Strong Pyro-Electro-Chemical Coupling of Elbaite/H2O2 System for Pyrocatalysis Dye Wastewater
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterization of Elbaite
2.2. Degradation Factors for Contaminant RhB
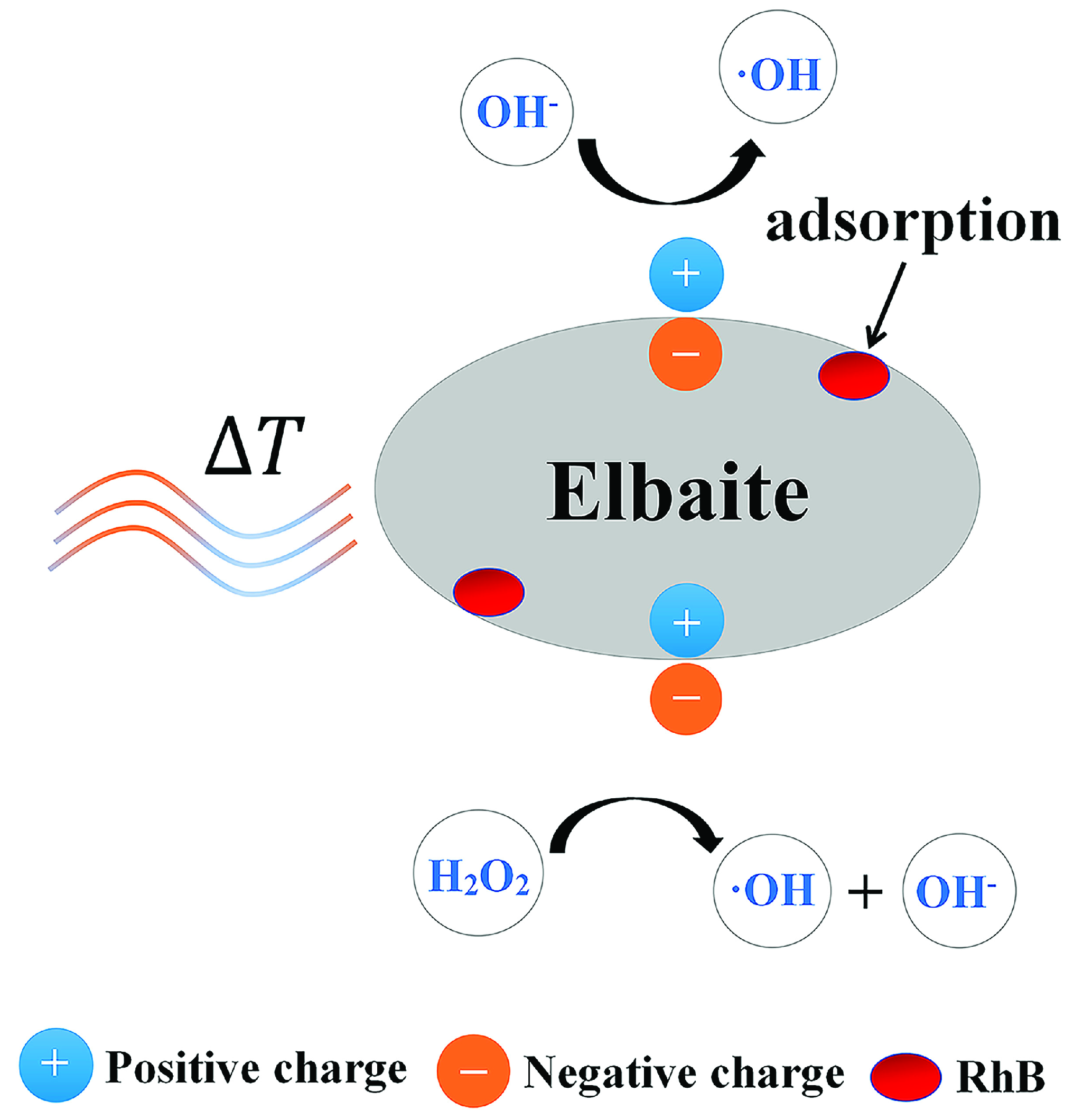
2.3. Detection of Hydroxyl Radicals
2.4. Degradation Factors for Contaminant RhB
2.5. Degradation Factors for Contaminant RhB
3. Materials and Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ahmad, A.; Mohd-Setapar, S.H.; Chuong, C.S.; Khatoon, A.; Wani, W.A.; Kumar, R.; Rafatullah, M. Recent advances in new generation dye removal technologies: Novel search for approaches to reprocess wastewater. RSC Adv. 2015, 5, 30801–30818. [Google Scholar] [CrossRef]
- Lin, H.; Wu, Z.; Jia, Y.; Li, W.; Zheng, R.K.; Luo, H. Piezoelectrically induced mechano-catalytic effect for degradation of dye wastewater through vibrating Pb (Zr0.52Ti0.48) O3 fibers. Appl. Phys. Lett. 2014, 104, 162907. [Google Scholar] [CrossRef]
- Krawczyk, K.; Wacławek, S.; Kudlek, E.; Silvestri, D.; Kukulski, T.; Grübel, K.; Černík, M.; Padil, V.V.T. UV-catalyzed persulfate oxidation of an anthraquinone based dye. Catalysts 2020, 10, 456. [Google Scholar] [CrossRef] [Green Version]
- Forgacs, E.; Cserhati, T.; Oros, G. Removal of synthetic dyes from wastewaters: A review. Environ. Int. 2004, 30, 953–971. [Google Scholar] [CrossRef]
- Xu, Y.; Li, X.; Cheng, X.; Sun, D.; Wang, X. Degradation of cationic red GTL by catalytic wet air oxidation over Mo–Zn–Al–O catalyst under room temperature and atmospheric pressure. Environ. Sci. Technol. 2012, 46, 2856–2863. [Google Scholar] [CrossRef] [PubMed]
- Mat Yasin, N.M.F.; Hossain, M.S.; HPS., A.K.; Zulkifli, M.; Al-Gheethi, A.; Asis, A.J.; Yahaya, A.N. Treatment of palm oil refinery effluent using tannin as a polymeric coagulant: Isotherm, kinetics, and thermodynamics analyses. Polymers 2020, 12, 2353. [Google Scholar] [CrossRef] [PubMed]
