Anomaly Negative Resistance Phenomena in Highly Epitaxial PrBa0.7Ca0.3Co2O5+δ Thin Films Induced from Superfast Redox Reactions
Abstract
:1. Introduction
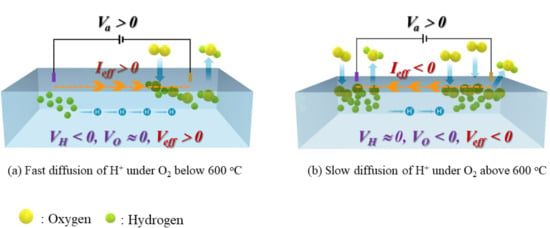
2. Results and Discussion
3. Materials and Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zeng, Q.N.; Zhang, X.Z.; Wang, W.; Zhang, D.D.; Jiang, Y.H.; Zhou, X.J.; Lin, B. A Zn-Doped Ba0.5Sr0.5Co0.8Fe0.2O3-delta Perovskite Cathode with Enhanced ORR Catalytic Activity for SOFCs. Catalysts 2020, 10, 235. [Google Scholar] [CrossRef] [Green Version]
- Chen, H.; Li, L.J.; Kemps, R.; Michielsen, B.; Jacobs, M.; Snijkers, F.; Middelkoop, V. Reactive air brazing for sealing mixed ionic electronic conducting hollow fibre membranes. Acta Mater. 2015, 88, 74–82. [Google Scholar] [CrossRef]
- Khandale, A.P.; Pahune, B.S.; Bhoga, S.S.; Kumar, R.V.; Tomov, R. Development of Pr2-xSrxCuO4+/-delta mixed ion-electron conducting system as cathode for intermediate temperature solid oxide fuel cell. Int. J. Hydrogen Energy 2019, 44, 15417–15435. [Google Scholar] [CrossRef]
- Qu, F.; Zhou, X.; Zhang, B.; Zhang, S.; Jiang, C.; Ruan, S.; Yang, M. Fe2O3 nanoparticles-decorated MoO3 nanobelts for enhanced chemiresistive gas sensing. J. Alloys Compd. 2019, 782, 672–678. [Google Scholar] [CrossRef]
- Hagedorn, K.; Li, W.; Liang, Q.; Dilger, S.; Noebels, M.; Wagner, M.R.; Reparaz, J.S.; Dollinger, A.; Guenne, J.S.A.D.; Dekorsy, T.; et al. Catalytically Doped Semiconductors for Chemical Gas Sensing: Aerogel-Like Aluminum-Containing Zinc Oxide Materials Prepared in the Gas Phase. Adv. Funct. Mater. 2016, 26, 3424–3437. [Google Scholar] [CrossRef]
- Shi, C.; Rani, A.; Thomson, B.; Debnath, R.; Motayed, A.; Ioannou, D.E.; Li, Q. High-performance room-temperature TiO2-functionalized GaN nanowire gas sensors. Appl. Phys. Lett. 2019, 115, 121602. [Google Scholar] [CrossRef]
- Shen, Q.W.; Dong, S.S.; Li, S.; Yang, G.G.; Pan, X.X. A Review on the Catalytic Decomposition of NO by Perovskite-Type Oxides. Catalysts 2021, 11, 622. [Google Scholar] [CrossRef]
- Joo, S.; Kwon, O.; Kim, K.; Kim, S.; Kim, H.; Shin, J.; Jeong, H.Y.; Sengodan, S.; Han, J.W.; Kim, G. Cation-swapped homogeneous nanoparticles in perovskite oxides for high power density. Nat. Commun. 2019, 10, 697. [Google Scholar] [CrossRef]
- Lin, Y.S.; Choi, E.M.; Lu, P.; Sun, X.; Wu, R.; Yun, C.; Zhu, B.N.; Wang, H.Y.; Li, W.W.; Maity, T.; et al. Vertical Strain-Driven Antiferromagnetic to Ferromagnetic Phase Transition in EuTiO3 Nanocomposite Thin Films. ACS Appl. Mater. Interfaces 2020, 12, 8513–8521. [Google Scholar] [CrossRef]
- Gao, Y.; Wang, J.J.; Wu, L.; Bao, S.Y.; Shen, Y.; Lin, Y.H.; Nan, C.W. Tunable magnetic and electrical behaviors in perovskite oxides by oxygen octahedral tilting. Sci. China Mater. 2015, 58, 302–312. [Google Scholar] [CrossRef] [Green Version]
