Phytochemical-Assisted Green Synthesis of Nickel Oxide Nanoparticles for Application as Electrocatalysts in Oxygen Evolution Reaction
Abstract
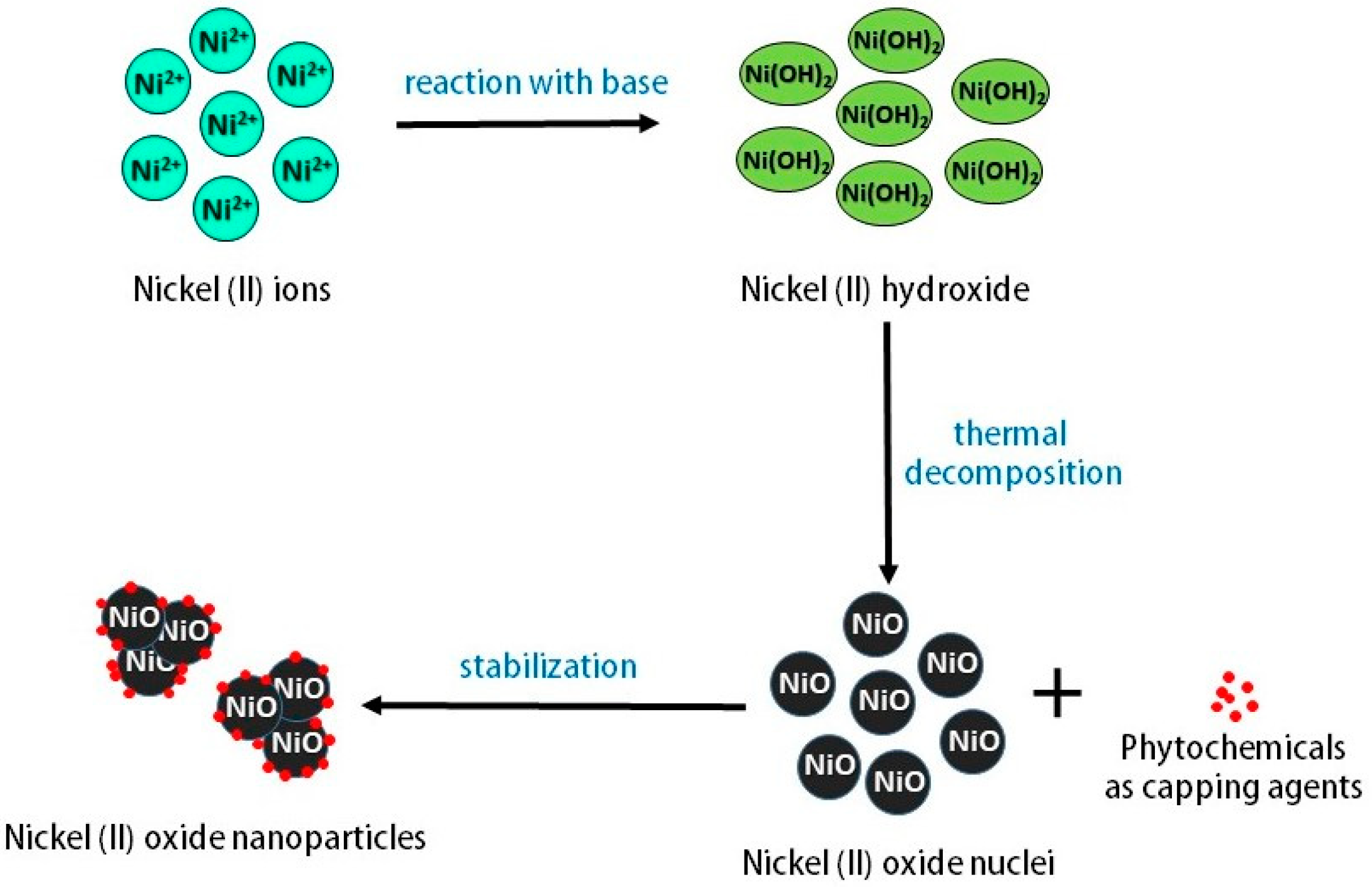
:1. Introduction
2. Results and Discussion
2.1. Structural Properties
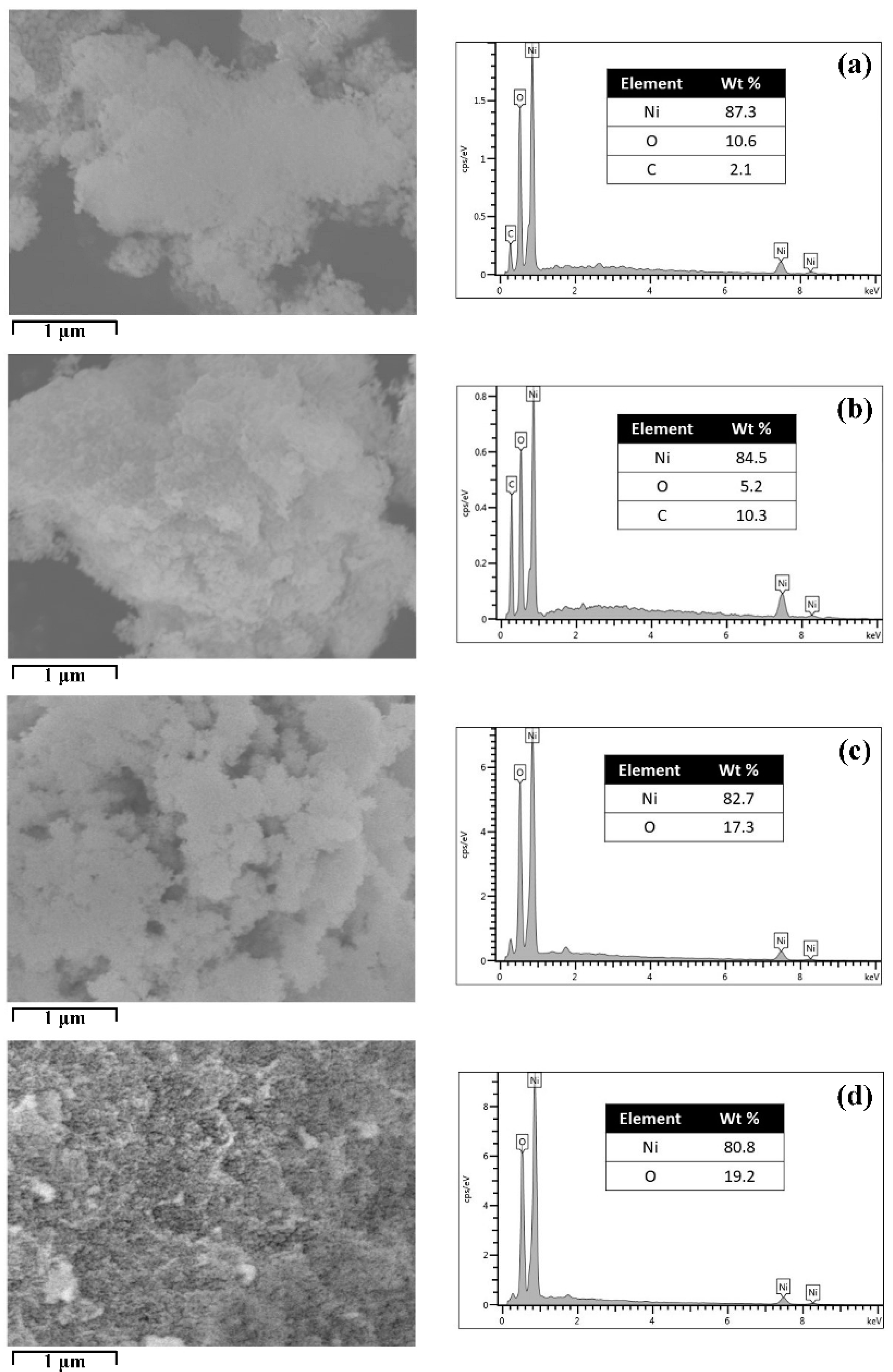
2.2. Morphological Properties
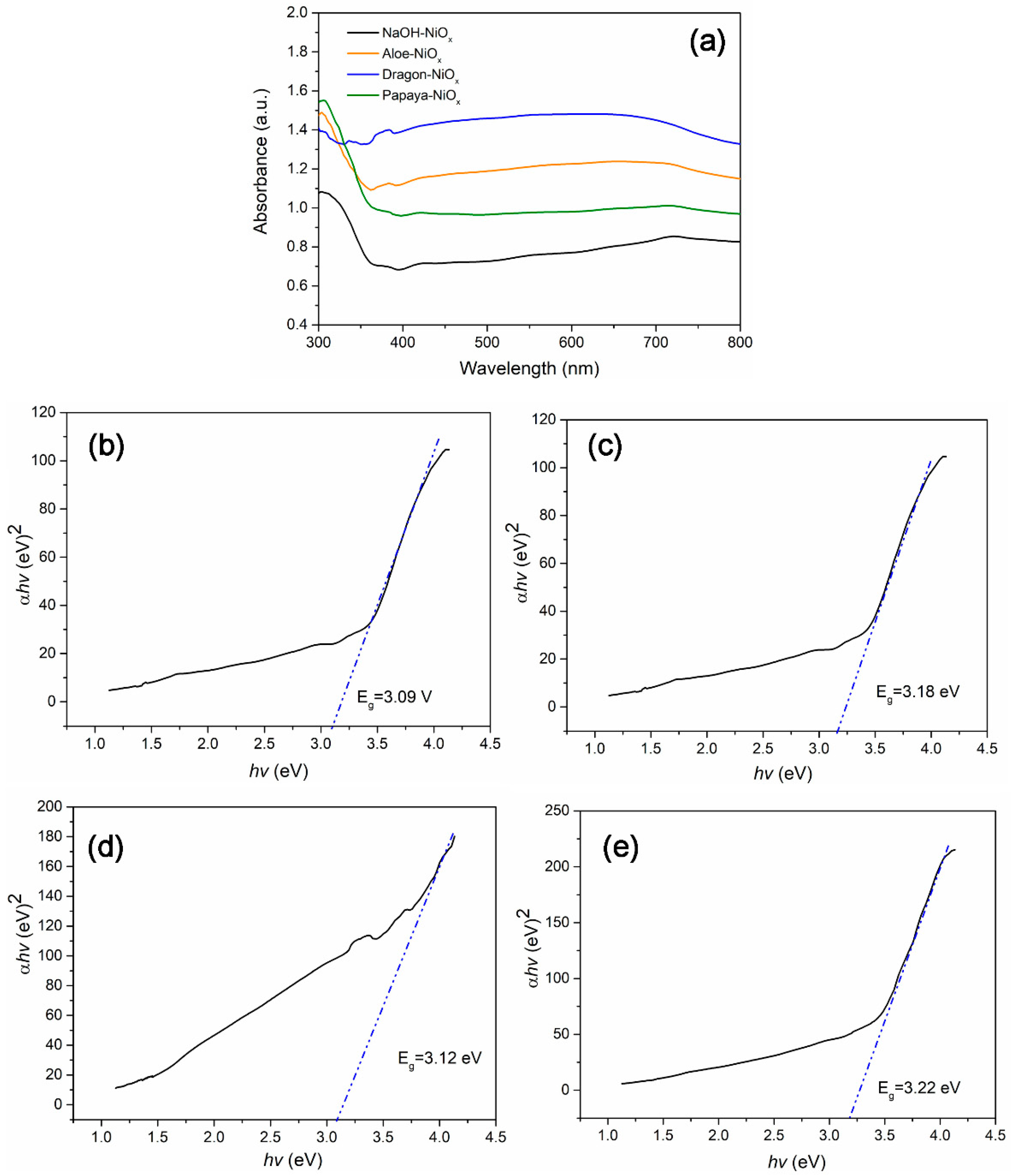
2.3. Optical Properties
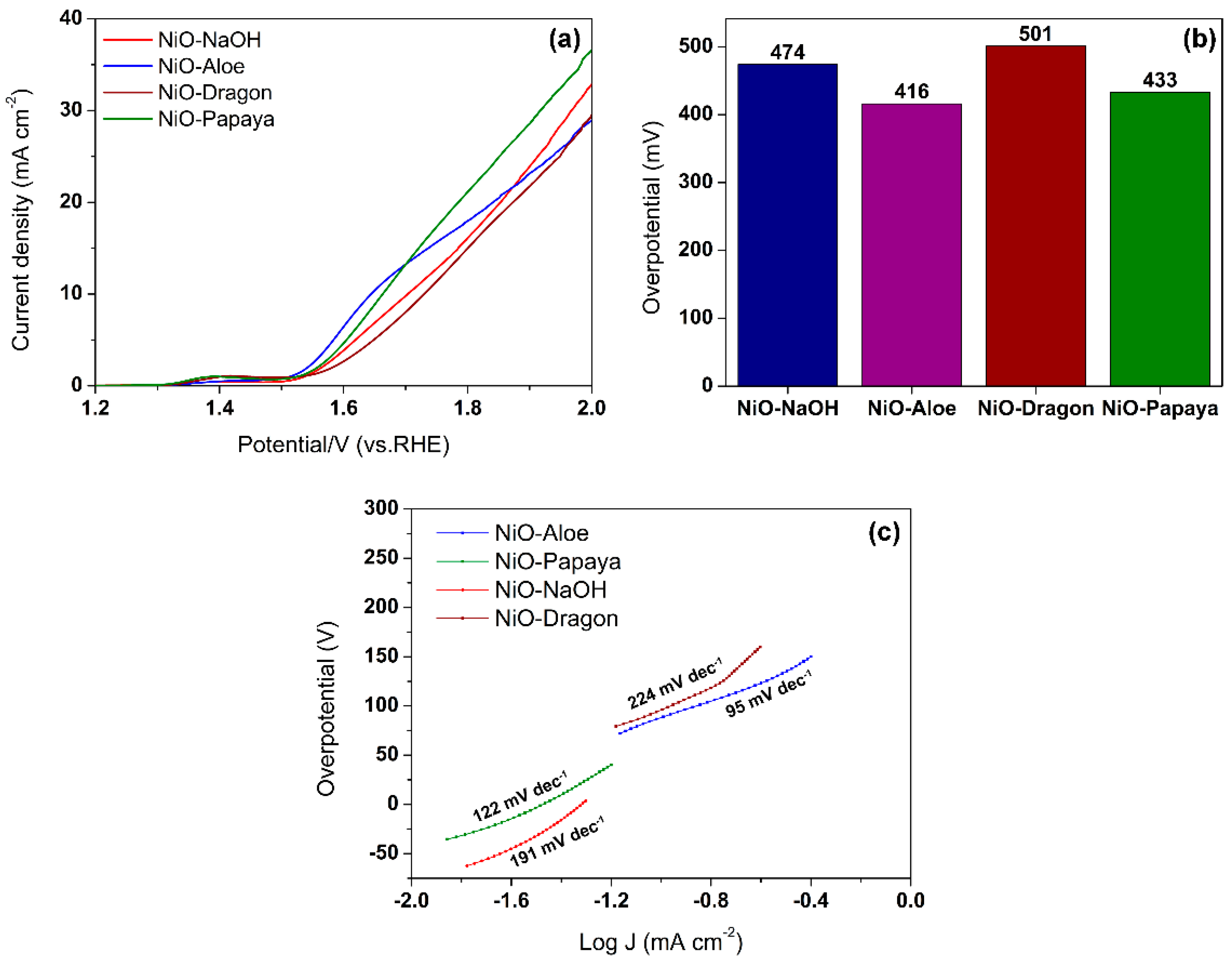
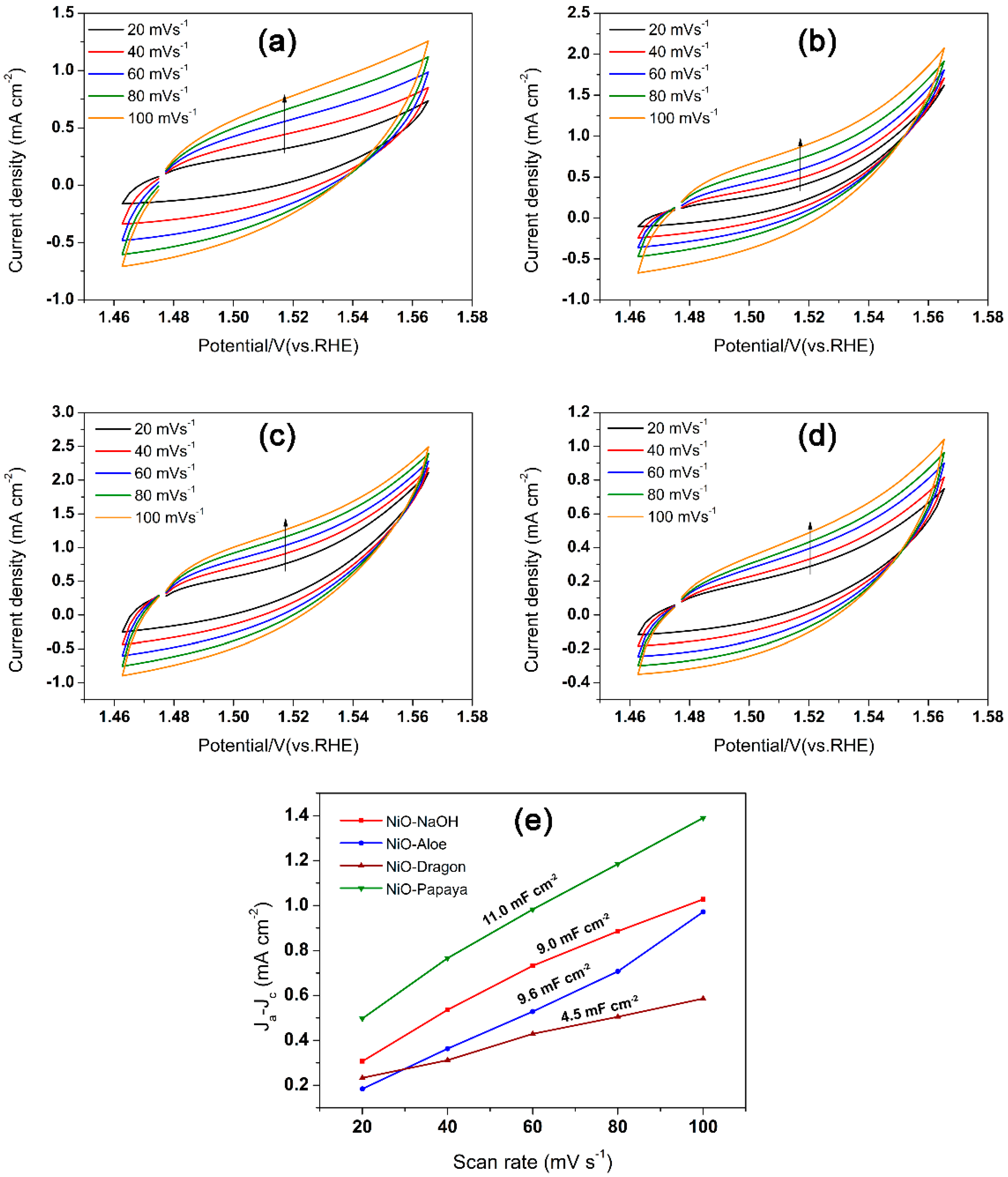
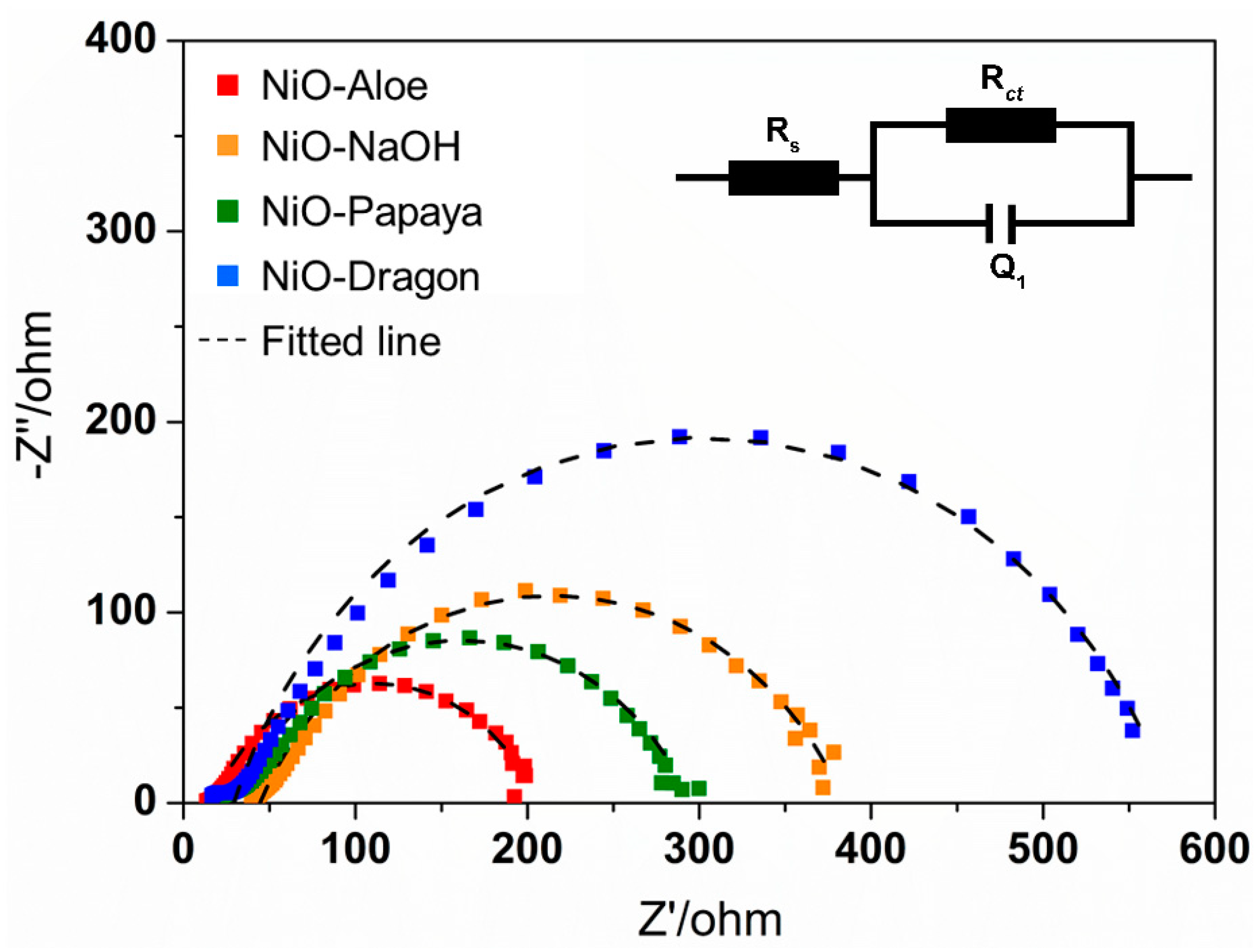
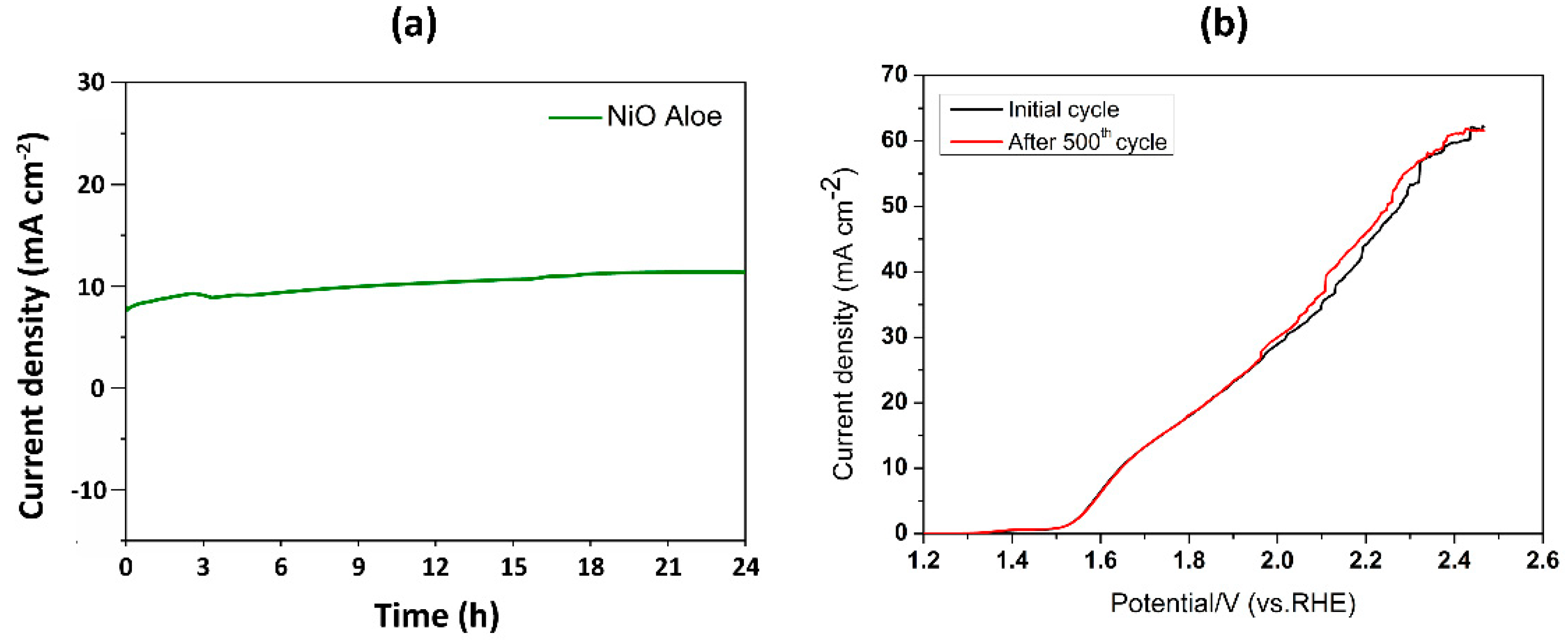
2.4. Electrocatalytic Properties for Oxygen Evolution Reaction (OER)
3. Materials and Methods
3.1. Chemicals
3.2. Preparation of Plant Extract
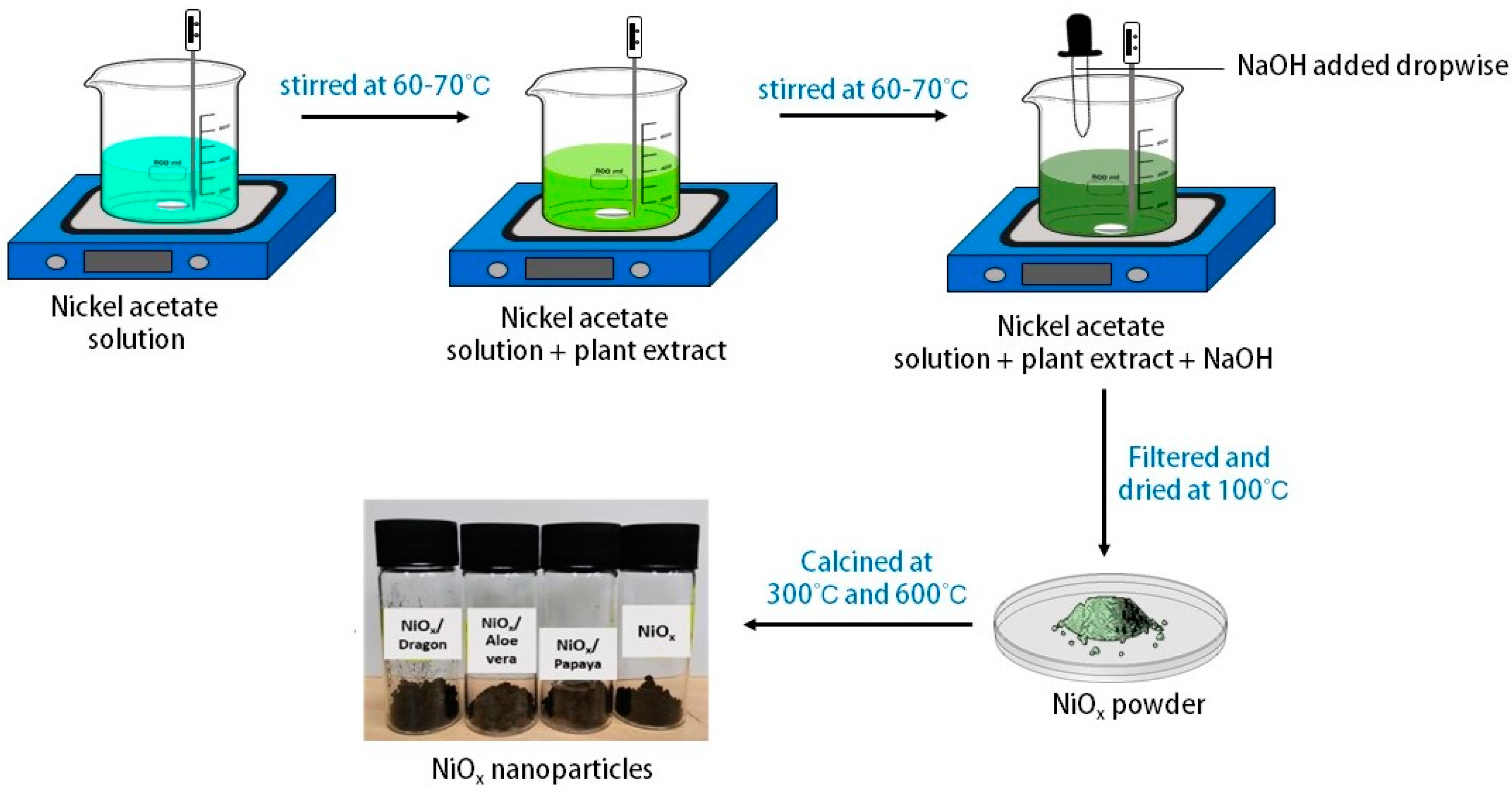
3.3. Synthesis of NiOx Nanoparticle
3.4. Characterization of Materials
3.5. Electrochemical Characterization
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- You, B.; Sun, Y. Innovative strategies for electrocatalytic water splitting. Acc. Chem. Res. 2018, 51, 1571–1580. [Google Scholar] [CrossRef]
- Pal, G.; Rai, P.; Pandey, A. Chapter 1—Green synthesis of nanoparticles: A greener approach for a cleaner future. In Green Synthesis, Characterization and Applications of Nanoparticles; Shukla, A.K., Iravani, S., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 1–26. [Google Scholar] [CrossRef]
