Radial TiO2 Nanorod-Based Mesocrystals: Synthesis, Characterization, and Applications
Abstract
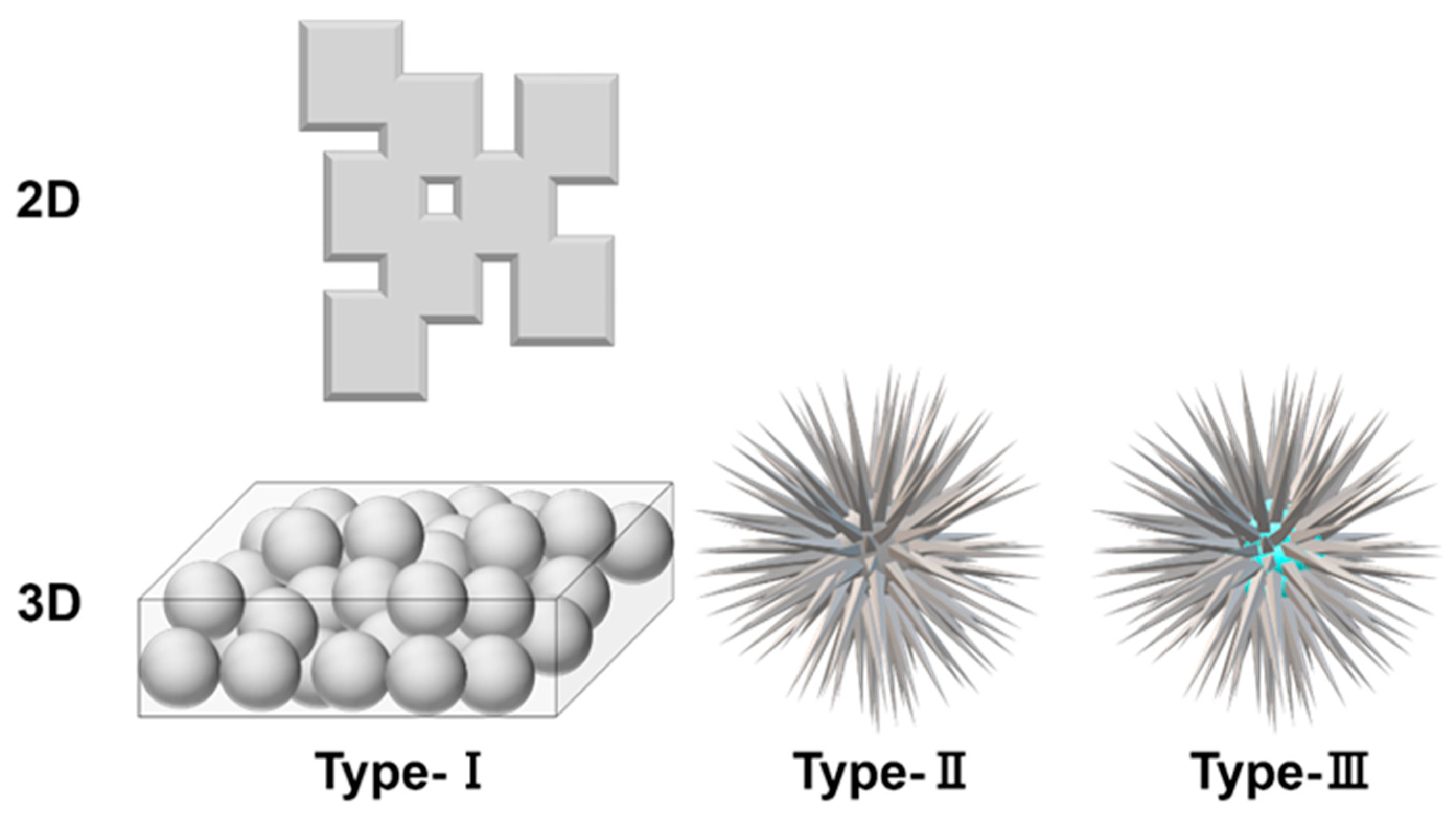
:1. Introduction
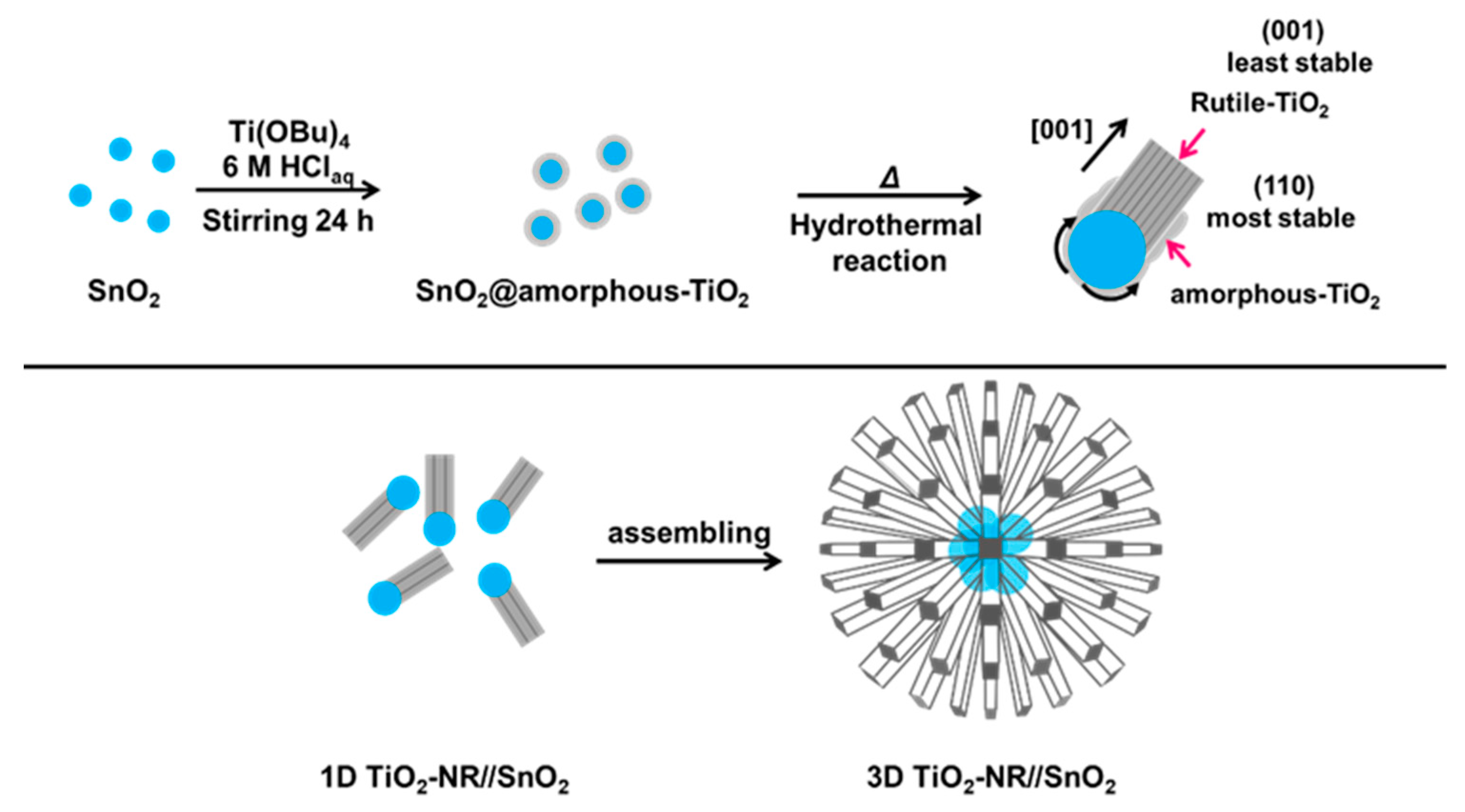
2. Synthesis and Characterization
3. Photocatalytic Applications
3.1. TiO2-Nanorod Homomesocrystals
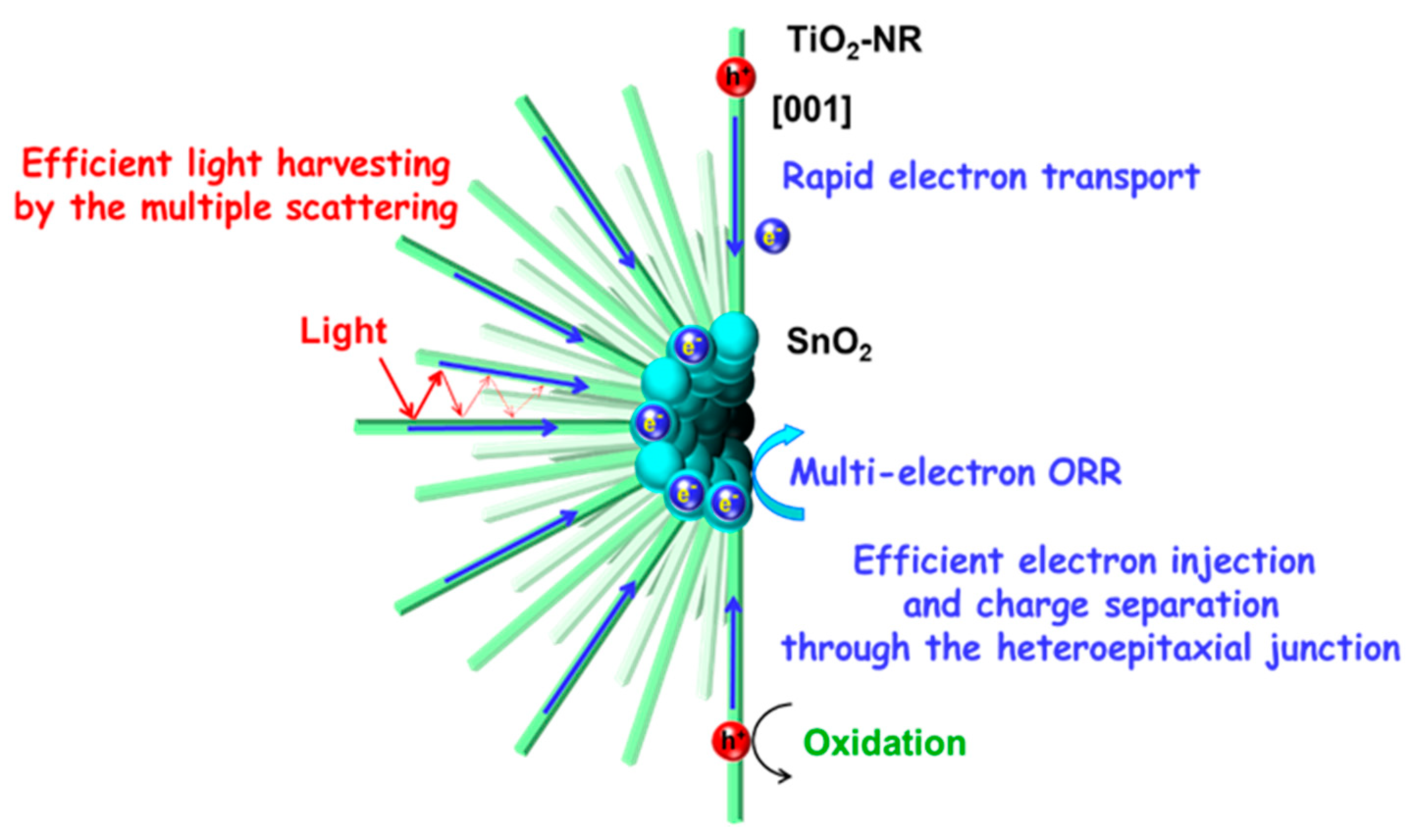
3.2. TiO2-Nanorod//SnO2 Heteromesocrystals
3.3. Photocatalytic Action Mechanism
4. Electrochemical Applications
4.1. Solar Cells
4.2. Lithium-Ion Batteries
5. Conclusions and Future Subjects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hashimoto, K.; Irie, H.; Fujishima, A. TiO2 photocatalysis: A historical overview and future prospects. Jpn. J. Appl. Phys. 2005, 44, 8269–8285. [Google Scholar] [CrossRef]
- Park, H.; Park, Y.; Kim, W.; Choi, W. Surface modification of TiO2 photocatalyst for environmental applications. J. Photochem. Photobiol. C 2013, 15, 1–20. [Google Scholar] [CrossRef]
- Sang, L.; Zhao, Y.; Burda, C. TiO2 nanoparticles as functional building blocks. Chem. Rev. 2014, 114, 9283–9318. [Google Scholar] [CrossRef]
- Chen, X.; Li, C.; Grätzel, M.; Kostecki, R.; Mao, S.S. Nanomaterials for renewable energy production and storage. Chem. Soc. Rev. 2012, 41, 7909–7937. [Google Scholar] [CrossRef] [PubMed]
- Bian, Z.; Tachikawa, T.; Zhang, P.; Fujitsuka, M.; Majima, T. Au/TiO2 Superstructure-based plasmonic photocatalysts exhibiting efficient charge separation and unprecedented activity. J. Am. Chem. Soc. 2014, 136, 458–465. [Google Scholar] [CrossRef]
- Tada, H. Overall water splitting and hydrogen peroxide synthesis by gold nanoparticle-based plasmonic photocatalysts. Nanoscale Adv. 2019, 1, 4238–4245. [Google Scholar] [CrossRef] [Green Version]
- O’Regan, B.; Grätzel, M. A low-cost, high-efficiency solar cell based on dye-sensitized colloidal titanium dioxide films. Nature 1991, 353, 737–740. [Google Scholar] [CrossRef]
