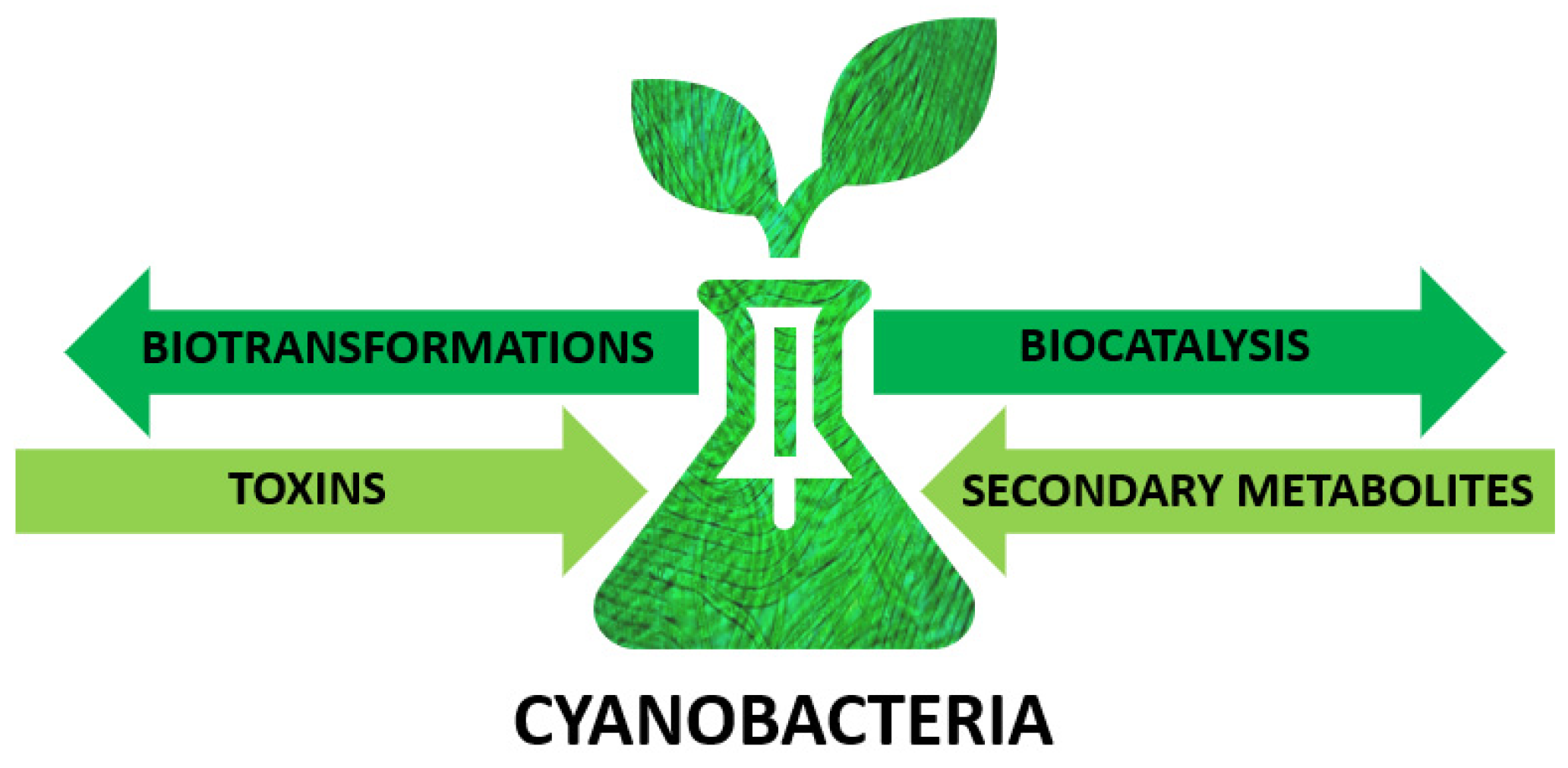
Cyanobacteria as Valuable Tool in Biotechnology
Abstract
1. Introduction
2. Cyanobacteria—Unique Microorganisms
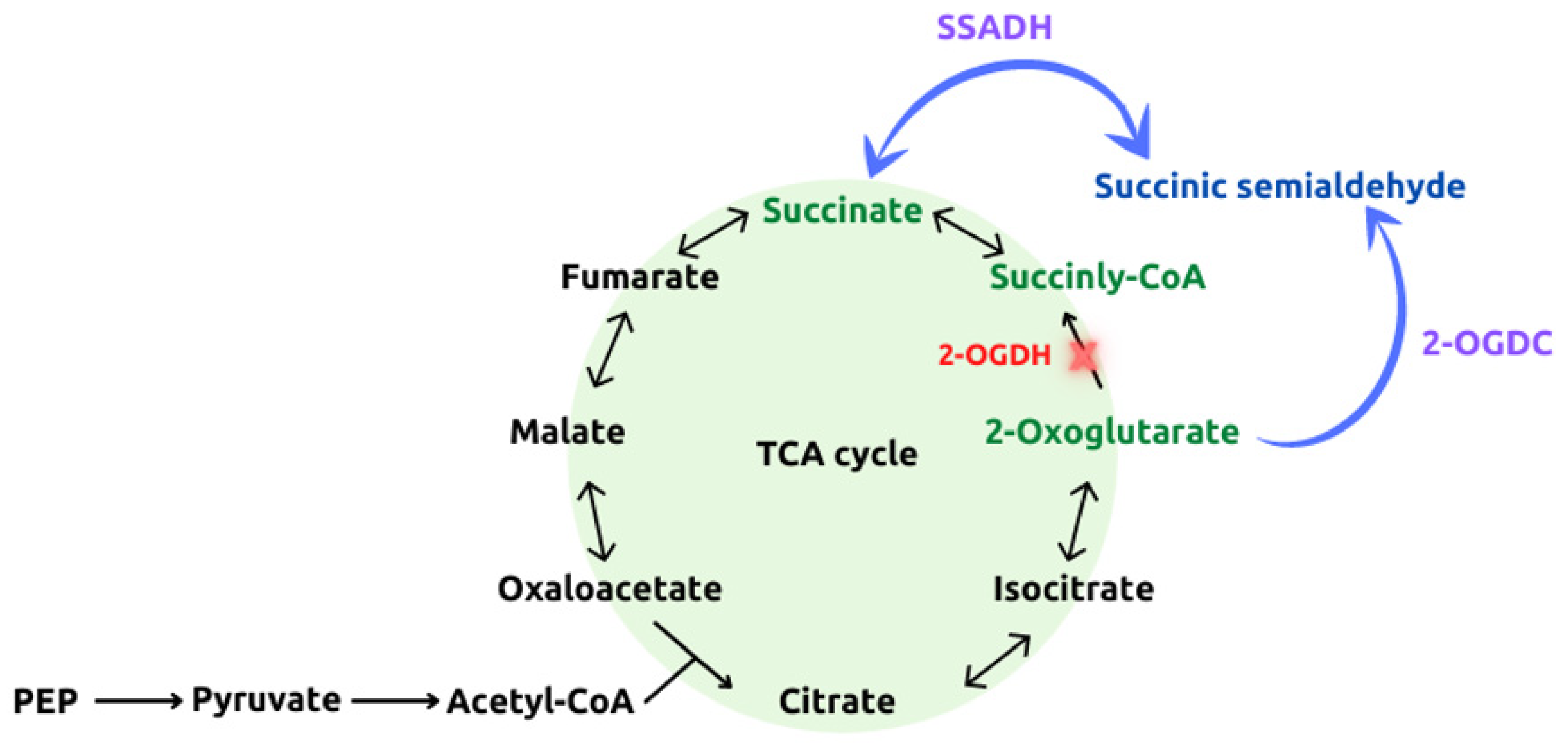
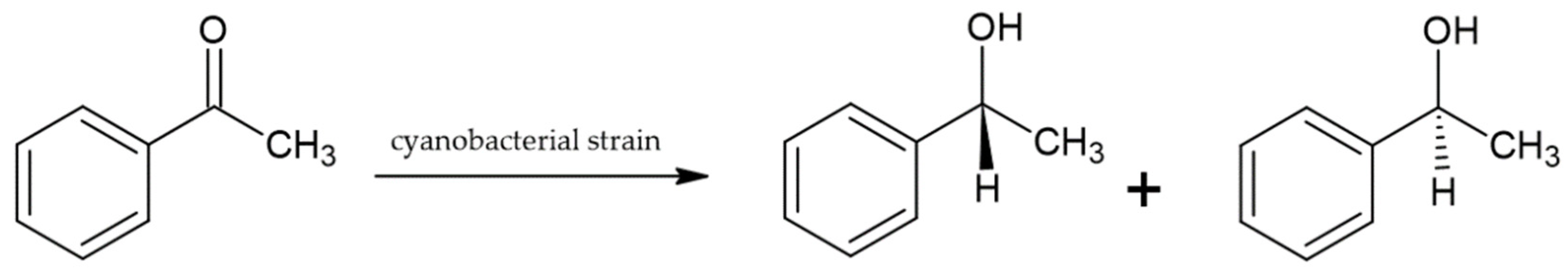
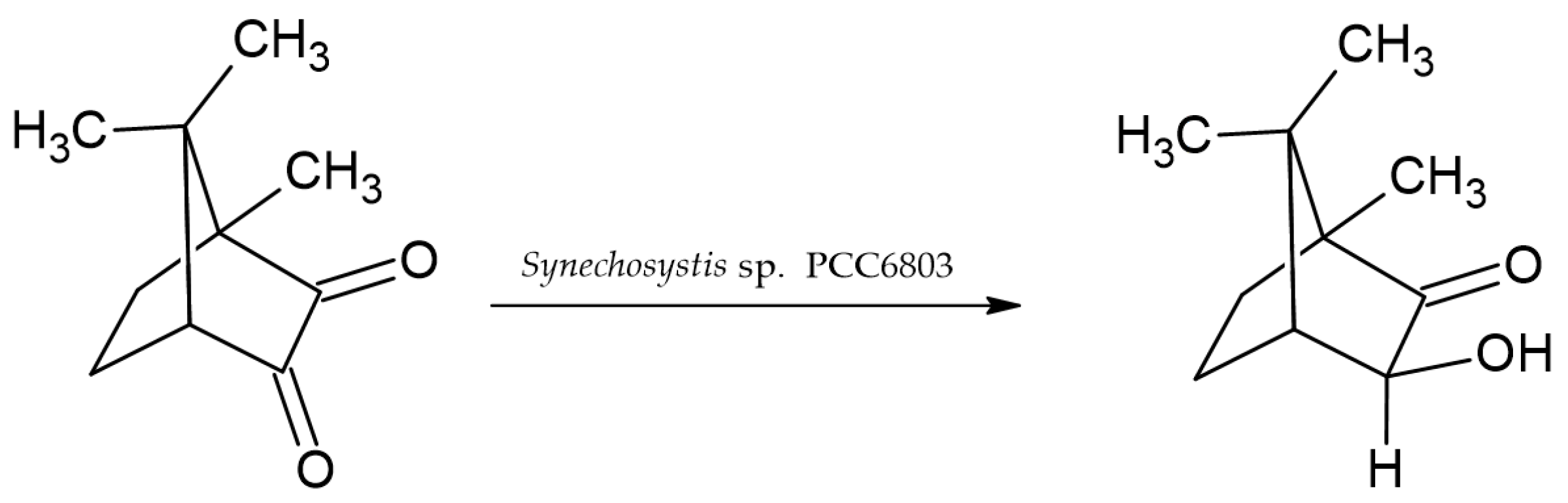
3. Cyanobacteria as Biocatalysts
4. Secondary Metabolites
4.1. Antimicrobial Compounds
4.2. Antifungal Compounds
4.3. Antiviral Compounds
4.4. Anticancer Compounds
4.5. Sunscreen Compounds
5. Cyanobacterial Toxins
5.1. Hepatotoxin
5.2. Neurotoxin
5.3. Dermatoxin
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Domínguez de María, P. Biocatalysis, sustainability, and industrial applications: Show me the metrics. Curr. Opin. Green Sustain. Chem. 2021, 31, 100514. [Google Scholar] [CrossRef]
- Sheldon, R.A. E factors, green chemistry and catalysis: An odyssey. Chem. Commun. 2008, 3352. [Google Scholar] [CrossRef] [PubMed]
- Jodlbauer, J.; Rohr, T.; Spadiut, O.; Mihovilovic, M.D.; Rudroff, F. Biocatalysis in Green and Blue: Cyanobacteria. Trends Biotechnol. 2021, 39, 875–889. [Google Scholar] [CrossRef]
- Ducat, D.C.; Way, J.C.; Silver, P.A. Engineering cyanobacteria to generate high-value products. Trends Biotechnol. 2011, 29, 95–103. [Google Scholar] [CrossRef]
- Vu, C.H.T.; Lee, H.-G.; Chang, Y.K.; Oh, H.-M. Axenic cultures for microalgal biotechnology: Establishment, assessment, maintenance, and applications. Biotechnol. Adv. 2018, 36, 380–396. [Google Scholar] [CrossRef] [PubMed]
- Abed, R.M.M.; Dobretsov, S.; Sudesh, K. Applications of cyanobacteria in biotechnology. J. Appl. Microbiol. 2009, 106, 1–12. [Google Scholar] [CrossRef]
- Blankenship, R.E.; Hartman, H. The origin and evolution of oxygenic photosynthesis. Trends Biochem. Sci. 1998, 23, 94–97. [Google Scholar] [CrossRef]
- Casella, S.; Huang, F.; Mason, D.; Zhao, G.-Y.; Johnson, G.N.; Mullineaux, C.W.; Liu, L.-N. Dissecting the Native Architecture and Dynamics of Cyanobacterial Photosynthetic Machinery. Mol. Plant 2017, 10, 1434–1448. [Google Scholar] [CrossRef]
- Lu, X. A perspective: Photosynthetic production of fatty acid-based biofuels in genetically engineered cyanobacteria. Biotechnol. Adv. 2010, 28, 742–746. [Google Scholar] [CrossRef] [PubMed]
- Steinhauser, D.; Fernie, A.R.; Araújo, W.L. Unusual cyanobacterial TCA cycles: Not broken just different. Trends Plant Sci. 2012, 17, 503–509. [Google Scholar] [CrossRef] [PubMed]
- Kieninger, A.-K.; Maldener, I. Cell–cell communication through septal junctions in filamentous cyanobacteria. Curr. Opin. Microbiol. 2021, 61, 35–41. [Google Scholar] [CrossRef]
- Singh, S.; Sinha, R.P.; Häder, D. Role of Lipids and Fatty Acids in Stress Tolerance in Cyanobacteria. Acta Protozool 2002, 41, 297–308. [Google Scholar]
- Ntambi, J.M. (Ed.) Stearoyl-CoA Desaturase Genes in Lipid Metabolism; Springer: New York, NY, USA, 2013; ISBN 978-1-4614-7968-0. [Google Scholar]
- de la Rosa, F.; De Troch, M.; Malanga, G.; Hernando, M. Differential sensitivity of fatty acids and lipid damage in Microcystis aeruginosa (cyanobacteria) exposed to increased temperature. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2020, 235, 108773. [Google Scholar] [CrossRef]
- Shimoda, K.; Kubota, N.; Hamada, H.; Kaji, M.; Hirata, T. Asymmetric reduction of enones with Synechococcus sp. PCC 7942. Tetrahedron Asymmetry 2004, 15, 1677–1679. [Google Scholar] [CrossRef]
- Żymańczyk-Duda, E.; Głąb, A.; Górak, M.; Klimek-Ochab, M.; Brzezińska-Rodak, M.; Strub, D.; Śliżewska, A. Reductive capabilities of different cyanobacterial strains towards acetophenone as a model substrate—Prospect of applications for chiral building blocks synthesis. Bioorg. Chem. 2019, 93, 102810. [Google Scholar] [CrossRef] [PubMed]
- Utsukihara, T.; Chai, W.; Kato, N.; Nakamura, K.; Horiuchi, C.A. Reduction of (+)- and (−)-camphorquinones by cyanobacteria. J. Mol. Catal. B Enzym. 2004, 31, 19–24. [Google Scholar] [CrossRef]
- Sandoval, B.A.; Hyster, T.K. Emerging strategies for expanding the toolbox of enzymes in biocatalysis. Curr. Opin. Chem. Biol. 2020, 55, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Obexer, R.; Godina, A.; Garrabou, X.; Mittl, P.R.E.; Baker, D.; Griffiths, A.D.; Hilvert, D. Emergence of a catalytic tetrad during evolution of a highly active artificial aldolase. Nat. Chem. 2017, 9, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Köninger, K.; Gómez Baraibar, Á.; Mügge, C.; Paul, C.E.; Hollmann, F.; Nowaczyk, M.M.; Kourist, R. Recombinant Cyanobacteria for the Asymmetric Reduction of C=C Bonds Fueled by the Biocatalytic Oxidation of Water. Angew. Chem. Int. Ed. 2016, 55, 5582–5585. [Google Scholar] [CrossRef] [PubMed]