- Anjaneyulu, Y.; Chary, N.S.; Raj, D.S.S. Decolourization of industrial efflfluents–Available methods and emerging technologies–A review. Rev. Environ. Sci. Bio/Technol. 2005, 4, 245–273. [Google Scholar] [CrossRef]
- Sutherland, J.; Adams, C.; Kekobad, J. Treatment of MTBE by air stripping, carbon adsorption, and advanced oxidation: Technical and economic comparison for five groundwaters. Water Res. 2004, 38, 193–205. [Google Scholar] [CrossRef]
- Chiang, K.Y.; Wang, K.S.; Lin, F.L.; Chu, W.T. Chloride effects on the speciation and partitioning of heavy metal during the municipal solid waste incineration process. Sci. Total Environ. 1997, 203, 129–140. [Google Scholar] [CrossRef]
- Cimini, S.; Prisciandaro, M.; Barba, D. Simulation of a waste incineration process with flue-gas cleaning and heat recovery sections using Aspen Plus. Waste Manag. 2005, 25, 171–175. [Google Scholar] [CrossRef]
- Zagklis, D.P.; Vavouraki, A.I.; Kornaros, M.E.; Paraskeva, C.A. Purification of olive mill wastewater phenols through membrane filtration and resin adsorption/desorption. J. Hazard. Mater. 2015, 285, 69–76. [Google Scholar] [CrossRef]
- Boucher, F.R.; Lee, G.F. Adsorption of lindane and dieldrin pesticides on unconsolidated aquifer sands. Environ. Sci. Technol. 1972, 6, 538–543. [Google Scholar] [CrossRef]
- Martinez-Huitle, C.A.; Ferro, S. Electrochemical oxidation of organic pollutants for the wastewater treatment: Direct and indirect processes. Chem. Soc. Rev. 2006, 35, 1324–1340. [Google Scholar] [CrossRef]
- Lin, S.H.; Chuang, T.S. Combined treatment of phenolic wastewater by wet air oxidation and activated sludge. Toxicol. Environ. Chem. 1994, 44, 243–258. [Google Scholar] [CrossRef]
- Scott, J.P.; Ollis, D.F. Integration of chemical and biological oxidation processes for water treatment: Review and recommendations. Environ. Prog. 1995, 14, 88–103. [Google Scholar] [CrossRef]
- Wang, Y.-T. Effect of chemical oxidation on anaerobic biodegradation of model phenolic compounds. Water Environ. Res. 1992, 64, 268–273. [Google Scholar] [CrossRef]