- Chen, M.F.; Bao, S.Y.; Zhang, Y.; Wang, Y.J.; Liang, Y.H.; Wu, J.L.; Huang, T.T.; Wu, L.; Yu, P.; Zhu, J.; et al. Physical and chemical strains co-tuned magnetic properties of double perovskite PrBaMn2O5.5+delta epitaxial films. Appl. Phys. Lett. 2019, 115, 081903. [Google Scholar] [CrossRef]
- Kim, S.; Jun, A.; Kwon, O.; Kim, J.; Yoo, S.; Jeong, H.Y.; Shin, J.; Kim, G. Nanostructured Double Perovskite Cathode with Low Sintering Temperature For Intermediate Temperature Solid Oxide Fuel Cells. Chemsuschem 2015, 8, 3153–3158. [Google Scholar] [CrossRef] [PubMed]
- Hua, B.; Zhang, Y.Q.; Yan, N.; Li, M.; Sun, Y.F.; Chen, J.; Li, J.; Luo, J.L. The Excellence of Both Worlds: Developing Effective Double Perovskite Oxide Catalyst of Oxygen Reduction Reaction for Room and Elevated Temperature Applications. Adv. Funct. Mater. 2016, 26, 4106–4112. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhang, L.; Zhao, B.; Chen, H.; Liu, X.; Zhao, R.; Wang, X.; Liu, J.; Chen, Y.; Liu, M. Improving the Activity for Oxygen Evolution Reaction by Tailoring Oxygen Defects in Double Perovskite Oxides. Adv. Funct. Mater. 2019, 29, 1901783. [Google Scholar] [CrossRef]
- Chen, T.; Zhao, H.; Xie, Z.; Xu, N.; Lu, Y. Oxygen permeability of Ce0.8Sm0.2O2-aEuro parts per thousand delta-LnBaCo2O(5+delta) (Ln=La, Nd, Sm, and Y) dual-phase ceramic membranes. Ionics 2015, 21, 1683–1692. [Google Scholar] [CrossRef]
- Jia, Z.; Wang, P.; Zhong, Y.; Mei, H. Optimization and Electrochemical Properties of Double Perovskite NdBaCo2O6−(delta).LaBaCo2O5+delta as Cathode Material for Solid Oxide Fuel Cell. J. Nanoelectron. Optoelectron. 2018, 13, 749–757. [Google Scholar] [CrossRef]
- Zhang, L.; Li, S.; Xia, T.; Sun, L.; Huo, L.; Zhao, H. Co-deficient PrBaCo2−xO6−delta perovskites as cathode materials for intermediate-temperature solid oxide fuel cells: Enhanced electrochemical performance and oxygen reduction kinetics. Int. J. Hydrogen Energ. 2018, 43, 3761–3775. [Google Scholar] [CrossRef]
- Kong, X.; Feng, S.; Sun, H.; Yi, Z.; Liu, G. Effect of Mn on the Characterization of Layered Perovskite NdBaCo2−xMnxO5+delta (x = 0.5, 1, 1.5, 2) as Cathode Materials for IT-SOFCs. Int. J. Electrochem. Sci. 2018, 13, 7939–7948. [Google Scholar] [CrossRef]
- Bernuy-Lopez, C.; Hoydalsvik, K.; Einarsrud, M.-A.; Grande, T. Effect of A-Site Cation Ordering on Chemical Stability, Oxygen Stoichiometry and Electrical Conductivity in Layered LaBaCo2O5+delta Double Perovskite. Materials 2016, 9, 154. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.B.; Bao, S.Y.; Liu, J.; Collins, G.; Ma, C.R.; Liu, M.; Chen, C.L.; Dong, C.; Whangbo, M.H.; Guo, H.M.; et al. Ultrafast chemical dynamic behavior in highly epitaxial LaBaCo2O5+delta thin films. J. Mater. Chem. C 2014, 2, 5660–5666. [Google Scholar] [CrossRef]
- Liu, J.; Collins, G.; Liu, M.; Chen, C. Superfast oxygen exchange kinetics on highly epitaxial LaBaCo2O5+delta thin films for intermediate temperature solid oxide fuel cells. Apl Mater. 2013, 1, 031101. [Google Scholar] [CrossRef] [Green Version]
