Acesulfame K Photodegradation over Nitrogen-Doped TiO2
Abstract
:1. Introduction
2. Results and Discussion
2.1. Catalysts Characterization
2.2. Physical Interaction of the Ace K Molecule and Catalyst Surface
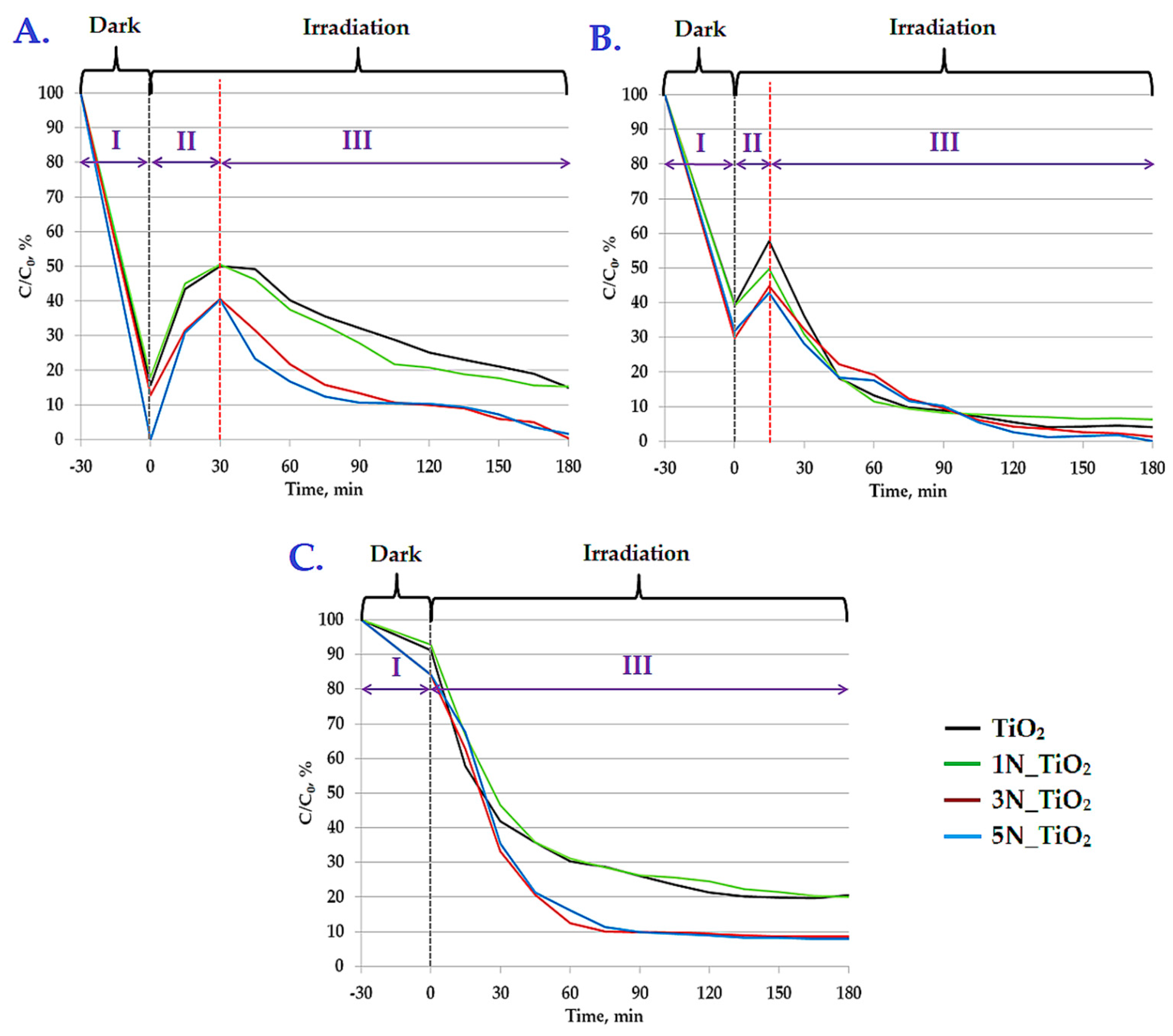
2.3. Acesulfame K Photodegradation
3. Materials and Methods
3.1. Un-Doped and N-Doped TiO2 Catalysts Synthesis
3.2. Catalysts Characterization
3.3. Photodegradation Procedure
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- CFR—Code of Federal Regulations Title 21, Chapter I, Subchapter B, Part 172, Subpart I, Section 172.800: Acesulfame Potassium; US Federal Government: Washington, DC, USA, 2016.
