Fractionation of Birch Wood by Integrating Alkaline-Acid Treatments and Hydrogenation in Ethanol over a Bifunctional Ruthenium Catalyst
Abstract
:1. Introduction
2. Results and Discussion
2.1. Hydrogenation of Initial and Pretreated Birch Wood in Ethanol
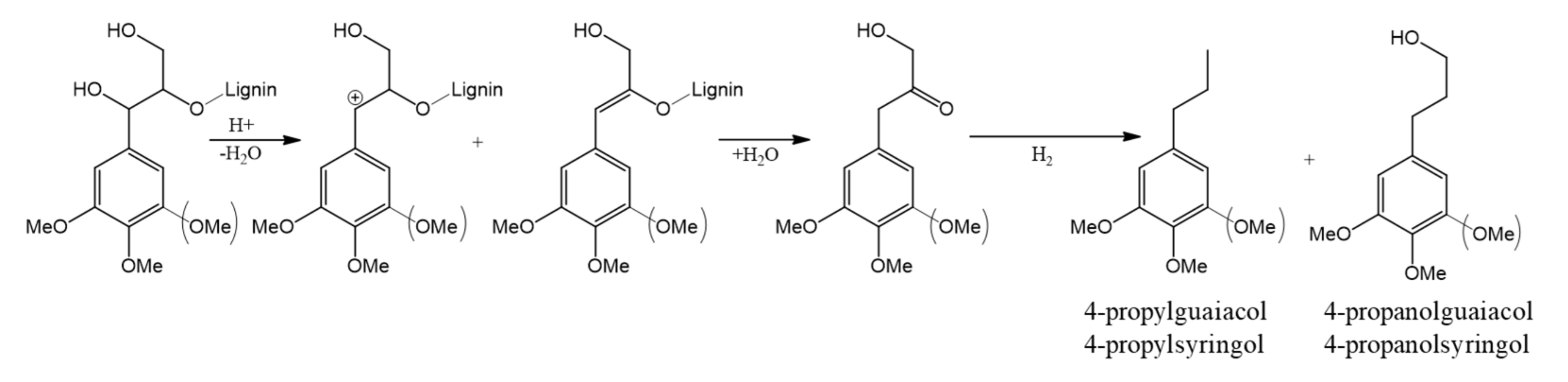
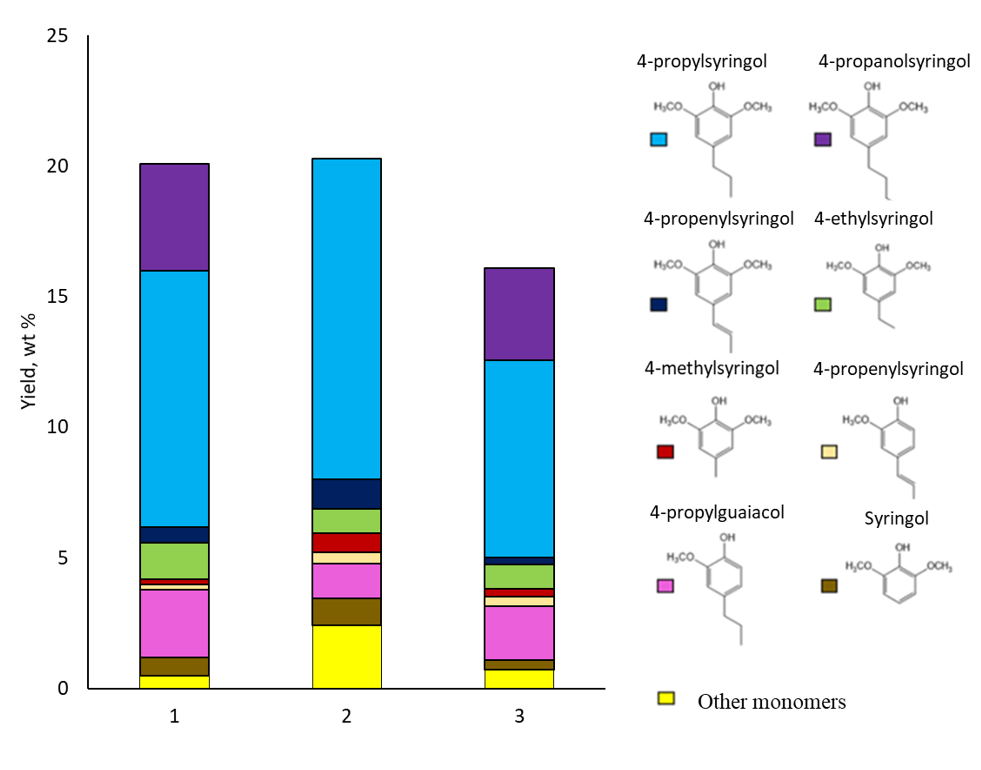
2.2. Composition of Liquid Products of Birch Wood Hydrogenation
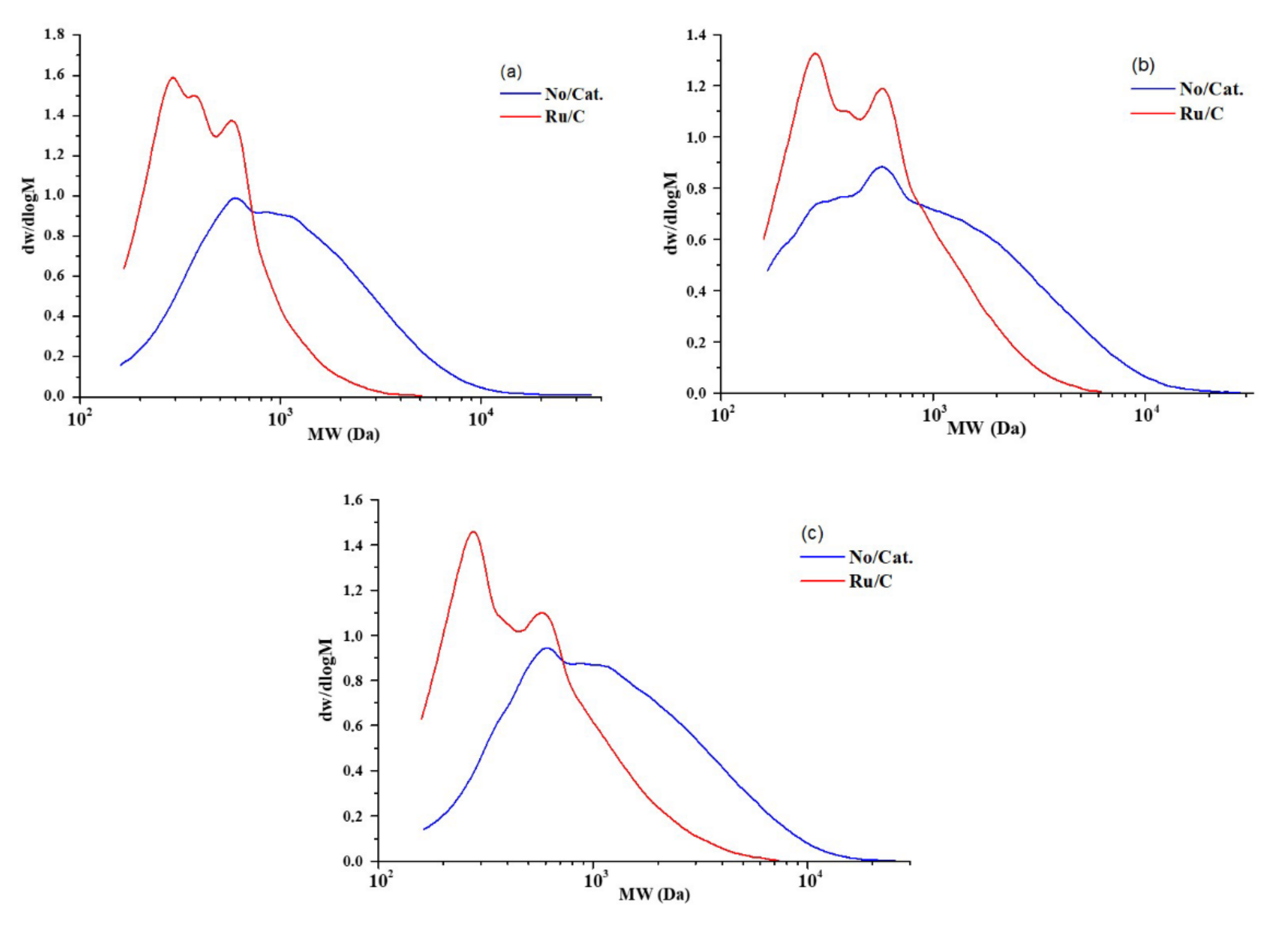
2.3. Composition and Structure of Solid Products of Birch Wood Hydrogenation
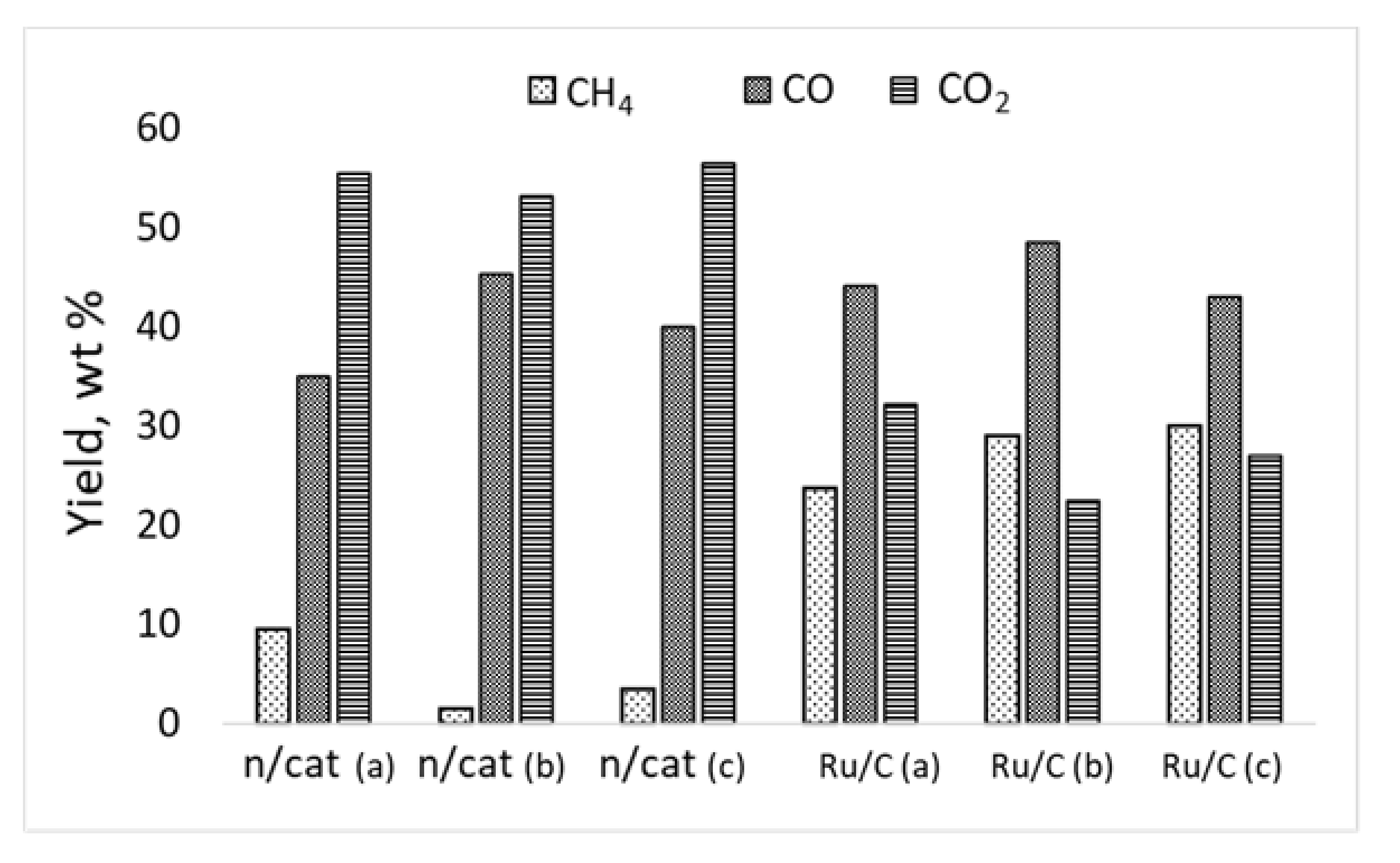
2.4. Composition of Gaseous Products of Hydrogenation of Birch Wood
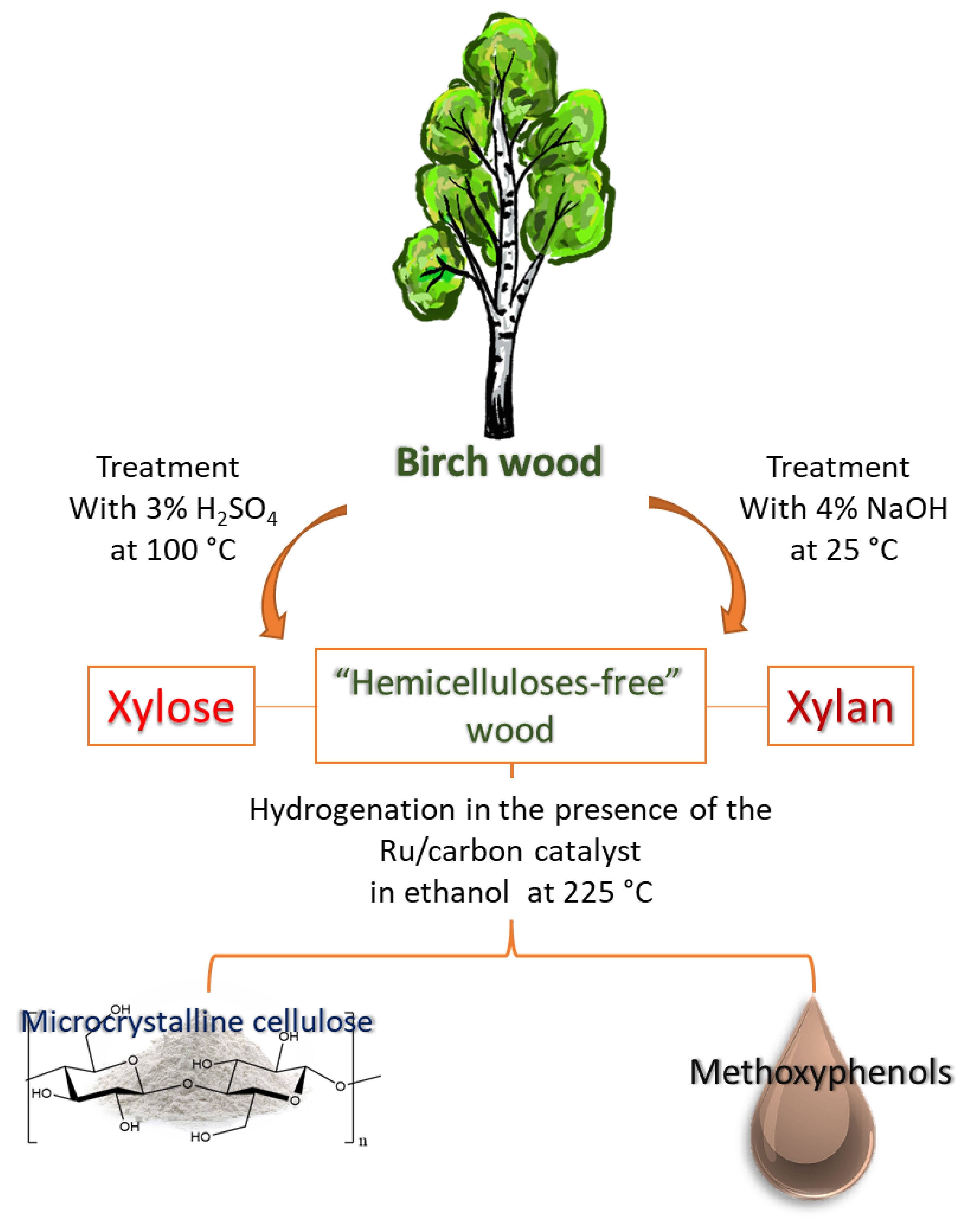
2.5. Fractionation of Birch Wood into Microcrystalline Cellulose, Xylose and Methoxyphenols
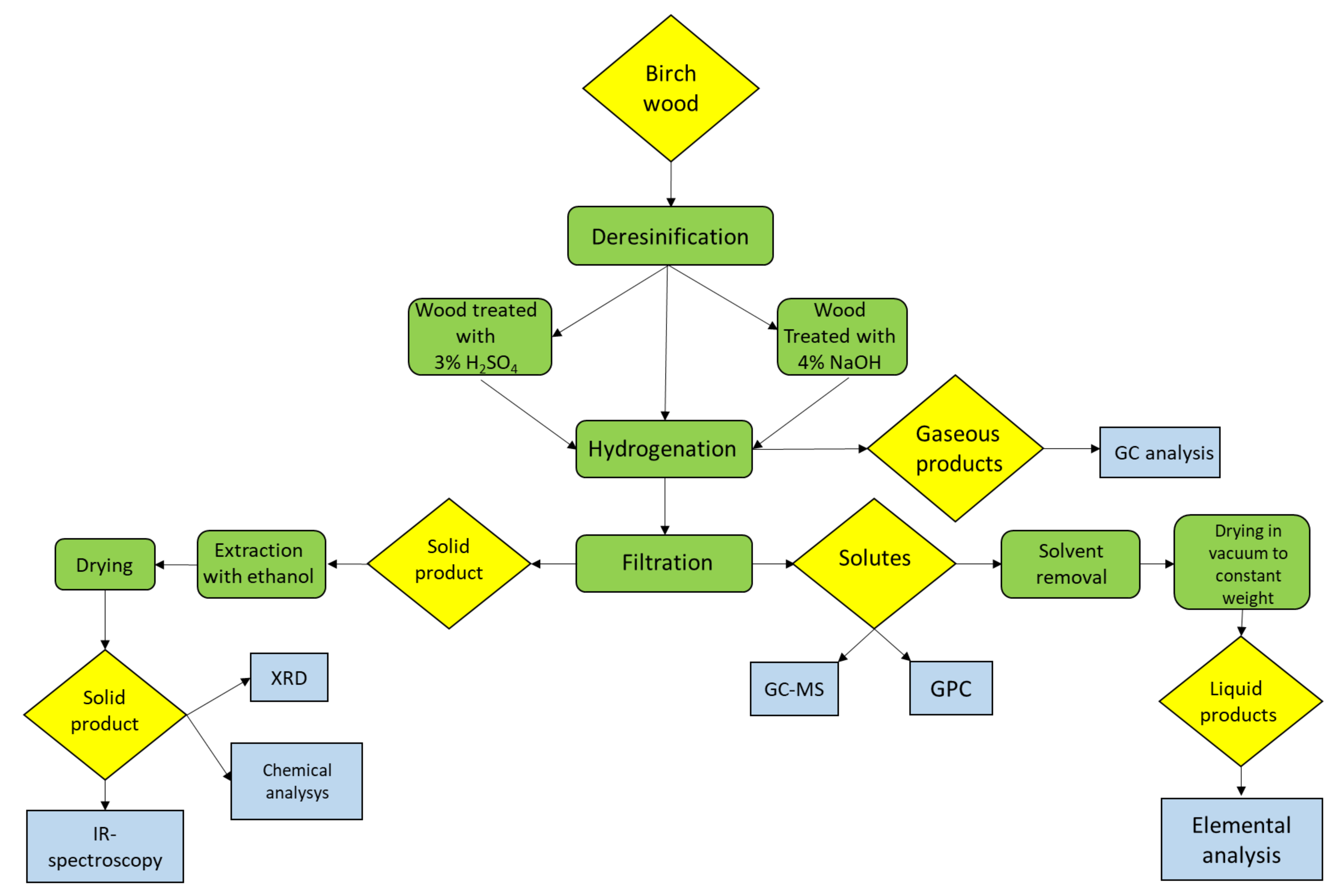
3. Materials and Methods
3.1. Initial Wood
3.2. Catalyst Ru/Carbon
3.3. Acid Hydrolysis of Hemicelluloses
3.4. Isolation of Xylan
3.5. Hydrogenation of Birch Wood
3.6. Analysis of Liquid Products
3.7. Analysis of Gaseous Products
3.8. Analysis of Solid Products
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rowell, R.M. Handbook of Wood Chemistry and Wood Composites, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2012. [Google Scholar]
- Gallezot, P. Conversion of biomass to selected chemical products. Chem. Soc. Rev. 2012, 41, 1538–1558. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Zhou, H.; Sikarwar, V.S.; Zhao, M.; Park, A.-H.A.; Fennell, P.S.; Shen, L.; Fan, L.-S. Biomass-based chemical looping technologies: The good, the bad and the future. Energy Environ. Sci. 2017, 10, 1885–1910. [Google Scholar] [CrossRef] [Green Version]
- Kamm, B.; Gruber, P.R.; Kamm, M. Biorefineries Industrial Processes and Products; Wiley-VCH: Weinheim, Germany, 2006; Volume 2. [Google Scholar]
- Sjostrom, E. Wood Chemistry. Fundamentals and Applications, 2nd ed.; Academic Press: Cambridge, MA, USA, 2013; p. 293. [Google Scholar]
- Naidu, D.S.; Hlangothi, S.P.; John, M.J. Bio-based products from xylan: A review. Carbohydr. Polym. 2018, 179, 28–41. [Google Scholar] [CrossRef] [PubMed]
