Abstract
Photocatalysis is proven to be the most efficient and environmentally friendly method for the degradation of organic pollutants in water purification. To meet the requirement of large-scale water treatment, there are two important points: One is the lifetime and chemical stability of the photocatalyst material, especially in the complex and harsh aqueous conditions. The other is the ease of synthesis of such photocatalysts with specific nano-morphology. In this work, two common photocatalyst materials, zinc oxide (ZnO) and titanium dioxide (TiO2), are selected to form more sustainable photocatalysts with high chemical stability. This involves the combination of both TiO2 and ZnO in a two-step simple synthesis method. It appears advantageous to exploit the conformal deposition of atomic layer deposition (ALD) to achieve nanometer-thick TiO2 coating on ZnO nanowires (NWs) with a high aspect ratio, which are firmly anchored to a substrate and exhibit a large specific surface area. The high chemical stability of the ALD TiO2 coating has been investigated in detail and proven to be effective under both strong acid and strong alkaline aqueous solutions. In addition, photocatalysis experiments with organic dyes show that via this simple two-step synthesis method, the produced ZnO/TiO2 tandem photocatalysts does indeed exhibit improved chemical stability in a harsh environment, while allowing efficient photodegradation.
1. Introduction
The combined effects of the growing human population, climate change, and increasing pollution are placing significant pressures on water resources. Water scarcity is steadily rising and has now become an urgent global issue. Thus, the development of new hygienically friendly water purification technologies has become urgent in order to ensure the sustainability of humanity’s water supplies. One of the more sustainable solutions for water purification is the photocatalytic process, a non-selective organic pollutant degradation technique using solely solar energy and requiring a wide-bandgap semiconductor photocatalyst, such as ZnO [1,2] or TiO2 [3,4], whose raw materials are abundant in nature and low-cost, and both have excellent photocatalytic properties. Both of these photocatalysts have been separately applied in water treatment research studies targeting wastewater with different organic pollutants [5,6]. However, in the real situation of water treatment, there are two main problems, resulting in the limitation of its development and large-scale application. One is the presence of an effluent that contains more complex pollutants and comprises a harsh aqueous condition, such as a strong acid or alkali solution environment. At the same time, the ease of synthesis of photocatalysts with a specific nano-morphology is another limitation for high-quality and large-scale water treatment applications.
The first problem has already been observed in many ZnO photocatalyst studies, wherein corrosion can cause damage to ZnO nanowires (NWs) during the photocatalysis process, thereby shortening their lifetime and decreasing device efficiency [7]. In addition, it has been reported that ZnO NWs can be damaged in harsh environments, such as in acidic or alkaline solutions [8]. In comparison, TiO2 has a better corrosion resistance performance than ZnO, which is thus one possible solution for water treatment, especially in harsh conditions.
Another problem comes with the complicated synthesis of a specific nanostructured photocatalysts. Normally, to increase the contact opportunity between the photocatalyst and the organic pollutant molecules to enable their degradation, and thus improve the photocatalytic efficiency of the device, photocatalysts are often employed in nanostructured form, such as nanoparticles [9], nanorods [10], or nanowires [11]. Nanostructured photocatalysts can be used by direct dispersion in water, such as with a powder [12]. However, once the purification is complete, the separation of the photocatalytic particles and purified water is far from easy, and it often requires further filtration of the effluents, adding significant time and energy costs, and reducing the yield of the water treatment process. To avoid those additional steps, an alternative solution is to rely on a surface substrate supporting nanowire arrays with a high surface-to-volume ratio. This strategy has been frequently used and proven as an efficiency-enhanced photocatalyst [13]. Compared to TiO2, whose NWs are usually synthesized in free form and dispersed in a growth solution, substrate-supported ZnO NW arrays can be easily synthesized onto a surface by chemical bath deposition such as a hydrothermal method. Such an alternative appears as a more environmentally friendly growth solution [14] and leads to ZnO NWs that are firmly anchored to the substrate, hence significantly reducing the risk of the detachment of the nanostructures to the surrounding environment. This is one of the reasons why ZnO NWs have drawn the attention of the research community, not only for their photocatalytic properties but also for their superior performances in different application fields, such as piezoelectric energy harvesting [15], photovoltaic solar cells [16], and chemical sensors [17,18].
