Constructing 1D Boron Chains in the Structure of Transition Metal Monoborides for Hydrogen Evolution Reactions
Abstract
:1. Introduction
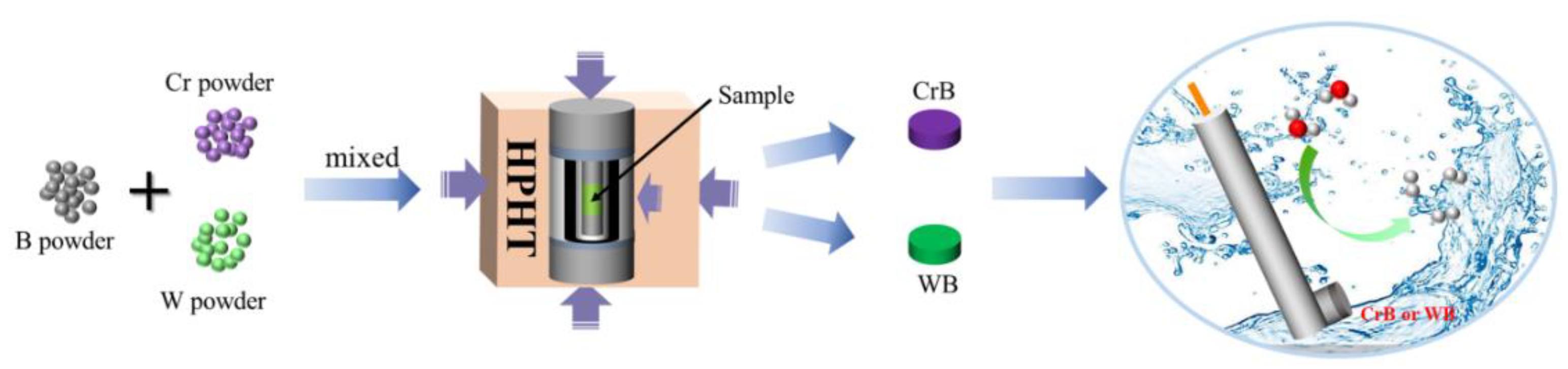
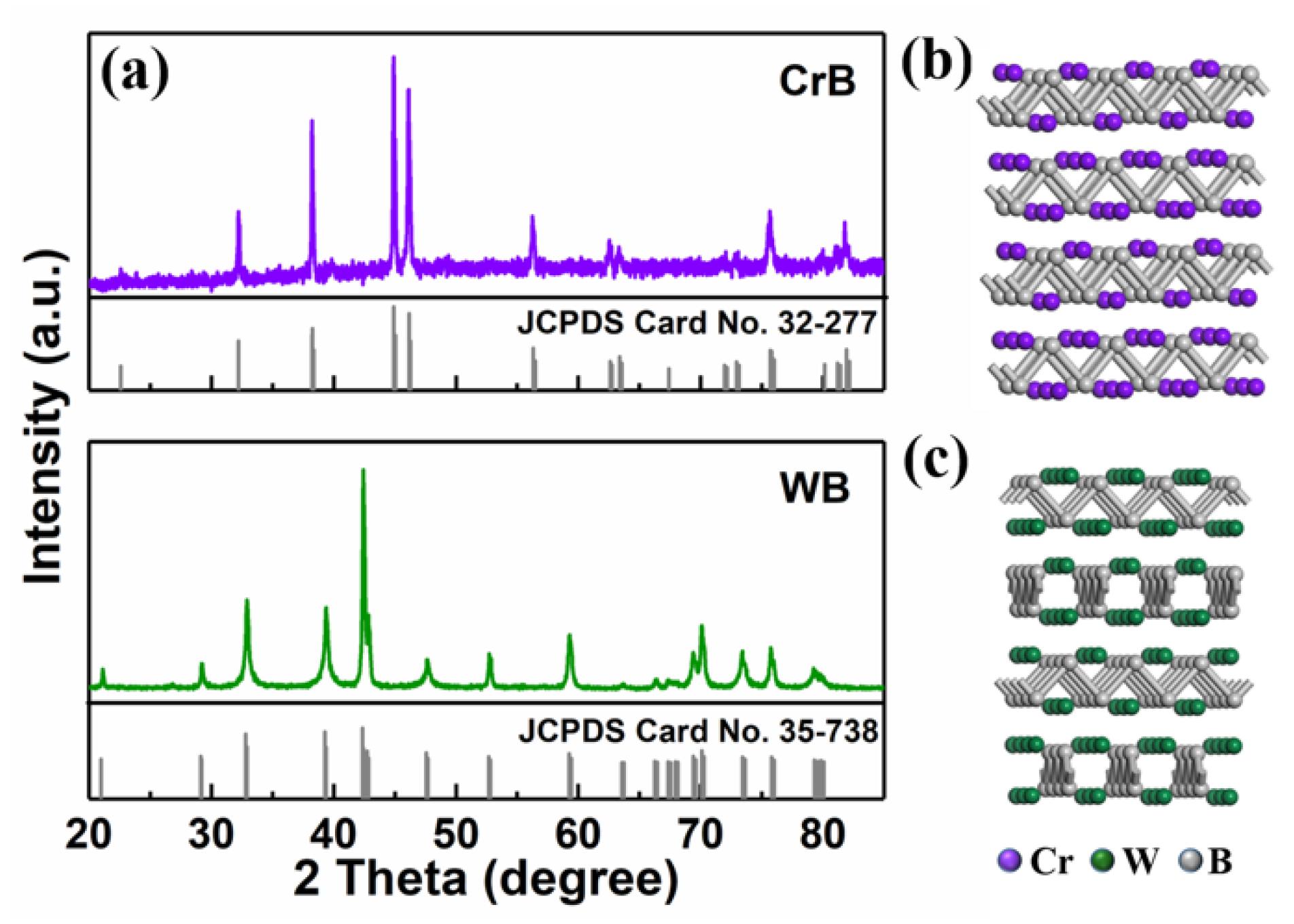
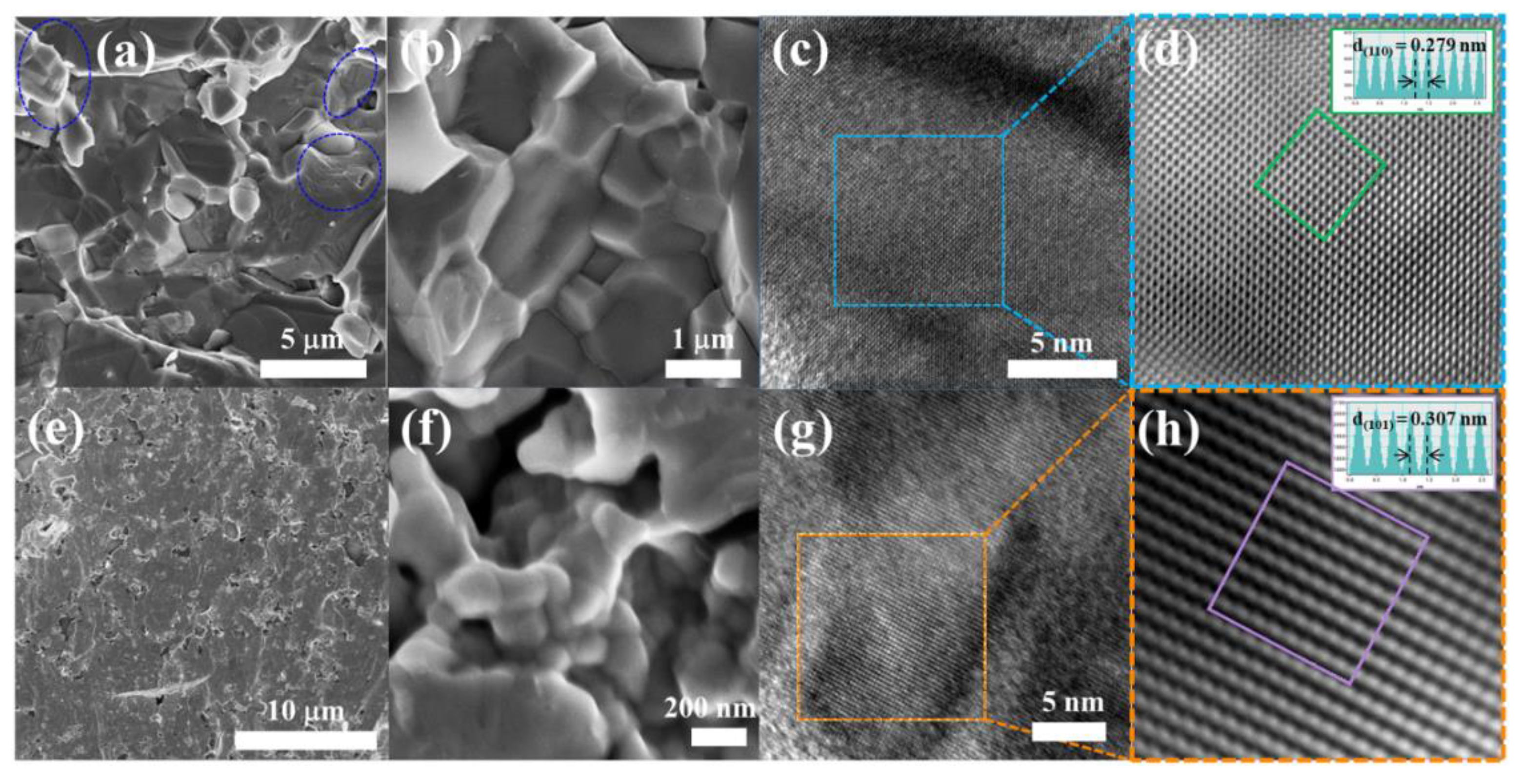
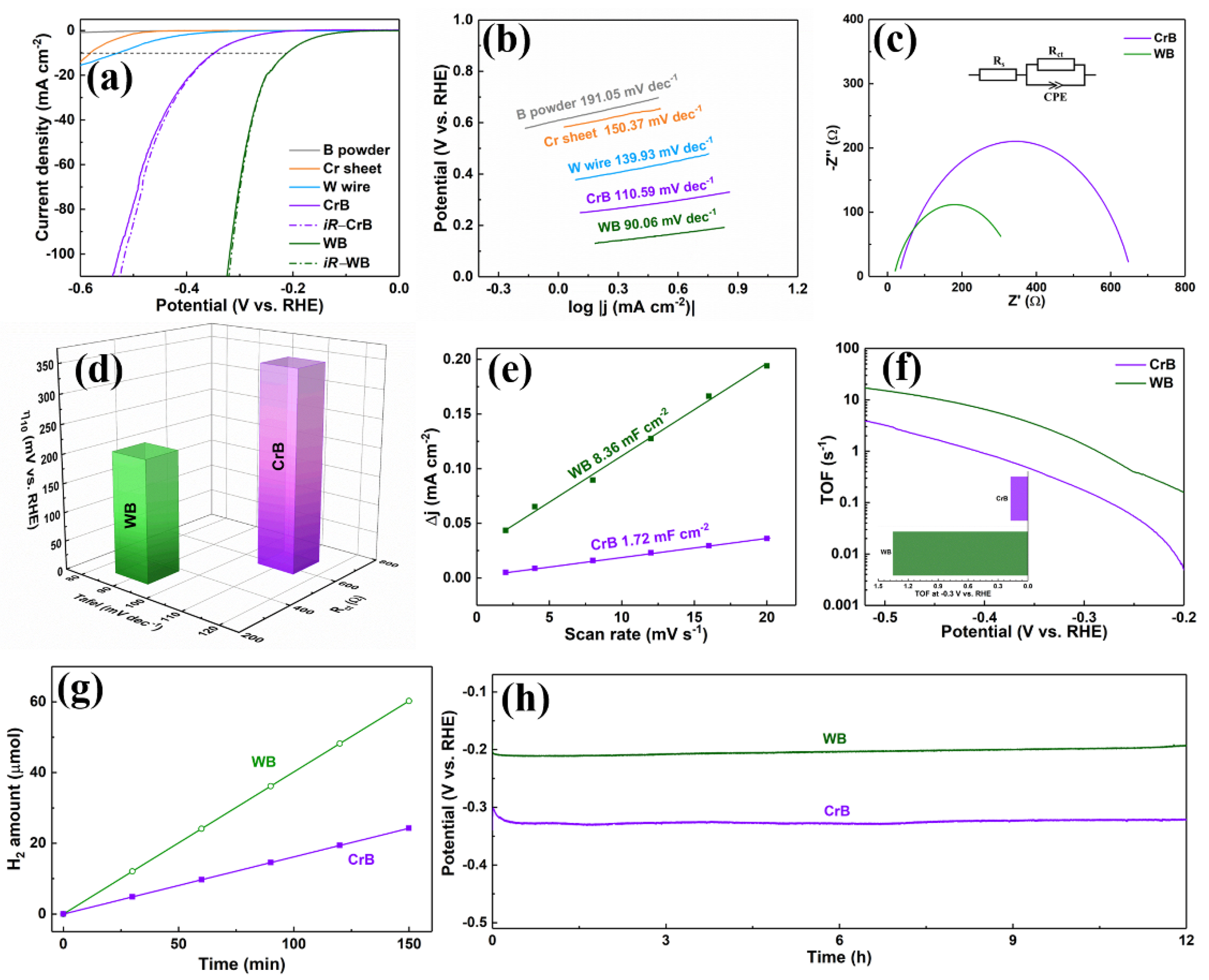
2. Results and Discussion
3. Materials and Methods
3.1. Materials
3.2. Materials Synthesis
3.3. Characterization
3.4. Preparation of Working Electrode
3.5. Electrochemical Measurements
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Schlapbach, L.; Züttel, A. Hydrogen-storage materials for mobile applications. Nature 2001, 414, 353–358. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.C.; Carlsson, J.M.; Domen, K.; Maeda, K.; Thomas, A.; Takanabe, K.; Xin, G.; Antonietti, M. A metal-free polymeric photocatalyst for hydrogen production from water under visible light. Nat. Mater. 2009, 8, 271–275. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Li, F.; Ma, L.P.; Cheng, H.M. Advanced materials for energy storage. Adv. Mater. 2010, 22, E28–E62. [Google Scholar] [CrossRef]
- Kanan, M.W.; Nocera, D.G. In Situ Formation of an Oxygen-Evolving Catalyst in Neutral Water Containing Phosphate and Co2+. Science 2008, 321, 1072–1075. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khaselev, O.; Turner, J.A. A Monolithic Photovoltaic-Photoelectrochemical Device for Hydrogen Production via Water Splitting. Science 1998, 280, 425–427. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.M.; Zhang, B. Recent advances in transition metal phosphide nanomaterials: Synthesis and applications in hydrogen evolution reaction. Chem. Soc. Rev. 2016, 45, 1529–1541. [Google Scholar] [CrossRef]
