Water Splitting on Multifaceted SrTiO3 Nanocrystals: Computational Study
Abstract
:1. Introduction
2. Methods and Computational Details
3. Results and Discussion
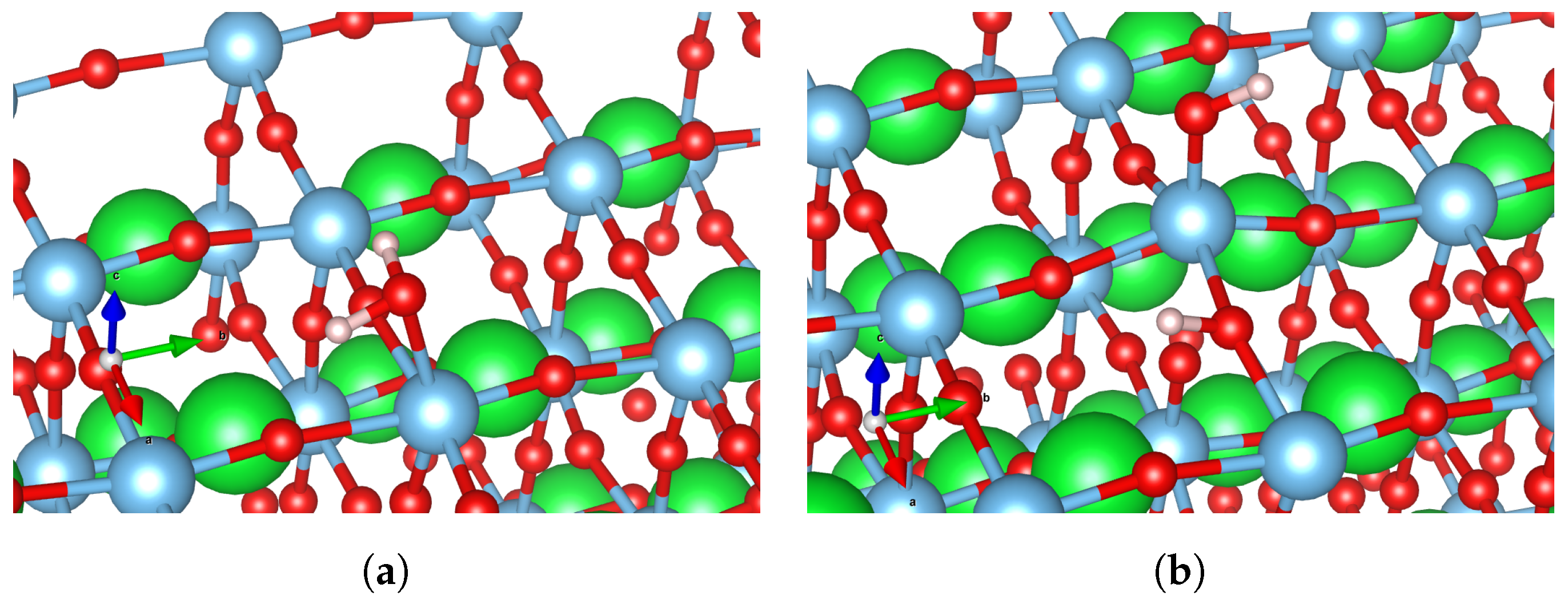
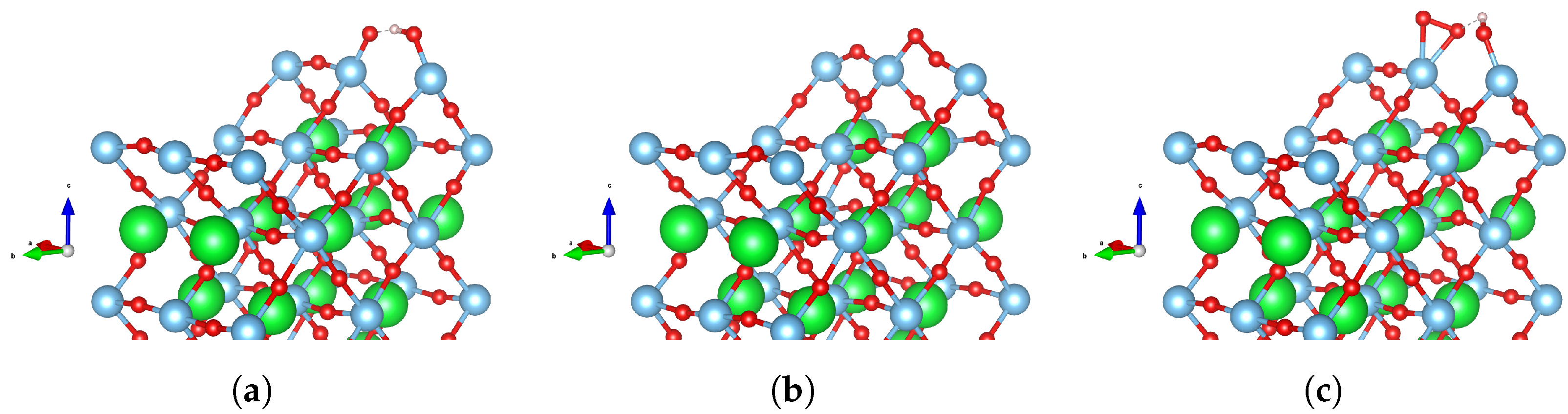
3.1. Water Adsorption
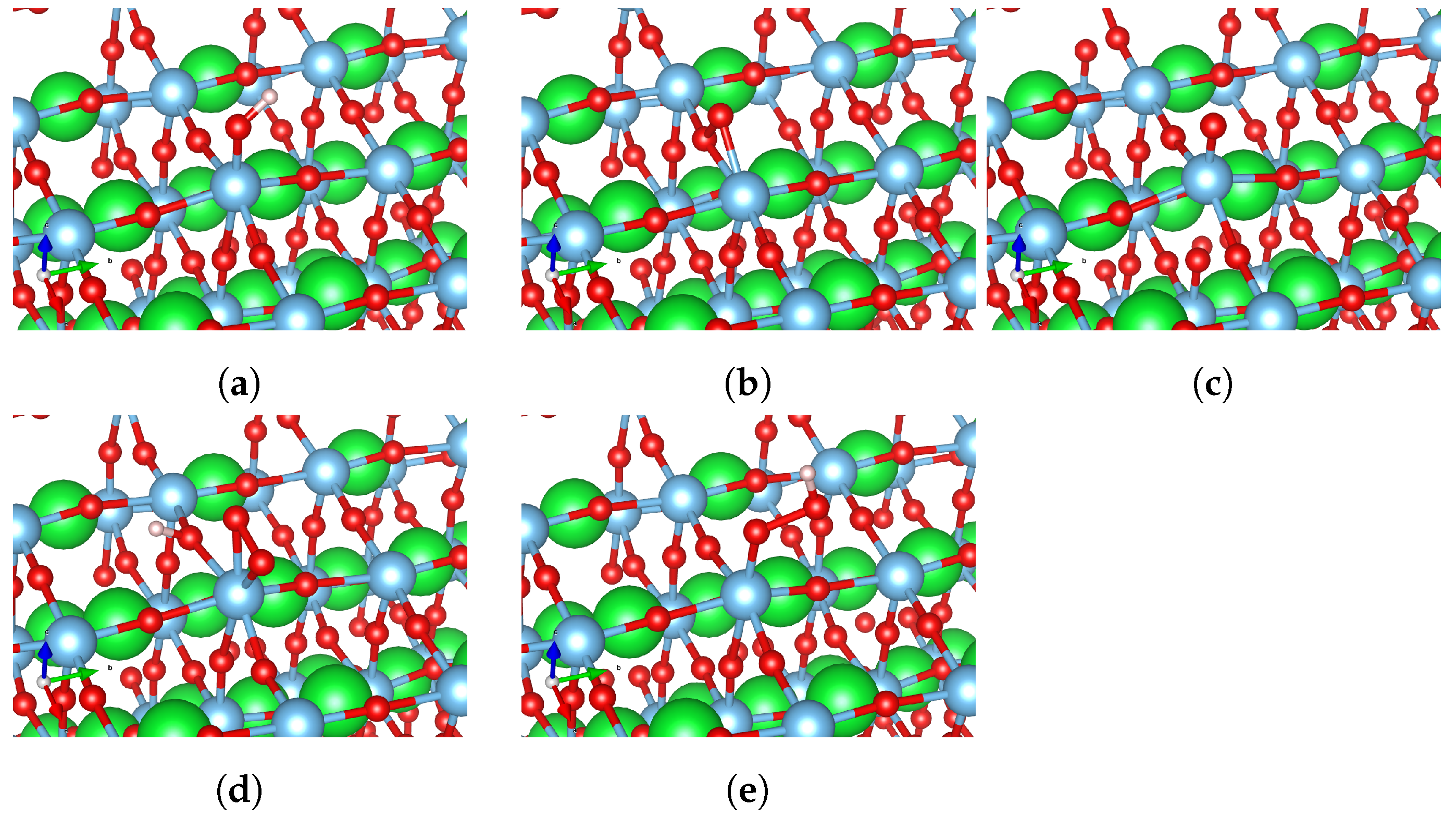
3.2. Oxygen Evolution Reaction (OER) Intermediates
4. Conclusions
- The ridge region permits dissociative water adsorption only, accompanied by spontaneous formation of oxygen vacancy;
- Results for the flat surface are in agreement with other computational studies [13];
- On the slope region, both molecular and dissociative adsorption modes are possible;
- Adsorption of both water and its intermediates on the slope region is similar to that on flat surfaces;
- Except for atomic hydrogen, no adsorption was observed on the gully region; and
- There are different adsorption configurations of OER intermediates possible on flat surfaces and slope regions.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kato, H.; Kudo, A. Visible-Light-Response and Photocatalytic Activities of TiO2 and SrTiO3 Photocatalysts Codoped with Antimony and Chromium. J. Phys. Chem. B 2002, 106, 5029–5034. [Google Scholar] [CrossRef]
- Miyauchi, M.; Nakajima, A.; Watanabe, T.; Hashimoto, K. Photocatalysis and Photoinduced Hydrophilicity of Various Metal Oxide Thin Films. Chem. Mater. 2002, 14, 2812–2816. [Google Scholar] [CrossRef]
