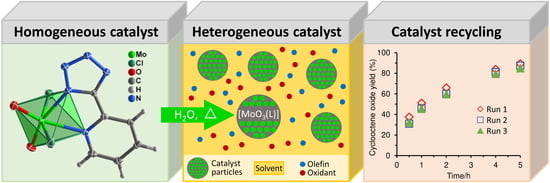
A 5-(2-Pyridyl)tetrazolate Complex of Molybdenum(VI), Its Structure, and Transformation to a Molybdenum Oxide-Based Hybrid Heterogeneous Catalyst for the Epoxidation of Olefins
Abstract
:1. Introduction
2. Results and Discussion
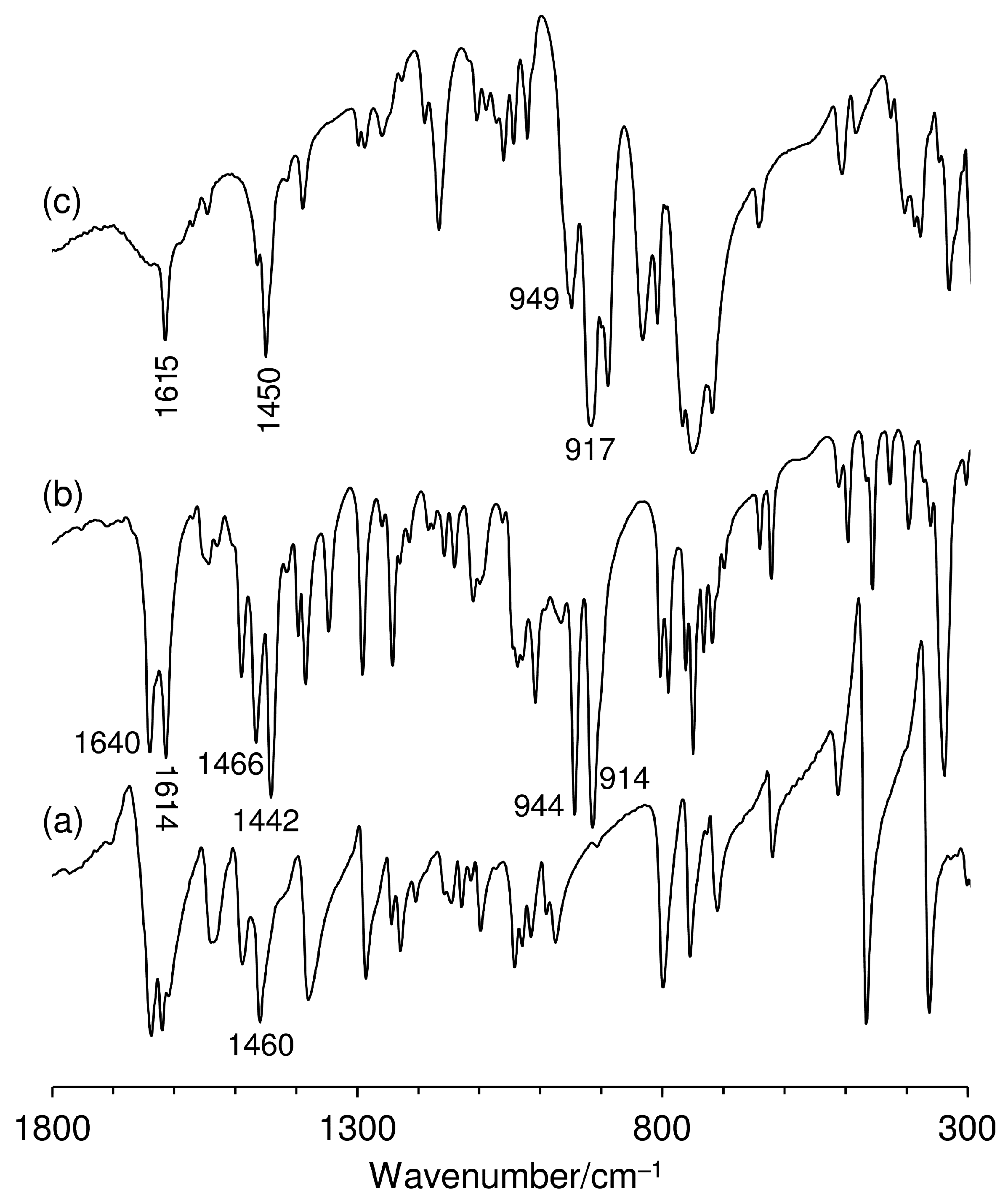
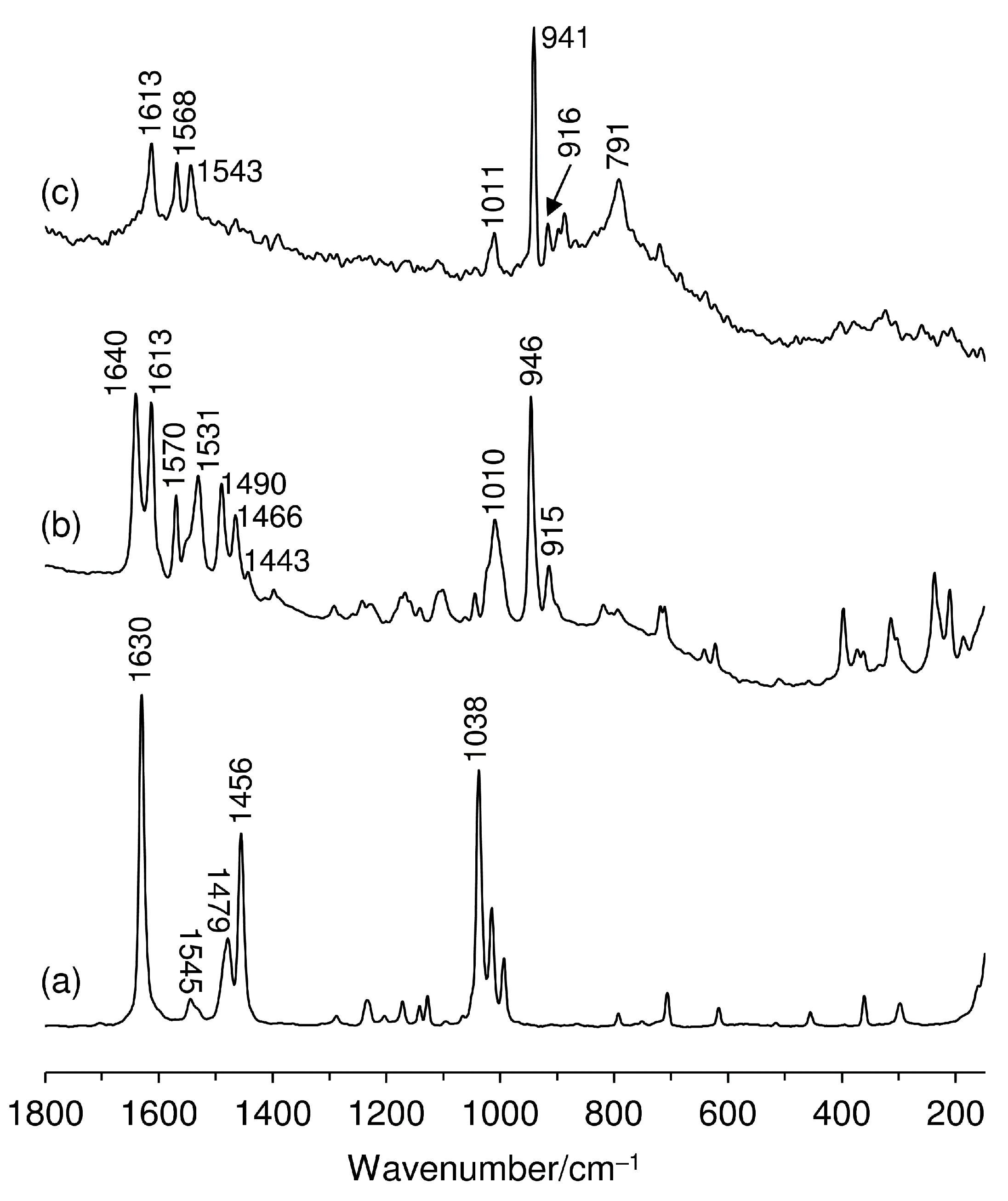
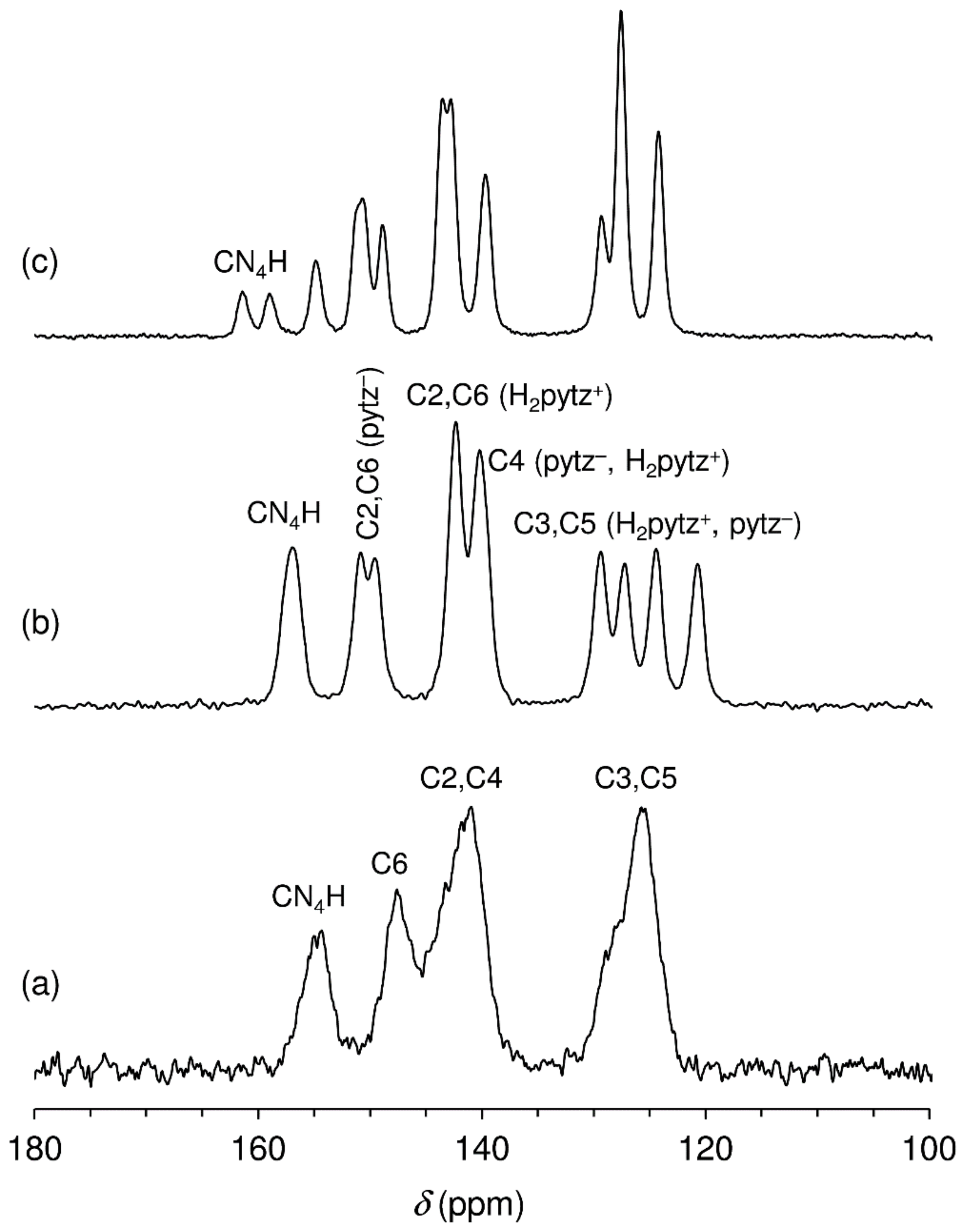
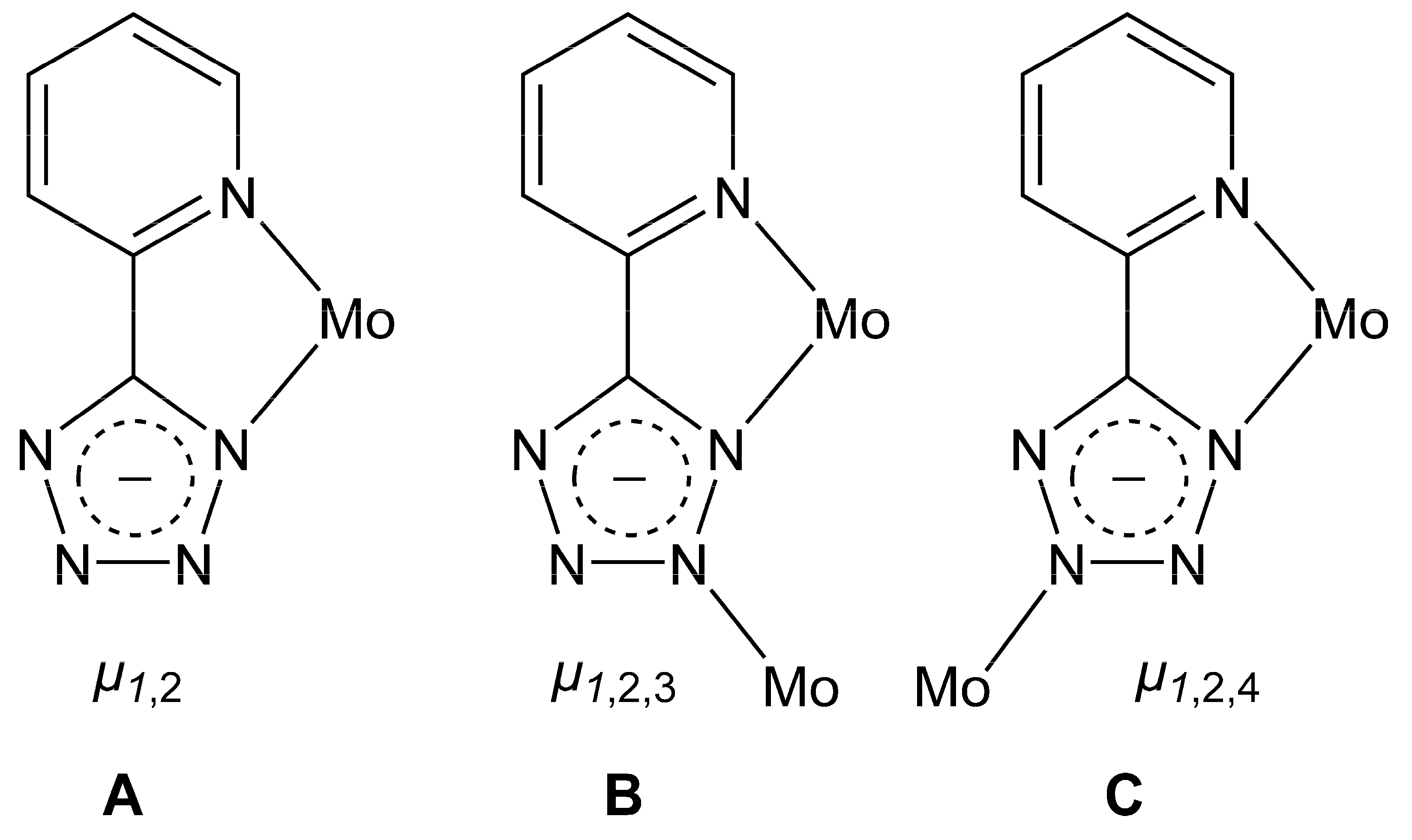
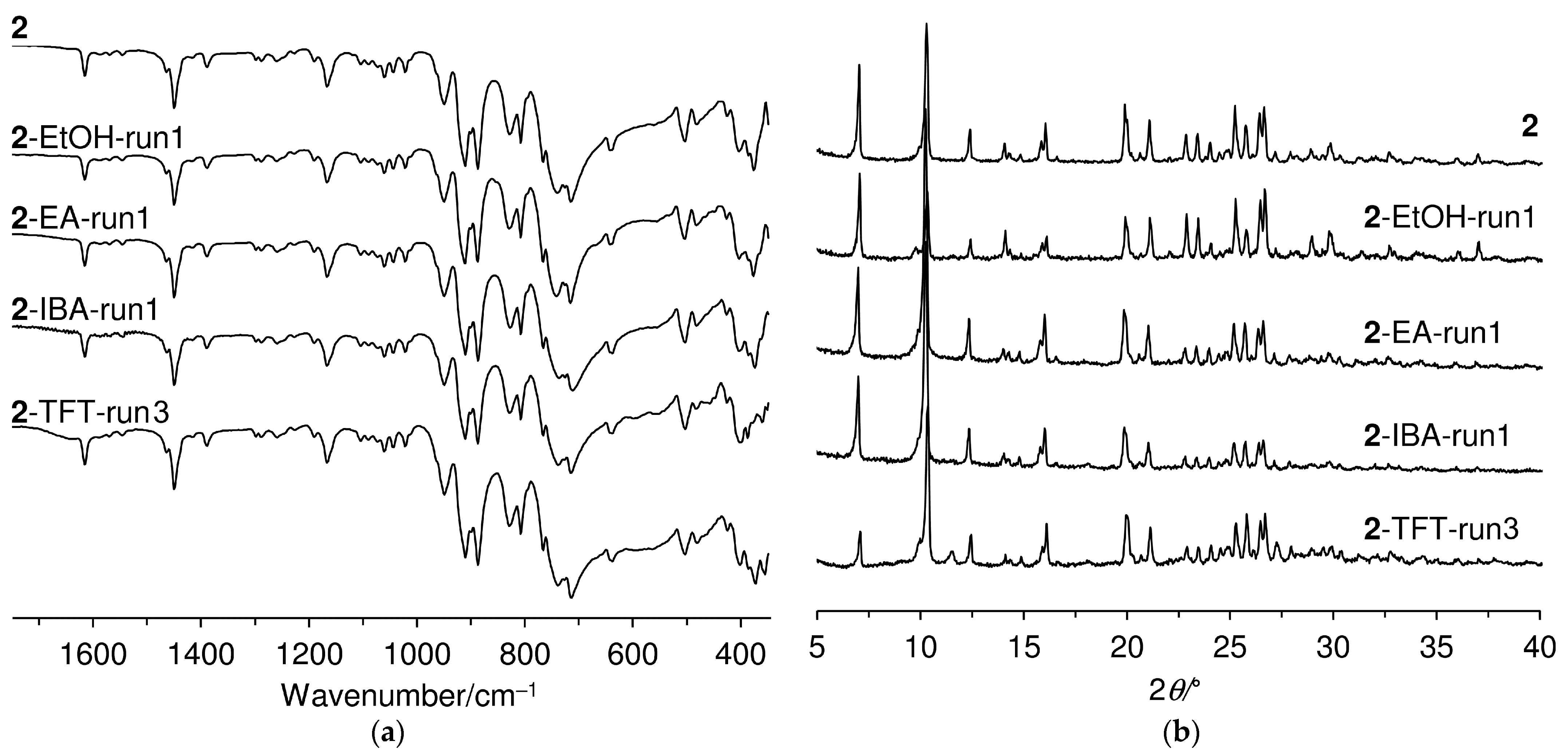
2.1. Catalyst Synthesis and Characterization
2.2. Catalytic Studies
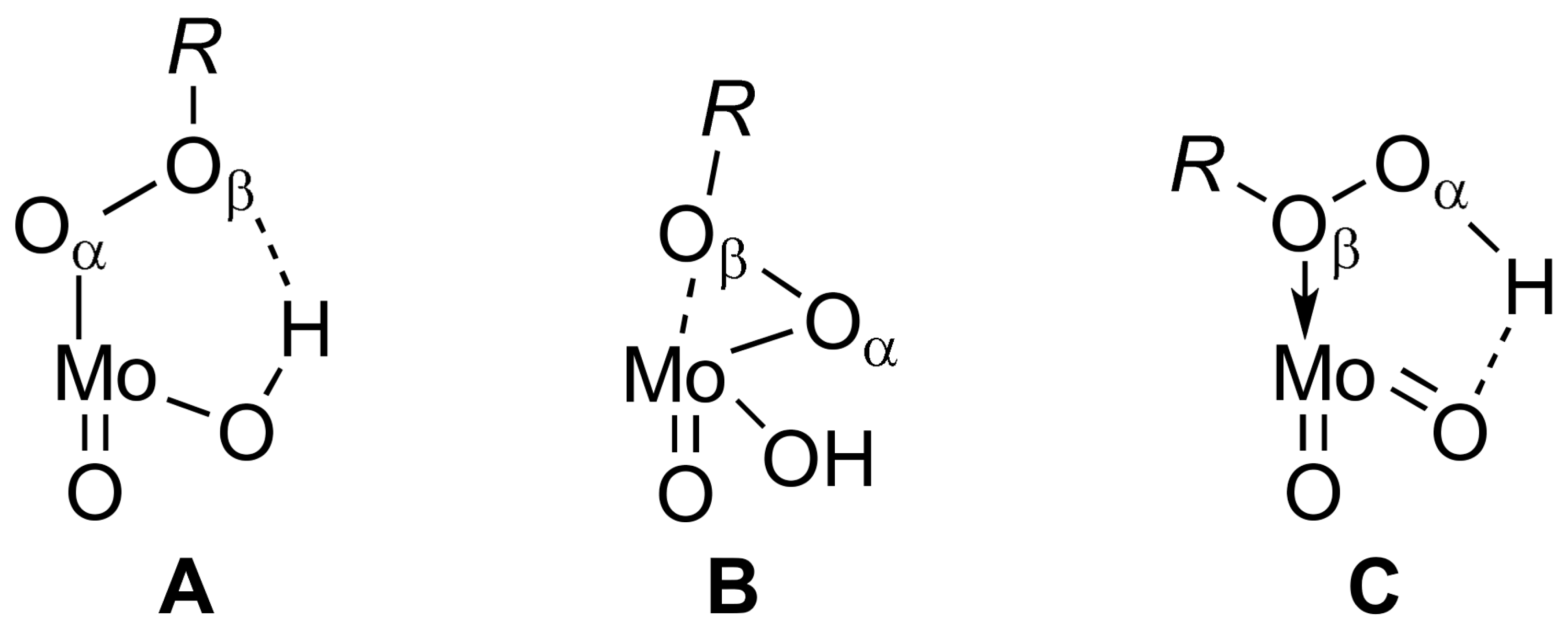
2.2.1. Model Reaction of cis-Cyclooctene Epoxidation
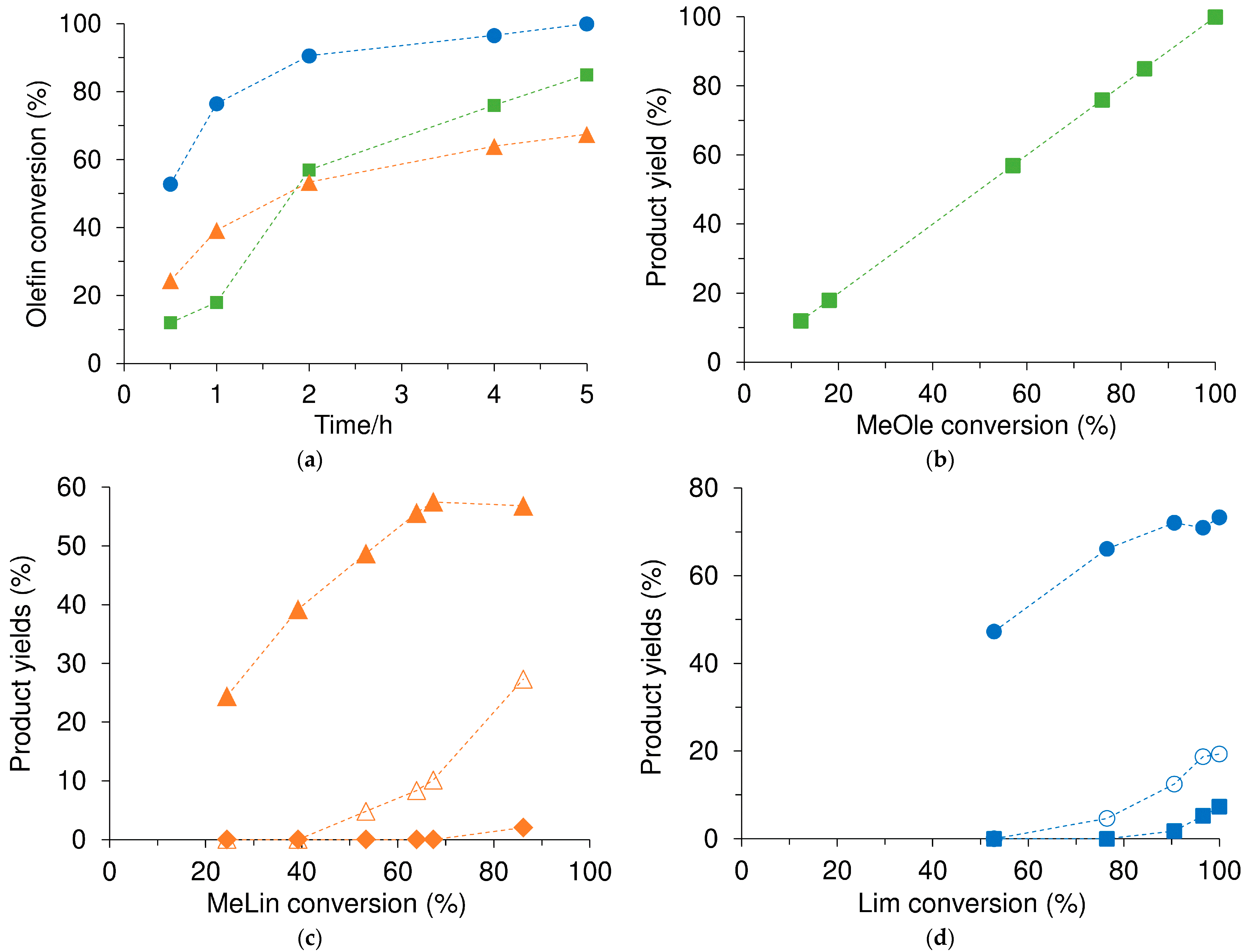
2.2.2. Epoxidation of Biomass-Derived Olefins
3. Materials and Methods
3.1. General Considerations
3.2. Synthesis of (H2pytz)[MoO2Cl2(pytz)] (1)
3.3. Synthesis of the Hybrid Material [MoO3(Hpytz)] (2)
3.3.1. Reflux Hydrolysis of 1 (Method A)
3.3.2. Autoclave Hydrolysis of 1 (Method B)
3.3.3. Direct Reaction of MoO3 with Hpytz (Method C)
3.3.4. Characterization Data for 2 (Method A)
3.4. Single-Crystal X-ray Diffraction Studies
3.5. Catalytic Tests
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Neochoritis, C.G.; Zhao, T.; Dömling, A. Tetrazoles via multicomponent reactions. Chem. Rev. 2019, 119, 1970–2042. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aromí, G.; Barrios, L.A.; Roubeau, O.; Gamez, P. Triazoles and tetrazoles: Prime ligands to generate remarkable coordination materials. Coord. Chem. Rev. 2011, 255, 485–546. [Google Scholar] [CrossRef]
- Roh, J.; Vávrová, K.; Hrabálek, A. Synthesis and functionalization of 5-substituted tetrazoles. Eur. J. Org. Chem. 2012, 6101–6118. [Google Scholar]
- Mittal, R.; Awasthi, S.K. Recent Advances in the synthesis of 5-substituted 1H-tetrazoles: A complete survey (2013–2018). Synthesis 2019, 51, 3765–3783. [Google Scholar] [CrossRef]
- Bladin, J.A. Ueber von Dicyanphenylhydrazin abgeleitete Verbindungen. Ber. Dtsch. Chem. Ges. 1885, 18, 1544–1551. [Google Scholar] [CrossRef]
- Herr, R.J. 5-Substituted-1H-tetrazoles as carboxylic acid isosteres: Medicinal chemistry and synthetic methods. Bioorg. Med. Chem. 2002, 10, 3379–3393. [Google Scholar] [CrossRef]
- Leyva-Ramos, S.; Cardoso-Ortiz, J. Recent developments in the synthesis of tetrazoles and their pharmacological relevance. Curr. Org. Chem. 2021, 25, 388–403. [Google Scholar] [CrossRef]
- Dhiman, N.; Kaur, K.; Jaitak, V. Tetrazoles as anticancer agents: A review on synthetic strategies, mechanism of action and SAR studies. Bioorg. Med. Chem. 2020, 28, 115599. [Google Scholar] [CrossRef]
- Gao, F.; Xiao, J.; Huang, G. Current scenario of tetrazole hybrids for antibacterial activity. Eur. J. Med. Chem. 2019, 184, 111744. [Google Scholar] [CrossRef]
- Wang, T.; Gao, H.; Shreeve, J.M. Functionalized tetrazole energetics: A route to enhanced performance. Z. Anorg. Allg. Chem. 2021, 647, 157–191. [Google Scholar] [CrossRef]
- Zhao, H.; Qu, Z.-R.; Ye, H.-Y.; Xiong, R.-G. In situ hydrothermal synthesis of tetrazole coordination polymers with interesting physical properties. Chem. Soc. Rev. 2008, 37, 84–100. [Google Scholar] [CrossRef] [PubMed]
