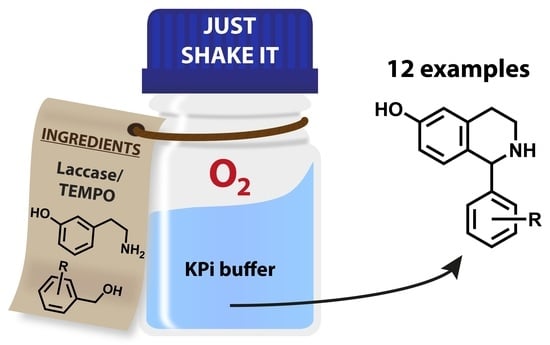
Chemoenzymatic One-Pot Process for the Synthesis of Tetrahydroisoquinolines
Abstract
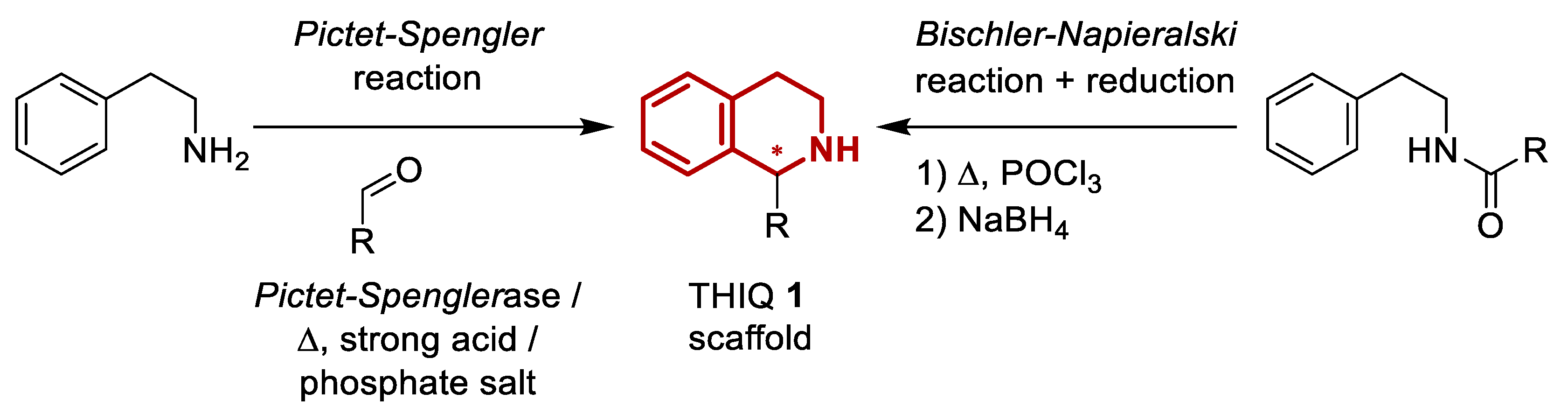
1. Introduction
2. Results and Discussion
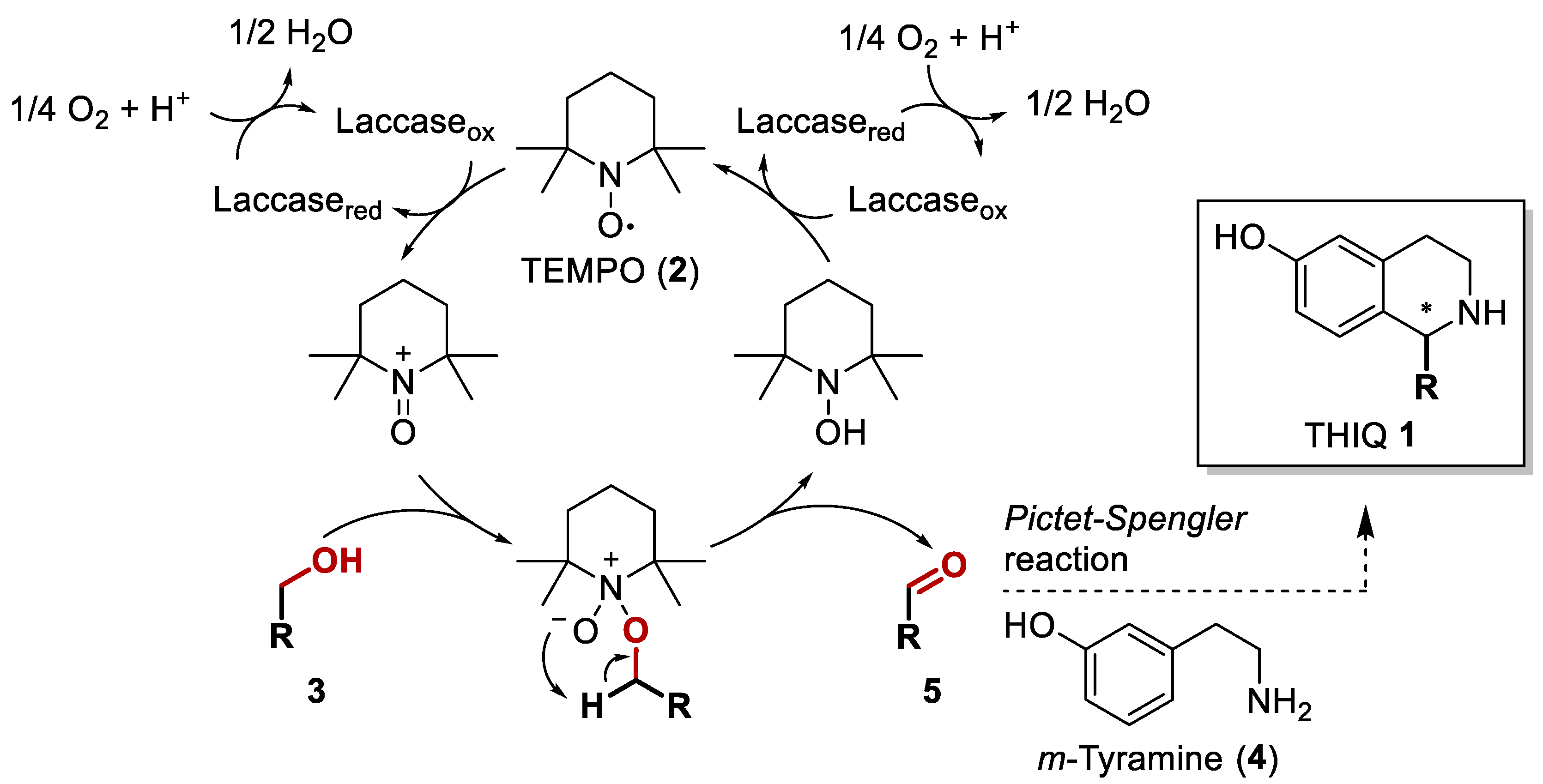