- Piekara, A.; Krzywonos, M.; Szymańska, A. Sweetening Agents and Sweeteners in Dietary Supplements for Children-Analysis of the Polish Market. Nutrients 2020, 12, 2387. [Google Scholar] [CrossRef]
- Salimi, M.; Esrafili, A.; Gholami, M.; Jonidi Jafari, A.; Rezaei Kalantary, R.; Farzadkia, M.; Kermani, M.; Sobhi, H.R. Contaminants of emerging concern: A review of new approach in AOP technologies. Environ. Monit. Assess. 2017, 189, 1–22. [Google Scholar] [CrossRef]
- Chappell, G.A.; Wikoff, D.S.; Doepker, C.L.; Borghoff, S.J. Lack of potential carcinogenicity for acesulfame potassium—Systematic evaluation and integration of mechanistic data into the totality of the evidence. Food Chem. Toxicol. 2020, 141, 111375. [Google Scholar] [CrossRef] [PubMed]
- Minella, M.; Giannakis, S.; Mazzavillani, A.; Maurino, V.; Minero, C.; Vione, D. Phototransformation of Acesulfame K in surface waters: Comparison of two techniques for the measurement of the second-order rate constants of indirect photodegradation, and modelling of photoreaction kinetics. Chemosphere 2017, 186, 185–192. [Google Scholar] [CrossRef]
- Cruz-Rojas, C.; SanJuan-Reyes, N.; Fuentes-Benites, M.P.A.G.; Dublan-García, O.; Galar-Martínez, M.; Islas-Flores, H.; Gómez-Oliván, L.M. Acesulfame potassium: Its ecotoxicity measured through oxidative stress biomarkers in common carp (Cyprinus carpio). Sci. Total Environ. 2019, 647, 772–784. [Google Scholar] [CrossRef] [PubMed]
- Shahriar, S.; Ahsan, T.; Khan, A.; Akhteruzzaman, S.; Shehreen, S.; Sajib, A.A. Aspartame, acesulfame K and sucralose- influence on the metabolism of Escherichia coli. Metab. Open 2020, 8, 100072. [Google Scholar] [CrossRef]
- Gan, Z.; Sun, H.; Wang, R.; Hu, H.; Zhang, P.; Ren, X. Transformation of acesulfame in water under natural sunlight: Joint effect of photolysis and biodegradation. Water Res. 2014, 64, 113–122. [Google Scholar] [CrossRef]
- Ghosh, M.; Chowdhury, P.; Ray, A.K. Study of solar photocatalytic degradation of Acesulfame K to limit the outpouring of artificial sweeteners. Sep. Purif. Technol. 2018, 207, 51–57. [Google Scholar] [CrossRef]