Therefore, the targeted technical solution for effective water purification needs to simultaneously meet the following two conditions: (i) robust chemical resistance in the photocatalysts is needed to increase its lifetime; (ii) simple synthesis of the photocatalysts, which can be achieved on a solid substrate while maintaining a high surface-to-volume ratio, so as to achieve an effective chemical reaction rate. To meet all these requirements, a thin protective TiO2 coating applied by atomic layer deposition onto the simple hydrothermally synthesized ZnO NWs could be straightforward, since atomic layer deposition (ALD) can produce conformal nanometer-thick coatings on all kinds of surfaces [19]. Several studies have reported that such an ALD TiO2 coating can be successfully applied to ZnO nanoparticles [20,21] and ZnO nanocrystallite [22] for different applications, such as dye-sensitized solar cells [23] and solar-powered hydrogen gas harvesters [24]. For this reason, we expect a TiO2/ZnO tandem photocatalyst to have robust chemical resistance under harsh conditions, while maintaining its high photocatalytic performance.
In this work, a TiO2/ZnO tandem photocatalyst is used for its high aspect ratio, together with its considerable chemical stability under strong-pH aqueous conditions, which is crucial in water purification applications. The structure and morphology of the original ZnO NW samples with different TiO2 coating thicknesses are compared before and after exposure to a solution with pH limit values, using high-resolution scanning electron microscopy (HR-SEM). The chemical compositions of the different TiO2-ZnO tandems and their uniformity are assessed using energy-dispersive X-ray (EDX) analysis integrated into the HR-SEM system. Finally, the photodegradation of organic dye under UV light illumination using TiO2-coated ZnO NWs is investigated for different TiO2 coating thicknesses, with a focus on efficiency and chemical stability.
2. Results and Discussion
2.1. Characterization Results
After synthesis, EDX analysis was used to characterize ALD TiO2-coated ZnO NW samples with different coating thicknesses, including 0 nm (bare ZnO NWs), 2 nm, 5 nm, and 10 nm. By focusing on a given position of the sample, the morphology and nanostructure as well as the composition of the sample can be obtained simultaneously at different positions. As shown in Figure 1, the EDX analysis with HR-SEM was carried out at three different positions on the ZnO NWs: bottom (●), middle (⯀), and top (▲). The observation points were 30 nm diameter spots on the sample’s surface. By comparing the chemical composition at these three different heights, the conformity of the TiO2 coating on ZnO NWs can be checked.
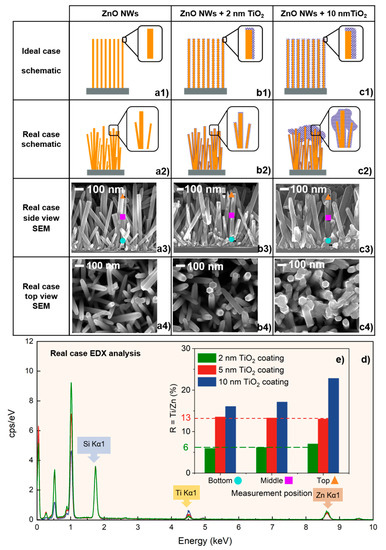
Figure 1.
Characterization of TiO2-coated ZnO NWs. (a1,b1,c1) Schematic descriptions of the ZnO NWs with TiO2 coating in ideal cases with different thickness values and (a2,b2,c2) more realistic schematic descriptions. (a3,b3,c3) Cross-section SEM images showing the morphologies as well as the 3 different measurement positions from bottom to top. (a4,b4,c4) Top-view SEM images. (d) EDX spectra of the top position recorded on the 2 nm, 5 nm, and 10 nm TiO2-coated ZnO NW samples. (e) The histogram of the Ti/Zn atomic ratio for different positions, bottom, middle, and top, on each of the 3 samples.
The schematic descriptions of the ZnO NWs with TiO2 coating in ideal cases with different thickness values are shown in Figure 1a1,b1,c1. For each thickness of the coating, since the purpose of the ALD coating on ZnO NW is to obtain a uniform film, we expect that all samples cover with the same thickness from top to bottom. However, the SEM characterization showed slightly different results than expected. From the SEM images shown in Figure 1a3,b3,c3, one can clearly see that the average diameter of bare ZnO NW is about 40 ± 10 nm, and the width of ZnO NWs gradually increases as the TiO2 coating thickness increases from 2 to 10 nm. A comparison with bare ZnO NWs shows that the 2 nm ALD TiO2 coating maintains the NWs’ high surface-to-volume ratio and good conformity. However, as the TiO2 coating thickness increases, it starts accumulating at the top of the ZnO NWs. The schematic descriptions of more realistic cases are shown in Figure 1a2,b2,c2. In order to compare the conformity and quality of different ALD TiO2 coatings, a coating coefficient R is defined in Equation (1), which is the ratio between the titanium and zinc atomic percentages for a given sample at a given position, where the titanium and zinc are provided by ALD TiO2 coating and ZnO NWs, respectively. For example, when one measures the bare ZnO NWs, the value of R should be 0.
