Abstract
The paper summarizes new information on the formation and functioning of Ni(acac)2-based multicomponent (Ziegler) systems for oligo- and polymerization of ethylene. It turns out that catalytically active sites formed in these systems are deposited on nickel-containing nanoparticles generated via the interaction of the starting components directly in the reaction system. It is shown that the activity, productivity, and composition of the target products depend on the water content in the initial solvent, all other things being equal. A model of nanosized nickel-containing particles formed during the interaction of Ni(acac)2 and diethylaluminum chloride or ethylaluminum sesquichloride is presented.
1. Introduction
Since 2004 we have been engaged in detailed investigations of the processes occurring during the formation and functioning of Ziegler hydrogenation catalytic systems [1,2,3,4,5,6,7,8,9,10,11,12,13,14,15]. Using M(acac)2–Red (where M = Co, Ni; Red = AlEt3, LiAlH4, AlEt2(OEt), LiAlH(tert-BuO)3) it was shown that the interaction of acetylacetonate complexes of cobalt, nickel, or palladium with AlEt3 or LiAlH4 produced nanoparticles consisting of a metal core (0.7–5 nm) stabilized by a ligand shell. The latter represented acetylacetonate compounds of aluminum and AlEt3 bound to surface metal atoms by the acid-base interaction. In LiAlH4-derived systems, the ligand shell contains Li(acac) as well as AlH4− and AlH63− anions. In addition, the nature of the ligand shells that stabilize transition metal nanoparticles depends on the amount and nature of the ligands incorporated into a precursor. The metal core can also contain decomposition products of complex hydrides (for example, aluminum for LiAlH4-based system or other cocatalysts). These particles are almost inactive in the catalysis of alkenes hydrogenation under mild conditions (T = 20–35 °C, PH2 = 2 atm), when the water content in the solution is less than 1.8 mmol/L (solvents with “obtainable” degree of purity [13]). Only after the introduction of proton-donating compounds (for example, water or alcohols) into the reaction system, these particles are activated due to the transformation of AlEt3 or LiAlH4 and the related products into alkoxy- or hydroxyl-derivatives of aluminum. Thus, aluminum-containing fragments on the surface of nanoparticles act as inhibitors of catalytic activity, while the addition of proton-donating substances contributes to the changing of the ligand shell nature and the activation of their catalytic properties. In the presence of crystallization water in a precursor (in the case of systems based on Ni(acac)2 or Co(acac)2), the addition of an activator is not required for the production of an efficient hydrogenation catalyst. Moreover, if AlEt2(OEt) or LiAlH(tert-BuO)3 are employed as reducing agents, the hydrogenation catalyst is also formed without an activator [4,7,8,12,13,15].
These results have allowed us to formulate a mechanistic concept of the formation of multicomponent (Ziegler) hydrogenation catalysts. The key point of the concept is as follows: it is necessary to select an optimal compound of the non-transition element (and its amount) as a reducing agent of the initial compounds of transition metals (for example, acetylacetonates). Such a compound, together with other specially introduced reagents (namely, proton-donating ones (for instance, alcohols, water, etc.)) should participate in the generation of nanosized particles that are active in the hydrogenation of unsaturated compounds [13,14,15].
In the present work, data on new aspects of the formation and functioning of Ziegler catalytic systems for the oligomerization of α-olefins, based on Ni(acac)2 and chlorine-containing organoaluminum compounds (diethylaluminum chloride (AlEt2Cl, DEAC) and ethylaluminum sesquichloride (Et2AlCl∙Cl2AlEt, EASC)) are summarized [13,16,17]. These data confirm that this concept can be considered a guideline for the directed design of any multicomponent Ziegler catalyst systems derived from transition metal compounds and non-transition elements formed in situ.
2. Physico-Chemical Aspects of the Formation and Nature of the Activity of Systems Based on Ni(acac)2 and DEAC or EASC
It is no coincidence that Ziegler systems based on Ni(acac)2 and chlorine-containing organoaluminium compounds (DEAC, EASC) were chosen as model reactants. For example, Ni(acac)2 is stable in a solution of aromatic solvents in the experiments with ethylene oligomerization, and is readily and completely dehydrated [13]. DEAC and EASC were employed as cocatalysts since, firstly, the catalytic systems formed on the basis of these cocatalysts are highly active in di- and oligomerization of olefins [18,19] and, secondly, the detailed experimental data on the interaction of both DEAC and EASC with H2O have been reported [20,21,22].
