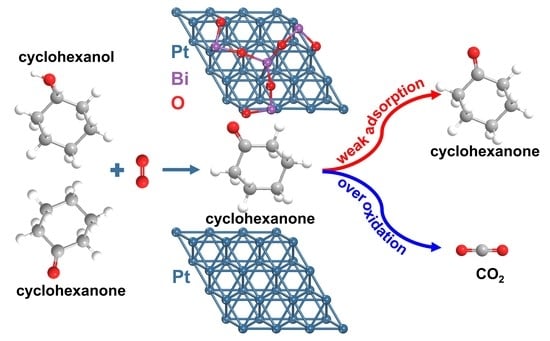
Engineering Pt-Bi2O3 Interface to Boost Cyclohexanone Selectivity in Oxidative Dehydrogenation of KA-Oil
Abstract
:1. Introduction
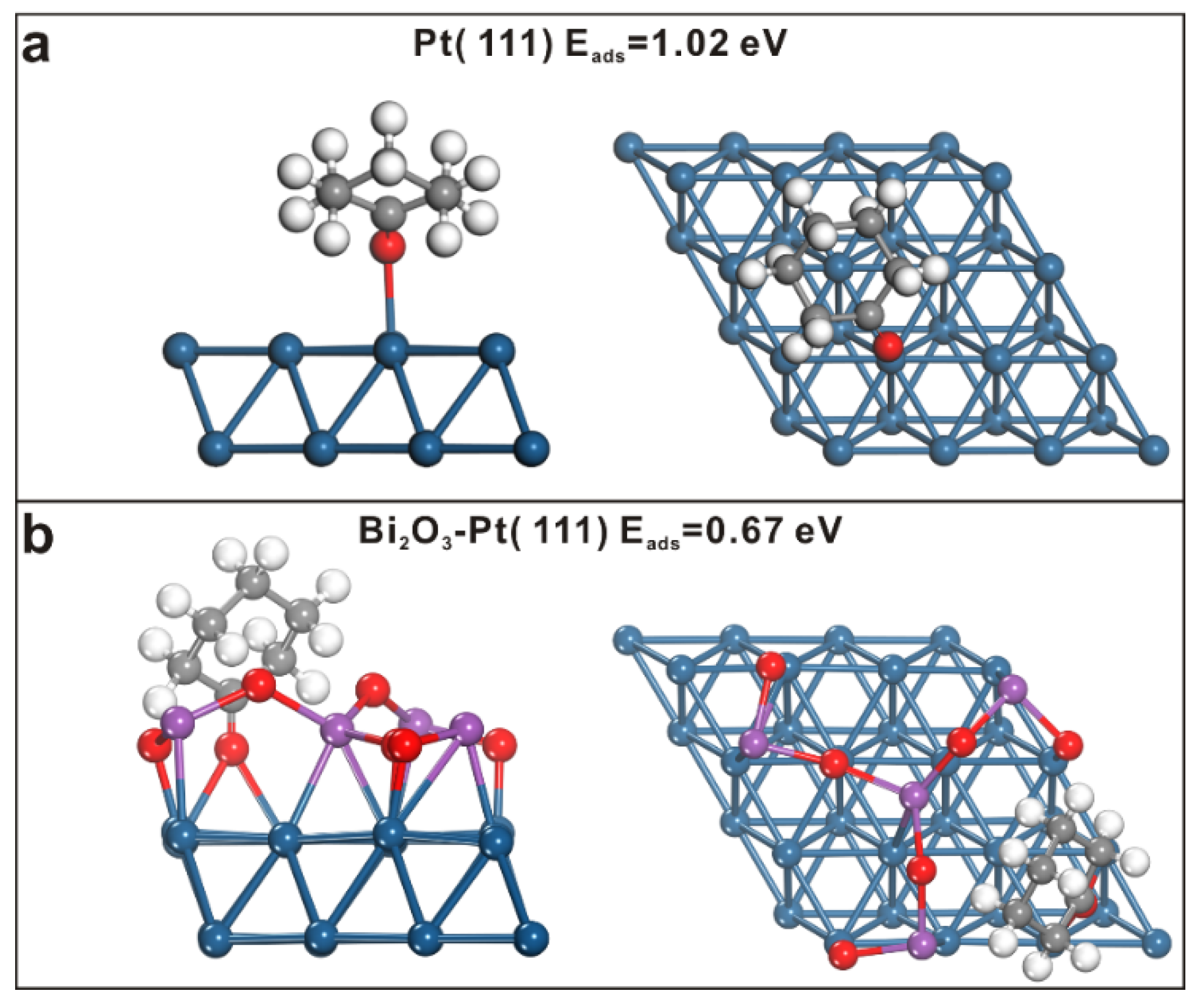
2. Results
3. Materials and Methods
3.1. Catalyst Preparation
3.1.1. Synthesis of Silica Nanospheres
3.1.2. Synthesis of Pt Nanoparticles
3.1.3. Preparation of Pt/SiO2 and PtBix/SiO2
3.2. Catalyst Characterization
3.3. Catalytic Measurement
3.4. DFT Calculations
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Liu, H.; Jiang, T.; Han, B.; Liang, S.; Zhou, Y. Selective Phenol Hydrogenation to Cyclohexanone Over a Dual Supported Pd–Lewis Acid Catalyst. Science 2009, 326, 1250–1252. [Google Scholar] [CrossRef]
- Li, Z.L.; Liu, J.H.; Xia, C.G.; Li, F.W. Nitrogen-Functionalized Ordered Mesoporous Carbons as Multifunctional Supports of Ultrasmall Pd Nanoparticles for Hydrogenation of Phenol. ACS Catal. 2013, 3, 2440–2448. [Google Scholar] [CrossRef]
- Dugal, M.; Sankar, G.; Raja, R.; Thomas, J.M. Designing a Heterogeneous Catalyst for the Production of Adipic Acid by Aerial Oxidation of Cyclohexane. Angew. Chem. Int. Ed. 2000, 39, 2310–2313. [Google Scholar] [CrossRef]
- Fridman, V.Z.; Davydov, A.A. Dehydrogenation of Cyclohexanol on Copper-Containing Catalysts I. The Influence of the Oxidation State of Copper on the Activity of Copper Sites. J. Catal. 2000, 195, 20–30. [Google Scholar] [CrossRef]
- Nagaraja, B.M.; Kumar, V.S.; Shashikala, V.; Padmasri, A.H.; Reddy, S.S.; Raju, B.D.; Rao, K.S.R. Effect of Method of Preparation of Copper-Magnesium Oxide Catalyst on the Dehydrogenation of Cyclohexanol. J. Mol. Catal. A Chem. 2004, 223, 339–345. [Google Scholar] [CrossRef]
- Tan, K.; Fujiii, K.; Nakamura, M.; Hanada, K. Process for Removing Impurities from the Mixture of Cyclohexanone and Cyclohexanol. U.S. Patent US5168983(A), 22 March 1991. [Google Scholar]
- Becker, C.L.; Davis, J.D.; Dakka, J.M.; Nadler, K.C. Process for Making Cyclohexanone. U.S. Patent US2017283353(A1), 8 September 2015. [Google Scholar]
- Gill, A.M.; Hinde, C.S.; Leary, R.K.; Potter, M.E.; Jouve, A.; Wells, P.P.; Midgley, P.A.; Thomas, J.M.; Raja, R. Design of Highly Selective Platinum Nanoparticle Catalysts for the Aerobic Oxidation of KA-Oil using Continuous-Flow Chemistry. ChemSusChem 2016, 9, 423–427. [Google Scholar] [CrossRef] [Green Version]
