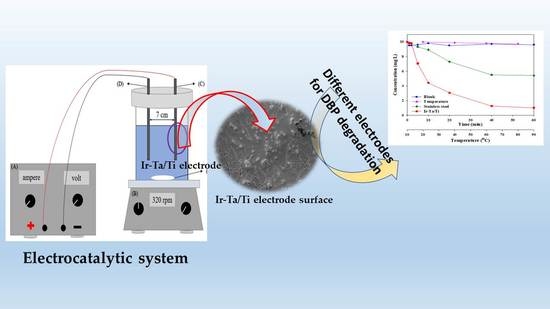
Degradation of Dibutyl Phthalate Plasticizer in Water by High-Performance Iro2-Ta2O5/Ti Electrocatalytic Electrode
Abstract
:1. Introduction
2. Results and Discussion
2.1. Identification of IrO2-Ta2O5/Ti Electrode
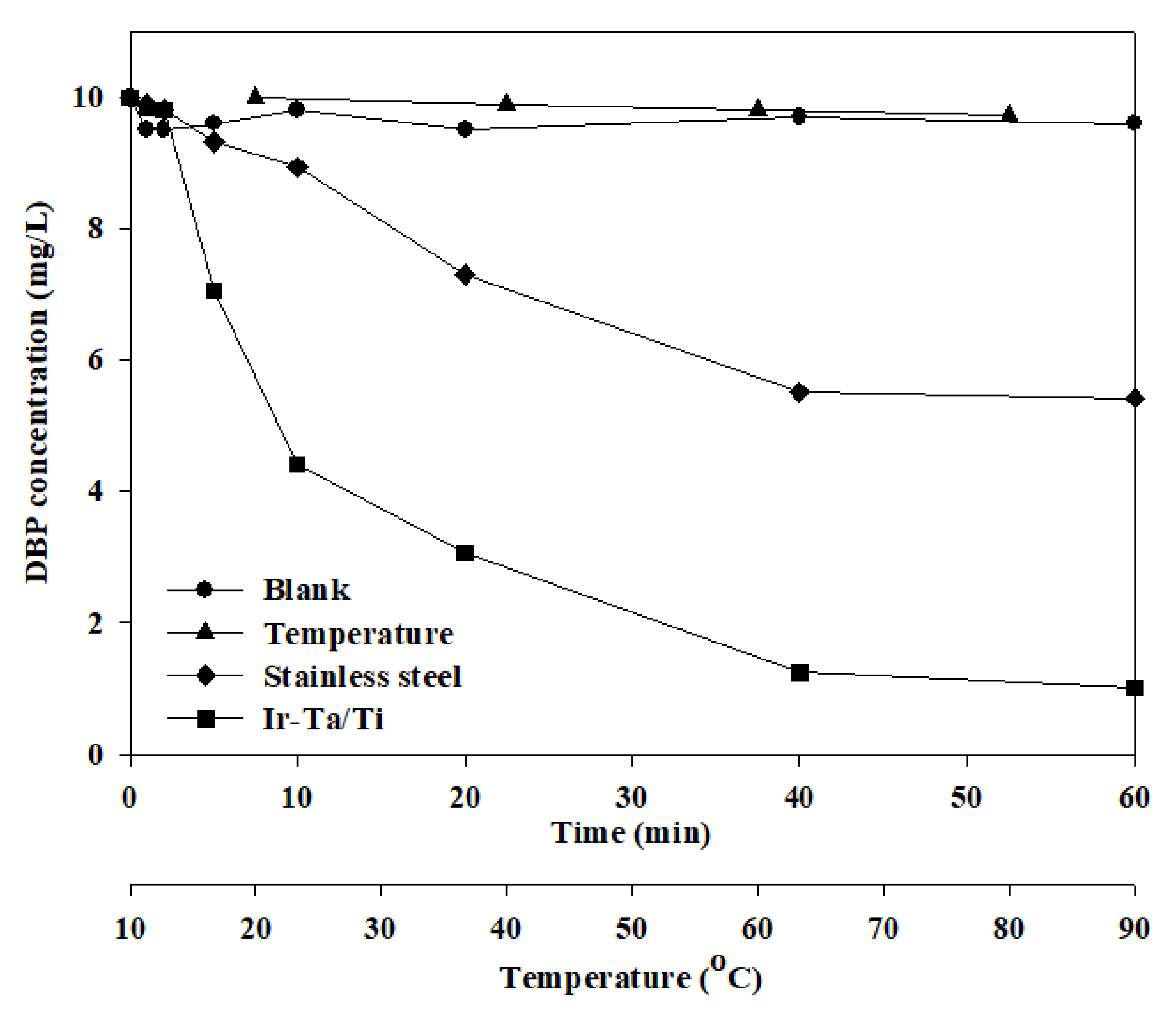
2.2. The influence of Electrode Material and Other Factors
