Advances in Nonprecious Metal Homogeneously Catalyzed Formic Acid Dehydrogenation
Abstract
:1. Introduction
2. Transition Metal-Based Catalysts
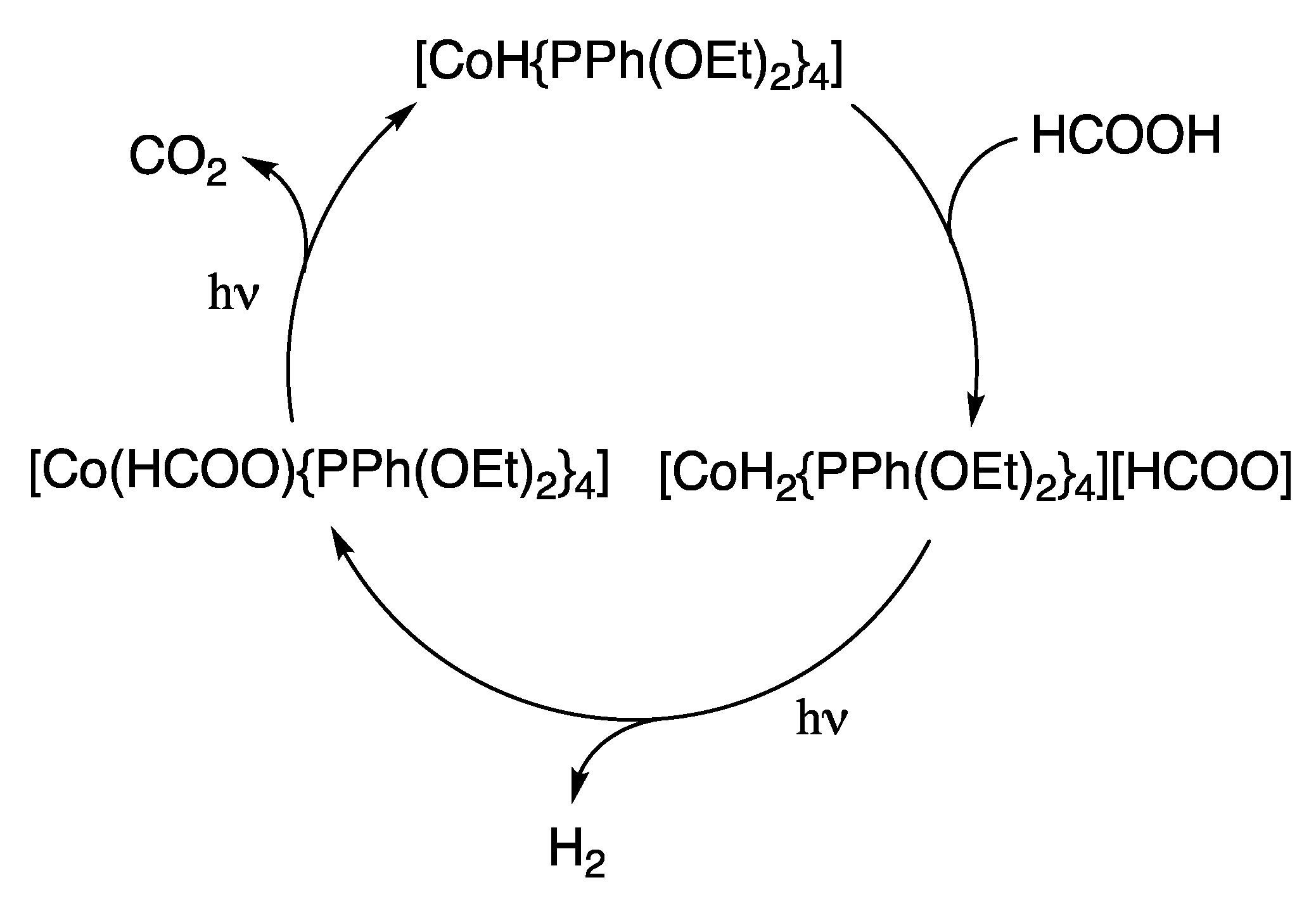
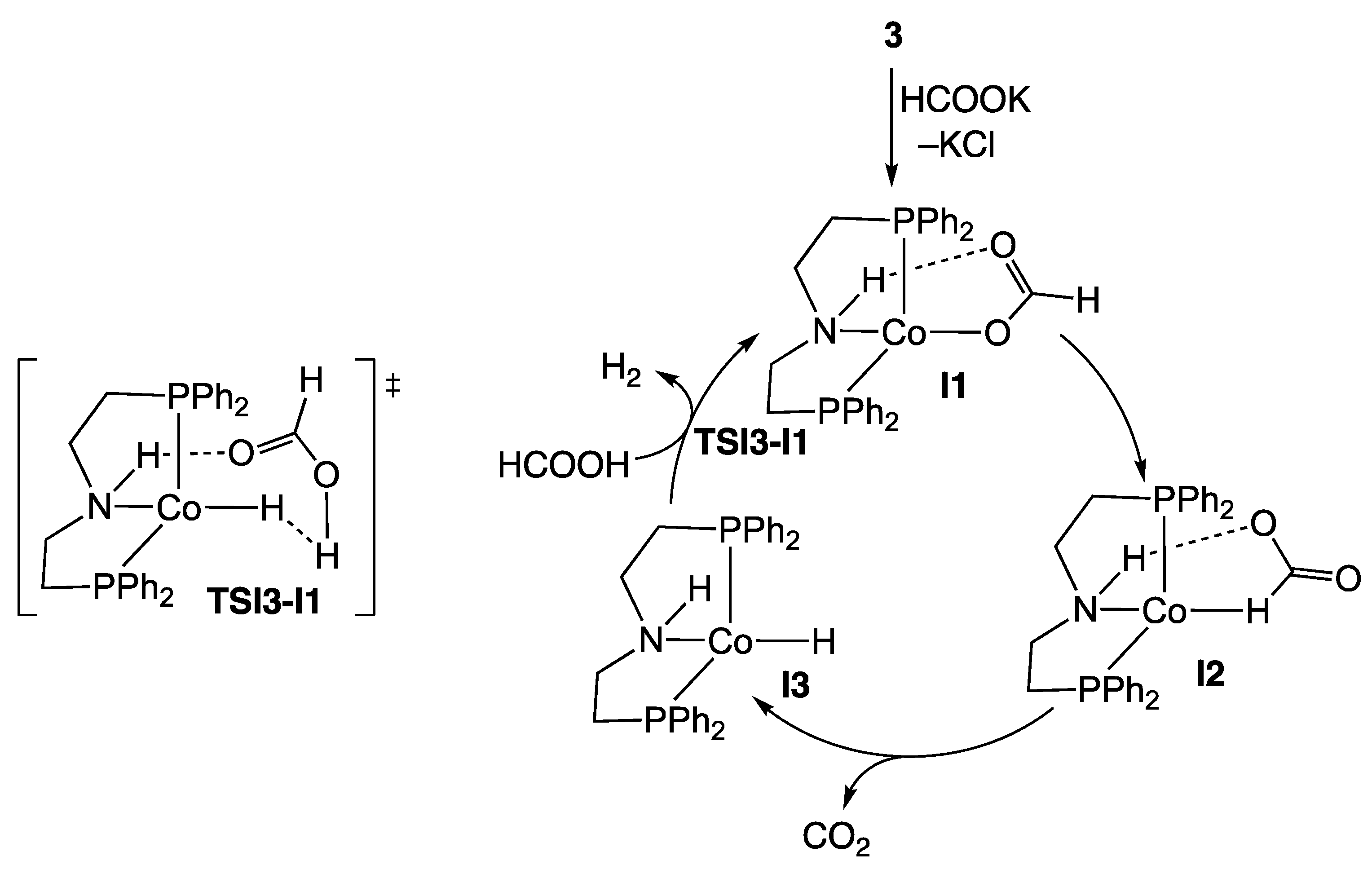
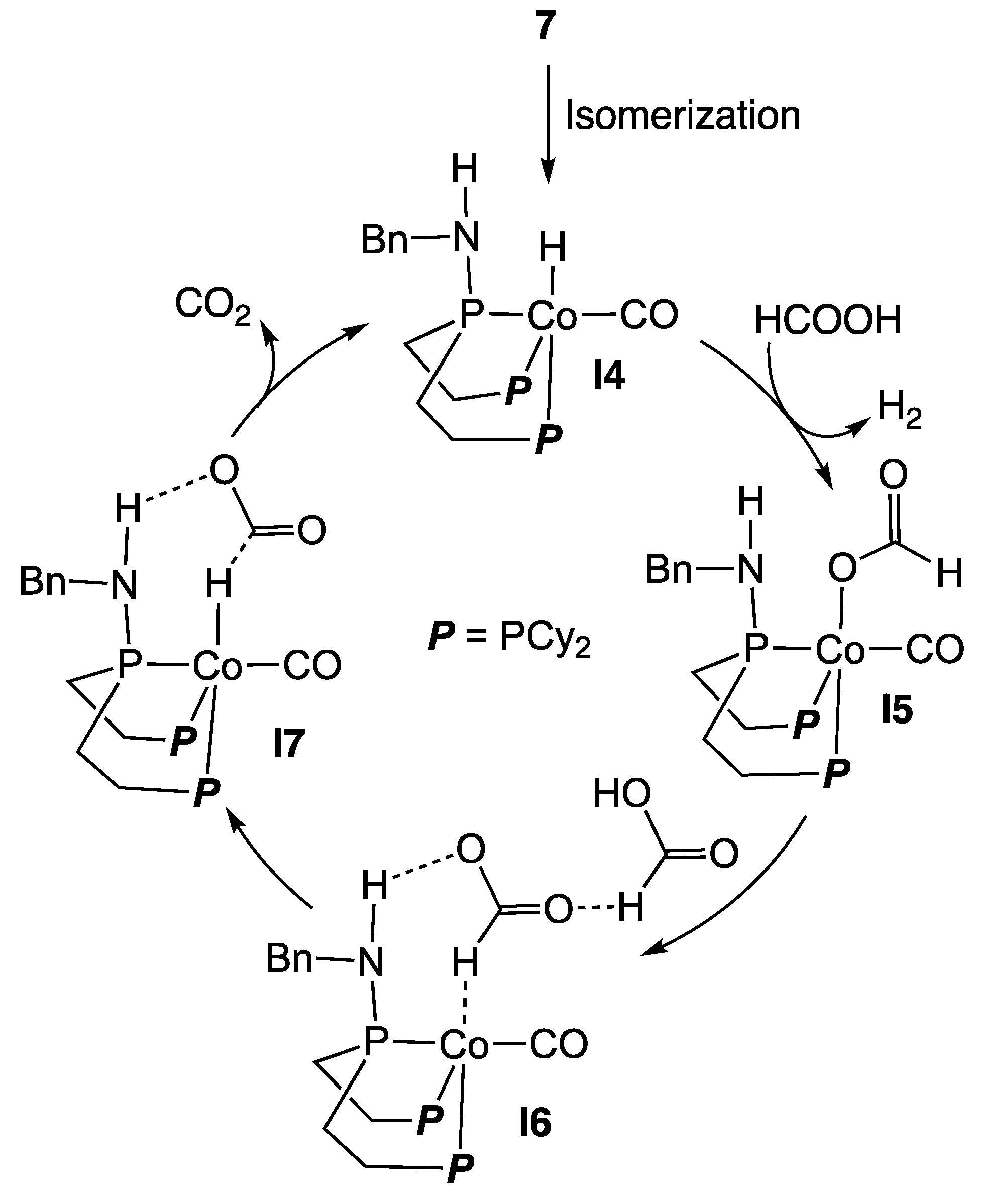
2.1. Cobalt Homogeneous Catalysts
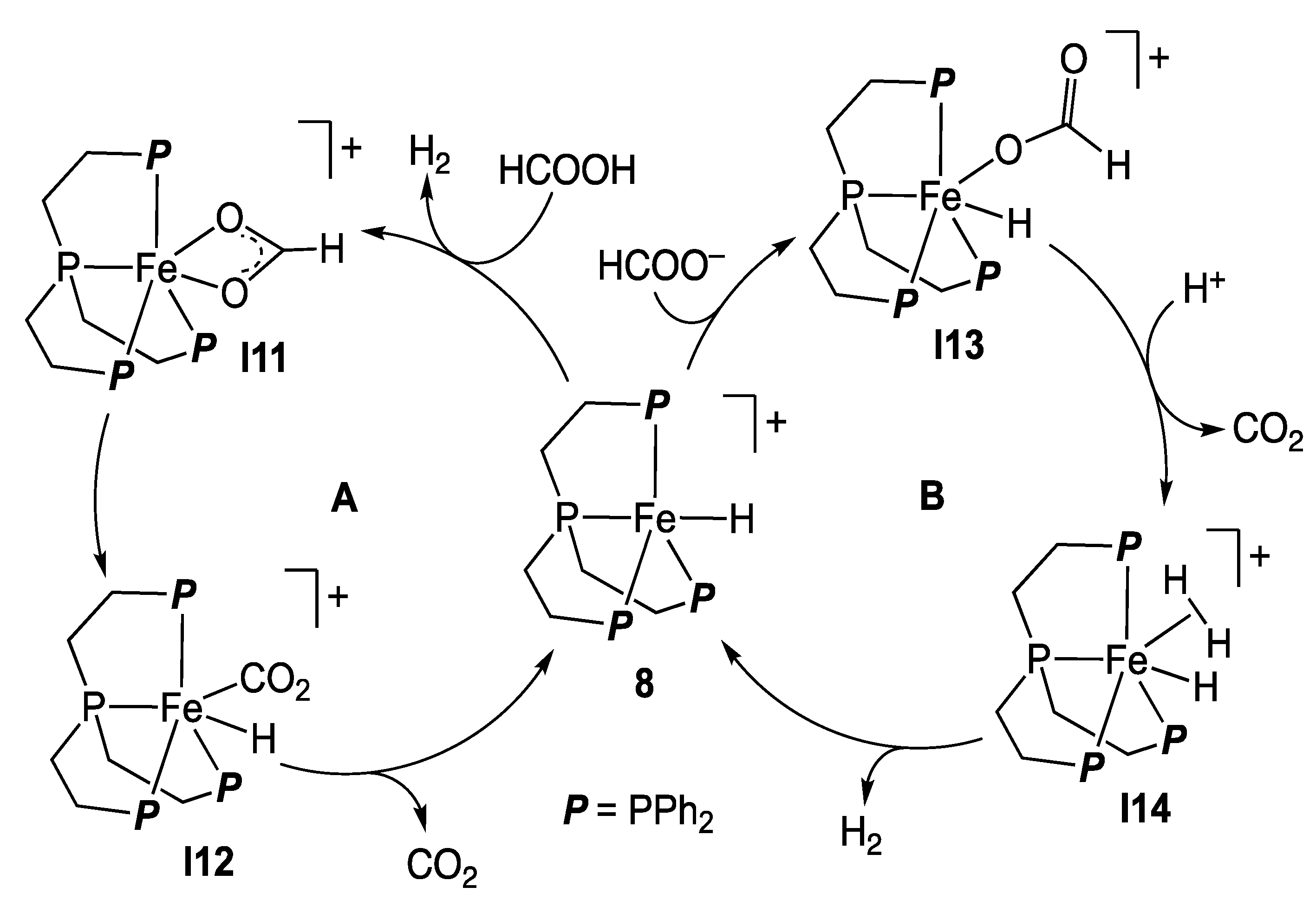
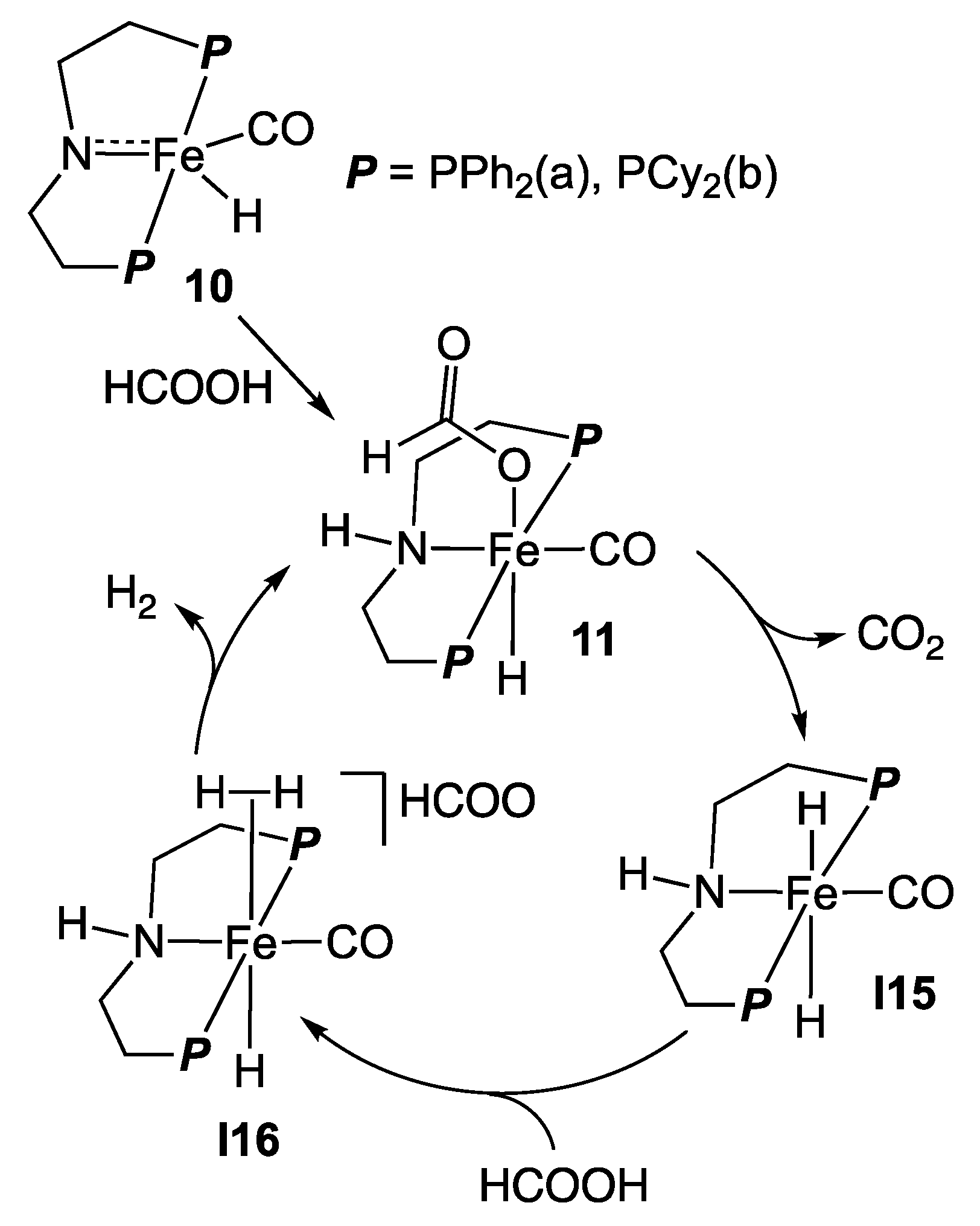
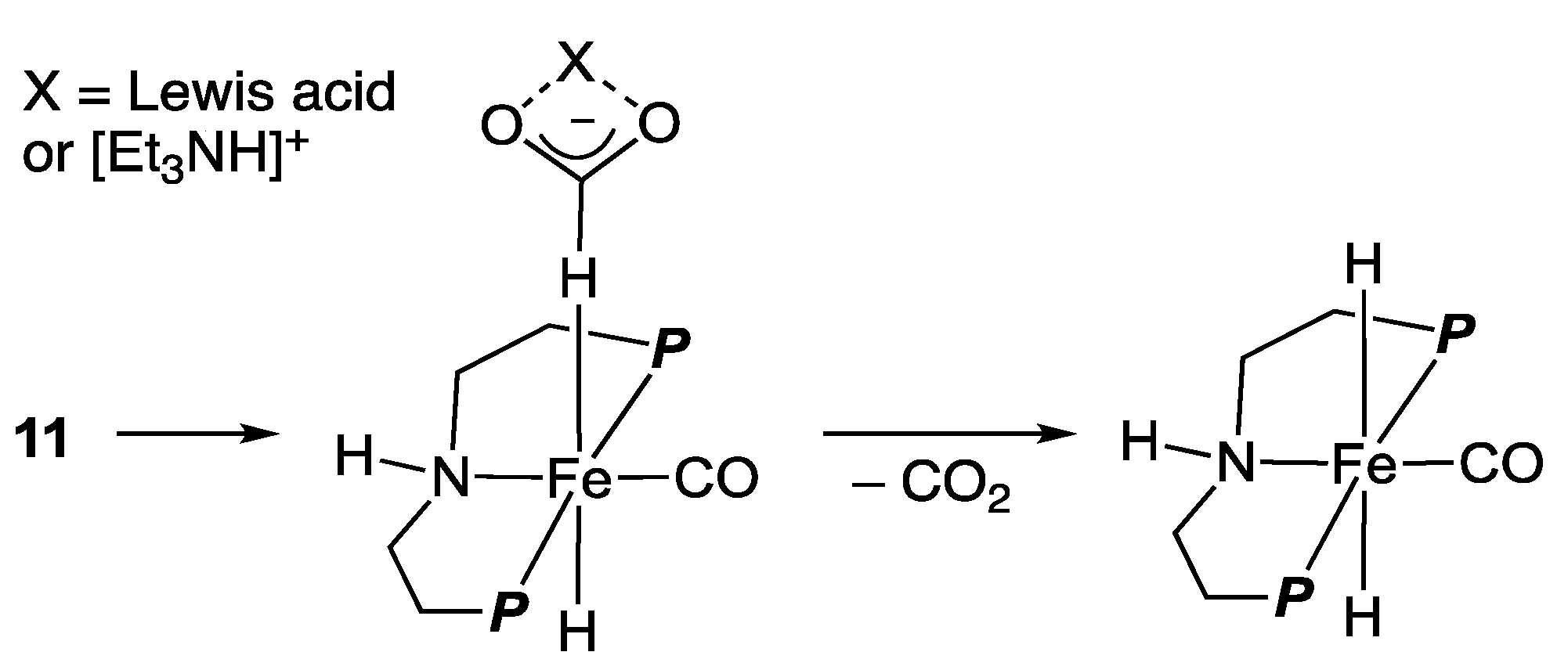
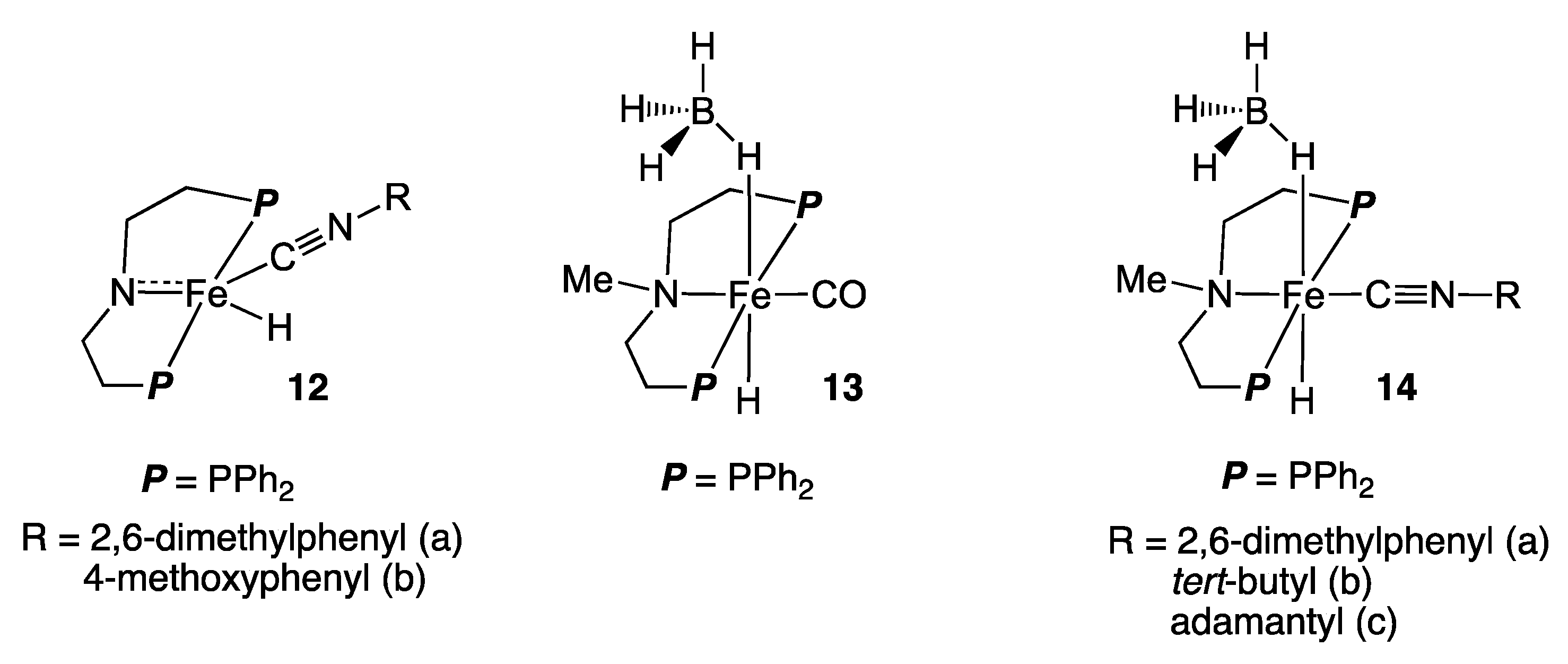
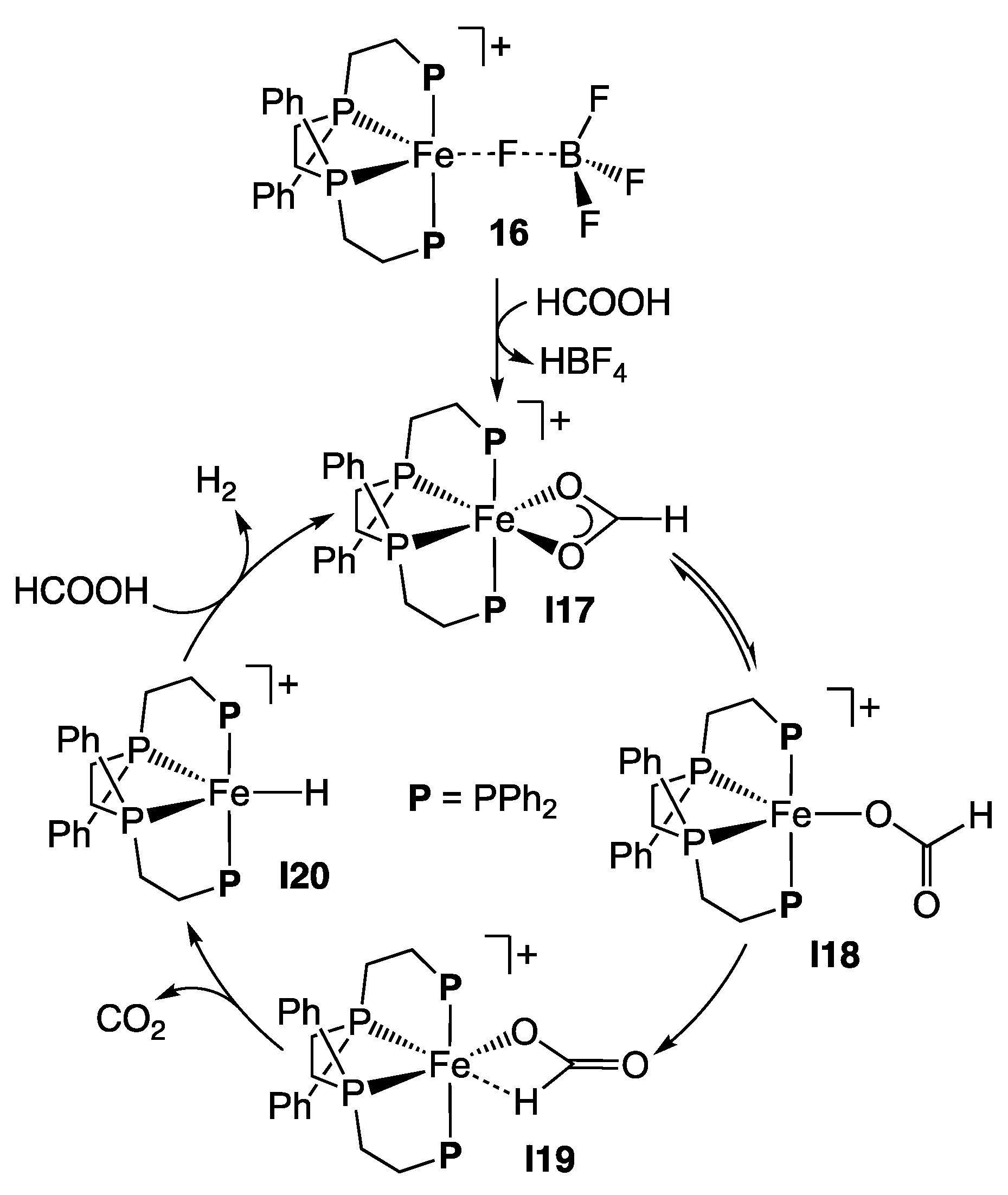
2.2. Iron Homogeneous Catalysts
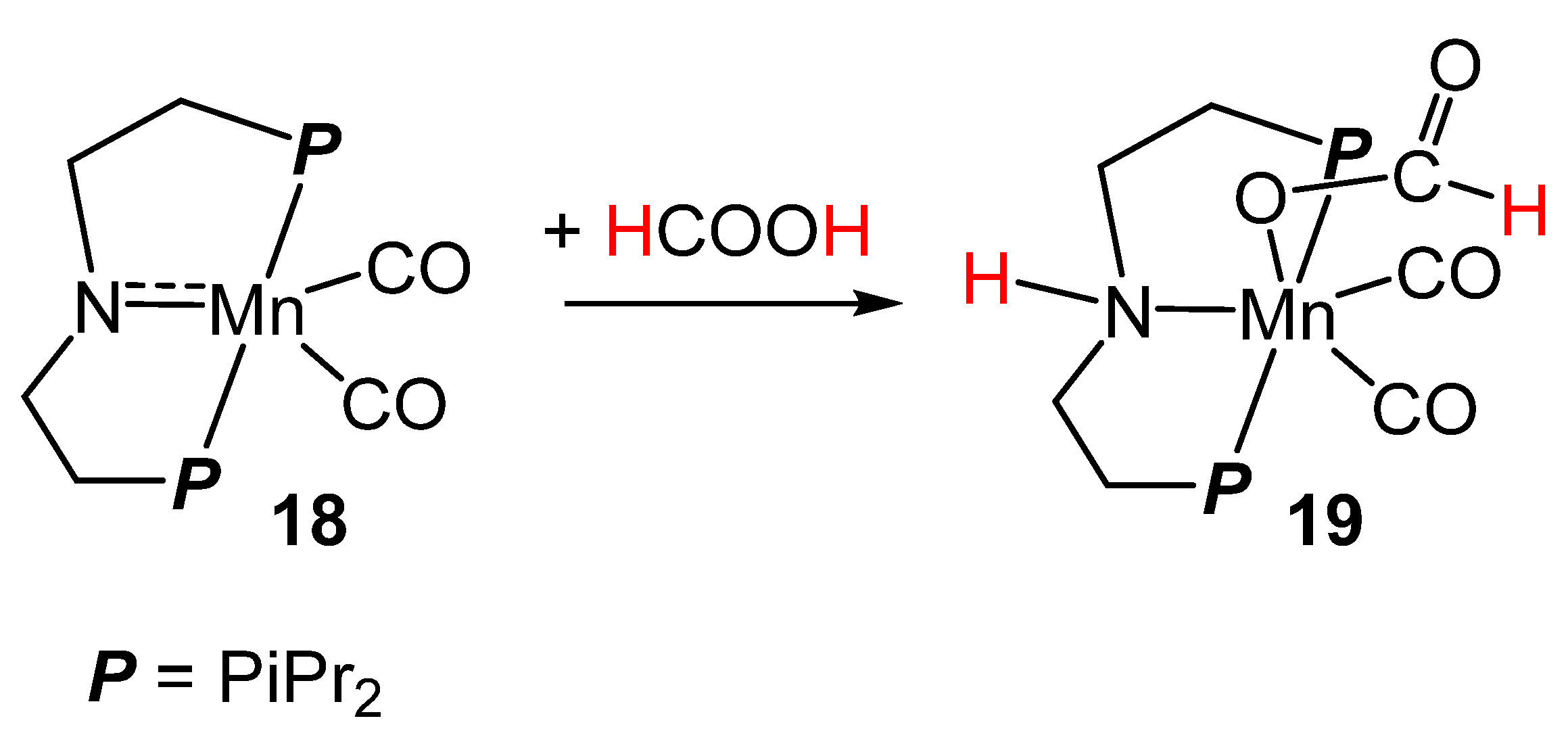
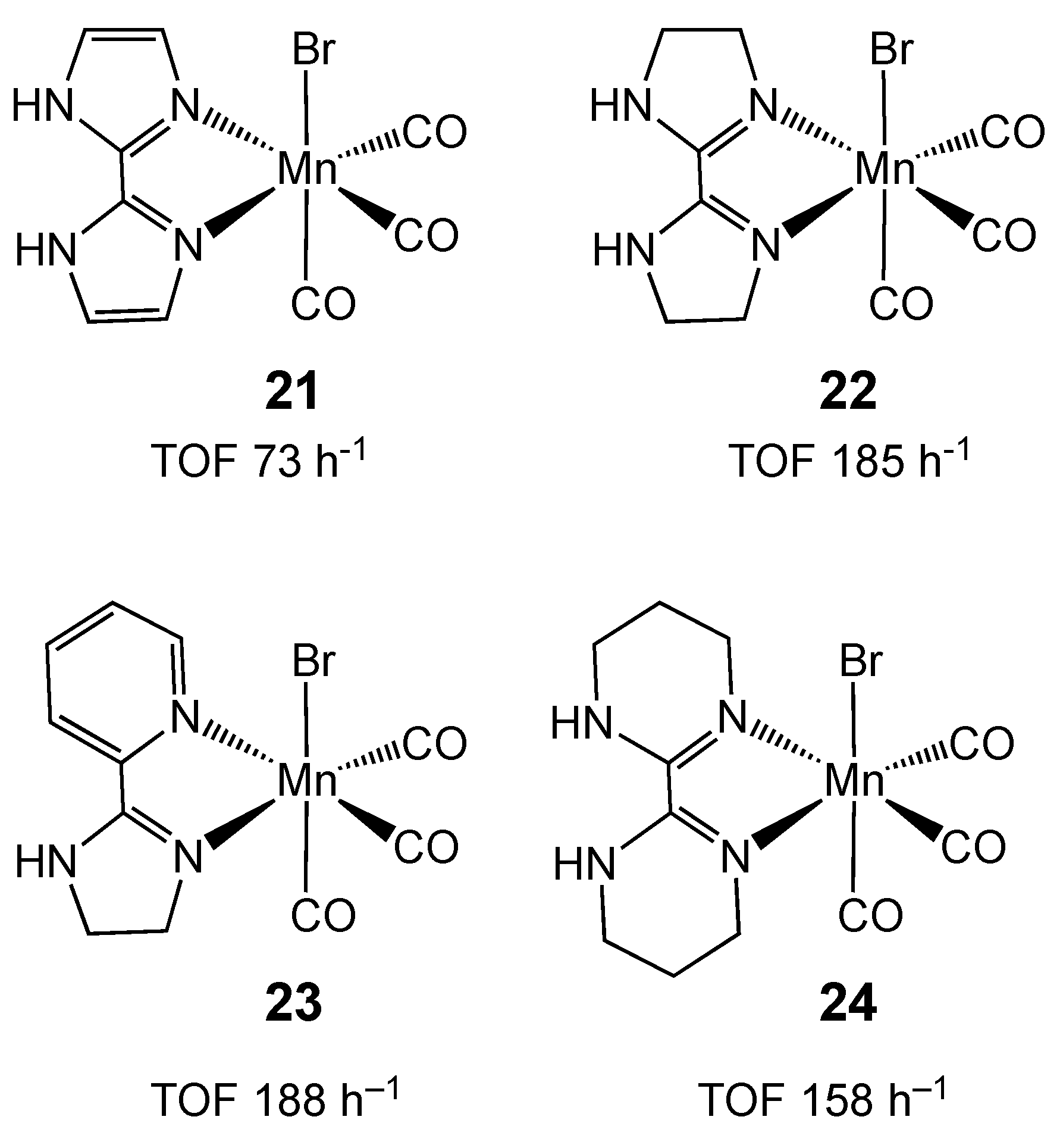
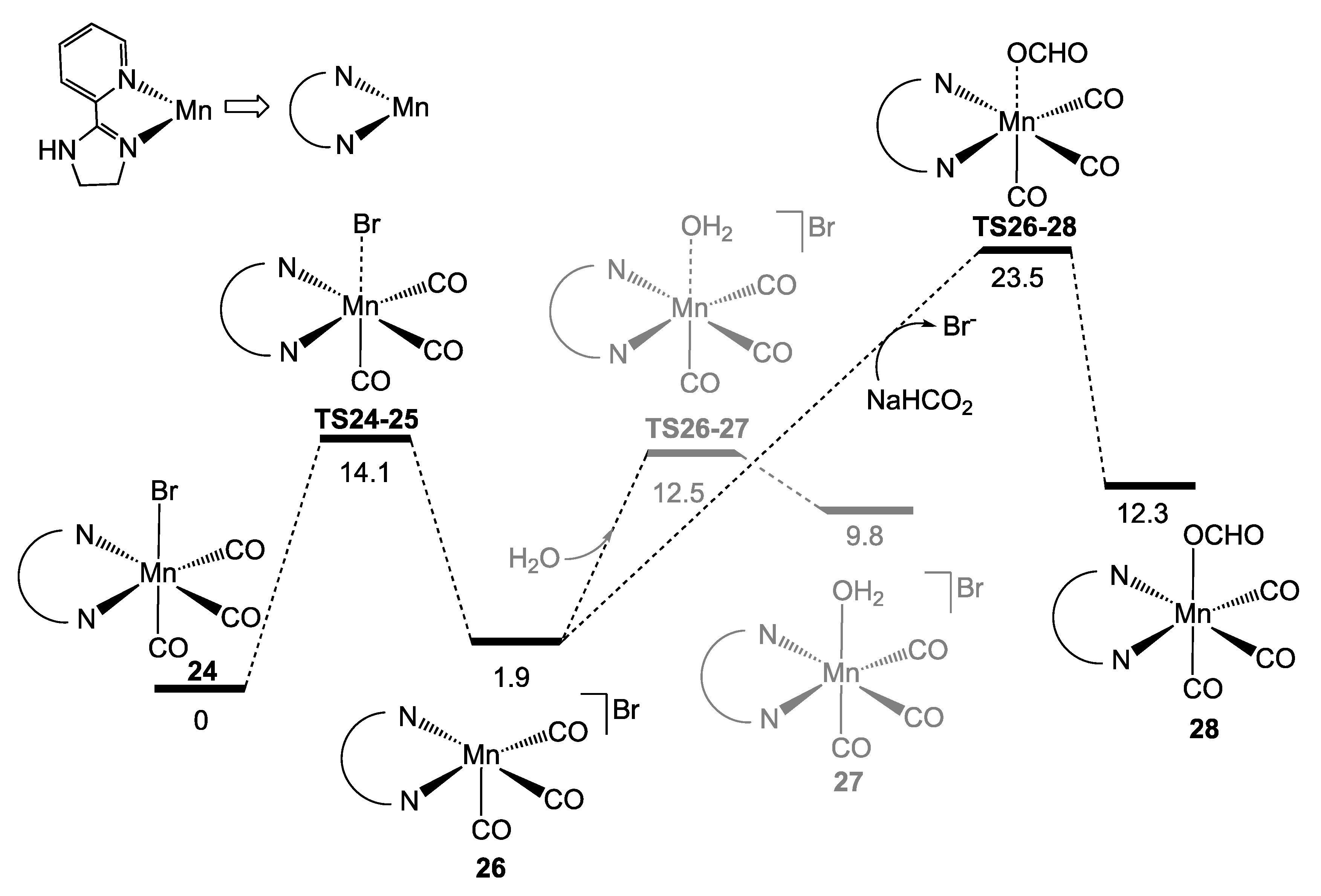
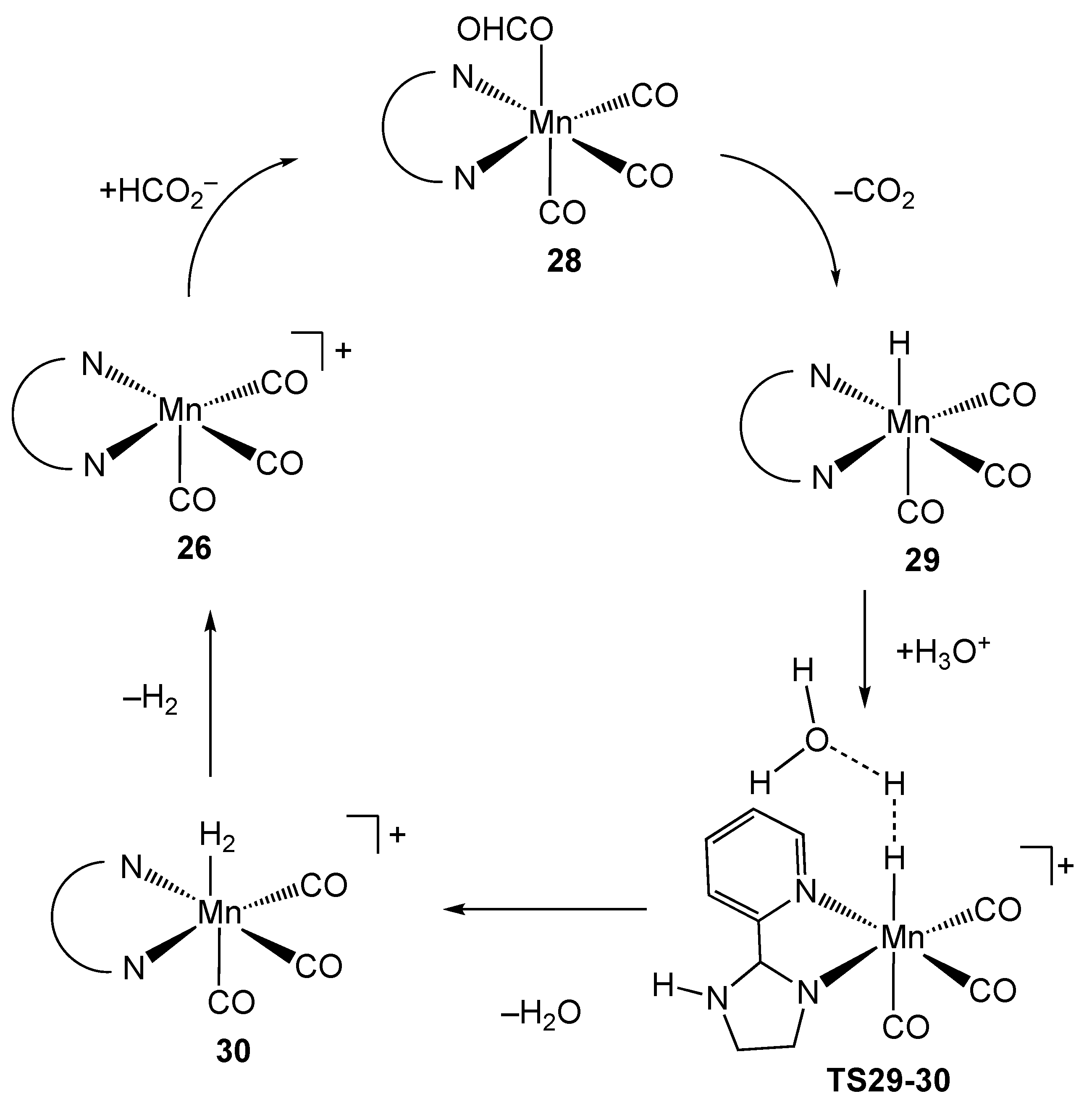
2.3. Manganese Homogeneous Catalysts
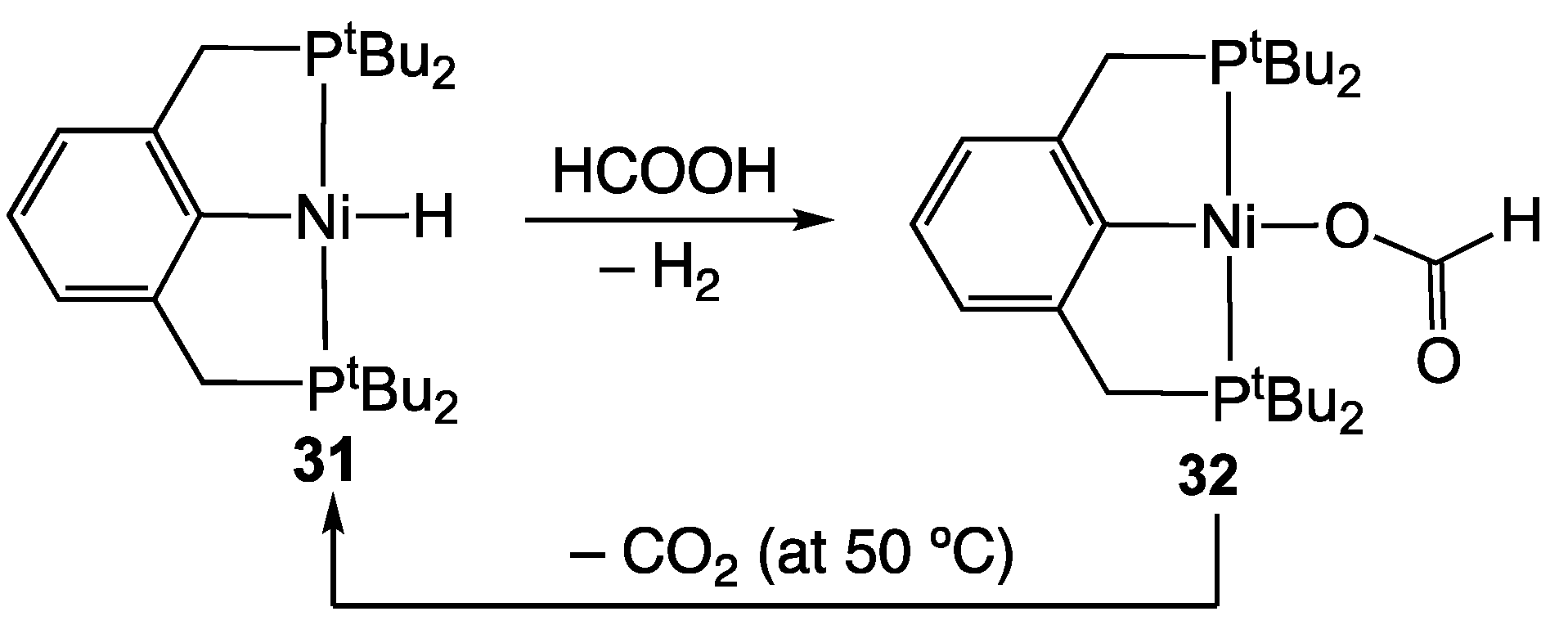
2.4. Nickel Homogeneous Catalysts
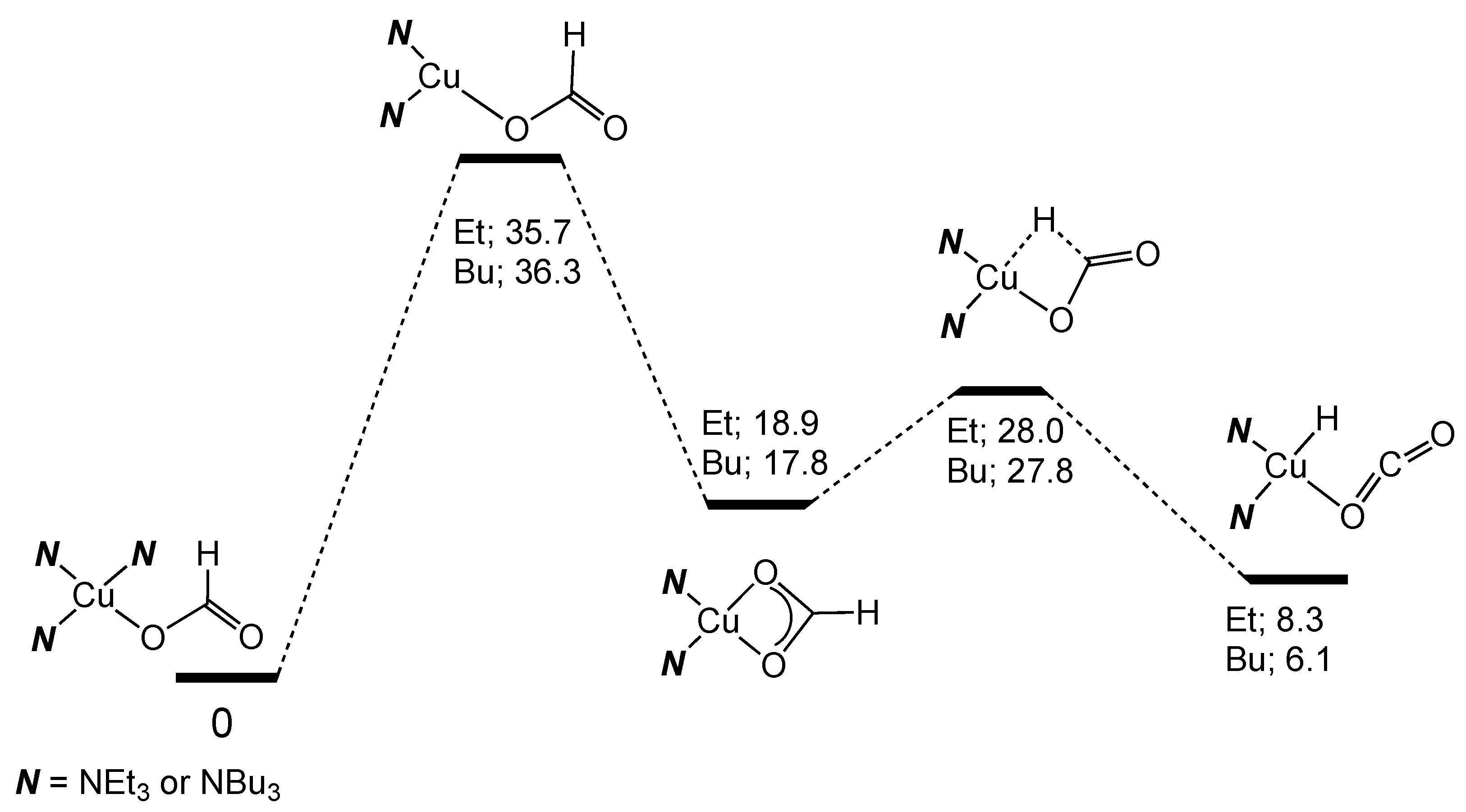
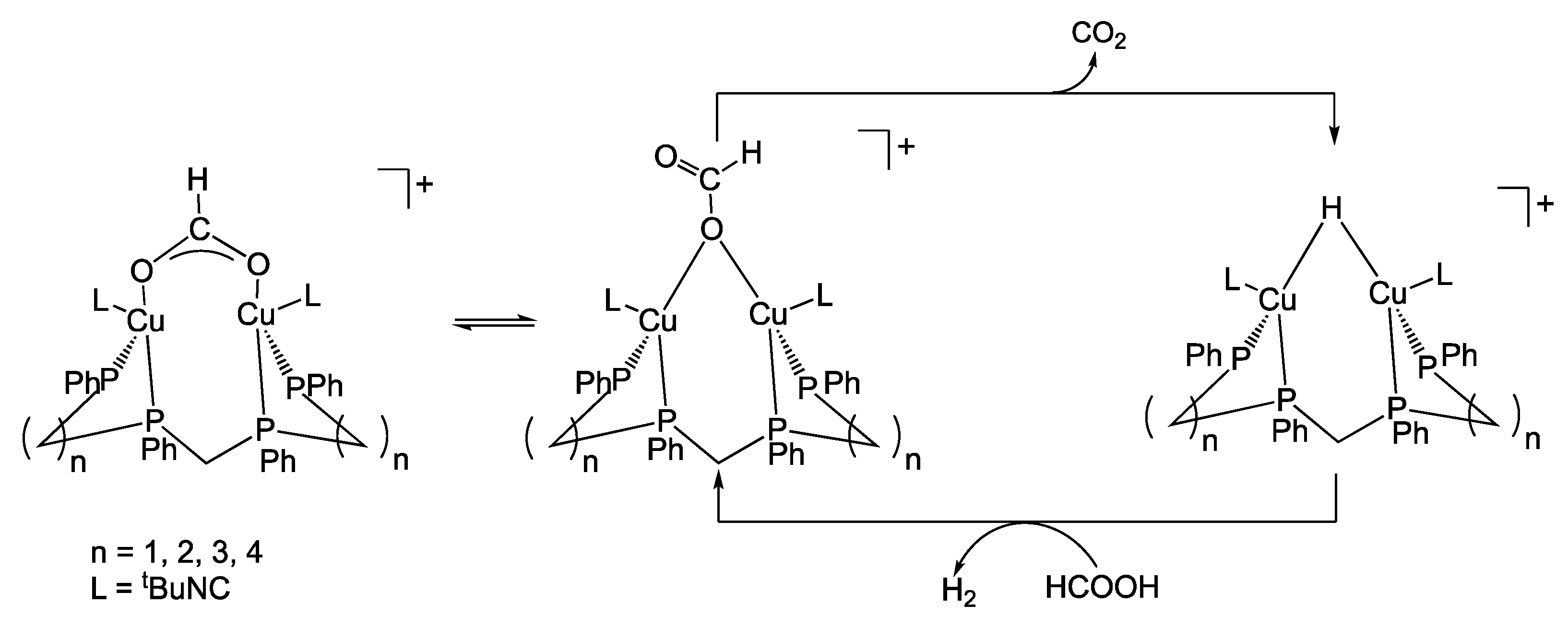
2.5. Copper Homogeneous Catalysts
3. Miscellaneous
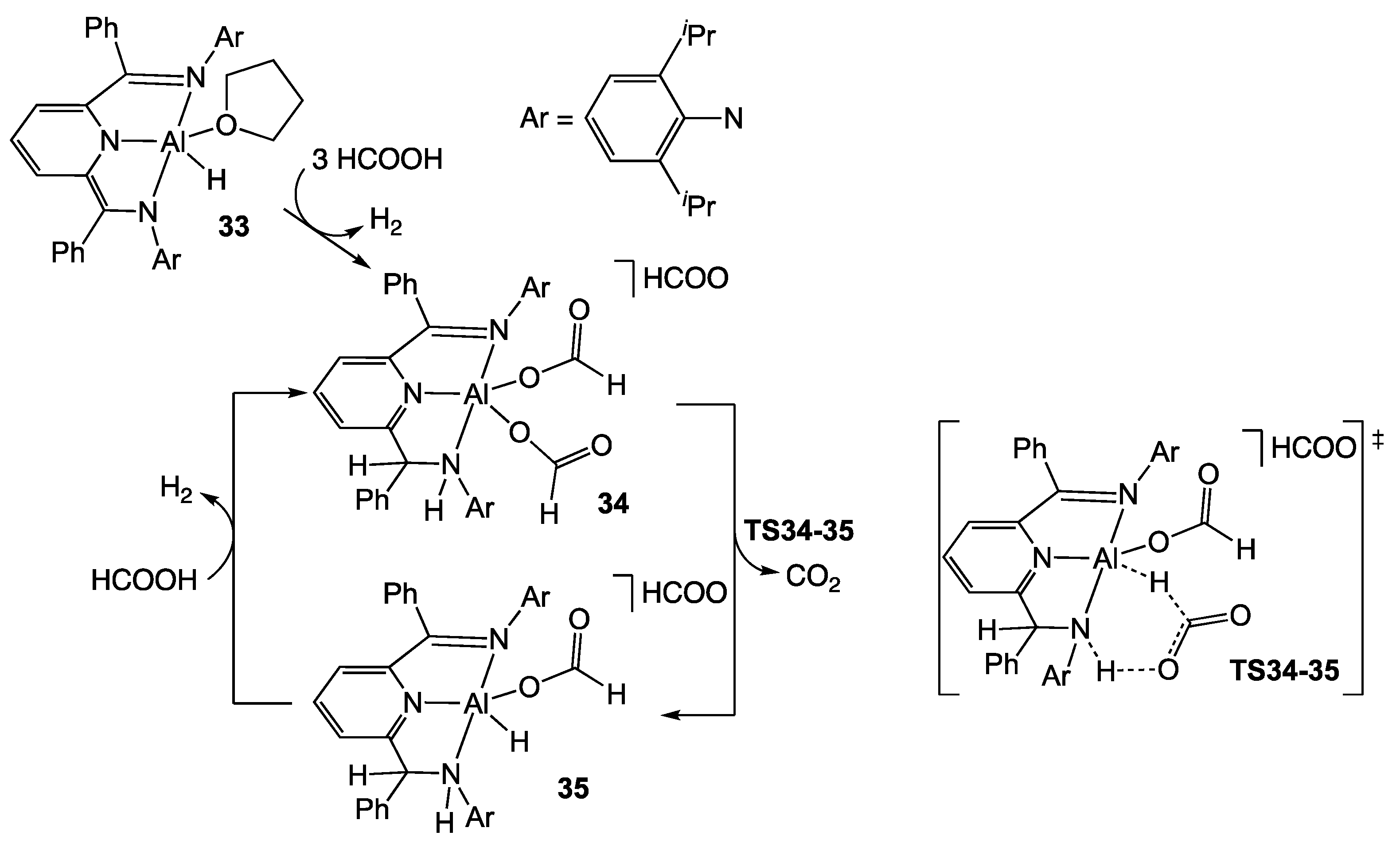
3.1. Aluminum and Boron Homogeneous Catalysts
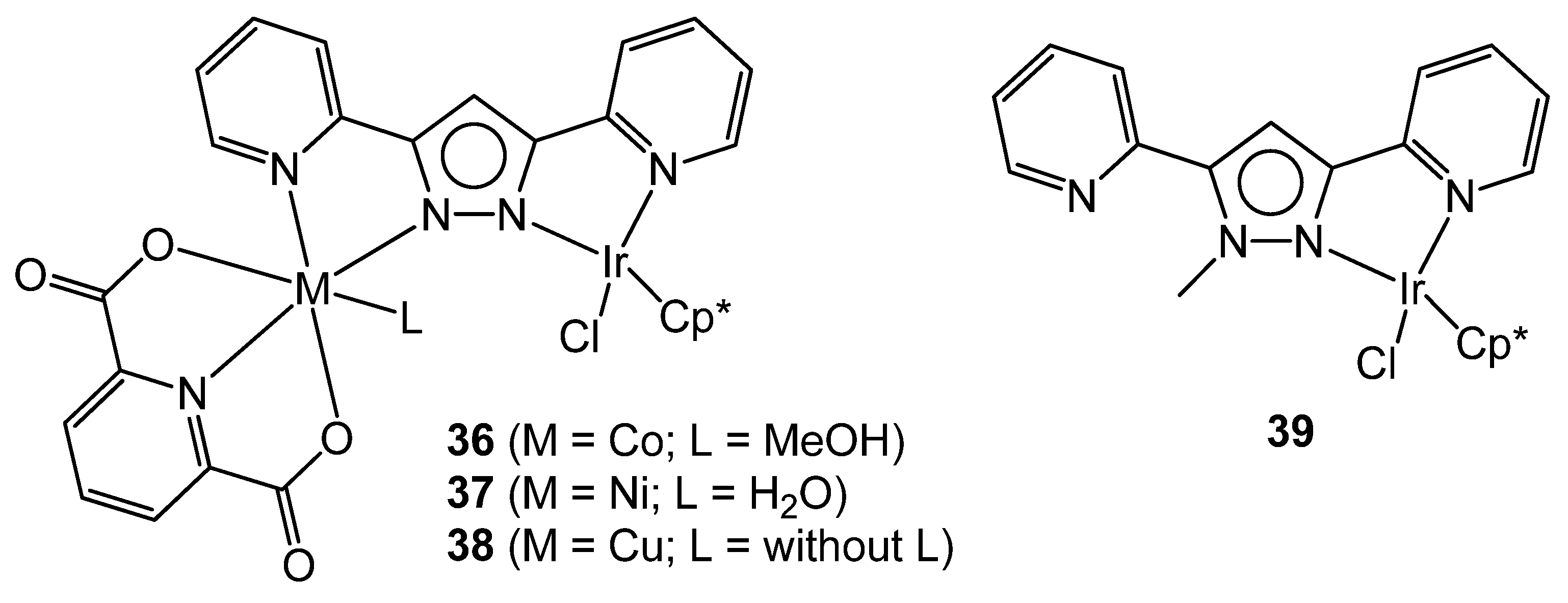
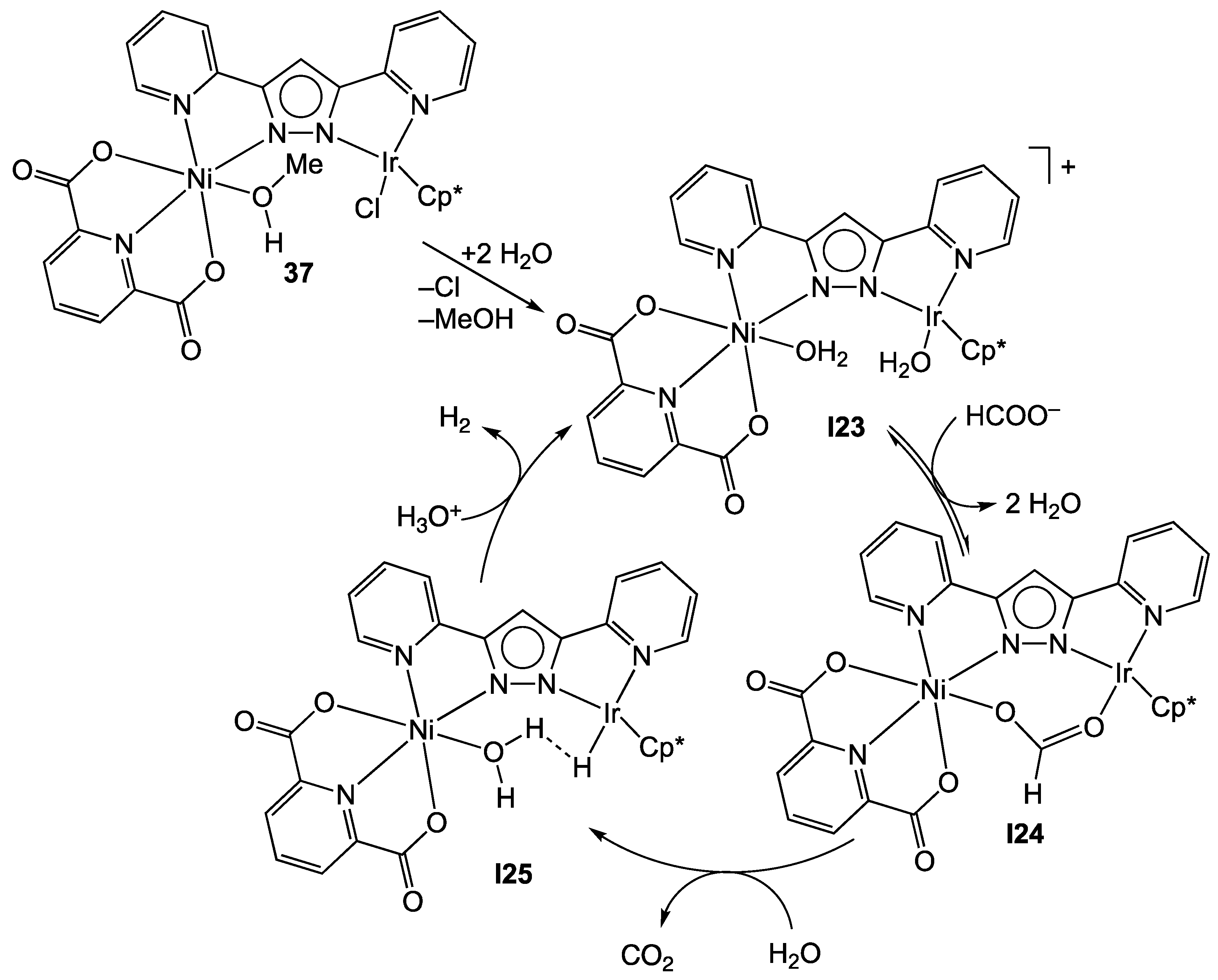
3.2. Heterodinuclear Homogeneous Catalysts
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bullock, R.M.; Chen, J.G.; Gagliardi, L.; Chirik, P.J.; Farha, O.K.; Hendon, C.H.; Jones, C.W.; Keith, J.A.; Klosin, J.; Minteer, S.D.; et al. Using nature’s blueprint to expand catalysis with Earth-abundant metals. Science 2020, 369, eabc3183. [Google Scholar] [CrossRef] [PubMed]
- Albrecht, M.; Bedford, R.; Plietker, B. Catalytic and Organometallic Chemistry of Earth-Abundant Metals. Organometallics 2014, 33, 5619–5621. [Google Scholar] [CrossRef] [Green Version]