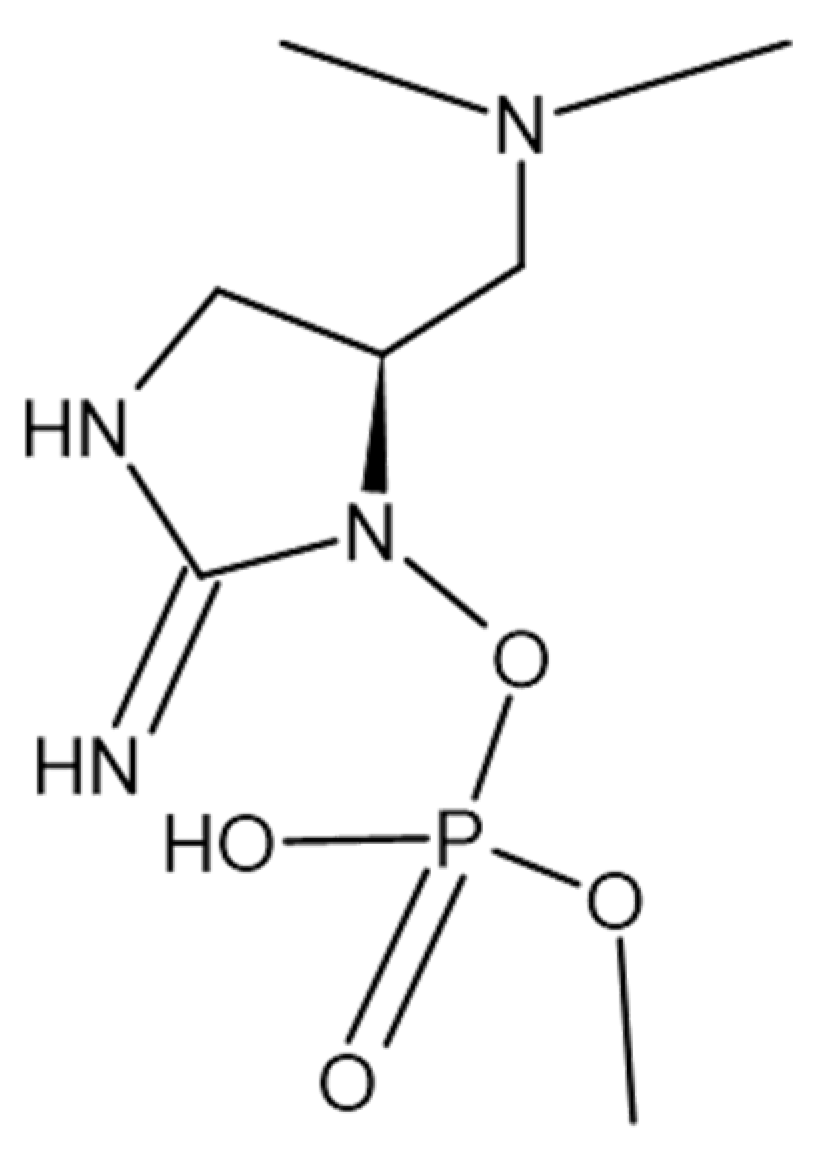
- Górak, M.; Żymańczyk-Duda, E. Application of cyanobacteria for chiral phosphonate synthesis. Green Chem 2015, 17, 4570–4578. [Google Scholar] [CrossRef]
- Wang, Z.; Cui, X.; Wang, C.; Huang, J.; Geng, D. Biotransformation of 6-deoxypseudoanisatin by Synechocystis sp. PCC6803. J. Tradit. Chin. Med. Sci. 2014, 1, 135–139. [Google Scholar] [CrossRef][Green Version]
- Demay, J.; Bernard, C.; Reinhardt, A.; Marie, B. Natural Products from Cyanobacteria: Focus on Beneficial Activities. Mar. Drugs 2019, 17, 320. [Google Scholar] [CrossRef]
- Haque, F.; Banayan, S.; Yee, J.; Chiang, Y.W. Extraction and applications of cyanotoxins and other cyanobacterial secondary metabolites. Chemosphere 2017, 183, 164–175. [Google Scholar] [CrossRef]
- Schopf, J.W. Microfossils of the Early Archean Apex Chert: New Evidence of the Antiquity of Life. Science 1993, 260, 640–646. [Google Scholar] [CrossRef]
- Cabanillas, A.H.; Tena Pérez, V.; Maderuelo Corral, S.; Rosero Valencia, D.F.; Martel Quintana, A.; Ortega Doménech, M.; Rumbero Sánchez, Á. Cybastacines A and B: Antibiotic Sesterterpenes from a Nostoc sp. Cyanobacterium. J. Nat. Prod. 2018, 81, 410–413. [Google Scholar] [CrossRef]
- Choi, H.; Engene, N.; Smith, J.E.; Preskitt, L.B.; Gerwick, W.H. Crossbyanols A−D, Toxic Brominated Polyphenyl Ethers from the Hawai’ian Bloom-Forming Cyanobacterium Leptolyngbya crossbyana. J. Nat. Prod. 2010, 73, 517–522. [Google Scholar] [CrossRef]
- Cheel, J.; Bogdanová, K.; Ignatova, S.; Garrard, I.; Hewitson, P.; Kolář, M.; Kopecký, J.; Hrouzek, P.; Vacek, J. Dimeric cyanobacterial cyclopent-4-ene-1,3-dione as selective inhibitor of Gram-positive bacteria growth: Bio-production approach and preparative isolation by HPCCC. Algal Res. 2016, 18, 244–249. [Google Scholar] [CrossRef][Green Version]
- Sarada, D.V.L.; Sreenath Kumar, C.; Rengasamy, R. Purified C-phycocyanin from Spirulina platensis (Nordstedt) Geitler: A novel and potent agent against drug resistant bacteria. World J. Microbiol. Biotechnol. 2011, 27, 779–783. [Google Scholar] [CrossRef]
- Jaki, B.; Orjala, J.; Heilmann, J.; Linden, A.; Vogler, B.; Sticher, O. Novel Extracellular Diterpenoids with Biological Activity from the Cyanobacterium Nostoc commune. J. Nat. Prod. 2000, 63, 339–343. [Google Scholar] [CrossRef] [PubMed]
- Ishida, K.; Matsuda, H.; Murakami, M.; Yamaguchi, K. Kawaguchipeptin B, an Antibacterial Cyclic Undecapeptide from the Cyanobacterium Microcystis aeruginosa. J. Nat. Prod. 1997, 60, 724–726. [Google Scholar] [CrossRef] [PubMed]
- Asthana, R.K.; Srivastava, A.; Singh, A.P.; Deepali; Singh, S.P.; Nath, G.; Srivastava, R.; Srivastava, B.S. Identification of an antimicrobial entity from the cyanobacterium Fischerella sp. isolated from bark of Azadirachta indica (Neem) tree. J. Appl. Phycol. 2006, 18, 33–39. [Google Scholar] [CrossRef]
- Zainuddin, E.N.; Jansen, R.; Nimtz, M.; Wray, V.; Preisitsch, M.; Lalk, M.; Mundt, S. Lyngbyazothrins A−D, Antimicrobial Cyclic Undecapeptides from the Cultured Cyanobacterium Lyngbya sp. J. Nat. Prod. 2009, 72, 1373–1378. [Google Scholar] [CrossRef]
- Gutiérrez, M.; Suyama, T.L.; Engene, N.; Wingerd, J.S.; Matainaho, T.; Gerwick, W.H. Apratoxin D, a Potent Cytotoxic Cyclodepsipeptide from Papua New Guinea Collections of the Marine Cyanobacteria Lyngbya majuscula and Lyngbya sordida. J. Nat. Prod. 2008, 71, 1099–1103. [Google Scholar] [CrossRef] [PubMed]
- Luesch, H.; Yoshida, W.Y.; Moore, R.E.; Paul, V.J.; Corbett, T.H. Total Structure Determination of Apratoxin A, a Potent Novel Cytotoxin from the Marine Cyanobacterium Lyngbya m ajuscula. J. Am. Chem. Soc. 2001, 123, 5418–5423. [Google Scholar] [CrossRef]
- Mevers, E.; Liu, W.-T.; Engene, N.; Mohimani, H.; Byrum, T.; Pevzner, P.A.; Dorrestein, P.C.; Spadafora, C.; Gerwick, W.H. Cytotoxic Veraguamides, Alkynyl Bromide-Containing Cyclic Depsipeptides from the Marine Cyanobacterium cf. Oscillatoria margaritifera. J. Nat. Prod. 2011, 74, 928–936. [Google Scholar] [CrossRef] [PubMed]