- Valente, A.; Lin, Z.; Brandao, P.; Portugal, I.; Anderson, M.; Rocha, J. Gas-phase Oxidative Dehydrogenation of Cyclohexanol over ETS-10 and Related Materials. J. Catal. 2001, 200, 99–105. [Google Scholar] [CrossRef]
- Zhao, G.F.; Yang, F.; Chen, Z.J.; Liu, Q.F.; Ji, Y.J.; Zhang, Y.; Niu, Z.Q.; Mao, J.J.; Bao, X.H.; Hu, P.J.; et al. Metal/Oxide Interfacial Effects on the Selective Oxidation of Primary Alcohols. Nat. Commun. 2017, 8, 14039–14046. [Google Scholar] [CrossRef] [Green Version]
- Yi, W.; Yuan, W.; Meng, Y.; Zou, S.; Zhou, Y.; Hong, W.; Che, J.; Hao, M.; Ye, B.; Xiao, L.; et al. A Rational Solid-State Synthesis of Supported Au-Ni Bimetallic Nanoparticles with Enhanced Activity for Gas-Phase Selective Oxidation of Alcohols. ACS Appl. Mater. Interfaces 2017, 9, 31853–31860. [Google Scholar] [CrossRef]
- Santhanaraj, D.; Suresh, C.; Vijayan, P.; Venkatathri, N.; Shanthi, K. Mn-MCM-41 Molecular Sieves: A Selective Gas-Phase Cyclohexanol Oxidation Catalyst. React. Kinet. Mech. Catal. 2010, 99, 439–446. [Google Scholar] [CrossRef]
- Hong, W.; Wang, Z.-Q.; Zou, S.; Zhou, Y.; Lu, L.; Zhou, Q.; Gong, X.-Q.; Fan, J. Calcination Atmosphere Regulated Morphology and Catalytic Performance of Pt/SiO2 in Gas-phase Oxidative Dehydrogenation of KA-oil. ChemCatChem 2018, 10, 5689–5697. [Google Scholar] [CrossRef]
- Chen, G.; Xu, C.; Huang, X.; Ye, J.; Gu, L.; Li, G.; Tang, Z.; Wu, B.; Yang, H.; Zhao, Z.; et al. Interfacial Electronic Effects Eontrol the Reaction Selectivity of Platinum Catalysts. Nat. Mater. 2016, 15, 564–569. [Google Scholar] [CrossRef] [PubMed]
- Lou, B.; Kang, H.; Yuan, W.; Ma, L.; Huang, W.; Wang, Y.; Jiang, Z.; Du, Y.; Zou, S.; Fan, J. Highly Selective Acetylene Semihydrogenation Catalyst with an Operation Window Exceeding 150 °C. ACS Catal. 2021, 11, 6073–6080. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhu, Y.; Wang, Z.Q.; Zou, S.; Ma, G.; Xia, M.; Kong, X.; Xiao, L.; Gong, X.Q.; Fan, J. Catalytic Activity Control via Crossover between Two Different Microstructures. J. Am. Chem. Soc. 2017, 139, 13740–13748. [Google Scholar] [CrossRef] [Green Version]
- Mallat, T.; Bodnar, Z.; Hug, P.; Baiker, A. Selective Oxidation of Cinnamyl Alcohol to Cinnamaldehyde with Air over Bi-Pt/Alumina Catalysts. J. Catal. 1995, 153, 131–143. [Google Scholar] [CrossRef]
- Villa, A.; Wang, D.; Veith, G.M.; Prati, L. Bismuth as a Modifier of Au–Pd Catalyst: Enhancing Selectivity in Alcohol Oxidation by Suppressing Parallel Reaction. J. Catal. 2012, 292, 73–80. [Google Scholar] [CrossRef]