- Su, B.; Cao, Z.-C.; Shi, Z.-J. Exploration of Earth-Abundant Transition Metals (Fe, Co, and Ni) as Catalysts in Unreactive Chemical Bond Activations. Acc. Chem. Res. 2015, 48, 886–896. [Google Scholar] [CrossRef]
- Thoi, V.S.; Sun, Y.; Long, J.R.; Chang, C.J. Complexes of earth-abundant metals for catalytic electrochemical hydrogen generation under aqueous conditions. Chem. Soc. Rev. 2013, 42, 2388–2400. [Google Scholar] [CrossRef] [PubMed]
- Reed-Berendt, B.G.; Polidano, K.; Morrill, L.C. Recent advances in homogeneous borrowing hydrogen catalysis using earth-abundant first row transition metals. Org. Biomol. Chem. 2019, 17, 1595–1607. [Google Scholar] [CrossRef] [Green Version]
- Gandeepan, P.; Cheng, C.-H. Cobalt Catalysis Involving π Components in Organic Synthesis. Acc. Chem. Res. 2015, 48, 1194–1206. [Google Scholar] [CrossRef] [PubMed]
- Chirik, P.; Morris, R. Getting Down to Earth: The Renaissance of Catalysis with Abundant Metals. Acc. Chem. Res. 2015, 48, 2495. [Google Scholar] [CrossRef] [Green Version]
- Eppinger, J.; Huang, K.-W. Formic Acid as a Hydrogen Energy Carrier. ACS Energy Lett. 2017, 2, 188–195. [Google Scholar] [CrossRef] [Green Version]
- Luque-Gómez, A.; García-Abellán, S.; Munarriz, J.; Polo, V.; Passarelli, V.; Iglesias, M. Impact of Green Cosolvents on the Catalytic Dehydrogenation of Formic Acid: The Case of Iridium Catalysts Bearing NHC-phosphane Ligands. Inorg. Chem. 2021, 60, 15497–15508. [Google Scholar] [CrossRef]
- Haider, R.; Wen, Y.; Ma, Z.-F.; Wilkinson, D.P.; Zhang, L.; Yuan, X.; Song, S.; Zhang, J. High temperature proton exchange membrane fuel cells: Progress in advanced materials and key technologies. Chem. Soc. Rev. 2021, 50, 1138–1187. [Google Scholar] [CrossRef]