- Williams, P.G.; Yoshida, W.Y.; Moore, R.E.; Paul, V.J. The Isolation and Structure Elucidation of Tasiamide B, a 4-Amino-3-hydroxy-5-phenylpentanoic Acid Containing Peptide from the Marine Cyanobacterium Symploca sp. J. Nat. Prod. 2003, 66, 1006–1009. [Google Scholar] [CrossRef]
- Williams, P.G.; Yoshida, W.Y.; Moore, R.E.; Paul, V.J. Tasiamide, a Cytotoxic Peptide from the Marine Cyanobacterium Symploca sp. J. Nat. Prod. 2002, 65, 1336–1339. [Google Scholar] [CrossRef] [PubMed]
- Eghtedari, M.; Jafari Porzani, S.; Nowruzi, B. Anticancer potential of natural peptides from terrestrial and marine environments: A review. Phytochem. Lett. 2021, 42, 87–103. [Google Scholar] [CrossRef]
- Li, B.; Gao, M.-H.; Zhang, X.-C.; Chu, X.-M. Molecular immune mechanism of C-phycocyanin from Spirulina platensis induces apoptosis in HeLa cells in vitro. Biotechnol. Appl. Biochem. 2006, 43, 155. [Google Scholar]
- Medina, R.A.; Goeger, D.E.; Hills, P.; Mooberry, S.L.; Huang, N.; Romero, L.I.; Ortega-Barría, E.; Gerwick, W.H.; McPhail, K.L. Coibamide A, a Potent Antiproliferative Cyclic Depsipeptide from the Panamanian Marine Cyanobacterium Leptolyngbya sp. J. Am. Chem. Soc. 2008, 130, 6324–6325. [Google Scholar] [CrossRef]
- Chen, X.; Smith, G.D.; Waring, P. Human cancer cell (Jurkat) killing by the cyanobacterial metabolite calothrixin A. J. Appl. Phycol. 2003, 15, 269–277. [Google Scholar] [CrossRef]
- Cai, W.; Matthew, S.; Chen, Q.-Y.; Paul, V.J.; Luesch, H. Discovery of new A- and B-type laxaphycins with synergistic anticancer activity. Bioorg. Med. Chem. 2018, 26, 2310–2319. [Google Scholar] [CrossRef]
- Taori, K.; Paul, V.J.; Luesch, H. Structure and Activity of Largazole, a Potent Antiproliferative Agent from the Floridian Marine Cyanobacterium Symploca sp. J. Am. Chem. Soc. 2008, 130, 1806–1807. [Google Scholar] [CrossRef]
- Luesch, H.; Moore, R.E.; Paul, V.J.; Mooberry, S.L.; Corbett, T.H. Isolation of Dolastatin 10 from the Marine Cyanobacterium Symploca Species VP642 and Total Stereochemistry and Biological Evaluation of Its Analogue Symplostatin 1. J. Nat. Prod. 2001, 64, 907–910. [Google Scholar] [CrossRef]
- Teruya, T.; Sasaki, H.; Fukazawa, H.; Suenaga, K. Bisebromoamide, a Potent Cytotoxic Peptide from the Marine Cyanobacterium Lyngbya sp.: Isolation, Stereostructure, and Biological Activity. Org. Lett. 2009, 11, 5062–5065. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.-S.; Santarsiero, B.D.; Kim, H.; Krunic, A.; Shen, Q.; Swanson, S.M.; Chai, H.; Kinghorn, A.D.; Orjala, J. Merocyclophanes A and B, antiproliferative cyclophanes from the cultured terrestrial Cyanobacterium Nostoc sp. Phytochemistry 2012, 79, 109–115. [Google Scholar] [CrossRef]
- Chang, Z.; Sitachitta, N.; Rossi, J.V.; Roberts, M.A.; Flatt, P.M.; Jia, J.; Sherman, D.H.; Gerwick, W.H. Biosynthetic Pathway and Gene Cluster Analysis of Curacin A, an Antitubulin Natural Product from the Tropical Marine Cyanobacterium Lyngbya m ajuscula. J. Nat. Prod. 2004, 67, 1356–1367. [Google Scholar] [CrossRef]
- Taniguchi, M.; Nunnery, J.K.; Engene, N.; Esquenazi, E.; Byrum, T.; Dorrestein, P.C.; Gerwick, W.H. Palmyramide A, a Cyclic Depsipeptide from a Palmyra Atoll Collection of the Marine Cyanobacterium Lyngbya majuscula. J. Nat. Prod. 2010, 73, 393–398. [Google Scholar] [CrossRef]
- Andrianasolo, E.H.; Gross, H.; Goeger, D.; Musafija-Girt, M.; McPhail, K.; Leal, R.M.; Mooberry, S.L.; Gerwick, W.H. Isolation of Swinholide A and Related Glycosylated Derivatives from Two Field Collections of Marine Cyanobacteria. Org. Lett. 2005, 7, 1375–1378. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, A.; Puddick, J.; Prinsep, M.R.; Lee, P.P.F.; Tan, L.T. Hantupeptin A, a Cytotoxic Cyclic Depsipeptide from a Singapore Collection of Lyngbya majuscula. J. Nat. Prod. 2009, 72, 29–32. [Google Scholar] [CrossRef] [PubMed]
- Dey, B.; Lerner, D.L.; Lusso, P.; Boyd, M.R.; Elder, J.H.; Berger, E.A. Multiple Antiviral Activities of Cyanovirin-N: Blocking of Human Immunodeficiency Virus Type 1 gp120 Interaction with CD4 and Coreceptor and Inhibition of Diverse Enveloped Viruses. J. Virol. 2000, 74, 4562–4569. [Google Scholar] [CrossRef] [PubMed]
- Xiong, C.; O’Keefe, B.R.; Botos, I.; Wlodawer, A.; McMahon, J.B. Overexpression and purification of scytovirin, a potent, novel anti-HIV protein from the cultured cyanobacterium Scytonema varium. Protein Expr. Purif. 2006, 46, 233–239. [Google Scholar] [CrossRef]
