Glycerol Hydrogenolysis to Produce 1,2-Propanediol in Absence of Molecular Hydrogen Using a Pd Promoted Cu/MgO/Al2O3 Catalyst
Abstract
:1. Introduction
2. Results and Discussion
2.1. Catalyst Screening
2.2. Catalyst Characterization
2.2.1. Temperature Programmed Reduction
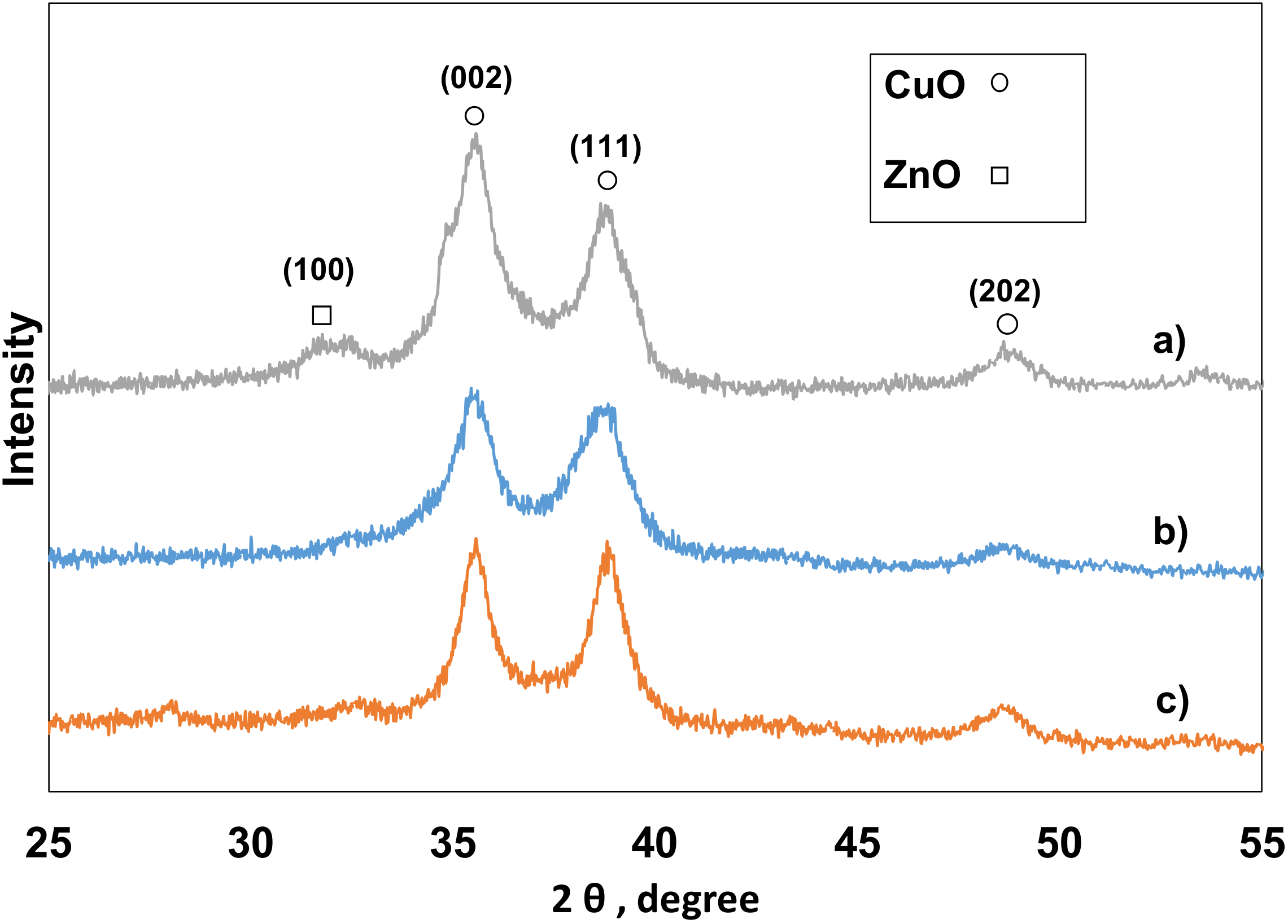
2.2.2. X-ray Diffraction
2.2.3. Transmission Electron Microscopy
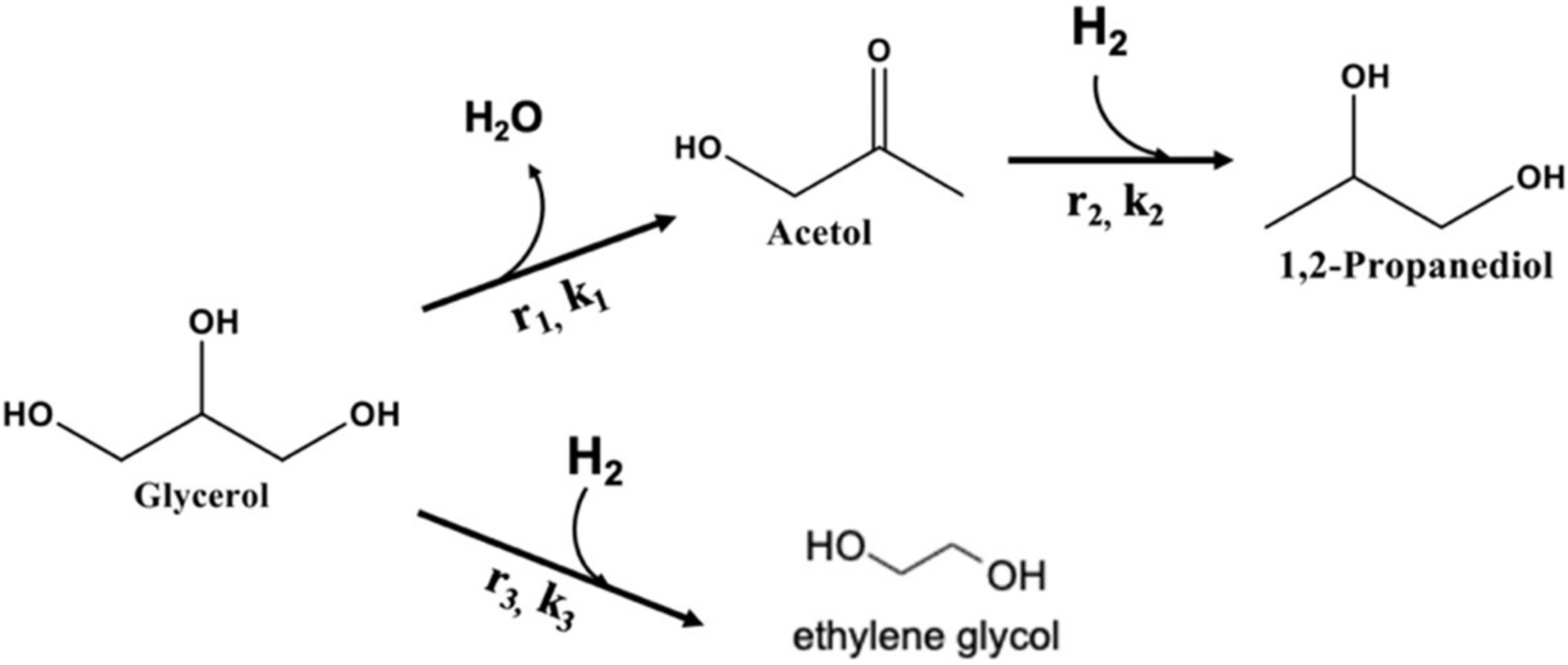
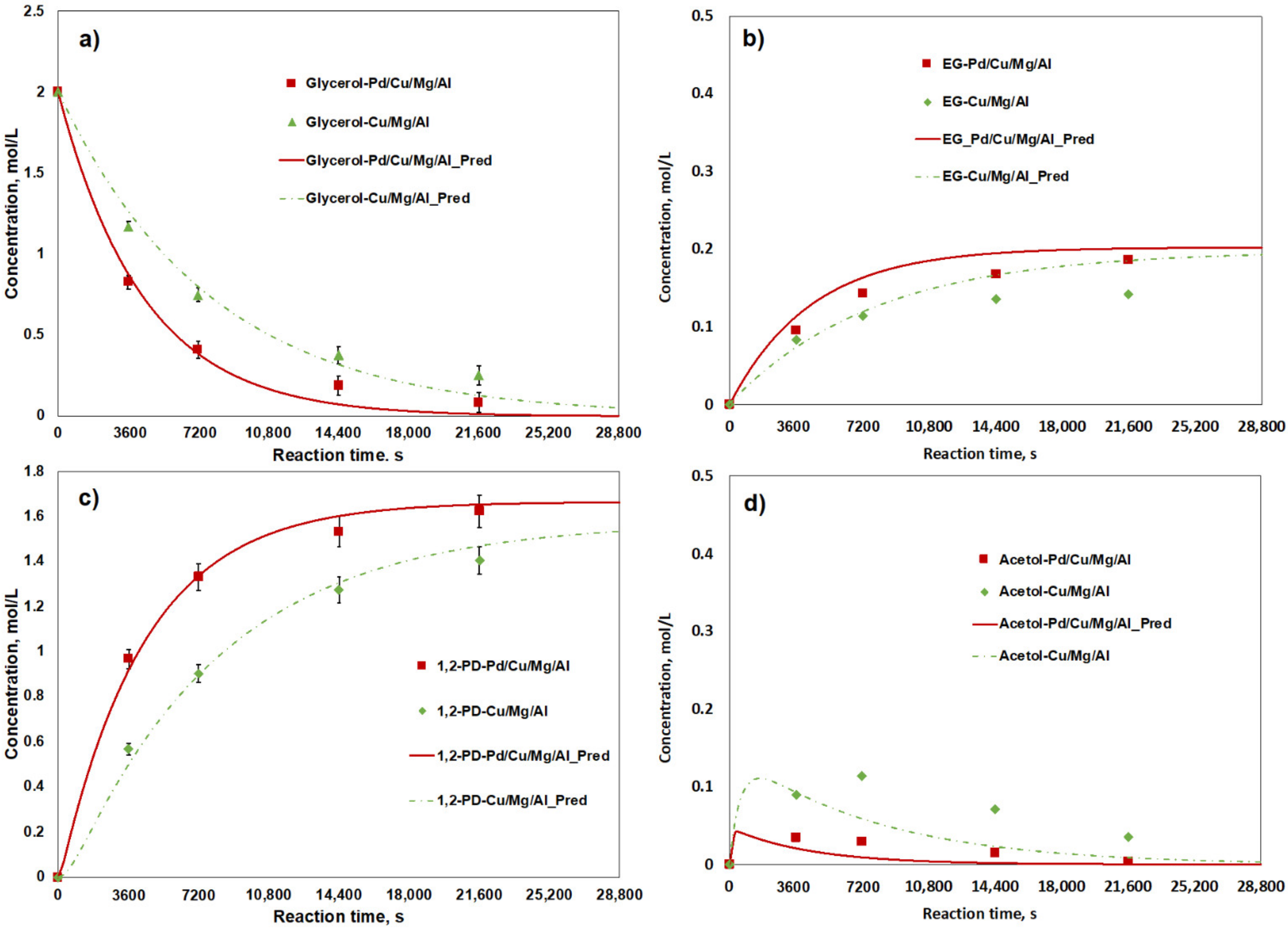
2.3. Kinetic Analysis
2.4. The Promoting Effect of Pd on Cu/MgO/Al2O3 Catalysts
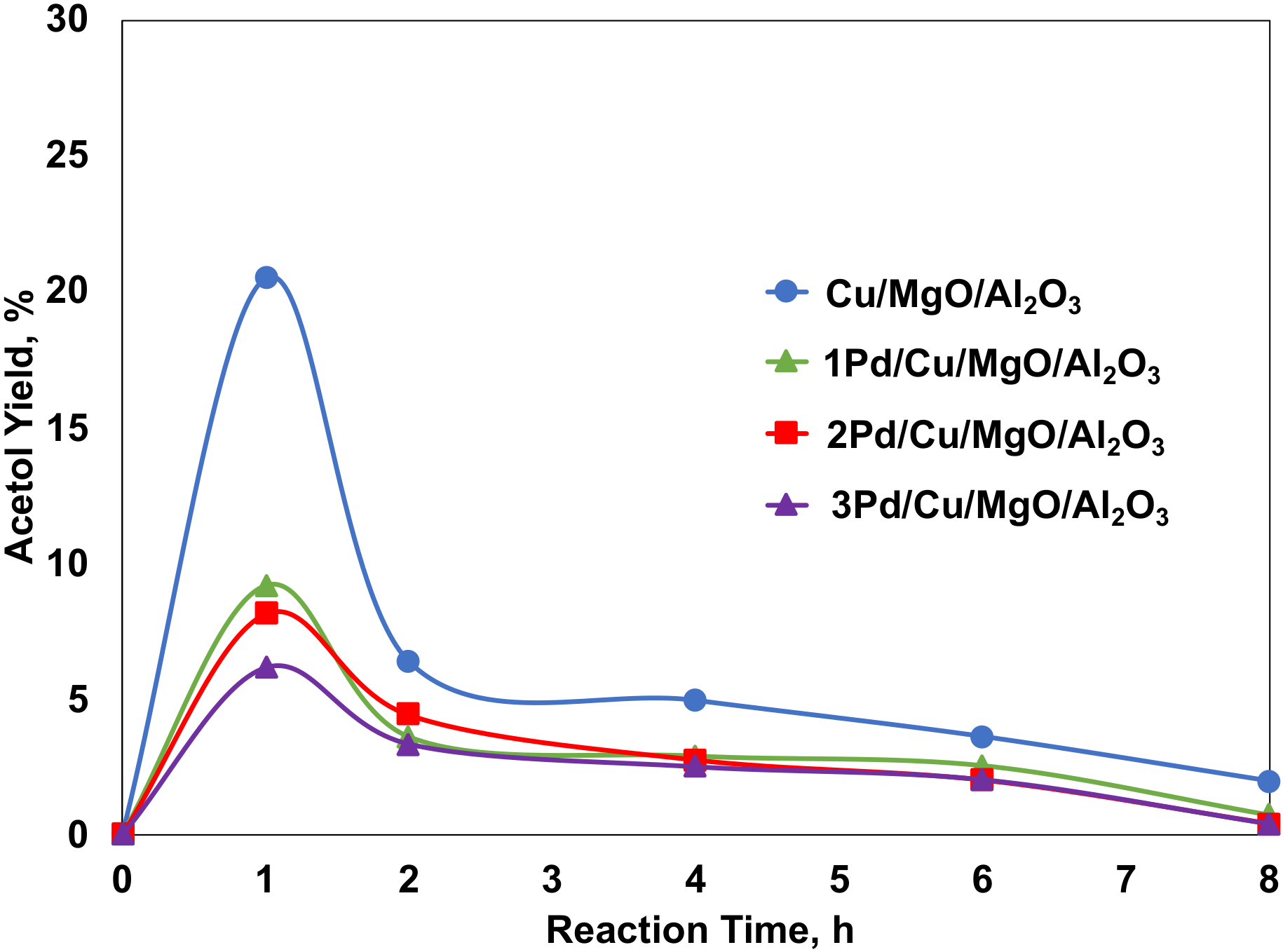
2.4.1. Effect of Pd Loadings on the Cu/MgO/Al2O3 Catalysts for the Glycerol Hydrogenolysis with In Situ Hydrogen Produced from Methanol Steam Reforming
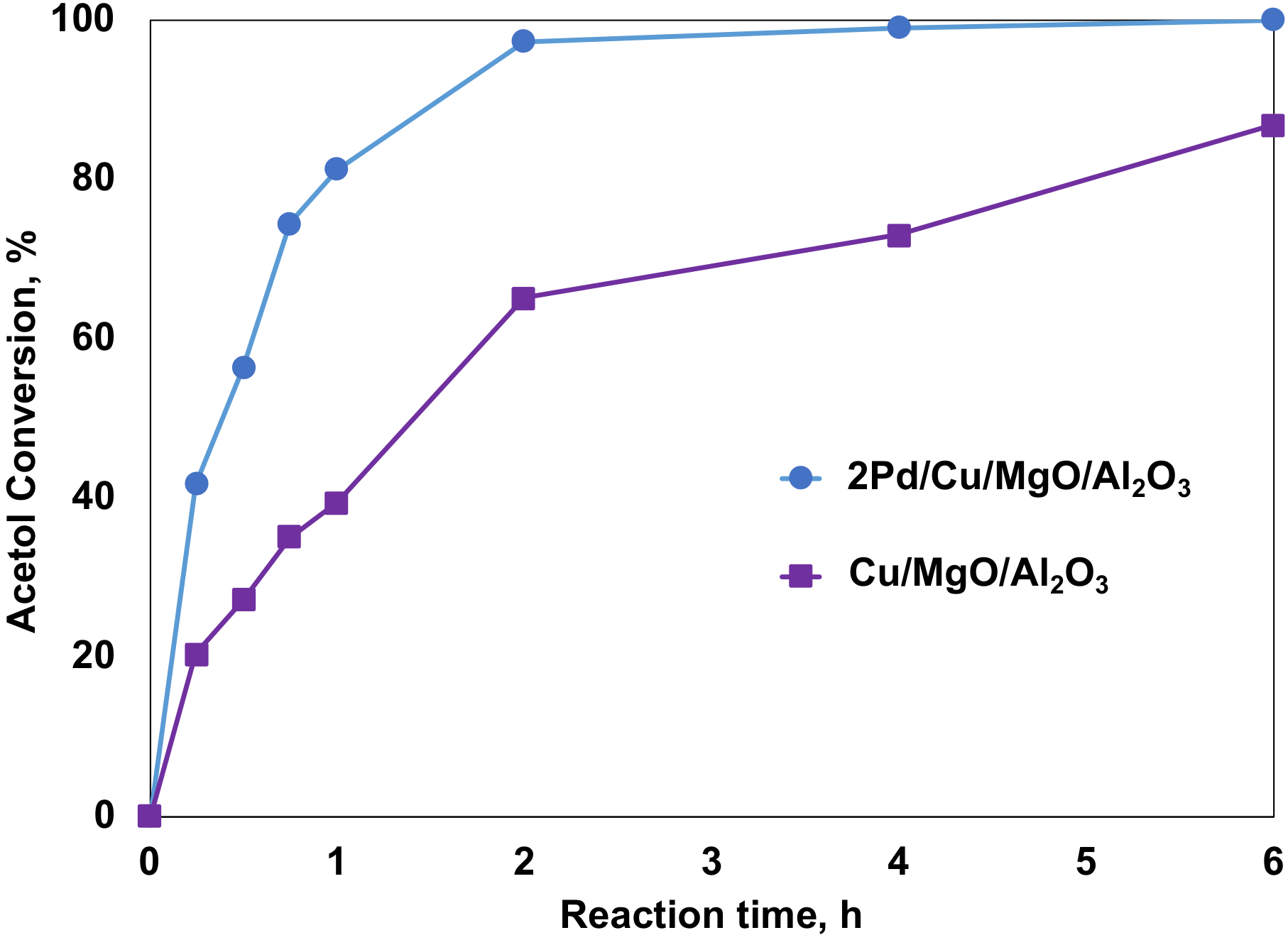
2.4.2. The Promoting Effect of Pd on Acetol Hydrogenation with Molecular Hydrogen
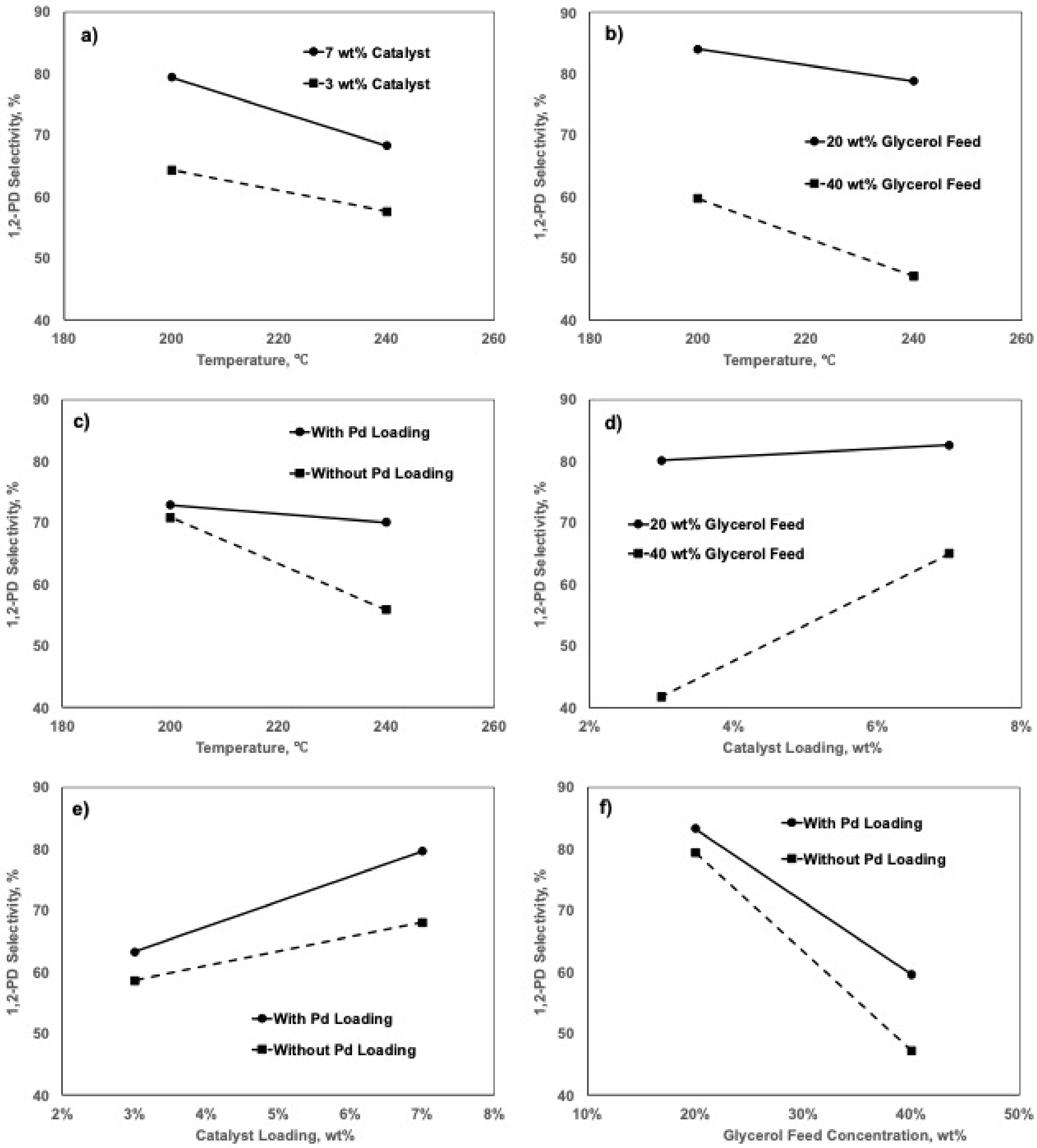
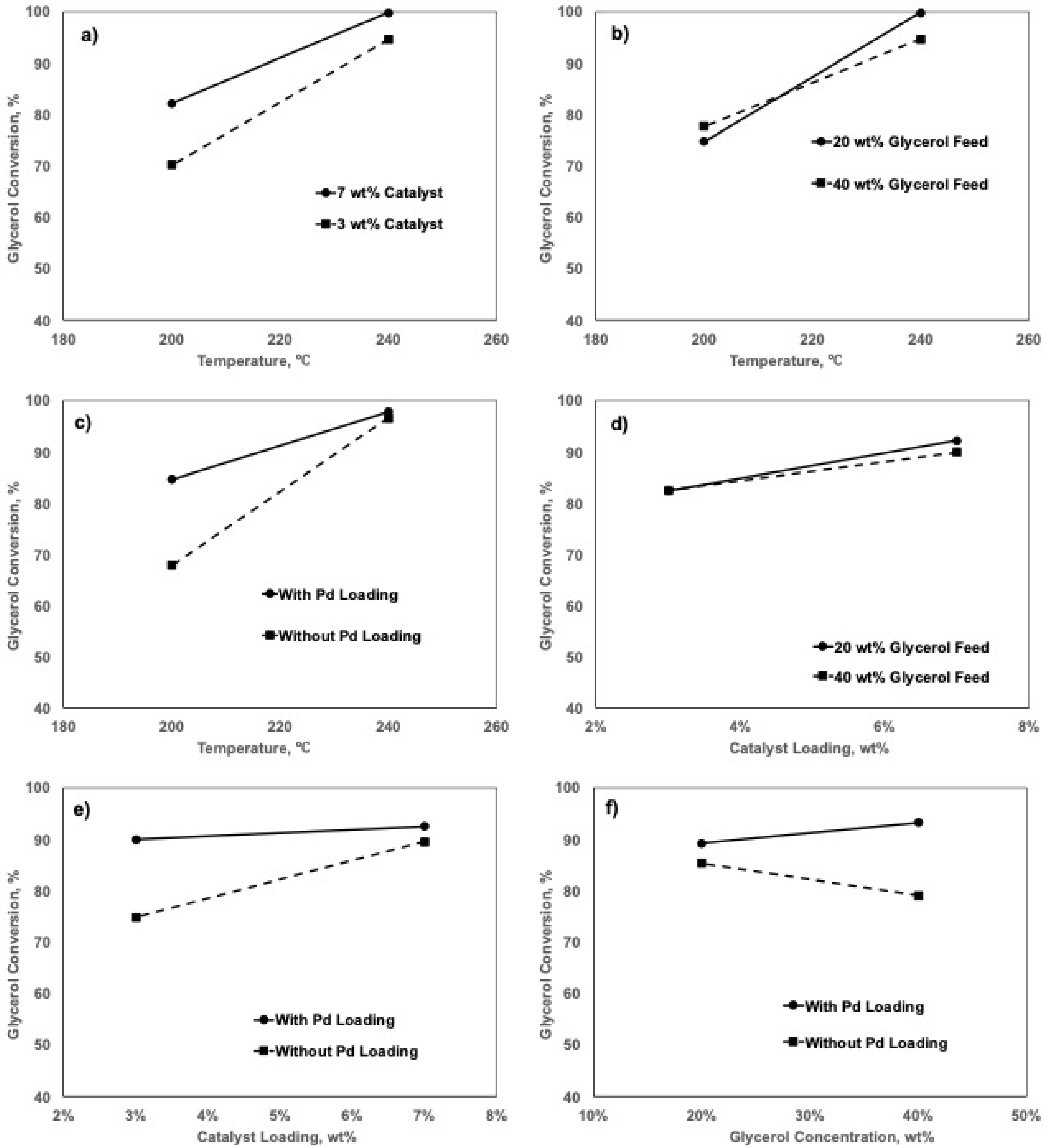
2.5. Factorial Design Analyses
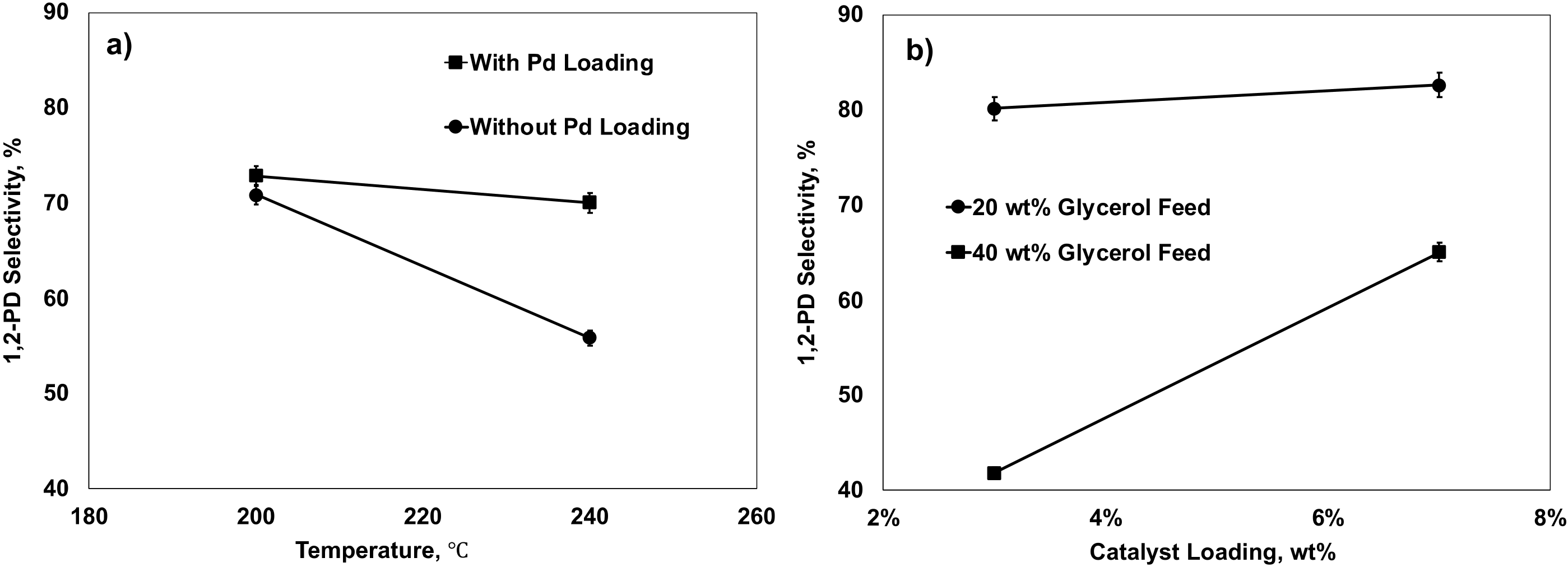
2.5.1. Effects of Factors on 1,2-PD Selectivity
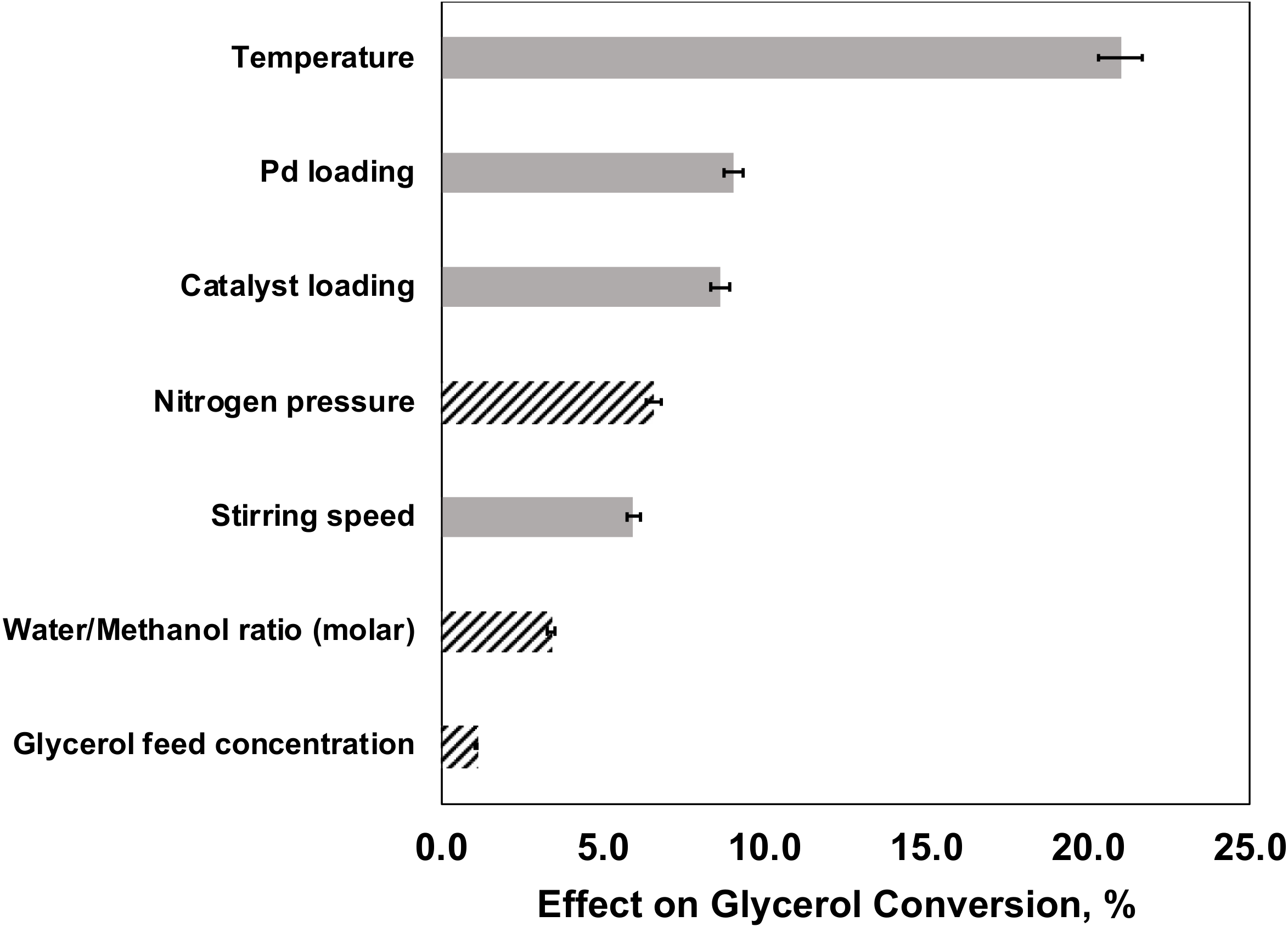
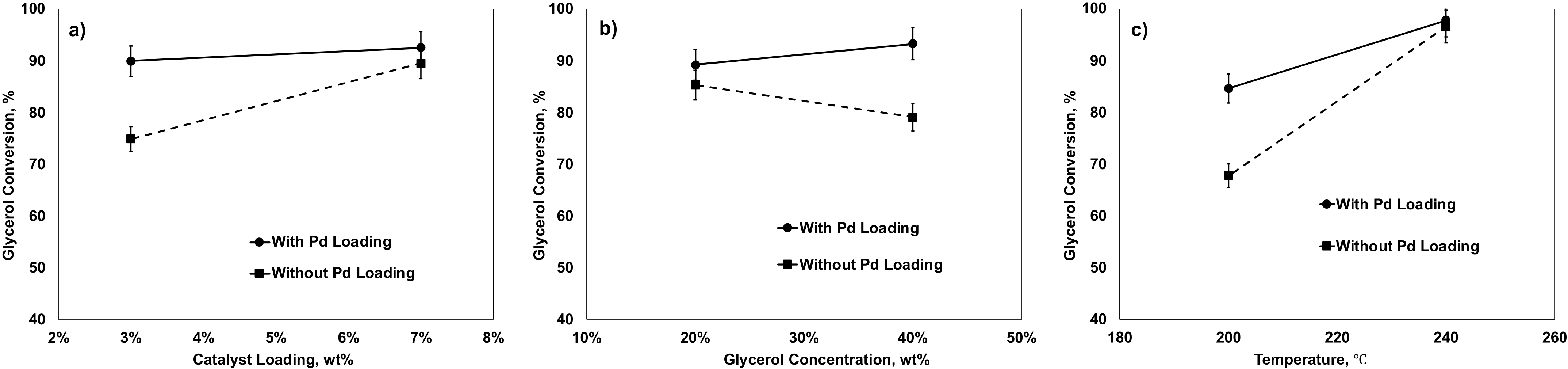
2.5.2. Effects of Factors on Glycerol Conversion
3. Materials and Methods
3.1. Catalyst Preparation
3.2. Catalyst Characterization
3.3. Catalyst Activity Test
3.4. Fractional Factorial Design and Analyses
- Ei—Effect of single factor i
- Ri—Response at factor i level (+/−)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Appendix A. Factorial Design Analysis
| Exp# | Glycerol Conversion % | Selectivities % | ||||
|---|---|---|---|---|---|---|
| 1,2-PD | Acetol | EG | Propanol | Others | ||
| 17 | 96.2 | 81.7 | 0.7 | 8.5 | 0.7 | 8.3 |
| 18 | 95.3 | 80.7 | 1.1 | 8.1 | 1.2 | 8.9 |
| 19 | 98.2 | 82.1 | 0.6 | 9.3 | 0.2 | 7.8 |
| 20 | 98.8 | 81.5 | 0.5 | 8.3 | 0.2 | 9.5 |
| mean | 97.1 | 81.5 | 0.7 | 8.6 | 0.6 | 8.6 |
| SD a | 1.7 | 0.6 | 0.3 | 0.5 | 0.5 | 0.7 |
| CoV b | 0.02 | 0.01 | 0.4 | 0.1 | 0.8 | 0.1 |
| Factors | DF | Effect | SS | MS | f-Value a | p-Value b |
|---|---|---|---|---|---|---|
| A | 1 | −8.9 | 315.95 | 315.95 | 4.48 | 0.058 |
| B | 1 | 12. 9 | 664.35 | 664.35 | 9.42 | 0.011 |
| C | 1 | −27.9 | 3122.02 | 3122.02 | 44.27 | 0.000 |
| D | 1 | 8.1 | 261.63 | 261.63 | 3.71 | 0.080 |
| E | 1 | −1.7 | 11.06 | 11.06 | 0.16 | 0.700 |
| F | 1 | 7.0 | 193.91 | 193.91 | 2.75 | 0.126 |
| G | 1 | 5.6 | 127.13 | 127.13 | 1.8 | 0.206 |
| Curvature | 1 | |||||
| Error | 11 | 775.78 | 70.53 | |||
| Total | 19 | 6110.83 |
| Factors | DF | Effect | SS | MS | f-Value a | p-Value b |
|---|---|---|---|---|---|---|
| A | 1 | 21.0 | 1761.90 | 1761.90 | 25.11 | 0.000 |
| B | 1 | 8.6 | 296.70 | 296.70 | 4.23 | 0.000 |
| C | 1 | −1.1 | 4.73 | 4.73 | 0.07 | 0.064 |
| D | 1 | 9.0 | 326.71 | 326.71 | 4.66 | 0.800 |
| E | 1 | 5.9 | 141.02 | 141.02 | 2.01 | 0.054 |
| F | 1 | −6.6 | 172.27 | 172.27 | 2.46 | 0.184 |
| G | 1 | −3.4 | 45.90 | 45.90 | 0.65 | 0.145 |
| Curvature | 1 | |||||
| Error | 11 | 771.79 | 70.16 | |||
| Total | 19 | 3870.04 |
Appendix B. Calculation of Kinetic Parameters for Glycerol Hydrogenolysis to 1,2-PD and Glycerol C–C Cleavage to EG with Molecular Hydrogen
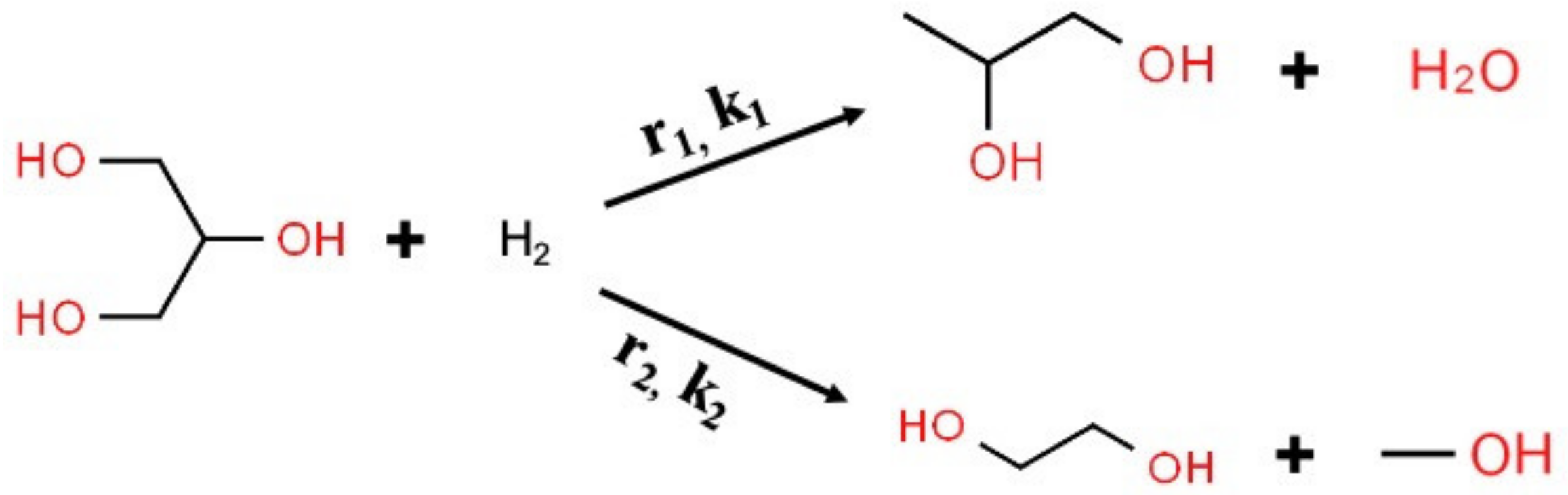
| (a) | |
|---|---|
| Reaction | Rate expression |
| Glycerol dehydration | |
