Current Trends and Future Prospects of Nanotechnology in Biofuel Production
Abstract
:1. Introduction
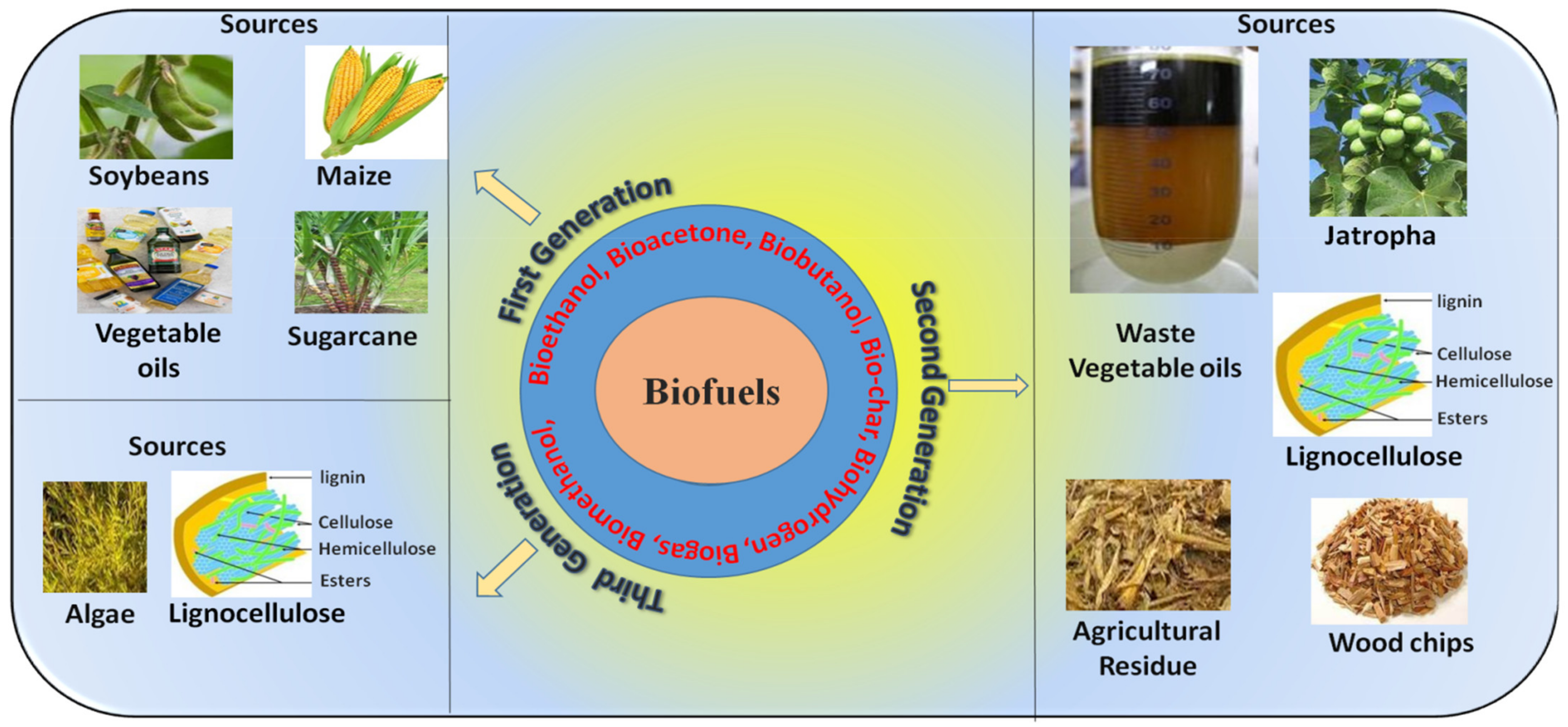
2. Biofuel Types
3. Different Nanoparticles in Biofuel Production
3.1. Carbon Nanotubes (CNTs)
3.2. Magnetic Nanoparticles
3.3. Acid Functionalized Nanoparticles
3.4. Metallic Nanoparticles
3.5. Metal-Oxide Nanoparticles
4. Nanoparticles in Heterogeneous Catalysis
5. Applications
5.1. Biohydrogen Production
5.2. Effectiveness of Nanoparticles in Biogas Generation for Industrial Benefits
5.3. Bioethanol
5.4. Biodiesel
6. Current Challenges and Future Perspectives for Biofuel Production with the Implementation of Nanotechnology
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rai, M.; Dos Santos, J.C.; Soler, M.F.; Franco Marcelino, P.R.; Brumano, L.P.; Ingle, A.P.; Gaikwad, S.; Gade, A.; Da Silva, S.S. Strategic Role of Nanotechnology for Production of Bioethanol and Biodiesel. Nanotechnol. Rev. 2016, 5, 231–250. [Google Scholar] [CrossRef]
- Bhattarai, K.; Stalick, W.M.; Mckay, S.; Geme, G.; Bhattarai, N. Biofuel: An Alternative to Fossil Fuel for Alleviating World Energy and Economic Crises. J. Environ. Sci. Health Part A Toxic 2011, 46, 1424–1442. [Google Scholar] [CrossRef]
- Shalaby, E.A. Biofuel: Sources, Extraction and Determination. In Liquid, Gaseous and Solid Biofuels; Fang, Z., Ed.; IntechOpen: Rijeka, Croatia, 2013. [Google Scholar]
- Folaranmi, J. Production of Biodiesel (B100) from Jatropha Oil Using Sodium Hydroxide as Catalyst. J. Pet. Eng. 2013, 2013, 1–6. [Google Scholar] [CrossRef]
- Forde, C.J.; Meaney, M.; Carrigan, J.B.; Mills, C.; Boland, S.; Hernon, A. Biobased Fats (Lipids) and Oils from Biomass as a Source of Bioenergy. Bioenergy Res. Adv. Appl. 2014, 185–201. [Google Scholar] [CrossRef]
- Ahmad, M.; Ajab, M.; Zafar, M.; Sult, S. Biodiesel from Non Edible Oil Seeds: A Renewable Source of Bioenergy. Econ. Eff. Biofuel Prod. 2011, 2005. [Google Scholar] [CrossRef] [Green Version]
- Mohadi, R.; Harahap, A.H.; Hidayati, N.; Lesbani, A. Transesterification of Tropical Edible Oils to Biodiesel Using Catalyst From Scylla Serrata. Sriwij. J. Environ. 2016, 1, 24–27. [Google Scholar] [CrossRef] [Green Version]
- Thirumarimurugan, M.; Sivakumar, V.M.; Xavier, A.M.; Prabhakaran, D.; Kannadasan, T. Preparation of Biodiesel from Sunflower Oil by Transesterification. IJBBB 2012, 441–444. [Google Scholar] [CrossRef]
- Ahmmed, B.; Samaddar, O.U.; Kibria, K.Q. Production of Biodiesel from Used Vegetable Oils Production of Biodiesel from Used Vegetable Oils. Int. J. Sci. Res. Sci. Technol. 2019, 6. [Google Scholar] [CrossRef]
- Do Nascimento, R.O.; Rebelo, L.M.; Sacher, E. Physicochemical Characterizations of Nanoparticles Used for Bioenergy and Biofuel Production. In Nanotechnology for Bioenergy and Biofuel Production; Rai, M., da Silva, S.S., Eds.; Springer: Cham, Switzerland, 2017; pp. 173–191. ISBN 978-3-319-45459-7. [Google Scholar]
- Dikshit, P.K.; Kumar, J.; Das, A.K.; Sadhu, S.; Sharma, S.; Singh, S.; Gupta, P.K.; Kim, B.S. Green Synthesis of Metallic Nanoparticles: Applications and Limitations. Catalysts 2021, 11, 902. [Google Scholar] [CrossRef]
- Saoud, K. Nanocatalyst for Biofuel Production: A Review. In Green Nanotechnology for Biofuel Production; Srivastava, N., Srivastava, M., Pandey, H., Mishra, P.K., Ramteke, P.W., Eds.; Springer: Cham, Switzerland, 2018; pp. 39–62. ISBN 978-3-319-75052-1. [Google Scholar]
- Singh, N.; Dhanya, B.S.; Verma, M.L. Nano-Immobilized Biocatalysts and Their Potential Biotechnological Applications in Bioenergy Production. Mater. Sci. Energy Technol. 2020, 3, 808–824. [Google Scholar] [CrossRef]
- Ali, S.; Shafique, O.; Mahmood, S.; Mahmood, T.; Khan, B.A.; Ahmad, I. Biofuels Production from Weed Biomass Using Nanocatalyst Technology. Biomass Bioenergy 2020, 139, 105595. [Google Scholar] [CrossRef]
- Hussain, S.T.; Ali, S.A.; Bano, A.; Mahmood, T. Use of Nanotechnology for the Production of Biofuels from Butchery Waste. Int. J. Phys. Sci. 2011, 6, 7271–7279. [Google Scholar] [CrossRef]
- Kumar, Y.; Yogeshwar, P.; Bajpai, S.; Jaiswal, P.; Yadav, S.; Pathak, D.P.; Sonker, M.; Tiwary, S.K. Nanomaterials: Stimulants for Biofuels and Renewables, Yield and Energy Optimization. Mater. Adv. 2021, 2, 5318–5343. [Google Scholar] [CrossRef]
- Malode, S.J.; Prabhu, K.K.; Mascarenhas, R.J.; Shetti, N.P.; Aminabhavi, T.M. Recent Advances and Viability in Biofuel Production. Energy Convers. Manag. X 2021, 10, 100070. [Google Scholar] [CrossRef]
- Hirani, A.H.; Javed, N.; Asif, M.; Basu, S.K.; Kumar, A. A Review on First- and Second-Generation Biofuel Productions. In Biofuels: Greenhouse Gas Mitigation and Global Warming: Next Generation Biofuels and Role of Biotechnology; Kumar, A., Ogita, S., Yau, Y.-Y., Eds.; Springer: New Delhi, India, 2018; pp. 141–154. ISBN 978-81-322-3763-1. [Google Scholar]
- Dahman, Y.; Syed, K.; Begum, S.; Roy, P.; Mohtasebi, B. 14-Biofuels: Their characteristics and analysis. In Biomass, Biopolymer-Based Materials, and Bioenergy; Verma, D., Fortunati, E., Jain, S., Zhang, X., Eds.; Woodhead Publishing Series in Composites Science and Engineering; Woodhead Publishing: Cambridge, UK, 2019; pp. 277–325. ISBN 978-0-08-102426-3. [Google Scholar]