- Ouellette, W.; Jones, S.; Zubieta, J. Solid state coordination chemistry of metal-1,2,4-triazolates and the related metal-4-pyridyltetrazolates. CrystEngComm 2011, 13, 4457–4485. [Google Scholar] [CrossRef]
- Rodríguez-Diéguez, A.; Mota, A.J.; Seco, J.M.; Palacios, M.A.; Romerosa, A.; Colacio, E. Influence of metal ions, coligands and reaction conditions on the structural versatility and properties of 5-pyrimidyl-tetrazolate containing complexes. Dalton Trans. 2009, 9578–9586. [Google Scholar] [CrossRef] [PubMed]
- Yang, E.-C.; Feng, Y.; Liu, Z.-Y.; Liu, T.-Y.; Zhao, X.-J. Diverse mixed-ligand metal complexes with in situ generated 5-(pyrazinyl) tetrazolate chelating-bridging ligand: In situ synthesis, crystal structures, magnetic and luminescent properties. CrystEngComm 2011, 13, 230–242. [Google Scholar] [CrossRef]
- Gaponik, P.N.; Voitekhovich, S.V.; Ivashkevich, O.A. Metal derivatives of tetrazoles. Russ. Chem. Rev. 2006, 75, 507–539. [Google Scholar] [CrossRef]
- Liu, M.-g.; Zhang, P.-p.; Peng, J.; Meng, H.-x.; Wang, X.; Zhu, M.; Wang, D.-d.; Meng, C.-l.; Alimaje, K. Organic−inorganic hybrids constructed from mixed-valence multinuclear copper complexes and templated by Keggin polyoxometalates. Cryst. Growth Des. 2012, 12, 1273–1281. [Google Scholar] [CrossRef]
- Wu, X.-Y.; Zhang, Q.-K.; Kuang, X.-F.; Yang, W.-B.; Yu, R.-M.; Lu, C.-Z. Two hybrid polyoxometalate-pillared metal–organic frameworks. Dalton Trans. 2012, 41, 11783–11787. [Google Scholar] [CrossRef] [PubMed]
- Darling, K.; Ouellette, W.; Prosvirin, A.; Freund, S.; Dunbar, K.R.; Zubieta, J. Solid state coordination chemistry of the copper(II)/pyridyl- and pyrazine-tetrazolate/sulfate system. Cryst. Growth Des. 2012, 12, 2662–2672. [Google Scholar] [CrossRef]
- Lin, P.; Clegg, W.; Harrington, R.W.; Henderson, R.A. Synthesis and structures of 5-(pyridyl) tetrazole complexes of Mn(II). Dalton Trans. 2005, 2388–2394. [Google Scholar] [CrossRef]
- Dong, P.; Zhang, Q.-K.; Wang, F.; Chen, S.-C.; Wu, X.-Y.; Zhao, Z.-G.; Lu, C.-Z. A new molybdenum-oxide-based organic-inorganic hybrid compound templated by 5-(2-pyridyl) tetrazole with new topology and canted antiferromagnetism. Cryst. Growth Des. 2010, 10, 3218–3221. [Google Scholar] [CrossRef]
- Mo, X.-J.; Gao, E.-Q.; He, Z.; Li, W.-J.; Yan, C.-H. An unexpected in situ formation of a substitute tetrazole ligand and its Mn (II) and Cu(II) complexes. Inorg. Chem. Commun. 2004, 7, 353–355. [Google Scholar] [CrossRef]
- Fiorini, V.; Ranieri, A.M.; Muzzioli, S.; Magee, K.D.M.; Zacchini, S.; Akabar, N.; Stefan, A.; Ogden, M.I.; Massi, M.; Stagni, S. Targeting divalent metal cations with Re(I) tetrazolato complexes. Dalton Trans. 2015, 44, 20597–20608. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, M.; Ma, S.-T.; Guo, L.-Q.; Fang, S.-M. Diaquabis [5-(2-pyridyl) tetrazolato-K2N1,N5] iron(II). Acta Crystallogr. Sect. E Struct. Rep. Online 2009, 65, m382. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghosh, K.; Banerjee, A.; Bauzá, A.; Frontera, A.; Chattopadhyay, S. One pot synthesis of two cobalt(III) schiff base complexes with chelating pyridyltetrazolate and exploration of their bio-relevant catalytic activities. RSC Adv. 2018, 8, 28216–28237. [Google Scholar] [CrossRef] [Green Version]