- Munter, R. Advanced oxidation processes–current status and prospects. Proc. Est. Acad. Sci. Chem. 2001, 50, 59–80. [Google Scholar] [CrossRef]
- Augugliaro, V.; Bellardita, M.; Loddo, V.; Palmisano, G.; Palmisano, L.; Yurdakal, S. Overview on oxidation mechanisms of organic compounds by TiO2 in heterogeneous photocatalysis. J. Photoch. Photobio. C Photochem. Rev. 2012, 13, 224–245. [Google Scholar] [CrossRef] [Green Version]
- Yurdakal, S.; Palmisano, G.; Loddo, V.; Augugliaro, V.; Palmisano, L. Nanostructured rutile TiO2 for selective photocatalytic oxidation of aromatic alcohols to aldehydes in water. J. Am. Chem. Soc. 2008, 130, 1568–1569. [Google Scholar] [CrossRef]
- Augugliaro, V.; Prevot, A.B.; Vázquez, J.C.; Garcıa-López, E.; Irico, A.; Loddo, V.; Malato Rodrıguez, S.; Marcı, G.; Palmisano, L.; Pramauro, E. Photocatalytic oxidation of acetonitrile in aqueous suspension of titanium dioxide irradiated by sunlight. Adv. Environ. Res. 2004, 8, 329–335. [Google Scholar] [CrossRef]
- Marin, M.L.; Santos-Juanes, L.; Arques, A.; Amat, A.M.; Miranda, M.A. Organic photocatalysts for the oxidation of pollutants and model compounds. Chem. Rev. 2012, 112, 1710–1750. [Google Scholar] [CrossRef]
- Martinez-Haya, R.; Luna, M.M.; Hijarro, A.; Martinez-Valero, E.; Miranda, M.A.; Marin, M.L. Photocatalytic degradation of phenolic pollutants using N-methylquinolinium and 9-mesityl-10-methylacridinium salts. Catal. Today 2019, 328, 243–251. [Google Scholar] [CrossRef]
- Martinez-Haya, R.; Gomis, J.; Arques, A.; Marin, M.L.; Amat, A.M.; Miranda, M.A. Time-resolved kinetic assessment of the role of singlet and triplet excited states in the photocatalytic treatment of pollutants at different concentrations. Appl. Catal. B-Environ. 2017, 203, 381–388. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhu, R.; Xi, Y.; Zhu, J.; Zhu, G.; He, H. Strategies for enhancing the heterogeneous Fenton catalytic reactivity: A review. Appl. Catal. B-Environ. 2019, 255, 117739. [Google Scholar] [CrossRef]
