norkus, E.; Kruusenberg, I. Biomass-derived Graphene-like Catalyst Material for Oxygen Reduction Reactio. ChemNanoMat 2021, 7, 307–313. [Google Scholar] [CrossRef]
- Ratso, S.; Zitolo, A.; Käärik, M.; Merisalu, M.; Kikas, A.; Kisand, V.; Rähn, M.; Paiste, P.; Leis, J.; Sammelselg, V.; et al. Non-precious metal cathodes for anion exchange membrane fuel cells from ball-milled iron and nitrogen doped carbide-derived carbons. Renew. Energy 2021, 167, 800–810. [Google Scholar] [CrossRef]
- Qu, L.; Liu, Y.; Baek, J.B.; Dai, L. Nitrogen-doped graphene as efficient metal-free electrocatalyst for oxygen reduction in fuel cells. ACS Nano. 2011, 4, 1321–1326. [Google Scholar] [CrossRef]
- Liang, J.; Jiao, Y.; Jaroniec, M.; Qiao, S.Z. Sulfur and nitrogen dual-doped mesoporous graphene electrocatalyst for oxygen reduction with synergistically enhanced performance. Angew. Chem. Int. Edit. 2012, 51, 11496–11500. [Google Scholar] [CrossRef]
- Wang, X.; Wang, J.; Wang, D.; Dou, S.; Ma, Z.; Wu, J.; Tao, L.; Shen, A.; Ouyang, C.; Liu, Q.; et al. One-pot synthesis of nitrogen and sulfur co-doped graphene as efficient metal-free electrocatalysts for the oxygen reduction reaction. Chem. Commun. 2014, 50, 4839–4842. [Google Scholar] [CrossRef]
- Liu, Z.; Nie, H.; Yang, Z.; Zhang, J.; Jin, Z.; Lu, Y.; Xiao, Z.; Huang, S. Sulfur-nitrogen co-doped three-dimensional carbon foams with hierarchical pore structures as efficient metal-free electrocatalysts for oxygen reduction reactions. Nanoscale 2013, 5, 3283–3288. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, L.; Xia, Z.; Roy, A.; Chang, D.W.; Baek, J.B.; Dai, L. BCN graphene as efficient metal-free electrocatalyst for the oxygen reduction reaction. Angew. Chem. Int. Edit. 2012, 51, 4209–4212. [Google Scholar] [CrossRef]
- Kumatani, A.; Miura, C.; Kuramochi, H.; Ohto, T.; Wakisaka, M.; Nagata, Y.; Ida, H.; Takahashi, Y.; Hu, K.; Jeong, S.; et al. Chemical dopants on edge of holey graphene accelerate electrochemical hydrogen evolution reaction. Adv. Sci. 2019, 6, 1900119. [Google Scholar] [CrossRef] [Green Version]
- Zhang, N.; Liu, F.; Xu, S.; Wang, F.; Yu, Q.; Liu, L. Nitrogen-phosphorus co-doped hollow carbon microspheres with hierarchical micro-meso-macroporous shells as efficient electrodes for supercapacitors. J. Mater. Chem. A 2017, 5, 22631–22640. [Google Scholar] [CrossRef]
- Yang, S.; Peng, L.; Huang, P.; Wang, X.; Sun, Y.; Cao, C.; Song, W. Nitrogen, phosphorus, and sulfur co-doped hollow carbon shell as superior metal-free catalyst for selective oxidation of aromatic alkanes. Angew. Chem. Int. Edit. 2016, 55, 4016–4020. [Google Scholar] [CrossRef]
- Zhang, X.; Lu, Z.; Xu, G.; Wang, T.; Ma, D.; Yang, Z.; Yang, L. Single Pt atom stabilized on nitrogen doped graphene: Co oxidation readily occurs via the tri-molecular Eley-Rideal mechanism. Chem. Phys. 2015, 17, 20006–20013. [Google Scholar] [CrossRef]