| Glycerol C-C cleavage | |
| (b) | |
| Compound | Rate expression |
| Glycerol | |
| 1,2-PD | |
| EG |
| Temperature °C | Conversion, % | Selectivity, % | Rate Constant, s−1 | ||||
|---|---|---|---|---|---|---|---|
| Glycerol | 1,2-PD | Acetol | EG | Others | k1 | k2 | |
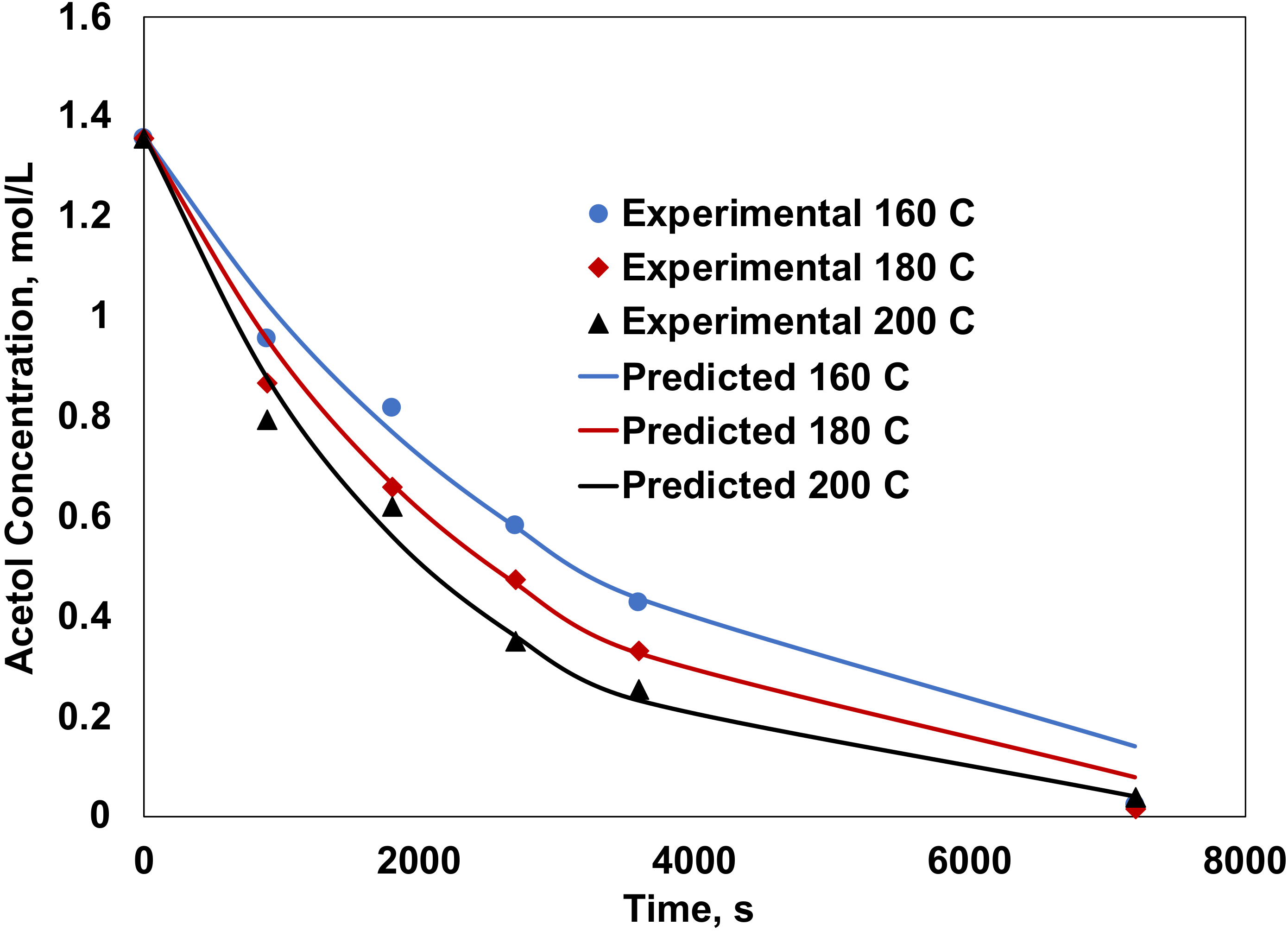
| 180 | 42.9 | 90.0 | 0.0 | 9.3 | 0.7 | 9.72 × 10−6 | 1.32 × 10−6 |
| 200 | 83.9 | 89.6 | 0.5 | 9.0 | 0.8 | 3.61 × 10−5 | 4.34 × 10−6 |
| 220 | 100.0 | 86.1 | 0.5 | 9.2 | 3.5 | 9.26 × 10−5 | 1.38 × 10−5 |

| Acetol Conversion (%) | 1,-2PD Selectivity (%) | Others Selectivity (%) | k0’ (s-1 gcat−1) | |
|---|---|---|---|---|
| 2% Pd-200 °C | 100.0 | 95.3 | 4.7 | 9.854 × 10−4 |
| 2% Pd-180 °C | 100.0 | 97.4 | 2.6 | 3.974 × 10−4 |
| 2% Pd-160 °C | 100.0 | 98.7 | 1.3 | 3.168 × 10−4 |

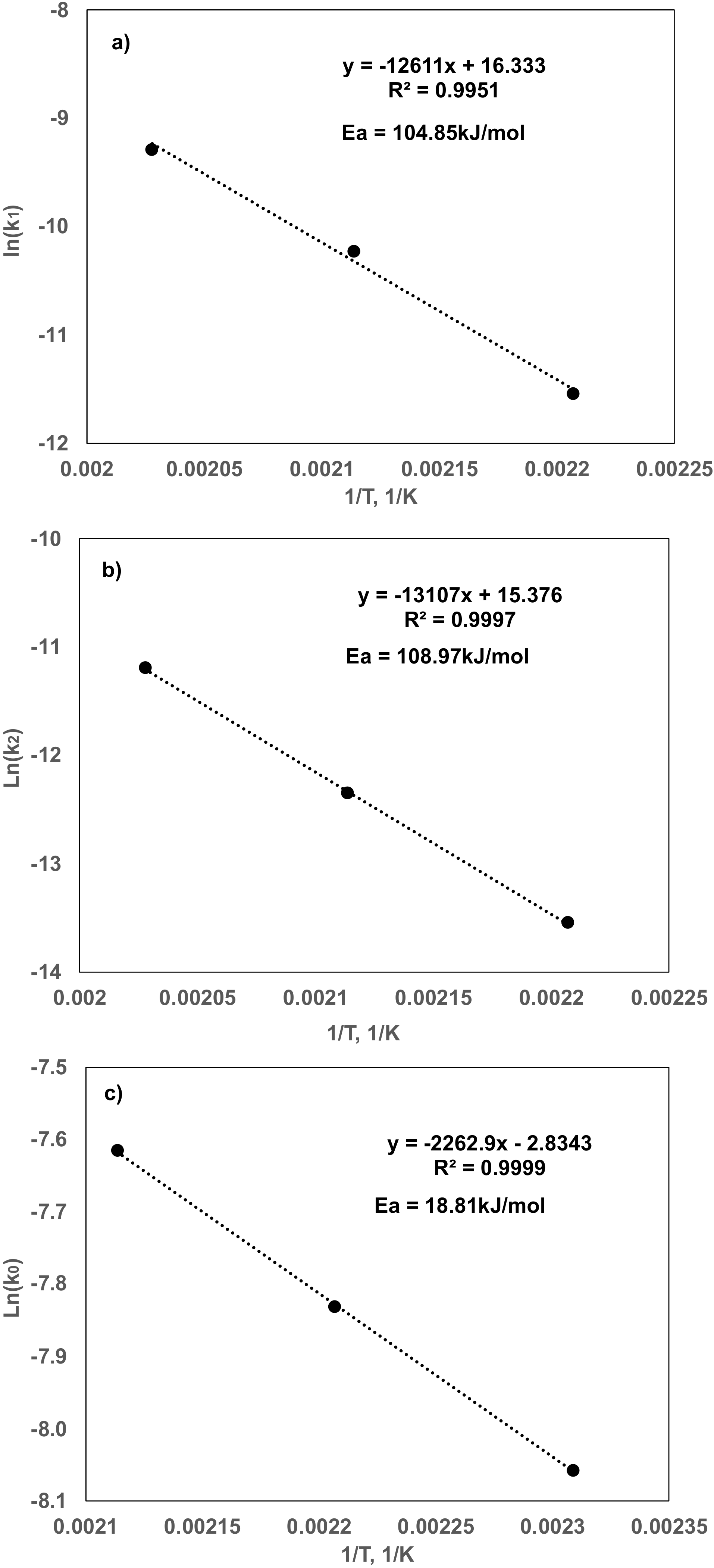
| Pre-Exponential Factor | Activation Energy kJ/mol | |
|---|---|---|
| Glycerol dehydration | 1.10 × 107 | 104.85 |
| Glycerol C–C cleavage | 5.8 × 106 | 108.97 |
| Acetol Hydrogenation | 0.092 | 18.81 |
References
- Veluturla, S.; Archna, N.; Subba Rao, D.; Hezil, N.; Indraja, I.S.; Spoorthi, S. Catalytic valorization of raw glycerol derived from biodiesel: A review. Biofuels 2018, 9, 305–314. [Google Scholar] [CrossRef]
- Pagliaro, M.; Rossi, M. The Future of Glycerol; The Royal Society of Chemistry: London, UK, 2010; pp. P001–P170. [Google Scholar]
- Liu, Y. Catalytic Glycerol Hydrogenolysis to Produce 1,2-propanediol with Molecular Hydrogen and in situ Hydrogen Produced from Steam Reforming. Ph.D. Thesis, University of Waterloo, Waterloo, ON, Canada, 2014. [Google Scholar]
- Cai, F.; Jin, F.; Hao, J.; Xiao, G. Selective hydrogenolysis of glycerol to 1,2-propanediol on Nb-modified Pd−Zr−Al catalysts. Catal. Commun. 2019, 131, 105801. [Google Scholar] [CrossRef]
- Jiang, T.; Zhou, Y.; Liang, S.; Liu, H.; Han, B. Hydrogenolysis of glycerol catalyzed by Ru-Cu bimetallic catalysts supported on clay with the aid of ionic liquids. Green Chem. 2009, 11, 1000–1006. [Google Scholar] [CrossRef]
- D’Hondt, E.; Van de Vyver, S.; Sels, B.F.; Jacobs, P.A. Catalytic glycerol conversion into 1,2-propanediol in absence of added hydrogen. Chem. Commun. 2008, 45, 6011–6012. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Tamura, M.; Shen, Z.; Zhang, Y.; Nakagawa, Y.; Tomishige, K. Hydrogenolysis of glycerol with in-situ produced H2 by aqueous-phase reforming of glycerol using Pt-modified Ir-ReOx/SiO2 catalyst. Catal. Today 2018, 303, 106–116. [Google Scholar] [CrossRef]
- Gandarias, I.; Arias, P.L.; Requies, J.; Güemez, M.B.; Fierro, J.L.G. Hydrogenolysis of glycerol to propanediols over a Pt/ASA catalyst: The role of acid and metal sites on product selectivity and the reaction mechanism. Appl. Catal. B 2010, 97, 248–256. [Google Scholar] [CrossRef]
- Pendem, C.; Gupta, P.; Chaudhary, N.; Singh, S.; Kumar, J.; Sasaki, T.; Datta, A.; Bal, R. Aqueous phase reforming of glycerol to 1,2-propanediol over Pt-nanoparticles supported on hydrotalcite in the absence of hydrogen. Green Chem. 2012, 14, 3107–3113. [Google Scholar] [CrossRef]
- Soares, A.V.; Perez, G.; Passos, F.B. Alumina supported bimetallic Pt–Fe catalysts applied to glycerol hydrogenolysis and aqueous phase reforming. Appl. Catal. B 2016, 185, 77–87. [Google Scholar] [CrossRef]
