Expeditious Asymmetric Synthesis of Polypropionates Relying on Sulfur Dioxide-Induced C–C Bond Forming Reactions
Abstract
1. Introduction
2. One-Pot Synthesis of Polypropionate Stereotriads Ready for Bidirectional Chain Elongations
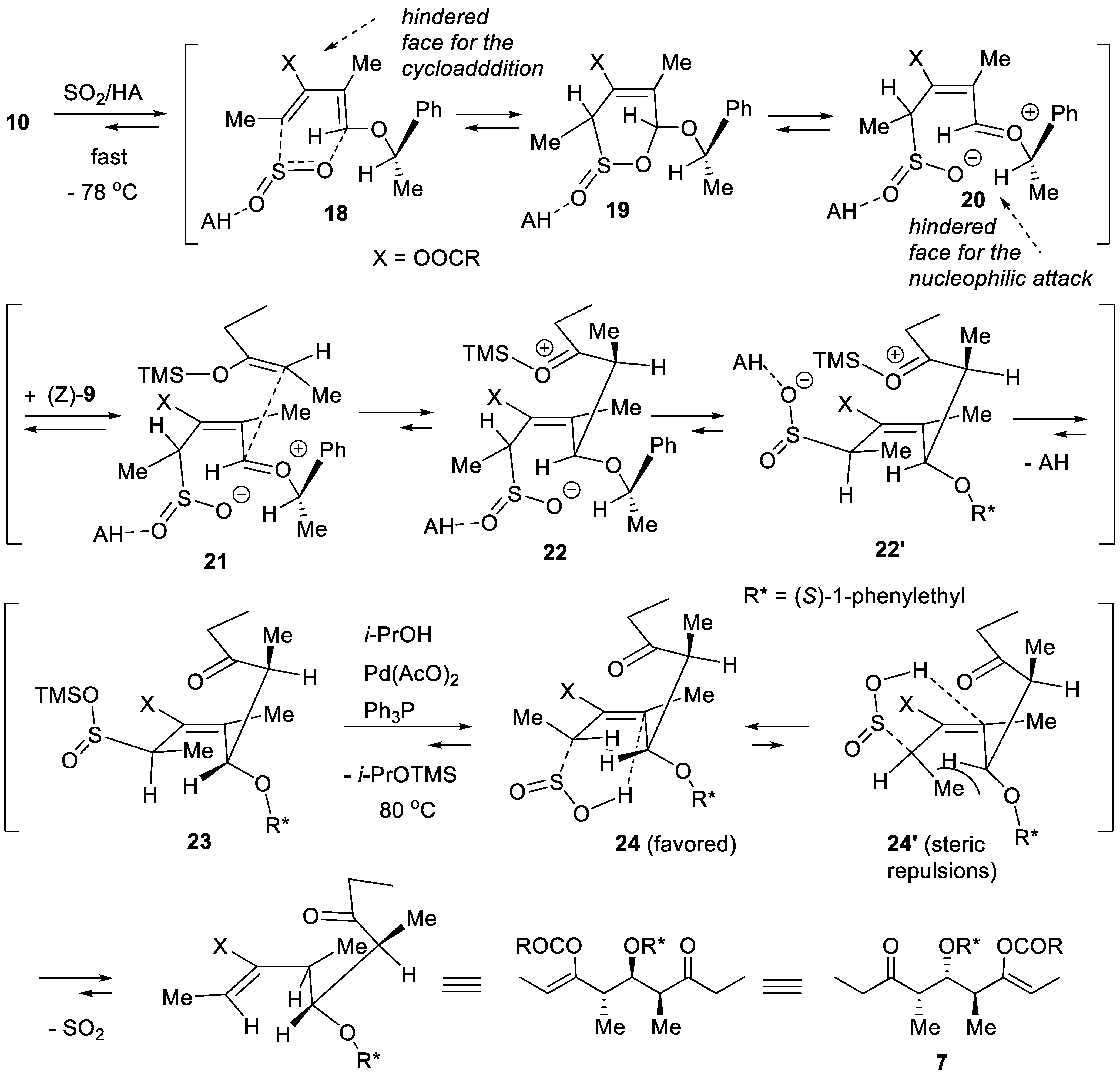
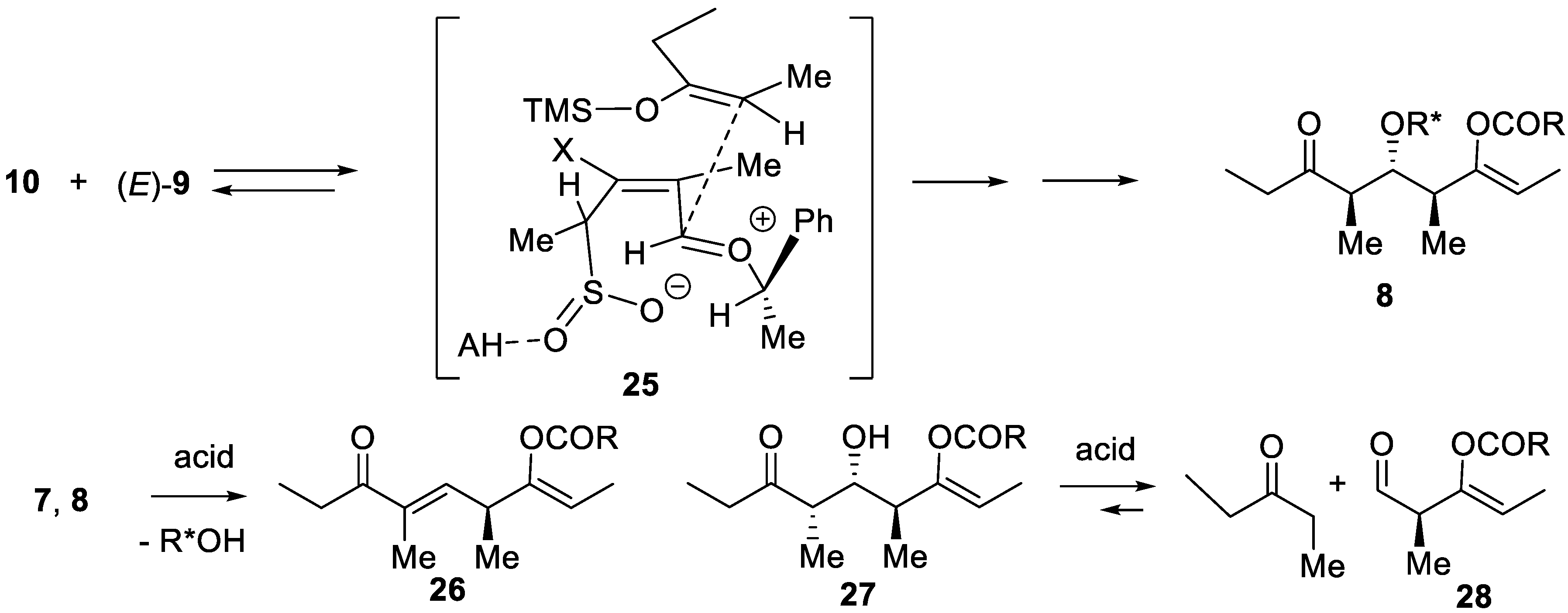
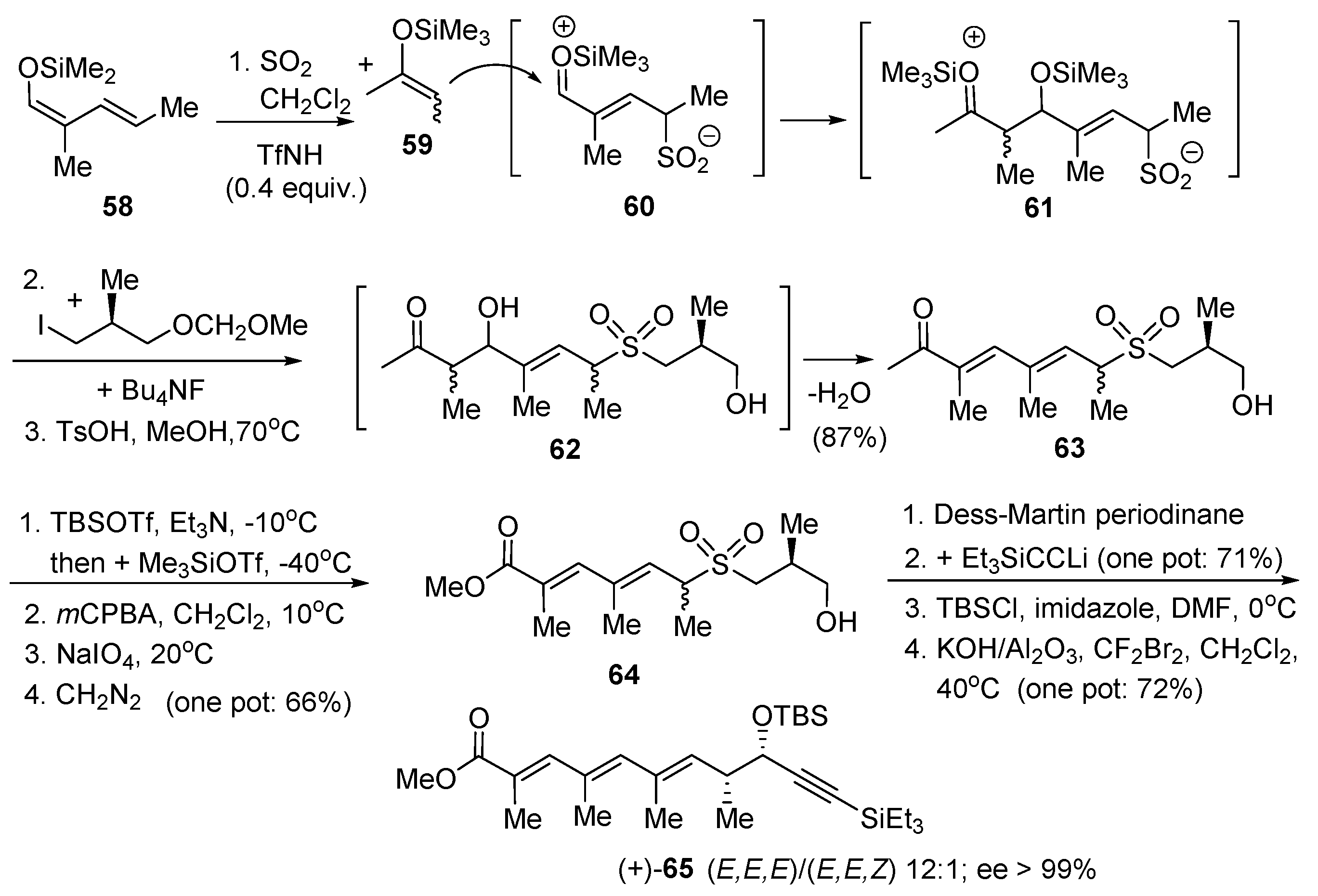
3. Sulfur Dioxide as Umpolung Agent to Promote Carbon–Carbon Bond Forming Reactions between Alkenes and Dienes
4. Long-Chain Polypropionates through Bidirectional Chain Elongation
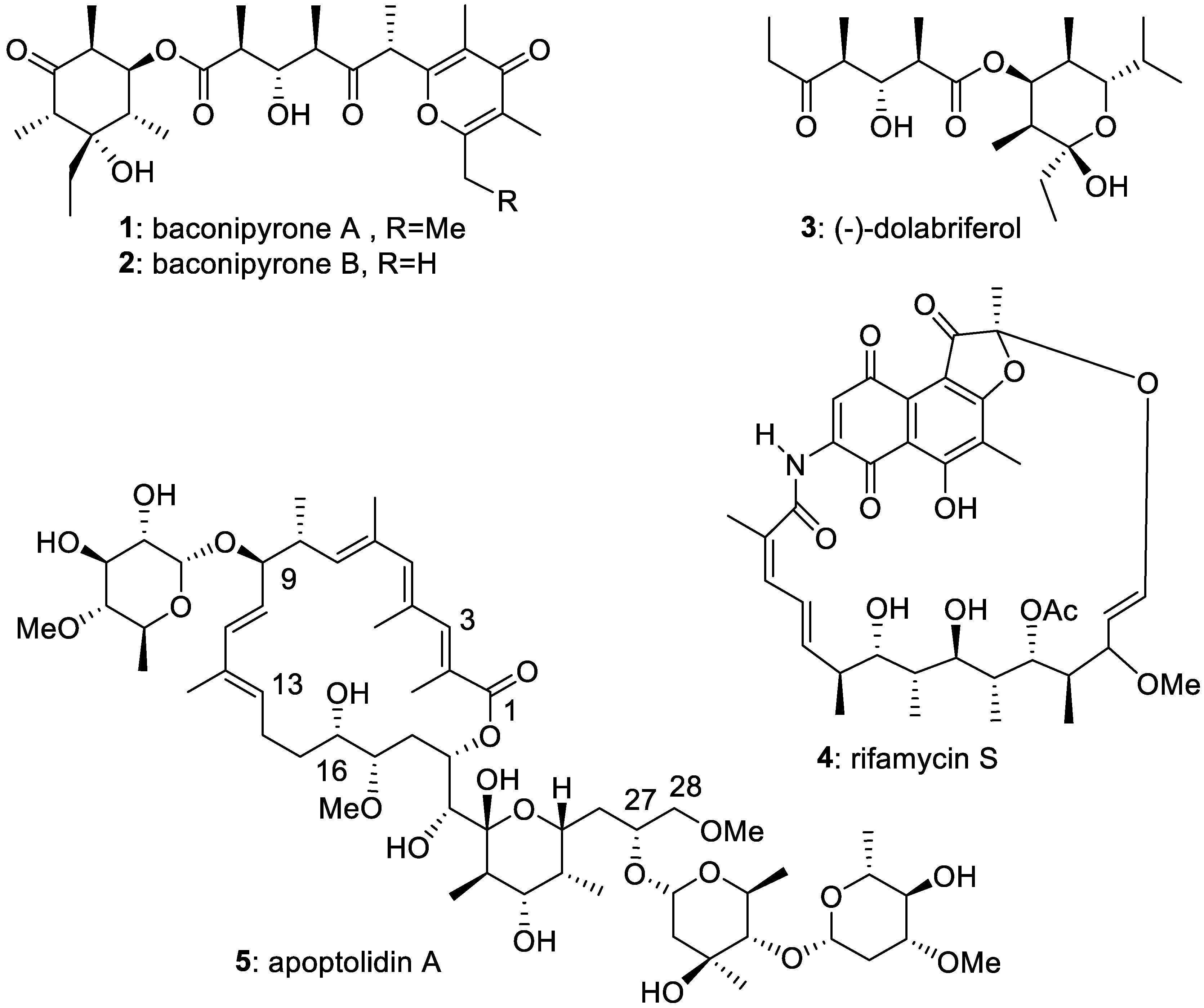
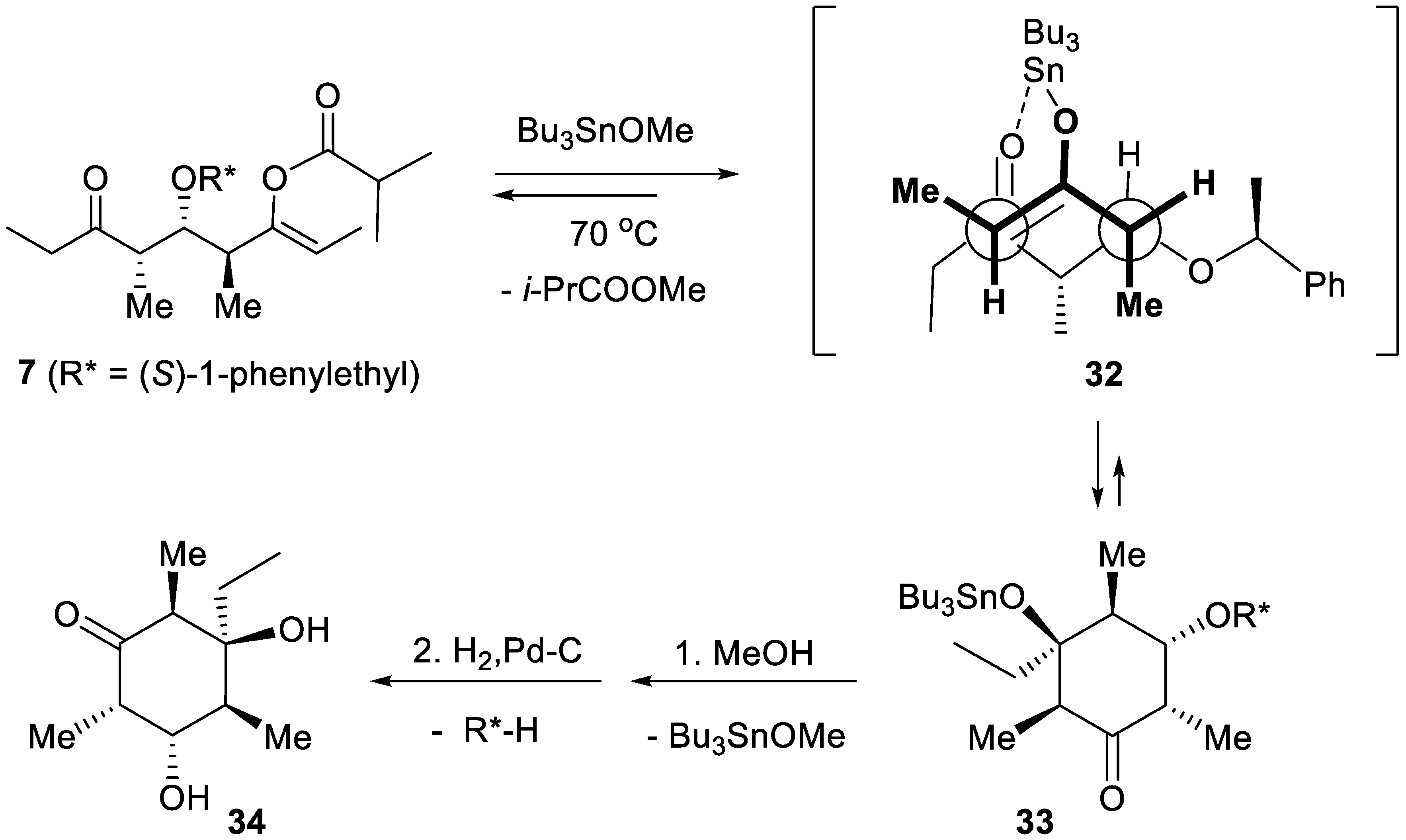
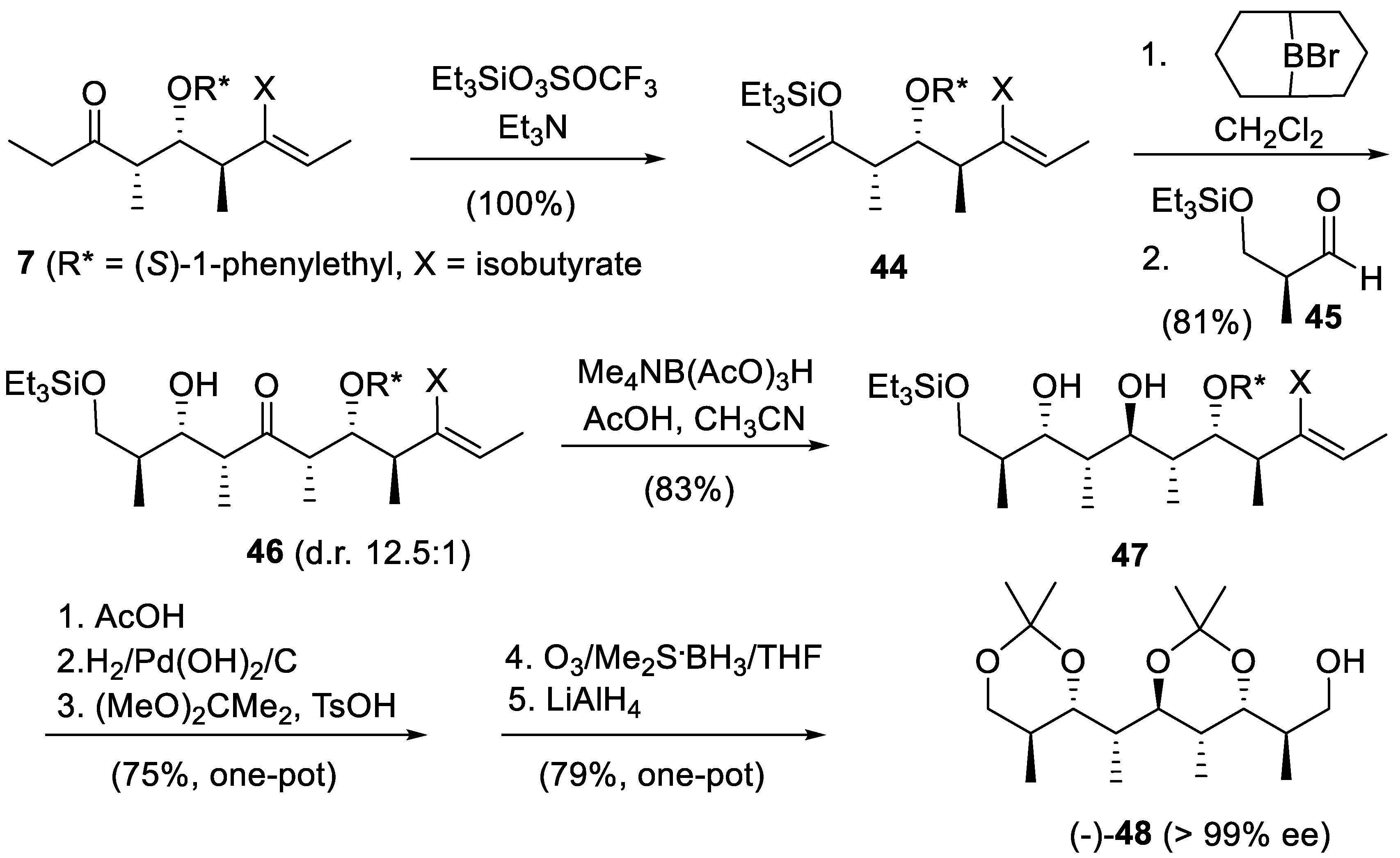
5. First Total Asymmetric Synthesis of the Cyclohexanol Subunit of Baconipyrones A and B
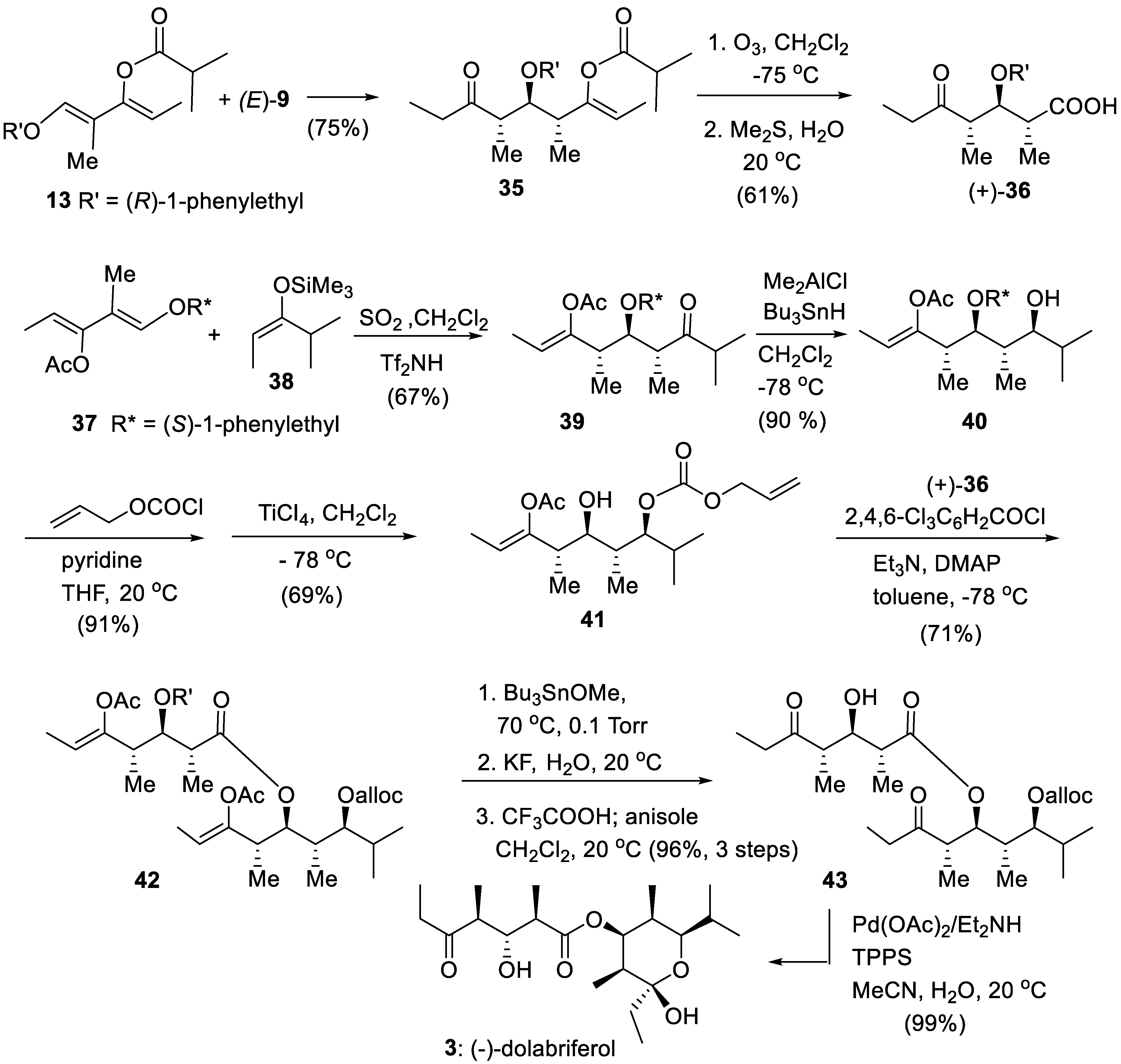
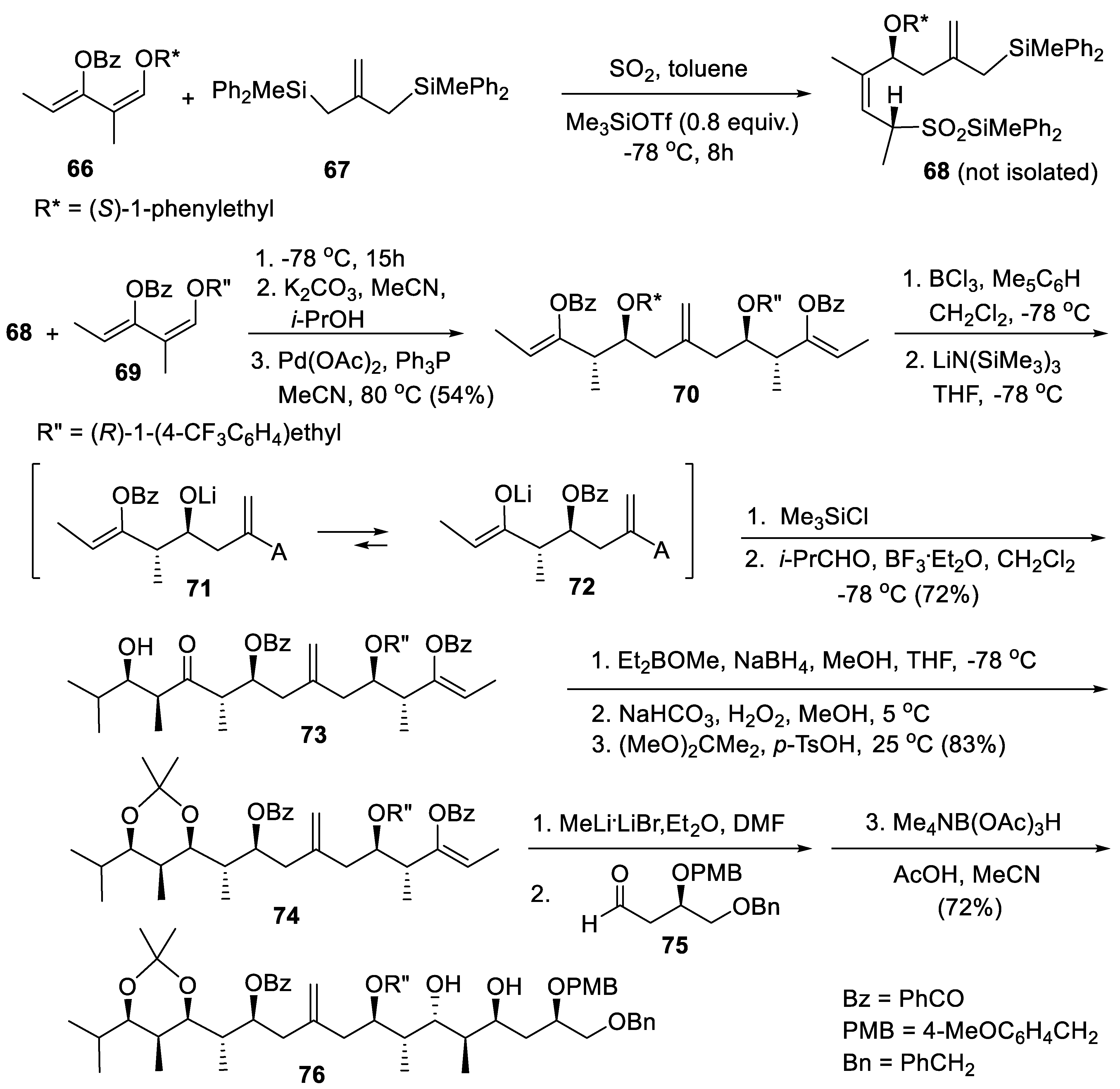
6. First Total Asymmetric Synthesis of (-)-Dolabriferol
7. Expeditious Asymmetric Synthesis of the Stereoheptad C19–C27 of Rifamycins: Formal Total Synthesis of Rifamycin S
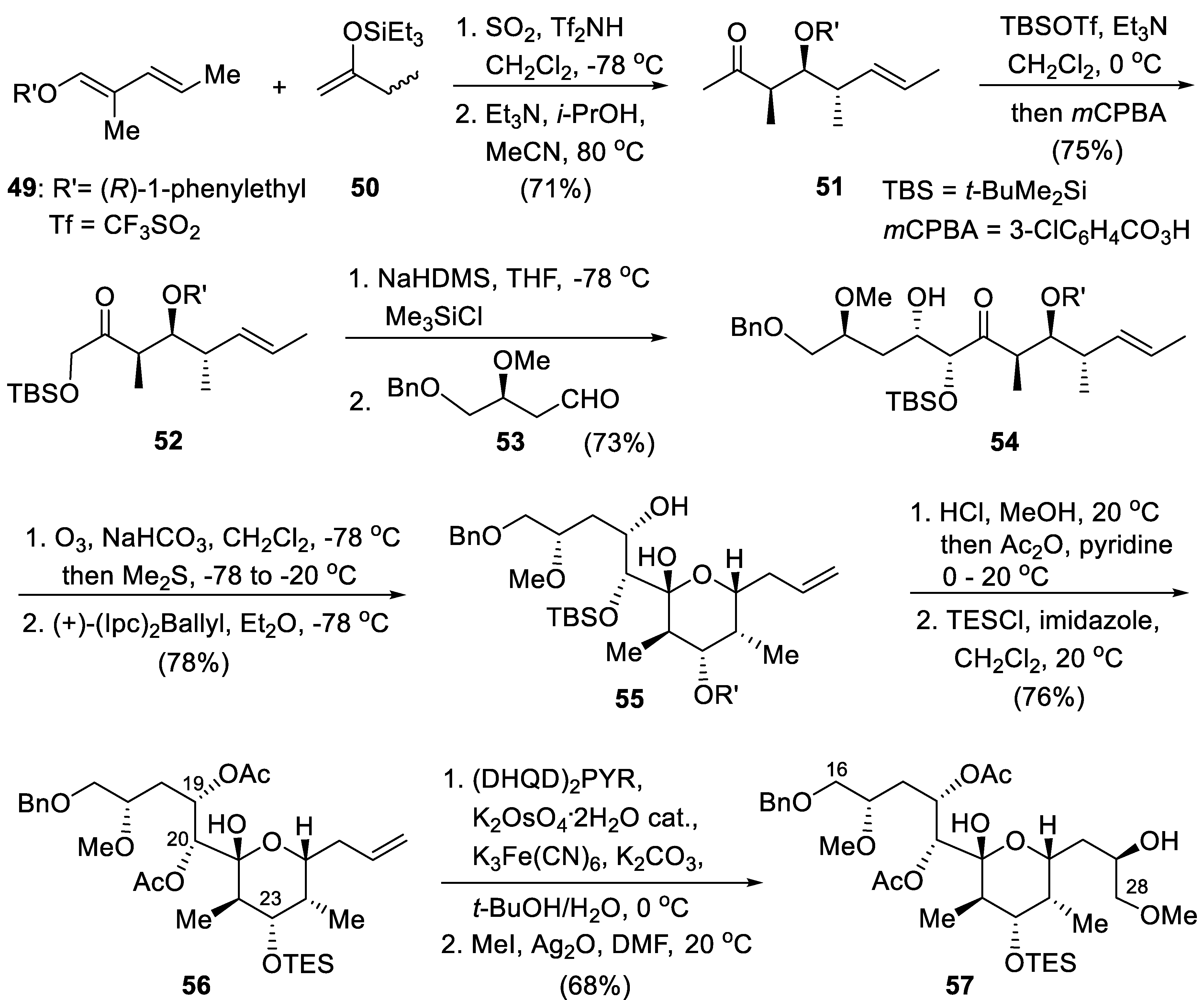
8. Generalization of the SO2-Induced Umpolung. Short Synthesis of the C16–C28 Fragment of Apoptolidinone: Formal Total Synthesis of Apoptolidin A
9. The One-Pot Four-Component Synthesis of Polyfunctional Sulfones: Application to a Short Synthesis of the C1–C11 Fragment of Apoptolidin A
10. Allylsilanes as Nucleophiles: Development of Two-Directional Polypropionate–Polyketide Synthesis
11. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Davies-Coleman, M.T.; Garson, M.J. Marine polypropionates. Nat. Prod. Rep. 1998, 15, 477–493. [Google Scholar] [CrossRef]
- Müller, W.E.G. Marine Molecular Biotechnology; Springer: Berlin, Germany, 2006; Chapter 1.2; Volume 71, pp. 570–575. [Google Scholar]
- Liu, Z.; Liu, H.; Zhang, W. Natural Polypropionates in 1999–2020: An Overview of Chemical and Biological Diversity. Mar. Drugs 2020, 18, 569. [Google Scholar] [CrossRef]
- Wu, Q.; Li, S.-W.; Xu, H.; Wang, H.; Hu, P.; Zhang, H.; Luo, C.; Chen, K.-X.; Nay, B.; Guo, Y.-W.; et al. Complex polypropionates from a South China Sea photosynthetic mollusk: Isolation and biomimetic synthesis highlighting novel rearrangements. Angew. Chem. Int. Ed. 2020, 59, 12105–12112. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Chen, D.; Yu, M.; Liu, Y.; Liu, P.; Zhang, X. A Review on Metabolites from Onchidium Genus: Chemistry and Bioactivity. Chem. Biodivers. 2020, 18, e2000580. [Google Scholar] [CrossRef]
- Lin, S.; Wu, Y.-Z.; Chen, K.-Y.; Ye, J.; Yang, X.-W.; Zhang, W.-D. Polyketides from the fungus Penicillium decumbens. J. Asian Nat. Prod. Res. 2018, 20, 445–450. [Google Scholar] [CrossRef]
- Koskinen, A.M.P.; Karisalmi, K. Polyketide stereotetrads in natural products. Chem. Soc. Rev. 2005, 34, 677–690. [Google Scholar] [CrossRef]
- Hertweck, C. The Biosynthetic Logic of Polyketide Diversity. Angew. Chem. Int. Ed. 2009, 48, 4688–4716. [Google Scholar] [CrossRef] [PubMed]
- Weissman, K.J.; Leadlay, P. Combinatorial biosynthesis of reduced polyketides. Nat. Rev. Genet. 2005, 3, 925–936. [Google Scholar] [CrossRef] [PubMed]
- Walsh, C.T. Nature Builds Macrocycles and Heterocycles into Its Antimicrobial Frameworks: Deciphering Biosynthetic Strategy. ACS Infect. Dis. 2018, 4, 1283–1299. [Google Scholar] [CrossRef] [PubMed]
- Cragg, G.M.; Grothaus, P.G.; Newman, D.J. Impact of Natural Products on Developing New Anti-Cancer Agents. Chem. Rev. 2009, 109, 3012–3043. [Google Scholar] [CrossRef] [PubMed]
- Panek, J.S.; Jain, N.F. Total Synthesis of Rutamycin B and Oligomycin C. J. Org. Chem. 2001, 66, 2747–2756. [Google Scholar] [CrossRef]
- Crossman, J.S.; Perkins, M.V. Total Synthesis and Structural Elucidation of (−)-Maurenone. J. Org. Chem. 2005, 71, 117–124. [Google Scholar] [CrossRef]
- Ward, D.E. The thiopyran route to polypropionates. Chem. Commun. 2011, 47, 11375–11393. [Google Scholar] [CrossRef]
- Ward, D.E.; Kazemeini, A. Aldol Reactions with Kinetic Resolution: Scope and Limitations of Ketal- and Dithioketal-Protected β-Ketoaldehydes. J. Org. Chem. 2012, 77, 10789–10803. [Google Scholar] [CrossRef]
- Turks, M.; Laclef, S.; Vogel, P. Construction of Polypropionate Fragments in Natural Product Synthesis. Stereoselective Synthesis of Drugs and Natural Products; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2013; Volume 1, pp. 271–318. [Google Scholar]
- Alagiri, K.; Lin, S.; Kumagai, N.; Shibasaki, M. Iterative Direct Aldol Strategy for Polypropionates: Enantioselective Total Synthesis of (−)-Membrenone A and B. Org. Lett. 2014, 16, 5301–5303. [Google Scholar] [CrossRef] [PubMed]
- Miles, W.H.; Madison, C.M.; Mastria, M.L.; Tang, P.-I. Synthesis of the C3–C7 fragment of tylonolide by the γ-hydroxybutenolide approach. Tetrahedron Lett. 2016, 57, 3929–3932. [Google Scholar] [CrossRef]
- Hosokawa, S. Remote Asymmetric Induction Reactions using a E,E-Vinylketene Silyl N,O-Acetal and the Wide Range Stereocontrol Strategy for the Synthesis of Polypropionates. Acc. Chem. Res. 2018, 51, 1301–1314. [Google Scholar] [CrossRef] [PubMed]