- Wang, A.; Wang, H.; Deng, H.; Wang, S.; Shi, W.; Yi, Z.; Yan, K. Controllable synthesis of mesoporous manganese oxide microsphere efficient for photo-Fenton-like removal of fluoroquinolone antibiotics. Appl. Catal. B-Environ. 2019, 248, 298–308. [Google Scholar] [CrossRef]
- Wang, J.; Wang, S. Activation of persulfate (PS) and peroxymonosulfate (PMS) and application for the degradation of emerging contaminants. Chem. Eng. J. 2018, 334, 1502–1517. [Google Scholar] [CrossRef]
- Jiang, L.; Zhang, Y.; Zhou, M.; Liang, L.; Li, K. Oxidation of Rhodamine B by persulfate activated with porous carbon aerogel through a non-radical mechanism. J. Hazard. Mater. 2018, 358, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, A.R.; Nunes, O.C.; Pereira, M.F.; Silva, A.M. An overview on the advanced oxidation processes applied for the treatment of water pollutants defined in the recently launched Directive 2013/39/EU. Environ. Int. 2015, 75, 33–51. [Google Scholar] [CrossRef] [Green Version]
- Mezyk, S.P.; Neubauer, T.J.; Cooper, W.J.; Peller, J.R. Free-radical-induced oxidative and reductive degradation of sulfa drugs in water: Absolute kinetics and efficiencies of hydroxyl radical and hydrated electron reactions. J. Phys. Chem. A 2007, 111, 9019–9024. [Google Scholar] [CrossRef] [PubMed]
- Sebald, G.; Guyomar, D.; Agbossou, A. On thermoelectric and pyroelectric energy harvesting. Smart Mater. Struct. 2009, 18, 125006. [Google Scholar] [CrossRef]
- Lang, S.B.; Muensit, S. Review of some lesser-known applications of piezoelectric and pyroelectric polymers. Appl. Phys. A 2006, 85, 125–134. [Google Scholar] [CrossRef]
- Xu, X.; Chen, S.; Wu, Z.; Jia, Y.; Xiao, L.; Liu, Y. Strong pyro-electro-chemical coupling of Ba0.7Sr0.3TiO3@Ag pyroelectric nanoparticles for room-temperature pyrocatalysis. Nano Energ. 2018, 50, 581–588. [Google Scholar] [CrossRef]