- Baschuk, J.J.; Li, X. Carbon monoxide poisoning of proton exchange membrane fuel cells. Int. J. Energy Res. 2001, 25, 695–713. [Google Scholar] [CrossRef]
- Iglesias, M.; Oro, L.A. Mechanistic Considerations on Homogeneously Catalyzed Formic Acid Dehydrogenation. Eur. J. Inorg. Chem. 2018, 2125–2138. [Google Scholar] [CrossRef]
- Li, J.; Li, J.; Zhang, D.; Liu, C. DFT Study on the Mechanism of Formic Acid Decomposition by a Well-Defined Bifunctional Cyclometalated Iridium(III) Catalyst: Self-Assisted Concerted Dehydrogenation via Long-Range Intermolecular Hydrogen Migration. ACS Catal. 2016, 6, 4746–4754. [Google Scholar] [CrossRef]
- Cohen, S.; Borin, V.; Schapiro, I.; Musa, S.; De-Botton, S.; Belkova, N.V.; Gelman, D. Ir(III)-PC(sp3)P Bifunctional Catalysts for Production of H2 by Dehydrogenation of Formic Acid: Experimental and Theoretical Study. ACS Catal. 2017, 7, 8139–8146. [Google Scholar] [CrossRef]
- Iturmendi, A.; Rubio-Pérez, L.; Pérez-Torrente, J.J.; Iglesias, M.; Oro, L.A. Impact of Protic Ligands in the Ir-Catalyzed Dehydrogenation of Formic Acid in Water. Organometallics 2018, 37, 3611–3618. [Google Scholar] [CrossRef] [Green Version]
- Matsunami, A.; Kuwata, S.; Kayaki, Y. A Bifunctional Iridium Catalyst Modified for Persistent Hydrogen Generation from Formic Acid: Understanding Deactivation via Cyclometalation of a 1,2-Diphenylethylenediamine Motif. ACS Catal. 2017, 7, 4479–4484. [Google Scholar] [CrossRef]
- Wang, W.-H.; Xu, S.; Manaka, Y.; Suna, Y.; Kambayashi, H.; Muckerman, J.T.; Fujita, E.; Himeda, Y. Formic Acid Dehydrogenation with Bioinspired Iridium Complexes: A Kinetic Isotope Effect Study and Mechanistic Insight. ChemSusChem 2014, 7, 1976–1983. [Google Scholar] [CrossRef] [PubMed]