- Santana, C.G.; Krische, M.J. From Hydrogenation to Transfer Hydrogenation to Hydrogen Auto-Transfer in Enantioselective Metal-Catalyzed Carbonyl Reductive Coupling: Past, Present, and Future. ACS Catal. 2021, 11, 5572–5585. [Google Scholar] [CrossRef] [PubMed]
- Dechert-Schmitt, A.-M.R.; Schmitt, D.C.; Gao, X.; Itoh, T.; Krische, M.J. Polyketide construction via hydrohydroxyalkylation and related alcohol C–H functionalizations: Reinventing the chemistry of carbonyl addition. Nat. Prod. Rep. 2014, 31, 504–513. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Kasun, Z.A.; Krische, M.J. Enantioselective Alcohol C–H Functionalization for Polyketide Construction: Unlocking Redox-Economy and Site-Selectivity for Ideal Chemical Synthesis. J. Am. Chem. Soc. 2016, 138, 5467–5478. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, K.D.; Park, B.Y.; Luong, T.; Sato, H.; Garza, V.J.; Krische, M.J. Metal-catalyzed reductive coupling of olefin-derived nucleophiles: Reinventing carbonyl addition. Science 2016, 354, aah5133. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.W.; Zhang, W.; Krische, M.J. Catalytic Enantioselective Carbonyl Allylation and Propargylation via Alcohol-Mediated Hydrogen Transfer: Merging the Chemistry of Grignard and Sabatier. Acc. Chem. Res. 2017, 50, 2371–2380. [Google Scholar] [CrossRef] [PubMed]
- Holmes, M.; Schwartz, L.A.; Krische, M.J. Intermolecular Metal-Catalyzed Reductive Coupling of Dienes, Allenes, and Enynes with Carbonyl Compounds and Imines. Chem. Rev. 2018, 118, 6026–6052. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Lin, S.; Jacobsen, K.M.; Poulsen, T.B. Chemical Syntheses and Chemical Biology of Carboxyl Polyether Ionophores: Recent Highlights. Angew. Chem. Int. Ed. 2019, 58, 13630–13642. [Google Scholar] [CrossRef] [PubMed]
- Doerksen, R.S.; Meyer, C.C.; Krische, M.J. Feedstock Reagents in Metal-Catalyzed Carbonyl Reductive Coupling: Minimizing Preactivation for Efficiency in Target-Oriented Synthesis. Angew. Chem. Int. Ed. 2019, 58, 14055–14064. [Google Scholar] [CrossRef] [PubMed]
- Siu, Y.-M.; Roane, J.; Krische, M.J. Total Synthesis of Leiodermatolide A via Transfer Hydrogenative Allylation, Crotylation, and Propargylation: Polyketide Construction beyond Discrete Allyl- or Allenylmetal Reagents. J. Am. Chem. Soc. 2021, 143, 10590–10595. [Google Scholar] [CrossRef]
- Li, J.; Menche, D. Direct methods for stereoselective polypropionate synthesis: A survey. Synthesis 2009, 14, 2293–2315. [Google Scholar]
- Solsona, J.G.; Romea, A.P.; Urpí, F. Studies Directed toward the Construction of the Polypropionate Fragment of Superstolide A. Org. Lett. 2003, 5, 4681–4684. [Google Scholar] [CrossRef]
- Yadav, J.S.; Srinivas, R.; Sathaiah, K. Total synthesis of natural (+)-membrenone C and its 7-epimer. Tetrahedron Lett. 2006, 47, 1603–1606. [Google Scholar] [CrossRef]
- Chandra, B.; Fu, D.; Nelson, S.G. Catalytic Asymmetric Synthesis of Complex Polypropionates: Lewis Base Catalyzed Aldol Equivalents in the Synthesis of Erythronolide B. Angew. Chem. Int. Ed. 2010, 49, 2591–2594. [Google Scholar] [CrossRef]
- Mochirian, P.; Godin, F.; Katsoulis, I.; Fontaine, I.; Brazeau, J.-F.; Guindon, Y. A Bidirectional Approach to the Synthesis of Polypropionates: Synthesis of C1–C13 Fragment of Zincophorin and Related Isomers. J. Org. Chem. 2011, 76, 7654–7676. [Google Scholar] [CrossRef]
- Brady, P.B.; Yamamoto, H. Rapid and Stereochemically Flexible Synthesis of Polypropionates: Super-Silyl-Governed Aldol Cascades. Angew. Chem. Int. Ed. 2012, 51, 1942–1946. [Google Scholar] [CrossRef]
- Becerril-Jiménez, F.; Ward, D.E. On the Origin of Siphonariid Polypropionates: Total Synthesis of Caloundrin B and Its Isomerization to Siphonarin B. Org. Lett. 2012, 14, 1648–1651. [Google Scholar] [CrossRef]
- Ward, D.E. Polypropionate synthesis via substrate-controlled stereoselective aldol couplings of chiral fragments. In Modern Methods in Stereoselective Aldol Reactions; Mahrwald, R., Ed.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2013; pp. 377–429. [Google Scholar]
- Oshima, M.; Yamazaki, H.; Shimizu, I.; Nisar, M.; Tsuji, J. Palladium-catalyzed selective hydrogenolysis of alkenyloxiranes with formic acid. Stereoselectivity and synthetic utility. J. Am. Chem. Soc. 1989, 111, 6280–6287. [Google Scholar] [CrossRef]
- Nagasawa, K.; Shimizu, I.; Nakata, T. ChemInform Abstract: Total Synthesis of Preswinholide A. Part 1. Stereoselective Synthesis of the C11-C23 Segment. ChemInform 2010, 28, 6881–6884. [Google Scholar] [CrossRef]
- Jung, M.E.; Chaumontet, M.; Salehi-Rad, R. Total Synthesis of Auripyrone B Using a Non-Aldol Aldol−Cuprate Opening Process. Org. Lett. 2010, 12, 2872–2875. [Google Scholar] [CrossRef][Green Version]
- Bandaru, A.; Kaliappan, K.P. Synthesis of the C1-C10 fragment of muamvatin. Chem. Asian J. 2020, 15, 2208–2211. [Google Scholar] [CrossRef]