- Du, X.; Pérez-Boada, M.; Fernández, C.; Rencoret, J.; del Río, J.C.; Jiménez-Barbero, J.; Li, J.; Gutiérrez, A.; Martínez, A.T. Analysis of lignin–carbohydrate and lignin–lignin linkages after hydrolase treatment of xylan–lignin, glucomannan–lignin and glucan–lignin complexes from spruce wood. Planta 2014, 239, 1079–1090. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.; Shakeel, U.; Saif Ur Rehman, M.; Li, H.; Xu, X.; Xu, J. Lignin-carbohydrate complexes (LCCs) and its role in biorefinery. J. Clean. Prod. 2020, 253, 120076. [Google Scholar] [CrossRef]
- Sun, S.; Sun, S.; Cao, X.; Sun, R. The role of pretreatment in improving the enzymatic hydrolysis of lignocellulosic materials. Bioresour. Technol. 2016, 199, 49–58. [Google Scholar] [CrossRef]
- Cao, S.; Pu, Y.; Studer, M.; Wyman, C.; Ragauskas, A.J. Chemical transformations of Populus trichocarpa during dilute acid pretreatment. RSC Adv. 2012, 2, 10925–10936. [Google Scholar] [CrossRef]
- Bali, G.; Meng, X.; Deneff, J.I.; Sun, Q.; Ragauskas, A.J. The Effect of Alkaline Pretreatment Methods on Cellulose Structure and Accessibility. ChemSusChem 2015, 8, 275–279. [Google Scholar] [CrossRef]
- Kuznetsov, B.N.; Sudakova, I.G.; Garyntseva, N.V.; Kondrasenko, A.A.; Pestunov, A.V.; Djakovitch, L.; Pinel, C. Catalytic peroxide fractionation processes for the green biorefinery of wood. Reaction Kinet. Mech. Catal. 2019, 126, 717–735. [Google Scholar] [CrossRef] [Green Version]
- Tarabanko, V.E.; Kaygorodov, K.L.; Skiba, E.A.; Tarabanko, N.E.; Chelbina, Y.V.; Baybakova, O.V.; Kuznetsov, B.N.; Djakovitch, L. Processing Pine Wood into Vanillin and Glucose by Sequential Catalytic Oxidation and Enzymatic Hydrolysis. Wood Chem. Technol. 2017, 37, 43–51. [Google Scholar] [CrossRef] [Green Version]
- Van den Bosch, S.; Schutyser, W.; Vanholme, R.; Driessen, T.; Koelewijn, S.F.; Renders, T.; De Meester, B.; Huijgen, W.J.J.; Dehaen, W.; Courtin, C.M.; et al. Reductive lignocellulose fractionation into soluble lignin-derived phenolic monomers and dimers and processable carbohydrate pulps. Energy Environ. Sci. 2015, 8, 1748–1763. [Google Scholar] [CrossRef] [Green Version]
- Baryshnikov, S.V.; Miroshnikova, A.V.; Kazachenko, A.S.; Malyar, Y.N.; Taran, O.P.; Lavrenov, A.V.; Djakovitch, L.; Kuznetsov, B.N. Hydrogenation of abies wood and ethanol lignin by hydrogen in supercritical ethanol in the presence of bifunctional catalyst Pt/ZrO2. J. Sib. Fed. Univ. Chem. 2019, 12, 550–561. [Google Scholar] [CrossRef]