- Liu, Z.; Lv, F.; Zheng, H.; Zhang, C.; Wei, F.; Xing, X.-H. Enhanced Hydrogen Production in a UASB Reactor by Retaining Microbial Consortium onto Carbon Nanotubes (CNTs). Int. J. Hydrog. Energy 2012, 37, 10619–10626. [Google Scholar] [CrossRef]
- Saifuddin, N.; Raziah, A.Z.; Junizah, A.R. Carbon Nanotubes: A Review on Structure and Their Interaction with Proteins. J. Chem. 2013, 2013, 1–18. [Google Scholar] [CrossRef]
- Ando, Y.; Zhao, X.; Shimoyama, H.; Sakai, G.; Kaneto, K. Physical Properties of Multiwalled Carbon Nanotubes. Int. J. Inorg. Mater. 1999, 1, 77–82. [Google Scholar] [CrossRef]
- Dresselhaus, M.S.; Dresselhaus, G.; Eklund, P.C.; Rao, A.M. Carbon Nanotubes. In The Physics of Fullerene-Based and Fullerene-Related Materials; Andreoni, W., Ed.; Springer: Dordrecht, The Netherlands, 2000; pp. 331–379. ISBN 978-94-011-4038-6. [Google Scholar]
- Peng, F.; Zhang, L.; Wang, H.; Lv, P.; Yu, H. Sulfonated Carbon Nanotubes as a Strong Protonic Acid Catalyst. Carbon 2005, 43, 2405–2408. [Google Scholar] [CrossRef]
- Boshagh, F.; Rostami, K.; Moazami, N. Biohydrogen Production by Immobilized Enterobacter Aerogenes on Functionalized Multi-Walled Carbon Nanotube. Int. J. Hydrog. Energy 2019, 44, 14395–14405. [Google Scholar] [CrossRef]
- Feng, W.; Ji, P. Enzymes Immobilized on Carbon Nanotubes. Biotechnol. Adv. 2011, 29, 889–895. [Google Scholar] [CrossRef]
- Lee, D.G.; Ponvel, K.M.; Kim, M.; Hwang, S.; Ahn, I.S.; Lee, C.H. Immobilization of Lipase on Hydrophobic Nano-Sized Magnetite Particles. J. Mol. Catal. B Enzym. 2009, 57, 62–66. [Google Scholar] [CrossRef]
- Pavlidis, I.V.; Tsoufis, T.; Enotiadis, A.; Gournis, D.; Stamatis, H. Functionalized Multi-Wall Carbon Nanotubes for Lipase Immobilization. Adv. Eng. Mater. 2010, 12, B179–B183. [Google Scholar] [CrossRef]
- Khan, M.; Anwer, T.; Mohammad, F. Sensing Properties of Sulfonated Multi-Walled Carbon Nanotube and Graphene Nanocomposites with Polyaniline. J. Sci. Adv. Mater. Devices 2019, 4, 132–142. [Google Scholar] [CrossRef]
- Deep, A.; Sharma, A.L.; Kumar, P. Lipase Immobilized Carbon Nanotubes for Conversion of Jatropha Oil to Fatty Acid Methyl Esters. Biomass Bioenergy 2015, 81, 83–87. [Google Scholar] [CrossRef]
- Verma, M.; Naebe, M.; Barrow, C.; Puri, M. Enzyme Immobilisation on Amino-Functionalised Multi-Walled Carbon Nanotubes: Structural and Biocatalytic Characterisation. PLoS ONE 2013, 8, e73642. [Google Scholar] [CrossRef] [PubMed]
- Nazaruk, E.; Sadowska, K.; Biernat, J.F.; Rogalski, J.; Ginalska, G.; Bilewicz, R. Enzymatic Electrodes Nanostructured with Functionalized Carbon Nanotubes for Biofuel Cell Applications. Anal. Bioanal. Chem. 2010, 398, 1651–1660. [Google Scholar] [CrossRef] [PubMed]
- Shu, Q.; Zhang, Q.; Xu, G.; Wang, J. Preparation of Biodiesel Using S-MWCNT Catalysts and the Coupling of Reaction and Separation. Food Bioprod. Process. 2009, 87, 164–170. [Google Scholar] [CrossRef]
- Ahmad, R.; Khare, S.K. Immobilization of Aspergillus Niger Cellulase on Multiwall Carbon Nanotubes for Cellulose Hydrolysis. Bioresour. Technol. 2018, 252, 72–75. [Google Scholar] [CrossRef]
- Mubarak, N.M.; Wong, J.R.; Tan, K.W.; Sahu, J.N.; Abdullah, E.C.; Jayakumar, N.S.; Ganesan, P. Immobilization of Cellulase Enzyme on Functionalized Multiwall Carbon Nanotubes. J. Mol. Catal. B Enzym. 2014, 107, 124–131. [Google Scholar] [CrossRef]
- Tran, D.T.; Chen, C.L.; Chang, J.S. Immobilization of Burkholderia Sp. Lipase on a Ferric Silica Nanocomposite for Biodiesel Production. J. Biotechnol. 2012, 158, 112–119. [Google Scholar] [CrossRef]
- Verma, M.L.; Chaudhary, R.; Tsuzuki, T.; Barrow, C.J.; Puri, M. Immobilization of β-Glucosidase on a Magnetic Nanoparticle Improves Thermostability: Application in Cellobiose Hydrolysis. Bioresour. Technol. 2013, 135, 2–6. [Google Scholar] [CrossRef]
- Puri, M.; Barrow, C.J.; Verma, M.L. Enzyme Immobilization on Nanomaterials for Biofuel Production. Trends Biotechnol. 2013, 31, 215–216. [Google Scholar] [CrossRef]
- Singh, O.V.; Chandel, A.K. Sustainable Biotechnology-Enzymatic Resources of Renewable Energy; Springer: Berlin/Heidelberg, Germany, 2018; ISBN 9783319954806. [Google Scholar]
- Alftrén, J.; Hobley, T.J. Covalent Immobilization of β-Glucosidase on Magnetic Particles for Lignocellulose Hydrolysis. Appl. Biochem. Biotechnol. 2013, 169, 2076–2087. [Google Scholar] [CrossRef] [PubMed]
- Huang, P.J.; Chang, K.L.; Hsieh, J.F.; Chen, S.T. Catalysis of Rice Straw Hydrolysis by the Combination of Immobilized Cellulase from Aspergillus Niger on β -Cyclodextrin-Fenanoparticles and Ionic Liquid. BioMed Res. Int. 2015, 2015, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Teo, S.H.; Islam, A.; Chan, E.S.; Thomas Choong, S.Y.; Alharthi, N.H.; Taufiq-Yap, Y.H.; Awual, M.R. Efficient Biodiesel Production from Jatropha Curcus Using CaSO4/Fe2O3-SiO2 Core-Shell Magnetic Nanoparticles. J. Clean. Prod. 2019, 208, 816–826. [Google Scholar] [CrossRef]
- Shakeel, N.; Ahamed, M.I.; Ahmed, A.; Rahman, M.M; Asiri, A.M. Functionalized Magnetic Nanoparticle-Reduced Graphene Oxide Nanocomposite for Enzymatic Biofuel Cell Applications. Int. J. Hydrog. Energy 2019, 44, 28294–28304. [Google Scholar] [CrossRef]
- Dantas, J.; Leal, E.; Mapossa, A.B.; Cornejo, D.R.; Costa, A.C.F.M. Magnetic nanocatalysts of Ni0.5Zn0.5Fe2O4 doped with Cu and performance evaluation in transesterification reaction for biodiesel production. Fuel 2017, 191, 463–471. [Google Scholar] [CrossRef]