- Jadoun, S.; Arif, R.; Jangid, N.K.; Meena, R.K. Green synthesis of nanoparticles using plant extracts: A review. Environ. Chem. Lett. 2021, 19, 355–374. [Google Scholar] [CrossRef]
- Shafey, A.M.E. Green synthesis of metal and metal oxide nanoparticles from plant leaf extracts and their applications: A review. Green Process. Synth. 2020, 9, 304–339. [Google Scholar] [CrossRef]
- Singh, J.; Dutta, T.; Kim, K.-H.; Rawat, M.; Samddar, P.; Kumar, P. ‘Green’ synthesis of metals and their oxide nanoparticles: Applications for environmental remediation. J. Nanobiotechnol. 2018, 16, 84. [Google Scholar] [CrossRef]
- Madkour, M.; Bumajdad, A.; Al-Sagheer, F. To what extent do polymeric stabilizers affect nanoparticles characteristics? Adv. Colloid Interface Sci. 2019, 270, 38–53. [Google Scholar] [CrossRef]
- Tippayawat, P.; Phromviyo, N.; Boueroy, P.; Chompoosor, A. Green synthesis of silver nanoparticles in aloe vera plant extract prepared by a hydrothermal method and their synergistic antibacterial activity. PeerJ 2016, 4, e2589. [Google Scholar] [CrossRef]
- Quispe, C.; Villalobos, M.; Bórquez, J.; Simirgiotis, M. Chemical composition and antioxidant activity of Aloe vera from the Pica Oasis (Tarapacá, Chile) by UHPLC-Q/Orbitrap/MS/MS. J. Chem. 2018, 2018, 6123850. [Google Scholar] [CrossRef] [Green Version]