- Kazachenko, A.S.; Tarabanko, V.E.; Miroshnikova, A.V.; Sychev, V.V.; Skripnikov, A.M.; Malyar, Y.N.; Mikhlin, Y.L.; Baryshnikov, S.V.; Taran, O.P. Reductive Catalytic Fractionation of Flax Shive over Ru/C Catalysts. Catalysts 2021, 11, 42. [Google Scholar] [CrossRef]
- Kuznetsov, B.N.; Chesnokov, N.V.; Sudakova, I.G.; Garyntseva, N.V.; Kuznetsova, S.A.; Malyar, Y.N.; Yakovlev, V.A.; Djakovitch, L. Green catalytic processing of native and organosolv lignins. Catal. Today 2018, 309, 18–30. [Google Scholar] [CrossRef] [Green Version]
- Kazachenko, A.S.; Miroshnikova, A.V.; Tarabanko, V.E.; Skripnikov, A.M.; Malyar, Y.N.; Borovkova, V.S.; Sychev, V.V.; Taran, O.P. Thermal Conversion of Flax Shives in Sub- and Supercritical Ethanol in the Presence of Ru/C Catalyst. Catalysts 2021, 11, 970. [Google Scholar] [CrossRef]
- Renders, T.; Van den Bossche, G.; Vangeel, T.; Van Aelst, K.; Sels, B. Reductive catalytic fractionation: State of the art of the lignin-first biorefinery. Curr. Opin. Biotechnol. 2019, 56, 193–201. [Google Scholar] [CrossRef]
- Schutyser, W.; Renders, T.; Van den Bosch, S.; Koelewijn, S.F.; Beckham, G.T.; Sels, B.F. Chemicals from lignin: An interplay of lignocellulose fractionation, depolymerisation, and upgrading. Chem. Soc. Rev. 2018, 47, 852–908. [Google Scholar] [CrossRef]
- Li, C.; Zhao, X.; Wang, A.; Huber, G.W.; Zhang, T. Catalytic Transformation of Lignin for the Production of Chemicals and Fuels. Chem. Rev. 2015, 115, 11559–11624. [Google Scholar] [CrossRef]
- Galkin, M.V.; Samec, J.S. Lignin Valorization through Catalytic Lignocellulose Fractionation: A Fundamental Platform for the Future Biorefinery. ChemSusChem 2016, 9, 1544–1558. [Google Scholar] [CrossRef]
- Renders, T.; Van den Bosch, S.; Koelewijn, S.F.; Schutyser, W.; Sels, B.F. Lignin-first biomass fractionation: The advent of active stabilisation strategies. Energy Environ. Sci. 2017, 10, 1551–1557. [Google Scholar] [CrossRef]
- Kuznetsov, B.N.; Sudakova, I.G.; Garyntseva, N.V.; Tarabanko, V.E.; Yatsenkova, O.V.; Djakovitch, L.; Rataboul, F. Processes of catalytic oxidation for the production of chemicals from softwood biomass. Catal. Today 2021, 375, 132–144. [Google Scholar] [CrossRef]
- Kuznetsov, B.N.; Sharypov, V.I.; Baryshnikov, S.V.; Miroshnikova, A.V.; Taran, O.P.; Yakovlev, V.A.; Lavrenov, A.V.; Djakovitch, L. Catalytic hydrogenolysis of native and organosolv lignins of aspen wood to liquid products in supercritical ethanol medium. Catal. Today 2021, 379, 114–123. [Google Scholar] [CrossRef]