- Sun, H.M.; Yan, Z.H.; Liu, F.M.; Xu, W.C.; Cheng, F.Y.; Chen, J. Self-Supported Transition-Metal-Based Electrocatalysts for Hydrogen and Oxygen Evolution. Adv. Mater. 2020, 32, 1806326. [Google Scholar] [CrossRef]
- Zhang, Y.Q.; Ouyang, B.; Xu, J.; Jia, G.C.; Chen, S.; Rawat, R.S.; Fan, H.J. Rapid Synthesis of Cobalt Nitride Nanowires: Highly Efficient and Low-Cost Catalysts for Oxygen Evolution. Angew. Chem. Int. Edit. 2016, 55, 8670–8674. [Google Scholar] [CrossRef]
- Vrubel, H.; Hu, X.L. Molybdenum boride and carbide catalyze hydrogen evolution in both acidic and basic solutions. Angew. Chem. Int. Edit. 2012, 51, 12703–12706. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.F.; Tai, G.A.; Wu, Z.H.; Hu, T.S.; Wang, R. Ultrathin molybdenum boride films for highly efficient catalysis of the hydrogen evolution reaction. J. Mater. Chem. A 2017, 5, 23471–23475. [Google Scholar] [CrossRef]
- Lu, W.B.; Liu, T.T.; Xie, L.S.; Tang, C.; Liu, D.N.; Hao, S.; Qu, F.L.; Du, G.; Ma, Y.J.; Asiri, A.M.; et al. In Situ Derived CoB Nanoarray: A High-Efficiency and Durable 3D Bifunctional Electrocatalyst for Overall Alkaline Water Splitting. Small 2017, 13, 1700805. [Google Scholar] [CrossRef]
- Zhang, P.L.; Wang, M.; Yang, Y.; Yao, T.Y.; Han, H.X.; Sun, L.C. Electroless plated Ni–B films as highly active electrocatalysts for hydrogen production from water over a wide pH range. Nano Energy 2016, 19, 98–107. [Google Scholar] [CrossRef]
- Li, H.; Wen, P.; Li, Q.; Dun, C.C.; Xing, J.H.; Lu, C.; Adhikari, S.; Jiang, L.; Carroll, D.L.; Geyer, S.M. Earth-Abundant Iron Diboride (FeB2) Nanoparticles as Highly Active Bifunctional Electrocatalysts for Overall Water Splitting. Adv. Energ. Mater. 2017, 7, 1700513. [Google Scholar] [CrossRef]