- Roy, D.; Subramaniam, B.; Chaudhari, R.V. Aqueous phase hydrogenolysis of glycerol to 1,2-propanediol without external hydrogen addition. Catal. Today 2010, 156, 31–37. [Google Scholar] [CrossRef]
- Von Held Soares, A.; Atia, H.; Armbruster, U.; Passos, F.B.; Martin, A. Platinum, palladium and nickel supported on Fe3O4 as catalysts for glycerol aqueous-phase hydrogenolysis and reforming. Appl. Catal. A 2017, 548, 179–190. [Google Scholar] [CrossRef]
- Seretis, A.; Tsiakaras, P. Crude bio-glycerol aqueous phase reforming and hydrogenolysis over commercial SiO2-Al2O3 nickel catalyst. Renewable Energy 2016, 97, 373–379. [Google Scholar] [CrossRef]
- Seretis, A.; Tsiakaras, P. Hydrogenolysis of glycerol to propylene glycol by in situ produced hydrogen from aqueous phase reforming of glycerol over SiO2–Al2O3 supported nickel catalyst. Fuel Process. Technol. 2016, 142, 135–146. [Google Scholar] [CrossRef]
- Yin, A.; Guo, X.; Dai, W.; Fan, K. The synthesis of propylene glycol and ethylene glycol from glycerol using Raney Ni as a versatile catalyst. Green Chem. 2009, 11, 1514–1516. [Google Scholar] [CrossRef]
- Zhou, C.H.; Deng, K.; Serio, M.D.; Xiao, S.; Tong, D.S.; Li, L.; Lin, C.X.; Beltramini, J.; Zhang, H.; Yu, W.H. Cleaner hydrothermal hydrogenolysis of glycerol to 1,2-propanediol over Cu/oxide catalysts without addition of external hydrogen. Mol. Catal. 2017, 432, 274–284. [Google Scholar] [CrossRef]
- Mane, R.B.; Rode, C.V. Simultaneous glycerol dehydration and in situ hydrogenolysis over Cu-Al oxide under an inert atmosphere. Green Chem. 2012, 14, 2780–2789. [Google Scholar] [CrossRef]
- Musolino, M.G.; Scarpino, L.A.; Mauriello, F.; Pietropaolo, R. Selective transfer hydrogenolysis of glycerol promoted by palladium catalysts in absence of hydrogen. Green Chem. 2009, 11, 1511–1513. [Google Scholar] [CrossRef]
- Gandarias, I.; Arias, P.L.; Fernández, S.G.; Requies, J.; El Doukkali, M.; Güemez, M.B. Hydrogenolysis through catalytic transfer hydrogenation: Glycerol conversion to 1,2-propanediol. Catal. Today 2012, 195, 22–31. [Google Scholar] [CrossRef]
- Mauriello, F.; Ariga, H.; Musolino, M.G.; Pietropaolo, R.; Takakusagi, S.; Asakura, K. Exploring the catalytic properties of supported palladium catalysts in the transfer hydrogenolysis of glycerol. Appl. Catal. B 2015, 166–167, 121–131. [Google Scholar] [CrossRef]
- Gandarias, I.; Arias, P.L.; Requies, J.; El Doukkali, M.; Güemez, M.B. Liquid-phase glycerol hydrogenolysis to 1,2-propanediol under nitrogen pressure using 2-propanol as hydrogen source. J. Catal. 2011, 282, 237–247. [Google Scholar] [CrossRef]
- Musolino, M.G.; Scarpino, L.A.; Mauriello, F.; Pietropaolo, R. Glycerol Hydrogenolysis Promoted by Supported Palladium Catalysts. ChemSusChem 2011, 4, 1143–1150. [Google Scholar] [CrossRef]
- Cai, F.; Pan, D.; Ibrahim, J.J.; Zhang, J.; Xiao, G. Hydrogenolysis of glycerol over supported bimetallic Ni/Cu catalysts with and without external hydrogen addition in a fixed-bed flow reactor. Appl. Catal. A 2018, 564, 172–182. [Google Scholar] [CrossRef]
- Xia, S.; Zheng, L.; Wang, L.; Chen, P.; Hou, Z. Hydrogen-free synthesis of 1,2-propanediol from glycerol over Cu-Mg-Al catalysts. RSC Adv. 2013, 3, 16569–16576. [Google Scholar] [CrossRef]