- Xiang, L.; Zhao, X. Wet-chemical preparation of TiO2-based composites with different morphologies and photocatalytic properties. Nanomaterials 2017, 7, 310. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, W.-Q.; Xu, Y.-F.; Liao, J.-F.; Wang, L.; Kuang, D.-B. Branched titania nanostructures for efficient energy conversion and storage: A review on design strategies, structural merits and multifunctionalities. Nano Energy 2019, 62, 791–809. [Google Scholar] [CrossRef]
- Ohno, T.; Sarukawa, K.; Matsumura, M. Photocatalytic activities of pure rutile particles isolated from TiO2 powder by dissolving the anatase component in HF solution. J. Phys. Chem. B 2001, 105, 2417. [Google Scholar] [CrossRef]
- Ohno, T.; Tokieda, K.; Higashida, S.; Matsumura, M. Synergism between rutile and anatase TiO2 particles in photocatalytic oxidation of naphthalene. Appl. Catal. A 2003, 244, 383. [Google Scholar] [CrossRef]
- Yang, S.; Gao, L. Fabrication and shape-evolution of nanostructured TiO2 via a sol-solvothermal process based on benzene-water interfaces. Mater. Chem. Phys. 2006, 99, 437–440. [Google Scholar] [CrossRef]
- Fattakhova-Rohlfing, D.; Zaleska-Medynska, A.; Bein, T. Three-dimensional titanium dioxide nanomaterials. Chem. Rev. 2014, 114, 9487–9558. [Google Scholar] [CrossRef] [PubMed]
- Akita, A.; Tada, H. Synthesis of 1D-anisotropic particles consisting of TiO2 nanorod and SnO2 with heteroepitaxial junctions and the self-assembling to 3D-microspheres. Langmuir 2019, 35, 17096–17102. [Google Scholar] [CrossRef]
- Akita, A.; Kobayashi, H.; Tada, H. Action of chloride ions as a habit modifier in the hydrothermal crystal growth of rutile TiO2 nanorod from SnO2 seed crystal. Chem. Phys. Lett. 2020, 761, 138003. [Google Scholar] [CrossRef]
- Liu, B.; Aydil, E.S. Growth of oriented single-crystalline rutile TiO2 nanorods on transparent conducting substrates for dye-sensitized solar cells. J. Am. Chem. Soc. 2009, 131, 3985–3990. [Google Scholar] [CrossRef] [PubMed]