- Zhang, T.; Song, F.Z.; Qian, Y.Q.; Gao, H.; Shaw, J.; Rao, Y. Elemental Engineering of High-Charge-Density Boron in Nickel as Multifunctional Electrocatalysts for Hydrogen Oxidation and Water Splitting. ACS Appl. Energy Mater. 2021, 4, 5434–5442. [Google Scholar] [CrossRef]
- Ma, X.Y.; Zhang, S.H.; He, Y.; He, T.; Li, H.J.; Zhang, Y.H.; Chen, J.Y. Boron and phosphorus co-doped NiVFe LDHs@NF as a highly efficient self-supporting electrocatalyst for the hydrogen evolution reaction. J. Electroanal. Chem. 2021, 886, 115107. [Google Scholar] [CrossRef]
- Gu, W.S.; Pan, Z.Q.; Tao, H.; Guo, Y.L.; Pu, J.; Zhong, C.L.; Li, J.C.; Ye, C.Q.; Zhou, Q.W. Boron-modulated surface of hollow nickel framework for improved hydrogen evolution. Chem. Commun. 2021, 57, 2404–2407. [Google Scholar] [CrossRef] [PubMed]
- Guan, J.T.; Zhang, X.R.; Li, Q.Y.; Deng, K.M.; Jena, P.; Kan, E.J. Two-dimensional metal-free boron chalcogenides B2X3 (X = Se and Te) as photocatalysts for water splitting under visible light. Nanoscale 2021, 13, 3627–3632. [Google Scholar] [CrossRef] [PubMed]
- Han, C.; Li, W.J.; Wang, J.Z.; Huang, Z.G. Boron leaching: Creating vacancy-rich Ni for enhanced hydrogen evolution. Nano Res. 2021, 14, 1–9. [Google Scholar] [CrossRef]
- Guo, X.W.; Chen, Z.C.; Huang, Y.P.; Lv, H.; Wang, Y.R.; Sun, L.Z.; Song, K.; Liu, B. Mesoporous Palladium-Boron-Sulfur Alloy Nanospheres for Efficient Hydrogen Evolution. Inorg. Chem. 2021, 60, 4380–4384. [Google Scholar] [CrossRef]
- Cheng, Z.F.; Pi, Y.C.; Shao, Q.; Huang, X.Q. Boron-doped amorphous iridium oxide with ultrahigh mass activity for acidic oxygen evolution reaction. Sci. China Mater. 2021, 64, 1–6. [Google Scholar] [CrossRef]