- Phang, Y.-K.; Aminuzzaman, M.; Akhtaruzzaman, M.; Muhammad, G.; Ogawa, S.; Watanabe, A.; Tey, L.-H. Green synthesis and characterization of CuO nanoparticles derived from papaya peel extract for the photocatalytic degradation of palm oil mill effluent (POME). Sustainability 2021, 13, 796. [Google Scholar] [CrossRef]
- Hendra, R.; Masdeatresa, L.; Abdulah, R.; Haryani, Y. Red dragon peel (Hylocereus polyrhizus) as antioxidant source. AIP Conf. Proc. 2020, 2243, 030007. [Google Scholar] [CrossRef]
- Manigandan, R.; Dhanasekaran, T.; Padmanaban, A.; Giribabu, K.; Suresh, R.; Narayanan, V. Bifunctional hexagonal Ni/NiO nanostructures: Influence of the core–shell phase on magnetism, electrochemical sensing of serotonin, and catalytic reduction of 4-nitrophenol. Nanoscale Adv. 2019, 1, 1531–1540. [Google Scholar] [CrossRef] [Green Version]
- Sekar, S.; Kim, D.Y.; Lee, S. Excellent oxygen evolution reaction of activated carbon-anchored NiO nanotablets prepared by green routes. Nanomaterials 2020, 10, 1382. [Google Scholar] [CrossRef]
- Munna, F.T.; Selvanathan, V.; Sobayel, K.; Muhammad, G.; Asim, N.; Amin, N.; Sopian, K.; Akhtaruzzaman, M. Diluted chemical bath deposition of CdZnS as prospective buffer layer in CIGS solar cell. Ceram. Int. 2021, 47, 11003–11009. [Google Scholar] [CrossRef]