- Si, D.; Kaliappan, K.P. Synthesis of C9-C13 and C15-C21 subunits of discodermolide. Asian J. Org. Chem. 2020, 9, 1205–1212. [Google Scholar] [CrossRef]
- Zacuto, M.J.; O’Malley, S.J.; Leighton, J.L. Tandem silylformylation–allyl(crotyl)silylation: A new approach to polyketide synthesis. Tetrahedron 2003, 59, 8889–8900. [Google Scholar] [CrossRef]
- Foley, C.N.; Leighton, J.L. Beyond the Roche Ester: A New Approach to Polypropionate Stereotriad Synthesis. Org. Lett. 2014, 16, 1180–1183. [Google Scholar] [CrossRef] [PubMed]
- Foley, C.N.; Leighton, J.L. A Highly stereoselective, efficient, and scalable synthesis of the C(1)–C(9) fragment of the epothilones. Org. Lett. 2015, 17, 5858–5861. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Kong, L.; Gu, Q.; Shao, S.; Lin, G.-Q.; Hong, R. Stereoselective Access to Polypropionates Expedited by the Double Hydroboration of Allenes: Total Synthesis of Antitumor (−)-Pironetin. CCS Chem. 2021, 3, 769–779. [Google Scholar] [CrossRef]
- Bode, J.W.; Fraefel, N.; Muri, D.; Carreira, E.M. A General solution to the modular synthesis of polyketide building blocks by Kanemasa hydroxy-directed nitrile oxide cycloadditions. Angew. Chem. Int. Ed. 2001, 40, 2082–2085. [Google Scholar] [CrossRef]
- Fader, L.D.; Carreira, E.M. Stereochemically Rich Pentaketides from Bis(isoxazolines): A General Strategy for Efficient Polyketide Synthesis. Org. Lett. 2004, 6, 2485–2488. [Google Scholar] [CrossRef]
- Muri, D.; Carreira, E. Stereoselective Synthesis of Erythronolide A via Nitrile Oxide Cycloadditions and Related Studies. J. Org. Chem. 2009, 74, 8695–8712. [Google Scholar] [CrossRef]
- Danishefsky, S.; Harvey, D.F. A new approach to polypropionates—Routes to subunits of nonensin and tirandamycin. J. Am. Chem. Soc. 1985, 107, 6647–6652. [Google Scholar] [CrossRef]
- Danishefsky, S.J.; Myles, D.C.; Harvey, D.F. Expeditious synthesis of the polypropionate sector of rifamycin S by reiterative Diene-Aldehyde cyclocondensation reactions. J. Am. Chem. Soc. 1987, 109, 862–867. [Google Scholar] [CrossRef]
- Vogel, P.; Sevin, A.-F.; Kernen, P.; Bialecki, M. ’Naked sugars of the second generation’: Asymmetric synthesis of long-chain polypropionates and analogues starting with acetone. Pure Appl. Chem. 1996, 68, 719–722. [Google Scholar] [CrossRef]
- Vogel, P.; Cossy, J.; Plumet, J.; Arjona, O. Derivatives of 7-oxabicyclo[2.2.1]heptane in nature and as useful synthetic intermediates. Tetrahedron 1999, 55, 13521–13642. [Google Scholar] [CrossRef]
- Arjona, O.; Menchaca, R.; Plumet, J. Building a small polypropionate library. Synthesis of all possible stereotetrads (building blocks for polyketide synthesis) from furan. J. Org. Chem. 2001, 66, 2400–2413. [Google Scholar] [CrossRef] [PubMed]
- Arjona, O.; Plumet, J. The Ring Opening of Oxabicyclic Compounds Controlled by a Phenylsulfonyl Group: Synthetic Applications. ChemInform 2003, 34, 571–595. [Google Scholar] [CrossRef]
- Hunt, K.W.; Grieco, P.A. Oxabicyclo[3.2.1]octenes in organic synthesis—Direct ring opening of oxabicyclo[3.2.1] systems employing silyl ketene acetals in concentrated solutions of lithium perchlorate-diethyl ether: Application to the synthesis of the C(19)-C(27) fragment of rifamycin S. Org. Lett. 2001, 3, 481–484. [Google Scholar] [PubMed]
- Hagenbuch, J.-P.; Vogel, P. Asymmetric induction in the rearrangement of monocyclic endoperoxides into gamma-hydroxy-alpha,beta-unsaturated aldehydes. J. Chem. Soc. Chem. Commun. 1980, 22, 1062–1063. [Google Scholar] [CrossRef]
- Gesinski, M.R.; Brenzovich, W.E.; Staben, S.T.; Srinilta, D.J.; Toste, F.D. A divergent/convergent approach to dolabriferol: The Kornblum-DeLaMare enantiomeric resolution. Tetraedron Lett. 2015, 56, 3643–3646. [Google Scholar] [CrossRef]
- Tokura, N. Olefin-sulfur dioxide copolymers. Encycl. Polym. Sci. Technol. 1968, 9, 460–485. [Google Scholar]
- Gray, D.N. The status of olefin-sulfur dioxide copolymers as biomaterials. Polym. Sci. Technol. 1981, 14, 21–27. [Google Scholar]
- Fawcett, A.H. Olefin-sulfur dioxide copolymers. Encycl. Polym. Sci. Eng. 1987, 10, 408–432. [Google Scholar]
- Masilamani, D.; Reuman, M.E.; Rogic, M.M. Ene-type reaction through the intermediacy of the 1,4-dipolar ion in the reaction of tetracyanoethylene with nucleophilic double-bonds in liquid sulfur dioxide. J. Org. Chem. 1980, 45, 4602–4605. [Google Scholar] [CrossRef]
- Masilamani, D.; Rogic, M.M. Organic reactions of sulfur dioxide. 4. Facile regiospecific hydrogen-deuterium exchange in olefins—Consequence of intermediacy of allylic sulfinic acids in ene reaction of sulfur dioxide with double bonds. J. Am. Chem. Soc. 1978, 100, 4634–4635. [Google Scholar] [CrossRef]
- Marković, D.; Vogel, P. Polysulfones: Catalysts for Alkene Isomerization. Angew. Chem. Int. Ed. 2004, 43, 2928–2930. [Google Scholar] [CrossRef]