- Cherian, E.; Dharmendirakumar, M.; Baskar, G. Immobilization of Cellulase onto MnO2 Nanoparticles for Bioethanol Production by Enhanced Hydrolysis of Agricultural Waste. Chin. J. Catal. 2015, 36, 1223–1229. [Google Scholar] [CrossRef]
- Ivanova, V.; Petrova, P.; Hristov, J. Application in the Ethanol Fermentation of Immobilized Yeast Cells in Matrix of Alginate/Magnetic Nanoparticles, on Chitosan-Magnetite Microparticles and Cellulose-Coated Magnetic Nanoparticles. arXiv preprint 2011, arXiv:1105.0619. [Google Scholar]
- Lee, K.H.; Choi, I.S.; Kim, Y.-G.; Yang, D.-J.; Bae, H.-J. Enhanced Production of Bioethanol and Ultrastructural Characteristics of Reused Saccharomyces Cerevisiae Immobilized Calcium Alginate Beads. Bioresour. Technol. 2011, 102, 8191–8198. [Google Scholar] [CrossRef]
- Duraiarasan, S.; Razack, S.A.; Manickam, A.; Munusamy, A.; Syed, M.B.; Ali, M.Y.; Ahmed, G.M.; Mohiuddin, M.S. Direct Conversion of Lipids from Marine Microalga C. Salina to Biodiesel with Immobilised Enzymes Using Magnetic Nanoparticle. J. Environ. Chem. Eng. 2016, 4, 1393–1398. [Google Scholar] [CrossRef]
- Mahmood, T.; Zada, B.; Malik, S.A. Effect of Iron Nanoparticles on Hyacinth’s Fermentation. Int. J. Sci. 2013, 2, 106–121. [Google Scholar]
- Taherdanak, M.; Zilouei, H.; Karimi, K. Investigating the Effects of Iron and Nickel Nanoparticles on Dark Hydrogen Fermentation from Starch Using Central Composite Design. Int. J. Hydrog. Energy 2015, 40, 12956–12963. [Google Scholar] [CrossRef]
- Nath, D.; Manhar, A.K.; Gupta, K.; Saikia, D.; Das, S.K.; Mandal, M. Phytosynthesized Iron Nanoparticles: Effects on Fermentative Hydrogen Production by Enterobacter Cloacae DH-89. Bull. Mater. Sci. 2015, 38, 1533–1538. [Google Scholar] [CrossRef] [Green Version]
- Engliman, N.S.; Abdul, P.M.; Wu, S.-Y.; Jahim, J.M. Influence of Iron (II) Oxide Nanoparticle on Biohydrogen Production in Thermophilic Mixed Fermentation. Int. J. Hydrog. Energy 2017, 42, 27482–27493. [Google Scholar] [CrossRef]
- Malik, S.N.; Pugalenthi, V.; Vaidya, A.N.; Ghosh, P.C.; Mudliar, S.N. Kinetics of Nano-Catalysed Dark Fermentative Hydrogen Production from Distillery Wastewater. Energy Procedia 2014, 54, 417–430. [Google Scholar] [CrossRef] [Green Version]
- Reddy, K.; Nasr, M.; Kumari, S.; Kumar, S.; Gupta, S.K.; Enitan, A.M.; Bux, F. Biohydrogen Production from Sugarcane Bagasse Hydrolysate: Effects of PH, S/X, Fe2+, and Magnetite Nanoparticles. Environ. Sci.Pollut. Res. 2017, 24, 8790–8804. [Google Scholar] [CrossRef]
- Wang, T.; Zhang, D.; Dai, L.; Chen, Y.; Dai, X. Effects of Metal Nanoparticles on Methane Production from Waste-Activated Sludge and Microorganism Community Shift in Anaerobic Granular Sludge. Sci. Rep. 2016, 6, 25857. [Google Scholar] [CrossRef]
- Wang, A.; Wang, J.; Lu, C.; Xu, M.; Lv, J.; Wu, X. Esterification for Biofuel Synthesis over an Eco-Friendly and Efficient Kao- 610 linite-Supported SO42−/ZnAl2O4 Macroporous Solid Acid Catalyst. Fuel 2018, 234, 430–440. [Google Scholar] [CrossRef]
- Wang, H.; Covarrubias, J.; Prock, H.; Wu, X.; Wang, D.; Bossmann, S.H. Acid-Functionalized Magnetic Nanoparticle as Heterogeneous Catalysts for Biodiesel Synthesis. J. Phys. Chem. C 2015, 119, 26020–26028. [Google Scholar] [CrossRef]
- Peña, L.; Hohn, K.L.; Li, J.; Sun, X.S.; Wang, D. Synthesis of Propyl-Sulfonic Acid-Functionalized Nanoparticles as Catalysts for Cellobiose Hydrolysis. J. Biomater. Nanobiotechnol. 2014, 5, 241–253. [Google Scholar] [CrossRef] [Green Version]
- Erdem, S.; Erdem, B.; Öksüzoğlu, R.M. Magnetic Nano-Sized Solid Acid Catalyst Bearing Sulfonic Acid Groups for Biodiesel Synthesis. Open Chem. 2018, 16, 923–929. [Google Scholar] [CrossRef]
- Lai, D.; Deng, L.; Guo, Q.; Fu, Y. Hydrolysis of Biomass by Magnetic Solid Acid. Energy Environ. Sci. 2011, 4, 3552–3557. [Google Scholar] [CrossRef]
- Antunes, F.A.F.; Gaikwad, S.; Ingle, A.P.; Pandit, R.; dos Santos, J.C.; Rai, M.; da Silva, S.S. Bioenergy and Biofuels: Nanotechnological Solutions for Sustainable Production. In Nanotechnology for Bioenergy and Biofuel Production; Rai, M., da Silva, S.S., Eds.; Springer: Cham, Switzerland, 2017; pp. 3–18. ISBN 978-3-319-45459-7. [Google Scholar]
- Vincent, K.A.; Li, X.; Blanford, C.F.; Belsey, N.A.; Weiner, J.H.; Armstrong, F.A. Enzymatic Catalysis on Conducting Graphite Particles. Nat. Chem. Biol. 2007, 3, 761–762. [Google Scholar] [CrossRef]
- Kwon, C.H.; Ko, Y.; Shin, D.; Kwon, M.; Park, J.; Bae, W.K.; Lee, S.W.; Cho, J. High-Power Hybrid Biofuel Cells Using Layer-by-Layer Assembled Glucose Oxidase-Coated Metallic Cotton Fibers. Nat. Commun. 2018, 9, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aquino Neto, S.; Almeida, T.S.; Palma, L.M.; Minteer, S.D.; De Andrade, A.R. Hybrid Nanocatalysts Containing Enzymes and Metallic Nanoparticles for Ethanol/O2 Biofuel Cell. J. Power Sources 2014, 259, 25–32. [Google Scholar] [CrossRef]
- Hebié, S.; Holade, Y.; Maximova, K.; Sentis, M.; Delaporte, P.; Kokoh, K.B.; Napporn, T.W.; Kabashin, A.V. Advanced Electrocatalysts on the Basis of Bare Au Nanomaterials for Biofuel Cell Applications. ACS Catal. 2015, 5, 6489–6496. [Google Scholar] [CrossRef]
- Zhang, Y.; Shen, J. Enhancement Effect of Gold Nanoparticles on Biohydrogen Production from Artificial Wastewater. Int. J. Hydrog. Energy 2007, 32, 17–23. [Google Scholar] [CrossRef]