- Kanekiyo, K.; Lee, J.-B.; Hayashi, K.; Takenaka, H.; Hayakawa, Y.; Endo, S.; Hayashi, T. Isolation of an Antiviral Polysaccharide, Nostoflan, from a Terrestrial Cyanobacterium, Nostoc flagelliforme. J. Nat. Prod. 2005, 68, 1037–1041. [Google Scholar] [CrossRef]
- Sato, Y.; Murakami, M.; Miyazawa, K.; Hori, K. Purification and characterization of a novel lectin from a freshwater cyanobacterium, Oscillatoria agardhii. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2000, 125, 169–177. [Google Scholar] [CrossRef]
- Chirasuwan, N.; Chaiklahan, R.; Kittakoop, P.; Chanasattru, W.; Ruengjitchatchawalya, M.; Tanticharoen, M.; Bunnag, B. Anti HSV-1 activity of sulphoquinovosyl diacylglycerol isolated from Spirulina platensis. ScienceAsia 2009, 35, 137. [Google Scholar] [CrossRef]
- Gupta, D.; Kaur, P.; Leong, S.; Tan, L.; Prinsep, M.; Chu, J. Anti-Chikungunya Viral Activities of Aplysiatoxin-Related Compounds from the Marine Cyanobacterium Trichodesmium erythraeum. Mar. Drugs 2014, 12, 115–127. [Google Scholar] [CrossRef] [PubMed]
- Frankmölle, W.P.; Knübel, G.; Moore, R.E.; Patterson, G.M.L. Antifungal cyclic peptides from the terrestrial blue-green alga Anabaena laxa. II. Structures of laxaphycins A, B, D and E. J. Antibiot. (Tokyo) 1992, 45, 1458–1466. [Google Scholar] [CrossRef] [PubMed]
- Marquez, B.L.; Watts, K.S.; Yokochi, A.; Roberts, M.A.; Verdier-Pinard, P.; Jimenez, J.I.; Hamel, E.; Scheuer, P.J.; Gerwick, W.H. Structure and Absolute Stereochemistry of Hectochlorin, a Potent Stimulator of Actin Assembly. J. Nat. Prod. 2002, 65, 866–871. [Google Scholar] [CrossRef]
- Milligan, K.E.; Marquez, B.L.; Williamson, R.T.; Gerwick, W.H. Lyngbyabellin B, a Toxic and Antifungal Secondary Metabolite from the Marine Cyanobacterium Lyngbya majuscula. J. Nat. Prod. 2000, 63, 1440–1443. [Google Scholar] [CrossRef]
- Carter, D.C.; Moore, R.E.; Mynderse, J.S.; Niemczura, W.P.; Todd, J.S. Structure of majusculamide C, a cyclic depsipeptide from Lyngbya majuscula. J. Org. Chem. 1984, 49, 236–241. [Google Scholar] [CrossRef]
- Bui, T.-H.; Wray, V.; Nimtz, M.; Fossen, T.; Preisitsch, M.; Schröder, G.; Wende, K.; Heiden, S.E.; Mundt, S. Balticidins A–D, Antifungal Hassallidin-Like Lipopeptides from the Baltic Sea Cyanobacterium Anabaena cylindrica Bio33. J. Nat. Prod. 2014, 77, 1287–1296. [Google Scholar] [CrossRef]
- Neuhof, T.; Schmieder, P.; Seibold, M.; Preussel, K.; von Döhren, H. Hassallidin B—Second antifungal member of the Hassallidin family. Bioorg. Med. Chem. Lett. 2006, 16, 4220–4222. [Google Scholar] [CrossRef]
- Fewer, D.P.; Jokela, J.; Rouhiainen, L.; Wahlsten, M.; Koskenniemi, K.; Stal, L.J.; Sivonen, K. The non-ribosomal assembly and frequent occurrence of the protease inhibitors spumigins in the bloom-forming cyanobacterium Nodularia spumigena. Mol. Microbiol. 2009, 73, 924–937. [Google Scholar] [CrossRef]
- Gunasekera, S.P.; Miller, M.W.; Kwan, J.C.; Luesch, H.; Paul, V.J. Molassamide, a Depsipeptide Serine Protease Inhibitor from the Marine Cyanobacterium Dichothrix utahensis. J. Nat. Prod. 2010, 73, 459–462. [Google Scholar] [CrossRef] [PubMed]
- Rouhiainen, L.; Jokela, J.; Fewer, D.P.; Urmann, M.; Sivonen, K. Two Alternative Starter Modules for the Non-Ribosomal Biosynthesis of Specific Anabaenopeptin Variants in Anabaena (Cyanobacteria). Chem. Biol. 2010, 17, 265–273. [Google Scholar] [CrossRef] [PubMed]
- Ziemert, N.; Ishida, K.; Liaimer, A.; Hertweck, C.; Dittmann, E. Ribosomal Synthesis of Tricyclic Depsipeptides in Bloom-Forming Cyanobacteria. Angew. Chem. Int. Ed. 2008, 47, 7756–7759. [Google Scholar] [CrossRef] [PubMed]
- Itou, Y.; Ishida, K.; Shin, H.J.; Murakami, M. Oscillapeptins A to F, serine protease inhibitors from the three strains of Oscillatoria agardhii. Tetrahedron 1999, 55, 6871–6882. [Google Scholar] [CrossRef]
- Niedermeyer, T. Anti-infective Natural Products from Cyanobacteria. Planta Med. 2015, 81, 1309–1325. [Google Scholar] [CrossRef]
- Flores, E.; Wolk, C.P. Production, by filamentous, nitrogen-fixing cyanobacteria, of a bacteriocin and of other antibiotics that kill related strains. Arch. Microbiol. 1986, 145, 215–219. [Google Scholar] [CrossRef]
- Vepritskiĭ, A.A.; Gromov, B.V.; Titova, N.N.; Mamkaeva, K.A. Production of the antibiotic-algicide cyanobacterin LU-2 by a filamentous cyanobacterium Nostoc sp. Mikrobiologiia 1991, 60, 21–25. [Google Scholar]