- Tripathi, R.M.; Bhadwal, A.S.; Gupta, R.K.; Singh, P.; Shrivastav, A.; Shrivastav, B.R. ZnO nanoflowers: Novel biogenic synthesis and enhanced photocatalytic activity. J. Photochem. Photobiol. B Biol. 2014, 141, 288–295. [Google Scholar] [CrossRef]
- Lamba, P.; Singh, P.; Singh, P.; Kumar, A.; Singh, P.; Bharti; Kumar, Y.; Gupta, M. Bioinspired synthesis of nickel oxide nanoparticles as electrode material for supercapacitor applications. Ionics 2021, 27, 5263–5276. [Google Scholar] [CrossRef]
- Cao, Y.; Hu, P.; Jia, D. Solvothermal synthesis, growth mechanism, and photoluminescence property of sub-micrometer PbS anisotropic structures. Nanoscale Res. Lett. 2012, 7, 668. [Google Scholar] [CrossRef] [Green Version]
- Talluri, B.; Prasad, E.; Thomas, T. Ultra-small (r < 2 nm), stable (>1 year) copper oxide quantum dots with wide band gap. Superlattices Microstruct. 2018, 113, 600–607. [Google Scholar] [CrossRef] [Green Version]
- Selvanathan, V.; Aminuzzaman, M.; Tey, L.-H.; Razali, S.A.; Althubeiti, K.; Alkhammash, H.I.; Guha, S.K.; Ogawa, S.; Watanabe, A.; Shahiduzzaman, M.; et al. Muntingia calabura leaves mediated green synthesis of CuO nanorods: Exploiting phytochemicals for unique morphology. Materials 2021, 14, 6379. [Google Scholar] [CrossRef]