- Konta, R.; Ishii, T.; Kato, H.; Kudo, A. Photocatalytic Activities of Noble Metal Ion Doped SrTiO3 under Visible Light Irradiation. J. Phys. Chem. B 2004, 108, 8992–8995. [Google Scholar] [CrossRef]
- Yu, S.C.; Huang, C.W.; Liao, C.H.; Wu, J.C.; Chang, S.T.; Chen, K.H. A novel membrane reactor for separating hydrogen and oxygen in photocatalytic water splitting. J. Membr. Sci. 2011, 382, 291–299. [Google Scholar] [CrossRef]
- Kato, H.; Kobayashi, M.; Hara, M.; Kakihana, M. Fabrication of SrTiO3 exposing characteristic facets using molten salt flux and improvement of photocatalytic activity for water splitting. Catal. Sci. Technol. 2013, 3, 1733. [Google Scholar] [CrossRef]
- Wang, B.; Shen, S.; Guo, L. SrTiO3 single crystals enclosed with high-indexed 023 facets and 001 facets for photocatalytic hydrogen and oxygen evolution. Appl. Catal. B Environ. 2015, 166–167, 320–326. [Google Scholar] [CrossRef]
- Ham, Y.; Hisatomi, T.; Goto, Y.; Moriya, Y.; Sakata, Y.; Yamakata, A.; Kubota, J.; Domen, K. Flux-mediated doping of SrTiO3 photocatalysts for efficient overall water splitting. J. Mater. Chem. A 2016, 4, 3027–3033. [Google Scholar] [CrossRef] [Green Version]
- Foo, G.S.; Hood, Z.D.; Wu, Z. Shape Effect Undermined by Surface Reconstruction: Ethanol Dehydrogenation over Shape-Controlled SrTiO3 Nanocrystals. ACS Catal. 2018, 8, 555–565. [Google Scholar] [CrossRef]
- Li, D.; Yu, J.C.C.; Nguyen, V.H.; Wu, J.C.; Wang, X. A dual-function photocatalytic system for simultaneous separating hydrogen from water splitting and photocatalytic degradation of phenol in a twin-reactor. Appl. Catal. B Environ. 2018, 239, 268–279. [Google Scholar] [CrossRef]
