Enhanced Thermostability of Pseudomonas nitroreducens Isoeugenol Monooxygenase by the Combinatorial Strategy of Surface Residue Replacement and Consensus Mutagenesis
Abstract
:1. Introduction
2. Results
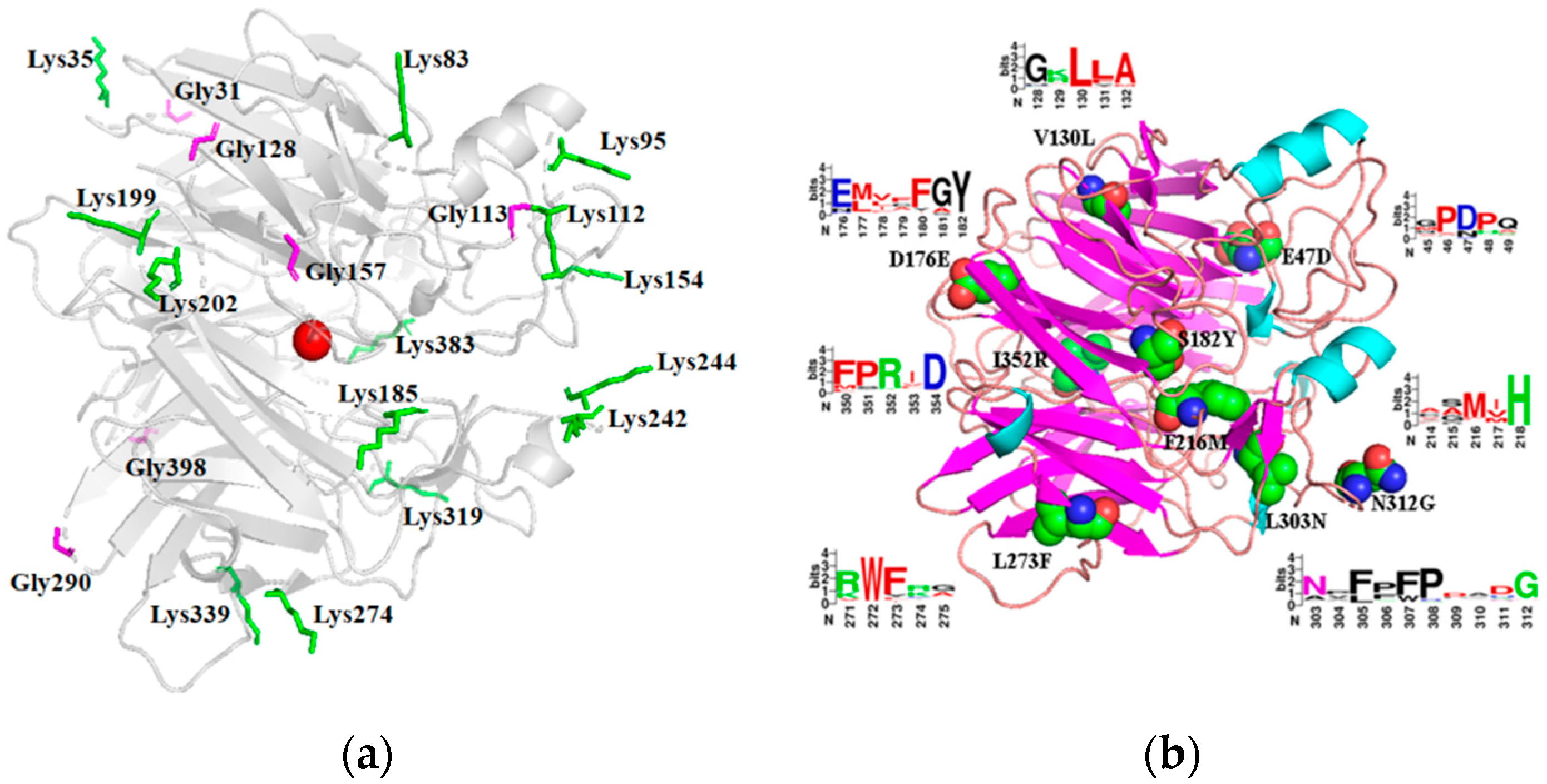
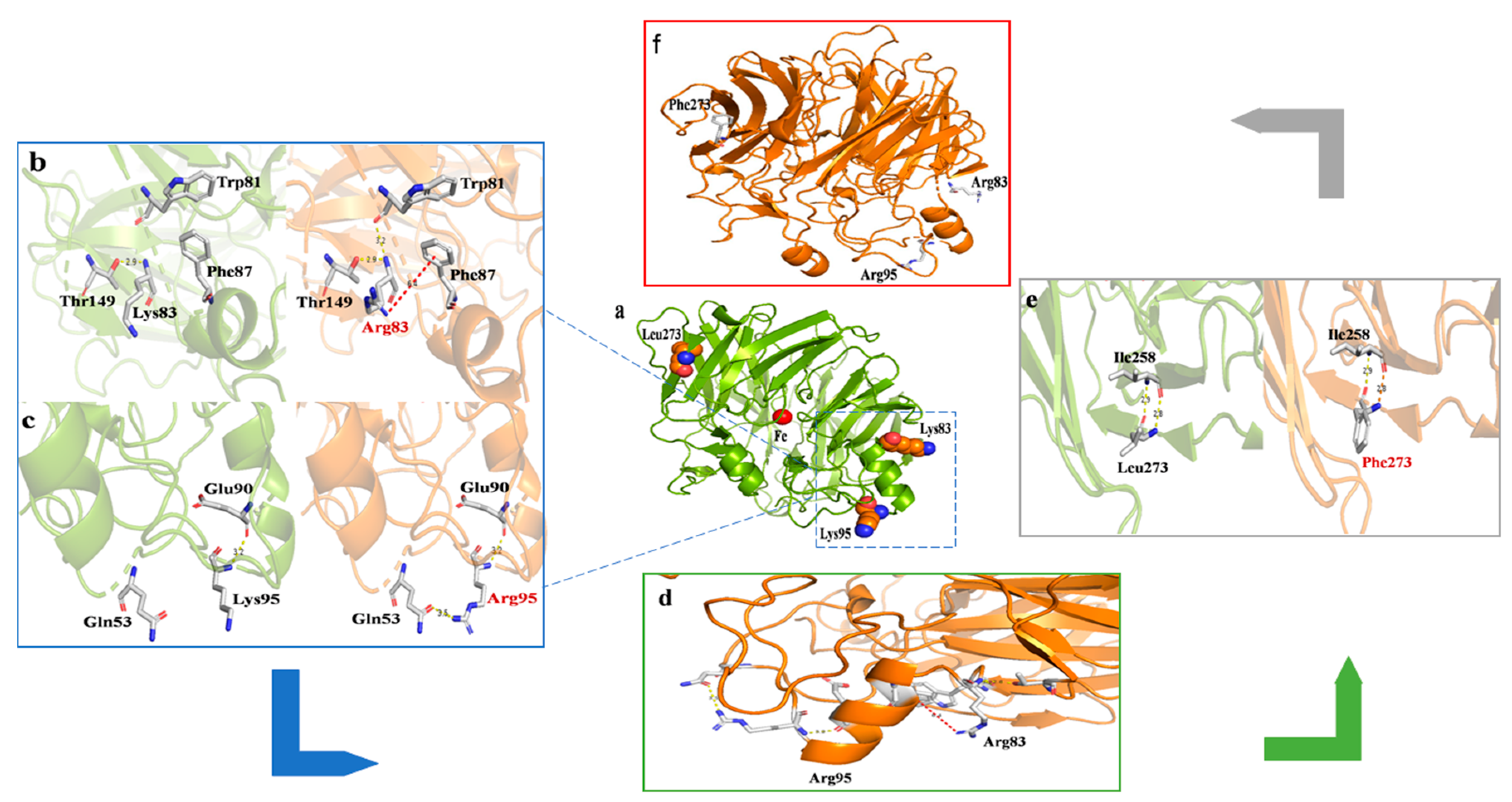
2.1. Selection of Sites for Surface Residue Replacement and Consensus Mutagenesis
2.2. Preliminary Selection of IEM Mutants with Improved Thermostability and Activity
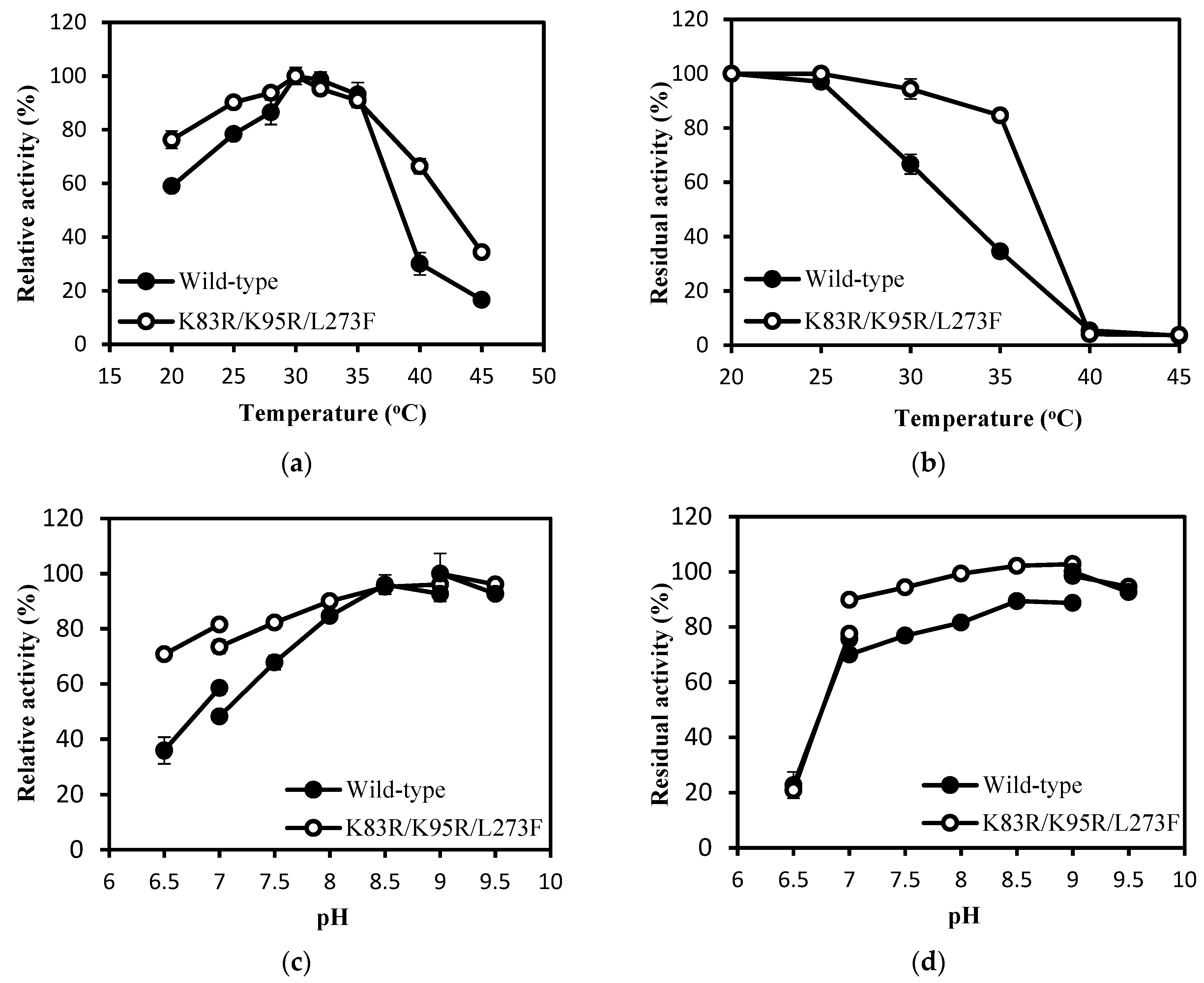
2.3. Thermostability and Kinetic Analysis of IEM and Its Mutants
2.4. Enzymatic Properties of IEM and Its Mutant IEMK83R/K95R/L273F
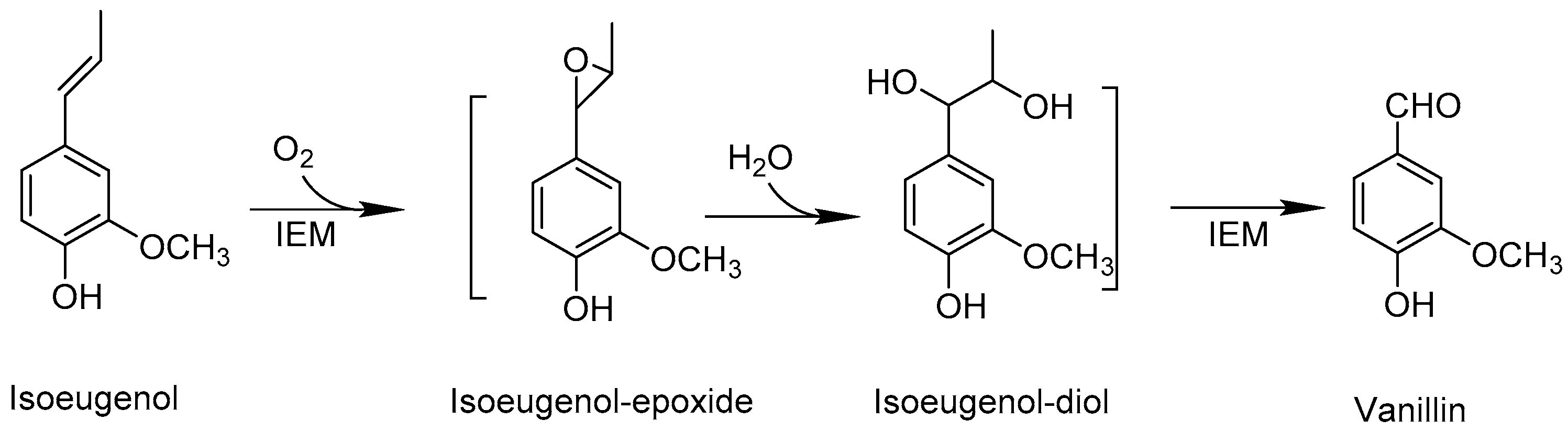
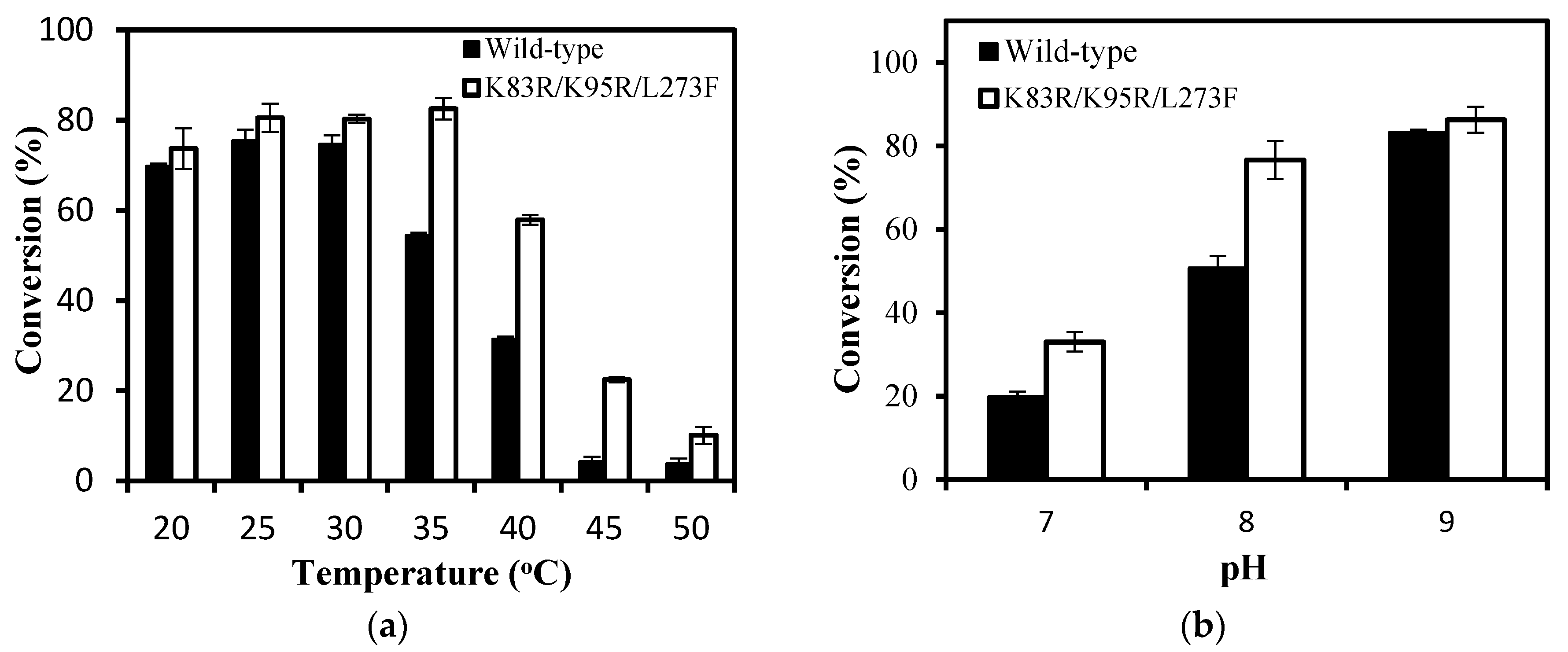
2.5. Biocatalytic Synthesis of Vanillin by IEM and Its Mutant IEMK83R/K95R/L273F
3. Discussion
4. Materials and Methods
4.1. Chemicals, Plasmids and Strains
4.2. Prediction of Potential Stable Variants and Site-Directed Mutagenesis
4.3. Enzyme Expression and Purification
4.4. Enzyme Activity Assay and Kinetic Analysis
4.5. Thermal Stability Assay
4.6. Effects of Temperature, pH, Metal Ions and Product-Inhibition on Enzyme Activity
4.7. Biosynthesis of Vanillin Using Whole-Cell Catalysis
4.8. Preparation of Natural Vanillin
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Priefert, H.; Rabenhorst, J.; Steinbüchel, A. Biotechnological production of vanillin. Appl. Microbiol. Biotechnol. 2001, 56, 296–314. [Google Scholar] [CrossRef] [PubMed]
- Converti, A.; Aliakbarian, B.; Domínguez, J.M.; BustosVázquez, G.; Perego1, P. Microbial production of biovanillin. Braz. J. Microbiol. 2010, 41, 519–530. [Google Scholar] [CrossRef] [Green Version]
- Sainsbury, P.D.; Hardiman, E.M.; Ahmad, M.; Otani, H.; Seghezzi, N.; Eltis, L.D.; Bugg, T.D.H. Breaking down lignin to high-value chemicals: The conversion of lignocellulose to vanillin in a gene deletion mutant of Rhodococcusjostii RHA1. ACS Chem. Biol. 2013, 8, 2151–2156. [Google Scholar] [CrossRef]