- Markovic, D.; Vogel, P. Allyl, Methallyl, Prenyl, and Methylprenyl Ethers as Protected Alcohols: Their Selective Cleavage with Diphenyldisulfone under Neutral Conditions. Org. Lett. 2004, 6, 2693–2696. [Google Scholar] [CrossRef]
- Marković, D.; Varela-Álvarez, A.; Sordo, J.A.; Vogel, P. Mechanism of the Diphenyldisulfone-Catalyzed Isomerization of Alkenes. Origin of the Chemoselectivity: Experimental and Quantum Chemistry Studies. J. Am. Chem. Soc. 2006, 128, 7782–7795. [Google Scholar] [CrossRef]
- Backer, H.J.; Strating, J. Cyclical sulphones, derivatives and butadienes. Recl. Trav. Chim. Pays-Bas 1934, 53, 525–543. [Google Scholar] [CrossRef]
- Heldeweg, R.F.; Hogeveen, H. (2+4) (π+π,π) and (2+4)(n+π-π) modes of addition in reaction between SO2 and a diene—Kinetic vs thermodynamic control. J. Am. Chem. Soc. 1976, 98, 2341–2342. [Google Scholar] [CrossRef]
- Durst, T.; Tetreault-Ryan, L. Reaction of ortho-quinodimethane with sulfur dioxide. Competition between π + π-π and n + π-π cycloadditions. Tetrahedron Lett. 1978, 26, 2353–2354. [Google Scholar] [CrossRef]
- Deguin, B.; Vogel, P. Hetero-Diels-Alder addition of sulfur dioxide to 1,3-dienes. Suprafaciality, regioselectivity, and stereoselectivity. J. Am. Chem. Soc. 1992, 114, 9210–9211. [Google Scholar] [CrossRef]
- Fernandez, T.; Sordo, J.A.; Monnat, F.; Deguin, B.; Vogel, P. Sulfur dioxide promotes its hetero-Diels-Alder and cheletropic additions to 1,2-dimethylenecyclohexane. J. Am. Chem. Soc. 1998, 120, 13276–13277. [Google Scholar] [CrossRef]
- Monnat, F.; Vogel, P.; Rayón, V.M.; Sordo, J.A. Ab Initio and Experimental Studies on the Hetero-Diels−Alder and Cheletropic Additions of Sulfur Dioxide to (E)-1-Methoxybutadiene: A Mechanism Involving Three Molecules of SO2. J. Org. Chem. 2002, 67, 1882–1889. [Google Scholar] [CrossRef] [PubMed]
- Vogel, P.; Sordo, J. Hetero-Diels-Alder and Cheletropic Additions of Sulfur Dioxide to Conjugated Dienes. Experimental Facts and Theoretical Analysis. Curr. Org. Chem. 2006, 10, 2007–2036. [Google Scholar] [CrossRef]
- Markovic, D.; Roversi, E.; Scoppelliti, R.; Vogel, P.; Meana, R.; Sordo, J.A. The hetero-Diels-Alder addition of sulfur dioxide: The pseudo-chair conformation of a 4,5-dialkylsultine. Chem. Eur. J. 2003, 9, 4911–4915. [Google Scholar] [CrossRef] [PubMed]
- Monnat, F.; Vogel, P.; Meana, R.; Sordo, J.A. Equilibrium and Kinetic Deuterium Isotope Effects on the Hetero-Diels–Alder Addition of Sulfur Dioxide. Angew. Chem. Int. Ed. 2003, 42, 3924–3927. [Google Scholar] [CrossRef] [PubMed]
- Roversi, E.; Scopelliti, R.; Solari, E.; Estoppey, R.; Vogel, P.; Braña, P.; Menéndez, B.; Sordo, J.A. The Hetero-Diels-Alder Addition of Sulfur Dioxide to 1-Fluorobuta-1,3-dienes: The Sofa Conformations Preferred by 6-Fluorosultines (6-Fluoro-3,6-dihydro-1,2-oxathiin-2-oxides) Enjoy Enthalpic and Conformational Anomeric Effects. Chem. A Eur. J. 2002, 8, 1336–1355. [Google Scholar] [CrossRef]
- Laclef, S.; Exner, C.J.; Turks, M.; Videtta, V.; Vogel, P. Synthesis of (E,Z)-1-Alkoxy-3-acyloxy-2-methylpenta-1,3-dienes via Danishefsky-Type Dienes or O-Acylation of Enones. J. Org. Chem. 2009, 74, 8882–8885. [Google Scholar] [CrossRef] [PubMed]
- Vogel, P.; Turks, M.; Exner, C.; Hamel, C. Umpolung with Sulfur Dioxide: Carbon-Carbon Cross-Coupling of Electron-Rich 1,3-Dienes and Alkenes; Application to the Enantioselective Synthesis of Long-Chain Polyketide Fragments. Synthesis 2009, 2009, 1065–1074. [Google Scholar] [CrossRef]
- Kanazawa, A.; Delair, P.; Pourashraf, M.; Greene, A.E. Convergent, enantioselective synthesis of the novel furanoditerpene (+)-taonianone through facially selective chiral olefin–ketene [2+2] cycloaddition. J. Chem. Soc. Perkin Trans. 1997, 13, 1911–1912. [Google Scholar] [CrossRef]
- Narkevitch, V.; Megevand, S.; Schenk, K.; Vogel, P. Development of a New Carbon−Carbon Bond Forming Reaction. New Organic Chemistry of Sulfur Dioxide. Asymmetric Four-Component Synthesis of Polyfunctional Sulfones. J. Org. Chem. 2001, 66, 5080–5093. [Google Scholar] [CrossRef] [PubMed]
- Vogel, P.; Huang, X. One-Pot, Four-Component Synthesis of Polyfunctional Sulfones. Synthesis 2002, 2002, 0232–0236. [Google Scholar] [CrossRef]
- Bouchez, L.C.; Dubbaka, S.R.; Turks, M.; Vogel, P. Sulfur Dioxide Mediated One-Pot, Three- and Four-Component Syntheses of Polyfunctional Sulfonamides and Sulfonic Esters: Study of the Stereoselectivity of the Ene Reaction of Sulfur Dioxide. J. Org. Chem. 2004, 69, 6413–6418. [Google Scholar] [CrossRef] [PubMed]
- Bouchez, L.C.; Turks, M.; Dubbaka, S.R.; Fonquerne, F.; Craita, C.; Laclef, S.; Vogel, P. Sulfur dioxide mediated one-pot, four-component synthesis of polyfunctional sulfones and sulfonamides, including medium-ring cyclic derivatives. Tetrahedron 2005, 61, 11473–11487. [Google Scholar] [CrossRef]
- Vogel, P.; Turks, M.; Bouchez, L.; Craita, C.; Murcia, M.C.; Fonquerne, F.; Didier, C.; Huang, X.; Flowers, C. Use of sultines in the asymmetric synthesis of polypropionate antibiotics. Pure Appl. Chem. 2008, 80, 791–805. [Google Scholar] [CrossRef]