- Yu, H.W.; Park, M.; Wu, S.; Lopez, I.J.; Ji, W.; Scheideler, J.; Snyder, S.A. Strategies for selecting indicator compounds to assess attenuation of emerging contaminants during UV advanced oxidation processes. Water Res. 2019, 166, 115030. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Thuy, G.N.S.T.; Srivastava, V.; Ambat, I.; Sillanpää, M. Photocatalytic degradation of an artificial sweetener (Acesulfame-K) from synthetic wastewater under UV-LED controlled illumination. Process Saf. Environ. Prot. 2019, 123, 206–214. [Google Scholar] [CrossRef]
- Chow, C.H.; Law, J.C.F.; Leung, K.S.Y. Degradation of acesulfame in UV/monochloramine process: Kinetics, transformation pathways and toxicity assessment. J. Hazard. Mater. 2021, 403, 123935. [Google Scholar] [CrossRef]
- Fu, Y.; Wu, G.; Geng, J.; Li, J.; Li, S.; Ren, H. Kinetics and modeling of artificial sweeteners degradation in wastewater by the UV/persulfate process. Water Res. 2019, 150, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Punturat, V.; Huang, K.L. Degradation pathways and organic matter transformation of acesulfame potassium electro-oxidation in real water matrices. J. Taiwan Inst. Chem. Eng. 2017, 80, 222–230. [Google Scholar] [CrossRef]
- Punturat, V.; Huang, K.L. Degradation of acesulfame in aqueous solutions by electro-oxidation. J. Taiwan Inst. Chem. Eng. 2016, 63, 286–294. [Google Scholar] [CrossRef]
- Shao, Y.; Pang, Z.; Wang, L.; Liu, X. Efficient Degradation of Acesulfame by Ozone/Peroxymonosulfate Advanced Oxidation Process. Molecules 2019, 24, 2874. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, R.; Lu, S.; Song, Y.; Ma, X.; Li, X.; Jia, J.; Deng, Y. Occurrence of Emerging Contaminant Acesulfame in Water Treatment System and Its Degradation during Ozone Oxidation. Ozone Sci. Eng. 2021, 43, 185–194. [Google Scholar] [CrossRef]
- López-Muňoz, M.J.; Daniele, A.; Zorzi, M.; Medana, C.; Calza, P. Investigation of the photocatalytic transformation of acesulfame K in the presence of different TiO2-based materials. Chemosphere 2018, 193, 151–159. [Google Scholar] [CrossRef]
- Appasamy, J.S.; Kurnia, J.C. Synthesis and Evaluation of Novel TiO2-based Self-cleaning Coating Layer for Buildings. IOP Conf. Ser. Earth Environ. Sci. 2019, 268, 102049. [Google Scholar] [CrossRef]
- Aba Guevara, C.G.; Sanjuan Galindo, R.; Gracia Pinilla, M.A.; Martínez Vargas, D.X.; Ramos Delgado, N.A. Water Disinfection Using Chitosan Microbeads With N-, C-, C-N/TiO2 By Photocatalysis Under Visible Light. Top. Catal. 2020, 64, 142–154. [Google Scholar] [CrossRef]