Then, the coating coefficient R is calculated at three different heights for all samples. The coating coefficient R of 2 nm and 5 nm TiO2-coated ZnO NWs samples remains essentially unchanged from the bottom to the top position, at around 6.5 and 13, respectively. This shows that the ALD TiO2 coatings cover the entire ZnO NW uniformly from bottom to top, as illustrated schematically in Figure 1a2,b2. The cross-section view SEM image of 5 nm TiO2-coated ZnO NWs shows their very similar behavior to 2 nm TiO2-coated ZnO NWs, which also indicates uniform coating (Figure S1, Supporting Information). When the coating thickness reaches 10 nm, the coating coefficient R from the bottom to the top is 16, 17, and 22, respectively. The R value at the top position is much larger than the R values at the middle and bottom, which confirms the morphological difference observed by HR-SEM of the sample shown in Figure 1c4. In addition, the EDX spectra of the samples with 2 nm, 5 nm and 10 nm TiO2 coatings are also presented in Figure 1d. To avoid the confusion caused by other elements attached to the sample during the measurement process, the spectra were normalized and only compared with the K-line for quantifying Zn and Ti, instead of the L-line. Zn Kα and Ti Kα peaks were present at about 8.63 keV and 4.508 keV, respectively. At these two positions, one can clearly see that the quantity of the Ti increases as the coating thickness increases, while the quantity of the Zn remains the same. By comparing the R value shown in Figure 1e, we see that as the TiO2 coating thickness increases, the ALD TiO2 coating starts to accumulate on the top of the ZnO NWs, which also reduces the uniformity of the coating in the middle and bottom positions.
2.2. Stability in Harsh Chemical Conditions
After characterization, an experiment was conducted to evaluate the stability of the TiO2/ZnO tandem photocatalysts under harsh aqueous conditions with different pH values. Sulfuric acid and sodium hydroxide were diluted in distilled (DI) water to prepare solutions from pH 3 to pH 11. Each sample was submitted to the pH solution bath for 30 min at room temperature and then rinsed with DI water three times to remove the residual pH solution. Severe damage was observed on a bare ZnO nanostructure at pH values lower than 4 and higher than 10. Thus, for the following study, pH 3 and pH 11 are chosen as harsh chemical conditions to compare the chemical corrosion resistance of bare ZnO NWs and TiO2-coated ZnO NWs.
After exposure to the pH solution, SEM examination of each sample was carried out to compare the morphologies of samples with different TiO2 coating thicknesses. The effect of the pH aqueous solution on TiO2-coated ZnO NWs also has been tested from pH 3 to pH 11 (See details in Figure S2, Supporting Information). As shown in Figure 2a2, significant damage to the nanostructure of bare ZnO NWs is observed after exposure to a pH 3 solution bath. With the increase in the ALD TiO2 coating’s thickness, there is an obvious protective effect. With a 2 nm TiO2 coating, parts of the samples can survive the acid solution. For samples with 5 nm and 10 nm TiO2 coating, this protective effect of the TiO2 coating is steadily improved, and almost all samples can withstand a pH 3 solution bath. In the alkaline solution test, the sample was less affected and damaged than in the acidic solution, and even the exposed non-coated ZnO NWs maintained their pristine nanostructure after treatment in a solution at pH 11.
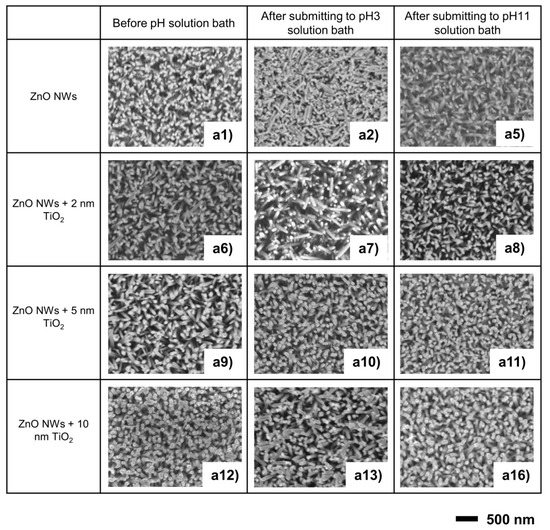
Figure 2.
SEM images of the effect of TiO2 coating thickness on ZnO NWs in strong acid/alkaline aqueous solutions.