- Oldenhof, S.; Lutz, M.; de Bruin, B.; van der Vlugt, J.I.; Reek, J.N.H. Dehydrogenation of formic acid by Ir–bisMETAMORPhos complexes: Experimental and computational insight into the role of a cooperative ligand. Chem. Sci. 2015, 6, 1027–1034. [Google Scholar] [CrossRef] [Green Version]
- Sordakis, K.; Tang, C.; Vogt, L.K.; Junge, H.; Dyson, P.J.; Beller, M.; Laurenczy, G. Homogeneous Catalysis for Sustainable Hydrogen Storage in Formic Acid and Alcohols. Chem. Rev. 2018, 118, 372–433. [Google Scholar] [CrossRef] [PubMed]
- Laurenczy, G.; Dyson, P.J. Homogeneous Catalytic Dehydrogenation of Formic Acid: Progress Towards a Hydrogen-Based Economy. J. Braz. Chem. Soc. 2014, 25, 2157–2163. [Google Scholar] [CrossRef]
- Guan, C.; Pan, Y.; Zhang, T.; Ajitha, M.J.; Huang, K.-W. An Update on Formic Acid Dehydrogenation by Homogeneous Catalysis. Chem. Asian J. 2020, 15, 937–946. [Google Scholar] [CrossRef] [PubMed]
- Grasemann, M.; Laurenczy, G. Formic acid as a hydrogen source—Recent developments and future trends. Energy Environ. Sci. 2012, 5, 8171–8181. [Google Scholar] [CrossRef]
- Stathi, P.; Solakidou, M.; Louloudi, M.; Deligiannakis, Y. From Homogeneous to Heterogenized Molecular Catalysts for H2 Production by Formic Acid Dehydrogenation: Mechanistic Aspects, Role of Additives, and Co-Catalysts. Energies 2020, 13, 733. [Google Scholar] [CrossRef] [Green Version]