- Kosmulski, M. pH-Dependent surface charging and points of zero charge. IV. Update and new approach. J. Colloid Interface Sci. 2009, 337, 439–448. [Google Scholar] [CrossRef]
- Israelachivili, J.N. Intermolecular and Surface Forces; Academic Press: London, UK, 1985. [Google Scholar]
- Nosaka, Y.; Nosaka, A. Nyumon Hikarisyokubai; Tokyo Tosho: Tokyo, Japan, 2004. [Google Scholar]
- Xiang, L.; Zhao, X.; Shang, C.; Yin, J. Au or Ag nanoparticle-decorated 3D urchin-like TiO2 nanostructures: Synthesis, characterization, and enhanced photocatalytic activity. J. Colloid Interface Sci. 2013, 403, 22–28. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Zhang, Y.; Wang, S.; Xu, J.; Ding, H.; Li, G. Structure-enhanced photocatalytic removal of CrVI by a TiO2 superstructure with ultrathin rutile nanorods and abundant {110} faces. Eur. J. Inorg. Chem. 2013, 2601–2607. [Google Scholar] [CrossRef]
- The Electrochemical Society of Japan. Denki Kagaku Binran, 5th ed.; Maruzen: Tokyo, Japan, 2000. [Google Scholar]
- Qiao, H.; Wang, Y.; Xiao, L.; Zhang, L. High lithium electroactivity of hierarchical porous rutile TiO2 nanorod microspheres. Electrochem. Commun. 2008, 10, 1280–1283. [Google Scholar] [CrossRef]
- Ahmed, A.Y.; Kandiel, T.A.; Oekermann, T.; Bahnemann, D. Photocatalytic Activities of Different Well-defined Single Crystal TiO2 Surfaces: Anatase versus Rutile. J. Phys. Chem. Lett. 2011, 2, 2461. [Google Scholar] [CrossRef]
- Pan, J.H.; Wang, X.Z.; Huang, Q.; Shen, C.; Koh, Z.Y.; Wang, Q.; Engel, A.; Bahnemann, D.W. Large-scale synthesis of urchin-like mesoporous TiO2 hllow spheres by targeted etching and their photoelectrochemical properties. Adv. Funct. Mater. 2014, 24, 95–104. [Google Scholar] [CrossRef]
- Sun, X.; Xu, S.; Gao, Y.; Yue, M.; Yue, Q.; Gao, B. 3D hierarchical golden wattle-like TiO2 microspheres: Polar acetone-based solvothermal synthesis and enhanced water purification performance. CrystEngComm 2017, 19, 2187–2194. [Google Scholar] [CrossRef]