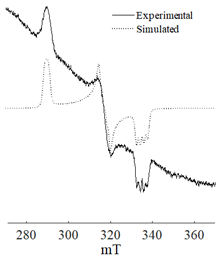
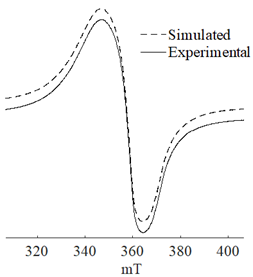
It is mainly assumed that hydride or alkyl complexes of Ni(II) act as active centers in the oligomerization of olefins [23,24,25,26,27,28,29], and the catalytic processes are predominantly of a homogeneous nature [30]. However, it is also noted that the electron spin resonance (ESR) monitoring of ethylene conversion in the presence of multicomponent Ziegler systems reveals a growing ferromagnetic resonance signal in the g ~ 2.2 region, which is associated with the formation of transition metal particles (for example, nickel ones) [11,31,32].
A series of preliminary experiments has demonstrated that each of the starting components separately, namely DEAC, EASC, and Ni(acac)2, has no catalytic activity in di- and oligomerization of ethylene. This fact allows one to exclude their participation in the catalytic transformations of ethylene described below under the action of Ziegler systems based on Ni(acac)2 and DEAC or EASC.
Table 1 shows the turnover frequency (TOF) and turnover number (TON) of the system derived from Ni(acac)2(subl.)–DEAC or EASC versus the Al/Ni ratio during ethylene oligomerization. Similar results were previously obtained for other Ziegler nickel catalytic systems [33,34]. The stationary section of the dependence at the Al/Ni ratio of 30-75 is apparently due to the fact that, at these ratios, concentration of the formed active nickel complexes remains approximately the same, and DEAC or EASC excess (at least at 40 ≤ Al/Ni ≥ 75) does not affect the catalytic activity of the system in ethylene oligomerization.

Table 1.
Ethylene oligomerization in the presence of Ni(acac)2–DEAC and Ni(acac)2–EASC catalytic systems at different Al/Ni ratios. Data were taken from ref. [13,16].
By analogy with hydrogenation catalysts based on Ni(acac)2·nH2O (where n = 0, 0.5, and 3.0) [10,11,13,15] three Ni(acac)2 samples were studied as the initial precursor. It was shown that when Ni(acac)2∙0.5H2O was used as the initial precursor under similar conditions, the TON values slightly increased, while TOF remained approximately the same as in the case of Ni (acac)2(subl.). Therefore, all further studies were carried out at the ratio of Al/Ni = 50 and with Ni(acac)2∙0.5H2O as a precursor.
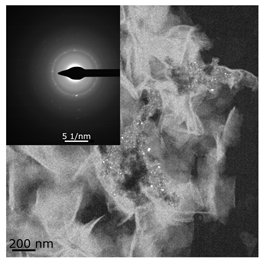
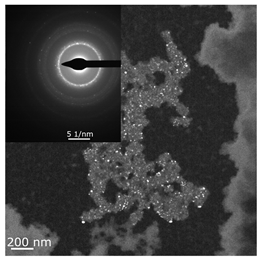
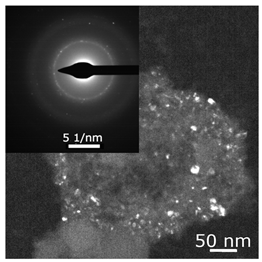
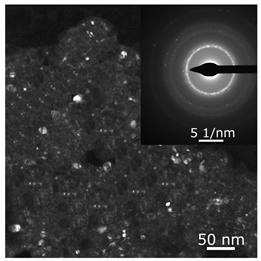
The combined ESR and transmission electronic microscopy (TEM) study revealed that during the interaction of Ni(acac)2 with DEAC or EASC in solvents with an “obtainable” degree of purity, immediately after mixing the initial components, only the formation of Ni(I) complexes was recorded (g1 = 2.003, g2 = 2.116 и g3 = 2.318). Moreover, the concentration of Ni(I) (calculated from intensity of the ESR signal) did not exceed 25–30% of the total amount of nickel loaded to the reactor for the Ni(acac)2–DEAC system, and 55–60% for the system based on Ni(acac)2–EASC (see Table 2, point 1).

Table 2.