- Kampouri, S.; Stylianou, K.C. Dual-Functional Photocatalysis for Simultaneous Hydrogen Production and Oxidation of Organic Substances. ACS Catal. 2019, 9, 4247–4270. [Google Scholar] [CrossRef]
- Kanazawa, T.; Nozawa, S.; Lu, D.; Maeda, K. Structure and Photocatalytic Activity of PdCrOx Cocatalyst on SrTiO3 for Overall Water Splitting. Catalysts 2019, 9, 59. [Google Scholar] [CrossRef] [Green Version]
- Saleem, Z.; Pervaiz, E.; Yousaf, M.U.; Niazi, M.B.K. Two-dimensional materials and composites as potential water splitting photocatalysts: A review. Catalysts 2020, 10, 464. [Google Scholar] [CrossRef]
- Guhl, H.; Miller, W.; Reuter, K. Water adsorption and dissociation on SrTiO3 (001) revisited: A density functional theory study. Phys. Rev. B Condens. Matter Mater. Phys. 2010, 81, 155455. [Google Scholar] [CrossRef] [Green Version]
- Cui, M.; Liu, T.; Li, Q.; Yang, J.; Jia, Y. Oxygen Evolution Reaction (OER) on Clean and Oxygen Deficient Low-Index SrTiO3 Surfaces: A Theoretical Systematic Study. ACS Sustain. Chem. Eng. 2019, 7, 15346–15353. [Google Scholar] [CrossRef]
- Holmström, E.; Spijker, P.; Foster, A.S. The interface of SrTiO3 and H2O from density functional theory molecular dynamics. Proc. R. Soc. A Math. Phys. Eng. Sci. 2016, 472. [Google Scholar] [CrossRef] [Green Version]