- Eroglu, E.; Eggers, P.K.; Winslade, M.; Smith, S.M.; Raston, C.L. Enhanced Accumulation of Microalgal Pigments Using Metal Nanoparticle Solutions as Light Filtering Devices. Green Chem. 2013, 15, 3155–3159. [Google Scholar] [CrossRef]
- Su, L.; Shi, X.; Guo, G.; Zhao, A.; Zhao, Y. Stabilization of Sewage Sludge in the Presence of Nanoscale Zero-Valent Iron (NZVI): Abatement of Odor and Improvement of Biogas Production. J Mater Cycles Waste Manag. 2013, 15, 461–468. [Google Scholar] [CrossRef]
- Karri, S.; Sierra-Alvarez, R.; Field, J.A. Zero Valent Iron as an Electron-Donor for Methanogenesis and Sulfate Reduction in Anaerobic Sludge. Biotechnol. Bioeng. 2005, 92, 810–819. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, H.; Hosaka, Y.; Hara, K.; Feng, B.; Hirosaki, Y.; Fukuoka, A. Control of Selectivity, Activity and Durability of Simple Supported Nickel Catalysts for Hydrolytic Hydrogenation of Cellulose. Green Chem. 2014, 16, 637–644. [Google Scholar] [CrossRef] [Green Version]
- Yigezu, Z.D.; Muthukumar, K. Catalytic Cracking of Vegetable Oil with Metal Oxides for Biofuel Production. Energy Convers. Manag. 2014, 84, 326–333. [Google Scholar] [CrossRef]
- Hashmi, S.; Gohar, S.; Mahmood, T.; Nawaz, U.; Farooqi, H. Biodiesel Production by Using CaO-Al2O3 Nano Catalyst. Int. J. Eng. Res. Sci. 2016, 2, 2395–6992. [Google Scholar]
- Kim, M.; DiMaggio, C.; Salley, S.O.; Ng, K.S. A New Generation of Zirconia Supported Metal Oxide Catalysts for Converting Low Grade Renewable Feedstocks to Biodiesel. Bioresour. Technol. 2012, 118, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Li, L.; Shitao, Y.; Liu, S.; Hailong, Y.; Qiong, W.; Ragauskas, A.J. Catalytic Conversion of Waste Cooking Oils for the Production of Liquid Hydrocarbon Biofuels Using In-Situ Coating Metal Oxide on SBA-15 as Heterogeneous Catalyst. J. Anal. Appl. Pyrolysis 2019, 138, 137–144. [Google Scholar] [CrossRef]
- Abbas, M.; Parvatheeswara Rao, B.; Nazrul Islam, M.; Naga, S.M.; Takahashi, M.; Kim, C. Highly Stable- Silica Encapsulating Magnetite Nanoparticles (Fe3O4/SiO2) Synthesized Using Single Surfactantless- Polyol Process. Ceram. Int. 2014, 40, 1379–1385. [Google Scholar] [CrossRef]
- Kunzmann, A.; Andersson, B.; Vogt, C.; Feliu, N.; Ye, F.; Gabrielsson, S.; Toprak, M.S.; Buerki-Thurnherr, T.; Laurent, S.; Vahter, M.; et al. Efficient Internalization of Silica-Coated Iron Oxide Nanoparticles of Different Sizes by Primary Human Macrophages and Dendritic Cells. Toxicol. Appl. Pharmacol. 2011, 253, 81–93. [Google Scholar] [CrossRef] [Green Version]
- Mohan, S.V.; Mohanakrishna, G.; Reddy, S.S.; Raju, B.D.; Rao, K.S.R.; Sarma, P.N. Self-Immobilization of Acidogenic Mixed Consortia on Mesoporous Material (SBA-15) and Activated Carbon to Enhance Fermentative Hydrogen Production. Int. J. Hydrog. Energy 2008, 33, 6133–6142. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, T.; He, H.; Liang, H. Fe3O4/ZnMg(Al)O Magnetic Nanoparticles for Efficient Biodiesel Production. Appl. Organomet. Chem. 2018, 32, e4330. [Google Scholar] [CrossRef]
- Chiang, Y.-D.; Dutta, S.; Chen, C.-T.; Huang, Y.-T.; Lin, K.-S.; Wu, J.C.S.; Suzuki, N.; Yamauchi, Y.; Wu, K.C.-W. Functionalized Fe3O4@Silica Core–Shell Nanoparticles as Microalgae Harvester and Catalyst for Biodiesel Production. ChemSusChem 2015, 8, 789–794. [Google Scholar] [CrossRef]
- Papadopoulou, A.; Zarafeta, D.; Galanopoulou, A.P.; Stamatis, H. Enhanced Catalytic Performance of Trichoderma Reesei Cellulase Immobilized on Magnetic Hierarchical Porous Carbon Nanoparticles. Protein J. 2019, 38, 640–648. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, R.; Menezes, R.; Sampaio, K.; Batista, E. Heterogeneous Catalysts for Biodiesel Production: A Review. Food Public Health 2019, 9, 125–137. [Google Scholar] [CrossRef]
- Semwal, S.; Arora, A.K.; Badoni, R.P.; Tuli, D.K. Biodiesel Production Using Heterogeneous Catalysts. Bioresour. Technol. 2011, 102, 2151–2161. [Google Scholar] [CrossRef]
- Narasimhan, M.; Chandrasekaran, M.; Govindasamy, S.; Aravamudhan, A. Heterogeneous Nanocatalysts for Sustainable Biodiesel Production: A Review. J. Environ. Chem. Eng. 2021, 9, 104876. [Google Scholar] [CrossRef]
- Akia, M.; Yazdani, F.; Motaee, E.; Han, D.; Arandiyan, H. A Review on Conversion of Biomass to Biofuel by Nanocatalysts. Biofuel Res. J. 2014, 1, 16–25. [Google Scholar] [CrossRef]
- Ibrahim, A.A.; Lin, A.; Zhang, F.; AbouZeid, K.M.; El-Shall, M.S. Palladium Nanoparticles Supported on Hybrid MOF-PRGO for Catalytic Hydrodeoxygenation of Vanillin as a Model for Biofuel Upgrade Reactions. ChemCatChem 2016, 9, 469–480. [Google Scholar] [CrossRef]
- Zhao, W.; Zhang, Y.; Du, B.; Wei, D.; Wei, Q.; Zhao, Y. Enhancement Effect of Silver Nanoparticles on Fermentative Biohydrogen Production Using Mixed Bacteria. Bioresour. Technol. 2013, 142, 240–245. [Google Scholar] [CrossRef]
- Khan, M.M.; Lee, J.; Cho, M.H. Electrochemically Active Biofilm Mediated Bio-Hydrogen Production Catalyzed by Positively Charged Gold Nanoparticles. Int. J. Hydrog. Energy 2013, 38, 5243–5250. [Google Scholar] [CrossRef]
- Mohanraj, S.; Anbalagan, K.; Rajaguru, P.; Pugalenthi, V. Effects of Phytogenic Copper Nanoparticles on Fermentative Hydrogen Production by Enterobacter Cloacae and Clostridium Acetobutylicum. Int. J. Hydrog. Energy 2016, 41, 10639–10645. [Google Scholar] [CrossRef]