TEM and ESR spectroscopic data for the Ni(acac)2–50DEAC or Ni(acac)2–50EASC systems, where probes (1) were selected over 1 min after components mixture, (2) t the moment of maximum ethylene absorption rate, (3) after a drop in the rate of ethylene absorption. Data were taken from ref. [13,17].
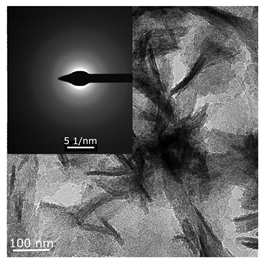
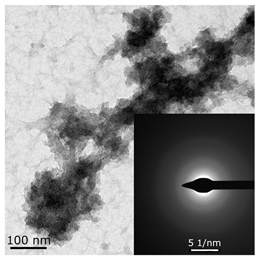
The hyperfine structure observed for g1 = 2.003 (≥4 lines) is owing to the presence of 58Ni and 35Cl atoms in a molecule of the paramagnetic complex. In addition, since g┴ < g║, it can be concluded that this Ni(I) complex has a tetragonal structure [35]. The ethylene conversion rate at this moment was insignificant, namely, about 35 min−1 for the system based on Ni(acac)2–DEAC and about 45–50 min−1 for Ni(acac)2–EASC. At the same time, the crystalline particles were not detected by TEM and electron diffraction, and energy dispersive X-ray analysis (EDAX) indicated the presence of such elements as C, H, Al, and Cl. It is likely that either by this time the crystal structures did not yet reach 0.7 nm in size (the resolution of the used electron microscope) or the thickness of the samples was not sufficient for the TEM studies.
When Ni(I) concentration dropped to zero, and the content of ferromagnetic nickel centers reached 80–90% from the initial amount of nickel for the Ni(acac)2–DEAC system and 90–95% for the Ni(acac)2–EASC system (Table 2, point 2), the catalytic activity achieved the maximum values: 210–215 min−1 for the Ni(acac)2–DEAC and 450 min−1 for the Ni(acac)2–EASC. In this case, the average size of the registered particles corresponded to about 3.5 nm for the Ni(acac)2–DEAC and 6.0 nm for Ni(acac)2–EASC. The results of atomic profiles analysis and electron diffraction, shown in Table 3, indicated that the particles mainly composed of nickel and/or nickel aluminide. ESR and TEM study of the reaction system after the decrease of ethylene conversion rate (35 min−1 for the Ni(acac)2–DEAC and 85 min−1 for Ni(acac)2–EASC) showed the presence of nickel-containing nanosized particles scattered in the matrix of organic compounds. The noticeable agglomeration of particles was observed, while aggregations of the average particle size were not detected (see Table 2, point 3).

Table 3.
Interplanar distances obtained during analysis of the atomic profiles and electron diffraction of Ni(acac)2–DEAC and Ni(acac)2–EASC samples presented in Table 2 (point 2). Data were taken from ref. [13,17].
In other words, the ferromagnetic resonance signal in the region of g ~ 2.2, as well as the appearance and growth of catalytic activity, are due to the formation of nickel-containing nanoparticles, the size of which depends on the nature of cocatalyst (all other things being equal). When passing from DEAC to EASC, the average particle size increases by almost two times. Apparently, nickel-containing nanoparticles act as carriers or substrates for catalytically active centers containing Ni(II)–H or Ni(II)–C fragments, which were previously recorded by 1H NMR spectroscopy [13,36].
In addition, the obtained data evidence that the Ni(I) complexes, recorded in the ESR spectrum immediately after mixing the initial components, are involved in the formation of catalytically active structures, but do not directly participate in the catalysis of ethylene oligomerization. It should be noted that in the course of the functioning of the catalytically active system, dimeric compounds Ni(I)–Ni(I) can be formed. They also can act as catalytically active centers and are not observed in the ESR spectrum [13]. To check this assumption, in a series of special experiments, an appropriate amount of P(OEt)3, a strong reducing agent, was added to the ESR samples. It was proved that in the systems formed on the basis of Ni(acac)2 and DEAC or EASC, the dimers were not formed under the narrow range of experimental conditions.