- Vasiliadou, S.E.; Lemonidou, A.A. Catalytic Glycerol Hydrodeoxygenation under Inert Atmosphere: Ethanol as a Hydrogen Donor. Catalysts 2014, 4, 397–413. [Google Scholar] [CrossRef] [Green Version]
- Yuan, J.; Li, S.; Yu, L.; Liu, Y.; Cao, Y. Efficient catalytic hydrogenolysis of glycerol using formic acid as hydrogen source. Chin. J. Catal. 2013, 34, 2066–2074. [Google Scholar] [CrossRef]
- Gandarias, I.; Requies, J.; Arias, P.L.; Armbruster, U.; Martin, A. Liquid-phase glycerol hydrogenolysis by formic acid over Ni–Cu/Al2O3 catalysts. J. Catal. 2012, 290, 79–89. [Google Scholar] [CrossRef]
- Yfanti, V.L.; Lemonidou, A.A. Effect of hydrogen donor on glycerol hydrodeoxygenation to 1,2-propanediol. Catal. Today 2019, 355, 727–736. [Google Scholar] [CrossRef]
- West, A.H.; Posarac, D.; Ellis, N. Assessment of four biodiesel production processes using HYSYS.Plant. Bioresour. Technol. 2008, 99, 6587–6601. [Google Scholar] [CrossRef]
- Andreatta, A.E.; Casás, L.M.; Hegel, P.; Bottini, S.B.; Brignole, E.A. Phase Equilibria in Ternary Mixtures of Methyl Oleate, Glycerol, and Methanol. Ind. Eng. Chem. Res. 2008, 47, 5157–5164. [Google Scholar] [CrossRef]
- Lee, M.; Lo, Y.; Lin, H. Liquid–liquid equilibria for mixtures containing water, methanol, fatty acid methyl esters, and glycerol. Fluid Phase Equilib. 2010, 299, 180–190. [Google Scholar] [CrossRef]
- Liu, Y.; Guo, X.; Rempel, L.G.; Ng, T.T.F. The Promoting Effect of Ni on Glycerol Hydrogenolysis to 1,2-Propanediol with In Situ Hydrogen from Methanol Steam Reforming Using a Cu/ZnO/Al2O3 Catalyst. Catalysts 2019, 9, 412. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Mai, C.T.Q.; Ng, F.T.T. Glycerol Hydrogenolysis with In Situ Hydrogen Produced via Methanol Steam Reforming: The Promoting Effect of Pd on a Cu/ZnO/Al2O3 Catalyst. Catalysts 2021, 11, 110. [Google Scholar] [CrossRef]
- Van Ryneveld, E.; Mahomed, A.S.; van Heerden, P.S.; Friedrich, H.B. Direct Hydrogenolysis of Highly Concentrated Glycerol Solutions Over Supported Ru, Pd and Pt Catalyst Systems. Catal. Lett. 2011, 141, 958–967. [Google Scholar] [CrossRef]
- Vasiliadou, E.S.; Yfanti, V.L.; Lemonidou, A.A. One-pot tandem processing of glycerol stream to 1,2-propanediol with methanol reforming as hydrogen donor reaction. Appl. Catal. B 2015, 163, 258–266. [Google Scholar] [CrossRef]
- Yfanti, V.L.; Vasiliadou, E.S.; Lemonidou, A.A. Glycerol hydro-deoxygenation aided by in situ H2 generation via methanol aqueous phase reforming over a Cu-ZnO-Al2O3 catalyst. Catal Sci. Technol. 2016, 6, 5415–5426. [Google Scholar] [CrossRef]
- Yfanti, V.L.; Ipsakis, D.; Lemonidou, A.A. Kinetic study of liquid phase glycerol hydrodeoxygenation under inert conditions over a Cu-based catalyst. React. Chem. Eng. 2018, 3, 559–571. [Google Scholar] [CrossRef]
- Yfanti, V.L.; Ipsakis, D.; Lemonidou, A.A. Kinetic model of glycerol hydrodeoxygenation under inert conditions over copper catalyst. Mater. Today Proc. 2018, 5, 27482–27490. [Google Scholar] [CrossRef]
- Yfanti, V.L.; Lemonidou, A.A. Mechanistic study of liquid phase glycerol hydrodeoxygenation with in-situ generated hydrogen. J. Catal. 2018, 368, 98–111. [Google Scholar] [CrossRef]