- Mo, S.; Krunic, A.; Santarsiero, B.D.; Franzblau, S.G.; Orjala, J. Hapalindole-related alkaloids from the cultured cyanobacterium Fischerella ambigua. Phytochemistry 2010, 71, 2116–2123. [Google Scholar] [CrossRef]
- Humisto, A.; Jokela, J.; Teigen, K.; Wahlsten, M.; Permi, P.; Sivonen, K.; Herfindal, L. Characterization of the interaction of the antifungal and cytotoxic cyclic glycolipopeptide hassallidin with sterol-containing lipid membranes. Biochim. Biophys. Acta BBA-Biomembr. 2019, 1861, 1510–1521. [Google Scholar] [CrossRef]
- Neuhof, T.; Schmieder, P.; Preussel, K.; Dieckmann, R.; Pham, H.; Bartl, F.; von Döhren, H. Hassallidin A, a Glycosylated Lipopeptide with Antifungal Activity from the Cyanobacterium Hassallia sp. J. Nat. Prod. 2005, 68, 695–700. [Google Scholar] [CrossRef] [PubMed]
- Neuhof, T.; Seibold, M.; Thewes, S.; Laue, M.; Han, C.-O.; Hube, B.; von Döhren, H. Comparison of susceptibility and transcription profile of the new antifungal hassallidin A with caspofungin. Biochem. Biophys. Res. Commun. 2006, 349, 740–749. [Google Scholar] [CrossRef] [PubMed]
- Xiong, C.; O’Keefe, B.R.; Byrd, R.A.; McMahon, J.B. Potent anti-HIV activity of scytovirin domain 1 peptide. Peptides 2006, 27, 1668–1675. [Google Scholar] [CrossRef]
- McFeeters, R.L.; Xiong, C.; O’Keefe, B.R.; Bokesch, H.R.; McMahon, J.B.; Ratner, D.M.; Castelli, R.; Seeberger, P.H.; Byrd, R.A. The Novel Fold of Scytovirin Reveals a New Twist For Antiviral Entry Inhibitors. J. Mol. Biol. 2007, 369, 451–461. [Google Scholar] [CrossRef]
- Garrison, A.R.; Giomarelli, B.G.; Lear-Rooney, C.M.; Saucedo, C.J.; Yellayi, S.; Krumpe, L.R.H.; Rose, M.; Paragas, J.; Bray, M.; Olinger, G.G.; et al. The cyanobacterial lectin scytovirin displays potent in vitro and in vivo activity against Zaire Ebola virus. Antiviral Res. 2014, 112, 1–7. [Google Scholar] [CrossRef]
- Nunnery, J.K.; Mevers, E.; Gerwick, W.H. Biologically active secondary metabolites from marine cyanobacteria. Curr. Opin. Biotechnol. 2010, 21, 787–793. [Google Scholar] [CrossRef] [PubMed]
- Malaker, A.; Ahmad, S.A.I. Therapeutic potency of anticancer peptides derived from marine organism. Int. J. Adv. Eng. Sci. Appl. Math. 2013, 2, 53–65. [Google Scholar]
- Zheng, L.-H.; Wang, Y.-J.; Sheng, J.; Wang, F.; Zheng, Y.; Lin, X.-K.; Sun, M. Antitumor Peptides from Marine Organisms. Mar. Drugs 2011, 9, 1840–1859. [Google Scholar] [CrossRef]
- Sithranga Boopathy, N.; Kathiresan, K. Anticancer Drugs from Marine Flora: An Overview. J. Oncol. 2010, 2010, 214186. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.; Luesch, H. Largazole: From discovery to broad-spectrum therapy. Nat. Prod. Rep. 2012, 29, 449. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, A.; Fang, W.; Leong, D.T.; Tan, L.T. Biochemical Studies of the Lagunamides, Potent Cytotoxic Cyclic Depsipeptides from the Marine Cyanobacterium Lyngbya majuscula. Mar. Drugs 2012, 10, 1126–1137. [Google Scholar] [CrossRef] [PubMed]
- Simmons, T.L.; Nogle, L.M.; Media, J.; Valeriote, F.A.; Mooberry, S.L.; Gerwick, W.H. Desmethoxymajusculamide C, a Cyanobacterial Depsipeptide with Potent Cytotoxicity in Both Cyclic and Ring-Opened Forms. J. Nat. Prod. 2009, 72, 1011–1016. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Salvador, L.A.; Byeon, S.; Ying, Y.; Kwan, J.C.; Law, B.K.; Hong, J.; Luesch, H. Anticolon Cancer Activity of Largazole, a Marine-Derived Tunable Histone Deacetylase Inhibitor. J. Pharmacol. Exp. Ther. 2010, 335, 351–361. [Google Scholar] [CrossRef] [PubMed]
- Pathak, J.; Ahmed, H.; Rajneesh; Singh, S.P.; Häder, D.-P.; Sinha, R.P. Genetic regulation of scytonemin and mycosporine-like amino acids (MAAs) biosynthesis in cyanobacteria. Plant Gene 2019, 17, 100172. [Google Scholar] [CrossRef]
- Grant, C.S.; Louda, J.W. Scytonemin-imine, a mahogany-colored UV/Vis sunscreen of cyanobacteria exposed to intense solar radiation. Org. Geochem. 2013, 65, 29–36. [Google Scholar] [CrossRef]
- Wada, N.; Sakamoto, T.; Matsugo, S. Multiple Roles of Photosynthetic and Sunscreen Pigments in Cyanobacteria Focusing on the Oxidative Stress. Metabolites 2013, 3, 463–483. [Google Scholar] [CrossRef]
- Funari, E.; Testai, E. Human Health Risk Assessment Related to Cyanotoxins Exposure. Crit. Rev. Toxicol. 2008, 38, 97–125. [Google Scholar] [CrossRef]