- Tamarany, R.; Shin, D.Y.; Kang, S.; Jeong, H.; Kim, J.; Kim, J.; Yoon, C.W.; Lim, D.-H. Formic acid dehydrogenation over PdNi alloys supported on N-doped carbon: Synergistic effect of Pd–Ni alloying on hydrogen release. Phys. Chem. Chem. Phys. 2021, 23, 11515–11527. [Google Scholar] [CrossRef] [PubMed]
- Liab, J.; Zhua, Q.-L.; Xu; Q. Dehydrogenation of Formic Acid by Heterogeneous Catalysts. Chimia 2015, 69, 348–352. [Google Scholar] [CrossRef]
- Onishi, M. Decomposition of formic acid catalyzed by hydrido (phosphonite) cobalt (I) under photoirradiation. J. Molec. Catal. 1993, 80, 145–149. [Google Scholar] [CrossRef]
- Zhou, W.; Wei, Z.; Spannenberg, A.; Jiao, H.; Junge, K.; Junge, H.; Beller, M. Cobalt-Catalyzed Aqueous Dehydrogenation of Formic Acid. Chem. Eur. J. 2019, 25, 8459–8464. [Google Scholar] [CrossRef] [PubMed]
- Junge, K.; Wendt, B.; Cingolani, A.; Spannenberg, A.; Wei, Z.; Jiao, H.; Beller, M. Cobalt Pincer Complexes for Catalytic Reduction of Carboxylic Acid Esters. Chem. Eur. J. 2018, 24, 1046–1052. [Google Scholar] [CrossRef]
- Lentz, N.; Aloisi, A.; Thuéry, P.; Nicolas, E.; Cantat, T. Additive-Free Formic Acid Dehydrogenation Catalyzed by a Cobalt Complex. Organometallics 2021, 40, 565–569. [Google Scholar] [CrossRef]
- Cook, A.W.; Emge, T.J.; Waldie, K.M. Insights into Formate Oxidation by a Series of Cobalt Piano-Stool Complexes Supported by Bis(phosphino)amine Ligands. Inorg. Chem. 2021, 60, 7372–7380. [Google Scholar] [CrossRef]
- Boddien, A.; Loges, B.; Gärtner, F.; Torborg, C.; Fumino, K.; Junge, H.; Ludwig, R.; Beller, M. Iron-Catalyzed Hydrogen Production from Formic Acid. J. Am. Chem. Soc. 2010, 132, 8924–8934. [Google Scholar] [CrossRef]