- Turks, M.; Fairweather, K.A.; Scopelliti, R.; Vogel, P. Efficient Asymmetric Synthesis of Long-Chain Polyketides Containing up to Ten Contiguous Stereogenic Centres by Double Chain Elongation. Eur. J. Org. Chem. 2011, 2011, 3317–3328. [Google Scholar] [CrossRef]
- Paterson, I.; Florence, G.J.; Gerlach, K.; Scott, J.; Sereinig, N. Total synthesis of the immunosuppressive agent (-)-discodermolide. J. Am. Chem. Soc. 2001, 123, 9535–9544. [Google Scholar] [CrossRef]
- Paterson, I.; Perkins, M.V. Total Synthesis of (−)-Denticulatins A and B Using Efficient Methods of Acyclic Stereocontrol. Tetrahedron 1996, 52, 1811–1834. [Google Scholar] [CrossRef]
- Stockdale, T.P.; Lam, N.Y.S.; Anketell, M.J.; Paterson, I. The Stereocontrolled Total Synthesis of Polyketide Natural Products: A Thirty-Year Journey. Bull. Chem. Soc. Jpn. 2021, 94, 713–731. [Google Scholar] [CrossRef]
- Evans, D.A.; Chapman, K.T. The directed reduction of β-hydroxy ketones employing Me4NB(OAc)3H. Tetrahedron Lett. 1986, 27, 5939–5942. [Google Scholar] [CrossRef]
- Evans, D.A.; Chapman, K.T.; Carreira, E.M. Directed reduction of beta.-hydroxy ketones employing tetramethylammonium triacetoxyborohydride. J. Am. Chem. Soc. 1988, 110, 3560–3578. [Google Scholar] [CrossRef]
- Manker, D.C.; Faulkner, D.J.; Stout, T.J.; Clardy, J. The baconipyrones. Novel polypropionates from the pulmonate Siphonaria baconi. J. Org. Chem. 1989, 54, 5371–5374. [Google Scholar] [CrossRef]
- Turks, M.; Murcia, M.C.; Scopelliti, R.; Vogel, P. First Asymmetric Synthesis of the Cyclohexanone Subunit of Baconipyrones A and B. Revision of Its Structure. Org. Lett. 2004, 6, 3031–3034. [Google Scholar] [CrossRef]
- Pereyre, M.; Bellegarde, B.; Mendelsohn, J.; Valade, J. Action of triorganotin alkaloids on enol esters—Problem of obtaining C-or O-stannylated compounds. J. Organomet. Chem. 1968, 11, 97–110. [Google Scholar] [CrossRef]
- Labadie, S.S.; Stille, J.K. Stereoselective aldol condensations of organotin reagents with aldehydes. Tetrahedron 1984, 40, 2329–2336. [Google Scholar] [CrossRef]
- Ciavatta, M.L.; Gavagnin, M.; Puliti, R.; Cimino, G.; Martinez, E.; Ortea, J.; Mattia, C.A. Dolabriferol: A new polypropionate from the skin of the anaspidean mollusc Dolabrifera dolabrifera. Tetrahedron 1996, 52, 12831–12838. [Google Scholar] [CrossRef]
- Paterson, I.; Chen, D.Y.K.; Acena, J.L.; Franklin, A.S. Studies in marine polypropionate synthesis: Total synthesis of (−)-baconipyrone C. Org. Lett. 2000, 2, 1513–1516. [Google Scholar] [CrossRef]
- Laclef, S.; Turks, M.; Vogel, P. Total Synthesis and Determination of the Absolute Configuration of (−)-Dolabriferol. Angew. Chem. Int. Ed. 2010, 49, 8525–8527. [Google Scholar] [CrossRef]
- Currie, R.H.; Goodman, J.M. In Silico Inspired Total Synthesis of (−)-Dolabriferol. Angew. Chem. Int. Ed. 2012, 51, 4695–4697. [Google Scholar] [CrossRef]
- Karagiannis, A.; Diddi, N.; Ward, D.E. On the Origin of Dolabriferol: Total Synthesis via Its Putative Contiguous Precursor. Org. Lett. 2016, 18, 3794–3797. [Google Scholar] [CrossRef] [PubMed]
- Gantasala, N.; Borra, S.; Pabbaraja, S.; Srihari, P. Stereoselective Total Synthesis of the Non-Contiguous Polyketide Natural Product (-)-Dolabriferol. Eur. J. Org. Chem. 2018, 2018, 1230–1240. [Google Scholar] [CrossRef]
- Bandaru, A.; Si, D.; Kaliappan, K.P. Synthesis of C1-C9 and C10-C21 fragments of (-)-dolabriferol. Asian J. Org. Chem. 2020, 9, 1045–1052. [Google Scholar] [CrossRef]
- Rinehart, K.L. Antibiotics with ansa rings. Acc. Chem. Res. 1972, 5, 57–64. [Google Scholar] [CrossRef]
- Rinehart, K.L.; Shield, S. Chemistry of the ansamycin antibiotics. Fortsch. Chem. Org. Naturst. 1976, 33, 231–307. [Google Scholar]
- Wehrli, W. Ansamycins Chemistry, biosynthesis and biological activity. Top. Curr. Chem. 1977, 72, 21–49. [Google Scholar]
- Prelog, V. Conformation and reactivity of medium-sized ring compounds. Pure Appl. Chem. 1963, 6, 545–560. [Google Scholar] [CrossRef][Green Version]
- Sensi, P.; Furesz, S.; Maggi, G.; Maffi, G. Chemical modifications and biological properties of rifamycins. Antimicrob. Agents Chemother. 1966, 6, 699–714. [Google Scholar]
- Arora, S.K. Correlation of structure and activity in ansamycins—Structure, conformation, and interactions of antibiotic rifamycin-S. J. Med. Chem. 1985, 28, 1099–1102. [Google Scholar] [CrossRef]
- Joss, U.R.; Hughes, A.M.; Calvin, M. Effect of dimethylbenzyldesmethyl-rifamycin (Dmb) on chemically induced mammary-tumors in rats. Nat.-New Biol. 1973, 242, 88–90. [Google Scholar] [CrossRef]
- Spisani, S.; Traniello, S.; Martuccio, C.; Rizzuti, O.; Cellai, L. Rifamycins inhibit human neutrophil functions: New derivatives with potential antiinflammatory activity. Inflammation 1997, 21, 391–400. [Google Scholar] [CrossRef]