- Liang, Q.; Brocks, G.; Bieberle-Hütter, A. Oxygen evolution reaction (OER) mechanism under alkaline and acidic conditions. J. Phys. Energy 2021, 3, 026001. [Google Scholar] [CrossRef]
- Jin, H.; Wang, X.; Tang, C.; Vasileff, A.; Li, L.; Slattery, A.; Qiao, S.-Z. Stable and highly efficient hydrogen evolution from seawater enabled by an unsaturated nickel surface nitride. Adv. Mater. 2021, 33, 2007508. [Google Scholar] [CrossRef]
- Wang, C.; Qi, L. Hollow nanosheet arrays assembled by ultrafine ruthenium–cobalt phosphide nanocrystals for exceptional pH-universal hydrogen evolution. ACS Mater. Lett. 2021, 3, 1695–1701. [Google Scholar] [CrossRef]
- Rani, B.J.; Ravi, G.; Yuvakkumar, R.; Ravichandran, S.; Ameen, F.; Al-Sabri, A. Efficient, highly stable Zn-doped NiO nanocluster electrocatalysts for electrochemical water splitting applications. J. Sol-Gel Sci. Technol. 2019, 89, 500–510. [Google Scholar] [CrossRef]
- Zhu, K.; Shi, F.; Zhu, X.; Yang, W. The roles of oxygen vacancies in electrocatalytic oxygen evolution reaction. Nano Energy 2020, 73, 104761. [Google Scholar] [CrossRef]
- Wang, Y.; Liang, Z.; Zheng, H.; Cao, R. Recent progress on defect-rich transition metal oxides and their energy-related applications. Chemistry 2020, 15, 3717–3736. [Google Scholar] [CrossRef]