- Yu, X.; Zhao, Z.; Zhang, J.; Guo, W.; Li, L.; Liu, H.; Wang, Z.L. One-step synthesis of ultrathin nanobelts-assembled urchin-like anatase TiO2 nanostructures for highly efficient photocatalysis. CrystEngComm 2017, 19, 129–136. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, Y.; Matsushima, S.; Hojo, H.; Einaga, H. Photocatalytic oxidation Process for treatment of gas phase benzene using Ti3+ self-doped TiO2 microsphere with sea urchin-like structure. Chem. Eng. J. 2020, 402, 126220. [Google Scholar] [CrossRef]
- Wittcoff, H.A.; Reuben, B.G.; Plotkin, J.S. Industrial Organic Chemicals; Wiley: Hoboken, NJ, USA, 2013. [Google Scholar]
- Jira, R. Acetaldehyde from ethylene—A retrospective on the discovery of the Wacker process. Angew. Chem. Int. Ed. 2009, 48, 9034–9037. [Google Scholar] [CrossRef]
- Takei, T.; Iguchi, N.; Haruta, M. Synthesis of acetoaldehyde, acetic acid, and others by the dehydrogenation and oxidation of ethanol. Catal. Surv. Asia 2011, 15, 80–88. [Google Scholar] [CrossRef]
- Akita, A.; Sugita, S.; Naya, S.; Tada, H. A heteromesocrystal photocatalyst consisting of SnO2(head)-TiO2(tail) nanorod hybrids. Catal. Commun. 2021, 154, 106301. [Google Scholar]
- Harvey, P.R.; Rudham, R.; Ward, S. Photocatalytic oxidation of liquid alcohols and binary alcohol mixtures by rutile. J. Chem. Soc. Faraday Trans. 1 1983, 79, 2975–2981. [Google Scholar] [CrossRef]
- Coutts, J.L.; Levine, L.H.; Richards, J.T.; Mazyck, D.W. The effect of photon source on heterogeneous photocatalytic oxidation of ethanol by a silica-titania composite. J. Photochem. Photobiol. A Chem. 2011, 225, 58–64. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Nosaka, Y. Photocatalytic oxidation mechanism of methanol and the other reactants in irradiated TiO2 aqueous suspension investigated by OH radical detection. Appl. Catal. B Environ. 2015, 166-167, 32–36. [Google Scholar] [CrossRef]