- Nasani, R.; Saha, M.; Mobin, S.M.; Mukhopadhyay, S. Microwave synthesis of mono- and bis-tetrazolato complexes via 1,3-dipolar cycloaddition of organonitriles with nickel(II)-bound azides: Isolation of 5-substituted tetrazoles from parent complex. Polyhedron 2013, 55, 24–36. [Google Scholar] [CrossRef]
- Das, M.; Chatterjee, S.; Harms, K.; Mondal, T.K.; Chattopadhyay, S. Formation of bis (μ-tetrazolato) dinickel(II) complexes with N,N,O-donor Schiff bases via in situ 1,3-dipolar cyclo-additions: Isolation of a novel bi-cyclic trinuclear nickel(II)–sodium(I)–nickel(II) complex. Dalton Trans. 2014, 2936–2947. [Google Scholar] [CrossRef] [PubMed]
- Mukhopadhyay, B.G.; Mukhopadhyay, S.; Guedes da Silva, M.F.C.; Charmier, M.A.J.; Pombeiro, A.J.L. Synthesis of mono- and bis-tetrazolato Complexes of Ni(II), Pt(II) and Cu(II) via 1,3-dipolar cycloadditions of 2-cyanopyridines with metal ligated azides in N,N,O-aminoiminophenolato complexes. Dalton Trans. 2009, 4778–4785. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.; Zhao, H.; Zhao, J. Unprecedented preparation of a unique 2D multinuclear copper metal-tetrazole polymer by in situ solvothermal reaction: Crystal structure and magnetic property. Inorg. Chem. Commun. 2013, 31, 1–4. [Google Scholar] [CrossRef]
- Wang, L.-Z.; Qu, Z.-R.; Zhao, H.; Wang, X.-S.; Xiong, R.-G.; Xue, Z.-L. Isolation and crystallographic characterization of a solid precipitate/intermediate in the preparation of 5-substituted 1H-tetrazoles from nitrile in water. Inorg. Chem. 2003, 42, 3969–3971. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L. Bis [μ-5-(2-pyridyl) tetrazolato]-K3N1,N5:N2;K3N2:N1,N5-bis [triaquazinc(II)] bis (trifluoroacetate) monohydrate. Acta Crystallogr. Sect. E Struct. Rep. Online 2009, 65, m871–m872. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.-Y.; Zhang, R.; Li, S.-L.; Yu, S.-H.; Gao, L.; Yan, W.-F.; Jin, J.; Luo, Y.-N. A new silver-organic coordination polymer: Synthesis, crystal structure, fluorescence and antibacterial activity. Inorg. Chem. Commun. 2020, 116, 107897. [Google Scholar] [CrossRef]
- Darling, K.; Ouellette, W.; Pellizzeri, S.; Smith, T.; Vargas, J.; Tomaszfski, S.; O’Connor, C.J.; Zubieta, J. One- and two-dimensional coordination polymers of substituted tetrazoles with cadmium(II). Inorg. Chim. Acta 2012, 392, 417–427. [Google Scholar] [CrossRef]
- Bhaumik, P.K.; Roy, S.; Harms, K.; Chattopadhyay, S. Formation of novel cadmium(II) tetrazolato complexes with Schiff bases as co-ligands via in situ [3+2] cyclo-addition. Polyhedron 2014, 81, 168–179. [Google Scholar] [CrossRef]
- Mrowiec, A.; Jurowska, A.; Hodorowicz, M.; Szklarzewicz, J. 5-(2-pyridil)-1H-tetrazole complexes with Mo(IV) and W(IV) cyanides. Dalton Trans. 2017, 46, 4030–4037. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.-X.; Chen, L.-J.; Lin, S.; Chen, X.-H.; Huang, H. Inorganic–organic hybrid compounds based on molybdenum oxide chains and tetrazolate-bridged polymeric silver cations. Dalton Trans. 2011, 40, 1866–1872. [Google Scholar] [CrossRef]