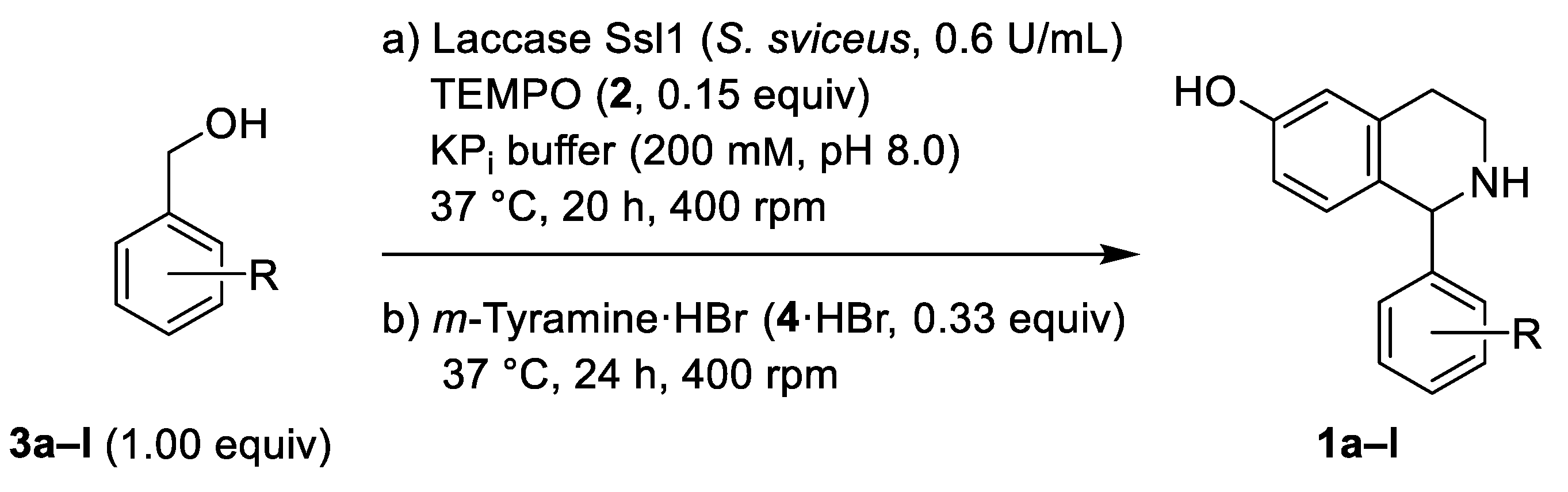
2.1. Oxidation of Benzylic Alcohols 3 via the Laccase/TEMPO (2) System
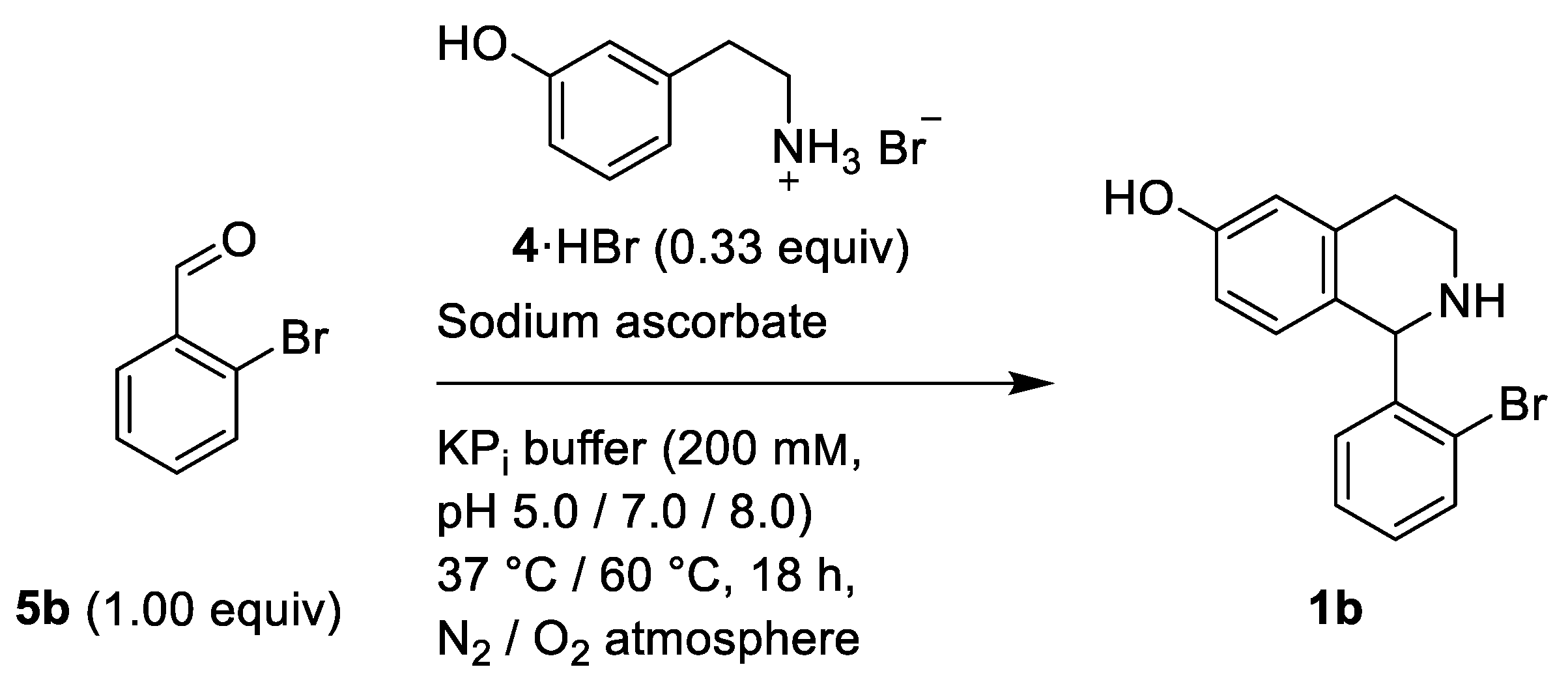
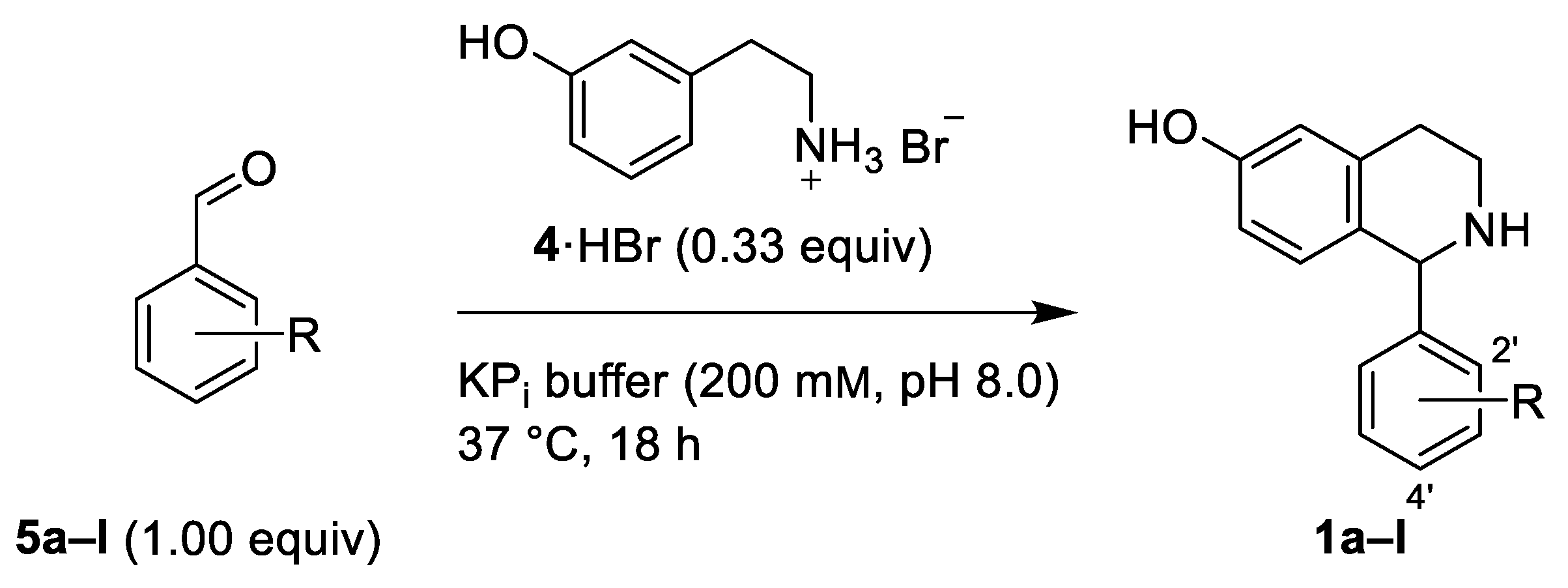