- Tada, H.; Kiyonaga, T.; Naya, S. Rational design and applications of highly efficient reaction systems photocatalyzed by noble metal nanoparticle-loaded titanium(iv) sioxide. Chem. Soc. Rev. 2009, 38, 1849–1858. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Chen, J.; Feng, Z.; Chen, T.; Lian, Y.; Wang, X.; Li, C. Photoluminescence characteristics of TiO2 and their relationship to the photoassisted reaction of water/methanol mixture. J. Phys. Chem. C 2007, 111, 693–699. [Google Scholar] [CrossRef]
- Nakamura, R.; Ohashi, N.; Imanishi, A.; Osawa, T.; Matsumoto, Y.; Koinuma, H.; Nakato, Y. Crystal-face dependences of surface band edges and hole reactivity, revealed by preparation of essentially atomically smooth and stable (110) and (100) n-TiO2 (rutile) surfaces. J. Phys. Chem. B 2005, 109, 1648–1651. [Google Scholar] [CrossRef] [PubMed]
- Awa, K.; Akashi, R.; Akita, A.; Naya, S.; Kobayashi, H.; Tada, H. Highly efficient and selective oxidation of ethanol to acetaldehyde by a hybrid photocatalyst consisting of SnO2 nanorod and rutile TiO2 with heteroepitaxial junction. ChemPhysChem 2019, 20, 2155–2161. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, X.; Liu, Y. Promotion of multi-electron transfer for enhanced photocatalysis: A review focused on oxygen reduction reaction. Appl. Surf. Sci. 2015, 358, 28–45. [Google Scholar] [CrossRef]
- Hou, H.; Zeng, X.; Zhang, X. Production of hydrogen peroxide by photocatalytic processes. Angew. Chem. Int. Ed. 2020, 59, 17356–17376. [Google Scholar] [CrossRef] [PubMed]
- Kawano, S.; Fujishima, M.; Tada, H. Size effect of zinc oxide-supported gold nanoparticles on the photocatalytic activity for two-electron oxygen reduction reaction. Catal. Commun. 2020, 144, 106076. [Google Scholar] [CrossRef]