- Ciriminna, R.; Fidalgo, A.; Meneguzzo, F.; Parrino, F.; Ilharco, L.M.; Pagliaro, M. Vanillin: The case for greener production driven by sustainability megatrend. ChemPubSoc Eur. 2019, 8, 660–667. [Google Scholar] [CrossRef]
- Fache, M.; Boutevin, B.; Caillol, S. Vanillin production from lignin and its use as a renewable chemical. ACS Sustain. Chem. Eng. 2016, 4, 35–46. [Google Scholar] [CrossRef]
- Mao, H.-F.; Wang, L.-Z.; Zhao, F.-F.; Wu, J.-X.; Huo, H.-H.; Yu, J. Cobalt-catalyzed aerobic oxidation of eugenol to vanillin and vanillic acid. J. Chin. Chem. Soc. 2016, 63, 261–266. [Google Scholar] [CrossRef]
- Zhang, R.; Maltari, R.; Guo, M.; Kontro, J.; Eronen, A.; Repo, T. Facile synthesis of vanillin from fractionated Kraft lignin. Ind. Crops. Prod. 2020, 145, 112095. [Google Scholar] [CrossRef]
- Yamada, M.; Okada, Y.; Yoshida, T.; Nagasawa, T. Biotransformation of isoeugenol to vanillin by Pseudomonas putida IE27 cells. Appl. Microbiol. Biotechnol. 2007, 73, 1025–1030. [Google Scholar] [CrossRef]
- Ashengroph, M.; Nahvi, I.; Zarkesh-Esfahani, H.; Momenbeik, F. Pseudomonas resinovorans SPR1, a newly isolated strain with potential of transforming eugenol to vanillin and vanillic acid. New Biotechnol. 2011, 28, 656–664. [Google Scholar] [CrossRef]
- Kaur, B.; Chakraborty, D. Statistical media and process optimization for biotransformation of rice bran to vanillin using Pediococcus acidilactici. India J. Exp. Biol. 2013, 51, 935–943. [Google Scholar]
- Suresh, B.; Ravishankar, G.A. Methyl jasmonate modulated biotransformation of phenylpropanoids to vanillin related metabolites using Capsicum frutescens root cultures. Plant Physiol. Biochem. 2005, 43, 125–131. [Google Scholar] [CrossRef]
- Yamada, M.; Okada, Y.; Yoshida, T.; Nagasawa, T. Vanillin production using Escherichia coli cells over-expressing isoeugenol monooxygenase of Pseudomonas putida. Biotechnol. Lett. 2008, 30, 665–670. [Google Scholar] [CrossRef]