3. The Water Role in the Catalysis of Ethylene Di- and Oligomerization Based on Ni(acac)2 and DEAC or EASC
About 18-fold growth of the water content in the initial toluene (see Table 4) changed the kinetic parameters. Namely, the TOF value was augmented ~9–10 times, and TON increased by 5–6 times [13,16]. Moreover, a fundamental difference between the sizes of nickel-containing particles formed at various concentrations of water in the solvent was not found (with TEM). In addition, the ESR spectrum also contained a signal from the Ni(I) complex in 1–2 min after mixing the components. This signal was further transformed into a ferromagnetic resonance signal, the intensity of which increased at higher rates of ethylene conversion. It is likely that the significant enhancement of catalytic activity is explained by the nature of the stabilizing ligand shell, which was formed in solvents with “obtainable” degree of purity and in the presence of excess water.

Table 4.
Dependence of the products composition formed in the ethylene oligomerization and toluene alkylation (toluene + alkylation products = 100%) in the presence of catalytic systems Ni(acac)2–50DEAC and Ni(acac)2–EASC on the concentration of water in toluene: CNi = 2.5 × 10−3 mol/L, T = 285 K, the solvent is toluene. Data were taken from ref. [13,16].
The obtained dependence between the rate of ethylene conversion and concentration of water in the initial solvent (see Table 4) for the Ni(acac)2–DEAC system is described by the equation:
y = a + bx + cx2 − dx3
Moreover, if x is a H2O concentration (mol/L), and y is TOF (min−1) or TON ((mol C2H4)/(mol Ni)) values, a corresponds to TOF = 41 min−1 and TON = 91.5 (mol C2H4)/(mol Ni). These values can be interpreted as quantitative characteristics of the “anhydrous” system. It is worthwhile to note that in this case, the value of the approximation accuracy refers to R2TOF = 0.9746 and R2TON = 0.9271, respectively.
The general character of the dependence of ethylene conversion rate on water concentration for the Ni(acac)2–EASC system is slightly different from that for the Ni(acac)2–DEAC system (see Table 4) and is described by larger values than in the case of the Ni(acac)2–DEAC (aTOF = 426 min−1, aTON = 1195 (mol C2H4)/(mol Ni)). This may be due to the fact that EASC is a stronger Lewis acid than DEAC [37]. The interaction of DEAC or EASC with water, contained in toluene, affords Bronsted acids, namely AlRxCl3−x∙H2O or H+[AlRxCl3−xOH]−, at the first stage, while at the second stage, alumoxane-like compounds (even stronger Lewis acid sites than EASC or DEAC) are formed [38]. Both H+[AlRxCl3−xOH]− and alumoxane-like substances can act as cocatalysts for ethylene oligomerization and stabilizers of cationic hydride complexes of Ni(II) [13,36]. It is likely that the formation and efficient functioning of Ni(II) hydride centers, catalytically active in ethylene oligomerization and produced on the basis of Ni(acac)2 and chlorine-containing organoaluminium compounds, require some optimum amount of cocatalyst with a certain acidity. Under otherwise equal conditions, this optimal amount is determined by the nature of the cocatalyst itself.
In addition, the cocatalyst nature also affects composition of the products of ethylene oligomerization (see Table 4) and toluene alkylation used as a solvent [13,16]. As seen from Table 4, higher concentrations of water in toluene effects the composition of products for the system Ni(acac)2–DEAC *namely, the fraction of methylpentenes and linear hexenes increases(, while the content of butenes-2 decreases and the amount of butenes-1 remains almost intact. This experimental fact probably evidences greater contribution of butenes-2 codimerization with ethylene during the formation of hexenes. Apparently, with the increased water concentration in toluene, the nature of anionic part of the nickel complex changes that enhances probability of coordination of already formed butenes-2 and ethylene with nickel.
For the system Ni(acac)2–50EASC (see Table 4), the products are also represented mainly by butenes (no more than 54–58%), methylpentenes (20–24%), linear hexenes (9.5–12.5%), and oligomers (6.5–9.7%), and the quantitative composition of the products formed at H2O concentration in toluene of 1.71 × 10−3 mol/L is approximately the same.
In conclusion, all of the obtained experimental data confirm the earlier suggestion [13] that the nature of ligand shells stabilizing nickel-containing particles, which are formed during the formation of Ziegler systems, depends, all other things being equal, not only on the cocatalyst but also on the water concentration in the initial solvent.