- Chen, K.; Wang, Z.M.; Wang, L.; Wu, X.Z.; Hu, B.J.; Liu, Z.; Wu, M.H. Boron Nanosheet-Supported Rh Catalysts for Hydrogen Evolution: A New Territory for the Strong Metal-Support Interaction Effect. Nano-Micro Lett. 2021, 13, 138. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.F.; Ali, R.; Ma, J.; Jiao, W.; Yin, L.J.; Mu, C.H.; Jian, X. Graphene-Decorated Boron−Carbon−Nitride-Based Metal-Free Catalysts for an Enhanced Hydrogen Evolution Reaction. ACS Appl. Energy Mater. 2021, 4, 3861–3868. [Google Scholar] [CrossRef]
- Bat-Erdene, M.; Batmunkh, M.; Sainbileg, B.; Hayashi, M.; Bati, A.S.R.; Qin, J.D.; Zhao, H.J.; Zhong, Y.L.; Shapter, J.G. Highly Dispersed Ru Nanoparticles on Boron-Doped Ti3C2Tx (MXene) Nanosheets for Synergistic Enhancement of Electrocatalytic Hydrogen Evolution. Small 2021, 17, 2102218. [Google Scholar] [CrossRef]
- Chen, Y.L.; Yu, G.T.; Chen, W.; Liu, Y.P.; Li, G.D.; Zhu, P.W.; Tao, Q.; Li, Q.J.; Liu, J.W.; Shen, X.P.; et al. Highly Active, Nonprecious Electrocatalyst Comprising Borophene Subunits for the Hydrogen Evolution Reaction. J. Am. Chem. Soc. 2017, 139, 12370–12373. [Google Scholar] [CrossRef]
- Tao, Q.; Chen, Y.L.; Lian, M.; Xu, C.H.; Li, L.; Feng, X.K.; Wang, X.; Cui, T.; Zheng, W.T.; Zhu, P.W. Modulating Hardness in Molybdenum Monoborides by Adjusting an Array of Boron Zigzag Chains. Chem. Mater. 2018, 31, 200–206. [Google Scholar] [CrossRef]
- Kiessling, R. The crystal structures of molybdenum and Tungsten borides. Acta Chem. Scand. 1947, 1, 893–916. [Google Scholar] [CrossRef]
- Kayhan, M.; Hildebrandt, E.; Frotscher, M.; Senyshyn, A.; Hofmann, K.; Alff, L.; Albert, B. Neutron diffraction and observation of superconductivity for tungsten borides, WB and W2B4. Solid State Sci. 2021, 14, 1656–1659. [Google Scholar] [CrossRef]