- Yan, Y.; Ye, B.; Chen, M.; Lu, L.; Yu, J.; Zhou, Y.; Wang, Y.; Liu, J.; Xiao, L.; Zou, S.; et al. Site-specific Deposition Creats Electron-Rich Pd Atoms for Unprecedented C-H Activation in Aerobic Alcohol Oxidation. Chin. J. Catal. 2020, 41, 1240–1247. [Google Scholar] [CrossRef]
- Liu, L.; Li, H.; Tan, Y.; Chen, X.; Lin, R.; Yang, W.; Huang, C.; Wang, S.; Wang, X.; Liu, X.Y.; et al. Metal-Support Synergy of Supported Gold Nanoclusters in Selective Oxidation of Alcohols. Catalysts 2020, 10, 107. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Zou, S.; Wu, J.; Kobayashi, H.; Zhao, H.; Fan, J. Green Catalytic Oxidation of Benzyl Alcohol over Pt/ZnO in Base-Free Aqueous Medium at Room Temperature. Chin. J. Catal. 2018, 39, 1081–1089. [Google Scholar] [CrossRef]
- Liu, J.; Han, F.; Chen, L.; Lu, L.; Zou, S.; Zhao, H. Remarkable Promoting Effects of BiOCl on the Room-Temperature Selective Oxidation of Benzyl Alcohol over Supported Pt Catalysts. New J. Chem. 2018, 42, 8979–8984. [Google Scholar] [CrossRef]
- Mondelli, C.; Grunwaldt, J.-D.; Ferri, D.; Baiker, A. Role of Bi Promotion and Solvent in Platinum-Catalyzed Acohol Oxidation Probed by in Situ X-ray Absorption and ATR-IR Spectroscopy. Phys. Chem. Chem. Phys. 2010, 12, 5307–5316. [Google Scholar] [CrossRef]
- Mallat, T.; Bodnar, Z.; Baiker, A.; Greis, O.; Strubig, H.; Reller, A. Preparation of Promoted Platinum Catalysts of Designed Geometry and the Role of Promoters in the Liquid-Phase Oxidation of 1-Methoxy-2-propanol. J. Catal. 1993, 142, 237–253. [Google Scholar] [CrossRef]
- Zhou, C.; Guo, Z.; Dai, Y.; Jia, X.; Yu, H.; Yang, Y. Promoting Role of Bismuth on Carbon Nanotube Supported Platinum Catalysts in Aqueous Phase Aerobic Oxidation of Benzyl Alcohol. Appl. Catal. B Environ. 2016, 181, 118–126. [Google Scholar] [CrossRef]
- Oganov, A.R.; Lyakhov, A.O.; Valle, M. How Evolutionary Crystal Structure Prediction Works—And Why. Acc. Chem. Res. 2011, 44, 227–237. [Google Scholar] [CrossRef]
- Wang, Q.; Oganov, A.R.; Zhu, Q.; Zhou, X.-F. New Reconstructions of the (110) Surface of Rutile TiO2 Predicted by an Evolutionary Method. Phys. Rev. Lett. 2014, 113, 266101. [Google Scholar] [CrossRef] [Green Version]
- Teranishi, T.; Hosoe, M.; Tanaka, T.; Miyake, M. Size Control of Monodispersed Pt Nanoparticles and Their 2D Organization by Electrophoretic Deposition. J. Phys. Chem. B 1999, 103, 3818–3827. [Google Scholar] [CrossRef]