- Furuya, T.; Miura, M.; Kuroiwa, M.; Kino, K. High-yield production of vanillin from ferulic acid by a coenzyme-independent decarboxylase/oxygenase two-stage process. New Biotechnol. 2015, 32, 335–339. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chakraborty, D.; Selvam, A.; Kaur, B.; Wong, J.W.C.; Karthikeyan, O.P. Application of recombinant Pediococcus acidilactici BD16 (fcs+/ech+) for bioconversion of agrowaste to vanillin. Appl. Microbiol. Biotechnol. 2017, 101, 5615–5626. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.-Q.; Xie, Y.-M.; Chen, L.-Y.; Xu, X.-F.; Zhao, C.X.-X.; Cheng, F. Efficient biotransformation of isoeugenol to vanillin in recombinant strains of Escherichia coli by using engineered isoeugenol monooxygenase and sol-gel chitosan membrane. Process. Biochem. 2018, 71, 76–81. [Google Scholar] [CrossRef]
- Singh, P.; Khan, S.; Pandey, S.; Singh, M.; Banerjee, S.; Kitamura, Y.; Rahman, L. Vanillin production in metabolically engineered Beta vulgaris hairy roots through heterologous expression in Pseudomonas Fuorescens HCHL gene. Ind. Crops. Prod. 2015, 74, 839–848. [Google Scholar] [CrossRef]
- Paz, A.; Outeiriño, D.; Oliveira, R.P.D.S.; Domínguez, J.M. Fed-batch production of vanillin by Bacillus aryabhattai BA03. New Biotechnol. 2018, 40, 186–191. [Google Scholar] [CrossRef] [PubMed]
- Kaur, B.; Chakraborty, D.; Kumar, B. Metabolic engineering of Pediococcus acidilactici BD16 for production of vanillin through ferulic acid catabolic pathway and process optimization using response surface methodology. Appl. Microbiol. Biotechnol. 2014, 98, 8539–8551. [Google Scholar] [CrossRef]
- García-Bofill, M.; Sutton, P.W.; Guillén, M.; Álvaro, G. Enzymatic synthesis of vanillin catalyzed by an eugenol oxidase. Appl. Catal. A-Gen. 2019, 582, 117117. [Google Scholar]
- Yamada, M.; Okada, Y.; Yoshida, T.; Nagasawa, T. Purification, characterization and gene cloning of isoeugenol-degrading enzyme from Pseudomonas putida IE27. Arch. Microbiol. 2007, 187, 511–517. [Google Scholar] [CrossRef]