- Taherdanak, M.; Zilouei, H.; Karimi, K. The Effects of Fe0 and Ni0 Nanoparticles versus Fe2+ and Ni2+ Ions on Dark Hydrogen Fermentation. Int. J. Hydrog. Energy 2016, 41, 167–173. [Google Scholar] [CrossRef]
- Elreedy, A.; Ibrahim, E.; Hassan, N.; El-Dissouky, A.; Fujii, M.; Yoshimura, C.; Tawfik, A. Nickel-Graphene Nanocomposite as a Novel Supplement for Enhancement of Biohydrogen Production from Industrial Wastewater Containing Mono-Ethylene Glycol. Energy Convers. Manag. 2017, 140, 133–144. [Google Scholar] [CrossRef]
- Mohanraj, S.; Kodhaiyolii, S.; Rengasamy, M.; Pugalenthi, V. Phytosynthesized Iron Oxide Nanoparticles and Ferrous Iron on Fermentative Hydrogen Production Using Enterobacter Cloacae: Evaluation and Comparison of the Effects. Int. J. Hydrog. Energy 2014, 39, 11920–11929. [Google Scholar] [CrossRef]
- Pandey, A.; Gupta, K.; Pandey, A. Effect of Nanosized TiO2 on Photofermentation by Rhodobacter Sphaeroides NMBL-02. Biomass Bioenergy 2015, 72, 273–279. [Google Scholar] [CrossRef]
- Nzila, A. Mini Review: Update on Bioaugmentation in Anaerobic Processes for Biogas Production. Anaerobe 2017, 46, 3–12. [Google Scholar] [CrossRef]
- Abdelsalam, E.; Samer, M.; Attia, Y.A.; Abdel-Hadi, M.A.; Hassan, H.E.; Badr, Y. Influence of Zero Valent Iron Nanoparticles and Magnetic Iron Oxide Nanoparticles on Biogas and Methane Production from Anaerobic Digestion of Manure. Energy 2017, 120, 842–853. [Google Scholar] [CrossRef]
- Ganzoury, M.A.; Allam, N.K. Impact of Nanotechnology on Biogas Production: A Mini-Review. Renew. Sustain. Energy Rev. 2015, 50, 1392–1404. [Google Scholar] [CrossRef]
- Suanon, F.; Sun, Q.; Li, M.; Cai, X.; Zhang, Y.; Yan, Y.; Yu, C.-P. Application of Nanoscale Zero Valent Iron and Iron Powder during Sludge Anaerobic Digestion: Impact on Methane Yield and Pharmaceutical and Personal Care Products Degradation. J. Hazard. Mater. 2017, 321, 47–53. [Google Scholar] [CrossRef]
- Amen, T.W.M.; Eljamal, O.; Khalil, A.M.E.; Matsunaga, N. Biochemical Methane Potential Enhancement of Domestic Sludge Digestion by Adding Pristine Iron Nanoparticles and Iron Nanoparticles Coated Zeolite Compositions. J. Environ. Chem. Eng. 2017, 5, 5002–5013. [Google Scholar] [CrossRef]
- Gonzalez-Estrella, J.; Sierra-Alvarez, R.; Field, J.A. Toxicity Assessment of Inorganic Nanoparticles to Acetoclastic and Hydrogenotrophic Methanogenic Activity in Anaerobic Granular Sludge. J. Hazard. Mater. 2013, 260, 278–285. [Google Scholar] [CrossRef]
- Zhang, L.; He, X.; Zhang, Z.; Cang, D.; Nwe, K.A.; Zheng, L.; Li, Z.; Cheng, S. Evaluating the Influences of ZnO Engineering Nanomaterials on VFA Accumulation in Sludge Anaerobic Digestion. Biochem. Eng. J. 2017, 125, 206–211. [Google Scholar] [CrossRef]
- Ünşar, E.K.; Çığgın, A.S.; Erdem, A.; Perendeci, N.A. Long and Short Term Impacts of CuO, Ag and CeO2 Nanoparticles on Anaerobic Digestion of Municipal Waste Activated Sludge. Environ. Sci. Process. Impacts 2016, 18, 277–288. [Google Scholar] [CrossRef]
- Kim, Y.K.; Lee, H. Use of Magnetic Nanoparticles to Enhance Bioethanol Production in Syngas Fermentation. Bioresour. Technol. 2016, 204, 139–144. [Google Scholar] [CrossRef]
- Lam, M.K.; Lee, K.T. Chapter 12—Bioethanol Production from Microalgae; Kim, S.-K., Ed.; Academic Press: Boston, MD, USA, 2015; pp. 197–208. ISBN 978-0-12-800776-1. [Google Scholar]
- De Farias Silva, C.E.; Bertucco, A. Bioethanol from Microalgae and Cyanobacteria: A Review and Technological Outlook. Process Biochem. 2016, 51, 1833–1842. [Google Scholar] [CrossRef]
- Velazquez, J.; Rodriguez-Jasso, R.; Colla, L.; Galindo, A.; Cervantes, D.; Aguilar, C.; Fernandes, B.; Ruiz, H. Microalgal Biomass Pretreatment for Bioethanol Production: A Review. Biofuel Res. J. 2018, 5, 780–791. [Google Scholar] [CrossRef]
- Parambil, L.K.; Sarkar, D. In Silico Analysis of Bioethanol Overproduction by Genetically Modified Microorganisms in Coculture Fermentation. Biotechnol. Res. Int. 2015, 2015, 238082. [Google Scholar] [CrossRef] [Green Version]
- Sanusi, I.A.; Suinyuy, T.N.; Kana, G.E.B. Impact of Nanoparticle Inclusion on Bioethanol Production Process Kinetic and Inhibitor Profile. Biotechnol. Rep. 2021, 29, e00585. [Google Scholar] [CrossRef] [PubMed]
- Sanusi, I.A.; Faloye, F.D.; Gueguim Kana, E.B. Impact of Various Metallic Oxide Nanoparticles on Ethanol Production by Saccharomyces Cerevisiae BY4743: Screening, Kinetic Study and Validation on Potato Waste. Catal. Lett. 2019, 149, 2015–2031. [Google Scholar] [CrossRef]
- Gupta, K.; Chundawat, T.S. Zinc Oxide Nanoparticles Synthesized Using Fusarium Oxysporum to Enhance Bioethanol Production from Rice-Straw. Biomass Bioenergy 2020, 143, 105840. [Google Scholar] [CrossRef]
- Bohlouli, A.; Mahdavian, L. Catalysts Used in Biodiesel Production: A Review. Biofuels 2018, 12, 885–898. [Google Scholar] [CrossRef]
- Kumar, L.R.; Ram, S.K.; Tyagi, R.D. Application of Nanotechnology in Biodiesel Production. In Biodiesel Production: Technologies, Challenges, and Future Prospects; American Society of Civil Engineers: Reston, VA, USA, 2021; pp. 397–419. [Google Scholar]
- Banković-Ilić, I.B.; Miladinović, M.R.; Stamenković, O.S.; Veljković, V.B. Application of Nano CaO–Based Catalysts in Biodiesel Synthesis. Renew. Sust. Energ. Rev. 2017, 72, 746–760. [Google Scholar] [CrossRef]