- Wang, H.; Xie, A.; Li, X.; Wang, Q.; Zhang, W.; Zhu, Z.; Wei, J.; Chen, D.; Peng, Y.; Luo, S. Three-dimensional petal-like graphene Co3.0Cu1.0 metal organic framework for oxygen evolution reaction. J. Alloys Comp. 2021, 884, 161144. [Google Scholar] [CrossRef]
- Santos, H.L.S.; Corradini, P.G.; Medina, M.; Dias, J.A.; Mascaro, L.H. NiMo–NiCu Inexpensive Composite with High Activity for Hydrogen Evolution Reaction. ACS Appl. Mater. Interfaces 2020, 12, 17492–17501. [Google Scholar] [CrossRef]
- Selvanathan, V.; Ruslan, M.H.; Aminuzzaman, M.; Muhammad, G.; Amin, N.; Sopian, K.; Akhtaruzzaman, M. Resorcinol-Formaldehyde (RF) as a Novel Plasticizer for Starch-Based Solid Biopolymer Electrolyte. Polymers 2020, 12, 2170. [Google Scholar] [CrossRef]
- Akbayrak, M.; Önal, A.M. Metal oxides supported cobalt nanoparticles: Active electrocatalysts for oxygen evolution reaction. Electrochim. Acta 2021, 393, 139053. [Google Scholar] [CrossRef]
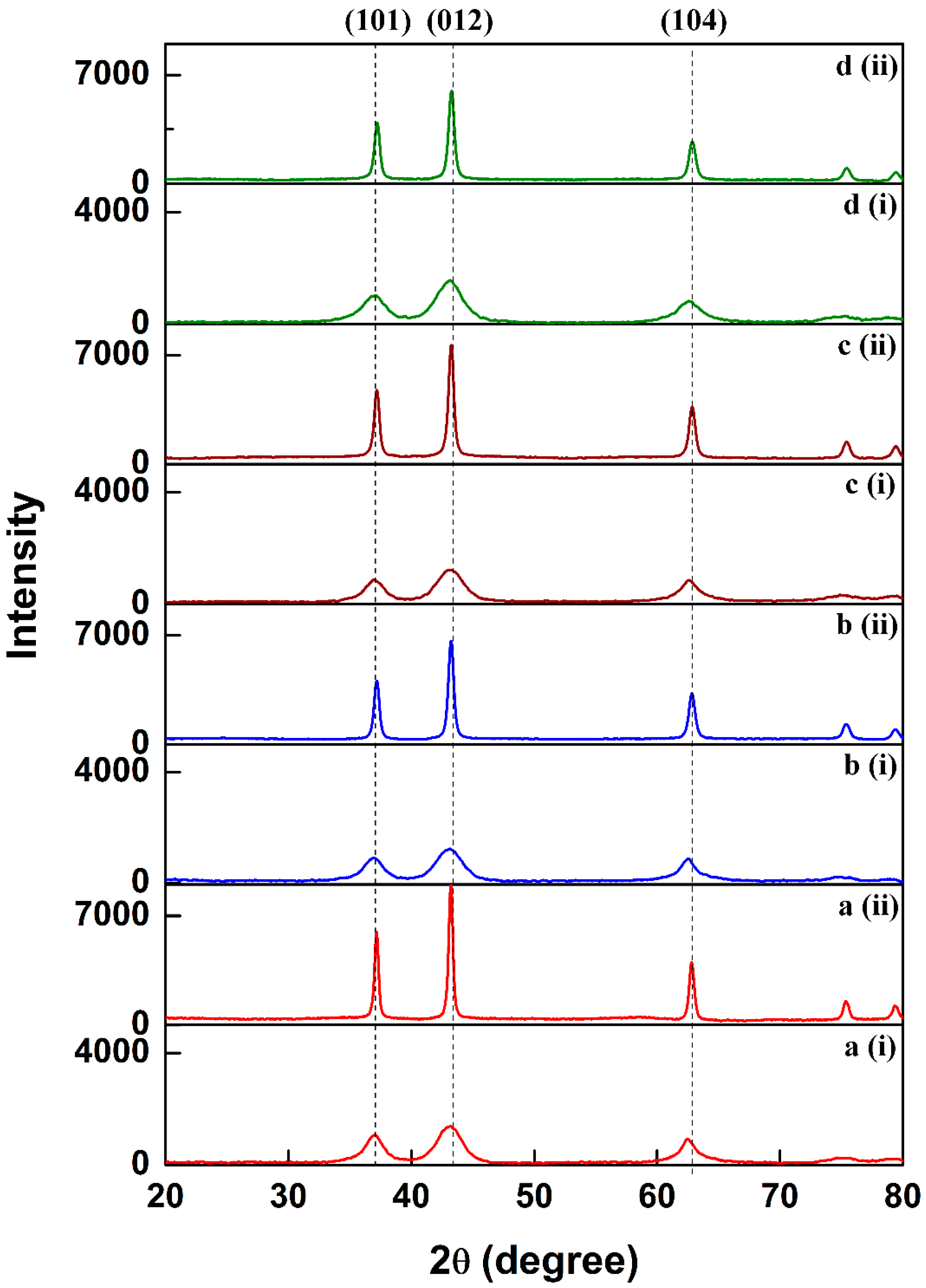

| Material | 2θ | FWHM | βcosθ | Crystallite Size (nm) | Average Crystallite Size (nm) |
|---|---|---|---|---|---|
| NiO–NaOH | 37.4 | 0.51 | 0.0085 | 16.3 | 15.9 |
| 43.2 | 0.51 | 0.0083 | 16.8 | ||
| 62.8 | 0.63 | 0.0094 | 14.7 | ||
| NiO–Aloe | 37.4 | 0.71 | 0.0117 | 11.8 | 11.5 |