2.2. Phosphate Salt-Mediated Pictet–Spengler Reaction
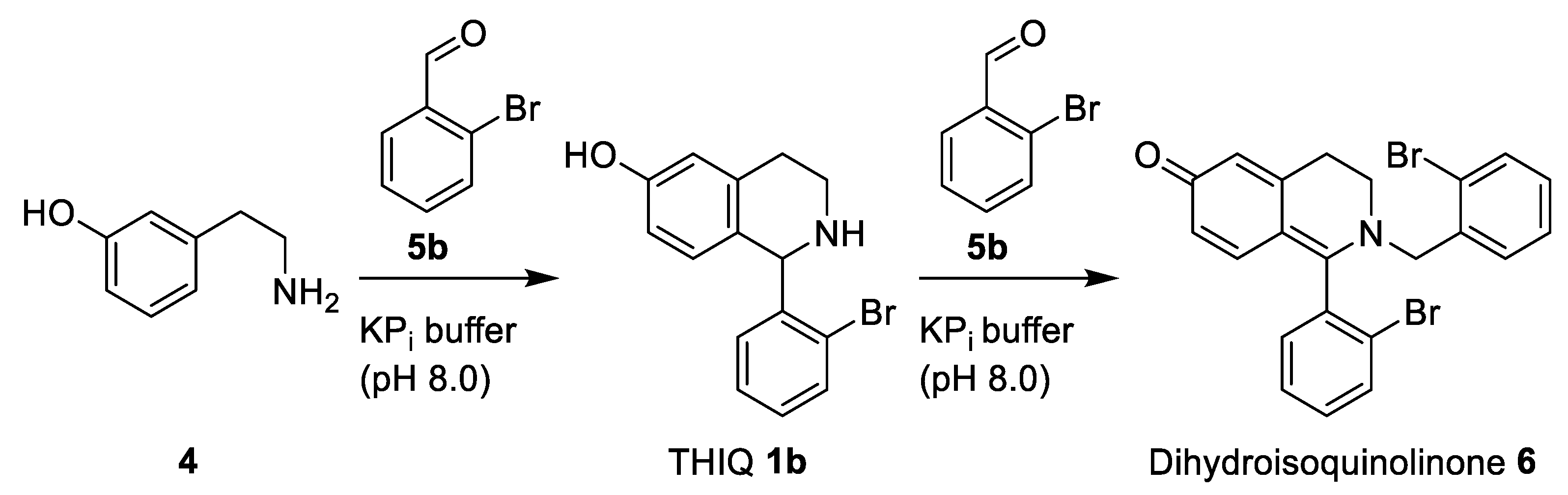
2.3. Chemoenzymatic One-Pot Process
- Laccase Ssl1 from S. sviceus (heat-treated clarified cell supernatant, prepared as a batch, aliquoted, thawed as needed).