- Zhang, Y.; Fugane, K.; Mori, T.; Niu, L.; Ye, J. Wet chemical synthesis of nitrogen-doped graphene towards oxygen reduction electrocatalysts without high-temperature pyrolysis. J. Mater. Chem. 2012, 22, 6575. [Google Scholar] [CrossRef]
- Zhang, X.; Lu, Z.; Fu, Z.; Tang, Y.; Ma, D.; Yang, Z. The mechanisms of oxygen reduction reaction on phosphorus doped grapheme. J. Power Sour. 2015, 276, 222–229. [Google Scholar] [CrossRef]
- Razmjooei, F.; Singh, K.P.; Song, M.; Yu, J. Enhanced Electrocatalytic activity due to additional phosphorous doping in nitrogen and sulfur-doped graphene: A comprehensive study. Carbon 2014, 78, 257–267. [Google Scholar] [CrossRef]
- Riel, A.M.S.; Jessop, M.J.; Decato, D.A.; Massena, C.J.; Nascimento, V.R.; Berryman, O.B. Experimental investigation of halogen-bond hard-soft acid-base complementarity. Acta Crystallogr. 2017, 73, 203–209. [Google Scholar] [CrossRef]
- Talluri, B.; Thomas, T. Indications of hard-soft-acid-base interactions governing formation of ultra-small (r < 3 nm) digestively ripened copper oxide quantum-dots. Chem. Phys. Lett. 2017, 685, 84–88. [Google Scholar] [CrossRef]
- Wang, Z.; Jin, H.; Meng, T.; Liao, K.; Meng, W.; Yang, J.; He, D.; Xiong, Y.; Mu, S. Fe, Cu-coordinated ZIF-derived carbon framework for efficient oxygen reduction reaction and zinc-air batteries. Adv. Funct. Mater. 2018, 28, 1802596. [Google Scholar] [CrossRef]
- Lee, S.H.; Kim, J.; Chung, D.Y.; Yoo, J.M.; Lee, H.S.; Kim, M.J.; Mun, B.S.; Kwon, S.G.; Sung, Y.E.; Hyeon, T. Design principle of Fe-N-C electrocatalysts: How to optimize multimodal porous structures. J. Am. Chem. Soc. 2019, 141, 2035–2045. [Google Scholar] [CrossRef]
- Geng, H.; Guo, Y.; Ding, X.; Wang, H.; Zhang, Y.; Wu, X.; Jiang, J.; Zheng, J.; Yang, Y.; Gu, H. Porous cubes constructed by cobalt oxide nanocrystals with graphene sheet coatings for enhanced lithium storage properties. Nanoscale 2016, 8, 7688–7694. [Google Scholar] [CrossRef]
- Kumar, K.; Canaff, C.; Rousseau, J.; Clacens, S.A.; Kokoh, B. Effect of the oxide-carbon heterointerface on the activity of Co3O4/NRGO nanocomposites toward ORR and OER. J. Phys. Chem. 2016, 120, 7949–7958. [Google Scholar] [CrossRef]
- Qiu, B.; Deng, Y.; Du, M.; Xing, Y.; Zhang, J. Ultradispersed cobalt ferrite nanoparticles assembled in graphene aerogel for continuous photo-Fenton reaction and enhanced lithium storage performance. Sci. Rep. 2016, 6, 29099. [Google Scholar] [CrossRef] [Green Version]
- Zou, J.; Kim, F. Diffusion driven layer-by-layer assembly of graphene oxide nanosheets into porous three-dimensional macrostructures. Nat. Commun. 2014, 5, 5254. [Google Scholar] [CrossRef] [Green Version]
- Yu, S.; Webster, R.D.; Zhou, Y.; Yan, X. Novel carboxylated graphene oxide-CuS-Ag nanocomposite glass coating for organic degradation under solar light. J. Chem. Technol. Biotechnol. 2017, 92, 2626–2634. [Google Scholar] [CrossRef]
- Chen, Y.; Li, Z.; Zhu, Y.; Sun, D.; Liu, X.; Xu, L.; Tang, Y. Atomic Fe dispersed on N-doped carbon hollow nanospheres for high-efficiency electrocatalytic oxygen reduction. Adv. Mater. 2019, 31, 1806312. [Google Scholar] [CrossRef]