By analogy with the previously proposed model of nickel nanoparticles formed via the interaction of Ni(acac)2 with AlEt3 [13,15], it can be assumed that an excess of DEAC or EASC cocatalyst (Al/Ni = 50) is involved in the formation of a stabilizing shell. Moreover, since nickel-containing nanoparticles have a weak Lewis acidity (electron deficiency), some of the aluminum-containing compounds can bind to the particles surface by the acid-base interaction mechanism (see Figure 1). Moreover, AlEt2(acac) coordinated to nickel-containing surface through the acetylacetonate ligand.
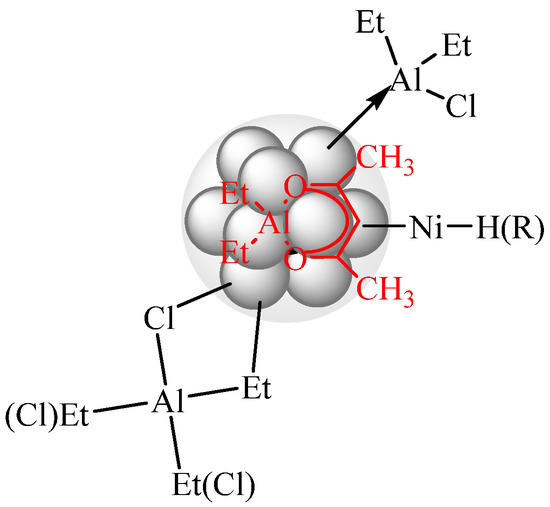
Figure 1.
Model of the nanosized particles formed by interaction of Ni(acac)2 and DEAC or EASC (Al/Ni = 50).
The next stage of the study was the application of the methods and approaches described in the present paper for ethylene polymerization, namely, using nickel Brookhart-type systems: α-diimine complexes of Ni(0) and Ni(II) with the general formula NiBr2(DAD–R) (R = –C3H7 or –CH3) or Ni(DAD–CH3)2 (DAD(–C3H7) = 1,4-bis(2,6-diiso-propylphenyl)-2,3-(dimethyl-1,4-diazabuta-1,3-diene), DAD(–CH3) = 1,4-bis(2,6-dimethylphenyl)-2,3-dimethyl-1,4-diazabuta-1,3-diene)), in combination with Lewis acids (AlEt3, AlEt2Cl, AlEtCl2).
Earlier [13,36,39,40], using these systems as an example, we showed that the interaction of α-diimine complexes of Ni(0) and Ni(II) with cocatalysts of various natures (AlEtnCl3−n (n = 1–3), Et2AlCl∙EtAlCl2, BF3·OEt2, B(C6F5)3, CF3COOH) afforded Ni(I) complexes, which are precursors during the formation of catalytically active particles.
It was found that during the formation of the catalytic system, a ferromagnetic resonance signal appears in the ESR spectrum at g = 2.2, which increases in the course of its functioning. In this case, the increase in the signal intensity at g = 2.2 as well as the decrease of signal intensity from the Ni(I) complexes allowed us to assume the formation of nanosized catalytically active center [13,39,40]. Unfortunately, the nature of the products did not permit us to perform high-resolution transmission electron microscopy (HR TEM) monitoring. First, the nickel-containing nanoparticles themselves have little contrast for TEM studies [41]. Second, the described nanoparticles are distributed inside the polymer matrix, which made it impossible to perform both the energy dispersive X-ray analysis of the particles and their electron diffraction. Moreover, even using a dark field TEM did not allow obtaining reliable images of nanoparticles. It remains unclear from analysis of the microphotographs whether the particles contain nickel or there are simply areas of thickening of the polymer matrix when analyzing micrographs. Thus, a detailed analysis of the nature and composition of such nickel-containing systems in the ethylene oligomerization requires further research.
Author Contributions
Y.Y.T. and F.K.S. conceived, designed, performed experiments, results interpretation, wrote the paper, conceptualized the overall idea of this research, and submitted the manuscript in the final form. All authors have read and agreed to the published version of the manuscript.
Funding
Research completed in the framework of State Contract No. AAAA-A19-119022690046-4 of the Program of Fundamental Research.
Data Availability Statement
Data are contained within the article.