- Hartmann, G.; Honikel, K.O.; Knüsel, F.; Nüesch, J. The specific inhibition of the DNA-directed RNA synthesis by rifamycin. Biochim. Biophys. Acta Nucleic Acids Protein Synth. 1967, 145, 843–844. [Google Scholar] [CrossRef]
- Bacchi, A.; Pelizzi, G.; Nebuloni, M.; Ferrari, P. Comprehensive Study on Structure−Activity Relationships of Rifamycins: Discussion of Molecular and Crystal Structure and Spectroscopic and Thermochemical Properties of Rifamycin O. J. Med. Chem. 1998, 41, 2319–2332. [Google Scholar] [CrossRef]
- Floss, H.G.; Yu, T.-W. Rifamycin-mode of action, resistance, and biosynthesis. Chem. Rev. 2005, 105, 621–632. [Google Scholar] [CrossRef]
- O’Brien, R.J.; Lyle, M.A.; Snider, J.D.E. Rifabutin (Ansamycin LM 427): A New Rifamycin-S Derivative for the Treatment of Mycobacterial Diseases. Clin. Infect. Dis. 1987, 9, 519–530. [Google Scholar] [CrossRef]
- Brogden, R.N.; Fitton, A. Rifabutin—A review of its antimicrobial activity, pharmacokinetic properties and therapeutic efficacy. Drugs 1994, 47, 983–1009. [Google Scholar] [CrossRef]
- Barluenga, J.; Aznar, F.; García, A.B.; Cabal, M.P.; Palacios, J.J.; Menéndez, M.A. New rifabutin analogs: Synthesis and biological activity against mycobacterium tuberculosis. Bioorg. Med. Chem. Lett. 2006, 16, 5717–5722. [Google Scholar] [CrossRef]
- Selva, E.; Lancini, G. Rifamycins, Antibacterial Antibiotics and Their New Applications; Fischer, J., Ganellin, C.R., Eds.; Analogue-Based Drug Discovery II; Wiley-VCH Verlag GmbH & CO. KGaA: Weinheim, Germany, 2010; pp. 173–187. [Google Scholar]
- Nagaoka, H.; Rutsch, W.; Schmid, G.; Iio, H.; Johnson, M.R.; Kishi, Y. Total synthesis of rifamycins. 1. Stereocontrolled synthesis of the aliphatic building block. J. Am. Chem. Soc. 1980, 102, 7962–7965. [Google Scholar] [CrossRef]
- Iio, H.; Nagaoka, H.; Kishi, Y. Total synthesis of rifamycins. 2. Total synthesis of racemic rifamycin-S. J. Am. Chem. Soc. 1980, 102, 7965–7967. [Google Scholar] [CrossRef]
- Kishi, Y. Total synthesis of rifamycin S. Pure Appl. Chem. 1981, 53, 1163–1180. [Google Scholar] [CrossRef]
- Nagaoka, H.; Kishi, Y. Further synthetic studies on rifamycin s. Tetrahedron 1981, 37, 3873–3888. [Google Scholar] [CrossRef]
- Hanessian, S.; Pougny, J.R.; Boessenkool, I.K. Total synthesis of the C19-C29 aliphatic segment of (+)-rifamycin-S. J. Am. Chem. Soc. 1982, 104, 6164–6166. [Google Scholar] [CrossRef]
- Katsuki, T.; Hanamoto, T.; Yamaguchi, M. Synthesis of C19–C27Fragment of Ansa Chain Part of Rifamycin S. Chem. Lett. 1989, 18, 117–118. [Google Scholar] [CrossRef]
- Paterson, I.; McClure, C.K.; Schumann, R.C. A short asymmetric synthesis of a C19–C27 segment of rifamycin S. Kinetic resolution in the aldol reactions of ethylketones using chiral boron reagents. Tetrahedron Lett. 1989, 30, 1293–1296. [Google Scholar] [CrossRef]
- Chênevert, R.; Rose, Y.S. Enzymatic Desymmetrization of a Meso Polyol Corresponding to the C(19)−C(27) Segment of Rifamycin S. J. Org. Chem. 2000, 65, 1707–1709. [Google Scholar] [CrossRef]
- Corey, E.J.; Hase, T. Studies on the total synthesis of rifamycin—Highly stereoselective synthesis of intermediates for construction of the C(15) to C(29) chain. Tetrahedron Lett. 1979, 4, 335–338. [Google Scholar] [CrossRef]
- Masamune, S.; Imperiali, B.; Garvey, D.S. Synthesis of ansamycins: The ansa chain of rifamycin S. J. Am. Chem. Soc. 1982, 104, 5528–5531. [Google Scholar] [CrossRef]
- Paterson, I.; Mansuri, M.M. Recent developments in the total synthesis of macrolide antibiotics. Chem. Informationsdienst 1985, 41, 3569–3624. [Google Scholar] [CrossRef]
- Rao, A.V.R.; Yadav, J.S.; Vidyasagar, V. Stereoselective synthesis of the C-21 to C-27 segment of rifamycin-S. J. Chem. Soc. Chem. Commun. 1985, 55–56. [Google Scholar] [CrossRef]
- Rao, A.V.R.; Yadav, J.S.; Vidyasagar, V. Stereoselective synthesis of the C-21 to C-27 segment of rifamycin-S. Tetrahedron Lett. 1986, 27, 3297–3298. [Google Scholar] [CrossRef]
- Roush, W.R.; Palkowitz, A.D. Applications of tartrate ester modified allylic boronates in organic synthesis—An efficient, highly stereoselective synthesis of the C(19)-C(29) segment of rifamycin-S. J. Am. Chem. Soc. 1987, 109, 953–955. [Google Scholar] [CrossRef]
- Ziegler, F.E.; Kneisley, A. 3-Methyl-gamma-butyrolactone as a source of 2-methyl-3-hydroxyketones and 2-methyl-1,3-diols—A synthesis of the C19-C27 fragment of rifamycin-S by linear iteration. Tetrahedron Lett. 1987, 28, 1725–1728. [Google Scholar] [CrossRef]
- Ziegler, F.E.; Cain, W.T.; Kneisley, A.; Stirchak, E.P.; Wester, R.T. Applications of the 3-methyl-gamma-butyrolactone strategy to the synthesis of polypropionates—the Prelog-Djerassi lactonic ester, ent-invictolide, and the C19-C27 fragment of rifamycin S. J. Am. Chem. Soc. 1988, 110, 5442–5452. [Google Scholar] [CrossRef]
- Tarara, G.; Hoppe, D. Total synthesis of protected D-altro-3,6-dideoxy-3-C-methylhexose and D-galacto-3,6-dideoxy-3-C-methylhexose—Key intermediates of a rifamycin S synthesis. Synthesis 1989, 2, 89–92. [Google Scholar] [CrossRef]