| 43.2 | 0.63 | 0.0101 | 13.6 | ||
| 62.8 | 1.03 | 0.0153 | 9.0 | ||
| NiO–Papaya | 37.4 | 0.90 | 0.0149 | 9.3 | 10.2 |
| 43.2 | 0.68 | 0.0109 | 12.6 | ||
| 62.8 | 1.08 | 0.0160 | 8.6 | ||
| NiO–Dragon | 37.4 | 1.62 | 0.0269 | 5.2 | 8.1 |
| 43.2 | 0.68 | 0.0109 | 12.6 | ||
| 62.8 | 1.45 | 0.0216 | 6.4 |
| Material | Onset Potential (V) | Overpotential at 10 mA cm−2 (mV) | Tafel Slope (mV dec−1) |
|---|---|---|---|
| NiO–NaOH | 1.57 | 474 | 191 |
| NiO–Aloe | 1.54 | 416 | 95 |
| NiO–Papaya | 1.56 | 433 | 122 |
| NiO–Dragon | 1.59 | 501 | 224 |
| Material | Cdl (mF cm−2) | ECSA (cm2) | Rf |
|---|---|---|---|
| NiO–NaOH | 4.50 | 112.50 | 1584 |
| NiO–Aloe | 4.80 | 120.00 | 1690 |
| NiO–Papaya | 5.50 | 137.50 | 1936 |
| NiO–Dragon | 2.25 | 56.25 | 792 |
| Material | RS (Ω) | RCT (Ω) | QDL (F) |
|---|---|---|---|
| NiO–NaOH | 44.5 | 339.9 | 3.04 × 10−4 |
| NiO–Aloe | 17.0 | 187.9 | 3.59 × 10−4 |
| NiO–Papaya | 29.2 | 265.6 | 2.72 × 10−4 |
| NiO–Dragon | 29.4 | 544.5 | 1.54 × 10−4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Selvanathan, V.; Shahinuzzaman, M.; Selvanathan, S.; Sarkar, D.K.; Algethami, N.; Alkhammash, H.I.; Anuar, F.H.; Zainuddin, Z.; Aminuzzaman, M.; Abdullah, H.; et al. Phytochemical-Assisted Green Synthesis of Nickel Oxide Nanoparticles for Application as Electrocatalysts in Oxygen Evolution Reaction. Catalysts 2021, 11, 1523. https://doi.org/10.3390/catal11121523