- Boddien, A.; Mellmann, D.; Gärtner, F.; Jackstell, R.; Junge, H.; Dyson, P.J.; Laurenczy, G.; Ludwig, R.; Beller, M. Efficient dehydrogenation of formic acid using an iron catalyst. Science 2011, 333, 1733–1736. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bielinski, E.A.; Lagaditis, P.O.; Zhang, Y.; Mercado, B.Q.; Würtele, C.; Bernskoetter, W.H.; Hazari, N.; Schneider, S. Lewis Acid-Assisted Formic Acid Dehydrogenation Using a Pincer-Supported Iron Catalyst. J. Am. Chem. Soc. 2014, 136, 10234–10237. [Google Scholar] [CrossRef]
- Curley, J.B.; Smith, N.E.; Bernskoetter, W.H.; Hazari, N.; Mercado, B.Q. Catalytic Formic Acid Dehydrogenation and CO2 Hydrogenation Using Iron PNRP Pincer Complexes with Isonitrile Ligands. Organometallics 2018, 37, 3846–3853. [Google Scholar] [CrossRef]
- Bertini, F.; Mellone, I.; Ienco, A.; Peruzzini, M.; Gonsalvi, L. Iron(II) Complexes of the Linear rac-Tetraphos-1 Ligand as Efficient Homogeneous Catalysts for Sodium Bicarbonate Hydrogenation and Formic Acid Dehydrogenation. ACS Catal. 2015, 5, 1254–1265. [Google Scholar] [CrossRef]
- Tondreu, A.M.; Boncella, J.M. 1,2-Addition of Formic or Oxalic Acid to −N{CH2CH2(PiPr2)}2-Supported Mn(I) Dicarbonyl Complexes and the Manganese-Mediated Decomposition of Formic Acid. Organometallics 2016, 35, 2049–2052. [Google Scholar] [CrossRef]
- Anderson, N.H.; Boncella, J.; Tondreau, A.M. Manganese-Mediated Formic Acid Dehydrogenation. Chem. Eur. J. 2019, 25, 10557–10560. [Google Scholar] [CrossRef] [PubMed]
- Lèval, A.; Agapova, A.; Steinlechner, C.; Alberico, E.; Junge, H.; Beller, M. Hydrogen production from formic acid catalyzed by a phosphine free manganese complex: Investigation and mechanistic insights. Green Chem. 2020, 22, 913–920. [Google Scholar] [CrossRef]