- Ryu, J.Y.; Seo, J.; Park, S.; Ahn, J.H.; Chong, Y.; Sadowsky, M.; Hur, H.G. Characterization of an isoeugenol monooxygenase (Iem) from Pseudomonas nitroreducens Jin1 that transforms isoeugenol to vanillin. Biosci. Biotech. Biochem. 2013, 77, 289–294. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Q.; Wu, X.-M.; Lu, X.-Y.; He, Y.-C.; Ma, B.-D.; Xu, Y. Efficient biosynthesis of vanillin from isoeugenol by recombinant isoeugenol monooxygenase from Pseudomonas nitroreducens Jin1. Appl. Biochem. Biotechnol. 2021, 193, 1116–1128. [Google Scholar] [CrossRef] [PubMed]
- Porter, J.L.; Rusli, R.A.; Ollis, D.L. Directed evolution of enzymes for industrial biocatalysis. ChemBioChem 2016, 17, 197–203. [Google Scholar] [CrossRef]
- Tu, T.; Wang, Y.; Huang, H.-Q.; Wang, Y.; Jiang, X.; Wang, Z.-X.; Yao, B.; Luo, H.-Y. Improving the thermostability and catalytic efficiency of glucose oxidase from Aspergillus niger by molecular evolution. Food Chem. 2019, 281, 163–170. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Su, L.-Q.; Xu, F.; Xia, Y.-M.; Wu, J. Improved thermostability of maltooligosyltrehalose synthase from Arthrobacter ramosus by directed evolution and site-directed mutagenesis. J. Agric. Food Chem. 2019, 67, 5587–5595. [Google Scholar] [CrossRef]
- Schmidt, S.; Genz, M.; Balke, K.; Bornscheuer, U.T. The effect of disulfide bond introduction and related Cys/Ser mutations on the stability of a cyclohexanone monooxygenase. J. Biotechnol. 2015, 214, 199–211. [Google Scholar] [CrossRef] [PubMed]
- Saab-Rincon, G.; Alwaseem, H.; Guzimán-Luna, V.; Olvera, L.; Fasan, R. Stabilization of the reductase domain in the catalytically self-sufficient cytochrome P450BM3 via consensus-guided mutagenesis. ChemBioChem 2018, 19, 622–632. [Google Scholar] [CrossRef]
- Hua, Y.-J.; Lyu, C.-J.; Liu, C.-Y.; Wang, H.-P.; Hu, S.; Zhao, W.-R.; Mei, J.-Q.; Huang, J.; Mei, L.-H. Improving the thermostability of glutamate decarboxylase from Lactobacillus brevis by consensus mutagenesis. Appl. Biochem. Biotechnol. 2020, 191, 1456–1469. [Google Scholar] [CrossRef]