- Bao, S.; Xu, X.; Enriquez, E.; Mace, B.E.; Chen, G.; Kelliher, S.P.; Chen, C.; Zhang, Y.; Whangbo, M.-H.; Dong, C.; et al. Atomically layer-by-layer diffusion of oxygen/hydrogen in highly epitaxial PrBaCo2O5.5+delta thin films. Appl. Phys. Lett. 2015, 107, 243903. [Google Scholar] [CrossRef] [Green Version]
- Bao, S.; Ma, C.; Chen, G.; Xu, X.; Enriquez, E.; Chen, C.; Zhang, Y.; Bettis, J.L., Jr.; Whangbo, M.-H.; Dong, C.; et al. Ultrafast Atomic Layer-by-Layer Oxygen Vacancy-Exchange Diffusion in Double-Perovskite LnBaCo2O(5.5+delta) Thin Films. Sci. Rep. 2014, 4, 4726. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qi, H.; Thomas, T.; Li, W.Y.; Li, W.; Xia, F.; Zhang, N.; Sabolsky, E.M.; Zondlo, J.; Hart, R.; Liu, X.B. Reduced Thermal Expansion and Enhanced Redox Reversibility of La0.5Sr1.5Fe1.5Mo0.5O6−delta Anode Material for Solid Oxide Fuel Cells. ACS Appl. Energy Mater. 2019, 2, 4244–4254. [Google Scholar] [CrossRef]
- Bu, Y.F.; Gwon, O.; Nam, G.; Jang, H.; Kim, S.; Zhong, Q.; Cho, J.; Kim, G. A Highly Efficient and Robust Cation Ordered Perovskite Oxide as a Bifunctional Catalyst for Rechargeable Zinc-Air Batteries. ACS Nano 2017, 11, 11594–11601. [Google Scholar] [CrossRef]
- Opherden, L.; Sieger, M.; Pahlke, P.; Huhne, R.; Schultz, L.; Meledin, A.; Van Tendeloo, G.; Nast, R.; Holzapfel, B.; Bianchetti, M.; et al. Large pinning forces and matching effects in YBa2Cu3O7−delta thin films with Ba2Y(Nb/Ta)O-6 nano-precipitates. Sci. Rep. 2016, 6, 21188. [Google Scholar] [CrossRef] [PubMed]
- Kleibeuker, J.E.; Choi, E.-M.; Jones, E.D.; Yu, T.-M.; Sala, B.; MacLaren, B.A.; Kepaptsoglou, D.; Hernandez-Maldonado, D.; Ramasse, Q.M.; Jones, L.; et al. Route to achieving perfect B-site ordering in double perovskite thin films. NPG Asia Mater. 2017, 9, 406. [Google Scholar] [CrossRef] [Green Version]
- Jin, F.; Li, J.; Wang, Y.; Chu, X.; Xu, M.; Zhai, Y.; Zhang, Y.; Fang, W.; Zou, P.; He, T. Evaluation of Fe and Mn co-doped layered perovskite PrBaCo2/3Fe2/3Mn1/2O5+delta as a novel cathode for intermediate-temperature solid-oxide fuel cell. Ceram. Int. 2018, 44, 22489–22496. [Google Scholar] [CrossRef]
- Huang, X.; Feng, J.; Abdellatif, H.R.S.; Zou, J.; Zhang, G.; Ni, C. Electrochemical evaluation of double perovskite PrBaCo2−xMn(x)O(5+delta) (x = 0, 0.5,1) as promising cathodes for IT-SOFCs. Int. J. Hydrogen Energ. 2018, 43, 8962–8971. [Google Scholar] [CrossRef]
- Enriquez, E.; Chen, A.; Harrell, Z.; Dowden, P.; Koskelo, N.; Roback, J.; Janoschek, M.; Chen, C.; Jia, Q. Oxygen Vacancy-Tuned Physical Properties in Perovskite Thin Films with Multiple B-site Valance States. Sci. Rep. 2017, 7, 46184. [Google Scholar] [CrossRef] [Green Version]