- Ahmad, M.A.; Yuesuo, Y.; Ao, Q.; Adeel, M.; Hui, Z.Y.; Javed, R. Appraisal of Comparative Therapeutic Potential of Undoped and Nitrogen-Doped Titanium Dioxide Nanoparticles. Molecules 2019, 24, 3916. [Google Scholar] [CrossRef] [Green Version]
- Bakre, P.V.; Tilve, S.G.; Shirsat, R.N. Influence of N sources on the photocatalytic activity of N-doped TiO2. Arab. J. Chem. 2020, 13, 7637–7651. [Google Scholar] [CrossRef]
- Cherni, D.; Moussa, N.; Nsib, M.F.; Olivo, A.; Signoretto, M.; Prati, L.; Villa, A. Photocatalytic degradation of ethylbenzene in gas phase over N or NF doped TiO2 catalysts. J. Mater. Sci. Mater. Electron. 2019, 30, 18919–18926. [Google Scholar] [CrossRef]
- Barkul, R.P.; Koli, V.B.; Shewale, V.B.; Patil, M.K.; Delekar, S.D. Visible active nanocrystalline N-doped anatase TiO2 particles for photocatalytic mineralization studies. Mater. Chem. Phys. 2016, 173, 42–51. [Google Scholar] [CrossRef]
- Khan, M.S.; Shah, J.A.; Arshad, M.; Halim, S.A.; Khan, A.; Shaikh, A.J.; Riaz, N.; Khan, A.J.; Arfan, M.; Shahid, M.; et al. Photocatalytic Decolorization and Biocidal Applications of Nonmetal Doped TiO2: Isotherm, Kinetic Modeling and In Silico Molecular Docking Studies. Molecules 2020, 25, 4468. [Google Scholar] [CrossRef] [PubMed]
- Yadav, V.; Saini, V.K.; Sharma, H. How different dopants leads to difference in photocatalytic activity in doped TiO2? Ceram. Int. 2020, 46, 27308–27317. [Google Scholar] [CrossRef]
- Gazulla, M.F.; Rodrigo, M.; Blasco, E.; Orduña, M. Nitrogen determination by SEM-EDS and elemental analysis. X-ray Spectrom. 2013, 42, 394–401. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef] [Green Version]
- Ansari, S.A.; Khan, M.M.; Ansari, M.O.; Cho, M.H. Nitrogen-doped titanium dioxide (N-doped TiO2) for visible light photocatalysis. New J. Chem. 2016, 40, 3000–3009. [Google Scholar] [CrossRef]
- Asahi, R.; Morikawa, T.; Irie, H.; Ohwaki, T. Nitrogen-doped titanium dioxide as visible-light-sensitive photocatalyst: Designs, developments, and prospects. Chem. Rev. 2014, 114, 9824–9852. [Google Scholar] [CrossRef] [PubMed]