- Xie, D.-F.; Yang, J.-X.; Lv, C.-J.; Mei, J.-Q.; Wang, H.-P.; Hu, S.; Zhao, W.-R.; Cao, J.-R.; Tu, J.-L.; Huang, J.; et al. Construction of stabilized (R)-selective amine transaminase from Aspergillusterreus by consensus mutagenesis. J. Biotechnol. 2019, 293, 8–16. [Google Scholar] [CrossRef]
- Wang, R.; Wang, S.; Xu, Y.; Yu, X.-W. Enhancing the thermostability of Rhizopuschinensis lipase by rational design and MD simulations. Int. J. Biol. Macromol. 2020, 160, 1189–1200. [Google Scholar] [CrossRef]
- Argos, P.; Rossmann, M.G.; Grau, U.M.; Zuber, H.; Frank, G.; Tratschin, J.D. Thermal stability and protein structure. Biochemistry 1979, 18, 5698–5703. [Google Scholar] [CrossRef] [PubMed]
- Mrabet, N.T.; Van den Broeck, A.; Van den Brande, I.; Stanssens, P.; Laroche, Y.; Lambeir, A.M.; Matthijssens, G.; Jenkins, J.; Chiadmi, M.; Van, T.H. Arginine residues as stabilizing elements in proteins. Biochemistry 1992, 31, 2239–2253. [Google Scholar] [CrossRef]
- Liu, Z.-M.; Zheng, W.-H.; Wang, C.; Gao, Y.; Cui, W.-J.; Zhou, Z.-M. Characterization of cysteine sulfinic acid decarboxylase from Tribolium castaneum and its application in the production of β-alanine. Appl. Microbiol. Biotechnol. 2019, 103, 9443–9453. [Google Scholar] [CrossRef] [PubMed]
- Jones, B.J.; Kan, C.N.E.; Luo, C.; Kazlauskas, R.J. Consensus Finder web tool to predict stabilizing substitutions in proteins. Methods Enzymol. 2020, 643, 129–148. [Google Scholar]
- Duan, X.-G.; Chen, J.; Wu, J. Improving the thermostability and catalytic efficiency of Bacillus deramificans Pullulanase by site-directed mutagenesis. Appl. Environ. Microbial. 2013, 79, 4072–4077. [Google Scholar] [CrossRef] [Green Version]
- Wu, I.; Arnold, F.H. Engineered thermostable fungal Cel6A and Cel7A cellobiohydrolases hydrolyze cellulose efficiency at elevated temperatures. Biotechnol. Bioeng. 2013, 110, 1874–1883. [Google Scholar] [CrossRef]
- Han, N.-Y.; Ma, Y.; Mu, Y.-L.; Tang, X.-H.; Li, J.-J.; Huang, Z.-X. Enhancing thermal tolerance of a fungal GH11 xylanase guided by B-factor analysis and multiple sequence alignment. Enzyme Microb. Technol. 2019, 131, 109422. [Google Scholar] [CrossRef] [PubMed]