- Wang, H.; Pu, Y.; Ragauskas, A.; Yang, B. From lignin to valuable products–strategies, challenges, and prospects. Bioresour. Technol. 2019, 271, 449–461. [Google Scholar] [CrossRef]
- Ullah, N.; Odda, A.H.; Liang, K.; Kombo, M.A.; Sahar, S.; Ma, L.-B.; Fang, X.-X.; Xu, A.-W. Metal–acid nanoplate-supported ultrafine Ru nanoclusters for efficient catalytic fractionation of lignin into aromatic alcohols. Green Chem. 2019, 21, 2739–2751. [Google Scholar] [CrossRef]
- Deuss, P.J.; Scott, M.; Tran, F.; Westwood, N.J.; de Vries, J.G.; Barta, K. Aromatic Monomers by in Situ Conversion of Reactive Intermediates in the Acid-Catalyzed Depolymerization of Lignin. J. Am. Chem. Soc. 2015, 137, 7456–7467. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Li, H.; Xiao, L.-P.; Sun, R.-C.; Song, G. Chemodivergent hydrogenolysis of eucalyptus lignin with Ni@ZIF-8 catalyst. Green Chem. 2019, 21, 1498–1504. [Google Scholar] [CrossRef]
- Van den Bosch, S.; Schutyser, W.; Koelewijn, S.F.; Renders, T.; Courtin, C.M.; Sels, B.F. Tuning the lignin oil OH-content with Ru and Pd catalysts during lignin hydrogenolysis on birch wood. Chem. Commun. 2015, 51, 13158–13161. [Google Scholar] [CrossRef] [Green Version]
- Zhang, K.; Li, H.; Xiao, L.-P.; Wang, B.; Sun, R.-C.; Song, G. Sequential utilization of bamboo biomass through reductive catalytic fractionation of lignin. Bioresour. Technol. 2019, 285, 121335. [Google Scholar] [CrossRef] [PubMed]
- Lawoko, M.; Henriksson, G.; Gellerstedt, G. Structural Differences between the Lignin−Carbohydrate Complexes Present in Wood and in Chemical Pulps. Biomacromolecules 2005, 6, 3467–3473. [Google Scholar] [CrossRef] [PubMed]
- Aminzadeh, S.; Zhang, L.; Henriksson, G. A possible explanation for the structural inhomogeneity of lignin in LCC networks. Wood Sci. Technol. 2017, 51, 1365–1376. [Google Scholar] [CrossRef] [Green Version]
- Teacă, C.A.; Roşu, D.; Bodîrlău, R.; Roşu, L. Structural Changes in Wood under Artificial UV Light Irradiation Determined by FTIR Spectroscopy and Color Measurements—A Brief Review. BioResources 2013, 8, 1478–1507. [Google Scholar] [CrossRef]
- Shi, J.-B.; Yang, Q.-L.; Lin, L.; Peng, L.-C. Fractionation and characterization of physicochemical and structural features of corn stalk hemicelluloses from yellow liquor of active oxygen cooking. Ind. Crops Prod. 2013, 44, 542–548. [Google Scholar] [CrossRef]