- Henschel, A.; Binnewies, M.; Schmidt, M.; Kçppe, R.; Burkhardt, U.; Grin, Y. Tungsten Borides: On the Reaction of Tungsten with Boron(III) Bromide. Chem. Eur. J. 2018, 24, 10109–10115. [Google Scholar] [CrossRef] [PubMed]
- Kumar, B.; Kumar, S.; Ganguli, A.K. Surface decoration through electrostatic interaction leading to enhanced reactivity: Low temperature synthesis of nanostructured chromium borides (CrB and CrB2). J. Solid State Chem. 2013, 200, 117–122. [Google Scholar]
- Homolová, V.; Čiripová, L.; Kepič, J. Isothermal Section of the B-Cr-Fe System at 873 K. J. Phase Equilib. Diffus. 2019, 40, 79–85. [Google Scholar] [CrossRef]
- Hartman, P.; Perdok, W.G. On the Relations Between Structure and Morphology of Crystals. I. Acta Cryst. 1955, 8, 49–52. [Google Scholar] [CrossRef]
- Hartman, P.; Perdok, W.G. On the Relations Between Structure and Morphology of Crystals. II. Acta Cryst. 1955, 8, 521–524. [Google Scholar] [CrossRef]
- Hartman, P. The Attachment Energy as a Habit Controlling Factor III. Application to Corundum. J. Cryst. Growth 1980, 49, 166–170. [Google Scholar] [CrossRef]
- Park, H.; Encinas, A.; Scheifers, J.P.; Zhang, Y.; Fokwa, B.P.T. Boron-Dependency of Molybdenum Boride Electrocatalysts for the Hydrogen Evolution Reaction. Angew. Chem. Int. Ed. 2017, 56, 5575–5578. [Google Scholar] [CrossRef] [PubMed]
- Vinoth, S.; Das, H.T.; Govindasamy, M.; Wang, S.F.; Alkadhi, N.S.; Ouladsmane, M. Facile solid-state synthesis of layered molybdenum boride-based electrode for efficient electrochemical aqueous asymmetric supercapacitor. J. Alloy. Compd. 2021, 877, 160192. [Google Scholar] [CrossRef]