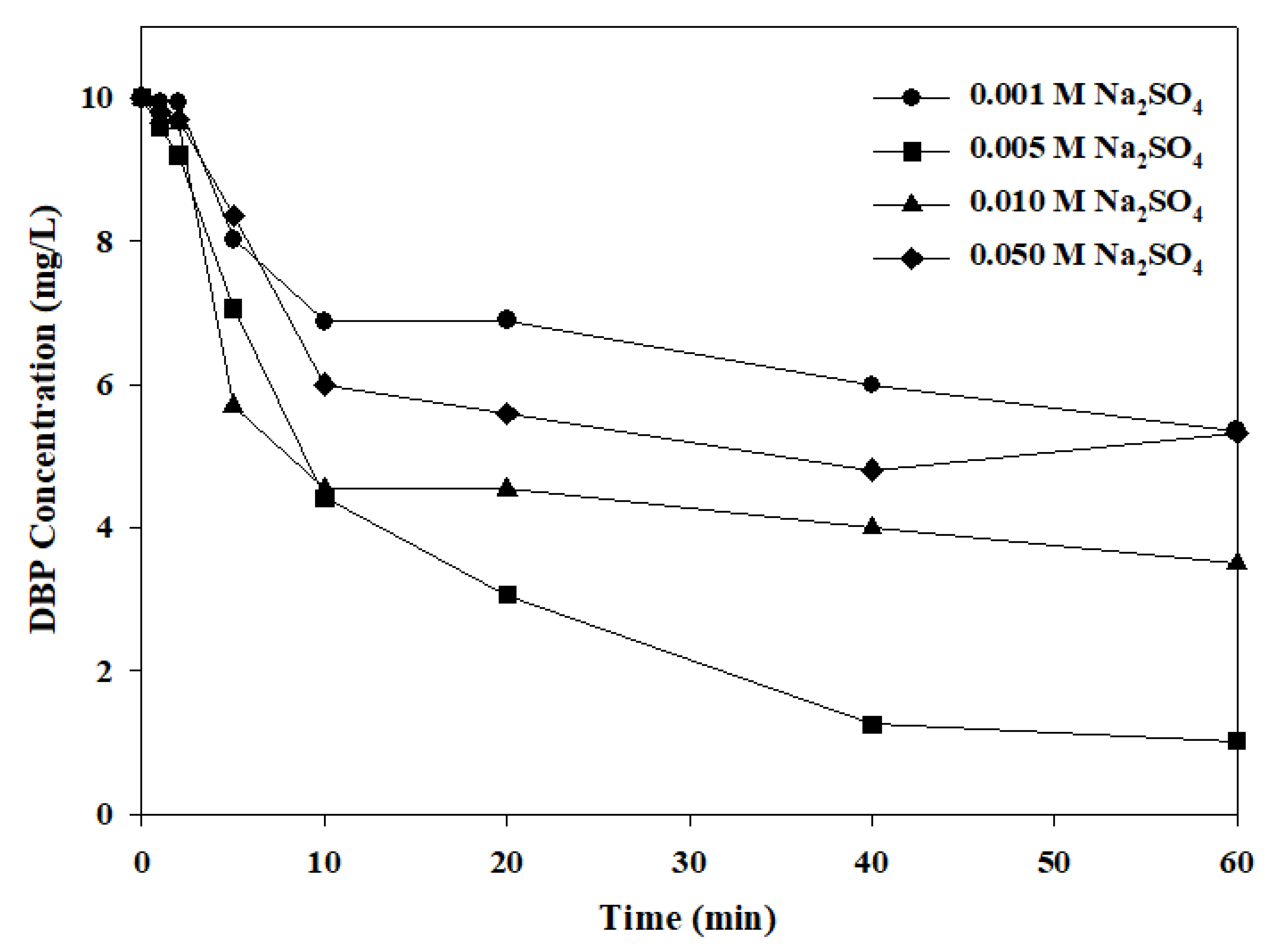
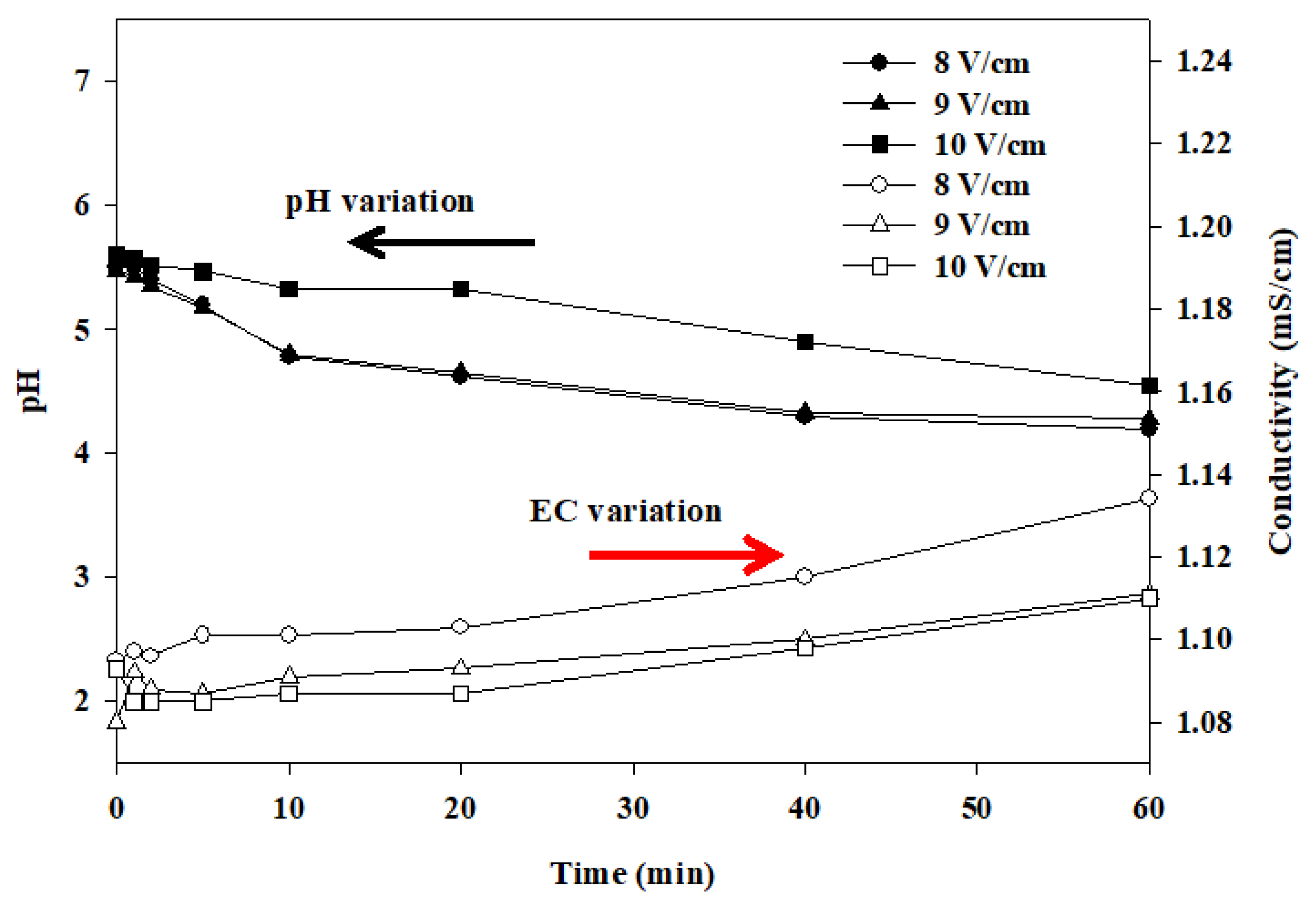
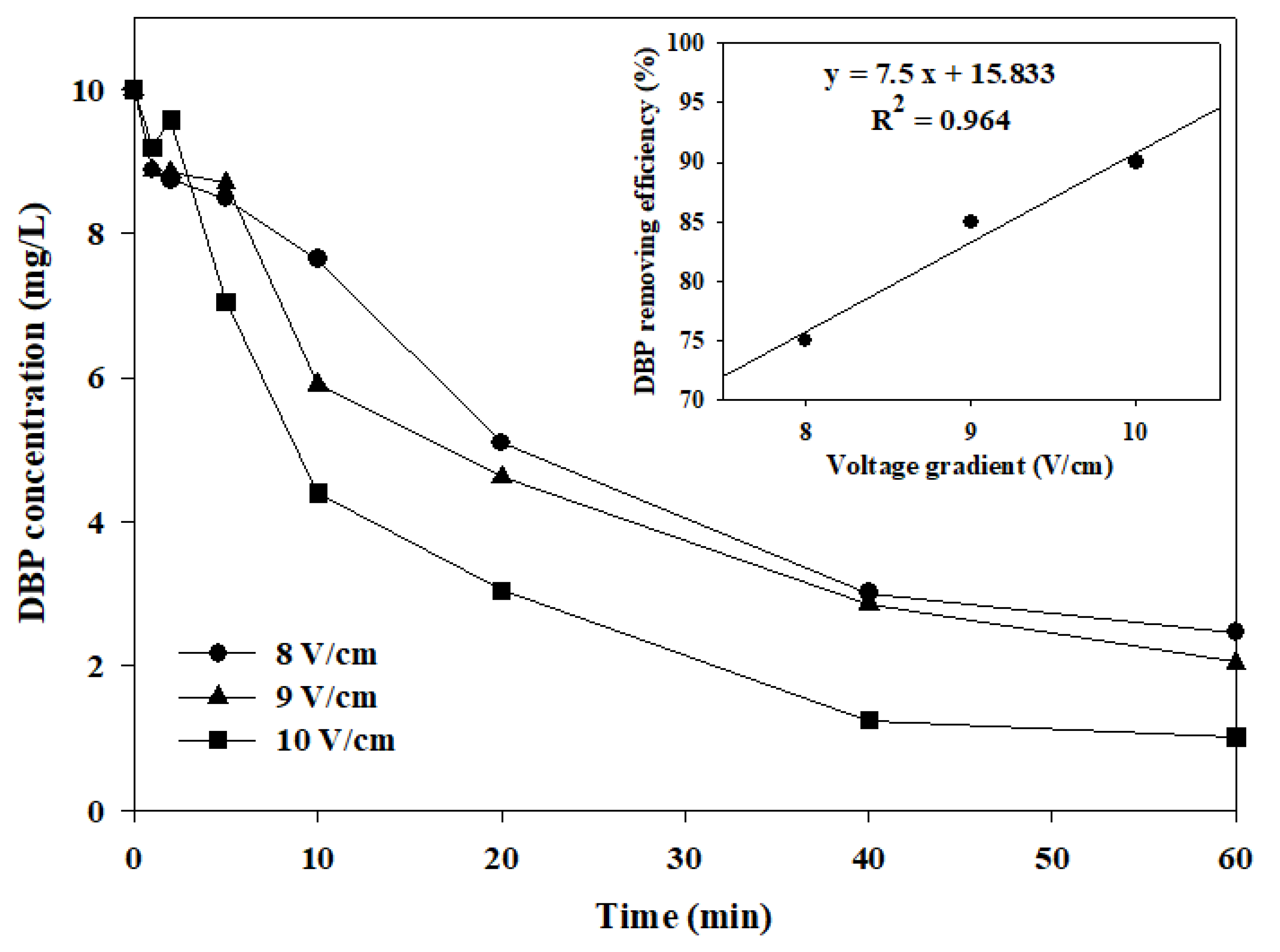
2.3. The Influence of Electrolyte and Voltage Gradient
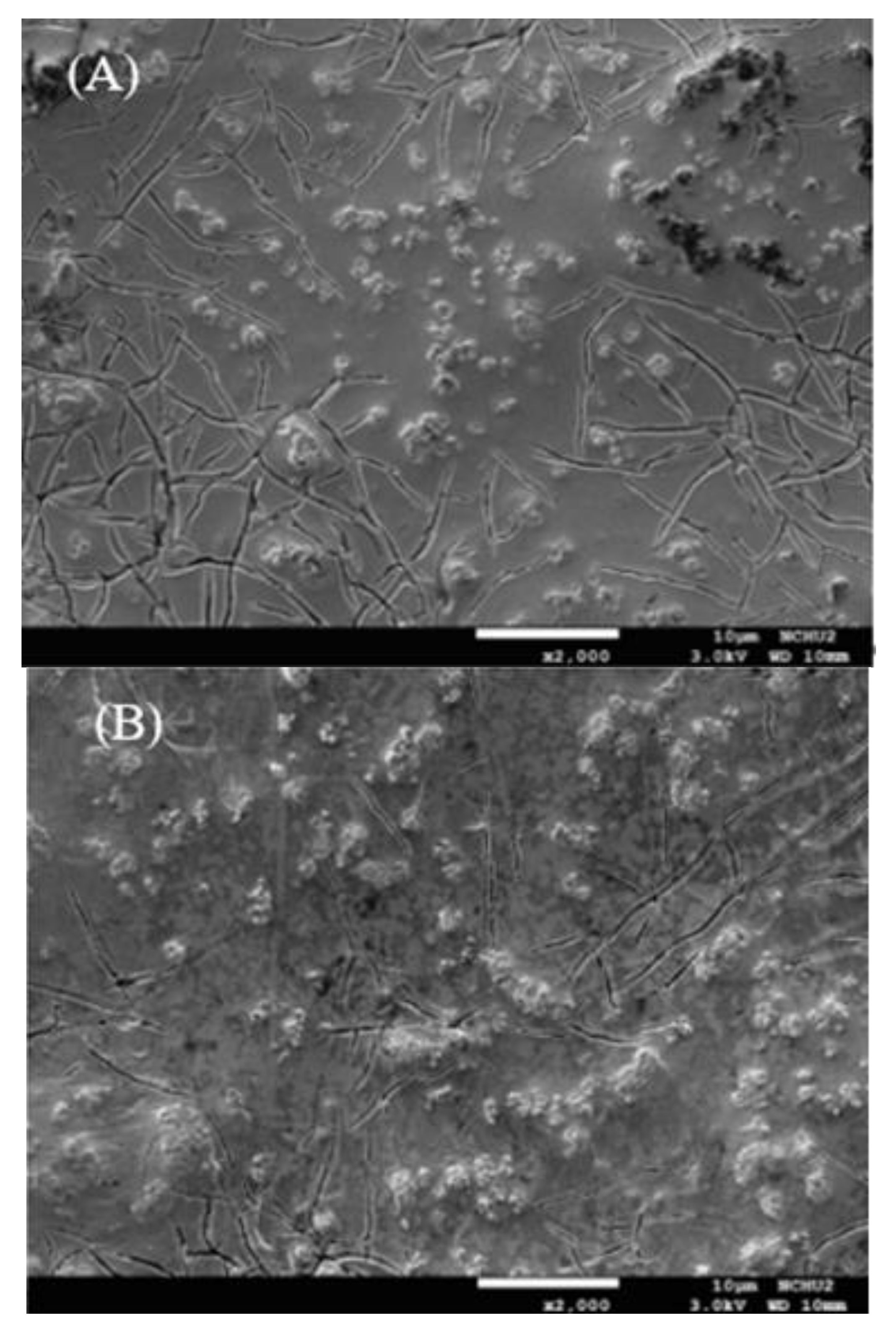
2.4. Surface Micrograph and Repeated Tests
3. Materials and Method
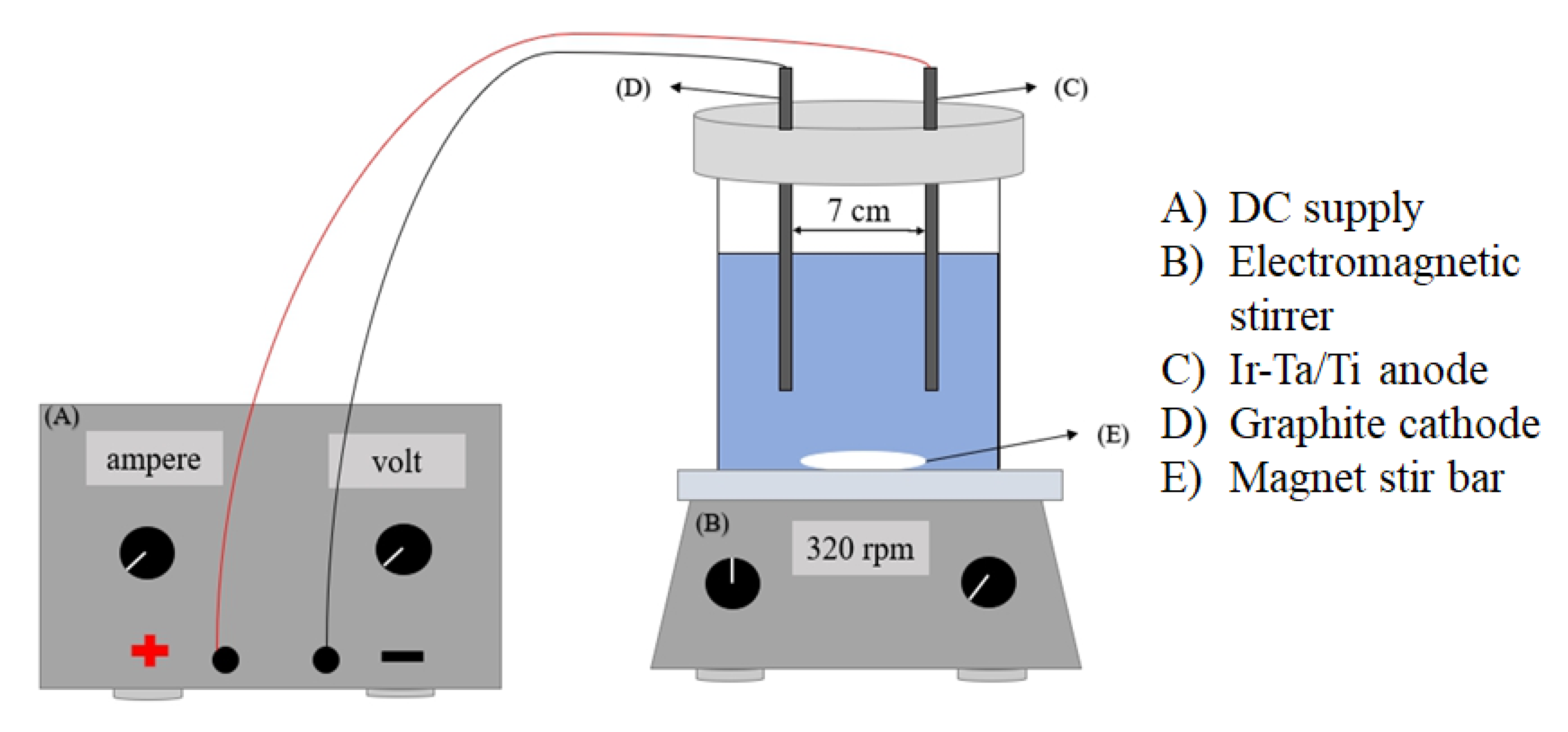
3.1. Electrocatalytic Reactor
3.2. Electrocatalytic Degradation of DBP
4. Conclusions
- The electrocatalytic oxidation (EO) technique treating water containing DBP, in which the pH and conductivity were slightly changed and eventually remained in a stable state.
- IrO2-Ta2O5/Ti electrode has the highest removal efficiency of DBP and can reach 90% under a voltage gradient of 10 V/cm and 0.005 M Na2SO4. The electric consumption has been calculated at about 0.046 KWh, and it is better than the current photocatalytic technique. It can provide a new approach for the degradation of DBP.
- The removal efficiency of TOC can reach about 55% after the IrO2-Ta2O5/Ti electrode for 60 min of treatment time. In addition, the mineralization reaction of DBP is increased with the voltage gradient.
- The removal efficiency of the DBP can still be maintained at about 90% when the IrO2-Ta2O5/Ti electrode is operated 3 times; the FESEM, EDS, and XRD patterns confirm that the surface structure of the IrO2-Ta2O5/Ti electrode was still stable after use of 100 h.