- Guo, W.; Guo, R.; Liu, L.; Cai, G.; Zhang, C.; Wu, C.; Liu, Z.; Jiang, H. Thermal and electrochemical properties of layered perovskite PrBaCo2−xMnxO5+delta (x = 0.1, 0.2 and 0.3) cathode materials for intermediate temperature solid oxide fuel cells. Int. J. Hydrogen Energ. 2015, 40, 12457–12465. [Google Scholar] [CrossRef]
- Saccoccio, M.; Jiang, C.; Gao, Y.; Chen, D.; Ciucci, F. Nb-substituted PrBaCo2O5+delta as a cathode for solid oxide fuel cells: A systematic study of structural, electrical, and electrochemical properties. Int. J. Hydrogen Energ. 2017, 42, 19204–19215. [Google Scholar] [CrossRef]
- Enriquez, E.; Xu, X.; Bao, S.; Harrell, Z.; Chen, C.; Choi, S.; Jun, A.; Kim, G.; Whangbo, M.-H. Catalytic Dynamics and Oxygen Diffusion in Doped PrBaCo2O5.5+delta Thin Films. ACS Appl. Mater. Interfaces 2015, 7, 24353–24359. [Google Scholar] [CrossRef]
- Pang, S.; Yang, G.; Su, Y.; Xu, J.; Shen, X.; Zhu, M.; Wu, X.; Li, S.; Chen, C. A-site cation deficiency tuned oxygen transport dynamics of perovskite Pr0.5Ba0.25−xCa0.25CoO3−delta oxide for intermediate temperature solid oxide fuel cells. Ceram. Int. 2019, 45, 14602–14607. [Google Scholar] [CrossRef]
- Bao, S.; Pang, S.; Wang, W.; Chen, J.; Chen, M.; Ma, J.; Nan, C.-W.; Chen, C. Ca doping effect on the magnetic and electronic transport properties in double perovskite PrBaCo2O5+delta films. Appl. Phys. Lett. 2017, 111, 232406. [Google Scholar] [CrossRef]
- Fan, M.; Wang, H.; Misra, S.; Zhang, B.; Qi, Z.; Sun, X.; Huang, J.; Wang, H. Microstructure, Magnetic, and Magnetoresistance Properties of La0.7Sr0.3MnO3:CuO Nanocomposite Thin Films. ACS Appl. Mater. Interfaces 2018, 10, 5779–5784. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, R.; Dutta, A.; Sinha, T.P. Dielectric Relaxation of Rare Earth Ordered Double Perovskite Oxide Ba2ErTaO6. J. Electron. Mater. 2016, 45, 846–852. [Google Scholar] [CrossRef]
- Wang, H.; Enriquez, E.; Collins, G.; Ma, C.; Liu, M.; Zhang, Y.; Dong, C.; Chen, C. Anomalous redox properties and ultrafast chemical sensing behavior of double perovskite CaBaCo2O5+δ thin films. J. Mater. 2015, 1, 113–117. [Google Scholar] [CrossRef] [Green Version]
- Gupta, P.; Sharma, S.K. A study of oxygen gas sensing in Zn-doped SnO2 nanostructures. Mater. Res. Express 2017, 4, 065010. [Google Scholar] [CrossRef]
- Rajput, J.K.; Pathak, T.K.; Kumar, V.; Swart, H.C.; Purohit, L.P. Controlled sol-gel synthesis of oxygen sensing CdO:ZnO hexagonal particles for different annealing temperatures. RSC Adv. 2019, 9, 31316–31324. [Google Scholar] [CrossRef] [Green Version]
- Rajput, J.K.; Pathak, T.K.; Kumar, V.; Swart, H.C.; Purohit, L.P. CdO:ZnO nanocomposite thin films for oxygen gas sensing at low. Mater. Sci. Eng. B 2018, 228, 241–248. [Google Scholar] [CrossRef]