- Zhang, X.; Yan, S.; Tyagi, R.D.; Surampalli, R.Y. Biodiesel Production from Heterotrophic Microalgae through Transesterification and Nanotechnology Application in the Production. Renew. Sustain. Energy Rev. 2013, 26, 216–223. [Google Scholar] [CrossRef]
- Demirbas, A. Biofuels Sources, Biofuel Policy, Biofuel Economy and Global Biofuel Projections. Energy Convers. Manag. 2008, 49, 2106–2116. [Google Scholar] [CrossRef]
- Feyzi, M.; Norouzi, L. Preparation and Kinetic Study of Magnetic Ca/Fe3O4@SiO2 Nanocatalysts for Biodiesel Production. Renew. Energy 2016, 94, 579–586. [Google Scholar] [CrossRef]
- Jeon, H.-S.; Park, S.E.; Ahn, B.; Kim, Y.-K. Enhancement of Biodiesel Production in Chlorella Vulgaris Cultivation Using Silica Nanoparticles. Biotechnol.Bioproc. E 2017, 22, 136–141. [Google Scholar] [CrossRef]
- Tahvildari, K.; Anaraki, Y.N.; Fazaeli, R.; Mirpanji, S.; Delrish, E. The Study of CaO and MgO Heterogenic Nano-Catalyst Coupling on Transesterification Reaction Efficacy in the Production of Biodiesel from Recycled Cooking Oil. J. Environ. Health Sci.Eng. 2015, 13, 73. [Google Scholar] [CrossRef] [Green Version]
- Baskar, G.; Aberna Ebenezer Selvakumari, I.; Aiswarya, R. Biodiesel Production from Castor Oil Using Heterogeneous Ni Doped ZnO Nanocatalyst. Bioresour. Technol. 2018, 250, 793–798. [Google Scholar] [CrossRef]
- Harsha Hebbar, H.R.; Math, M.C.; Yatish, K.V. Optimization and Kinetic Study of CaO Nano-Particles Catalyzed Biodiesel Production from Bombax Ceiba Oil. Energy 2018, 143, 25–34. [Google Scholar] [CrossRef]
- Bet-Moushoul, E.; Farhadi, K.; Mansourpanah, Y.; Nikbakht, A.M.; Molaei, R.; Forough, M. Application of CaO-Based/Au Nanoparticles as Heterogeneous Nanocatalysts in Biodiesel Production. Fuel 2016, 164, 119–127. [Google Scholar] [CrossRef]
- Rahmani Vahid, B.; Haghighi, M.; Toghiani, J.; Alaei, S. Hybrid-Coprecipitation vs. Combustion Synthesis of Mg-Al Spinel Based Nanocatalyst for Efficient Biodiesel Production. Energy Convers. Manag. 2018, 160, 220–229. [Google Scholar] [CrossRef]
- Deng, X.; Fang, Z.; Liu, Y.; Yu, C.-L. Production of Biodiesel from Jatropha Oil Catalyzed by Nanosized Solid Basic Catalyst. Energy 2011, 36, 777–784. [Google Scholar] [CrossRef]
- Madhuvilakku, R.; Piraman, S. Biodiesel Synthesis by TiO2–ZnO Mixed Oxide Nanocatalyst Catalyzed Palm Oil Transesterification Process. Bioresour. Technol. 2013, 150, 55–59. [Google Scholar] [CrossRef]
- Mazaheri, H.; Ong, H.C.; Masjuki, H.H.; Amini, Z.; Harrison, M.D.; Wang, C.-T.; Kusumo, F.; Alwi, A. Rice Bran Oil Based Biodiesel Production Using Calcium Oxide Catalyst Derived from Chicoreus Brunneus Shell. Energy 2018, 144, 10–19. [Google Scholar] [CrossRef]
- Pandit, P.R.; Fulekar, M.H. Egg Shell Waste as Heterogeneous Nanocatalyst for Biodiesel Production: Optimized by Response Surface Methodology. J. Environ. Manag. 2017, 198, 319–329. [Google Scholar] [CrossRef] [PubMed]
- Varghese, R.; Henry, J.P.; Irudayaraj, J. Ultrasonication-Assisted Transesterification for Biodiesel Production by Using Heterogeneous ZnO Nanocatalyst. Environ. Prog. Sustain. Energy 2018, 37, 1176–1182. [Google Scholar] [CrossRef]
- Cheah, W.Y.; Sankaran, R.; Show, P.L.; Ibrahim, T.N.B.T.; Chew, K.W.; Culaba, A.; Chang, J.S. Pretreatment Methods for Lig- 781 nocellulosic Biofuels Production: Current Advances, Challenges and Future Prospects. Biofuel Res. J. 2020, 7, 1115–1127. [Google Scholar] [CrossRef] [Green Version]
- Pattakrine, M.V.; Pattakrine, V.M. Nanotechnology for Algal Biofuels. In The Science of Algal Fuels; Springer: Dordrecht, The Netherlands, 2012; Volume 25, pp. 147–163. [Google Scholar]


| Nanoparticles | Substrate/ Feedstock | Reaction Conditions | Summary | Reference |
|---|---|---|---|---|
| Ag | Glucose | Mixed culture; pH–8.5; temperature–35 °C; rotation–120 rpm; | Higher hydrogen yield (2.48 mol/mol glucose) observed compared to blank. Reduction in lag phase observed with addition of Ag NPs. Reduction in ethanol production observed in presence of Ag NPs. | [86] |
| Au | Acetate | Anaerobic sludge; pH–7.2; temperature–35 °C | The hydrogen production rate reached 105 2 mL/L per day with the addition of Ag NPs. | [87] |
| Au | Artificial wastewater | Anaerobic culture; pH–7.2; temperature–35 °C | Maximum cumulative hydrogen production 4.48 mol per mol sucrose achieved with 5 nm Au NPs. The conversion efficiency of sucrose to hydrogen reached 56%. | [66] |
| Cu | Glucose | Enterobacter cloacae 811101 and Clostridium acetobutylicum NCIM 2337; pH–7.0 (E. cloacae), 6.0 (C.acetobutylicum);temperature–37 °C;duration–24 h | The Cu-NPs were found to have a more inhibitory effect on biohydrogen production. Addition of Cu NPs in fermentative process showed higher inhibitory effect than the CuSO4 supplementation. Cu NPs with concentration less than 2.5 mg/L enhanced hydrogen production. | [88] |
| Fe | Glucose | Anaerobic sludge, pH–5.5; temperature–37 °C | The hydrogen and biogas yield of the control test were 247 and 391 mL/g VS, respectively. Addition Ni2+ ions improved hydrogen production by 55%. | [89] |
| Fe | Water hyacinth | Mixed culture and Clostridium butyricum TISTR, temperature–35 °C; duration–4 days | A maximum hydrogen yield 57mL/g of the plant biomass equal to 85.50% of the theoretical maximum is obtained. | [35] |