- Chrzanowska-Giżyńska, J.; Denis, P.; Woźniacka, S.; Kurpaska, Ł. Mechanical properties and thermal stability of tungsten boride films deposited by radio frequency magnetron sputtering. Ceram. Int. 2018, 44, 19603–19611. [Google Scholar] [CrossRef]
- Biesinger, M.C.; Payne, B.P.; Grosvenor, A.P.; Lau, L.W.M.; Gerson, A.R.; Smart, R.S.C. Resolving surface chemical states in XPS analysis of first row transition metals, oxides and hydroxides: Cr, Mn, Fe, Co and Ni. Appl. Surf. Sci. 2011, 257, 2717–2730. [Google Scholar] [CrossRef]
- Korotin, M.A.; Zhidkov, I.S.; Kukharenko, A.I.; Cholakh, S.O.; Kamenetskikh, A.S.; Gavrilov, N.V.; Kurmaev, E.Z. Interfacial reactions in Al2O3/Cr2O3 layers: Electronic structure calculations and X-ray photoelectron spectra. Thin Solid Films 2018, 665, 6–8. [Google Scholar] [CrossRef]
- Moulder, J.F.; Stickle, W.F.; Sobol, P.E.; Bomben, K.D. Handbook of X-ray Photoelectron Spectroscopy. In Perkin-Elmer Corporation Physical Electronics Division; Perkin-Elmer: Eden Prairie, MN, USA, 1992; p. 261. [Google Scholar]
- Luo, J.Y.; Gong, L.; Tan, H.D.; Deng, S.Z.; Xu, N.S.; Zeng, Q.G.; Wang, Y. Study of the catalyst poisoning and reactivation of Pt nanoparticles on the surface of WO3 nanowire in gasochromic coloration. Sensor. Actuat. B Chem. 2012, 171–172, 1117–1124. [Google Scholar] [CrossRef]
- Li, Q.J.; Wang, L.N.; Ai, X.; Chen, H.; Zou, J.Y.; Li, G.D.; Zou, X.X. Multiple crystal phases of intermetallic tungsten borides and phase-dependent electrocatalytic property for hydrogen evolution. Chem. Commun. 2020, 56, 13983–13986. [Google Scholar] [CrossRef]