- Peighambardoust, N.S.; Khameneh Asl, S.; Mohammadpour, R.; Asl, S.K. Band-gap narrowing and electrochemical properties in N-doped and reduced anodic TiO2 nanotube arrays. Electrochim. Acta 2018, 270, 245–255. [Google Scholar] [CrossRef]
- Katoueizadeh, E.; Zebarjad, S.M.; Janghorban, K. Synthesis and enhanced visible-light activity of N-doped TiO2 nano-additives applied over cotton textiles. J. Mater. Res. Technol. 2018, 7, 204–211. [Google Scholar] [CrossRef]
- Gomes, J.; Lincho, J.; Domingues, E.; Quinta-Ferreira, R.M.; Martins, R.C. N-TiO2 photocatalysts: A review of their characteristics and capacity for emerging contaminants removal. Water 2019, 11, 373. [Google Scholar] [CrossRef] [Green Version]
- Chiu, Y.H.; Chang, T.F.M.; Chen, C.Y.; Sone, M.; Hsu, Y.J. Mechanistic insights into photodegradation of organic dyes using heterostructure photocatalysts. Catalysts 2019, 9, 430. [Google Scholar] [CrossRef] [Green Version]
- Colmenares, J.C.; Luque, R.; Campelo, J.M.; Colmenares, F.; Karpiński, Z.; Romero, A.A. Nanostructured photocatalysts and their applications in the photocatalytic transformation of lignocellulosic biomass: An overview. Materials 2009, 2, 2228–2258. [Google Scholar] [CrossRef]
- Lorenc-Grabowska, E.; Diez, M.A.; Gryglewicz, G. Influence of pore size distribution on the adsorption of phenol on PET-based activated carbons. J. Colloid Interface Sci. 2016, 469, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Gopinath, K.P.; Madhav, N.V.; Krishnan, A.; Malolan, R.; Rangarajan, G. Present applications of titanium dioxide for the photocatalytic removal of pollutants from water: A review. J. Environ. Manag. 2020, 270, 110906. [Google Scholar] [CrossRef] [PubMed]
- Nolan, N.T.; Synnott, D.W.; Seery, M.K.; Hinder, S.J.; Van Wassenhoven, A.; Pillai, S.C. Effect of N-doping on the photocatalytic activity of sol–gel TiO2. J. Hazard. Mater. 2012, 211–212, 88–94. [Google Scholar] [CrossRef] [Green Version]
- Patterson, A.L. The Scherrer Formula for X-Ray Particle Size Determination. Phys. Rev. 1939, 56, 978–982. [Google Scholar] [CrossRef]
- López, R.; Gómez, R. Band-gap energy estimation from diffuse reflectance measurements on sol-gel and commercial TiO2: A comparative study. J. Sol-Gel Sci. Technol. 2012, 61, 1–7. [Google Scholar] [CrossRef]
- Moreno-Castilla, C.; López-Ramón, M.V.; Carrasco-Marín, F. Changes in surface chemistry of activated carbons by wet oxidation. Carbon 2000, 38, 1995–2001. [Google Scholar] [CrossRef]