- Rajput, J.K.; Pathak, T.K.; Purohit, L.P. Impact of Sputtering Power on Properties of CdO:ZnO Thin Films Synthesized by Composite Method for Oxygen Gas Sensing Application. J. Electron. Mater. 2019, 48, 6640–6646. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, W.; Yu, H.; Zhong, X.; Wang, L.; Singh, A.; Lin, Y. Preparation and oxygen sensing properties of Ti3O5 submicron rods. Micro Nano Lett. 2016, 11, 811–813. [Google Scholar] [CrossRef]
- Raghu, A.V.; Karuppanan, K.K.; Pullithadathil, B. Highly Sensitive, Temperature-Independent Oxygen Gas Sensor Based on Anatase TiO2 Nanoparticle Grafted, 2D Mixed Valent VOx Nanoflakelets. ACS Sens. 2018, 3, 1811–1821. [Google Scholar] [CrossRef]
- Hong, D.; Pan, T.; Feng, D.; Huang, Z.; Liao, F.; Li, X.; Zhang, Y.; Gao, M. The oxygen sensitive properties of (LaBa)Co2O5+delta thin films fabricated by polymer-assisted deposition technique. J. Sol-Gel Sci. Technol. 2014, 71, 464–469. [Google Scholar] [CrossRef]
- Yuan, Z.; Liu, J.; Chen, C.L.; Wang, C.H.; Luo, X.G.; Chen, X.H.; Kim, G.T.; Huang, D.X.; Wang, S.S.; Jacobson, A.J.; et al. Epitaxial behavior and transport properties of PrBaCo2O5 thin films on (001) SrTiO3. Appl. Phys. Lett. 2007, 90, 212111. [Google Scholar] [CrossRef] [Green Version]





| Sample | a (Å) | b (Å) | c (Å) | V(Å3) |
|---|---|---|---|---|
| As-prepared PBCC | 3.927 | 3.927 | 7.660 | 118.1 |
| Annealed PBCC | 3.933 | 3.933 | 7.642 | 118.2 |
| Oxide | Response Time (s) (t90%) | Temperature (°C) | Ref |
|---|---|---|---|
| 1.5 wt% Zn-doped SnO2 | 141 | 250 | [39] |
| CdO-ZnO hexagonal particles | 43 | 200 | [40] |
| CdO:ZnO (3:1)nanostructure | 18 | 32–200 | [41] |
| CdO:ZnO (3:1) nanocomposite thin film | 10–20 | 25–200 | [42] |
| Ti3O5 | 67 | 200 | [43] |
| VOx/TiO2 | 3–5 | 100 | [44] |
| (LaBa)Co2O5+δ thin film | ~2.2 | 580 | [45] |
| PrBa0.7Ca0.3Co2O5+δthin film | <2 | 350–725 | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, Y.; Xu, X.; Xia, Y.; Pang, S.; Xu, F.; Whangbo, M.-H.; Sun, L.; Chen, C. Anomaly Negative Resistance Phenomena in Highly Epitaxial PrBa0.7Ca0.3Co2O5+δ Thin Films Induced from Superfast Redox Reactions. Catalysts 2021, 11, 1441. https://doi.org/10.3390/catal11121441
Luo Y, Xu X, Xia Y, Pang S, Xu F, Whangbo M-H, Sun L, Chen C. Anomaly Negative Resistance Phenomena in Highly Epitaxial PrBa0.7Ca0.3Co2O5+δ Thin Films Induced from Superfast Redox Reactions. Catalysts. 2021; 11(12):1441. https://doi.org/10.3390/catal11121441
Chicago/Turabian StyleLuo, Yumei, Xing Xu, Yudong Xia, Shengli Pang, Fen Xu, Myung-Hwan Whangbo, Lixian Sun, and Chonglin Chen. 2021. "Anomaly Negative Resistance Phenomena in Highly Epitaxial PrBa0.7Ca0.3Co2O5+δ Thin Films Induced from Superfast Redox Reactions" Catalysts 11, no. 12: 1441. https://doi.org/10.3390/catal11121441
APA StyleLuo, Y., Xu, X., Xia, Y., Pang, S., Xu, F., Whangbo, M.-H., Sun, L., & Chen, C. (2021). Anomaly Negative Resistance Phenomena in Highly Epitaxial PrBa0.7Ca0.3Co2O5+δ Thin Films Induced from Superfast Redox Reactions. Catalysts, 11(12), 1441. https://doi.org/10.3390/catal11121441