- Zhang, M.L.; Zhang, Y.Q.; Ye, L.; Guo, B.W.; Gong, Y.Q. Hierarchically constructed Ag nanowires shelled with ultrathin Co-LDH nanosheets for advanced oxygen evolution reaction. Appl. Catal. B Environ. 2021, 298, 120601. [Google Scholar] [CrossRef]
- Xie, X.Q.; Liu, J.P.; Gu, C.N.; Li, J.J.; Zhao, Y.; Liu, C.S. Hierarchical structured CoP nanosheets/carbon nanofibers bifunctional eletrocatalyst for high-efficient overall water splitting. J. Energ. Chem. 2022, 64, 503–510. [Google Scholar] [CrossRef]
- Shamloofard, M.; Shahrokhian, S.; Amini, M.K. Mesoporous nanostructures of NiCo-LDH/ZnCo2O4 as an efficient electrocatalyst for oxygen evolution reaction. J. Colloid Interf. Sci. 2021, 604, 832–843. [Google Scholar] [CrossRef]
- Kim, C.; Kim, S.H.; Lee, S.; Kwon, I.; Kim, S.H.; Kim, S.; Seok, C.; Park, Y.S.; Kim, Y. Boosting overall water splitting by incorporating sulfur into NiFe(oxy) hydroxide. J. Energ. Chem. 2022, 64, 364–371. [Google Scholar] [CrossRef]
- Chen, Y.L.; Rong, J.S.; Tao, Q.; Xing, C.; Lian, M.; Cheng, J.E.; Liu, X.Y.; Cao, J.; Lv, S.Q.; Yang, L.L.; et al. Modifying microscopic structures of MoS2 by high pressure and high temperature used in hydrogen evolution reaction. Electrochim. Acta 2020, 357, 136868. [Google Scholar] [CrossRef]
- Wu, Z.P.; Lu, X.F.; Zang, S.Q.; Lou, X.W. Non-Noble-Metal-Based Electrocatalysts toward the Oxygen Evolution Reaction. Adv. Funct. Mater. 2020, 30, 1910274. [Google Scholar] [CrossRef]
- Wu, L.L.; Chen, X.H.; Zhang, Q.; Luo, J.; Fu, H.C.; Shen, L.; Luo, H.Q.; Li, N.B. Formation of hierarchical NiFe Prussian blue analogues/Prussian blue on nickel foam for superior water oxidation. Appl. Surf. Sci. 2021, 567, 150835. [Google Scholar] [CrossRef]