Acknowledgments
The studies were carried out using the equipment of the Center for Collective Use of Analytical Equipment of the Irkutsk State University (http://ckp-rf.ru/ckp/3264/). The authors are grateful to the Baikal Analytical Center for Collective Uses, SB RAS.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Belykh, L.B.; Goremyka, T.V.; Rokhin, A.V.; Titova, Y.Y.; Umanets, V.A.; Schmidt, F.K. Reactions of Pd Betta-Diketonate Complexes with Triethylaluminium. Russ. J. Coord. Chem. Khimiya 2005, 31, 719–724. [Google Scholar] [CrossRef]
- Belykh, L.B.; Titova, Y.Y.; Umanets, V.A.; Shmidt, F.K. Palladium Hydrogenation Catalysts Modified with Aluminum- and Phosphorus-Containing Compounds and with Alcohols: Effect of Modifiers. Russ. J. Appl. Chem. 2006, 79, 1271–1277. [Google Scholar] [CrossRef]
- Belykh, L.B.; Titova, Y.Y.; Rokhin, A.V.; Belonogova, L.N.; Shmidt, F.K. Hydrogenation Catalysts Based on Palladium Bisacetylacetonate and Lithium Tetrahydroaluminate: Formation Mechanism and Reasons for Modified Effect of Water. Russ. J. Appl. Chem. 2008, 81, 917–925. [Google Scholar] [CrossRef]
- Belykh, L.B.; Titova, Y.Y.; Rokhin, A.V.; Shmidt, F.K. Generation and Properties of Hydrogenation Catalysts Based on Palladium Bisacetylacetonate and Lithium Alkoxyhydroaluminates. Russ. J. Appl. Chem. 2008, 81, 1153–1159. [Google Scholar] [CrossRef]
- Belykh, L.B.; Titova, Y.Y.; Rokhin, A.V.; Shmidt, F.K. Formation, Nature of Activity, and Hydrogenation Catalysis by Nickel Bis(Acetylacetonate)–Lithium Tetrahydroaluminate Systems. Russ. J. Appl. Chem. 2010, 83, 1911–1918. [Google Scholar] [CrossRef]
- Belykh, L.B.; Titova, Y.Y.; Umanets, V.A.; Rokhin, A.V.; Schmidt, F.K. The Role of Lithium Tetrahydroaluminate in the Formation of Nanodimensional Nickel Hydrogenation Catalysts. Appl. Catal. A Gen. 2011, 401, 65–72. [Google Scholar] [CrossRef]
- Schmidt, F.K.; Titova, Y.; Belykh, L.B.; Umanets, V.A.; Khutsishvili, S.S. Formation of the Cobalt Hydrogenation Catalysts at the Action of Lithium Aluminum Hydride and Lithium Tri(Tert-Butoxy)Aluminohydride and Their Properties. Russ. J. Gen. Chem. 2012, 82, 1334–1341. [Google Scholar] [CrossRef]
- Titova, Y.Y.; Belykh, L.B.; Rokhin, A.V.; Umanets, V.A.; Schmidt, F.K. Formation and Properties of Nickel Catalysts for Hydrogenation under the Action of Lithium Di- and Tris(Tert-Butoxy)Hydroaluminates. Kinet. Catal. 2012, 53, 577–584. [Google Scholar] [CrossRef]
- Shmidt, F.K.; Titova, Y.Y.; Belykh, L.B. Functions of Organoaluminum and Proton Donor Compounds in the Formation and Functioning of Nanosized Ziegler-Type Nickel-Containing Hydrogenation Catalysts. Kinet. Catal. 2015, 56, 574–583. [Google Scholar] [CrossRef]
- Schmidt, F.K.; Titova, Y.Y.; Kolesnikov, S.S.; Belykh, L.B. Nanosized Nickel Ziegler-Type Hydrogenation Catalysts: The Role of Organoaluminum and Proton-Donating Compounds in Their Formation and Optimum Catalysis. Appl. Catal. A Gen. 2015, 499, 177–187. [Google Scholar] [CrossRef]
- Titova, Y.Y.; Belykh, L.B.; Shmidt, F.K. Ziegler-Type Nickel-Based Hydrogenation Catalysts: The Effect of the Water Content of the Nickel Precursor on the Size and Nature of the Resulting Particles. Kinet. Catal. 2016, 57, 388–393. [Google Scholar] [CrossRef]