- Born, M.; Tamm, C. Stereoselective Synthesis of the C(19)-to-C(27) Segment of Rifamycin S. Helvetica Chim. Acta 1990, 73, 2242–2250. [Google Scholar] [CrossRef]
- Roush, W.R.; Palkowitz, A.D.; Ando, K. Acyclic diastereoselective synthesis using tartrate ester-modified crotylboronates. Double asymmetric reactions with alpha.-methyl chiral aldehydes and synthesis of the C(19)-C(29) segment of rifamycin S. J. Am. Chem. Soc. 1990, 112, 6348–6359. [Google Scholar] [CrossRef]
- Harada, T.; Kagamihara, Y.; Tanaka, S.; Sakamoto, K.; Oku, A. A highly convergent asymmetric synthesis of the C(19)-C(27) segment of rifamycin S: An application of enantiodifferentiating acetalization with menthone. J. Org. Chem. 1992, 57, 1637–1639. [Google Scholar] [CrossRef]
- Lautens, M.; Belter, R.K. The effect of remote oxygens on the ring-opening reactions of oxabicyclic compounds with organolithium reagents. Synthesis of the C21–C27 segment of rifamycin S. Tetrahedron Lett. 1992, 33, 2617–2620. [Google Scholar] [CrossRef]
- Miyashita, M.; Yoshihara, K.; Kawamine, K.; Hoshino, M.; Irie, H. Synthetic studies on polypropionate antibiotics based on the stereospecific methylation of gamma,delta-epoxy acrylates by trimethylaluminum—A highly stereoselective construction of the 8 contiguous chiral centers of ansa-chains of rifamycins. Tetrahedron Lett. 1993, 34, 6285–6288. [Google Scholar] [CrossRef]
- Harada, T.; Oku, A. Enantiodifferentiating transformation of prochiral polyols by using menthone as chiral template. Synlett 1994, 2, 95–104. [Google Scholar] [CrossRef]
- Yadav, J.S.; Rao, C.S.; Chandrasekhar, S.; Rao, A.V.R. Asymmetric synthesis of C-19 to C-27 fragment of rifamycin-S. Tetrahedron Lett. 1995, 36, 7717–7720. [Google Scholar] [CrossRef]
- Hanessian, S.; Wang, W.; Gai, Y.; Olivier, E. A General and Stereocontrolled Strategy for the Iterative Assembly of Enantiopure Polypropionate Subunits: Synthesis of the C19−C28 Segment of Rifamycin S from a Single Chiron. J. Am. Chem. Soc. 1997, 119, 10034–10041. [Google Scholar] [CrossRef]
- Marshall, J.A.; Palovich, M.R. Synthesis of Stereopentad Subunits of Zincophorin and Rifamycin-S through Use of Chiral Allenyltin Reagents. J. Org. Chem. 1998, 63, 3701–3705. [Google Scholar] [CrossRef]
- Turks, M.; Huang, X.; Vogel, P. Expeditious Asymmetric Synthesis of a Stereoheptad Corresponding to the C(19)-C(27)-Ansa Chain of Rifamycins: Formal Total Synthesis of Rifamycin S. Chem. A Eur. J. 2004, 11, 465–476. [Google Scholar] [CrossRef]
- Kim, J.W.; Adachi, H.; Shin, Y.K.; Hayakawa, Y.; Seto, H. Apoptolidin, a new apoptosis inducer in transformed cells from Nocardiopsis sp. J. Antibiot. 1997, 50, 628–630. [Google Scholar] [CrossRef][Green Version]
- Hayakawa, Y.; Kim, J.W.; Adachi, H.; Shin-Ya, K.; Fujita, A.K.-I.; Seto, H. Structure of Apoptolidin, a Specific Apoptosis Inducer in Transformed Cells. J. Am. Chem. Soc. 1998, 120, 3524–3525. [Google Scholar] [CrossRef]
- Wender, P.A.; Sukopp, M.; Longcore, K. Apoptolidins B and C: Isolation, Structure Determination, and Biological Activity. Org. Lett. 2005, 7, 3025–3028. [Google Scholar] [CrossRef]
- Wender, P.A.; Longcore, K.E. Isolation, Structure Determination, and Anti-Cancer Activity of Apoptolidin D. Org. Lett. 2007, 9, 691–694. [Google Scholar] [CrossRef]
- Wender, P.A.; Longcore, K.E. Apoptolidins E and F, New Glycosylated Macrolactones Isolated from Nocardiopsis sp. Org. Lett. 2009, 11, 5474–5477. [Google Scholar] [CrossRef]
- Salomon, A.R.; Voehringer, D.W.; Herzenberg, L.A.; Khosla, C. Understanding and exploiting the mechanistic basis for selectivity of polyketide inhibitors of F0F1-ATPase. Proc. Natl. Acad. Sci. USA 2000, 97, 14766–14771. [Google Scholar] [CrossRef]
- Benitez-Bribiesca, L. Assessment of apoptosis in tumor growth: Importance in clinical oncology and cancer therapy. In When Cells Die; Lockshin, R.A., Zakeri, Z., Tilly, J.L., Eds.; Wiley-Liss: New York, NK, USA, 1998; pp. 453–492. [Google Scholar]
- Salomon, A.R.; Vöhringer, D.W.; Herzenberg, L.A.; Khosla, C. Apoptolidin, a selective cytotoxic agent, is an inhibitor of F0F1-ATPase. Chem. Biol. 2001, 8, 71–80. [Google Scholar] [CrossRef]
- Salomon, A.R.; Zhang, Y.; Seto, H.; Khosla, C. Structure−Activity Relationships within a Family of Selectively Cytotoxic Macrolide Natural Products. Org. Lett. 2000, 3, 57–59. [Google Scholar] [CrossRef]
- Nicolaou, K.C.; Li, Y.; Fylaktakidou, K.C.; Mitchell, H.J.; Sugita, K. Total Synthesis of Apoptolidin: Part 2. Coupling of Key Building Blocks and Completion of the Synthesis. Angew. Chem. Int. Ed. 2001, 40, 3854–3857. [Google Scholar] [CrossRef]
- Nicolaou, K.C.; Fylaktakidou, K.C.; Monenschein, H.; Li, Y.; Weyershausen, B.; Mitchell, H.J.; Wei, H.-X.; Guntupalli, P.; Hepworth, D.; Sugita, K. Total Synthesis of Apoptolidin: Construction of Enantiomerically Pure Fragments. J. Am. Chem. Soc. 2003, 125, 15433–15442. [Google Scholar] [CrossRef]