- Rutkowska, M.; Płotka-Wasylka, J.; Majchrzak, T.; Wojnowski, W.; Mazur-Marzec, H.; Namieśnik, J. Recent trends in determination of neurotoxins in aquatic environmental samples. TrAC Trends Anal. Chem. 2019, 112, 112–122. [Google Scholar] [CrossRef]
- Tillett, D.; Dittmann, E.; Erhard, M.; von Döhren, H.; Börner, T.; Neilan, B.A. Structural organization of microcystin biosynthesis in Microcystis aeruginosa PCC7806: An integrated peptide–polyketide synthetase system. Chem. Biol. 2000, 7, 753–764. [Google Scholar] [CrossRef]
- van Apeldoorn, M.E.; van Egmond, H.P.; Speijers, G.J.A.; Bakker, G.J.I. Toxins of cyanobacteria. Mol. Nutr. Food Res. 2007, 51, 7–60. [Google Scholar] [CrossRef]
- Chen, Y.; Shen, D.; Fang, D. Nodularins in poisoning. Clin. Chim. Acta 2013, 425, 18–29. [Google Scholar] [CrossRef]
- Merel, S.; Walker, D.; Chicana, R.; Snyder, S.; Baurès, E.; Thomas, O. State of knowledge and concerns on cyanobacterial blooms and cyanotoxins. Environ. Int. 2013, 59, 303–327. [Google Scholar] [CrossRef] [PubMed]
- Cox, P.A.; Banack, S.A.; Murch, S.J.; Rasmussen, U.; Tien, G.; Bidigare, R.R.; Metcalf, J.S.; Morrison, L.F.; Codd, G.A.; Bergman, B. Diverse taxa of cyanobacteria produce -N-methylamino-L-alanine, a neurotoxic amino acid. Proc. Natl. Acad. Sci. USA 2005, 102, 5074–5078. [Google Scholar] [CrossRef] [PubMed]
- Kellmann, R.; Ploux, O.; Neilan, B.A. Neurotoxic Alkaloids from Cyanobacteria. In Natural Products; Ramawat, K.G., Mérillon, J.-M., Eds.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 39–83. ISBN 978-3-642-22143-9. [Google Scholar]
- Skulberg, O.M.; Skulberg, R.; Carmichael, W.W.; Andersen, R.A.; Matsunaga, S.; Moore, R.E. Investigations of a neurotoxic oscillatorialean strain (Cyanophyceae) and its toxin. Isolation and characterization of homoanatoxin-a. Environ. Toxicol. Chem. 1992, 11, 321–329. [Google Scholar] [CrossRef]
- Mahmood, N.A.; Carmichael, W.W. Anatoxin-a(s), an anticholinesterase from the cyanobacterium Anabaena flos-aquae NRC-525-17. Toxicon 1987, 25, 1221–1227. [Google Scholar] [CrossRef]
- Lajeunesse, A.; Segura, P.A.; Gélinas, M.; Hudon, C.; Thomas, K.; Quilliam, M.A.; Gagnon, C. Detection and confirmation of saxitoxin analogues in freshwater benthic Lyngbya wollei algae collected in the St. Lawrence River (Canada) by liquid chromatography–tandem mass spectrometry. J. Chromatogr. A 2012, 1219, 93–103. [Google Scholar] [CrossRef]
- Edwards, D.J.; Gerwick, W.H. Lyngbyatoxin Biosynthesis: Sequence of Biosynthetic Gene Cluster and Identification of a Novel Aromatic Prenyltransferase. J. Am. Chem. Soc. 2004, 126, 11432–11433. [Google Scholar] [CrossRef] [PubMed]
- Chorus, I.; Bartram, J. (Eds.) Toxic Cyanobacteria in Water: A Guide to Their Public Health Consequences, Monitoring, and Management; E & FN Spon: London, UK; New York, NY, USA, 1999; ISBN 978-0-419-23930-7. [Google Scholar]
- Barco, M.; Rivera, J.; Caixach, J. Analysis of cyanobacterial hepatotoxins in water samples by microbore reversed-phase liquid chromatography–electrospray ionisation mass spectrometry. J. Chromatogr. A 2002, 959, 103–111. [Google Scholar] [CrossRef]
- Heresztyn, T. Determination of cyanobacterial hepatotoxins directly in water using a protein phosphatase inhibition assay. Water Res. 2001, 35, 3049–3056. [Google Scholar] [CrossRef]
- MacKintosh, C.; Beattie, K.A.; Klumpp, S.; Cohen, P.; Codd, G.A. Cyanobacterial microcystin-LR is a potent and specific inhibitor of protein phosphatases 1 and 2A from both mammals and higher plants. FEBS Lett. 1990, 264, 187–192. [Google Scholar] [CrossRef]
- Codd, G.; Bell, S.; Kaya, K.; Ward, C.; Beattie, K.; Metcalf, J. Cyanobacterial toxins, exposure routes and human health. Eur. J. Phycol. 1999, 34, 405–415. [Google Scholar] [CrossRef]
- Buratti, F.M.; Manganelli, M.; Vichi, S.; Stefanelli, M.; Scardala, S.; Testai, E.; Funari, E. Cyanotoxins: Producing organisms, occurrence, toxicity, mechanism of action and human health toxicological risk evaluation. Arch. Toxicol. 2017, 91, 1049–1130. [Google Scholar] [CrossRef] [PubMed]
- Pearson, L.; Mihali, T.; Moffitt, M.; Kellmann, R.; Neilan, B. On the Chemistry, Toxicology and Genetics of the Cyanobacterial Toxins, Microcystin, Nodularin, Saxitoxin and Cylindrospermopsin. Mar. Drugs 2010, 8, 1650–1680. [Google Scholar] [CrossRef]