In Figure 3a–c, three SEM images of the same sample under different magnifications are shown along with a schematic to illustrate the protective effect of the ALD TiO2 coating on ZnO NWs. The whole sequence illustrates what happens in a specific region of the sample that is exposed to mechanical damage (Figure 3d). In those specific damaged regions only, the NWs are broken, and consequently, there is no more protective TiO2 coating on the topmost part of the broken NWs. This lack of protective TiO2 coating caused the ZnO NWs to react with the acid solution, eventually resulting in the complete dissolution of the ZnO NWs’ core. Only the ALD TiO2 shells were left, which appear translucent on the left side of Figure 3c. In contrast, in the absence of mechanical damage (right side of Figure 3c and other regions of the sample, except those with stripes), all the ZnO NWs protected by a fully conformal coating remained intact, and appear opaque in the SEM image.
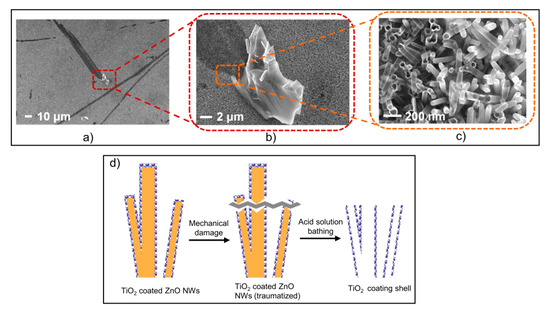
Figure 3.
(a–c) Zoomed SEM views of 10 nm ALD TiO2 coating on ZnO NWs after bathing in pH 3 aqueous solution for 30 min. (d) A schematic of the protective effect of the TiO2 coating.
2.3. Photodegradation of Organic Dye for Water Purification
UV-Vis spectroscopy was used to evaluate the photodegradation efficiency of the organic dye MB (Certistain®, SigmaAldrich, Burlington, MA, USA), which is one of the most common pollutants in industrial wastewater [25], and the process of the photodegradation experiment was basically the same as in our previous research [26,27]. The evolution of the MB concentration has been assessed by absorption spectroscopy at 665 nm, corresponding to the strongest absorption peak. The photodegradation efficiency at each sampling time is expressed by Equation (2):
where A0 and At are the intensities of the absorption peaks of the original solution and the treated solution (in arbitrary units) after UV exposure, and the photocatalysis process was suspended during the sampling. The recorded spectra are given in Figure S3 of the Supporting Information.
As shown in Figure 4a, the best photodegradation efficiency of MB was obtained with samples of bare ZnO NWs. Almost 100% degradation was achieved using such bare ZnO NWs in less than 120 min, while the samples with TiO2 ALD coatings showed slower degradation kinetics and required longer reaction times to achieve the 100% degradation of MB. To evaluate the kinetic degradation loss, a characteristic time constant τ has been defined, as the time needed to achieve 63.2% degradation efficiency (η = 1 − 1/e). As the thickness of the TiO2 coating on the ZnO NWs increased from 0 (bare ZnO NWs) to 10 nm, the characteristic time constant τ also increased, to values of 43, 83, 106, and 91 min, respectively. This indicates that the ALD TiO2 coating on ZnO NWs is very useful for ensuring chemical robustness; it also has the side effect of weakening and limiting the degradation process.
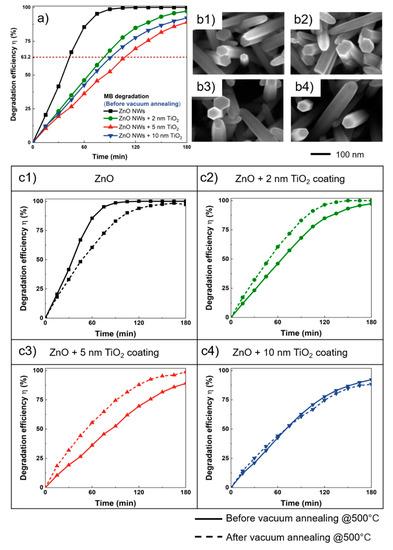
Figure 4.
(a) Comparison of the photodegradation efficiency of MB among the different TiO2 coating thicknesses from 0 to 10 nm. (b) The SEM photos of the ZnO NWs before (b1) and after vacuum-annealing (b2), 2 nm TiO2-coated ZnO NWs before (b3) and after vacuum-annealing (b4). (c1–c4) Comparison of photodegradation efficiency of different samples before and after vacuum-annealing.