- Titova, Y.Y.; Belykh, L.B.; Shmidt, F.K. Preparation Method Effect on the Properties of Ziegler-Type Hydrogenation Catalysts Based on Bis(Acetylacetonato)Cobalt. Kinet. Catal. 2016, 57, 344–353. [Google Scholar] [CrossRef]
- Titova, Y.Y. Physico-Chemical Aspects of the Formation and Nature of the Activity of Systems Based on Cobalt, Nickel or Palladium Complexes in Hydrogenation and Oligomerization Reactions. ScD Thesis, Irkutsk State University, Irkutsk, Russia, 2018. [Google Scholar]
- Titova, Y.Y.; Sukhov, B.G.; Schmidt, F.K. Nano-Size Bimetallic Ternary Hydrogenation Catalysts Based on Nickel and Copper Complexes. J. Organomet. Chem. 2020, 925, 121485. [Google Scholar] [CrossRef]
- Titova, Y.Y.; Schmidt, F.K. Directed Design of Hydrogenation Ziegler Systems. New J. Chem. 2021, 45, 4525–4533. [Google Scholar] [CrossRef]
- Titova, Y.Y.; Shmidt, F.K. Role of Water in the Catalysis of Ethylene Di- and Oligomerization and Toluene Alkylation Reactions Based on Nickel Bis(Acetylacetonate) Systems. Kinet. Catal. 2017, 58, 749–757. [Google Scholar] [CrossRef]
- Titova, Y.Y.; Kon’kova, V.; Sukhov, G.; Schmidt, F.K. Nickel-Containing Nanophases as the Carriers of Catalytic Active Sites in the Ethylene Oligomerization in the Presence of Systems Based on Ni(Acac)2 and Organoaluminum Compounds. Mendeleev Commun. 2020, 30, 465–467. [Google Scholar] [CrossRef]
- Schmidt, F.K. Hydrogenation and Dimerization Catalyzed by Complexes of First Row Transition Metals; Gos. University: Irkutsk, Russia, 1986; p. 231. [Google Scholar]
- O’Connor, C.T.; Kojima, M. Alkene Oligomerization. Catal. Today 1990, 6, 329–349. [Google Scholar] [CrossRef]
- Petrova, V.D.; Rzhevskaya, N.N.; Shcherbakova, N.V.; Sangalov, Y.A.; Minsker, K.S. On the complexation of organochlorine compounds and water. Dokl. Akad. Nauk SSSR 1977, 233, 602–605. [Google Scholar]
- Petrova, V.D.; Rzhevskaya, N.N.; Shcherbakova, N.V.; Sangalov, Y.A.; Minsker, K.S. Interaction of alkylaluminium chlorides with water. Izv. Akad. Nauk SSSR Ser. Khim. 1978, 6, 1373–1379. [Google Scholar]
- Yamasaki, T. Structure and Lewis Acid Sites in Alumoxane Compounds. Catal. Today 1995, 23, 425–429. [Google Scholar] [CrossRef]
- Dötterl, M.; Alt, H.G. Heavy Metal with a Heavy Impact Olefin Dimerization Reactions in Triphenylbismuth Buffered Chloroaluminate Ionic Liquids.Pdf. ChemCatChem 2011, 3, 1799–1804. [Google Scholar] [CrossRef]
- Dçtterl, M.; Alt, H.G. Buffered Aluminum Chloride as a Highly Efficient Cocatalyst for Olefin Dimerization and Polymerization. ChemCatChem 2012, 4, 370–378. [Google Scholar] [CrossRef]
- Speiser, F.; Speiser, F.; Braunstein, P.; Braunstein, P.; Saussine, L.; Saussine, L. Dimerization and Oligomerization: Recent Developments with Nickel Complexes Containing. Acc. Chem. Res. 2005, 38, 784–793. [Google Scholar] [CrossRef]
- Chen, E.Y.-X.; Marks, T.J. Cocatalysts for Metal-Catalyzed Olefin Polymerization: Activators, Activation Processes, and Structure-Activity Relationships. Chem. Rev. 2000, 100, 1391–1434. [Google Scholar] [CrossRef]
- Deng, L.; Woo, T.K.; Cavallo, L.; Margl, P.M.; Ziegler, T.; January, R.V.; Re, V.; Recei, M.; April, V. The Role of Bulky Substituents in Brookhart-Type Ni(II) Diimine Catalyzed Olefin Polymerization: A Combined Density Functional Theory and Molecular Mechanics Study. J. Am. Chem. Soc. 1997, 119, 6177–6186. [Google Scholar] [CrossRef]