- Cheng, S.; Huang, A.; Wang, S.; Zhang, Q. Effect of Different Heat Treatment Temperatures on the Chemical Composition and Structure of Chinese Fir Wood. BioResources 2016, 11, 4006–4016. [Google Scholar] [CrossRef] [Green Version]
- Shi, J.; Xing, D.; Lia, J. FTIR Studies of the Changes in Wood Chemistry from Wood Forming Tissue under Inclined Treatment. Energy Proc. 2012, 16, 758–762. [Google Scholar] [CrossRef] [Green Version]
- Nelson, M.L.; O’Connor, R.T. Relation of certain infrared bands to cellulose crystallinity and crystal lattice type. Part II. A new infrared ratio for estimation of crystallinity in celluloses I and II. J. Appl. Polymer Sci. 1964, 8, 1325–1341. [Google Scholar] [CrossRef]
- Šturcová, A.; His, I.; Apperley, D.C.; Sugiyama, J.; Jarvis, M.C. Structural Details of Crystalline Cellulose from Higher Plants. Biomacromolecules 2004, 5, 1333–1339. [Google Scholar] [CrossRef]
- Park, S.; Baker, J.O.; Himmel, M.E.; Parilla, P.A.; Johnson, D.K. Cellulose crystallinity index: Measurement techniques and their impact on interpreting cellulase performance. Biotechnol. Biofuels 2010, 3, 10. [Google Scholar] [CrossRef] [Green Version]
- Sudakova, I.G.; Garyntseva, N.V.; Chudina, A.I.; Kuznetsov, B.N. Experimental and Mathematical Optimization of the Peroxide Delignification of Larch Wood in the Presence of MnSO4 Catalyst. Catal. Industry 2020, 12, 265–272. [Google Scholar] [CrossRef]
- Rinaldi, R.; Jastrzebski, R.; Clough, M.T.; Ralph, J.; Kennema, M.; Bruijnincx, P.C.A.; Weckhuysen, B.M. Paving the Way for Lignin Valorisation: Recent Advances in Bioengineering, Biorefining and Catalysis. Angew. Chem. Int. Ed. 2016, 55, 8164–8215. [Google Scholar] [CrossRef] [Green Version]
- Boonyasuwat, S.; Omotoso, T.; Resasco, D.E.; Crossley, S.P. Conversion of Guaiacol over Supported Ru Catalysts. Catal. Lett. 2013, 143, 783–791. [Google Scholar] [CrossRef]
- Yatsenkova, O.V.; Chudina, A.I.; Skripnikov, A.M.; Chesnokov, N.V.; Kuznetsov, B.N. The Influence of Sulfuric Acid Catalyst Concentration on Hydrolysis of Birch Wood Hemicelluloses. J. Sib. Federal Univ. Chem. 2015, 2, 211–221. [Google Scholar] [CrossRef]