- Yuan, Z.; Wang, L.; Wang, J.; Xia, S.; Chen, P.; Hou, Z.; Zheng, X. Hydrogenolysis of glycerol over homogenously dispersed copper on solid base catalysts. Appl. Catal. B 2011, 101, 431–440. [Google Scholar] [CrossRef]
- Yuan, Z.; Wang, J.; Wang, L.; Xie, W.; Chen, P.; Hou, Z.; Zheng, X. Biodiesel derived glycerol hydrogenolysis to 1,2-propanediol on Cu/MgO catalysts. Bioresour. Technol. 2010, 101, 7088–7092. [Google Scholar] [CrossRef] [PubMed]
- Xia, S.; Nie, R.; Lu, X.; Wang, L.; Chen, P.; Hou, Z. Hydrogenolysis of glycerol over Cu0.4/Zn5.6−xMgxAl2O8.6 catalysts: The role of basicity and hydrogen spillover. J. Catal. 2012, 296, 1–11. [Google Scholar] [CrossRef]
- Xia, S.; Yuan, Z.; Wang, L.; Chen, P.; Hou, Z. Hydrogenolysis of glycerol on bimetallic Pd-Cu/solid-base catalysts prepared via layered double hydroxides precursors. Appl. Catal. A 2011, 403, 173–182. [Google Scholar] [CrossRef]
- Mitta, H.; Devunuri, N.; Sunkari, J.; Mutyala, S.; Balla, P.; Perupogu, V. A highly active dispersed copper oxide phase on calcined Mg9Al2.7-Ga2.3O2 catalysts in glycerol hydrogenolysis. Catal. Today 2020, 375, 204–215. [Google Scholar] [CrossRef]
- Xia, S.; Yuan, Z.; Wang, L.; Chen, P.; Hou, Z. Catalytic production of 1,2-propanediol from glycerol in bio-ethanol solvent. Bioresour. Technol. 2012, 104, 814–817. [Google Scholar] [CrossRef]
- Mallesham, B.; Sudarsanam, P.; Reddy, B.V.S.; Reddy, B.M. Development of cerium promoted copper–magnesium catalysts for biomass valorization: Selective hydrogenolysis of bioglycerol. Appl. Catal. B 2016, 181, 47–57. [Google Scholar] [CrossRef]
- Yue, C.; Zhang, Q.; Gu, L.; Su, Y.; Zhu, S. Oxides-modified Raney copper as catalysts for selective hydrogenolysis of glycerol. Asia-Pac. J. Chem. Eng. 2014, 9, 581–590. [Google Scholar] [CrossRef]
- Rekha, V.; Raju, N.; Sumana, C.; Douglas, S.P.; Lingaiah, N. Selective Hydrogenolysis of Glycerol Over Cu–ZrO2–MgO Catalysts. Catal. Lett. 2016, 146, 1487–1496. [Google Scholar] [CrossRef]
- Pudi, S.M.; Biswas, P.; Kumar, S. Selective hydrogenolysis of glycerol to 1,2-propanediol over highly active copper–magnesia catalysts: Reaction parameter, catalyst stability and mechanism study. J. Chem. Technol. Biotechnol. 2016, 91, 2063–2075. [Google Scholar] [CrossRef]
- Hou, X.; Qing, S.; Liu, Y.; Li, L.; Gao, Z.; Qin, Y. Enhancing effect of MgO modification of Cu–Al spinel oxide catalyst for methanol steam reforming. Int. J. Hydrogen Energy 2020, 45, 477–489. [Google Scholar] [CrossRef]
- Phongboonchoo, Y.; Thouchprasitchai, N.; Pongstabodee, S. Hydrogen production with a low carbon monoxide content via methanol steam reforming over CuxCeyMgz/Al2O3 catalysts: Optimization and stability. Int. J. Hydrogen Energy 2017, 42, 12220–12235. [Google Scholar] [CrossRef]
- Kim, N.D.; Park, J.R.; Park, D.S.; Kwak, B.K.; Yi, J. Promoter effect of Pd in CuCr2O4 catalysts on the hydrogenolysis of glycerol to 1,2-propanediol. Green Chem. 2012, 14, 2638–2646. [Google Scholar] [CrossRef]
- Hu, B.; Yin, Y.; Liu, G.; Chen, S.; Hong, X.; Tsang, S.C.E. Hydrogen spillover enabled active Cu sites for methanol synthesis from CO2 hydrogenation over Pd doped CuZn catalysts. J. Catal. 2018, 359, 17–26. [Google Scholar] [CrossRef]