In fact, the catalytic activity of ZnO catalysts is slightly higher than that of TiO2. Even though the bandgaps of titanium and zinc oxide are very similar, the mobility of the charge carriers of ZnO is higher (by a factor of 100) than that of TiO2 [28,29]. At the same time, the decreasing of the effective surface-to-volume ratio could also be another reason behind the reduction in TiO2-coated ZnO photodegradation efficiency. In addition, the formation of the ZnO-TiO2 interface may prevent the separation of charge carriers, thereby increasing the electron–hole recombination rate and hence affecting the photocatalytic activity. However, as the thickness of the ALD increased, the photodegradation efficiency was shown to exhibit a slight increase. This can be explained as TiO2 is another well-known photocatalysis material that also contributes to the degradation of the MB. The band gap measurements of the samples also confirm the contribution of the 10 nm TiO2 coating to ZnO NWs (Figure S4, Supporting Information). In fact, the results show that the band gaps of the ZnO NWs, as well as the 2 nm and 5 nm TiO2-coated ZnO NWs, remained almost unchanged, at around 3.18 eV, whereas the band gaps of the 10 nm TiO2-coated ZnO NWs were around 3.21 eV. After 180 min of reaction, the degradation rate of MB reached 100%, 97.24%, 89.02%, and 92.20% for 0 nm, 2 nm, 5 nm, and 10 nm, respectively.
Since the ALD TiO2-coated ZnO NWs have some weakening effect on the degradation efficiency, we explored another strategy to further increase the photodegradation efficiency of the TiO2-coated ZnO NWs. It has been already proven that in the photocatalysis process, oxygen vacancies can be used as important adsorption and active sites, thereby affecting the efficiency of photodegradation [30]. Depending on the different methods, the oxygen vacancies formed at different positions have different effects on electric charge separation [31,32]. Considering that the photocatalysis degradation is almost entirely a surface chemical reaction process, annealing at 500 °C under vacuum for 1 h was applied to the samples in order to increase the surface oxygen vacancy. From Figure 4b1,b2, compared with before vacuum-annealing, increases in the tiny pore structure of the exposed ZnO nanowires can be observed, and these are probably formed by the oxygen vacancy under the vacuum conditions. However, when vacuum-annealing was applied to the 2 nm TiO2-coated ZnO NWs samples, as shown in Figure 4b3,b4, there is no obvious increase in the tiny pore structure of the samples. In Figure 4c, one can see the photodegradation efficiency of the samples with different TiO2 coating thicknesses before and after annealing. The ZnO NWs samples coated with 2 nm and 5 nm TiO2 exhibit better photodegradation efficiencies after annealing, of 100% and 98.83%. In order to evaluate the formation of defects in the sample, the photoluminescence spectrum was recorded before and after vacuum-annealing (Figure S5, Supporting Information), which shows a good agreement with the increase in oxygen vacancy defects benefiting from the vacuum-annealing process. The introduction of surface oxygen vacancies can not only form a defect state under the conduction band of the photocatalyst to reduce the bandgap and increase the absorption of visible light, but it can also serve as a trapping center for photogenerated electrons in order to inhibit the recombination of photogenerated electron–hole pairs, thereby improving the photocatalytic [33]. For the bare ZnO NWs sample, the dynamic degradation efficiency was slightly lower than previously. This difference might be due to the different processes of oxygen vacancy formation. When the concentration of oxygen vacancies is greater than a given threshold, the oxygen vacancies behave as charge recombination centers, and reduce the mobility of free charges and photocatalytic activity [34].
3. Experimental Section
3.1. Sample Fabrication and pH Solution Preparation
All the photocatalyst samples were synthesized in a simple 2-step method. Firstly, the ZnO NWs samples were prepared via a hydrothermal synthesis method slightly adapted from our previous works [14].
Then, a uniform and conformal coating of TiO2 can be deposited on the ZnO samples via a precise chemical vapor deposition process at 300 °C. A first titanium tetrachloride (TiCl4) precursor was injected into the reactor for 0.2 s. This was intended to be adsorbed by the sample’s surface until saturation. Then, the reactor was purged with nitrogen (N2) gas long enough to remove any trace of titanium tetrachloride. A purge duration of 6 s was used. Next, water was injected into the reactor for 0.2 s to start the self-limiting reaction with the titanium tetrachloride. Finally, the reactor was purged again with nitrogen for 6 s to finish the first deposition cycle. This cycle was repeated to reach the targeted TiO2 thickness. The sample information is listed in Table 1.

Table 1.
Sample information.
To test the protective effect of the ALD TiO2 coating in both acid and alkaline aqueous environments, a series of aqueous solutions with different pH values was prepared to simulate harsh aqueous conditions. Sulfuric acid and sodium hydroxide were diluted into the DI water to prepare solutions with different pH values from pH 3 to pH 11. Each sample was submitted to the pH solution bath for 30 min at room temperature and then rinsed with DI water three times to remove the residual pH solution.
3.2. Characterization and Photodegradation Experiment
For characterization, EDX analysis (HR-SEM) was used to characterize ALD TiO2-coated ZnO NW samples using a Zeiss Merlin Field Emission Scanning Electron Microscope operating at 5 kV (Oberkochen, Germany).