- Banker, R.; Carmeli, S.; Werman, M.; Teltsch, B.; Porat, R.; Sukenik, A. Uracil Moiety is Required for Toxicity of the Cyanobacterial Hepatotoxin Cylindrospermopsin. J. Toxicol. Environ. Health A 2001, 62, 281–288. [Google Scholar] [CrossRef]
- Colas, S.; Marie, B.; Lance, E.; Quiblier, C.; Tricoire-Leignel, H.; Mattei, C. Anatoxin-a: Overview on a harmful cyanobacterial neurotoxin from the environmental scale to the molecular target. Environ. Res. 2021, 193, 110590. [Google Scholar] [CrossRef]
- Ha, M.-H.; Pflugmacher, S. Phytotoxic effects of the cyanobacterial neurotoxin anatoxin-a: Morphological, physiological and biochemical responses in aquatic macrophyte, Ceratophyllum demersum. Toxicon 2013, 70, 1–8. [Google Scholar] [CrossRef]
- Christensen, V.G.; Khan, E. Freshwater neurotoxins and concerns for human, animal, and ecosystem health: A review of anatoxin-a and saxitoxin. Sci. Total Environ. 2020, 736, 139515. [Google Scholar] [CrossRef]
- Addante-Moya, L.G.; Agulló, C.; Quiñones-Reyes, G.; Mercader, J.V.; Abad-Fuentes, A.; Abad-Somovilla, A. A unified approach to the synthesis of both enantiomers of anatoxin-a and homoanatoxin-a cyanotoxins. Tetrahedron 2018, 74, 5022–5031. [Google Scholar] [CrossRef]
- Aas, P.; Eriksen, S.; Kolderup, J.; Lundy, P.; Haugen, J.E.; Skulberg, O.M.; Fonnum, F. Enhancement of acetylcholine release by homoanatoxin-a from Oscillatoria formosa. Environ. Toxicol. Pharmacol. 1996, 2, 223–232. [Google Scholar] [CrossRef]
- Wood, S.A.; Selwood, A.I.; Rueckert, A.; Holland, P.T.; Milne, J.R.; Smith, K.F.; Smits, B.; Watts, L.F.; Cary, C.S. First report of homoanatoxin-a and associated dog neurotoxicosis in New Zealand. Toxicon 2007, 50, 292–301. [Google Scholar] [CrossRef]
- Lima-Filho, C.M.; Nogaroli, L.; Hedin-Pereira, C.; Azevedo, S.M.F.O.; Soares, R.M. Effects of saxitoxins exposure on oligodendrocyte development in mouse neonates. Toxicon 2020, 188, 89–94. [Google Scholar] [CrossRef]
- Schantz, E.J.; Ghazarossian, V.E.; Schnoes, H.K.; Strong, F.M.; Springer, J.P.; Pezzanite, J.O.; Clardy, J. Structure of saxitoxin. J. Am. Chem. Soc. 1975, 97, 1238–1239. [Google Scholar] [CrossRef]
- Etheridge, S.M. Paralytic shellfish poisoning: Seafood safety and human health perspectives. Toxicon 2010, 56, 108–122. [Google Scholar] [CrossRef] [PubMed]
- Cardellina, J.; Marner, F.; Moore, R. Seaweed dermatitis: Structure of lyngbyatoxin A. Science 1979, 204, 193–195. [Google Scholar] [CrossRef] [PubMed]
- Cosp, A.; Llàcer, E.; Romea, P.; Urpí, F. Synthesis of the C9–C21 fragment of debromoaplysiatoxin and oscillatoxins A and D. Tetrahedron Lett. 2006, 47, 5819–5823. [Google Scholar] [CrossRef]
- Nagai, H.; Yasumoto, T.; Hokama, Y. Aplysiatoxin and debromoaplysiatoxin as the causative agents of a red alga Gracilaria coronopifolia poisoning in hawaii. Toxicon 1996, 34, 753–761. [Google Scholar] [CrossRef]
- Tønder, J.E.; Tanner, D. Toward the enantioselective total synthesis of lyngbyatoxin A: On the stereocontrolled introduction of the quaternary stereogenic centre. Tetrahedron 2003, 59, 6937–6945. [Google Scholar] [CrossRef]
- Werner, K.A.; Marquart, L.; Norton, S.A. Lyngbya dermatitis (toxic seaweed dermatitis): Lyngbya dermatitis (toxic seaweed dermatitis). Int. J. Dermatol. 2012, 51, 59–62. [Google Scholar] [CrossRef]
- Nagai, H.; Watanabe, M.; Sato, S.; Kawaguchi, M.; Xiao, Y.-Y.; Hayashi, K.; Watanabe, R.; Uchida, H.; Satake, M. New aplysiatoxin derivatives from the Okinawan cyanobacterium Moorea producens. Tetrahedron 2019, 75, 2486–2494. [Google Scholar] [CrossRef]





| Biological Activity | Active Compound | Cyanobacteria | Ref. |
|---|---|---|---|
| antibacterial | cybastacines A and B | Nostoc sp. | [26] |
| crossbyanols B,C,D | Leptolyngbya crossbyana | [27] | |
| nostotrebin 6 | Nostoc sp. str. Lukešová 27/97 | [28] | |
| c-phycocyanin | Spirulina platensis | [29] | |
| comnostins A–E | Nostoc commune | [30] | |
| kawaguchipeptin B | Microcystis aeruginosa NIES-88 | [31] | |
| hapalindole T | Fischerella sp. | [32] | |
| lyngbyazothrins A−D | Lyngbya sp. 36.91 | [33] | |
| anticancer | apratoxin A,D | Lyngba majuscula, Lyngba sordida | [34,35] |
| veraguamides A-C and H-L | cf. Oscillatoria margaritifera | [36] | |
| tasiamide, tasiamide B | Symploca sp. | [37,38] | |
| desmethoxymajusculamide C (DMMC) | Lyngba majuscula | [39] | |
| c-phycocyanin | Spirulina platensis | [40] | |