- Chen, H.C.; Huang, C.W.; Wu, J.C.S.; Lin, S.T. Theoretical Investigation of the Metal-Doped SrTiO3 Photocatalysts for Water Splitting. J. Phys. Chem. C 2012, 116, 7897–7903. [Google Scholar] [CrossRef]
- Afroz, K.; Moniruddin, M.; Bakranov, N.; Kudaibergenov, S.; Nuraje, N. A heterojunction strategy to improve the visible light sensitive water splitting performance of photocatalytic materials. J. Mater. Chem. A 2018, 6, 21696–21718. [Google Scholar] [CrossRef]
- Maček Kržmanc, M.; Daneu, N.; Čontala, A.; Santra, S.; Kamal, K.M.; Likozar, B.; Spreitzer, M. SrTiO3/Bi4Ti3O12Nanohetero structural Platelets Synthesized by Topotactic Epitaxy as Effective Noble-Metal-Free Photocatalysts for pH-Neutral Hydrogen Evolution. ACS Appl. Mater. Interfaces 2021, 13, 370–381. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Yu, J.; Jaroniec, M. Hierarchical photocatalysts. Chem. Soc. Rev. 2016, 45, 2603–2636. [Google Scholar] [CrossRef]
- Takata, T.; Jiang, J.; Sakata, Y.; Nakabayashi, M.; Shibata, N.; Nandal, V.; Seki, K.; Hisatomi, T.; Domen, K. Photocatalytic water splitting with a quantum efficiency of almost unity. Nature 2020, 581, 411–414. [Google Scholar] [CrossRef]
- Mu, L.; Zhao, Y.; Li, A.; Wang, S.; Wang, Z.; Yang, J.; Wang, Y.; Liu, T.; Chen, R.; Zhu, J.; et al. Enhancing charge separation on high symmetry SrTiO3 exposed with anisotropic facets for photocatalytic water splitting. Energy Environ. Sci. 2016, 9, 2463–2469. [Google Scholar] [CrossRef]