- Wu, X.-Y.; Dong, P.; Yu, R.; Zhang, Q.-K.; Kuang, X.; Chen, S.-C.; Lin, Q.-P.; Lu, C.-Z. A 2D polyoxometalate-based complex: Spin-canting and metamagnetism. CrystEngComm 2011, 13, 3686–3688. [Google Scholar] [CrossRef]
- Wang, X.; Li, T.; Tian, A.; Zhang, R. The rigid isomeric 5-(x-pyridyl)-1H-tetrazole ligands-directed various isopolymolybdate-based compounds: Assembly, structures, and properties. J. Coord. Chem. 2016, 69, 1–11. [Google Scholar] [CrossRef]
- Wang, X.-L.; Li, T.-J.; Tian, A.-X.; Li, N.; Yang, Y.; Ning, Y.-L.; Hou, X. Influence of N-donor sites in 5-(x-pyridyl)-1H-tetrazole ligands (x = 2, 4) on assembly of polyoxometalate-based compounds modified by multinuclear metal clusters and infinite chains. CrystEngComm 2015, 17, 3257–3267. [Google Scholar] [CrossRef]
- Xue, C.-M.; Li, S.-X.; Zhang, L.; Sha, J.-Q.; Zheng, T.-Y.; Zhang, Q.-N.; Li, L. Hydrothermal synthesis, characterization and electrocatalytic/photocatalytic activities of new polyoxometalate based hybrid compound. J. Inorg. Organomet. Polym. 2013, 23, 1468–1476. [Google Scholar] [CrossRef]
- Neves, P.; Amarante, T.R.; Gomes, A.C.; Coelho, A.C.; Gago, S.; Pillinger, M.; Gonçalves, I.S.; Silva, C.M.; Valente, A.A. Heterogeneous oxidation catalysts formed in situ from molybdenum tetracarbonyl complexes and tert-butyl hydroperoxide. Appl. Catal. A: Gen. 2011, 395, 71–77. [Google Scholar] [CrossRef]
- Amarante, T.R.; Neves, P.; Gomes, A.C.; Nolasco, M.; Ribeiro-Claro, P.; Coelho, A.C.; Valente, A.A.; Paz, F.A.A.; Smeets, S.; McCusker, L.B.; et al. Synthesis, structural elucidation, and catalytic properties in olefin epoxidation of the polymeric hybrid material [Mo3O9(2-[3(5)-pyrazolyl]pyridine)]n. Inorg. Chem. 2014, 53, 2652–2665. [Google Scholar] [CrossRef] [PubMed]
- Coelho, A.C.; Nolasco, M.; Balula, S.S.; Antunes, M.M.; Pereira, C.C.L.; Paz, F.A.A.; Valente, A.A.; Pillinger, M.; Ribeiro-Claro, P.; Klinowski, J.; et al. Chemistry and catalytic activity of molybdenum(VI)-pyrazolylpyridine complexes in olefin epoxidation. Crystal structures of monomeric dioxo, dioxo-μ-oxo, and oxodiperoxo derivatives. Inorg. Chem. 2011, 50, 525–538. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Liu, Y.; Wei, P.; Hu, B.; Zhang, X. Tetra-μ-oxido-tetrakis {dioxide [3-(2-pyridyl)-1H-pyrazole] molybdenum(VI)}. Acta Crystallogr. Sect. E Struct. Rep. Online 2009, 65, m1074. [Google Scholar]
- Groom, C.R.; Bruno, I.J.; Lightfoot, M.P.; Ward, S.C. The Cambridge Structural Database. Acta Crystallogr. Sect. B Struct. Sci. Cryst. Eng. Mater. 2016, 72, 171–179. [Google Scholar] [CrossRef]
- Klein, A.; Krest, A.; Nitsche, S.; Stirnat, K.; Valldor, M. First homoleptic complexes of the tridentate pyridine-2,6-ditetrazolate ligand. Eur. J. Inorg. Chem. 2013, 2757–2767. [Google Scholar] [CrossRef]