For the photodegradation experiment, MB (methylene blue) was dissolved in deionized water at a concentration of 10 µmol L−1. The dye solution was then placed beneath a UV light (Hamamatsu LC8, Janpan, 4500 mW/cm2, λ = 365 nm) with a vertical incidence, and the distance between the surface of the solution and the light source was 10 cm; therefore, the received irradiation on the solution surface was about 35 mW/cm2, measured by a UV light meter (Lutron, Taiwan, UV-340A). Photodegradation was carried out in dynamic mode, i.e., under slight magnetic stirring to enhance the mass transport in the solution [27]. The photodegradation efficiency was recorded via the decrease in the absorbance of the solution over time, which was measured via UV-Vis spectroscopy (Lambda 35, Perkin Elmer, Waltham, MA, USA).
4. Conclusions
In summary, an investigation is reported on a simple two-step synthesis method that can provide high-aspect ratio TiO2/ZnO tandem photocatalysts, with the aim of enhancing their lifetime and chemical stability under harsh aqueous solution pH conditions, with applications mainly in water purification. It is demonstrated that the ALD TiO2 coating offers a sustainable protective layer thanks to its good conformity on the ZnO NWs’ surface, even with a thin TiO2 coating. With the help of EDX analysis via HR-SEM measurements, this study also revealed that as the coating thickness increases, the coating starts to accumulate on the top surface of the ZnO NWs, which limits the conformal coating on the middle and bottom positions, leading to an optimal value of around 5 nm for the TiO2 coating. There is no doubt that even for pH 3 solutions, the ALD TiO2 coating has a significant protective effect on ZnO NWs; in particular, when there are no mechanical defects in the coating, the protected ZnO NWs remain intact. The photodegradation efficiency of different thicknesses of TiO2-coated ZnO NWs has also been tested, whereby the TiO2-coated ZnO NWs were shown to undergo a slight reduction when the TiO2 coating thickness increased, compared to bare ZnO NWs. We finally proved that this degradation efficiency reduction can be minimized by simple vacuumannealing. Although more research is needed to reveal the photocatalytic mechanism of TiO2-coated ZnO NWs, our findings will provide a good reference for researchers interested in using ZnO NWs for practical applications.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/catal11111289/s1. Figure S1: The cross-section view SEM of 5 nm TiO2 coated ZnO NWs, Figure S2: Effect of pH aqueous solution on bare ZnO NWs and 10 nm TiO2-coated ZnO NWs, Figure S3: UV-Vis spectra recorded during MB photocatalysis degradation. (a) Bare ZnO NWs, (b) 2 nm, (c) 5 nm, (d) 10 nm TiO2-coated ZnO NWs, Figure S4: Band gap measurement results. Figure S5: The photoluminescence spectrum comparison before and after vacuum annealing at 500 °C.
Author Contributions
Y.L.-W. and T.B. devised the project, the main conceptual ideas and proof outline. L.G. is the main contributor to this study as part of her Ph.D. work. L.G. conducted the experiments of ZnO NWs growth and photodegradation. F.M. contributed to atomic layer deposition layer fabrication. S.B. and M.E. characterized the samples with energy-dispersive X-ray (EDX) analysis-integrated high-resolution scanning electron microscopy. E.N. contributed to the reviewing of the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by the I-SITE FUTURE Initiative (Reference ANR-16-IDEX-0003) in the frame of the Project NANO-4-WATER.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Qin, H.; Li, W.; Xia, Y.; He, T. Photocatalytic Activity of Heterostructures Based on ZnO and N-Doped ZnO. ACS Appl. Mater. Interfaces 2011, 3, 3152–3156. [Google Scholar] [CrossRef]