- Chen, Y.; Shi, Z.; Li, S.; Feng, J.; Pang, B.; Yu, L.; Zhang, W.; Dong, L. N, S-codoped graphene supports for Ag-MnFe2O4 nanoparticles with improved performance for oxygen reduction and oxygen evolution reactions. J. Electroanal. Chem. 2020, 860, 113930. [Google Scholar] [CrossRef]
- Zhu, Y.; Cao, C.; Jiang, W.; Yang, S.; Hu, J.; Song, W.; Wan, L. Nitrogen, phosphorus and sulfur co-doped ultrathin carbon nanosheets as a metal-free catalyst for selective oxidation of aromatic alkanes and the oxygen reduction reaction. J. Mater. Chem. 2016, 4, 18470–18477. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, S.; Shi, Z.; Yu, J.; Dong, H.; Qin, Y.; Yu, L.; Dong, L. Effect of electronic coupling on the electrocatalytic performance of Ag-MFe2O4 (M = Co, Mn) nanocomposites. J. Electrochem. Soc. 2017, 164, F1483–F1488. [Google Scholar] [CrossRef]
- Hummers, W.S., Jr.; Offeman, R.E. Preparation of graphitic oxide. J. Am. Chem. Soc. 1958, 80, 1339. [Google Scholar] [CrossRef]

| Samples | Content (at. %) | ||||||
|---|---|---|---|---|---|---|---|
| N 1s | S 2p | P 2p | C 1s | Ag 3d | Mn 2p | Fe 2p | |
| Ag-MnFe2O4/NPG | 2.65 | / | 0.89 | 91.38 | 1.59 | 1.25 | 2.24 |
| Ag-MnFe2O4/SPG | / | 2.55 | 0.86 | 91.85 | 1.53 | 1.28 | 1.93 |
| Ag-MnFe2O4/NSPG | 1.65 | 1.72 | 0.93 | 90.92 | 1.48 | 1.22 | 2.08 |
| Elemental Concentration (ppm) | |||
|---|---|---|---|
| Ag | Mn | Fe | |
| Ag-MnFe2O4/NPG | 183.5 | 94.3 | 173.6 |
| Ag-MnFe2O4/SPG | 177.9 | 100.4 | 164.8 |
| Ag-MnFe2O4/NSPG | 182.8 | 101.6 | 169.4 |
| Peak Potentials(V) in CV | Onset Potential(V) in LSV | Half-Wave Potential(V) in LSV | |
|---|---|---|---|
| Ag-MnFe2O4/NPG | 0.723 | 0.869 | 0.741 |
| Ag-MnFe2O4/SPG | 0.733 | 0.871 | 0.769 |
| Ag-MnFe2O4/NSPG | 0.775 | 0.887 | 0.831 |
| Pt/C | / | 0.879 | 0.827 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dong, H.; Chen, Y.; Gong, C.; Sui, L.; Sun, Q.; Lv, K.; Dong, L. N, S, P-Codoped Graphene-Supported Ag-MnFe2O4 Heterojunction Nanoparticles as Bifunctional Oxygen Electrocatalyst with High Efficiency. Catalysts 2021, 11, 1550. https://doi.org/10.3390/catal11121550
Dong H, Chen Y, Gong C, Sui L, Sun Q, Lv K, Dong L. N, S, P-Codoped Graphene-Supported Ag-MnFe2O4 Heterojunction Nanoparticles as Bifunctional Oxygen Electrocatalyst with High Efficiency. Catalysts. 2021; 11(12):1550. https://doi.org/10.3390/catal11121550
Chicago/Turabian StyleDong, Hongzhou, Yingjie Chen, Chong Gong, Lina Sui, Qiong Sun, Kangle Lv, and Lifeng Dong. 2021. "N, S, P-Codoped Graphene-Supported Ag-MnFe2O4 Heterojunction Nanoparticles as Bifunctional Oxygen Electrocatalyst with High Efficiency" Catalysts 11, no. 12: 1550. https://doi.org/10.3390/catal11121550
APA StyleDong, H., Chen, Y., Gong, C., Sui, L., Sun, Q., Lv, K., & Dong, L. (2021). N, S, P-Codoped Graphene-Supported Ag-MnFe2O4 Heterojunction Nanoparticles as Bifunctional Oxygen Electrocatalyst with High Efficiency. Catalysts, 11(12), 1550. https://doi.org/10.3390/catal11121550