- Lèval, A.; Junge, H.; Beller, M. Manganese(i) κ2-NN complex-catalyzed formic acid dehydrogenation. Catal. Sci. Technol. 2020, 10, 3931–3937. [Google Scholar] [CrossRef]
- Britto, N.J.; Jaccob, M. Deciphering the Mechanistic Details of Manganese-Catalyzed Formic Acid Dehydrogenation: Insights from DFT Calculations. Inorg. Chem. 2021, 60, 11038–11047. [Google Scholar] [CrossRef]
- Enthaler, S.; Brück, A.; Kammer, A.; Junge, H.; Irran, E.; Gülak, S. Exploring the Reactivity of Nickel Pincer Complexes in the Decomposition of Formic Acid to CO2/H2 and the Hydrogenation of NaHCO3 to NaOOCH. ChemCatChem 2015, 7, 65–69. [Google Scholar] [CrossRef]
- Neary, M.C.; Parkin, G. Nickel-catalyzed release of H2 from formic acid and a new method for the synthesis of zerovalent Ni(PMe3)4. Dalton Trans. 2016, 45, 14645–14650. [Google Scholar] [CrossRef] [PubMed]
- Scotti, N.; Psaro, R.; Ravasio, N.; Zaccheria, F. A new Cu-based system for formic acid dehydrogenation. RSC Adv. 2014, 4, 61514–61517. [Google Scholar] [CrossRef]
- Correa, A.; Cascella, M.; Scotti, N.; Zacheria, F.; Ravasio, N.; Psaro, R. Mechanistic insights into formic acid dehydrogenation promoted by Cu-amino based systems. Inorg. Chim. Acta. 2018, 470, 290–294. [Google Scholar] [CrossRef]
- O’Hair, R.A.J.; Mravak, A.; Krstić, M.; Bonačić-Koutecký, V. Models Facilitating the Design of a New Metal-Organic Framework Catalyst for the Selective Decomposition of Formic Acid into Hydrogen and Carbon Dioxide. ChemCatChem 2019, 11, 2443–2448. [Google Scholar] [CrossRef]
- Nakajima, T.; Kamiryo, Y.; Kishimoto, M.; Nakamae, K.; Ura, Y.; Tanase, T. Synergistic Cu2 Catalysts for Formic Acid Dehydrogenation. J. Am. Chem. Soc. 2019, 141, 8732–8736. [Google Scholar] [CrossRef]