- Thoorens, G.; Krier, F.; Leclercq, B.; Carlin, B.; Evrard, B. Microcrystalline cellulose, a direct compression binder in a quality by design environment—A review. Int. J. Pharm. 2014, 473, 64–72. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghanbarzadeh, B.; Oleyaei, S.A.; Almasi, H. Nano-Structured Materials Utilized in Biopolymer based Plastics for Food Packaging Applications. Crit. Rev. Food Sci. Nutr. 2014, 55, 1699–1723. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Fridrich, B.; de Santi, A.; Elangovan, S.; Barta, K. Bright Side of Lignin Depolymerization: Toward New Platform Chemicals. Chem. Rev. 2018, 118, 614–678. [Google Scholar] [CrossRef] [Green Version]
- Walker, J.C.F. Primary Wood Processing: Principles and Practice; Springer: Dordrecht, The Netherlands, 1993; pp. 1–596. [Google Scholar]
- Taran, O.P.; Polyanskaya, E.M.; Ogorodnikova, O.L.; Descorme, C.; Besson, M.; Parmon, V.N. Sibunit-based catalytic materials for the deep oxidation of organic ecotoxicants in aqueous solution: I. Surface properties of the oxidized sibunit samples. Catal. Ind. 2010, 2, 381–386. [Google Scholar] [CrossRef]
- Taran, O.P.; Descorme, C.; Polyanskaya, E.M.; Ayusheev, A.B.; Besson, M.; Parmon, V.N. Sibunit-based catalytic materials for the deep oxidation of organic ecotoxicants in aqueous solutions. III: Wet air oxidation of phenol over oxidized carbon and Rr/C catalysts. Catal. Ind. 2013, 5, 164–174. [Google Scholar] [CrossRef]
- Meng, X.; Bhagia, S.; Wang, Y.; Zhou, Y.; Pu, Y.; Dunlap, J.R.; Shuai, L.; Ragauskas, A.J.; Yoo, C.G. Effects of the advanced organosolv pretreatment strategies on structural properties of woody biomass. Ind. Crops Prod. 2020, 146, 112144. [Google Scholar] [CrossRef]
- Liu, X.; Feng, S.; Fang, Q.; Jiang, Z.; Hu, C. Reductive catalytic fractionation of lignin in birch sawdust to monophenolic compounds with high selectivity. Mol. Catal. 2020, 495, 111164. [Google Scholar] [CrossRef]
- Sluiter, J.B.; Ruiz, R.O.; Scarlata, C.J.; Sluiter, A.D.; Templeton, D.W. Compositional Analysis of Lignocellulosic Feedstocks. 1. Review and Description of Methods. J. Agricult. Food Chem. 2010, 58, 9043–9053. [Google Scholar] [CrossRef]
- Ruiz-Matute, A.I.; Hernández-Hernández, O.; Rodríguez-Sánchez, S.; Sanz, M.L.; Martínez-Castro, I. Derivatization of carbohydrates for GC and GC–MS analyses. J. Chromatogr. B 2011, 879, 1226–1240. [Google Scholar] [CrossRef]



| Sample of Wood | * Yield wt% | * Chemical Composition, wt% | ||