- Feng, L.; Li, B.; Xiao, Y.; Li, L.; Zhang, Y.; Zhao, Q.; Zuo, G.; Meng, X.; Roy, V.A.L. Au modified Bi2O3-TiO2 hybrid for photocatalytic synthesis of hydrogen peroxide. Catal. Commun. 2021, 155, 106315. [Google Scholar] [CrossRef]
- Xu, W.; Sheng, X.; Zhou, H.; Wang, D.; Ding, Z.; Feng, X. Enhanced plasmonic photocatalytic synthesis of hydrogen peroxide at an air-liquid-solid triphasic interface. Chem. Eng. J. 2021, 410, 128342. [Google Scholar] [CrossRef]
- Adams, J.S.; Chembulkar, A.; Priyadarshini, P.; Ricciardulli, T.; Lu, Y.; Maliekkal, V.; Sampath, A.; Winikoff, S.; Karim, A.M.; Neurock, M.; et al. Solvent molecules from surface redox mediators in situ and cocatalyze O2 reduction on Pd. Science 2021, 371, 626–632. [Google Scholar] [CrossRef]
- Sui, S.; Wang, X.; Zhou, X.; Su, Y.; Riffat, S.; Liu, C.-j. A comprehensive review of Pt electrocatalysts for the oxygen reduction reaction: Nanostructure, activity, mechanism and carbon support in PEM fuel cells. J. Mater. Chem. A 2017, 5, 1808–18025. [Google Scholar] [CrossRef]
- Abe, R.; Takami, H.; Murakami, N.; Ohtani, B. Pristine simple oxides as visible light driven photocatalysts: Highly efficient decomposition of organic compounds over platinum-loaded tungsten oxide. J. Am. Chem. Soc. 2008, 130, 7780–7781. [Google Scholar] [CrossRef] [PubMed]
- Tada, H.; Naya, S. Atomic level interface control of SnO2-TiO2 nanohybrids for the photocatalytic activity enhancement. Catalysts 2021, 11, 205. [Google Scholar] [CrossRef]
- Sun, Q.; Wu, S.; Li, K.; Han, B.; Chen, Y.; Pang, B.; Yu, L.; Dong, L. The favorable synergistic operation of photocatalysis and catalytic oxygen reduction reaction by a novel heterogeneous CoFe2O4-TiO2 nanocomposite. Appl. Surf. Sci. 2020, 516, 146142. [Google Scholar] [CrossRef]
- Byl, O.; Yates, J.T., Jr. Anisotropy in the electrical conductivity of rutile TiO2 in the (110) plane. J. Phys. Chem. B 2006, 110, 22966–22967. [Google Scholar] [CrossRef]