- 1.00 equiv benzylic alcohol 3, 0.15 equiv TEMPO (2), 0.33 equiv m-tyramine hydrobromide (4·HBr).
- KPi buffer (0.2 m, pH 8).
- Glass bottle, constant shaking in incubator at 37 °C, oxygen atmosphere, no additives.
3. Materials and Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Stöckigt, J.; Antonchick, A.P.; Wu, F.; Waldmann, H. The Pictet-Spengler reaction in nature and in organic chemistry. Angew. Chem. Int. Ed. 2011, 50, 8538–8564. [Google Scholar] [CrossRef]
- Dostert, P.; Strolin Benedetti, M.; Dordain, G. Dopamine-derived alkaloids in alcoholism and in Parkinson’s and Huntington’s diseases. J. Neural Transm. 1988, 74, 61–74. [Google Scholar] [CrossRef]
- Christinat, A.; Leyvraz, S. Role of trabectedin in the treatment of soft tissue sarcoma. Onco Targets Ther. 2009, 2, 105–113. [Google Scholar] [CrossRef][Green Version]
- Truax, V.M.; Zhao, H.; Katzman, B.M.; Prosser, A.R.; Alcaraz, A.A.; Saindane, M.T.; Howard, R.B.; Culver, D.; Arrendale, R.F.; Gruddanti, P.R.; et al. Discovery of Tetrahydroisoquinoline-Based CXCR4 Antagonists. ACS Med. Chem. Lett. 2013, 4, 1025–1030. [Google Scholar] [CrossRef]
- Antkiewicz-Michaluk, L.; Wąsik, A.; Możdżeń, E.; Romańska, I.; Michaluk, J. Antidepressant-like Effect of Tetrahydroisoquinoline Amines in the Animal Model of Depressive Disorder Induced by Repeated Administration of a Low Dose of Reserpine: Behavioral and Neurochemical Studies in the Rat. Neurotox. Res. 2014, 26, 85–98. [Google Scholar] [CrossRef]
- Hanna, J.N.; Ntie-Kang, F.; Kaiser, M.; Brun, R.; Efange, S.M.N. 1-Aryl-1,2,3,4-tetrahydroisoquinolines as potential antimalarials: Synthesis, in vitro antiplasmodial activity and in silico pharmacokinetics evaluation. RSC Adv. 2014, 4, 22856–22865. [Google Scholar] [CrossRef]
- Wadworth, A.N.; Brogden, R.N. Quinapril. Drugs 1991, 41, 378–399. [Google Scholar] [CrossRef]
- DeBono, A.; Capuano, B.; Scammells, P.J. Progress Toward the Development of Noscapine and Derivatives as Anticancer Agents. J. Med. Chem. 2015, 58, 5699–5727. [Google Scholar] [CrossRef]
- Welsch, M.E.; Snyder, S.A.; Stockwell, B.R. Privileged scaffolds for library design and drug discovery. Curr. Opin. Chem. Biol. 2010, 14, 347–361. [Google Scholar] [CrossRef]
- Roddan, R.; Ward, J.; Keep, N.; Hailes, H.C. Pictet–Spenglerases in alkaloid biosynthesis: Future applications in biocatalysis. Curr. Opin. Chem. Biol. 2020, 55, 69–76. [Google Scholar] [CrossRef]
- Samanani, N.; Liscombe, D.K.; Facchini, P.J. Molecular cloning and characterization of norcoclaurine synthase, an enzyme catalyzing the first committed step in benzylisoquinoline alkaloid biosynthesis. Plant. J. 2004, 40, 302–313. [Google Scholar] [CrossRef]
- Pasquo, A.; Bonamore, A.; Franceschini, S.; Macone, A.; Boffi, A.; Ilari, A. Cloning, expression, crystallization and preliminary X-ray data analysis of norcoclaurine synthase from Thalictrum flavum. Acta Crystallogr. Sect. F Struct. Biol. Cryst. Commun. 2008, 64, 281–283. [Google Scholar] [CrossRef]
- Pesnot, T.; Gershater, M.C.; Ward, J.M.; Hailes, H.C. The Catalytic Potential of Coptis japonica NCS2 Revealed-Development and Utilisation of a Fluorescamine-Based Assay. Adv. Synth. Catal. 2012, 354, 2997–3008. [Google Scholar] [CrossRef]