- Mosalkova, A.P.; Voitekhovich, S.V.; Lyakhov, A.S.; Ivashkevich, L.S.; Gaponik, P.N.; Ivashkevich, O.A. Direct synthesis and characterization of new copper(II) and zinc(II) 5-R-tetrazolato complexes [R = Me, Ph, 4-Py] with ethylenediamine and DMSO as coligands. Z. Anorg. Allg. Chem. 2012, 638, 103–110. [Google Scholar] [CrossRef]
- Nunes, M.S.; Neves, P.; Gomes, A.C.; Cunha-Silva, L.; Valente, A.A.; Pillinger, M.; Gonçalves, I.S. A Silicododecamolybdate/pyridinium-tetrazole hybrid molecular salt as a catalyst for the epoxidation of bio-derived olefins. Inorg. Chim. Acta 2021, 516, 120129. [Google Scholar] [CrossRef]
- Paciorek, P.; Szklarzewicz, J.; Nitek, W. A simple and safe method for the preparation of bis [2-(2H-tetrazol-5-yl)-pyridinium] tetrachloridozincate(II). Acta Crystallogr. Sect. C Cryst. Struct. Commun. 2013, 69, 513–516. [Google Scholar] [CrossRef]
- Demko, Z.P.; Sharpless, K.B. Preparation of 5-substituted 1H-tetrazoles from nitriles in water. J. Org. Chem. 2001, 66, 7945–7950. [Google Scholar] [CrossRef]
- Andrews, P.C.; Beck, T.; Fraser, B.H.; Junk, P.C.; Massi, M. Synthesis and structural characterisation of cationic, neutral and hydroxo-bridged lanthanoid (La, Gd, Ho, Yb, Y) bis 5-(2-pyridyl)tetrazolate complexes. Polyhedron 2007, 26, 5406–5413. [Google Scholar] [CrossRef]
- Stagni, S.; Orselli, E.; Palazzi, A.; Cola, L.D.; Zacchini, S.; Femoni, C.; Marcaccio, M.; Paolucci, F.; Zanarini, S. Polypyridyl ruthenium(II) complexes with tetrazolate-based chelating ligands. Synthesis, reactivity, and electrochemical and photophysical properties. Inorg. Chem. 2007, 46, 9126–9138. [Google Scholar] [CrossRef]
- Guan, Y.; Sowndarya, S.V.S.; Gallegos, L.C.; St. John, P.C.; Paton, R.S. Real-time prediction of 1H and 13C chemical shifts with DFT accuracy using a 3D graph neural network. Chem. Sci. 2021, 12, 12012–12026. [Google Scholar] [CrossRef] [PubMed]
- Andrews, P.C.; Junk, P.C.; Massi, M.; Silberstein, M. Gelation of La(III) cations promoted by 5-(2-pyridyl) tetrazolate and water. Chem. Commun. 2006, 3317–3319. [Google Scholar] [CrossRef]
- Abrantes, M.; Amarante, T.R.; Antunes, M.M.; Gago, S.; Paz, F.A.A.; Margiolaki, I.; Rodrigues, A.E.; Pillinger, M.; Valente, A.A.; Gonçalves, I.S. Synthesis, structure, and catalytic performance in cyclooctene epoxidation of a molybdenum oxide/bipyridine hybrid material: {[MoO3(bipy)][MoO3(H2O)]}n. Inorg. Chem. 2010, 49, 6865–6873. [Google Scholar] [CrossRef] [PubMed]
- Amarante, T.R.; Neves, P.; Tomé, C.; Abrantes, M.; Valente, A.A.; Paz, F.A.A.; Pillinger, M.; Gonçalves, I.S. An octanuclear molybdenum(VI) complex containing coordinatively bound 4,4′-di-tert-butyl-2,2′-bipyridine, [Mo8O22(OH)4(di-tBu-bipy)4]: Synthesis, structure, and catalytic epoxidation of bio-derived olefins. Inorg. Chem. 2012, 51, 3666–3676. [Google Scholar] [CrossRef]
- Figueiredo, S.; Gomes, A.C.; Neves, P.; Amarante, T.R.; Paz, F.A.A.; Soares, R.; Lopes, A.D.; Valente, A.A.; Pillinger, M.; Gonçalves, I.S. Synthesis, structural elucidation, and application of a pyrazolylpyridine–molybdenum oxide composite as a heterogeneous catalyst for olefin epoxidation. Inorg. Chem. 2012, 51, 8629–8635. [Google Scholar] [CrossRef] [PubMed]