- Cooper, G.; Turner, J.A.; Nozik, A.J. Mott-Schottky plots and flatband potentials for single crystal rutile electrodes. J. Electrochem. Soc. 1982, 129, 1973–1977. [Google Scholar] [CrossRef]
- Tada, H.; Hattori, A.; Tokihisa, Y.; Imai, K.; Tohge, N.; Ito, S. A patterned-TiO2/SnO2 bilayer type photocatalyst. J. Phys. Chem. B 2000, 104, 4585–4587. [Google Scholar] [CrossRef]
- Kim, W.; Tachikawa, T.; Moon, G.-H.; Majima, T.; Choi, W. Molecular-level understanding of the photocatalytic activity difference between anatase and rutile nanoparticles. Angew. Chem. Int. Ed 2014, 53, 14036–14041. [Google Scholar] [CrossRef] [PubMed]
- Nikawa, T.; Naya, S.; Kimura, T.; Tada, H. Rapid removal and subsequent low-temperature mineralization of gaseous acetaldehyde by the dual thermocatalysis of gold nanoparticle-loaded titanium(IV) oxide. J. Catal. 2015, 326, 9–14. [Google Scholar] [CrossRef]
- Kong, E.-H.; Chang, Y.-J.; Park, Y.-C.; Yoon, Y.-H.; Park, H.-J.; Jang, H.M. Sea urchin TiO2-nanoparticle hybrid composite photoelectrodes for CdS/CdSe/ZnS quantum-dot-sensitized solar cells. Phys. Chem. Chem. Phys. 2012, 14, 4620–4625. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, Y.; Wang, Y.; Li, M.; Li, X.; Yi, Q.; Deng, P.; Wu, H. Solvothermal synthesis and high optical performance of three-dimensional sea-urchin-like TiO2. Mater. Res. Bull. 2015, 66, 115–122. [Google Scholar] [CrossRef]
- Ri, J.H.; Wu, S.; Jin, J.; Peng, T. Growth of a sea urchin-like rutile TiO2 hierarchical microsphere frilm on Ti foil for a quai-solid-state dye-sensitized solar cell. Nanoscale 2017, 9, 18498–18506. [Google Scholar] [CrossRef] [PubMed]
- Ri, J.H.; Wu, S.; Jin, J.; Peng, T.; Kim, B.; Sonu, K.S. Preparation of Ti foil-based film containing rutile sea urchin-like microspheres covered with anatase nanotubes self-organized layer and its application in dye-sensitized solar cells. Electrochim. Acta 2017, 247, 754–763. [Google Scholar] [CrossRef]