- Myers, T.W.; Berben, L.A. Aluminium–ligand cooperation promotes selective dehydrogenation of formic acid to H2 and CO2. Chem. Sci. 2014, 5, 2771–2777. [Google Scholar] [CrossRef]
- Chauvier, C.; Tlili, A.; Das Neves Gomes, C.; Thuéry, P.; Cantat, T. Metal-free dehydrogenation of formic acid to H2 and CO2 using boron-based catalysts. Chem. Sci. 2015, 6, 2938–2942. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hong, D.; Shimoyama, Y.; Ohgomori, Y.; Kanega, R.; Kotani, H.; Ishizuka, Y.; Kon, Y.; Himeda, Y.; Kojima, T. Cooperative Effects of Heterodinuclear IrIII−MII Complexes on Catalytic H2 Evolution from Formic Acid Dehydrogenation in Water. Inorg. Chem. 2020, 59, 11976–11985. [Google Scholar] [CrossRef]



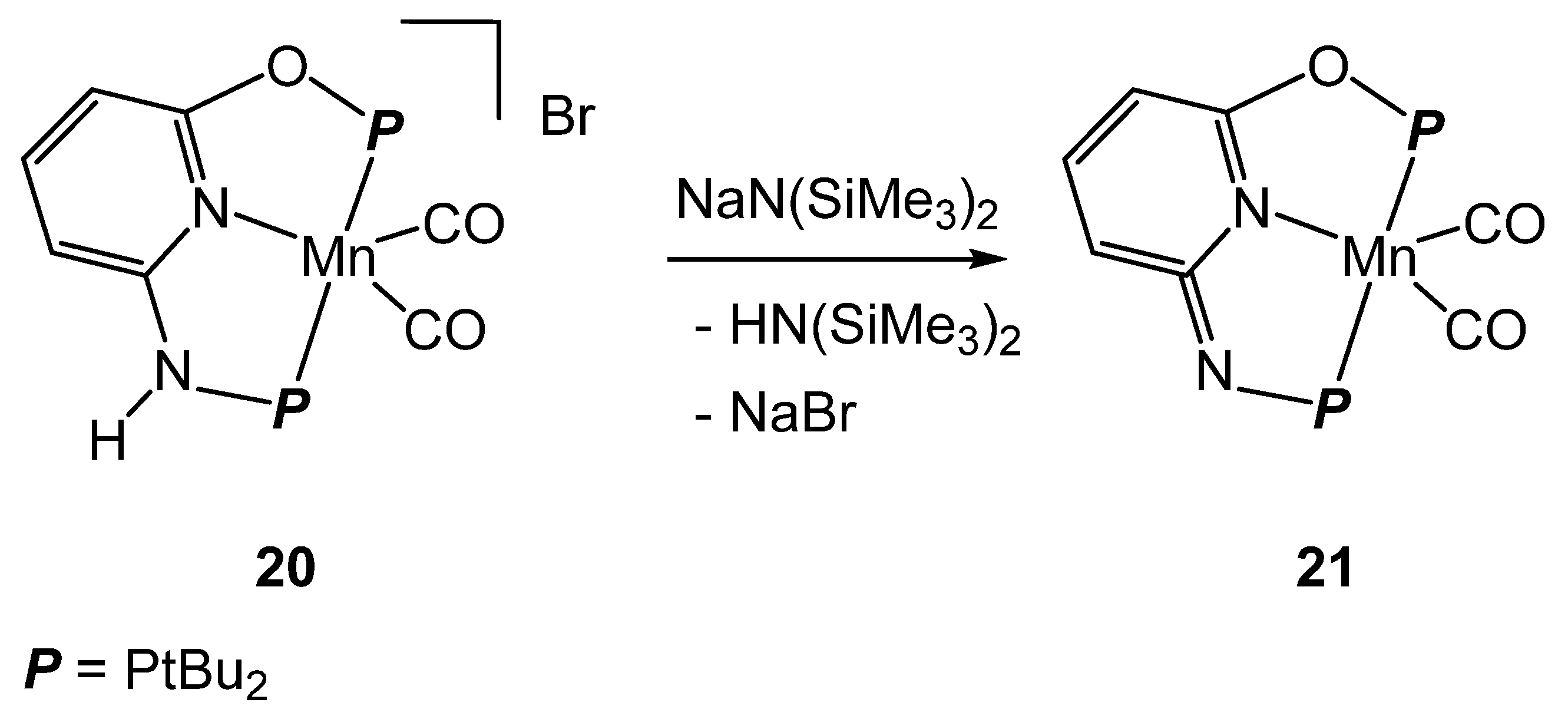


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iglesias, M.; Fernández-Alvarez, F.J. Advances in Nonprecious Metal Homogeneously Catalyzed Formic Acid Dehydrogenation. Catalysts 2021, 11, 1288. https://doi.org/10.3390/catal11111288
Iglesias M, Fernández-Alvarez FJ. Advances in Nonprecious Metal Homogeneously Catalyzed Formic Acid Dehydrogenation. Catalysts. 2021; 11(11):1288. https://doi.org/10.3390/catal11111288
Chicago/Turabian StyleIglesias, Manuel, and Francisco J. Fernández-Alvarez. 2021. "Advances in Nonprecious Metal Homogeneously Catalyzed Formic Acid Dehydrogenation" Catalysts 11, no. 11: 1288. https://doi.org/10.3390/catal11111288
APA StyleIglesias, M., & Fernández-Alvarez, F. J. (2021). Advances in Nonprecious Metal Homogeneously Catalyzed Formic Acid Dehydrogenation. Catalysts, 11(11), 1288. https://doi.org/10.3390/catal11111288