- You, H.; Wu, Z.; Wang, L.; Jia, Y.; Li, S.; Zou, J. Highly efficient pyrocatalysis of pyroelectric NaNbO3 shape-controllable nanoparticles for room-temperature dye decomposition. Chemosphere 2018, 199, 531–537. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Jia, Y.; Qian, W.; Xu, X.; Wu, Z.; Han, Z.; Hong, Y.; You, H.; Ismail, M.; Bai, G. Pyroelectrically induced pyro-electro-chemical catalytic activity of BaTiO3 nanofibers under room-temperature cold–hot cycle excitations. Metals 2017, 7, 122. [Google Scholar] [CrossRef] [Green Version]
- Qian, W.; Wu, Z.; Jia, Y.; Hong, Y.; Xu, X.; You, H.; Zheng, Y.; Xia, Y. Thermo-electro-chemical coupling for room temperature thermocatalysis in pyroelectric ZnO nanorods. Electrochem. Commun. 2017, 81, 124–127. [Google Scholar] [CrossRef]
- Ma, J.; Chen, L.; Wu, Z.; Chen, J.; Jia, Y.; Hu, Y. Pyroelectric Pb (Zr0.52Ti0.48) O3 polarized ceramic with strong pyro-driven catalysis for dye wastewater decomposition. Ceram. Int. 2019, 45, 11934–11938. [Google Scholar] [CrossRef]
- Zhou, G.; Liu, H.; Chen, K.; Gai, X.; Zhao, C.; Liao, L.; Shan, Y. The origin of pyroelectricity in tourmaline at varying temperature. J. Alloys Compd. 2018, 744, 328–336. [Google Scholar] [CrossRef]
- Liu, N.; Wang, H.; Weng, C.H.; Hwang, C.C. Adsorption characteristics of Direct Red 23 azo dye onto powdered tourmaline. Arab. J. Chem. 2018, 11, 1281–1291. [Google Scholar] [CrossRef] [Green Version]
- Shi, J.; Yin, D.; Xu, Z.; Song, D.; Cao, F. Fosfomycin removal and phosphorus recovery in a schorl/H2O2 system. RSC Adv. 2016, 6, 68185–68192. [Google Scholar] [CrossRef]
- Zhang, Y.; Shi, J.; Xu, Z.; Chen, Y.; Song, D. Degradation of tetracycline in a schorl/H2O2 system: Proposed mechanism and intermediates. Chemosphere 2018, 202, 661–668. [Google Scholar] [CrossRef] [PubMed]