- Fraczkiewicz, R.; Braun, W. Exact and efficient analytical calculation of the accessible surface areas and their gradients for macromolecules. J. Comput. Chem. 1998, 19, 319–333. [Google Scholar] [CrossRef]
- Jones, B.J.; Kan, C.N.E.; Luo, C.; Kazlauskas, R.J. Comparison of five protein engineering strategies to stabilize an α/β-hydrolase. Biochemistry 2017, 56, 6521–6532. [Google Scholar] [CrossRef]
- Eijsink, V.; Bjork, A.; Gaseidnes, S.; Sirevag, R.; Synstad, B.; Burg, B.; Vriend, G. Rational engineering of enzyme stability. J. Biotechnol. 2004, 113, 105–120. [Google Scholar] [CrossRef]
- Li, W.-F.; Zhou, X.-X.; Lu, P. Structural features of thermozymes. Biotechnol. Adv. 2005, 23, 271–281. [Google Scholar] [CrossRef]
- Mahadevi, A.S.; Sastry, G.N. Cation-π Interaction: Its role and relevance in chemistry, biology and material science. Chem. Rev. 2013, 113, 2100–2138. [Google Scholar] [CrossRef]
- Kabsch, W.; Sander, C. Dictionary of protein secondary structure: Pattern of hydrogen-bonded and geometrical features. Biopolymers 1983, 22, 2577–2637. [Google Scholar] [CrossRef]
- Polizzi, K.M.; Bommarius, A.S.; Broering, J.M.; Chaparro-Riggers, J.F. Stability of biocatalysts. Curr. Opin. Chem. Biol. 2007, 11, 220–225. [Google Scholar] [CrossRef]
- Ashengroph, M.; Nahvi, I.; Zarkesh-Esfahani, H.; Momenbeik, F. Candida galli strains PGO6: A novel isolated yeast strain capable of transformation of isoeugenol to vanillin and vanillic acid. Curr. Microbiol. 2011, 62, 990–998. [Google Scholar] [CrossRef]
- Ashengroph, M.; Nahvi, I.; Zarkesh-Esfahani, H.; Momenbeik, F. Use of growing cells of Pseudomonas aeruginosa for synthesis of the natural vanillin via conversion of isoeugenol. Iran. J. Pharm. Res. 2011, 10, 749–757. [Google Scholar]
- Li, Y.-H.; Sun, Z.-H.; Zhao, L.-Q.; Xu, Y. Bioconversion of isoeugenol into vanillin by crude enzyme extracted from soybean. Appl. Biochem. Biotechnol. 2005, 125, 1–10. [Google Scholar] [CrossRef]