- Ruff, B.M.; Bräse, S.; O’Connor, S.E. Biocatalytic production of tetrahydroisoquinolines. Tetrahedron Lett 2012, 53, 1071–1074. [Google Scholar] [CrossRef]
- Lechner, H.; Soriano, P.; Poschner, R.; Hailes, H.C.; Ward, J.M.; Kroutil, W. Library of Norcoclaurine Synthases and Their Immobilization for Biocatalytic Transformations. Biotechnol. J. 2018, 13, e1700542. [Google Scholar] [CrossRef]
- Luk, L.Y.P.; Bunn, S.; Liscombe, D.K.; Facchini, P.J.; Tanner, M.E. Mechanistic Studies on Norcoclaurine Synthase of Benzylisoquinoline Alkaloid Biosynthesis: An Enzymatic Pictet−Spengler Reaction. Biochemistry 2007, 46, 10153–10161. [Google Scholar] [CrossRef]
- Lichman, B.R.; Zhao, J.; Hailes, H.C.; Ward, J.M. Enzyme catalysed Pictet-Spengler formation of chiral 1,1′-disubstituted- and spiro-tetrahydroisoquinolines. Nat. Commun. 2017, 8, 14883. [Google Scholar] [CrossRef]
- Sheng, X.; Himo, F. Enzymatic Pictet–Spengler Reaction: Computational Study of the Mechanism and Enantioselectivity of Norcoclaurine Synthase. J. Am. Chem. Soc. 2019, 141, 11230–11238. [Google Scholar] [CrossRef]
- Lichman, B.R.; Sula, A.; Pesnot, T.; Hailes, H.C.; Ward, J.M.; Keep, N.H. Structural Evidence for the Dopamine-First Mechanism of Norcoclaurine Synthase. Biochemistry 2017, 56, 5274–5277. [Google Scholar] [CrossRef]
- Erdmann, V.; Lichman, B.R.; Zhao, J.; Simon, R.C.; Kroutil, W.; Ward, J.M.; Hailes, H.C.; Rother, D. Enzymatic and Chemoenzymatic Three-Step Cascades for the Synthesis of Stereochemically Complementary Trisubstituted Tetrahydroisoquinolines. Angew. Chem. Int. Ed. 2017, 56, 12503–12507. [Google Scholar] [CrossRef]
- Roddan, R.; Gygli, G.; Sula, A.; Méndez-Sánchez, D.; Pleiss, J.; Ward, J.M.; Keep, N.H.; Hailes, H.C. Acceptance and Kinetic Resolution of α-Methyl-Substituted Aldehydes by Norcoclaurine Synthases. ACS Catal 2019, 9, 9640–9649. [Google Scholar] [CrossRef]
- Roddan, R.; Sula, A.; Méndez-Sánchez, D.; Subrizi, F.; Lichman, B.R.; Broomfield, J.; Richter, M.; Andexer, J.N.; Ward, J.M.; Keep, N.H.; et al. Single step syntheses of (1S)-aryl-tetrahydroisoquinolines by norcoclaurine synthases. Commun. Chem. 2020, 3, 170. [Google Scholar] [CrossRef]
- Pictet, A.; Spengler, T. Über die Bildung von Isochinolin-derivaten durch Einwirkung von Methylal auf Phenyl-äthylamin, Phenyl-alanin und Tyrosin. Ber. Dtsch. Chem. Ges. 1911, 44, 2030–2036. [Google Scholar] [CrossRef]
- Nicoletti, M.; O’Hagan, D.; Slawin, A.M.Z. The asymmetric Bischler–Napieralski reaction: Preparation of 1,3,4-trisubstituted 1,2,3,4-tetrahydroisoquinolines. J. Chem. Soc. Perkin Trans. 1 2002, 116–121. [Google Scholar] [CrossRef]
- Youn, S.W. The Pictet-Spengler reaction: Efficient carbon-carbon bond forming reaction in heterocyclic synthesis. Org. Prep. Proced. Int. 2006, 38, 505–591. [Google Scholar] [CrossRef]
- Awuah, E.; Capretta, A. Strategies and Synthetic Methods Directed Toward the Preparation of Libraries of Substituted Isoquinolines. J. Org. Chem. 2010, 75, 5627–5634. [Google Scholar] [CrossRef]