Author Contributions
Funding
Conflicts of Interest
References
- Pignatello, J.J.; Oliveros, E.; MacKay, A. Advanced Oxidation Processes for Organic Contaminant Destruction Based on the Fenton Reaction and Related Chemistry. Crit. Rev. Environ. Sci. Technol. 2006, 36, 1–84. [Google Scholar] [CrossRef]
- Gao, D.W.; Wen, Z.D. Phthalate esters in the environment: A critical review of their occurrence, biodegradation, and removal during wastewater treatment processes. Sci. Total Environ. 2016, 541, 986–1001. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z. Efficient adsorption of dibutyl phthalate from aqueous solution by activated carbon developed from phoenix leaves. Int. J. Environ. Sci. Technol. 2015, 12, 1923–1932. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Chen, L. Adsorption characteristics of dibutyl phthalate from aqueous solution using ginkgo leaves-activated carbon by chemical activation with zinc chloride. Desalin. Water Treat. 2018, 54, 1969–1980. [Google Scholar] [CrossRef]
- Wu, Q.; Liu, H.; Ye, L.; Li, P.; Wang, Y. Biodegradation of Di-n-butyl phthalate esters by Bacillus sp. SASHJ under simulated shallow aquifer condition. Int. Biodeterior. Biodegrad. 2013, 76, 102–107. [Google Scholar] [CrossRef]
- Gao, M.; Xu, Y.; Liu, Y.; Wang, S.; Wang, C.; Dong, Y.; Song, Z. Effect of polystyrene on di-butyl phthalate (DBP) bioavailability and DBP-induced phytotoxicity in lettuce. Environ. Pollut. 2021, 268, 115870. [Google Scholar] [CrossRef]
- Sin, J.C.; Lam, S.M.; Mohamed, A.R.; Lee, K.T. Degrading Endocrine Disrupting Chemicals from Wastewater by TiO2 Photocatalysis: A Review. Int. J. Photoenergy 2011, 2012, 185159. [Google Scholar]
- Martínez-Huitle, C.A.; Brillas, E. Decontamination of wastewaters containing synthetic organic dyes by electrochemical methods: A general review. Appl. Catal. B Environ. 2009, 87, 105–145. [Google Scholar] [CrossRef]
- Panizza, M.; Cerisola, G. Direct and Mediated Anodic Oxidation of Organic Pollutants. Chem. Rev. 2009, 109, 6541–6569. [Google Scholar] [CrossRef]
- Martínez-Huitle, C.A.; Rodrigo, M.A.; Sirés, I.; Scialdone, O. Single and Coupled Electrochemical Processes and Reactors for the Abatement of Organic Water Pollutants: A Critical Review. Chem. Rev. 2015, 115, 13362–13407. [Google Scholar] [CrossRef]
- Martínez-Huitle, C.A.; Brillas, E. Decontamination of wastewaters containing synthetic organic dyes by electrochemical methods. An updated review. Appl. Catal. B Environ. 2015, 166, 603–643. [Google Scholar]
- Tung, C.H.; Chang, J.H.; Hsieh, Y.H.; Hsu, J.C.; Ellis, A.V.; Liu, W.C.; Yan, R.H. Comparison of hydroxyl radical yields between photo- and electro-catalyzed water treatments. J. Taiwan Inst. Chem. Eng. 2014, 45, 1649–1654. [Google Scholar] [CrossRef]
- Ma, C.; Zhang, Y. Spinel CuxCo1-xMn2O4 electrode for effectively cleaning organic wastewater via electrocatalytic oxidation. Sep. Purif. Technol. 2021, 258, 118024. [Google Scholar] [CrossRef]
- Rodríguez-Narváez, O.M.; Picos, A.R.; Bravo-Yumi, N.; Pacheco-Alvarez, M.; Martínez-Huitle, C.A.; Peralta-Hernández, J.M. Electrochemical oxidation technology to treat textile wastewaters. Curr. Opin. Electrochem. 2021, 29, 100806. [Google Scholar] [CrossRef]
- Ganzoury, M.A.; Ghasemian, S.; Zhang, N.; Yagar, M.; De Lannoy, C.F. Mixed metal oxide anodes used for the electrochemical degradation of a real mixed industrial wastewater. Chemosphere 2022, 286, 131600. [Google Scholar] [CrossRef]
- Chanikya, P.; Nidheesha, P.V.; Babu, D.S.; Gopinath, A.; Kumar, M.S. Treatment of dyeing wastewater by combined sulfate radical based electrochemical advanced oxidation and electrocoagulation processes. Sep. Purif. Technol. 2021, 254, 117570. [Google Scholar] [CrossRef]
- Lissens, G.; Pieters, J.; Verhaege, M.; Pinoy, L.; Verstraete, W. Electrochemical degradation of surfactants by intermediates of water discharge at carbon-based electrodes. Electrochim. Acta 2003, 48, 1655–1663. [Google Scholar] [CrossRef]