- Arnold, K.; Bordoli, L.; Kopp, J.; Schwede, T. The SWISS-MODEL workspace: A web-based environment for protein structure homology modeling. Bioinformatics 2006, 22, 195–201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Loewen, P.C.; Switala, J.; Wells, J.P.; Huang, F.; Zara, A.T.; Allingham, J.S.; Loewen, M.C. Structure and function of a lignostilbene-α,β-dioxygenase orthologue from Pseudomonas brassicacearum. BMC Biochem. 2018, 19, 8. [Google Scholar] [CrossRef] [PubMed]
- Laskowski, R.A.; Jablońska, J.; Pravda, L.; Vařeková, R.S.; Thornton, J.M. PDBsum: Structural summaries of PDB enteries. Protein Sci. 2018, 27, 129–134. [Google Scholar] [CrossRef] [PubMed]
- Bowie, J.U.; Lüthy, R.; Eisenberg, D. A method to identify protein sequences that fold into a known three-dimensional structure. Science 1991, 253, 164–170. [Google Scholar] [CrossRef] [Green Version]
- Larkin, M.A.; Blackshields, G.; Brown, N.P.; Chenna, R.; Mcgettigan, P.A.; Mcwilliam, H.; Valentin, F.; Wallace, I.M.; Wilm, A.; Lopez, R. Clustal W and Clustal X version 2.0. Bioinformatics 2007, 23, 2947–2948. [Google Scholar] [CrossRef] [Green Version]
- Crooks, G.E.; Hon, G.; Chandonia, J.M.; Brenner, S.E. WebLogo: A sequence logo generator. Genome Res. 2004, 14, 1188–1190. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ho, S.N.; Hunt, H.D.; Horton, R.M.; Pullen, J.K.; Pease, L.R. Site-directed mutagenesis by overlap extension using the polymerase chain reaction. Gene 1989, 77, 51–59. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
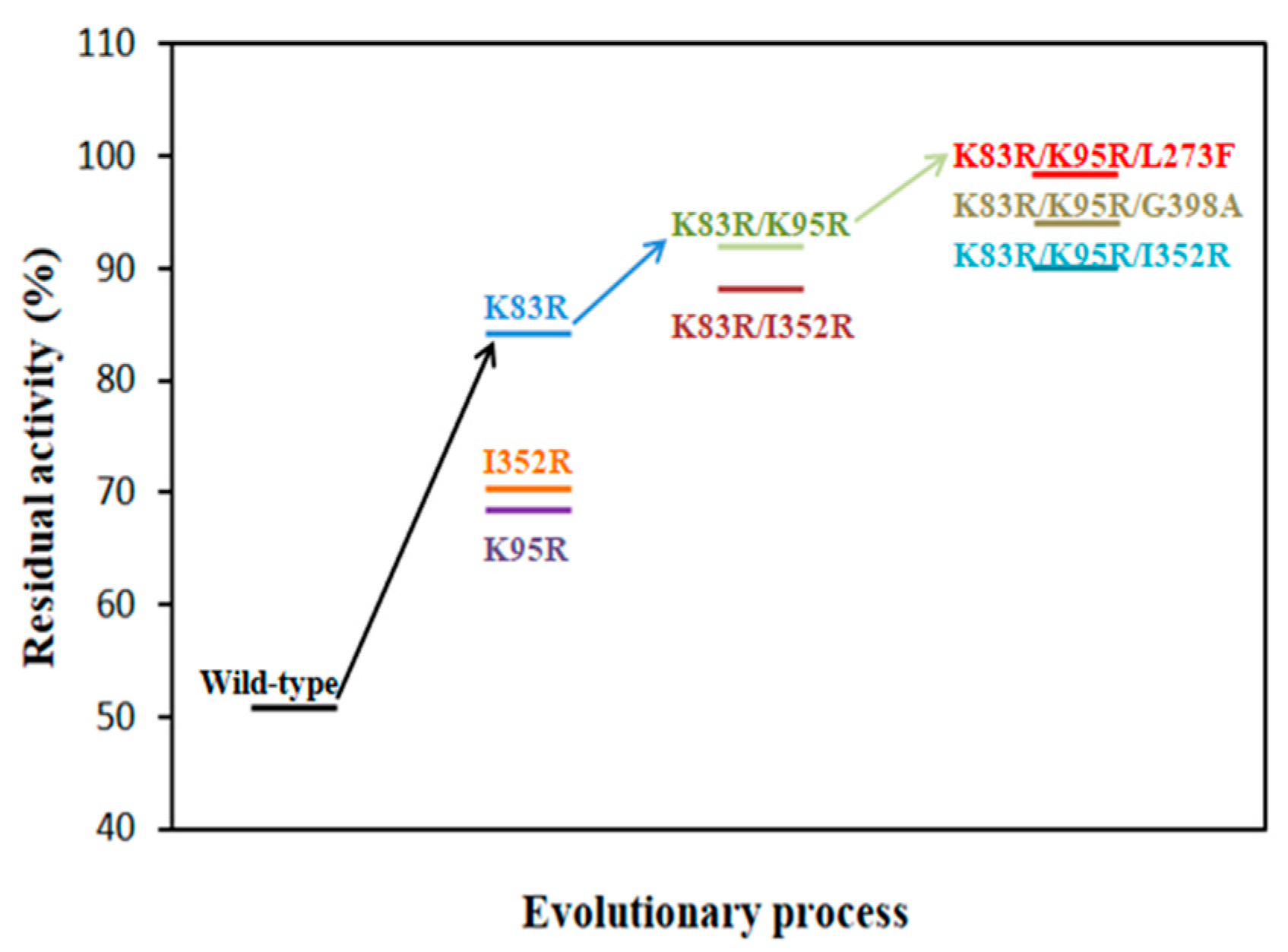
| Strategy | Mutants | Relative Activity (%) # | Residual Activity (%) * |
|---|---|---|---|
| - | IEM | 100.0 ± 8.0 | 53.2 ± 2.4 |
| Surface residue replacement | K83R | 103.9 ± 3.8 | 84.2 ± 2.7 |
| K95R | 106.6 ± 3.7 | 68.0 ± 0.4 | |
| G398A | 130.4 ± 1.5 | 55.6 ± 1.5 | |
| Consensus mutagenesis | I352R | 99.7 ± 1.3 | 70.4 ± 2.4 |
| L273F | 117.2 ± 0.6 | 50.4 ± 0.6 | |
| Combinatorial mutagenesis | K83R/K95R | 104.8 ± 1.0 | 92.1 ± 0.9 |
| K83R/I352R | 99.2 ± 4.2 | 87.8 ± 3.0 | |
| K83R/K95R/I352R | 114.3 ± 2.1 | 90.1 ± 2.5 | |