- Tomio, T.; Miki, H.; Tabata, H.; Kawai, T.; Kawai, S. Control of electrical conductivity in laser deposited SrTiO3 thin films with Nb doping. J. Appl. Phys. 1994, 76, 5886–5890. [Google Scholar] [CrossRef]
- Noguera, C. Polar oxide surfaces. J. Phys. Condens. Matter 2000, 12, R367–R410. [Google Scholar] [CrossRef]
- Bachelet, R.; Valle, F.; Infante, I.C.; Sánchez, F.; Fontcuberta, J. Step formation, faceting, and bunching in atomically flat SrTiO3 (110) surfaces. Appl. Phys. Lett. 2007, 91, 251904. [Google Scholar] [CrossRef]
- Man, I.C.; Su, H.Y.; Calle-Vallejo, F.; Hansen, H.A.; Martínez, J.I.; Inoglu, N.G.; Kitchin, J.; Jaramillo, T.F.; Nørskov, J.K.; Rossmeisl, J. Universality in Oxygen Evolution Electrocatalysis on Oxide Surfaces. ChemCatChem 2011, 3, 1159–1165. [Google Scholar] [CrossRef]
- Kresse, G.; Hafner, J. Ab initio molecular dynamics for liquid metals. Phys. Rev. B 1993, 47, 558–561. [Google Scholar] [CrossRef]
- Kresse, G.; Hafner, J. Ab initio molecular-dynamics simulation of the liquid-metal–amorphous-semiconductor transition in germanium. Phys. Rev. B 1994, 49, 14251–14269. [Google Scholar] [CrossRef] [PubMed]
- Kresse, G.; Furthmüller, J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comput. Mater. Sci. 1996, 6, 15–50. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 1996, 54, 11169–11186. [Google Scholar] [CrossRef]