- Panizza, M.; Martinez-Huitle, C.A. Role of electrode materials for the anodic oxidation of a real landfill leachate-comparison between Ti-Ru-Sn ternary oxide, PbO2 and boron-doped diamond anode. Chemosphere 2013, 90, 1455–1460. [Google Scholar] [CrossRef]
- Chang, J.H.; Yang, T.J.; Tung, C.H. Performance of nano- and nonnano-catalytic electrodes for decontaminating municipal wastewater. J. Hazard. Mater. 2009, 163, 152–157. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, S.; Jaiganesh, P.S.; Karunakaran, A.; Kumarasamy, K.; Lin, M.L. The effect of pH on the photocatalytic degradation of cationic and anionic dyes using polyazomethine/ZnO and polyazomethine/TiO2 nanocomposites. Int. J. Appl. Sci. Eng. 2021, 18, 2020247. [Google Scholar] [CrossRef]
- Samet, Y.; Agengui, L.; Abdelhedi, R. Electrochemical degradation of chlorpyrifos pesticide in aqueous solutions by anodic oxidation at boron-doped diamond electrodes. Chem. Eng. J. 2010, 161, 167–172. [Google Scholar] [CrossRef]
- Tang, W.W.; Li, Y.F.; Li, W.H.; Chen, X.J.; Zeng, X.P. Preparation of a coated Ti anode for producing acidic electrolyzed oxidizing water. LWT-Food Sci. Technol. 2016, 66, 606–614. [Google Scholar] [CrossRef]
- Jiang, M.; Wang, H.; Li, Y.J.; Zhang, H.C.; Zhang, G.X.; Lu, Z.Y.; Sun, X.M.; Jiang, L. Superaerophobic RuO2-based nanostructured electrode for high-performance chlorine evolution reaction. Small 2017, 13, 1602240. [Google Scholar] [CrossRef]
- Ren, Z.D.; Quan, S.S.; Gao, J.; Li, W.Y.; Zhu, Y.C.; Liu, Y.; Chai, B.; Wang, Y.R. The electrocatalytic activity of IrO2-Ta2O5 anode materials and electrolyzed oxidizing water preparation and sterilization effect. RSC Adv. 2015, 5, 8778–8786. [Google Scholar] [CrossRef]
- Zeradjanin, A.R.; Menzel, N.; Schuhmann, W.; Strasser, P. On the faradaic selectivity and the role of surface inhomogeneity during the chlorine evolution reaction on ternary Ti-Ru-Ir mixed metal oxide electrocatalysts. Phys. Chem. Chem. Phys. 2014, 16, 13741–13747. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Samet, Y.; Chaabane Elaoud, S.; Ammar, S.; Abdelhedi, R. Electrochemical degradation of 4-chloroguaiacol for wastewater treatment using PbO2 anodes. J. Hazard. Mater. 2006, 138, 614–619. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.; Chen, K.; Wu, W.; Wang, J.; Lee, S. Effect of IrO2 loading on RuO2-IrO2-TiO2 anodes a study of microstructure and working life for the chlorine evolution reaction. Ceram. Int. 2007, 33, 1087–1091. [Google Scholar]
- Deng, L.; Liu, Y.; Zhao, G.; Chen, J.; He, S.; Zhu, Y.; Chai, B.; Ren, Z. Preparation of electrolyzed oxidizing water by TiO2 doped IrO2-Ta2O5 electrode with high selectivity and stability for chlorine evolution. J. Electroanal. Chem. 2019, 832, 459–466. [Google Scholar] [CrossRef]
- Wang, H.; Wang, J.; Bo, G.; Wu, S.; Luo, L. Degradation of pollutants in polluted river water using Ti/IrO2-Ta2O5 coating electrode and evaluation of electrode characteristics. J. Clean. Prod. 2020, 273, 123019. [Google Scholar] [CrossRef]
- Babaei, T.; Zarei, M.; Hosseini, M.G.; Hosseini, M.M. Electrochemical advanced oxidation process of Phenazopyridine drug waste using different Ti-based IrO2-Ta2O5 anodes. J. Taiwan Inst. Chem. Eng. 2020, 117, 103–111. [Google Scholar] [CrossRef]
- Borbón, B.; Oropeza-Guzman, M.T.; Brillas, E.; Sirés, I. Sequential electrochemical treatment of dairy wastewater using aluminum and DSA-type anodes. Environ. Sci. Pollut. Res. 2014, 21, 8573–8584. [Google Scholar] [CrossRef]
- Li, B.S.; Lin, A.; Gan, F.X. Preparation and electrocatalytic properties of Ti/IrO2-Ta2O5 anodes for oxygen evolution. Trans. Nonferrous Met. Soc. China 2006, 16, 1193–1199. [Google Scholar] [CrossRef]
- Herrada, R.A.; Rodil, S.E.; Sepúlveda-Guzmán, S.; Manríquez, J.; Exner, K.S.; Bustos, E. Characterization of Ti electrodes electrophoretically coated with IrO2-Ta2O5 films with different Ir:Ta molar ratios. J. Alloys Compd. 2021, 862, 158015. [Google Scholar] [CrossRef]
- Salverda, A.; Dondapati, J.S.; Thiruppathi, A.R.; Chen, A. Effect of Reduced Graphene Oxide on the Ta2O5-IrO2 Electrocatalyst for Water Splitting. J. Electrochem. Soc. 2020, 167, 146506. [Google Scholar] [CrossRef]