Selvanathan V, Shahinuzzaman M, Selvanathan S, Sarkar DK, Algethami N, Alkhammash HI, Anuar FH, Zainuddin Z, Aminuzzaman M, Abdullah H, et al. Phytochemical-Assisted Green Synthesis of Nickel Oxide Nanoparticles for Application as Electrocatalysts in Oxygen Evolution Reaction. Catalysts. 2021; 11(12):1523. https://doi.org/10.3390/catal11121523
Chicago/Turabian StyleSelvanathan, Vidhya, M. Shahinuzzaman, Shankary Selvanathan, Dilip Kumar Sarkar, Norah Algethami, Hend I. Alkhammash, Farah Hannan Anuar, Zalita Zainuddin, Mohammod Aminuzzaman, Huda Abdullah, and et al. 2021. "Phytochemical-Assisted Green Synthesis of Nickel Oxide Nanoparticles for Application as Electrocatalysts in Oxygen Evolution Reaction" Catalysts 11, no. 12: 1523. https://doi.org/10.3390/catal11121523
APA StyleSelvanathan, V., Shahinuzzaman, M., Selvanathan, S., Sarkar, D. K., Algethami, N., Alkhammash, H. I., Anuar, F. H., Zainuddin, Z., Aminuzzaman, M., Abdullah, H., & Akhtaruzzaman, M. (2021). Phytochemical-Assisted Green Synthesis of Nickel Oxide Nanoparticles for Application as Electrocatalysts in Oxygen Evolution Reaction. Catalysts, 11(12), 1523. https://doi.org/10.3390/catal11121523