- Calcaterra, A.; Mangiardi, L.; Delle Monache, G.; Quaglio, D.; Balducci, S.; Berardozzi, S.; Iazzetti, A.; Franzini, R.; Botta, B.; Ghirga, F. The Pictet-Spengler Reaction Updates Its Habits. Molecules 2020, 25, 414. [Google Scholar] [CrossRef]
- Manabe, K.; Nobutou, D.; Kobayashi, S. Catalytic Pictet–Spengler reactions using Yb(OTf)3. Bioorg. Med. Chem 2005, 13, 5154–5158. [Google Scholar] [CrossRef]
- Eynden, M.J.V.; Stambuli, J.P. Calcium-Catalyzed Pictet−Spengler Reactions. Org. Lett. 2008, 10, 5289–5291. [Google Scholar] [CrossRef]
- Pesnot, T.; Gershater, M.C.; Ward, J.M.; Hailes, H.C. Phosphate mediated biomimetic synthesis of tetrahydroisoquinoline alkaloids. Chem. Commun. 2011, 47, 3242–3244. [Google Scholar] [CrossRef]
- Maresh, J.J.; Crowe, S.O.; Ralko, A.A.; Aparece, M.D.; Murphy, C.M.; Krzeszowiec, M.; Mullowney, M.W. Facile one-pot synthesis of tetrahydroisoquinolines from amino acids via hypochlorite-mediated decarboxylation and Pictet–Spengler condensation. Tetrahedron Lett. 2014, 55, 5047–5051. [Google Scholar] [CrossRef]
- Parra, R.D.; Maresh, J. Structural and energetics aspects of a proposed mechanism for the phosphate-mediated Pictet–Spengler cyclization reaction: A computational study. Comput. Theor. Chem. 2016, 1082, 1–10. [Google Scholar] [CrossRef]
- Zhao, J.; Méndez-Sánchez, D.; Ward, J.; Hailes, H.C. Biomimetic Phosphate-Catalyzed Pictet–Spengler Reaction for the Synthesis of 1,1′-Disubstituted and Spiro-Tetrahydroisoquinoline Alkaloids. J. Org. Chem. 2019, 84, 7702–7710. [Google Scholar] [CrossRef]
- Siedentop, R.; Claaßen, C.; Rother, D.; Lütz, S.; Rosenthal, K. Getting the Most Out of Enzyme Cascades: Strategies to Optimize In Vitro Multi-Enzymatic Reactions. Catalysts 2021, 11, 1183. [Google Scholar] [CrossRef]
- Classen, T.; Pietruszka, J. Complex molecules, clever solutions—Enzymatic approaches towards natural product and active agent syntheses. Bioorg. Med. Chem. 2018, 26, 1285–1303. [Google Scholar] [CrossRef]
- Schrittwieser, J.H.; Velikogne, S.; Hall, M.; Kroutil, W. Artificial Biocatalytic Linear Cascades for Preparation of Organic Molecules. Chem. Rev. 2018, 118, 270–348. [Google Scholar] [CrossRef]
- Sankar, M.; Nowicka, E.; Carter, E.; Murphy, D.M.; Knight, D.W.; Bethell, D.; Hutchings, G.J. The benzaldehyde oxidation paradox explained by the interception of peroxy radical by benzyl alcohol. Nat. Commun. 2014, 5, 3332. [Google Scholar] [CrossRef]
- Fabbrini, M.; Galli, C.; Gentili, P.; Macchitella, D. An oxidation of alcohols by oxygen with the enzyme laccase and mediation by TEMPO. Tetrahedron Lett. 2001, 42, 7551–7553. [Google Scholar] [CrossRef]
- d’Acunzo, F.; Baiocco, P.; Fabbrini, M.; Galli, C.; Gentili, P. A Mechanistic Survey of the Oxidation of Alcohols and Ethers with the Enzyme Laccase and Its Mediation by TEMPO. Eur. J. Org. Chem. 2002, 2002, 4195–4201. [Google Scholar] [CrossRef]
- Arends, I.W.C.E.; Li, Y.-X.; Ausan, R.; Sheldon, R.A. Comparison of TEMPO and its derivatives as mediators in laccase catalysed oxidation of alcohols. Tetrahedron 2006, 62, 6659–6665. [Google Scholar] [CrossRef]