| Fe | Glucose | Enterobacter cloacae DH–89, pH–7.0; temperature–37 °C | Supplementation of Fe NPs significantly improved the hydrogen yield. A maximum H2 yield 1.9 mol mol−1 glucose utilized was observed with addition of 100 mg/L FeNPs, which increases the glucose conversion by two-fold. | [51] |
| Ni | Industrial wastewater | Anaerobic sludge; pH–7.0; temperature–55 °C; rotation–180 rpm | Ni-Gr NC dose of 60 mg/L exhibited the highest improvement (105%) in H2 production. H2 production was improved by 67% compared with supplementation of Ni nanoparticles. | [90] |
| Iron oxide | Glucose | E. cloacae 811101; pH–7.0; temperature–37 °C; duration–24 h; | Maximum hydrogen yields 2.07 mol H2/mol glucose and 5.44 mol H2/mol sucrose were achieved with addition of 125 mg/L and 200 mg/L iron oxide NPs. Enhancement of hydrogen production was higher with addition of iron oxide NPs compared to ferrous iron supplementation. | [91] |
| Fe2O3 | Glucose | Anaerobic sludge; pH–5.5; temperature–60 °C; rotation–150 rpm | Maximum hydrogen yield reached 1.92 mol H2/mol glucose with a hydrogen content of 51%. Metal NPs are not consumed by the microbes and only act as hydrogen production enhancer. | [52] |
| Fe3O4 | Wastewater | Mixed culture; pH–6.0; temperature–37 °C; rotation–200 rpm | The maximum hydrogen production rate and specific hydrogen yield reached 80.7 mL/h and 44.28 mL H2/g COD with supplementation of NPs. Highest cumulative volume of hydrogen (380 mL), hydrogen content (62.14%) and % COD reduction (72.5) was obtained under the optimal conditions. | [53] |
| Fe3O4 | Sugarcane bagasse | Anaerobic sludge; pH–5.0; temperature–30 °C | Addition of 200 mg/LFe2+ and magnetite NPs enhanced the HY by 62.1% and 69.6%, respectively. Highest hydrogenase gene activity was confirmed by immobilized cultures on magnetite nanoparticles. | [54] |
| TiO2 | Malate | R. sphaeroides NMBL–02; pH–8.0; temperature–32 °C | Hydrogen production rate enhanced by 1.54 fold and duration by 1.88 fold in the presence of 60 mg/mL of TiO2 NPs in comparison to the control. Maximum hydrogen production 1900 mL/L with 63.27% malate conversion achieved. | [92] |
| Nanoparticles | Substrate/Feedstock | Reaction Conditions | Summary | Reference |
|---|---|---|---|---|
| Ni | Manure slurry | Temperature–37 °C; rotation–20 rpm (in 1 min interval) | Addition of 2 mg/L Ni NPs enhanced the biogas production by 1.74 times in comparison to control. The methane volume increased by 2.01 times. Highest specific biogas (614.5 mL per g VS) and methane (361.6 mL per g VS) production were attained with 2 mg/L Ni NPs. | [94] |
| Nano zero–valent iron (nZVI) | Waste activated sludge | Temperature–35 °C; rotation–120 rpm; duration–30 days | Addition of 10 mg/g total suspended solids (TSS)nZVI increased methane production to 120% of the control. Low concentrations of nZVI promoted a number of microbes (Bacteria and Archaea) and activities of key enzymes. | [55] |
| nZVI | Sewage sludge | pH–7.0; temperature–37 °C; duration–30 days | Methane yield enhanced by 25.2% in the presence of nZVI. COD removal efficiency was 54.4% in presence of nZVI, higher compared to control (44.6%). The addition of nZVI showed positive impact on the removal of chlorinated pharmaceutical and personal care products. | [96] |
| nZVI | Domestic sludge | Temperature–37 °C; duration–14 days | Methane content was stimulated up to 88% with addition of nZVI. | [97] |
| Co | Manure slurry | Temperature–37 °C; rotation–20 rpm (in 1 min interval) | Addition of 1 mg/L Ni NPs enhanced the biogas production by 1.64 times in comparison to control. The methane volume increased by 1.86 times. | [94] |
| Cu | Granular sludge | pH–7.2; temperature–30 °C; rotation–120 rpm | Cu NPs caused severe methanogenic inhibition. The 50% inhibiting concentrations determined towards aceto-clastic and hydrogenotrophic methanogens were 62 and 68 mg/L. | [98] |
| ZnO | Waste activated Sludge | Temperature–37 °C; duration–14 days | 100 mg/L Zn2+ exhibited 53.7% reduction in methane production compared to control. Less VFA consumed during methanogenesis when more ZnO ENMs were present. | [99] |
| ZnO | Granular sludge | pH–7.2; temperature–30 °C; rotation–120 rpm | The 50% inhibiting concentrations determined towards aceto-clastic and hydrogenotrophic methanogens were 87 and 250 mg/L. Methanogenic inhibition is due to the release of toxic divalent Zn ions caused by corrosion and dissolution of the NPs. | [98] |
| CuO | Municipal waste activated sludge | Temperature–35 °C | Increase in CuO NP concentration from 5 to 1000 mg per gTS, and an increase in the inhibition of AD from 5.8 to 84.0% was observed. EC50 values of short- and long-term inhibitions were calculated as 224.2 mg CuO per g TS and 215.1 mg CuO per g TS, respectively. | [100] |
| Nanoparticles | Substrate/ Feedstock | Reaction Conditions | Summary | Reference |
|---|---|---|---|---|
| NiO | Potato peel waste | S. cerevisiae BY4743; Instantaneous saccharificationfermentation (NIISF); temperature–37 °C, rotation–120 rpm, duration–24 h |
| [106] |
| NiOand Fe3O4 | Potato peel waste | Saccharomyces cerevisiae BY4743; temperature–30 °C; rotation–120 rpm; duration–72 h |
| [107] |
| ZnO | Rice straw | Fusariumoxysporum;temperature–20 to 25 °C; pH–6.0 to 8.0; rotation –100 to 200 rpm; duration –72 h |
| [108] |
| Magnetic nanoparticles | Corn starch | Immobilized Saccharomyces cerevisiae; pH–4.0; temperature –60 °C |
| [47] |