- Musaev, D.G.; Froese, R.D.J.; Svensson, M.; Morokuma, K. A Density Functional Study of the Mechanism of the Diimine—Nickel-Catalyzed Ethylene Polymerization Reaction. J. Am. Chem. Soc. 1997, 119, 367–374. [Google Scholar] [CrossRef]
- Siegbahn, P.E.M. Intrinsic Aptitude of Cationic Methyl- and Ethylpalladium To Associate Ethylene and To Further Undergo Subsequent Migratory Insertion. A Theoretical Study. Organometallics 1996, 15, 5542–5550. [Google Scholar] [CrossRef]
- van Leeuwen, P.W.N.M. Homogeneous Catalysis: Understanding the Art; Kluwer Academic Publishers: Amsterdam, The Netherlands, 2008; p. 423. [Google Scholar]
- Selim, M.M.; Abd El-Maksoud, I.H.; El-maksoud, I.H.A. Spectroscopic and Catalytic Characterization of Ni Nano-Size Catalyst for Edible Oil Hydrogenation. Microporous Mesoporous Mater. 2005, 85, 273–278. [Google Scholar] [CrossRef]
- Brückner, A.; Bentrup, U.; Zanthoff, H.; Maschmeyer, D. The Role of Different Ni Sites in Supported Nickel Catalysts for Butene Dimerization under Industry-like Conditions. J. Catal. 2009, 266, 120–128. [Google Scholar] [CrossRef]
- Onsager, O.-T.; Wang, H.; Blindheim, U. Niederdruck-Oligomerisation von Mono-Olefinen Mit Löslichen Nickel/Aluminium-Bimetallkatalysatoren, Teil I Entwicklung Neuer Katalysatorsysteme Auf Der Basis von π-Cyclobutadien-Nickel(II)-Verbindungen. Helv. Chim. Acta 1969, 52, 187–196. [Google Scholar] [CrossRef]
- Shmidt, F.K.; Mironova, L.V.; Kalabin, G.A.; Proidakov, A.G.; Kalabina, A.V. Dimerization of propylene in the presence of catalytic systems based on complexes of nickel with organophosphorus ligands. Neftekhimiya 1976, 16, 547–554. [Google Scholar]
- Wilkinson, G.; Gillard, R.D.; McCleverty, J.A. (Eds.) Comprehensive Coordination Chemistry; Pergamon: Oxford, UK, 1987; p. 533. [Google Scholar]
- Titova, Y.Y.; Belykh, L.B.; Rokhin, A.V.; Soroka, O.G.; Schmidt, F.K. Catalysis of Dimerization and Oligomerization Reactions of Lower Alkenes by Systems Based on Ni(PPh3)2(C2H4) and Ni(PPh3)nCl (n = 2 or 3). Kinet. Catal. 2014, 55, 35–46. [Google Scholar] [CrossRef]
- Vedejs, E.; Denmark, S.E. Lewis Base Catalysis in Organic Synthesis; Set-Wiley-VCH: Weinheim, Germany, 2016; Volume 1–3, p. 1441. [Google Scholar]
- Yamamoto, H. (Ed.) Lewis Acid Reagents. A Practical Approach; Oxford University Press: New York, NY, USA, 1999; p. 270. [Google Scholar]
- Titova, Y.Y.; Belykh, L.B.; Shmidt, F.K. Comparison of Catalytic Properties of Systems Based on Nickel Complexes with 1,4-Diaza-1,3-Butadiene Ligands in Reactions of Styrene Hydrogenation and Ethylene Polymerization. Russ. J. Appl. Chem. 2016, 89, 216–226. [Google Scholar] [CrossRef]
- Shmidt, F.K.; Titova, Y.Y.; Belykh, L.B. The Role of Phosphine and 1,2-Diimine Complexes of Nickel in the Oxidation States 0, +1, and +2 in the Catalyzed Di-, Oligo-, and Polymerization of Ethylene. Kinet. Catal. 2016, 57, 61–71. [Google Scholar] [CrossRef]
- Alley, W.M.; Hamdemir, I.K.; Wang, Q.; Frenkel, A.I.; Li, L.; Yang, J.C.; Menard, L.D.; Nuzzo, R.G.; Ozkar, S.; Yih, K.-H.; et al. Industrial Ziegler-Type Hydrogenation Catalysts Made from Co(Neodecanoate)2 or Ni(2-Ethylhexanoate)2 and AlEt3: Evidence for Nanoclusters and Sub-Nanocluster or Larger Ziegler-Nanocluster Based Catalysis. Langmuir 2011, 27, 6279–6294. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).