- Dondapati, J.S.; Thiruppathi, A.R.; Salverda, A.; Chen, A. Comparison of Pt and IrO2-Ta2O5/Ti as a counter electrode in acidic media. Electrochem. Commun. 2021, 124, 106946. [Google Scholar] [CrossRef]
- Kaneco, S.; Katsumata, H.; Suzuki, T.; Ohta, K. Titanium dioxide mediated photocatalytic degradation of dibutyl phthalate in aqueous solution-kinetics, mineralization and reaction mechanism. Chem. Eng. J. 2006, 125, 59–66. [Google Scholar] [CrossRef]
- Bajt, O.; Mailhot, G.; Bolte, M. Degradation of dibutyl phthalate by homogeneous photocatalysis with Fe (III) in aqueous solution. Appl. Catal. B Environ. 2001, 33, 239–248. [Google Scholar] [CrossRef]
- Wang, G.; Zhang, Q.; Ma, X.; Xin, Y.; Zhu, X.X.; Ma, D.; Chen, Q.; Zhang, J.; Xiao, Z.; Cui, C. Photocatalytic degradation performance and mechanism of dibutyl phthalate by graphene/TiO2 nanotube array photoelectrodes. Chem. Eng. J. 2019, 358, 1083–1090. [Google Scholar] [CrossRef]
- Tung, C.H.; Shen, S.Y.; Chang, J.H.; Hsu, Y.M.; Lai, Y.C. Treatment of real printing wastewater with an electrocatalytic process. Sep. Purif. Technol. 2013, 117, 131–136. [Google Scholar] [CrossRef] [Green Version]
- Sanromán, M.A.; Pazos, M.; Ricart, M.T.; Cameselle, C. Electrochemical decolourisation of structurally different dyes. Chemosphere 2004, 57, 233–239. [Google Scholar] [CrossRef]
- Costa, C.R.; Olivi, P. Effect of chloride concentration on the electrochemical treatment of a synthetic tannery wastewater. Electrochim. Acta 2009, 54, 2046–2052. [Google Scholar] [CrossRef]


| Treatment Method | Optimum Initial pH | Treatment Time (h) | Required Energy Consumption | % Pollutant Removal | Reference |
|---|---|---|---|---|---|
| Physical absorption | 13 | 2.0 | - | 97 | Wang and Chen (2015) [4] |
| Biological cultivation | 8 | 190 | - | 80 | Wu et al. (2013) [5] |
| Fe(III) Photocatalysis | 3 | 2 | Mercury lamp 125 W | 85 | Bajt et al. (2001) [37] |
| TiO2 Photocatalysis | 6 | 1.0 | Xenon lamp 990 W | 90 | Kaneco et al. (2006) [36] |
| graphene/TiO2 nanotube Photocatalysis | 11.5 | 1.5 | Xenon lamp 150 W | 95 | Wang et al. (2019) [38] |
| IrO2-Ta2O5/Ti electrocatalysis | - | 0.67 | 70 W | 90 | Present work |
| Element | Weight (%) before Operation | Weight (%) after Operation | Difference (%) |
|---|---|---|---|
| C | 3.57 | 4.58 | 1.01 |
| O | 21.36 | 22.80 | 1.44 |
| Ti | 0.33 | 0.65 | 0.32 |
| Ta | 47.21 | 45.31 | 1.9 |
| Ir | 27.53 | 26.66 | 0.87 |
| Total | 100 | 100 | 1.01 |
| Structure | 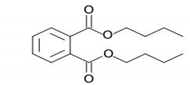 |
|---|---|
| Formula | C16H22O4 |
| Molecular weight | 278.34 |
| Density | 1.043 g/cm3 |
| Dye content | 85–95% |
| Solubility | 13 mg/L |
| CAS NO. | 84-74-2 |
| Source | Riedel-deHaen |
| Item | Parameters |
|---|---|
| Electrode | Anode: IrO2-Ta2O5/Ti Cathode: graphite |
| Voltage gradient (V/cm) | 8, 9, 10 |
| Electrolyte (M) | 0.001, 0.005, 0.01, 0.05 |
| DBP concentration (mg/L) | 10 |
| Treatment time (min) | 60 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, J.-M.; Chou, S.-H.; Zhang, Y.; Kumar, M.; Shen, S.-Y. Degradation of Dibutyl Phthalate Plasticizer in Water by High-Performance Iro2-Ta2O5/Ti Electrocatalytic Electrode. Catalysts 2021, 11, 1368. https://doi.org/10.3390/catal11111368
Xu J-M, Chou S-H, Zhang Y, Kumar M, Shen S-Y. Degradation of Dibutyl Phthalate Plasticizer in Water by High-Performance Iro2-Ta2O5/Ti Electrocatalytic Electrode. Catalysts. 2021; 11(11):1368. https://doi.org/10.3390/catal11111368
Chicago/Turabian StyleXu, Jia-Ming, Shu-Hsien Chou, Ying Zhang, Mohanraj Kumar, and Shan-Yi Shen. 2021. "Degradation of Dibutyl Phthalate Plasticizer in Water by High-Performance Iro2-Ta2O5/Ti Electrocatalytic Electrode" Catalysts 11, no. 11: 1368. https://doi.org/10.3390/catal11111368
APA StyleXu, J.-M., Chou, S.-H., Zhang, Y., Kumar, M., & Shen, S.-Y. (2021). Degradation of Dibutyl Phthalate Plasticizer in Water by High-Performance Iro2-Ta2O5/Ti Electrocatalytic Electrode. Catalysts, 11(11), 1368. https://doi.org/10.3390/catal11111368