- Wehlan, H.; Dauber, M.; Fernaud, M.-T.M.; Schuppan, J.; Mahrwald, R.; Ziemer, B.; Garcia, M.-E.J.; Koert, U. Total Synthesis of Apoptolidin. Angew. Chem. Int. Ed. 2004, 43, 4597–4601. [Google Scholar] [CrossRef]
- Wehlan, H.; Dauber, M.; Fernaud, M.T.M.; Schuppan, J.; Keiper, S.; Mahrwald, R.; Garcia, M.-E.J.; Koert, U. Apoptolidin A: Total Synthesis and Partially Glycosylated Analogues. Chem.—A Eur. J. 2006, 12, 7378–7397. [Google Scholar] [CrossRef]
- Wu, B.; Liu, Q.; Sulikowski, G.A. Total Synthesis of Apoptolidinone. Angew. Chem. Int. Ed. 2004, 43, 6673–6675. [Google Scholar] [CrossRef]
- Ghidu, V.P.; Wang, J.Q.; Wu, B.; Liu, Q.; Jacobs, A.; Marnett, L.J.; Sulikowski, G.A. Synthesis and Evaluation of the Cytotoxicity of Apoptolidinones A and D. J. Org. Chem. 2008, 73, 4949–4955. [Google Scholar] [CrossRef]
- Crimmins, M.T.; Christie, H.S.; Chaudhary, A.K.; Long, A. Enantioselective Synthesis of Apoptolidinone: Exploiting the Versatility of Thiazolidinethione Chiral Auxiliaries. J. Am. Chem. Soc. 2005, 127, 13810–13812. [Google Scholar] [CrossRef]
- Chau, S.T.; Sulikowski, G.A.; Wu, B. Studies on the synthesis of the apoptolidins. In Strategies and Tactics in Organic Synthesis; Harmata, M., Ed.; Elsevier Science Publ. Co. Inc.: San Diego, CA, USA, 2012; Volume 8, pp. 375–394. [Google Scholar]
- Craita, C.; Didier, C.; Vogel, P. Short synthesis of the C16–C28polyketide fragment of apoptolidin A aglycone. Chem. Commun. 2007, 23, 2411–2413. [Google Scholar] [CrossRef] [PubMed]
- Rubottom, G.M.; Vazquez, M.A.; Pelegrina, D.R. Peracid oxidation of trimethylsilyl enol ethers: A facile α-hydroxylation procedure. Tetrahedron Lett. 1974, 15, 4319–4322. [Google Scholar] [CrossRef]
- Bonini, C.; Chiummiento, L.; Pullez, M.; Solladie, G.; Colobert, F. Convergent Highly Stereoselective Preparation of the C12−C24 Fragment of Macrolactin A. J. Org. Chem. 2004, 69, 5015–5022. [Google Scholar] [CrossRef] [PubMed]
- Hartung, I.V.; Niess, B.; Haustedt, L.O.; Hoffmann, H.M.R. Toward the Total Synthesis of Disorazole A1 and C1: Asymmetric Synthesis of a Masked Southern Segment. Org. Lett. 2002, 4, 3239–3242. [Google Scholar] [CrossRef]
- Brown, H.C.; Bhat, K.S.; Randad, R.S. Chiral synthesis via organoboranes. 21. Allylboration and crotylboration of alpha-chiral aldehydes with diisopinocampheylboron as the chiral auxiliary. J. Org. Chem. 1989, 54, 1570–1576. [Google Scholar] [CrossRef]
- Kolb, H.C.; VanNieuwenhze, M.; Sharpless, K.B. Catalytic Asymmetric Dihydroxylation. Chem. Rev. 1994, 94, 2483–2547. [Google Scholar] [CrossRef]
- Bouchez, L.C.; Vogel, P. Synthesis of the C(1)-C(11) Polyene Fragment of Apoptolidin with a New Sulfur Dioxide-Based Organic Chemistry. Chem. A Eur. J. 2005, 11, 4609–4620. [Google Scholar] [CrossRef]
- Ramberg, L.; Bäcklung, B. The reactions of some monohalogen derivatives of diethyl sulfone. Ark. Kemi. Mineral. Geol. 1940, 13A, 1–50. [Google Scholar]
- Chan, T.L.; Fong, S.; Li, Y.; Man, T.O.; Poon, C.D. A new one-flask Ramberg-Bäcklund reaction. J. Chem. Soc. 1994, 15, 1771–1772. [Google Scholar] [CrossRef]
- Cao, X.P. Stereoselective synthesis of substituted all-trans-1,3,5,7-octatetraenes by a modified Ramberg-Bäcklund reaction. Tetrahedron 2002, 58, 1301–1307. [Google Scholar] [CrossRef]
- Narasaka, K.; Pai, H.C. ChemInform Abstract: STEREOSELECTIVE SYNTHESIS OF MESO (OR ERYTHRO) 1,3-DIOLS FROM β-HYDROXYKETONES. Chem. Inf. 1981, 12. [Google Scholar] [CrossRef]
- Exner, C.J.; Laclef, S.; Poli, F.; Turks, M.; Vogel, P. Total asymmetric syntheses of β-hydroxy-δ-lactones via Umpolung with sulfur dioxide. J. Org. Chem. 2011, 76, 840–845. [Google Scholar] [CrossRef]
- Exner, C.J.; Turks, M.; Fonquerne, F.; Vogel, P. Concise synthesis of complicated polypropionates through one-pot disymmetrical two-directional chain elongation. Chem. Eur. J. 2011, 17, 4246–4253. [Google Scholar] [CrossRef] [PubMed]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vogel, P.; Sordo Gonzalo, J.A. Expeditious Asymmetric Synthesis of Polypropionates Relying on Sulfur Dioxide-Induced C–C Bond Forming Reactions. Catalysts 2021, 11, 1267. https://doi.org/10.3390/catal11111267
Vogel P, Sordo Gonzalo JA. Expeditious Asymmetric Synthesis of Polypropionates Relying on Sulfur Dioxide-Induced C–C Bond Forming Reactions. Catalysts. 2021; 11(11):1267. https://doi.org/10.3390/catal11111267
Chicago/Turabian StyleVogel, Pierre, and José Angel Sordo Gonzalo. 2021. "Expeditious Asymmetric Synthesis of Polypropionates Relying on Sulfur Dioxide-Induced C–C Bond Forming Reactions" Catalysts 11, no. 11: 1267. https://doi.org/10.3390/catal11111267
APA StyleVogel, P., & Sordo Gonzalo, J. A. (2021). Expeditious Asymmetric Synthesis of Polypropionates Relying on Sulfur Dioxide-Induced C–C Bond Forming Reactions. Catalysts, 11(11), 1267. https://doi.org/10.3390/catal11111267