- Hariharan, C. Photocatalytic Degradation of Organic Contaminants in Water by ZnO Nanoparticles: Revisited. Appl. Catal. A Gen. 2006, 304, 55–61. [Google Scholar] [CrossRef]
- Shayegan, Z.; Lee, C.-S.; Haghighat, F. TiO2 Photocatalyst for Removal of Volatile Organic Compounds in Gas Phase–A Review. Chem. Eng. J. 2018, 334, 2408–2439. [Google Scholar] [CrossRef] [Green Version]
- Konstantinou, I.K.; Albanis, T.A. TiO2-Assisted Photocatalytic Degradation of Azo Dyes in Aqueous Solution: Kinetic and Mechanistic Investigations: A Review. Appl. Catal. B Environ. 2004, 49, 1–14. [Google Scholar] [CrossRef]
- Roy, N.; Chakraborty, S. ZnO as Photocatalyst: An Approach to Waste Water Treatment. Mater. Today Proc. 2021, 46, 6399–6403. [Google Scholar] [CrossRef]
- Fiorenza, R.; Bellardita, M.; Scirè, S.; Palmisano, L. Photocatalytic H2 Production over Inverse Opal TiO2 Catalysts. Catal. Today 2019, 321, 113–119. [Google Scholar] [CrossRef] [Green Version]
- Spathis, P.; Poulios, I. The Corrosion and Photocorrosion of Zinc and Zinc Oxide Coatings. Corros. Sci. 1995, 37, 673–680. [Google Scholar] [CrossRef]
- Zhou, J.; Xu, N.S.; Wang, Z.L. Dissolving Behavior and Stability of ZnO Wires in Biofluids: A Study on Biodegradability and Biocompatibility of ZnO Nanostructures. Adv. Mater. 2006, 18, 2432–2435. [Google Scholar] [CrossRef]
- Hong, R.Y.; Li, J.H.; Chen, L.L.; Liu, D.Q.; Li, H.Z.; Zheng, Y.; Ding, J. Synthesis, Surface Modification and Photocatalytic Property of ZnO Nanoparticles. Powder Technol. 2009, 189, 426–432. [Google Scholar] [CrossRef]
- Ren, C.; Yang, B.; Wu, M.; Xu, J.; Fu, Z.; Guo, T.; Zhao, Y.; Zhu, C. Synthesis of Ag/ZnO Nanorods Array with Enhanced Photocatalytic Performance. J. Hazard. Mater. 2010, 182, 123–129. [Google Scholar] [CrossRef]
- Kuo, T.-J.; Lin, C.-N.; Kuo, C.-L.; Huang, M.H. Growth of Ultralong ZnO Nanowires on Silicon Substrates by Vapor Transport and Their Use as Recyclable Photocatalysts. Chem. Mater. 2007, 19, 5143–5147. [Google Scholar] [CrossRef]
- Youssef, Z.; Colombeau, L.; Yesmurzayeva, N.; Baros, F.; Vanderesse, R.; Hamieh, T.; Toufaily, J.; Frochot, C.; Roques-Carmes, T.; Acherar, S. Dye-Sensitized Nanoparticles for Heterogeneous Photocatalysis: Cases Studies with TiO2, ZnO, Fullerene and Graphene for Water Purification. Dye. Pigment. 2018, 159, 49–71. [Google Scholar] [CrossRef]
- Le Pivert, M.; Zerelli, B.; Martin, N.; Capochichi-Gnambodoe, M.; Leprince-Wang, Y. Smart ZnO Decorated Optimized Engineering Materials for Water Purification under Natural Sunlight. Constr. Build. Mater. 2020, 257, 119592. [Google Scholar] [CrossRef]
- Chevalier-César, C.; Capochichi-Gnambodoe, M.; Leprince-Wang, Y. Growth Mechanism Studies of ZnO Nanowire Arrays via Hydrothermal Method. Appl. Phys. A 2014, 115, 953–960. [Google Scholar] [CrossRef]
- Leprince-Wang, Y. Piezoelectric ZnO Nanostructure for Energy Harvesting; John Wiley & Sons: London, UK, 2015; Volume 1. [Google Scholar]
- Kao, M.-C.; Chen, H.-Z.; Young, S.-L.; Lin, C.-C.; Kung, C.-Y. Structure and Photovoltaic Properties of ZnO Nanowire for Dye-Sensitized Solar Cells. Nanoscale Res. Lett. 2012, 7, 260. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fathy, A.; Le Pivert, M.; Kim, Y.J.; Ba, M.O.; Erfan, M.; Sabry, Y.M.; Khalil, D.; Leprince-Wang, Y.; Bourouina, T.; Gnambodoe-Capochichi, M. Continuous Monitoring of Air Purification: A Study on Volatile Organic Compounds in a Gas Cell. Sensors 2020, 20, 934. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Umar, A.; Rahman, M.M.; Hahn, Y.-B. Ultra-Sensitive Hydrazine Chemical Sensor Based on High-Aspect-Ratio ZnO Nanowires. Talanta 2009, 77, 1376–1380. [Google Scholar] [CrossRef] [PubMed]