- Liu, Y.; He, Y.; Zhou, D.; Feng, J.; Li, D. Catalytic performance of Pd-promoted Cu hydrotalcite-derived catalysts in partial hydrogenation of acetylene: Effect of Pd-Cu alloy formation. Catal. Sci. Technol. 2016, 6, 3027–3037. [Google Scholar] [CrossRef]
- Feng, Y.; Liu, C.; Kang, Y.; Zhou, X.; Liu, L.; Deng, J.; Xu, H.; Fu, Y. Selective hydrogenolysis of glycerol to 1,2-propanediol catalyzed by supported bimetallic PdCu-KF/γ-Al2O3. Chem. Eng. J. 2015, 281, 96–101. [Google Scholar] [CrossRef]
- Bichon, P.; Asheim, M.; Sperle, A.J.T.; Fathi, M.; Holmen, A.; Blekkan, E.A. Hydrogen from methanol steam-reforming over Cu-based catalysts with and without Pd promotion. Int. J. Hydrogen Energy 2007, 32, 1799–1805. [Google Scholar] [CrossRef]
- Sá, S.; Silva, H.; Brandão, L.; Sousa, J.M.; Mendes, A. Catalysts for methanol steam reforming—A review. Appl. Catal. B 2010, 99, 43–57. [Google Scholar] [CrossRef]
- Wang, Y.; Zhou, J.; Guo, X. Catalytic hydrogenolysis of glycerol to propanediols: A review. RSC Adv. 2015, 5, 74611–74628. [Google Scholar] [CrossRef]
- Sun, D.; Yamada, Y.; Sato, S.; Ueda, W. Glycerol hydrogenolysis into useful C3 chemicals. Appl. Catal. B 2016, 193, 75–92. [Google Scholar] [CrossRef] [Green Version]
- Feng, Y.; Yin, H.; Wang, A.; Shen, L.; Yu, L.; Jiang, T. Gas phase hydrogenolysis of glycerol catalyzed by Cu/ZnO/MOx (MOx=Al2O3, TiO2, and ZrO2) catalysts. Chem. Eng. J. 2011, 168, 403–412. [Google Scholar] [CrossRef]
- Matter, P.H.; Ozkan, U.S. Effect of pretreatment conditions on Cu/Zn/Zr-based catalysts for the steam reforming of methanol to H2. J. Catal. 2005, 234, 463–475. [Google Scholar] [CrossRef]
- Lindström, B.; Pettersson, L.J.; Menon, P.G. Activity and characterization of Cu/Zn, Cu/Cr and Cu/Zr on γ-alumina for methanol reforming for fuel cell vehicles. Appl. Catal. A 2002, 234, 111–125. [Google Scholar] [CrossRef]
- Cai, F.; Zhu, W.; Xiao, G. Promoting effect of zirconium oxide on Cu-Al2O3 catalyst for the hydrogenolysis of glycerol to 1,2-propanediol. Catal. Sci. Technol. 2016, 6, 4889–4900. [Google Scholar] [CrossRef]
- Yazdani, P.; Wang, B.; Du, Y.; Kawi, S.; Borgna, A. Lanthanum oxycarbonate modified Cu/Al2O3 catalysts for selective hydrogenolysis of glucose to propylene glycol: Base site requirements. Catal. Sci. Technol. 2017, 7, 4680–4690. [Google Scholar] [CrossRef]
- Khani, Y.; Bahadoran, F.; Soltanali, S.; Ahari, J.S. Hydrogen production by methanol steam reforming on a cordierite monolith reactor coated with Cu-Ni/LaZnAlO4 and Cu-Ni/γ -Al2O3 catalysts. Res. Chem. Intermed. 2018, 44, 925–942. [Google Scholar] [CrossRef]
- Chiu, C.; Dasari, M.A.; Suppes, G.J.; Sutterlin, W.R. Dehydration of glycerol to acetol via catalytic reactive distillation. AIChE J. 2006, 52, 3543–3548. [Google Scholar] [CrossRef]
- Pala Rosas, I.; Contreras, J.L.; Salmones, J.; Tapia, C.; Zeifert, B.; Navarrete, J.; Vázquez, T.; García, D.C. Catalytic Dehydration of Glycerol to Acrolein over a Catalyst of Pd/LaY Zeolite and Comparison with the Chemical Equilibrium. Catalysts 2017, 7, 73. [Google Scholar] [CrossRef]
- Bienholz, A.; Blume, R.; Knop-Gericke, A.; Girgsdies, F.; Behrens, M.; Claus, P. Prevention of Catalyst Deactivation in the Hydrogenolysis of Glycerol by Ga2O3-Modified Copper/Zinc Oxide Catalysts. J. Phys. Chem. C 2011, 115, 999–1005. [Google Scholar] [CrossRef]