- Amarante, T.R.; Neves, P.; Paz, F.A.A.; Valente, A.A.; Pillinger, M.; Gonçalves, I.S. Investigation of a dichlorodioxomolybdenum(VI)-pyrazolylpyridine complex and a hybrid derivative as catalysts in olefin epoxidation. Dalton Trans. 2014, 43, 6059–6069. [Google Scholar] [CrossRef]
- Neves, P.; Nogueira, L.S.; Gomes, A.C.; Oliveira, T.S.M.; Lopes, A.D.; Valente, A.A.; Gonçalves, I.S.; Pillinger, M. Chemistry and catalytic performance of pyridyl-benzimidazole oxidomolybdenum(VI) compounds in (bio)olefin epoxidation. Eur. J. Inorg. Chem. 2017, 2617–2627. [Google Scholar] [CrossRef]
- Wright, P.J.; Muzzioli, S.; Skelton, B.W.; Raiteri, P.; Lee, J.; Koutsantonis, G.; Silvester, D.S.; Stagni, S.; Massi, M. One-step assembly of Re(I) tricarbonyl 2-pyridyltetrazolato metallacalix[3]arene with aqua emission and reversible three-electron oxidation. Dalton Trans. 2013, 42, 8188–8191. [Google Scholar] [CrossRef] [Green Version]
- Morlot, J.; Uyttebroeck, N.; Agustin, D.; Poli, R. Solvent-free epoxidation of olefins catalyzed by “[MoO2(SAP)]”: A new mode of tert-butylhydroperoxide activation. ChemCatChem 2013, 5, 601–611. [Google Scholar] [CrossRef]
- Comas-Vives, A.; Lledós, A.; Poli, R. A Computational study of the olefin epoxidation mechanism catalyzed by cyclopentadienyloxidomolybdenum(VI) complexes. Chem. Eur. J. 2010, 16, 2147–2158. [Google Scholar] [CrossRef] [PubMed]
- Thiel, W.R.; Priermeier, T. The first olefin-substituted peroxomolybdenum complex: Insight into a new mechanism for the Molybdenum-catalyzed epoxidation of olefins. Angew. Chem. Int. Ed. Engl. 1995, 34, 1737–1738. [Google Scholar] [CrossRef]
- Thiel, W.R.; Eppinger, J. Molybdenum-catalyzed olefin epoxidation: Ligand effects. Chem. Eur. J. 1997, 3, 696–705. [Google Scholar] [CrossRef]
- Calhorda, M.J.; Costa, P.J. Unveiling the mechanisms of catalytic oxidation reactions mediated by oxo-molybdenum complexes: A computational overview. Curr. Org. Chem. 2012, 16, 65–72. [Google Scholar] [CrossRef]
- Veiros, L.F.; Prazeres, Â.; Costa, P.J.; Romão, C.C.; Kühn, F.E.; Calhorda, M.J. Olefin epoxidation with tert-butyl hydroperoxide catalyzed by MoO2X2L complexes: A DFT mechanistic study. Dalton Trans. 2006, 1383–1389. [Google Scholar] [CrossRef] [PubMed]
- Costa, P.J.; Calhorda, M.J.; Kühn, F.E. Olefin epoxidation catalyzed by η5-cyclopentadienyl molybdenum compounds: A computational study. Organometallics 2010, 29, 303–311. [Google Scholar] [CrossRef]
- Kühn, F.E.; Groarke, M.; Bencze, É.; Herdtweck, E.; Prazeres, A.; Santos, A.M.; Calhorda, M.J.; Romão, C.C.; Gonçalves, I.S.; Lopes, A.D.; et al. Octahedral bipyridine and bipyrimidine dioxomolybdenum(VI) complexes: Characterization, application in catalytic epoxidation, and density functional mechanistic study. Chem. Eur. J. 2002, 8, 2370–2383. [Google Scholar] [CrossRef]
- Chniti, S.; Hassouna, M. Ethanol fuel from biomass: A review. Eur. J. Chem. Environ. Eng. Sci. 2017, 1, 1–7. [Google Scholar]
- Dielectric Constant. Available online: https://www.flowline.com/dielectric-constant (accessed on 5 July 2021).