| coibamide A | Leptolyngbya sp. | [41] | |
| calothrixin A | Calothrix | [42] | |
| laxaphycins B4 and A2 | Hormothamnion enteromorphoides | [43] | |
| largazole | Symploca sp. | [44] | |
| dolastatin 10 | Symploca sp. VP642 | [45] | |
| bisebromoamide | Lyngba sp. | [46] | |
| merocyclophanes A,B | Nostoc sp. UIC 10062 | [47] | |
| curacin A | Lyngba majuscula | [48] | |
| palmyramide A | Lyngba majuscula | [49] | |
| ankaraholides A | Geitlerinema sp. | [50] | |
| hantupeptin A | Lyngba majuscula | [51] | |
| antiviral | cyanovirin-N (CV-N) | Nostoc ellipsosporum | [52] |
| scytovirin | Scytonema varium | [53] | |
| nostoflan | Nostoc flagelliforme | [54] | |
| o. agardhii agglutinin (OAA) | Oscillatoria agardhii NIES-204 | [55] | |
| sulphoquinovosyl diacylglycerol | Spirulina platensis | [56] | |
| debromoaplysiatoxin | Trichodesmium erythraeum | [57] | |
| 3-methoxydebromoaplysiatoxin | Trichodesmium erythraeum | [27] | |
| antifungal | laxaphycins A and B | Anabaena laxa FK-1-2 | [58] |
| hectochlorin | Lyngbya majuscula | [59] | |
| lyngbyabellin B | Lyngbya majuscula | [60] | |
| majusculamide C | Lyngbya majuscula | [61] | |
| balticidins A−D hassallidin B | Anabaena cylindrica Bio33 Hassallia sp. | [62] [63] | |
| protease inhibitor | spumigins | Nodularia spumigena AV1 Nodularia spumigena CCY9414 | [64] |
| molassamide | Dichothrix utahensis | [65] | |
| anabaenopeptin NZ 857 | Nostoc punctiforme PCC 73102 | [66] | |
| nostamide A | Nostoc punctiforme PCC 73102 | [66] | |
| anabaenopeptin A, B, C | Anabaena sp. strain 90 | [66] | |
| nodulapeptin B, C | Nodularia spumigena CCY9414 | [66] | |
| microviridin J | Microcystis UOWOCC MRC | [67] | |
| microviridin B | Microcystis aeruginosa NIES298 | [67] | |
| oscillapeptins B | Oscillatoria agardhii NIES-204 | [68] | |
| oscillapeptins C-E | Oscillatoria agardhii NIES-205 | [68] | |
| oscillapeptins F | Oscillatoria agardhii NIES-596 | [68] | |
| sunscreen | mycosporine-like amino acids (MAAs) | Synechocystis sp. PCC 6803, Gloeocapsa sp. CU-2556, Aphanothece halophytica, Gloeocapsa sp., Euhalothece sp., Microcystis aeruginosa, Arthrospira sp. CU2556, Lyngbya sp. CU2555, Leptolyngbya sp., Phormidium sp., Lyngbya cf. aestuarii, Microcoleus chthonoplastes, Microcoleus sp., Oscillatoria spongelidae, Trichodesmium spp., Anabaena sp., Anabaena doliolum, Anabaena variabilis PCC 7937, Nostoc sp., Nostoc commune var. Vaucher, Nostoc commune, Scytonema sp., Nostoc punctiforme ATCC 29133, Nostoc sp. HKAR-2 and HKAR-6, Nodularia baltica, Nodularia harveyana, Nodularia spumigena, Aphanizomenon flos-aquae, Chlorogloeopsis PCC 6912 | [23] |
| carotenoids | all | [23] |
| Biological Activity | Active Compound | Cyanobacteria | Ref. |
|---|---|---|---|
| hepatotoxin | microcystin | Microcystis aeruginosa PCC 7806 | [92,93] |
| Anabaena spp. | |||
| Oscillatoria agardhii | |||
| nodularins | Nodularia spumigena | [94] | |
| cylindrospermopsin | Cylindrospermopsis raciborskii Aphanizomenon ovalisporum, Raphidiopsis curvata Umezakia natans | [95] | |
| neurotoxin | β -N-methylamino-L-alanine | Nostoc PCC 7107 Anabaena variabilis ATCC 29413 | [96] |
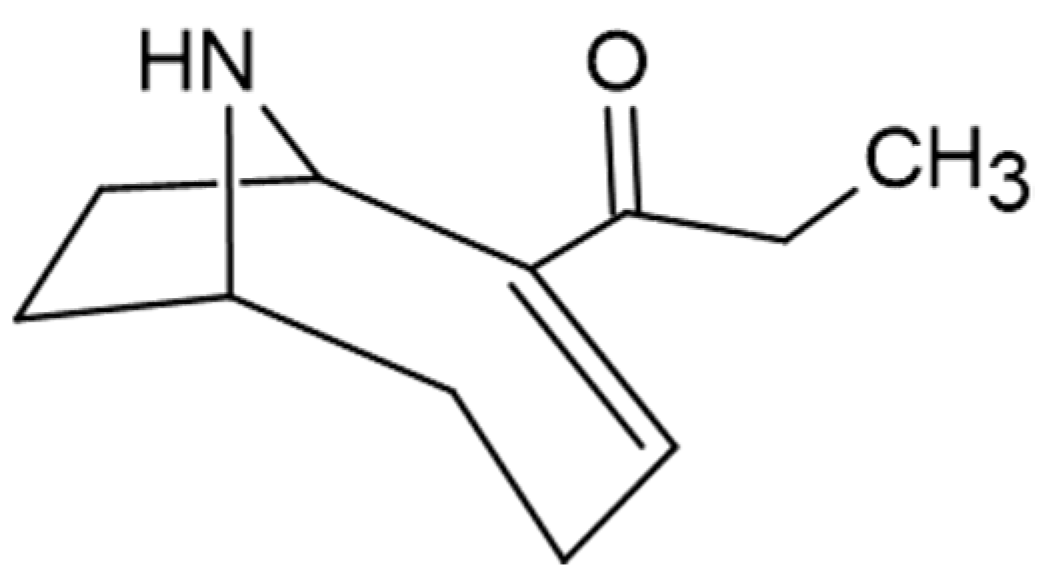
| anatoxin-a | Anabaena flos-aquae | [97] | |
| homoanatoxin-a | Oscillatoria formosa | [98] | |
| anatoxin-a(s) | Anabaena flos-aquae NRC-525-17 | [99] | |
| saxitoxins | Anabaena circinalis Lyngbya wollei | [100] | |
| dermatoxin | lyngbyatoxins A-C | Lyngbya majuscula | [101] |
| debromoaplysiatoxin | Lyngbya majuscula Oscillatoria nigroviridis Schizothrix calcicole | [102] | |
| aplysiatoxins | Lyngbya Schizothrix Planktothrix (Oscillatoria) | [102] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Śliżewska, A.; Żymańczyk-Duda, E. Cyanobacteria as Valuable Tool in Biotechnology. Catalysts 2021, 11, 1259. https://doi.org/10.3390/catal11111259
Śliżewska A, Żymańczyk-Duda E. Cyanobacteria as Valuable Tool in Biotechnology. Catalysts. 2021; 11(11):1259. https://doi.org/10.3390/catal11111259
Chicago/Turabian StyleŚliżewska, Agnieszka, and Ewa Żymańczyk-Duda. 2021. "Cyanobacteria as Valuable Tool in Biotechnology" Catalysts 11, no. 11: 1259. https://doi.org/10.3390/catal11111259
APA StyleŚliżewska, A., & Żymańczyk-Duda, E. (2021). Cyanobacteria as Valuable Tool in Biotechnology. Catalysts, 11(11), 1259. https://doi.org/10.3390/catal11111259