- Tromp, S.A.; Matijošytė, I.; Sheldon, R.A.; Arends, I.W.C.E.; Mul, G.; Kreutzer, M.T.; Moulijn, J.A.; de Vries, S. Mechanism of Laccase–TEMPO-Catalyzed Oxidation of Benzyl Alcohol. ChemCatChem 2010, 2, 827–833. [Google Scholar] [CrossRef]
- Larson, T.M.; Anderson, A.M.; Rich, J.O. Combinatorial evaluation of laccase-mediator system in the oxidation of veratryl alcohol. Biotechnol. Lett. 2013, 35, 225–231. [Google Scholar] [CrossRef] [PubMed]
- Albarrán-Velo, J.; Gotor-Fernández, V.; Lavandera, I. One-pot two-step chemoenzymatic deracemization of allylic alcohols using laccases and alcohol dehydrogenases. Mol. Catal. 2020, 493, 111087. [Google Scholar] [CrossRef]
- Albarrán-Velo, J.; Lavandera, I.; Gotor-Fernández, V. Sequential Two-Step Stereoselective Amination of Allylic Alcohols through the Combination of Laccases and Amine Transaminases. ChemBioChem 2020, 21, 200–211. [Google Scholar] [CrossRef]
- Arends, I.W.C.E.; Li, Y.-X.; Sheldon, R.A. Stabilities and rates in the laccase/TEMPO-catalyzed oxidation of alcohols. Biocatal. Biotransform. 2006, 24, 443–448. [Google Scholar] [CrossRef]
- Gross, J.; Tauber, K.; Fuchs, M.; Schmidt, N.G.; Rajagopalan, A.; Faber, K.; Fabian, W.M.F.; Pfeffer, J.; Haas, T.; Kroutil, W. Aerobic oxidation of isosorbide and isomannide employing TEMPO/laccase. Green Chem. 2014, 16, 2117–2121. [Google Scholar] [CrossRef]
- Jiang, J.; Chen, H.; Yu, J.; Liu, L.; Fan, Y.; Saito, T.; Isogai, A. Rate-Limited Reaction in TEMPO/Laccase/O2 Oxidation of Cellulose. Macromol. Rapid Commun. 2021, 42, 2000501. [Google Scholar] [CrossRef]
- Cheng, A.-D.; Zong, M.-H.; Lu, G.-H.; Li, N. Solvent-Promoted Oxidation of Aromatic Alcohols/Aldehydes to Carboxylic Acids by a Laccase-TEMPO System: Efficient Access to 2,5-Furandicarboxylic Acid and 5-Methyl-2-Pyrazinecarboxylic Acid. Adv. Sustain. Syst. 2021, 5, 2000297. [Google Scholar] [CrossRef]
- Risi, C.; Zhao, F.; Castagnolo, D. Chemo-Enzymatic Metathesis/Aromatization Cascades for the Synthesis of Furans: Disclosing the Aromatizing Activity of Laccase/TEMPO in Oxygen-Containing Heterocycles. ACS Catal. 2019, 9, 7264–7269. [Google Scholar] [CrossRef]
- Zippilli, C.; Botta, L.; Bizzarri, B.M.; Baratto, M.C.; Pogni, R.; Saladino, R. Biomimetic synthesis of galantamine via laccase/TEMPO mediated oxidative coupling. RSC Adv. 2020, 10, 10897–10903. [Google Scholar] [CrossRef]
- Bassanini, I.; Ferrandi, E.E.; Riva, S.; Monti, D. Biocatalysis with Laccases: An Updated Overview. Catalysts 2021, 11, 26. [Google Scholar] [CrossRef]
- Gunne, M.; Urlacher, V.B. Characterization of the Alkaline Laccase Ssl1 from Streptomyces sviceus with Unusual Properties Discovered by Genome Mining. PLoS ONE 2012, 7, e52360. [Google Scholar] [CrossRef]
- Gunne, M.; Höppner, A.; Hagedoorn, P.-L.; Urlacher, V.B. Structural and redox properties of the small laccase Ssl1 from Streptomyces sviceus. FEBS J. 2014, 281, 4307–4318. [Google Scholar] [CrossRef]
- Suljić, S.; Mortzfeld, F.B.; Gunne, M.; Urlacher, V.B.; Pietruszka, J. Enhanced Biocatalytic Performance of Bacterial Laccase from Streptomyces sviceus: Application in the Michael Addition Sequence Towards 3-Arylated 4-Oxochromanes. ChemCatChem 2015, 7, 1380–1385. [Google Scholar] [CrossRef]