| K83R/K95R/G398A | 130.1 ± 3.0 | 94.1 ± 1.5 | |
| K83R/K95R/L273F | 168.2 ± 7.3 | 98.5 ± 0.3 |
| Enzymes * | t1/2 | ||
|---|---|---|---|
| 25 °C (min) | 30 °C (min) | 35 °C (min) | |
| Wild-type | 967.2 | 49.8 | 7.8 |
| K83R | 1094.4 | 267.2 | 31.8 |
| K95R | 1108.8 | 198.0 | 28.8 |
| L273F | 602.7 | 133.2 | 12.6 |
| K83R/K95R | 2224.2 | 491.4 | 94.8 |
| K83R/K95R/L273F | 2848.8 | 594.0 | 192.6 |
| Enzymes | Km (mM) | kcat (s−1) | kcat/Km (mM−1 s−1) | Specific Activity * (U mg−1) |
|---|---|---|---|---|
| Wild-type | 0.1 ± 0.02 | 2.1 ± 0.09 | 21.0 | 3.4 ± 0.15 |
| K83R | 0.6 ± 0.12 | 5.5 ± 0.41 | 9.2 | 6.1 ± 0.04 |
| K95R | 0.4 ± 0.12 | 6.1 ± 0.55 | 15.3 | 5.3 ± 0.02 |
| L273F | 0.3 ± 0.11 | 6.9 ± 0.49 | 23.0 | 5.5 ± 0.09 |
| K83R/K95R | 0.5 ± 0.11 | 10.5 ± 0.69 | 21.0 | 6.0 ± 0.14 |
| K83R/K95R/L273F | 0.4 ± 0.12 | 10.1 ± 0.92 | 25.3 | 7.0 ± 0.04 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, X.-Y.; Wu, X.-M.; Ma, B.-D.; Xu, Y. Enhanced Thermostability of Pseudomonas nitroreducens Isoeugenol Monooxygenase by the Combinatorial Strategy of Surface Residue Replacement and Consensus Mutagenesis. Catalysts 2021, 11, 1199. https://doi.org/10.3390/catal11101199
Lu X-Y, Wu X-M, Ma B-D, Xu Y. Enhanced Thermostability of Pseudomonas nitroreducens Isoeugenol Monooxygenase by the Combinatorial Strategy of Surface Residue Replacement and Consensus Mutagenesis. Catalysts. 2021; 11(10):1199. https://doi.org/10.3390/catal11101199
Chicago/Turabian StyleLu, Xin-Yi, Xiao-Mei Wu, Bao-Di Ma, and Yi Xu. 2021. "Enhanced Thermostability of Pseudomonas nitroreducens Isoeugenol Monooxygenase by the Combinatorial Strategy of Surface Residue Replacement and Consensus Mutagenesis" Catalysts 11, no. 10: 1199. https://doi.org/10.3390/catal11101199
APA StyleLu, X.-Y., Wu, X.-M., Ma, B.-D., & Xu, Y. (2021). Enhanced Thermostability of Pseudomonas nitroreducens Isoeugenol Monooxygenase by the Combinatorial Strategy of Surface Residue Replacement and Consensus Mutagenesis. Catalysts, 11(10), 1199. https://doi.org/10.3390/catal11101199