- Tokumura, M.; Znad, H.T.; Kawase, Y. Modeling of an external light irradiation slurry photoreactor: UV light or sunlight-photoassisted Fenton discoloration of azo-dye Orange II with natural mineral tourmaline powder. Chem. Eng. Sci. 2006, 61, 6361–6371. [Google Scholar] [CrossRef]
- Li, J.; Wang, C.; Wang, D.; Zhou, Z.; Sun, H.; Zhai, S. A novel technology for remediation of PBDEs contaminated soils using tourmaline-catalyzed Fenton-like oxidation combined with P. chrysosporium. Chem. Eng. J. 2016, 296, 319–328. [Google Scholar] [CrossRef]
- Zhang, X.H.; Wu, R.H. Mechanism and experimental Research on improvement in conductive performances of ZnO coated tourmaline powder. Adv. Mat. Res. 2013, 750, 2108–2112. [Google Scholar] [CrossRef]
- Zhang, H.; Wu, J.; Wang, Z.; Zhang, D. Electrochemical oxidation of crystal violet in the presence of hydrogen peroxide. J. Chem. Technol. Biotechnol. 2010, 85, 1436–1444. [Google Scholar] [CrossRef]
- Li, Y.; Lu, Y.; Zhu, X. Photo-Fenton discoloration of the azo dye X-3B over pillared bentonites containing iron. J. Hazard. Mater. 2006, 132, 196–201. [Google Scholar] [CrossRef]
- Xu, T.; Cai, Y.; O’Shea, K.E. Adsorption and photocatalyzed oxidation of methylated arsenic species in TiO2 suspensions. Environ. Sci. Technol. 2007, 41, 5471–5477. [Google Scholar] [CrossRef] [Green Version]
- Yu, J.; Wang, W.; Cheng, B.; Su, B.L. Enhancement of photocatalytic activity of mesporous TiO2 powders by hydrothermal surface fluorination treatment. J. Mater. Chem. C 2009, 113, 6743–6750. [Google Scholar] [CrossRef]
- Xu, X.; Wu, Z.; Xiao, L.; Jia, Y.; Ma, J.; Wang, F.; Huang, H. Strong piezo-electro-chemical effect of piezoelectric BaTiO3 nanofibers for vibration-catalysis. J. Alloys Compd. 2018, 762, 915–921. [Google Scholar] [CrossRef]
- Li, G.; Chen, D.; Zhao, W.; Zhang, X. Efficient adsorption behavior of phosphate on La-modified tourmaline. J. Environ. Chem. Eng. 2015, 3, 515–522. [Google Scholar] [CrossRef]
- Damjanovic, D. Ferroelectric, dielectric and piezoelectric properties of ferroelectric thin films and ceramics. Rep. Prog. Phys. 1998, 61, 1267. [Google Scholar] [CrossRef] [Green Version]