- Zhao, J.; Lichman, B.R.; Ward, J.M.; Hailes, H.C. One-pot chemoenzymatic synthesis of trolline and tetrahydroisoquinoline analogues. Chem. Commun. 2018, 54, 1323–1326. [Google Scholar] [CrossRef] [PubMed]
- Suljić, S.; Pietruszka, J. Synthesis of 3-Arylated 3,4-Dihydrocoumarins: Combining Continuous Flow Hydrogenation with Laccase-Catalysed Oxidation. Adv. Synth. Catal. 2014, 356, 1007–1020. [Google Scholar] [CrossRef]
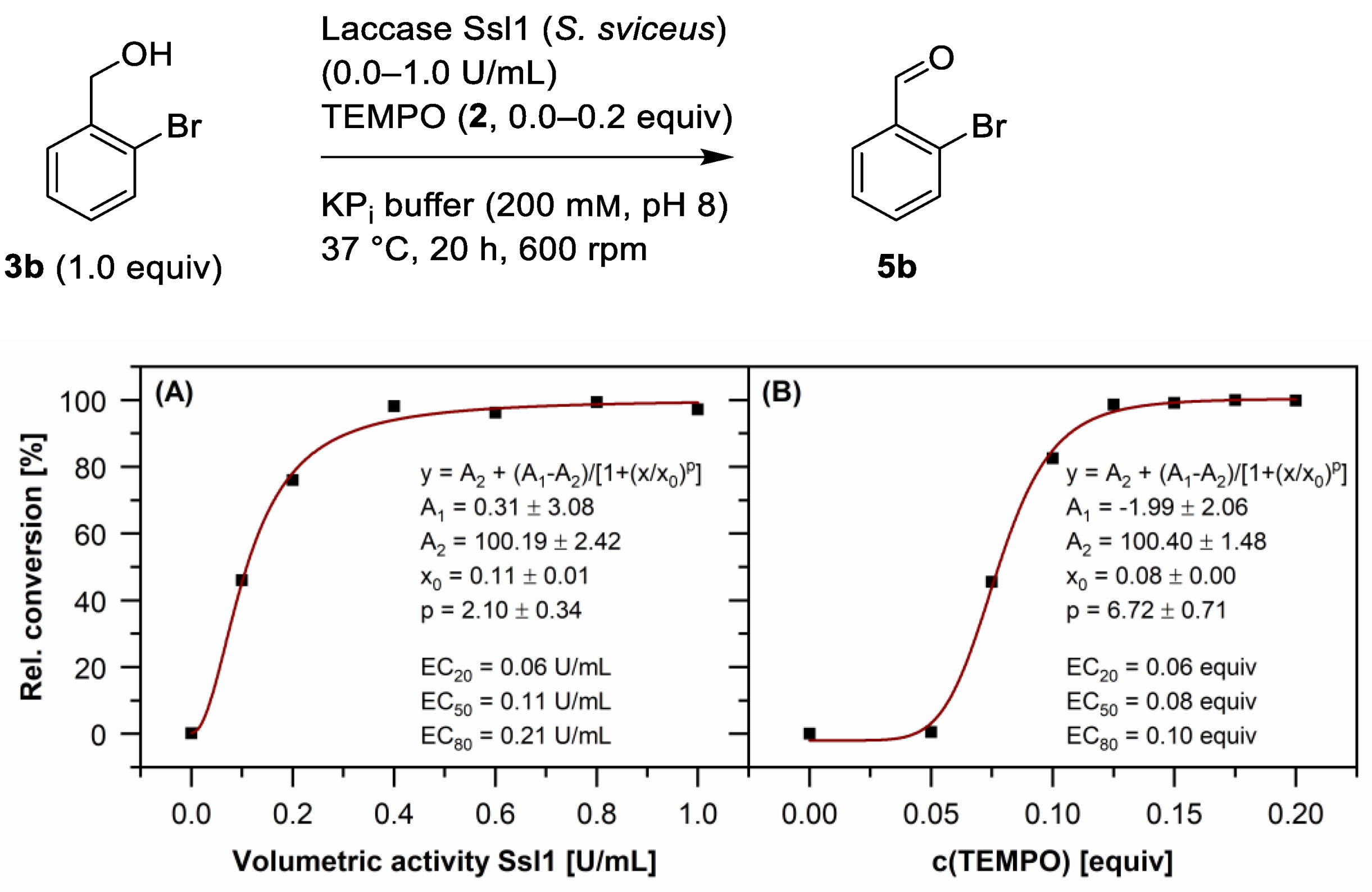
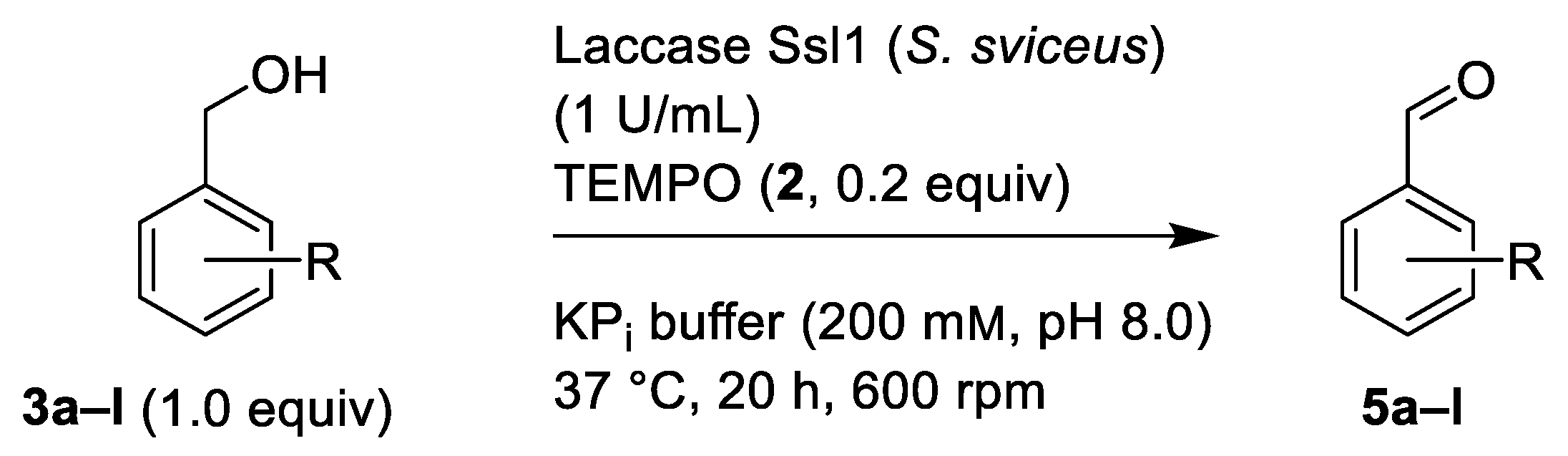

| Benzylic Alcohol | Benzaldehyde | R | Conversion [%] 1 |
|---|---|---|---|
| 3a | 5a | H | 28 |
| 3b | 5b | 2-Br | 100 |
| 3c | 5c | 2-F | 73 |
| 3d | 5d | 2-NO2 | 100 |
| 3e | 5e | 2-OMe | 72 |
| 3f | 5f | 4-Br | 100 |
| 3g | 5g | 4-Cl | 92 |
| 3h | 5h | 4-F | 98 |
| 3i | 5i | 4-CF3 | 100 |
| 3j | 5j | 4-NO2 | 84 |
| 3k | 5k | 4-Me | 100 |
| 3l | 5l | 4-OMe | 90 |
| pH | T [°C] | Sodium Ascorbate | Atmosphere | Yield [%] 1 |
|---|---|---|---|---|
| 5 | 60 | + | N2 | 2 |
| 7 | 60 | + | N2 | 74 |
| 7 | 37 | + | N2 | 65 |
| 8 | 37 | + | N2 | 67 |
| 8 | 37 | - | N2 | 65 |
| 8 | 37 | - | O2 | 68 |
| THIQ | R | Yield [%] 1 |
|---|---|---|
| 1a | H | 93 |
| 1b | 2’-Br | 76 |
| 1c | 2’-F | 92 |
| 1d | 2’-NO2 | 76 |
| 1e | 2’-OMe | 86 |
| 1f | 4’-Br | 79 |
| 1g | 4’-Cl | 84 |
| 1h | 4’-F | 88 |
| 1i | 4’-CF3 | 52 |
| 1j | 4’-NO2 | 91 |
| 1k | 4’-Me | 60 |
| 1l | 4’-OMe | 65 |
| Benzylic Alcohol | R | THIQ | Yield [%] 1 |
|---|---|---|---|
| 3a | H | 1a | 322 |
| 3b | 2-Br | 1b | 493 |
| 3c | 2-F | 1c | 64 |
| 3d | 2-NO2 | 1d | 473 |
| 3e | 2-OMe | 1e | 572 |
| 3f | 4-Br | 1f | 70 |
| 3g | 4-Cl | 1g | 552 |
| 3h | 4-F | 1h | 71 |
| 3i | 4-CF3 | 1i | 37 |
| 3j | 4-NO2 | 1j | 872 |
| 3k | 4-Me | 1k | 583 |
| 3l | 4-OMe | 1l | 43 |
| R | Conversion 1 1st step [%] | Yield 2 2nd step | Theor. Yield 3 Cascade [%] | Yield [%] 1 | THIQ |
|---|---|---|---|---|---|
| H | 28 | 93 | 26 | 32 | 1a |
| 2-Br | 100 | 76 | 76 | 49 | 1b |
| 2-F | 73 | 92 | 67 | 64 | 1c |
| 2-NO2 | 100 | 76 | 76 | 47 | 1d |
| 2-OMe | 72 | 86 | 62 | 57 | 1e |
| 4-Br | 100 | 79 | 79 | 70 | 1f |
| 4-Cl | 92 | 84 | 77 | 55 | 1g |
| 4-F | 98 | 88 | 86 | 71 | 1h |
| 4-CF3 | 100 | 52 | 52 | 37 | 1i |
| 4-NO2 | 84 | 91 | 76 | 87 | 1j |
| 4-Me | 100 | 60 | 60 | 58 | 1k |
| 4-OMe | 90 | 65 | 59 | 43 | 1l |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Klein, A.S.; Albrecht, A.C.; Pietruszka, J. Chemoenzymatic One-Pot Process for the Synthesis of Tetrahydroisoquinolines. Catalysts 2021, 11, 1389. https://doi.org/10.3390/catal11111389
Klein AS, Albrecht AC, Pietruszka J. Chemoenzymatic One-Pot Process for the Synthesis of Tetrahydroisoquinolines. Catalysts. 2021; 11(11):1389. https://doi.org/10.3390/catal11111389
Chicago/Turabian StyleKlein, Andreas Sebastian, Anna Christina Albrecht, and Jörg Pietruszka. 2021. "Chemoenzymatic One-Pot Process for the Synthesis of Tetrahydroisoquinolines" Catalysts 11, no. 11: 1389. https://doi.org/10.3390/catal11111389
APA StyleKlein, A. S., Albrecht, A. C., & Pietruszka, J. (2021). Chemoenzymatic One-Pot Process for the Synthesis of Tetrahydroisoquinolines. Catalysts, 11(11), 1389. https://doi.org/10.3390/catal11111389