| Nanoparticles | Substrate/Feedstock | Reaction Conditions | Summary | Reference |
|---|---|---|---|---|
| Fe3O4/ZnMg(Al)O | Microalgal oil | Temperature–65 °C;duration–3 h;methanol to oil ratio: 12:1 | Biodiesel yield reached 94% under the optimal conditions. 82% biodiesel yield was observed after 7 times regeneration. Increase of the molar ratio of methanol to oil increased biodiesel yield. | [78] |
| SiO2 and SiO2–CH3 | Chlorella vulgaris | Methanol/sulfuric acid–85:15 v/v;temperature–70 °C;duration–40 min | Dry cell weight increased by 177% and 210% by adding SiO2 and SiO2–CH3 NPs. Addition of NPs increased CO2 mass transfer rate. | [115] |
| CaO and MgO | Waste cooking oil | For CaO: weight–1.5%; methanol to oil ratio–1:7; duration–6 h. For MgO: weight–3% (0.7 g of Nano CaO and 0.5 g of Nano MgO); alcohol to oil ratio–1:7; duration–6 h. | Nano MgO alone is not capable of catalysing the transesterification reaction due to weaker affinity. Nano MgO in combination with CaO increased the transesterification yield. The biodiesel yield reached 98.95% of weight. | [116] |
| Ni doped ZnOnanocatalyst | Castor oil | Methanol to oil ratio–1:8; catalyst loading –11% (w/w); temperature–55 °C, duration–60 min | 95.20% higher biodiesel yield was observed under optimum conditions. The reusability study of nano-catalysts showed efficient for 3 cycles. | [117] |
| Ni0.5Zn0.5Fe2O4 doped with Cu | Soybean oil | Methanol to oil ratio–1:20; catalyst loading–4% (wt); temperature–180 °C, duration– 1 h, | Presence of Cu ions facilitated an increase of 5.5–85% in the conversion values in methyl esters. Cu2+ ions doping influenced in the structure, morphology and magnetic properties of nano-ferrites. | [44] |
| CaO | Bombaxceiba oil | Methanol to oil ratio–30.37:1; catalystloading–1.5% (wt); temperature–65 °C;duration– 70.52 min | 96.2% yield of methyl ester was achieved under optimum conditions. CaO-NPs reused for five consecutive cycles with minimum loss of activity. | [118] |
| Calcite/Au | Sunflower oil | Methanol to oil ratio–9:1; catalyst loading: 0.3% (wt); temperature–65 °C;duration– 6 h | The oil conversion was in the range of 90–97% under optimum conditions. The nano-catalysts were stable up to 10 cycles without loss of activity. | [119] |
| MgO/MgAl2O4 | Sunflower oil | Methanol to oil ratio–12:1; catalyst loading– 3% (wt); temperature–110 °C; time–3 h | 95.7% conversion of sunflower oil achieved. The prepared catalyst was stable for 6 cycles. Size, shape and crystallinity of catalysts are important parameters affecting biodiesel production. | [120] |
| Hydrotalcite particles with Mg/Al | Jatropha oil | Methanol to oil ratio–0.4:1 (v/v); catalyst loading– 1% (wt); temperature–44.85 °C;duration– 1.5 h; anhydrous methanol–40 mL; sulfuric acid–4 mL | 95.2% biodiesel yield was achieved under optimal conditions. The catalyst showed reliable performance for 8 consecutive cycles. | [121] |
| TiO2–ZnO | Palm oil | Methanol to oil ratio –6:1; temperature–50–80 °C; duration– 5 h | 92.2% FAME conversion and 92% yield was attained within 5 h at 60 °C. The synthesized catalysts were characterized by XRD, FT–IR, and FE–SEM. | [122] |
| CaO | Rice bran oil | Methanol to oil ratio–30:1; temperature–65 °C; duration–120 min; catalyst loading = 0.4%(wt) | 93% FAME yield observed after 120 min under optimum conditions. The reusability of catalyst revealed that the FAME yield decreased significantly after fifth cycle. | [123] |
| CaO | Microalgae oil | Methanol to oil ratio–10:1; temperature–70 °C, duration– 3.6 h, methanol/oil; catalyst loading–1.7% (wt) | The nanoparticles are of spherical shape with average particle size of 75 nm. 86.41% microalgal biodiesel yield reported under optimal conditions. Reusability study of catalyst revealed 86.41% to 67.87% loss in biodiesel production after the sixth cycle. | [124] |
| ZnO | Waste cooking oil | Methanol to oil ratio– 6:1; temperature–60 °C; duration– 15 min; catalyst loading–1.5% (wt) | FAME conversions yield up to 96% achieved under ultrasonic irradiation. Synthesized biodiesel properties such as density and viscosity were at par with standard biodiesel. | [125] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arya, I.; Poona, A.; Dikshit, P.K.; Pandit, S.; Kumar, J.; Singh, H.N.; Jha, N.K.; Rudayni, H.A.; Chaudhary, A.A.; Kumar, S. Current Trends and Future Prospects of Nanotechnology in Biofuel Production. Catalysts 2021, 11, 1308. https://doi.org/10.3390/catal11111308
Arya I, Poona A, Dikshit PK, Pandit S, Kumar J, Singh HN, Jha NK, Rudayni HA, Chaudhary AA, Kumar S. Current Trends and Future Prospects of Nanotechnology in Biofuel Production. Catalysts. 2021; 11(11):1308. https://doi.org/10.3390/catal11111308
Chicago/Turabian StyleArya, Indrajeet, Asha Poona, Pritam Kumar Dikshit, Soumya Pandit, Jatin Kumar, Himanshu Narayan Singh, Niraj Kumar Jha, Hassan Ahmed Rudayni, Anis Ahmad Chaudhary, and Sanjay Kumar. 2021. "Current Trends and Future Prospects of Nanotechnology in Biofuel Production" Catalysts 11, no. 11: 1308. https://doi.org/10.3390/catal11111308
APA StyleArya, I., Poona, A., Dikshit, P. K., Pandit, S., Kumar, J., Singh, H. N., Jha, N. K., Rudayni, H. A., Chaudhary, A. A., & Kumar, S. (2021). Current Trends and Future Prospects of Nanotechnology in Biofuel Production. Catalysts, 11(11), 1308. https://doi.org/10.3390/catal11111308