|---|---|---|---|---|
| Cellulose | Lignin | Hemicelluloses | ||
| Initial | - | 47.3 | 19.0 | 28.5 |
| Alkali treatment | 74.3 | 64.2 | 28.2 | 7.6 |
| Acid treatment 2.5 h | 86.7 | 63.4 | 29.7 | 7.0 |
| Acid treatment 5 h | 70.0 | 63.6 | 30.2 | 6.2 |
| Hydrogenation Conditions | Yield, wt% * | ||
|---|---|---|---|
| Liquid Products | Solid Product | Gases | |
| * non-catalytic a | 50.0 | 33.0 | 10.5 |
| ** non-catalytic b | 48.0 | 44.5 | 9.8 |
| ** non-catalytic c | 42.0 | 58.0 | 5.6 |
| * Ru/C a | 49.0 | 29.0 | 16.0 |
| ** Ru/C b | 52.0 | 41.6 | 8.4 |
| ** Ru/C c | 47.5 | 49.0 | 3.5 |
| Hydrogenation Conditions | C, wt% | H, wt% | O, wt% |
|---|---|---|---|
| non-catalytic a | 59.2 | 8.3 | 32.5 |
| non-catalytic b | 60.3 | 6.9 | 32.8 |
| non-catalytic c | 61.1 | 8.2 | 30.7 |
| Ru/C a | 60.8 | 8.8 | 30.4 |
| Ru/C b | 62.9 | 7.3 | 29.8 |
| Ru/C c | 68.0 | 8.5 | 23.5 |
| Initial wood | 49.9 | 6.1 | 44.0 |
| Hydrogenation Conditions | Number-Average Molecular Mass Mn(Da) | Mass-Average Molecular Mass Mw(Da) | Polydispersity PD |
|---|---|---|---|
| non-catalytic a | 698 | 1588 | 2.275 |
| non-catalytic b | 573 | 1508 | 2.632 |
| non-catalytic c | 701 | 1481 | 1.481 |
| Ru/C a | 374 | 524 | 1.401 |
| Ru/C b | 401 | 668 | 1.666 |
| Ru/C c | 390 | 664 | 1.703 |
| RT | Compound | Content, wt% * | ||||||
|---|---|---|---|---|---|---|---|---|
| Non- cat. a | Non- cat. b | Non- cat. c | Ru/C a | Ru/C b | Ru/C c | |||
| 24.39 | 4-ethylguaiacol | 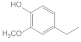 | 0.05 | 0.26 | 0.03 | 0.2 | 0.42 | 0.24 |
| 26.53 | Syringol | 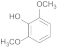 | no | 4.65 | 0.43 | 0.7 | 0.58 | 0.37 |
| 26.84 | 4-propylguaiacol | 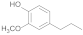 | 0.02 | 0.27 | 0.05 | 2.6 | 1.65 | 2.07 |
| 27.93 | 4-propenylguaiacol | 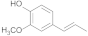 | 0.31 | 0.67 | 0.77 | 0.2 | 0.02 | 0.36 |
| 28.97 | 4-methylsyringol | 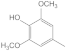 | 0.04 | 0.35 | 0.15 | 0.2 | 0.04 | 0.29 |
| 30.92 | 4-ethylsyringol | 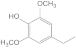 | 0.11 | 1.16 | 0.2 | 1.4 | 1.33 | 0.93 |
| 32.72 | 4-propenylsyringol | 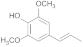 | 1.32 | 1.5 | 2.97 | 0.6 | no | 0.27 |
| 32.88 | 4-propylsyringol | 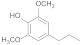 | 3.18 | 2.58 | 0.54 | 9.8 | 8.64 | 7.55 |