- Momma, K.; Izumi, F. VESTA 3 for three-dimensional visualization of crystal, volumetric and morphology data. J. Appl. Crystallogr. 2011, 44, 1272–1276. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vanderbilt, D. Soft self-consistent pseudopotentials in a generalized eigenvalue formalism. Phys. Rev. B 1990, 41, 7892–7895. [Google Scholar] [CrossRef]
- Kresse, G.; Hafner, J. Norm-conserving and ultrasoft pseudopotentials for first-row and transition elements. J. Phys. Condens. Matter 1994, 6, 8245–8257. [Google Scholar] [CrossRef]
- Blöchl, P.E. Projector augmented-wave method. Phys. Rev. B 1994, 50, 17953–17979. [Google Scholar] [CrossRef] [Green Version]
- Kresse, G.; Joubert, D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B 1999, 59, 1758–1775. [Google Scholar] [CrossRef]
- Monkhorst, H.J.; Pack, J.D. Special points for Brillouin-zone integrations. Phys. Rev. B 1976, 13, 5188–5192. [Google Scholar] [CrossRef]


| Software | VASP 6 [27,28,29] |
| Exchange-correlation functional | GGA-PBE [31] |
| Pseudopotentials | Ultra Soft [32,33] potentials using the Projector Augmented Wave (PAW) method [34,35] |
| Smearing | Gaussian smearing |
| Ti-valence configuration | , valence 10, energy cutoff
222 eV, generated 07.09.2000 |
| Sr-valence configuration | , valence 10, energy cutoff
229 eV, generated 07.09.2000 |
| O-valence configuration | , valence 6, energy cutoff
400 eV, generated 08.04.2002 |
| H-valence configuration | , valence 1, energy cutoff
250 eV, generated 15.06.2001 |
| Spin polarization | Non-spin polarized calculation |
| Plane wave basis set cut-off | 520 eV |