- Pstrowska, K.; Szyja, B.M.; Czapor-Irzabek, H.; Kiersnowski, A.; Walendziewski, J. The Properties and Activity of TiO2/beta-SiC Nanocomposites in Organic Dyes Photodegradation. Photochem. Photobiol. 2017, 93, 558–568. [Google Scholar] [CrossRef]
- Turak, F.; Ozgur, M.U. Validated spectrophotometric methods for simultaneous determination of food colorants and sweeteners. J. Chem. 2013, 2013, 127847. [Google Scholar] [CrossRef] [Green Version]

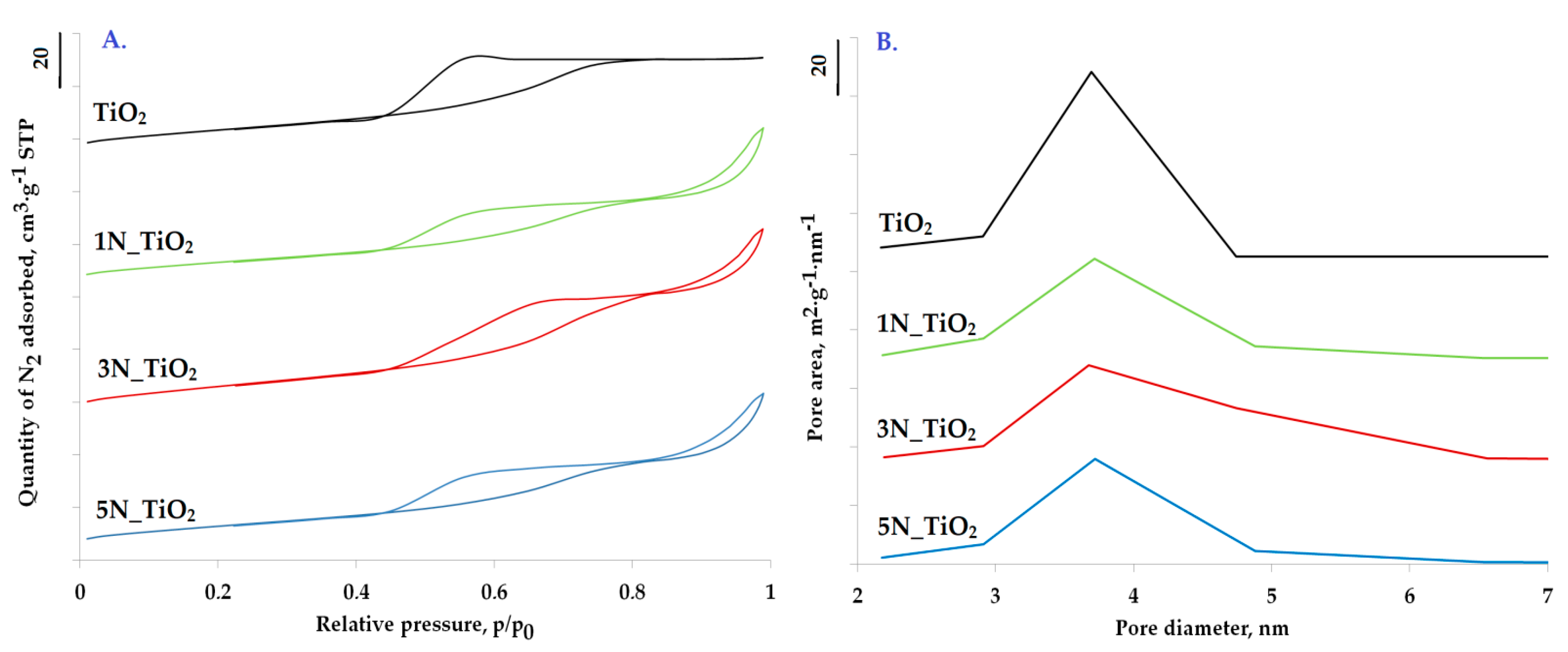

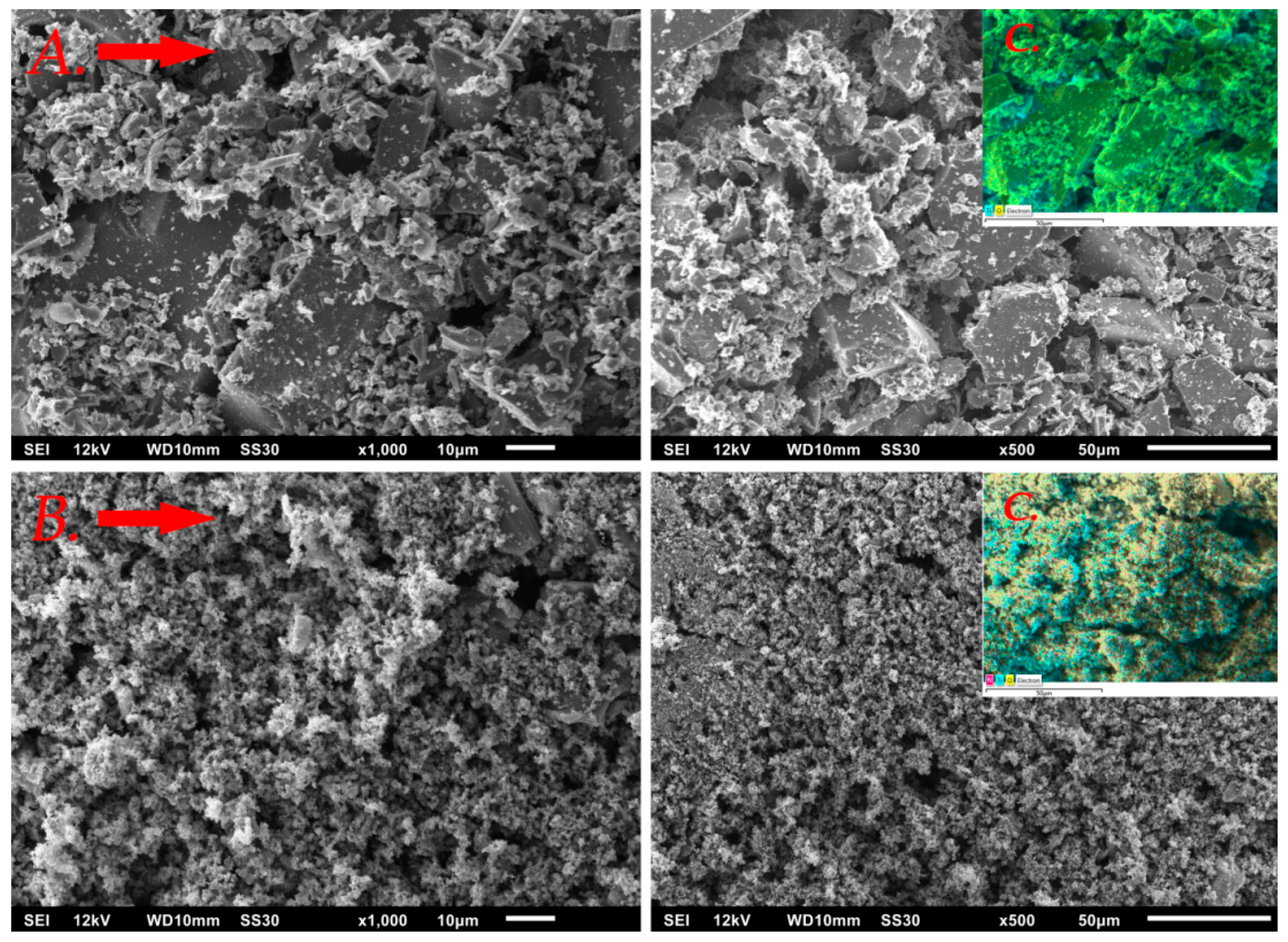
| Catalyst | SBET | VTOT | ADP | Average Crystallite Size | Band Gap Energy | pHPZC | Nitrogen Content |
|---|---|---|---|---|---|---|---|
| m2∙g−1 | cm3∙g−1 | nm | nm | eV | - | wt % | |
| TiO2 | 48.55 | 0.063 | 3.71 | 15.43 | 3.02 | 5.72 | - |
| 1N_TiO2 | 49.41 | 0.081 | 6.86 | 15.38 | 3.18 | 5.69 | 1.55 |
| 3N_TiO2 | 58.23 | 0.111 | 6.36 | 15.00 | 3.18 | 5.60 | 4.83 |
| 5N_TiO2 | 56.92 | 0.092 | 6.61 | 15.17 | 3.17 | 5.05 | 6.29 |
| Characteristic | 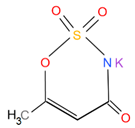 |
|---|---|
| Acesulfame K | |
| Molecular weight, g mol−1 | 163.15 |
| Solubility in water at 20 °C, g dm−3 | 270 |
| The longest spatial dimension of the dissociated form of the molecule *, nm | 0.574 |
| pKa | 2.0 |
| 100 ppm solution pH | 6.019 |
| 50 ppm solution pH | 6.202 |
| 20 ppm solution pH | 6.420 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pstrowska, K.; Czapor-Irzabek, H.; Borowiak, D.; Burchacka, E. Acesulfame K Photodegradation over Nitrogen-Doped TiO2. Catalysts 2021, 11, 1193. https://doi.org/10.3390/catal11101193
Pstrowska K, Czapor-Irzabek H, Borowiak D, Burchacka E. Acesulfame K Photodegradation over Nitrogen-Doped TiO2. Catalysts. 2021; 11(10):1193. https://doi.org/10.3390/catal11101193
Chicago/Turabian StylePstrowska, Katarzyna, Hanna Czapor-Irzabek, Daniel Borowiak, and Ewa Burchacka. 2021. "Acesulfame K Photodegradation over Nitrogen-Doped TiO2" Catalysts 11, no. 10: 1193. https://doi.org/10.3390/catal11101193
APA StylePstrowska, K., Czapor-Irzabek, H., Borowiak, D., & Burchacka, E. (2021). Acesulfame K Photodegradation over Nitrogen-Doped TiO2. Catalysts, 11(10), 1193. https://doi.org/10.3390/catal11101193