- Zhang, M.L.; Wang, J.L.; Zhang, Y.P.; Ye, L.; Gong, Y.Q. Ultrafine CoRu alloy nanoparticles in situ embedded in Co4N porous nanosheets as high-efficient hydrogen evolution electrocatalysts. Dalton Trans. 2021, 50, 2973–2980. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.T.; Guo, X.S.; Zhang, J.Y.; Xiao, W.; Xi, P.X.; Peng, S.L.; Gao, D.Q. Electronic structure modulation of NiS2 by transition metal doping for accelerating the hydrogen evolution reaction. J. Mater. Chem. A 2019, 7, 4971–4976. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.; Ye, Y.; Tao, Q.; Yang, L.; Cheng, J.; Liu, X.; Cao, J.; Fan, H.; Wei, M.; Zhu, P.; et al. Constructing 1D Boron Chains in the Structure of Transition Metal Monoborides for Hydrogen Evolution Reactions. Catalysts 2021, 11, 1265. https://doi.org/10.3390/catal11111265
Chen Y, Ye Y, Tao Q, Yang L, Cheng J, Liu X, Cao J, Fan H, Wei M, Zhu P, et al. Constructing 1D Boron Chains in the Structure of Transition Metal Monoborides for Hydrogen Evolution Reactions. Catalysts. 2021; 11(11):1265. https://doi.org/10.3390/catal11111265
Chicago/Turabian StyleChen, Yanli, Yanping Ye, Qiang Tao, Lihua Yang, Jiaen Cheng, Xiaoyan Liu, Jian Cao, Hougang Fan, Maobin Wei, Pinwen Zhu, and et al. 2021. "Constructing 1D Boron Chains in the Structure of Transition Metal Monoborides for Hydrogen Evolution Reactions" Catalysts 11, no. 11: 1265. https://doi.org/10.3390/catal11111265
APA StyleChen, Y., Ye, Y., Tao, Q., Yang, L., Cheng, J., Liu, X., Cao, J., Fan, H., Wei, M., Zhu, P., Yang, L., & Yang, J. (2021). Constructing 1D Boron Chains in the Structure of Transition Metal Monoborides for Hydrogen Evolution Reactions. Catalysts, 11(11), 1265. https://doi.org/10.3390/catal11111265