- Knez, M.; Nielsch, K.; Niinistö, L. Synthesis and Surface Engineering of Complex Nanostructures by Atomic Layer Deposition. Adv. Mater. 2007, 19, 3425–3438. [Google Scholar] [CrossRef]
- Sridharan, K.; Jang, E.; Park, Y.M.; Park, T.J. Superior Photostability and Photocatalytic Activity of ZnO Nanoparticles Coated with Ultrathin TiO2 Layers through Atomic-Layer Deposition. Chem.–A Eur. J. 2015, 21, 19136–19141. [Google Scholar] [CrossRef]
- Mousa, H.M.; Alenezi, J.F.; Mohamed, I.M.; Yasin, A.S.; Hashem, A.-F.M.; Abdal-hay, A. Synthesis of TiO2@ZnO Heterojunction for Dye Photodegradation and Wastewater Treatment. J. Alloys Compd. 2021, 886, 161169. [Google Scholar] [CrossRef]
- Park, K.; Zhang, Q.; Garcia, B.B.; Zhou, X.; Jeong, Y.-H.; Cao, G. Effect of an Ultrathin TiO2 Layer Coated on Submicrometer-Sized ZnO Nanocrystallite Aggregates by Atomic Layer Deposition on the Performance of Dye-Sensitized Solar Cells. Adv. Mater. 2010, 22, 2329–2332. [Google Scholar] [CrossRef]
- Chandiran, A.K.; Abdi-Jalebi, M.; Nazeeruddin, M.K.; Grätzel, M. Analysis of Electron Transfer Properties of ZnO and TiO2 Photoanodes for Dye-Sensitized Solar Cells. ACS Nano 2014, 8, 2261–2268. [Google Scholar] [CrossRef] [PubMed]
- Kao, E.; Park, H.S.; Zang, X.; Lin, L. Atomic Layer Deposition of TiO2 Nanocoatings on ZnO Nanowires for Improved Photocatalytic Stability. Int. J. Photoenergy 2019, 2019, 8982672. [Google Scholar] [CrossRef]
- Leprince-Wang, Y.; Martin, N.; Habba, Y.G.; Le Pivert, M.; Capochichi-Gnambodoe, M. ZnO Nanostructure Based Photocatalysis for Water Purification. NanoWorld J. 2020, 6, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Houas, A.; Lachheb, H.; Ksibi, M.; Elaloui, E.; Guillard, C.; Herrmann, J.-M. Photocatalytic Degradation Pathway of Methylene Blue in Water. Appl. Catal. B Environ. 2001, 31, 145–157. [Google Scholar] [CrossRef]
- Habba, Y.G.; Capochichi-Gnambodoe, M.; Leprince-Wang, Y. Enhanced Photocatalytic Activity of Iron-Doped ZnO Nanowires for Water Purification. Appl. Sci. 2017, 7, 1185. [Google Scholar] [CrossRef] [Green Version]
- Janisch, R.; Gopal, P.; Spaldin, N.A. Transition Metal-Doped TiO2 and ZnO—Present Status of the Field. J. Phys. Condens. Matter 2005, 17, R657. [Google Scholar] [CrossRef]
- Quintana, M.; Edvinsson, T.; Hagfeldt, A.; Boschloo, G. Comparison of Dye-Sensitized ZnO and TiO2 Solar Cells: Studies of Charge Transport and Carrier Lifetime. J. Phys. Chem. C 2007, 111, 1035–1041. [Google Scholar] [CrossRef]
- Pan, X.; Yang, M.-Q.; Fu, X.; Zhang, N.; Xu, Y.-J. Defective TiO2 with Oxygen Vacancies: Synthesis, Properties and Photocatalytic Applications. Nanoscale 2013, 5, 3601–3614. [Google Scholar] [CrossRef]
- Gonullu, M.P.; Ates, H. Investigation of the Impact of Annealing on the Structural, Optical and Morphological Evolution of Mixture-Phase ALD-TiO2 Films Containing Brookite. Superlattices Microstruct. 2020, 147, 106699. [Google Scholar] [CrossRef]
- Won, S.; Go, S.; Lee, W.; Jeong, K.; Jung, H.; Lee, C.; Lee, E.; Lee, J. Effects of Defects Generated in ALD TiO2 Films on Electrical Properties and Interfacial Reaction in TiO2/SiO2/Si System upon Annealing in Vacuum. Met. Mater. Int. 2008, 14, 759–765. [Google Scholar] [CrossRef]
- Huygh, S.; Bogaerts, A.; Neyts, E.C. How Oxygen Vacancies Activate CO2 Dissociation on TiO2 Anatase (001). J. Phys. Chem. C 2016, 120, 21659–21669. [Google Scholar] [CrossRef]
- Tan, H.; Zhao, Z.; Zhu, W.; Coker, E.N.; Li, B.; Zheng, M.; Yu, W.; Fan, H.; Sun, Z. Oxygen Vacancy Enhanced Photocatalytic Activity of Pervoskite SrTiO3. ACS Appl. Mater. Interfaces 2014, 6, 19184–19190. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).