- Zhan, H.; Tian, H. Photocatalytic degradation of acid azo dyes in aqueous TiO2 suspension I, The effect of substituents. Dye. Pigment. 1998, 37, 231–239. [Google Scholar] [CrossRef]
- Houas, A.; Lachheb, H.; Ksibi, M.; Elaloui, E.; Guillard, C.; Herrmann, J.M. Photocatalytic degradation pathway of methylene blue in water. Appl. Catal. B 2001, 31, 145–157. [Google Scholar] [CrossRef]
- Xiao, Q.; Si, Z.; Zhang, J.; Xiao, C.; Tan, X. Photoinduced hydroxyl radical and photocatalytic activity of samarium-doped TiO2 nanocrystalline. J. Hazard. Mater. 2008, 150, 62–67. [Google Scholar] [CrossRef] [PubMed]
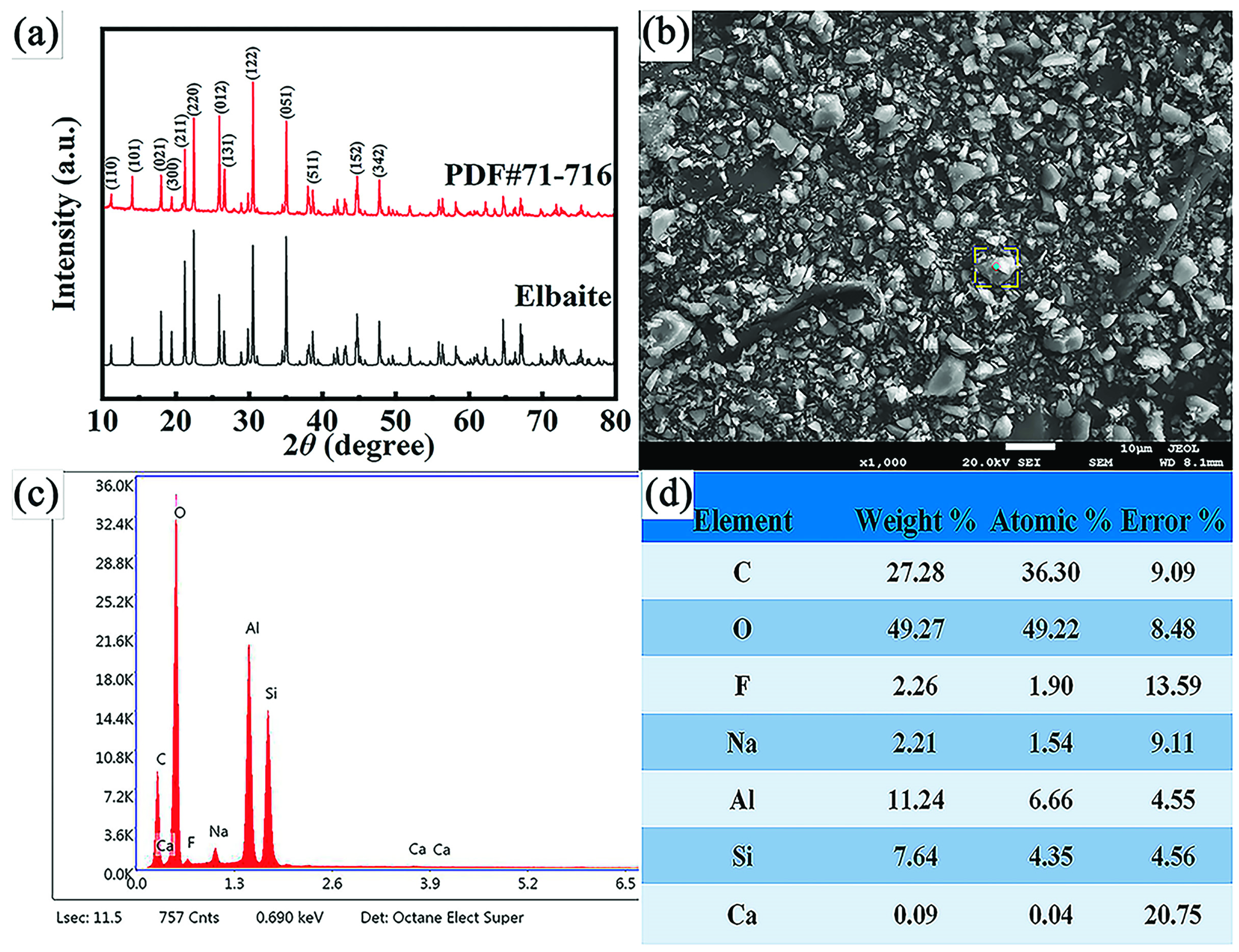



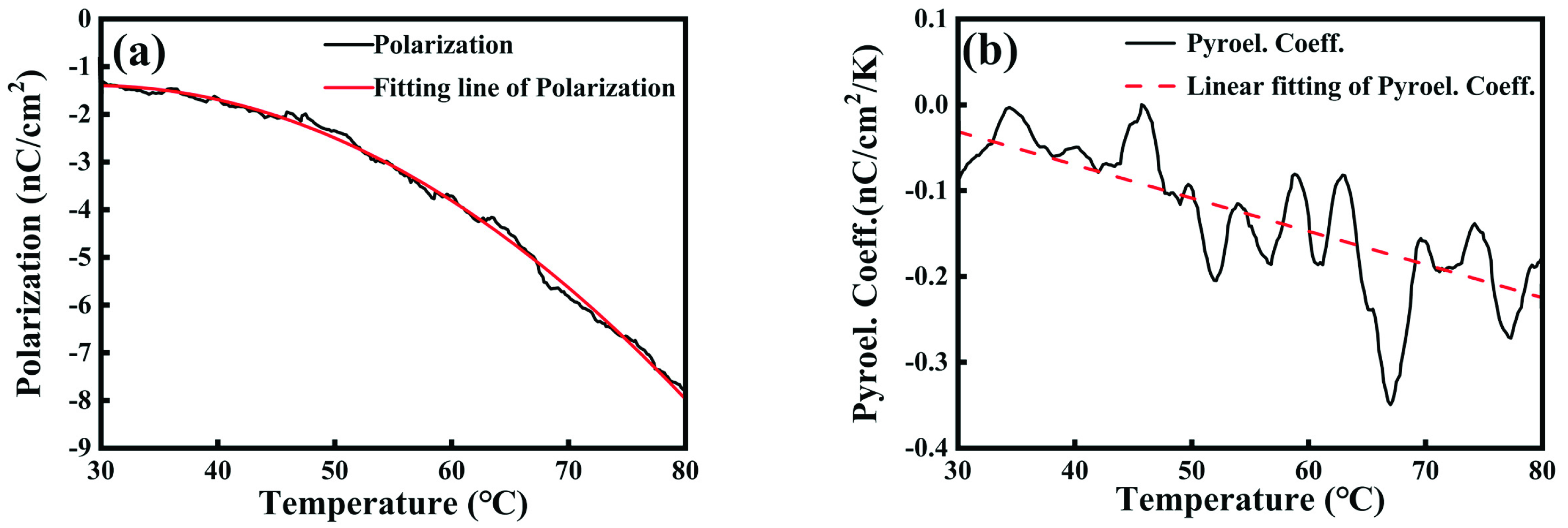
| Component | SiO2 | Al2O3 | SO3 | MnO | CaO | K2O | Lu2O3 | Li | PbO | Fe | Total |
|---|---|---|---|---|---|---|---|---|---|---|---|
| wt% | 51.80 | 40.50 | 3.16 | 1.83 | 1.68 | 0.31 | 0.25 | 0.23 | 0.10 | 0.07 | 99.93 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, F.; Guo, J.; Meng, D.; Wu, Y.; Sun, R.; Zhao, C. Strong Pyro-Electro-Chemical Coupling of Elbaite/H2O2 System for Pyrocatalysis Dye Wastewater. Catalysts 2021, 11, 1370. https://doi.org/10.3390/catal11111370
Chen F, Guo J, Meng D, Wu Y, Sun R, Zhao C. Strong Pyro-Electro-Chemical Coupling of Elbaite/H2O2 System for Pyrocatalysis Dye Wastewater. Catalysts. 2021; 11(11):1370. https://doi.org/10.3390/catal11111370
Chicago/Turabian StyleChen, Fei, Jiesen Guo, Dezhong Meng, Yuetong Wu, Ruijin Sun, and Changchun Zhao. 2021. "Strong Pyro-Electro-Chemical Coupling of Elbaite/H2O2 System for Pyrocatalysis Dye Wastewater" Catalysts 11, no. 11: 1370. https://doi.org/10.3390/catal11111370
APA StyleChen, F., Guo, J., Meng, D., Wu, Y., Sun, R., & Zhao, C. (2021). Strong Pyro-Electro-Chemical Coupling of Elbaite/H2O2 System for Pyrocatalysis Dye Wastewater. Catalysts, 11(11), 1370. https://doi.org/10.3390/catal11111370