- Chen, J.S.; Tan, Y.L.; Li, C.M.; Cheah, Y.L.; Luan, D.; Madhavi, S.; Boey, F.Y.C.; Archer, L.A.; Lou, X.W. Constructing hierarchical spheres from large ultrathin anataseTiO2 nanosheets with nearly 100% exposed (001) facets for fast reversible lithium storage. J. Am. Chem. Soc. 2010, 132, 6124–6130. [Google Scholar] [CrossRef] [Green Version]
- Ambade, R.B.; Koh, K.H.; Ambade, S.B.; Eom, W.; Noh, S.H.; Koo, C.M.; Kim, S.H.; Han, T.H. Kinetically controlled low-temperature solution-processed mesoporous rutile TiO2 for high performance lithium-ion batteries. J. Ind. Eng. Chem. 2019, 80, 667–676. [Google Scholar] [CrossRef]
- Nguyen-Phan, T.-D.; Luo, S.; Vovchok, D.; Llorca, J.; Sallis, S.; Kattel, S.; Xu, W.; Piper, L.F.J.; Polyansky, D.E.; Senanayake, S.D.; et al. Three-dimensional Ruthenium-Doped TiO2 Sea Urchins for Enhanced Visible-Light-Responsive H2 Production. Phys. Chem. Chem. Phys. 2016, 18, 15972–15979. [Google Scholar] [CrossRef]
- Tada, H.; Naya, S.; Fujishima, M. Nanohybrid crystals with heteroepitaxial junctions for solar-to-chemical transformations. J. Phys. Chem. C 2020, 124, 25657–25666. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akita, A.; Tada, H. Radial TiO2 Nanorod-Based Mesocrystals: Synthesis, Characterization, and Applications. Catalysts 2021, 11, 1298. https://doi.org/10.3390/catal11111298
Akita A, Tada H. Radial TiO2 Nanorod-Based Mesocrystals: Synthesis, Characterization, and Applications. Catalysts. 2021; 11(11):1298. https://doi.org/10.3390/catal11111298
Chicago/Turabian StyleAkita, Atsunobu, and Hiroaki Tada. 2021. "Radial TiO2 Nanorod-Based Mesocrystals: Synthesis, Characterization, and Applications" Catalysts 11, no. 11: 1298. https://doi.org/10.3390/catal11111298
APA StyleAkita, A., & Tada, H. (2021). Radial TiO2 Nanorod-Based Mesocrystals: Synthesis, Characterization, and Applications. Catalysts, 11(11), 1298. https://doi.org/10.3390/catal11111298