- Lysenko, A.B.; Senchyk, G.A.; Domasevitch, K.V.; Hauser, J.; Fuhrmann, D.; Kobalz, M.; Krautscheid, H.; Neves, P.; Valente, A.A.; Gonçalves, I.S. Synthesis and structural elucidation of triazolylmolybdenum(VI) oxide hybrids and their behavior as oxidation catalysts. Inorg. Chem. 2015, 54, 8327–8338. [Google Scholar] [CrossRef] [PubMed]
- Amarante, T.R.; Neves, P.; Paz, F.A.A.; Gomes, A.C.; Pillinger, M.; Valente, A.A.; Gonçalves, I.S. Heterogeneous catalysis with an organic–inorganic hybrid based on MoO3 chains decorated with 2,2′-biimidazole ligands. Catal. Sci. Technol. 2021, 11, 2214–2228. [Google Scholar] [CrossRef]
- Asgharpour, Z.; Farzaneh, F.; Abbasi, A.; Ghiasi, M. Synthesis, crystal structure and DFT studies of a new dioxomolybdenum(VI) Schiff base complex as an olefin epoxidation catalyst. Polyhedron 2015, 101, 282–289. [Google Scholar] [CrossRef]
- Rayati, S.; Abdolalian, P. Sonochemical syntheses of nano-sized dioxomolybdenum complexes: An efficient, selective and reusable heterogeneous nanocatalyst for oxidation of alkenes. Appl. Catal. A Gen. 2013, 456, 240–248. [Google Scholar] [CrossRef]
- Bagherzadeh, M.; Zare, M. Synthesis, characterization, and catalysis of recyclable new piperazine-bridged Mo(VI) polymers [MoO2 (Salen) (piperazine)]n in highly selective oxygenation of alkenes and sulfides. J. Coord. Chem. 2013, 66, 2885–2900. [Google Scholar] [CrossRef]
- Du, J.; Yu, J.; Tang, J.; Wang, J.; Zhang, W.; Thiel, W.R.; Jia, M. Supramolecular assemblies directed by hydrogen bonds and π–π interactions and based on N-heterocyclic-ligand-modified β-octamolybdate—Structure and catalytic application in olefin epoxidation. Eur. J. Inorg. Chem. 2011, 2361–2365. [Google Scholar] [CrossRef]
- Abdalghani, I.; Biancalana, L.; Aschi, M.; Pampaloni, G.; Marchetti, F.; Crucianelli, M. Dioxomolybdenum(VI) compounds with α-amino acid donor ligands as catalytic precursors for the selective oxyfunctionalization of olefins. Mol. Catal. 2018, 446, 39–48. [Google Scholar] [CrossRef] [Green Version]
- Hosseinyzade, S.S.; Zonoz, F.M.; Bahramian, B. Synthesis, characterization, and application of a new nanohybrid Schiff base polyoxometalate in epoxidation of olefins in the presence of tert-butyl hydroperoxide. J. Coord. Chem. 2020, 73, 243–254. [Google Scholar] [CrossRef]
- Armylisas, A.H.N.; Hazirah, M.F.S.; Yeong, S.K.; Hazimah, A.H. Modification of olefinic double bonds of unsaturated fatty acids and other vegetable oil derivatives via epoxidation: A review. Grasas Y Aceites 2017, 68, e174. [Google Scholar] [CrossRef] [Green Version]
- Corma, A.; Iborra, S.; Velty, A. Chemical routes for the transformation of biomass into chemicals. Chem. Rev. 2007, 107, 2411–2502. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, M.S.; Mendonça, M.A.; Pinho, D.M.M.; Resck, I.S.; Suarez, P.A.Z. Chromatographic analyses of fatty acid methyl esters by HPLC-UV and GC-FID. J. Braz. Chem. Soc. 2012, 23, 763–769. [Google Scholar] [CrossRef]
- Nameer, S.; Deltin, T.; Sundell, P.-E.; Johansson, M. Bio-based multifunctional fatty acid methyl esters as reactive diluents in coil coatings. Prog. Org. Coatings 2019, 136, 105277. [Google Scholar] [CrossRef]
- Lv, N.; He, W.; Fang, Z.; Sun, Q.; Qiu, C.; Guo, K. Epoxidation of methyl oleate and subsequent ring-opening catalyzed by lipase from candida sp. 99–125. Eur. J. Lipid Sci. Technol. 2018, 120, 1700257. [Google Scholar] [CrossRef]
- Wang, J.; Zhao, X.; Liu, D. Preparation of epoxidized fatty acid methyl ester with in situ auto-catalyzed generation of performic acid and the influence of impurities on epoxidation. Waste Biomass Valor. 2018, 9, 1881–1891. [Google Scholar] [CrossRef]
- Doll, K.M.; Erhan, S.Z. Synthesis and performance of surfactants based on epoxidized methyl oleate and glycerol. J. Surfact. Deterg. 2006, 9, 377–383. [Google Scholar] [CrossRef]
- Tang, J.; Zhang, J.; Lu, J.; Huang, J.; Zhang, F.; Hu, Y.; Liu, C.; An, R.; Miao, H.; Chen, Y.; et al. Preparation and properties of plant-oil-based epoxy acrylate-like resins for UV-curable coatings. Polymers 2012, 12, 2165. [Google Scholar] [CrossRef] [PubMed]
- Borugadda, V.B.; Goud, V.V. Epoxidation of castor oil fatty acid methyl esters (COFAME) as a lubricant base stock using heterogeneous ion-exchange resin (IR-120) as a catalyst. Energy Procedia 2014, 54, 75–84. [Google Scholar] [CrossRef] [Green Version]
- Hölderich, W.F.; Rios, L.A.; Weckes, P.P. Investigations into the epoxidation and alcoholysis of oleochemicals for use as lubricants. J. Synth. Lubr. 2004, 20, 289–301. [Google Scholar] [CrossRef]
- Lligadas, G.; Ronda, J.C.; Galià, M.; Biermann, U.; Metzger, J.O. Synthesis and characterization of polyurethanes from epoxidized methyl oleate based polyether polyols as renewable resources. J. Polym. Sci. Part A Polym. Chem. 2006, 44, 634–645. [Google Scholar] [CrossRef]
- Miao, S.; Wang, P.; Su, Z.; Zhang, S. Vegetable-oil-based polymers as future polymeric biomaterials. Acta Biomater. 2014, 10, 1692–1704. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Zhu, G.; Gao, Y.; Wu, H.; Fang, Z.; Guo, K. Green plasticizers derived from epoxidized soybean oil for poly (vinyl chloride): Continuous synthesis and evaluation in PVC films. Chem. Eng. J. 2020, 380, 122532. [Google Scholar] [CrossRef]
- Debal, A.; Rafaralahitsimba, G.; Bonfan, A.; Ucciani, E. Catalytic epoxidation of methyl linoleate—cyclisation products of the epoxyacid esters. Fat Sci. Technol. 1995, 97, 269–273. [Google Scholar] [CrossRef]
- Borhan, B.; Nourooz-Zadeh, J.; Uematsu, T.; Hammock, B.D.; Kurth, M.J. Stereochemical aspects of cytosolic epoxide hydrolase hydration of methyl diepoxystearates. Tetrahedron 1993, 49, 2601–2612. [Google Scholar] [CrossRef]
- Piazza, G.J.; Nuñez, A.; Foglia, T.A. Hydrolysis of mono- and diepoxyoctadecanoates by alumina. J. Am. Oil Chem. Soc. 2003, 80, 901–904. [Google Scholar] [CrossRef]
- Bantchev, G.B.; Doll, K.M.; Biresaw, G.; Vermillion, K.E. Formation of furan fatty alkyl esters from their bis-epoxide fatty esters. J. Am. Oil Chem. Soc. 2014, 91, 2117–2123. [Google Scholar] [CrossRef]
- Costa, V.V.; da Silva Rocha, K.A.; Kozhevnikov, I.V.; Kozhevnikova, E.F.; Gusevskaya, E.V. Heteropoly acid catalysts for the synthesis of fragrance compounds from biorenewables: Isomerization of limonene oxide. Catal. Sci. Technol. 2013, 3, 244–250. [Google Scholar] [CrossRef]
- Nguyen, T.-T.T.; Chau, D.-K.N.; Duus, F.; Le, T.N. Green synthesis of carvenone by montmorillonite-catalyzed isomerization of 1,2-limonene oxide. Int. J. Org. Chem. 2013, 3, 206–209. [Google Scholar] [CrossRef] [Green Version]
- Ferrarini, S.R.; Graebin, C.S.; Limberger, J.; Canto, R.F.S.; Dias, D.O.; da Rosa, R.G.; Madeira, M.D.F.; Eifler-lima, V.L. Synthesis of limonene β-amino alcohol derivatives in support of new antileishmanial therapies. Mem. Inst. Oswaldo Cruz 2008, 103, 773–777. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, C.; Veldhuis, T.; Reuvers, B.; Sablong, R.J.; Koning, C.E. Fully renewable limonene-derived polycarbonate as a high-performance alkyd resin. Polym. Int. 2020, 69, 24–30. [Google Scholar] [CrossRef]
- Ciriminna, R.; Lomeli-Rodrigues, M.; Lopez-Sanchez, J.A.; Pagliaro, M. Limonene: A versatile chemical of the bioeconomy. Chem. Commun. 2014, 50, 15288–15296. [Google Scholar] [CrossRef] [PubMed]
- Dipentene Dioxide. Available online: http://chemicalland21.com/specialtychem/nh/DIPENTENE%20DIOXIDE.htm (accessed on 29 October 2021).