| 33.92 | 4-propanolguaiacol | 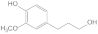 | no | no | no | no | 2.07 | no |
| 39.17 | 4-propanolsyringol | 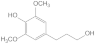 | no | 0.65 | no | 4.1 | 9.71 | 3.54 |
| Total content of alkylphenols | 5.03 | 12.09 | 5.14 | 19.8 | 24.46 | 15.62 | ||
| Other methoxyphenols | 0.68 | 1.94 | 0.8 | 0.28 | 0.41 | 0.48 | ||
| Hydrogenation Conditions | Composition of the Solid Product, wt% | ||
|---|---|---|---|
| Cellulose | Lignin | Hemicelluloses | |
| non-cat. a | 71.8 | 18.8 | 9.4 |
| non-cat. b | 78.7 | 14.2 | 7.1 |
| non-cat.c | 84.4 | 12.7 | 2.9 |
| Ru/C a | 87.0 | 7.8 | 5.2 |
| Ru/C b | 94.7 | 5.3 | 1.4 |
| Ru/C c | 95.0 | 3.8 | 1.2 |
| Sample | Crystallinity Index |
|---|---|
| Birch wood a | 0.59 |
| Birch wood b | 0.51 |
| Birch wood a | 0.69 |
| Solid product b | 0.64 |
| Solid product b | 0.54 |
| Solid product c | 0.70 |
| Solid product Ru/C a | 0.70 |
| Solid product Ru/C b | 0.62 |
| Solid product Ru/C c | 0.74 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuznetsov, B.N.; Baryshnikov, S.V.; Miroshnikova, A.V.; Kazachenko, A.S.; Malyar, Y.N.; Skripnikov, A.M.; Taran, O.P. Fractionation of Birch Wood by Integrating Alkaline-Acid Treatments and Hydrogenation in Ethanol over a Bifunctional Ruthenium Catalyst. Catalysts 2021, 11, 1362. https://doi.org/10.3390/catal11111362
Kuznetsov BN, Baryshnikov SV, Miroshnikova AV, Kazachenko AS, Malyar YN, Skripnikov AM, Taran OP. Fractionation of Birch Wood by Integrating Alkaline-Acid Treatments and Hydrogenation in Ethanol over a Bifunctional Ruthenium Catalyst. Catalysts. 2021; 11(11):1362. https://doi.org/10.3390/catal11111362
Chicago/Turabian StyleKuznetsov, Boris N., Sergey V. Baryshnikov, Angelina V. Miroshnikova, Aleksandr S. Kazachenko, Yuriy N. Malyar, Andrey M. Skripnikov, and Oxana P. Taran. 2021. "Fractionation of Birch Wood by Integrating Alkaline-Acid Treatments and Hydrogenation in Ethanol over a Bifunctional Ruthenium Catalyst" Catalysts 11, no. 11: 1362. https://doi.org/10.3390/catal11111362
APA StyleKuznetsov, B. N., Baryshnikov, S. V., Miroshnikova, A. V., Kazachenko, A. S., Malyar, Y. N., Skripnikov, A. M., & Taran, O. P. (2021). Fractionation of Birch Wood by Integrating Alkaline-Acid Treatments and Hydrogenation in Ethanol over a Bifunctional Ruthenium Catalyst. Catalysts, 11(11), 1362. https://doi.org/10.3390/catal11111362