- Mondal, S.; Janardhan, R.; Meena, M.L.; Biswas, P. Highly active Cu-Zn-Mg-Al-O catalyst derived from layered double hydroxides (LDHs) precursor for selective hydrogenolysis of glycerol to 1,2-propanediol. J. Environ. Chem. Eng. 2017, 5, 5695–5706. [Google Scholar] [CrossRef]
- Iwasa, N.; Mayanagi, T.; Nomura, W.; Arai, M.; Takezawa, N. Effect of Zn addition to supported Pd catalysts in the steam reforming of methanol. Appl. Catal. A 2003, 248, 153–160. [Google Scholar] [CrossRef]
- Kyriakou, G.; Boucher, M.B.; Jewell, A.D.; Lewis, E.A.; Lawton, T.J.; Baber, A.E.; Tierney, H.L.; Flytzani-Stephanopoulos, M.; Sykes, E.C. Isolated Metal Atom Geometries as a Strategy for Selective Heterogeneous Hydrogenations. Science 2012, 335, 1209–1212. [Google Scholar] [CrossRef] [PubMed]
- Rehman, S.; Mumtaz, A.; Hasanain, S.K. Size effects on the magnetic and optical properties of CuO nanoparticles. J. Nanopart. Res. 2011, 13, 2497–2507. [Google Scholar] [CrossRef]
- Ma, L.; Lv, C.; Wang, G. A DFT study and micro-kinetic analysis of acetylene selective hydrogenation on Pd-doped Cu(111) surfaces. Appl. Surf. Sci. 2017, 410, 154–165. [Google Scholar] [CrossRef]
- Tierney, H.L.; Baber, A.E.; Kitchin, J.R.; Sykes, E.C. Hydrogen Dissociation and Spillover on Individual Isolated Palladium Atoms. Phys. Rev. Lett. 2009, 103, 246102. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Pasupulety, N.; Gunda, K.; Rempel, G.L.; Ng, F.T. Glycerol Hydrogenolysis to 1,2-Propanediol by Cu/ZnO/Al2O3 Catalysts. Top. Catal. 2014, 57, 1454–1462. [Google Scholar] [CrossRef]
- Boucher, M.B.; Zugic, B.; Cladaras, G.; Kammert, J.; Marcinkowski, M.D.; Lawton, T.J.; Sykes, E.C.; Flytzani-Stephanopoulos, M. Single atom alloy surface analogs in Pd0.18Cu15 nanoparticles for selective hydrogenation reactions. Phys. Chem. Chem. Phys. 2013, 15, 12187–12196. [Google Scholar] [CrossRef]
- Montgomery, D.C.; Runger, G.C. Design of Experiments with Several Factors. In Applied Statistics and Probability for Engineers; John Wiley & Sons Inc.: Hoboken, NJ, USA, 2002; p. 549. [Google Scholar]



| Entry | Catalysts | Conversion | Selectivity | ||||
|---|---|---|---|---|---|---|---|
| % | % | ||||||
| Glycerol | 1,2-PD | Acetol | EG | Propanol | Others | ||
| 1 | Cu/ZnO/ZrO2 | 75.2 | 57.3 | 13.7 | 3.7 | 0.7 | 24.6 |
| 2 | Cu/ZrO2/Al2O3 | 59.1 | 52.9 | 20.6 | 5.2 | 0.8 | 20.5 |
| 3 | Cu/La2O3/Al2O3 | 29.7 | 58.0 | 30.0 | 6.1 | 0.0 | 6.1 |
| 4 | Cu/ZnO/Al2O3 2 | 87.1 | 70.7 | 6.0 | 3.3 | 0.8 | 19.2 |
| 5 | 5Ni/Cu/ZnO/Al2O3 2 | 70.0 | 85.5 | 5.0 | 5.0 | 0.9 | 3.7 |
| 6 | Cu/MgO/Al2O3 | 95.6 ± 3.2 | 79.5 ± 1.19 | 1.0 ± 0.72 | 8.1 ± 0.93 | 0.8 | 10.6 ± 1.72 |
| 7 | 1Pd/Cu/ZnO/Al2O3 3 | 94.9 | 74.3 | 4.1 | 3.9 | 0.8 | 16.8 |
| 8 | 1Pd/Cu/MgO/Al2O3 | 97.2 ± 3.2 | 83.4 ± 1.25 | 0.0 | 9.5 ± 1.09 | 0.7 | 6.4 ± 1.04 |
| 9 | 2Pd/ZnO 3 | 83.0 | 49.7 | 11.6 | 2.9 | 0.0 | 35.8 |
| 10 | 5Ni/ZnO/Al2O3 2 | 4.8 | 0.0 | 47.9 | 0.0 | 0.0 | 52.1 |
| 11 | ZnO/MgO/Al2O3 4 | 2.9 | 59.2 | N/A | 29.2 | N/A | N/A |
| 12 | Cu/MgO | 80.6 | 62.3 | 2.7 | 7.1 | 1.1 | 26.7 |
| (a) | |
|---|---|
| Reaction | Rate Expression |
| Glycerol dehydration | |
| Acetol hydrogenation | |
| Glycerol C–C cleavage | |
| (x): molar concentration of each compound (mol L−1) ri: rate of the reaction (mol L−1 s−1 gcat−1) ki: rate constant of each reaction (s−1 gcat−1) | |