- Li, C.; Sablong, R.J.; Koning, C.E. Chemoselective alternating copolymerization of limonene dioxide and carbon Dioxide: A new highly functional aliphatic epoxy polycarbonate. Angew. Chem. Int. Ed. 2016, 55, 11572–11576. [Google Scholar] [CrossRef] [Green Version]
- Schutz, L.; Kazemi, F.; Mackenzie, E.; Bergeron, J.-Y.; Gagnon, E.; Claverie, J.P. Trans-limonene dioxide, a promising bio-based epoxy monomer. J. Polym. Sci. 2021, 59, 321–328. [Google Scholar] [CrossRef]
- Bähr, M.; Bitto, A.; Mülhaupt, R. Cyclic limonene dicarbonate as a new monomer for non-isocyanate oligo- and polyurethanes (NIPU) based upon terpenes. Green Chem. 2012, 14, 1447–1454. [Google Scholar] [CrossRef]
- Kottke, T.; Stalke, D. Crystal handling at low temperatures. J. Appl. Crystallogr. 1993, 26, 615–619. [Google Scholar] [CrossRef] [Green Version]
- SAINT+. Data Integration Engine v. 8.37a©; Bruker AXS: Madison, WI, USA, 2015. [Google Scholar]
- Krause, L.; Herbst-Irmer, R.; Sheldrick, G.M.; Stalke, D. Comparison of silver and molybdenum microfocus X-ray sources for single-crystal structure determination. J. Appl. Crystallogr. 2015, 48, 3–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sheldrick, G.M. SHELXT—Integrated space-group and crystal-structure determination. Acta Crystallogr. Sect. A Found. Adv. 2015, 71, 3–8. [Google Scholar] [CrossRef] [Green Version]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Hübschle, C.B.; Sheldrick, G.M.; Dittrich, B. ShelXle: A Qt graphical user interface for SHELXL. J. Appl. Crystallogr. 2011, 44, 1281–1284. [Google Scholar] [CrossRef] [Green Version]
- Brandenburg, K. Diamond, Version 3.2f; Crystal Impact GbR: Bonn, Germany, 2010. [Google Scholar]







| Entry | Catalyst a | Reaction Conditions b | Conv. (%) c | Sel. (%) d | Ref. | ||
|---|---|---|---|---|---|---|---|
| Solv./T (°C) | Mo:Cy8:TBHP | t/h | |||||
| 1 | 2 | TFT/70 | 1:100:152 | 5/24 | c1-89/100 | c1-100/100 | This work |
| c2-87/100 | c2-100/100 | ||||||
| c3-85/100 | c3-100/100 | ||||||
| 2 | EtOH/70 | 1:100:152 | 5/24 | 85/100 | 100/100 | ||
| 3 | [Mo4O12(pypz)4] | -/55 | 1:100:152 | 6/24 | c1-55/92 | c1-100/100 | [40] |
| c2-57/89 | c2-100/100 | ||||||
| 4 | [Mo8O24(pypzEA)4] | -/55 | 1:100:152 | 6/24 | c1-89/100 | c1-100/100 | [40] |
| c2-91/100 | c2-100/100 | ||||||
| 5 | [Mo2O6(HpypzA)] | decane/55 | 1:100:152 | 24 | 26 | 100 | [56] |
| 6 | hexane/55 | 1:100:152 | 24 | 24 | 100 | ||
| 7 | DCE/55 | 1:100:152 | 24 | 74 | 100 | ||
| 8 | {[MoO3(bpy)][MoO3(H2O)]}n | hexane/55 | 1:50:76 | 24/48 | c1-22/34 | c1-100/100 | [54] |
| c2-~38/~50 | c2-100/100 | ||||||
| c3-~30/~45 | c3-100/100 | ||||||
| c4-~30/~45 | c4-100/100 | ||||||
| 9 | hexane/75 | 1:50:76 | 6 | 45 | 100 | ||
| 10 | [MoO3(biim)] | TFT/70 | 1:100:153 | 6/24 | c1-83/99 | c1-100/100 | [71] |
| c2-87/- | c2-100/- | ||||||
| c3-99/- | c3-100/- | ||||||
| 11 | [MoO2(Naph-His)] | CCl4/reflux | 1:174:244 | 8 | c1-100 | c1-100 | [72] |
| c2-100 | c2-100 | ||||||
| c3-100 | c3-100 | ||||||
| c5-98 | c5-100 | ||||||
| 12 | MoL1-nano, MoL2-nano | CHCl3/45 | - e | 1 | MoL1-nano: | MoL1-nano: | [73] |
| c1-100 | c1-100 | ||||||
| c2-100 | c2-100 | ||||||
| c3-97 | c3-100 | ||||||
| c4-94 | c4-100 | ||||||
| c5-93 | c5-100 | ||||||
| MoL2-nano: | MoL2-nano: | ||||||
| c1-100 | c1-100 | ||||||
| c2-100 | c2-100 | ||||||
| c3-99 | c3-100 | ||||||
| c4-98 | c4-100 | ||||||
| c5-97 | c5-100 | ||||||
| 13 | [piperazinCH2{MoO2(Salen)}] | DCE/75 | 1:200:200 | c1-12 | c1-95 | c1-98 | [74] |
| c2-7 | c2-98 | c2-98 | |||||
| c3-5 | c3-99 | c3-98 | |||||
| c4-4 | c4-97 | c4-98 | |||||
| c5-4 | c5-98 | c5-98 | |||||
| 14 | [piperazinCH2{MoO2(Salophen)}] | DCE/75 | 1:200:200 | 12 | 89 | 97 | |
| 15 | [piperazinCH2{MoO2(Salpn)}] | DCE/75 | 1:200:200 | 12 | 93 | 97 | |
| 16 | (Himi)4[(imi)2(Mo8O26)]·H2O | DCE/35 | 1:21:21 | 12 | c1-97 | c1-100 | [75] |
| c2~97 | c2-100 | ||||||
| c3~97 | c3-100 | ||||||
| c4~97 | c4-100 | ||||||
| c5~97 | c5-100 | ||||||
| 17 | [Mo2O4(OH)4(AA)] | DCE/50 | 1:50:100 | 1 | >99 | 100 | [76] |
| For Gly catalyst: | |||||||
| c2-96 | |||||||
| c3-96 | |||||||
| 18 | nanohybrid Schiff base POM | DCE/reflux | 1:13:29 | 0.5 | 99 | c1- ≥ 99 | [77] |
| c2 to c5-93-99 | c2- ≥ 99 | ||||||
| c3- ≥ 99 | |||||||
| c4- ≥ 99 | |||||||
| c5- ≥ 99 | |||||||
| Entry | Catalyst | Reaction Conditions a | Conv. (%) b | Sel. (%) c | Ref. | ||
|---|---|---|---|---|---|---|---|
| Olefin | Mo:olefin:TBHP | t/h | |||||
| 1 | 2 | MeOle | 1:100:210 | 5/24 | 85/100 | 100/100 | This work |
| 2 | - | MeLin | 1:100:210 | 5/24 | 67/86 | 86/66(0.2/0.5) | |
| 3 | - | Lim | 1:100:210 | 5/24 | 100/100 | 73/51(0.3/0.3) | |
| 4 | [MoO3(biim)] | MeOle | 1:100:226 | 6/24 | 72/97 | 99/97 | [71] |
| 5 | - | MeLin | 1:100:226 | 6/24 | 66/88 | 86/65(0.2/0.5) | |
| 6 | - | Lim | 1:100:226 | 6/24 | 97/100 | 80/55(0.2/0.6) | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nunes, M.S.; Gomes, D.M.; Gomes, A.C.; Neves, P.; Mendes, R.F.; Paz, F.A.A.; Lopes, A.D.; Valente, A.A.; Gonçalves, I.S.; Pillinger, M. A 5-(2-Pyridyl)tetrazolate Complex of Molybdenum(VI), Its Structure, and Transformation to a Molybdenum Oxide-Based Hybrid Heterogeneous Catalyst for the Epoxidation of Olefins. Catalysts 2021, 11, 1407. https://doi.org/10.3390/catal11111407
Nunes MS, Gomes DM, Gomes AC, Neves P, Mendes RF, Paz FAA, Lopes AD, Valente AA, Gonçalves IS, Pillinger M. A 5-(2-Pyridyl)tetrazolate Complex of Molybdenum(VI), Its Structure, and Transformation to a Molybdenum Oxide-Based Hybrid Heterogeneous Catalyst for the Epoxidation of Olefins. Catalysts. 2021; 11(11):1407. https://doi.org/10.3390/catal11111407
Chicago/Turabian StyleNunes, Martinique S., Diana M. Gomes, Ana C. Gomes, Patrícia Neves, Ricardo F. Mendes, Filipe A. Almeida Paz, André D. Lopes, Anabela A. Valente, Isabel S. Gonçalves, and Martyn Pillinger. 2021. "A 5-(2-Pyridyl)tetrazolate Complex of Molybdenum(VI), Its Structure, and Transformation to a Molybdenum Oxide-Based Hybrid Heterogeneous Catalyst for the Epoxidation of Olefins" Catalysts 11, no. 11: 1407. https://doi.org/10.3390/catal11111407
APA StyleNunes, M. S., Gomes, D. M., Gomes, A. C., Neves, P., Mendes, R. F., Paz, F. A. A., Lopes, A. D., Valente, A. A., Gonçalves, I. S., & Pillinger, M. (2021). A 5-(2-Pyridyl)tetrazolate Complex of Molybdenum(VI), Its Structure, and Transformation to a Molybdenum Oxide-Based Hybrid Heterogeneous Catalyst for the Epoxidation of Olefins. Catalysts, 11(11), 1407. https://doi.org/10.3390/catal11111407