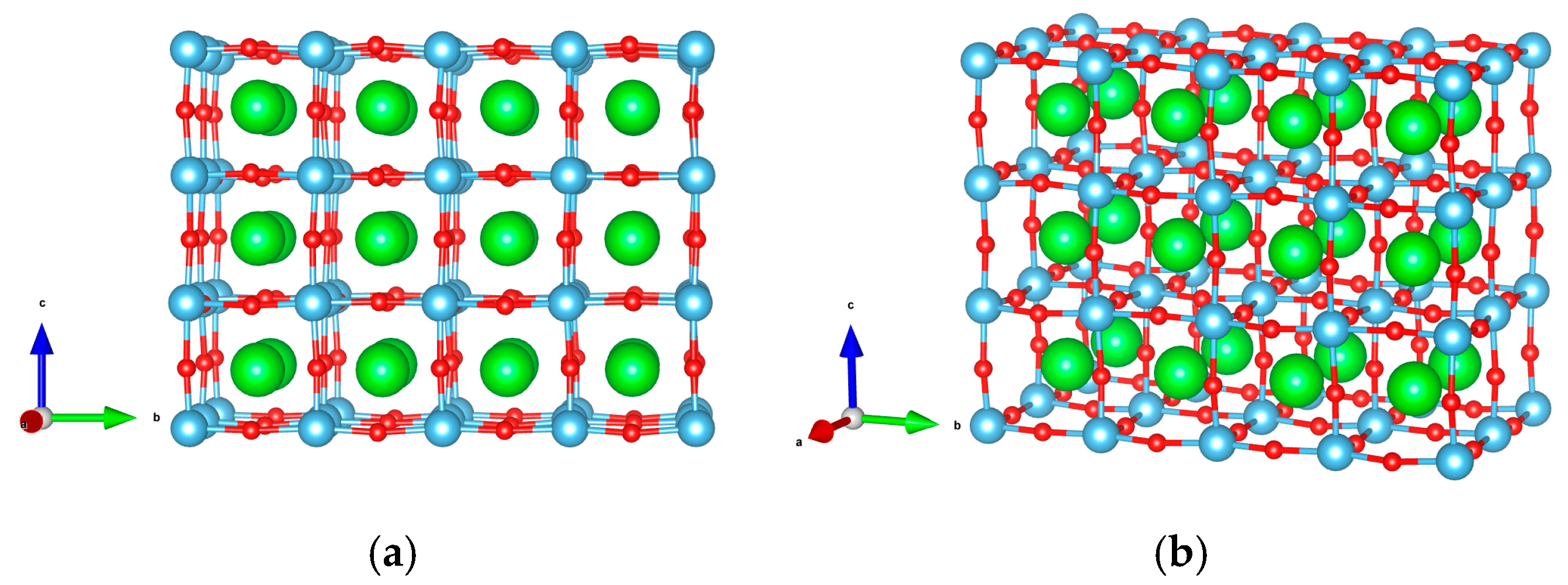
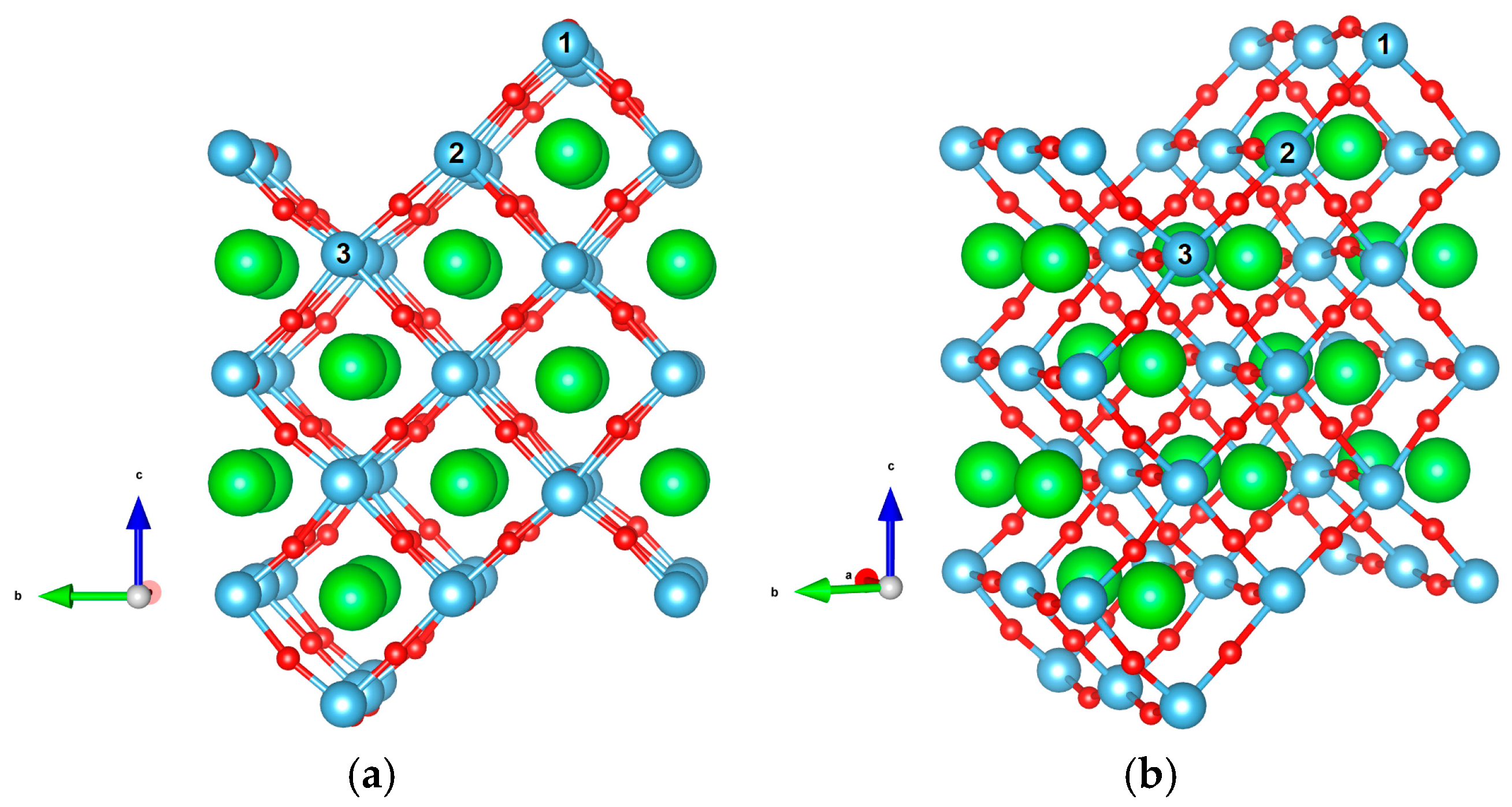
| Flat surface geometry (Figure 1) | surface cell, seven layers-thick,
20 Å vacuum gap, 144 atoms |
| Stepped surface geometry (Figure 2) | , thickness,
10 Å vacuum gap, 104 atoms |
| Flat surface k-point mesh | Monkhorst-Pack [36] |
| Stepped surface k-point mesh | Monkhorst-Pack [36] |
| Surface Type | HO*, eV | O*, eV | HOO*, eV |
|---|---|---|---|
| Flat surface | 0.77 (Figure 5a) | 2.87 (Figure 5b)/ 3.52 (Figure 5c) | 3.64 (Figure 5d)/ 4.24 (Figure 5e) |
| Slope | 1.35 (Figure 6a) | 2.93 (Figure 6b)/ 4.11 (Figure 6c) | 3.48 (Figure 6d)/ 4.52 (Figure 6e) |
| Ridge | 1.24 (Figure 7a) | 2.40 (Figure 7b) | 3.13 (Figure 7c) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sokolov, M.; Mastrikov, Y.A.; Zvejnieks, G.; Bocharov, D.; Kotomin, E.A.; Krasnenko, V. Water Splitting on Multifaceted SrTiO3 Nanocrystals: Computational Study. Catalysts 2021, 11, 1326. https://doi.org/10.3390/catal11111326
Sokolov M, Mastrikov YA, Zvejnieks G, Bocharov D, Kotomin EA, Krasnenko V. Water Splitting on Multifaceted SrTiO3 Nanocrystals: Computational Study. Catalysts. 2021; 11(11):1326. https://doi.org/10.3390/catal11111326
Chicago/Turabian StyleSokolov, Maksim, Yuri A. Mastrikov, Guntars Zvejnieks, Dmitry Bocharov, Eugene A. Kotomin, and Veera Krasnenko. 2021. "Water Splitting on Multifaceted SrTiO3 Nanocrystals: Computational Study" Catalysts 11, no. 11: 1326. https://doi.org/10.3390/catal11111326