| (b) | |
| Compound | Rate Expression |
| Glycerol | |
| Acetol | |
| 1,2-PD | |
| EG | |
| (i): molar concentration of each compound per g of catalyst (mol L−1) ri: rate of the reaction (mol L−1 s−1 gcat−1) w: weight of catalyst (g) | |
| Catalyst | k1 (s−1 gcat−1) | k2 (s−1 gcat−1) | k3 (s−1 gcat−1) |
|---|---|---|---|
| 1Pd/Cu/MgO/Al2O3 | 6.850 × 10−5 | 2.752 × 10−3 | 7.707 × 10−6 |
| Cu/MgO/Al2O3 | 3.843 × 10−5 | 4.897 × 10−4 | 4.203 × 10−6 |
| Catalysts | Conversion | Selectivity | ||||
|---|---|---|---|---|---|---|
| % | % | |||||
| Glycerol | 1,2-PD | Acetol | EG | Propanol | Others | |
| Cu/MgO/Al2O3 | 97.4 | 54.9 | 2.0 | 4.5 | 2.1 | 36.4 |
| 1Pd/Cu/MgO/Al2O3 | 100.0 | 66.8 | 0.7 | 5.5 | 0.7 | 26.3 |
| 2Pd/Cu/MgO/Al2O3 | 100.0 | 73.2 | 0.4 | 6.6 | 1.4 | 18.4 |
| 3Pd/Cu/MgO/Al2O3 | 100.0 | 73.6 | 0.4 | 6.6 | 1.2 | 18.2 |
| 2Pd/Cu/MgO/Al2O3 2 | 100.0 | 85.3 | 0.7 | 7.8 | 0.3 | 5.9 |
| Catalysts | Acetol Conversion (%) | 1,2-PD Selectivity (%) | Others Selectivity (%) | k2 (s−1 gcat−1) |
|---|---|---|---|---|
| Cu/MgO/Al2O3 | 86.8 | 68.6 | 31.5 | 2.262 × 10−4 |
| 2Pd/Cu/MgO/Al2O3 | 100.0 | 95.3 | 4.7 | 9.854 × 10−4 |
| Conditions | Code | −1 | 0 | +1 |
|---|---|---|---|---|
| Temperature (°C) | A | 200 | 220 | 240 |
| Catalyst loading (wt%) | B | 3 | 5 | 7 |
| Glycerol feed concentration (wt%) | C | 20 | 30 | 40 |
| Pd loading (wt%) | D | 0 | 1 | 2 |
| Stirring Speed (RPM) | E | 400 | 500 | 600 |
| Nitrogen Pressure (bar) | F | 15 | 25 | 35 |
| Water/Methanol (molar) | G | 1 | 1.2 | 1.4 |
| Exp | A | B | C | D | E = ABC | F = BCD | G = ACD |
|---|---|---|---|---|---|---|---|
| 1 | −1 | −1 | −1 | −1 | −1 | −1 | −1 |
| 2 | 1 | −1 | −1 | −1 | 1 | −1 | 1 |
| 3 | −1 | 1 | −1 | −1 | 1 | 1 | −1 |
| 4 | 1 | 1 | −1 | −1 | −1 | 1 | 1 |
| 5 | −1 | −1 | 1 | −1 | 1 | 1 | 1 |
| 6 | 1 | −1 | 1 | −1 | −1 | 1 | −1 |
| 7 | −1 | 1 | 1 | −1 | −1 | −1 | 1 |
| 8 | 1 | 1 | 1 | −1 | 1 | −1 | −1 |
| 9 | −1 | −1 | −1 | 1 | −1 | 1 | 1 |
| 10 | 1 | −1 | −1 | 1 | 1 | 1 | −1 |
| 11 | −1 | 1 | −1 | 1 | 1 | −1 | 1 |
| 12 | 1 | 1 | −1 | 1 | −1 | −1 | −1 |
| 13 | −1 | −1 | 1 | 1 | 1 | −1 | −1 |
| 14 | 1 | −1 | 1 | 1 | −1 | −1 | 1 |
| 15 | −1 | 1 | 1 | 1 | −1 | 1 | −1 |
| 16 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| 17 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 18 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 19 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 20 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Wu, M.; Rempel, G.L.; Ng, F.T.T. Glycerol Hydrogenolysis to Produce 1,2-Propanediol in Absence of Molecular Hydrogen Using a Pd Promoted Cu/MgO/Al2O3 Catalyst. Catalysts 2021, 11, 1299. https://doi.org/10.3390/catal11111299
Liu Y, Wu M, Rempel GL, Ng FTT. Glycerol Hydrogenolysis to Produce 1,2-Propanediol in Absence of Molecular Hydrogen Using a Pd Promoted Cu/MgO/Al2O3 Catalyst. Catalysts. 2021; 11(11):1299. https://doi.org/10.3390/catal11111299
Chicago/Turabian StyleLiu, Yuanqing, Michael Wu, Garry L. Rempel, and Flora T.T. Ng. 2021. "Glycerol Hydrogenolysis to Produce 1,2-Propanediol in Absence of Molecular Hydrogen Using a Pd Promoted Cu/MgO/Al2O3 Catalyst" Catalysts 11, no. 11: 1299. https://doi.org/10.3390/catal11111299