- Liu, S.; Tian, N.; Xie, A.-Y.; Du, J.-H.; Xiao, J.; Liu, L.; Sun, H.-Y.; Cheng, Z.-Y.; Zhou, Z.-Y.; Sun, S.-G. Electrochemically Seed-Mediated Synthesis of Sub-10 nm Tetrahexahedral Pt Nanocrystals Supported on Graphene with Improved Catalytic Performance. J. Am. Chem. Soc. 2016, 138, 5753–5756. [Google Scholar] [CrossRef]
- Ning, X.; Li, Y.; Yu, H.; Peng, F.; Wang, H.; Yang, Y. Promoting Role of Bismuth and Antimony on Pt Catalysts for the Selective Oxidation of Glycerol to Dihydroxyacetone. J. Catal. 2016, 335, 95–104. [Google Scholar] [CrossRef]
- Huang, Y.; Cai, J.; Guo, Y. A High-Efficiency Microwave Approach to Synthesis of Bi-Modified Pt Nanoparticle Catalysts for Ethanol Electro-Oxidation in Alkaline Medium. Appl. Catal. B Environ. 2013, 129, 549–555. [Google Scholar] [CrossRef]
- Nyholm, R.; Berndtsson, A.; Martensson, N. Core Level Binding Energies for the Elements Hf to Bi (Z = 72–83). J. Phys. C Solid State Phys. 1980, 13, L1091–L1096. [Google Scholar] [CrossRef]
- Hou, D.; Hu, X.; Hu, P.; Zhang, W.; Zhang, M.; Huang, Y. Bi4Ti3O12 nanofibers–BiOI nanosheets p–n junction: Facile synthesis and enhanced visible-light photocatalytic activity. Nanoscale 2013, 5, 9764–9772. [Google Scholar] [CrossRef]
- Borchert, H.; Fenske, D.; Kolny-Olesiak, J.; Parisi, J.; Al-Shamery, K.; Bäumer, M. Ligand-Capped Pt Nanocrystals as Oxide-Supported Catalysts: FTIR Spectroscopic Investigations of the Adsorption and Oxidation of CO. Angew. Chem. Int. Ed. 2007, 46, 2923–2926. [Google Scholar] [CrossRef] [PubMed]
- Hollins, P. The Influence of Surface Defects on the Infrared Spectra of Adsorbed Species. Surf. Sci. Rep. 1992, 16, 51–94. [Google Scholar] [CrossRef]
- Xie, J.; Huang, B.; Yin, K.; Pham, H.N.; Unocic, R.R.; Datye, A.K.; Davis, R.J. Influence of Dioxygen on the Promotional Effect of Bi during Pt-Catalyzed Oxidation of 1, 6-Hexanediol. ACS Catal. 2016, 6, 4206–4217. [Google Scholar] [CrossRef]
- Morales-Torres, S.; Pérez-Cadenas, A.F.; Kapteijn, F.; Carrasco-Marín, F.; Maldonado-Hódar, F.J.; Moulijn, J.A. Palladium and Platinum Catalysts Supported on Carbon Nanofiber Coated Monoliths for Low-Temperature Combustion of BTX. Appl. Catal. B Environ. 2009, 89, 411–419. [Google Scholar] [CrossRef]
- Manta, C.M.; Bozga, G.; Bercaru, G.; Bîldea, C.S. Kinetics of o-Xylene Combustion over a Pt/Alumina Catalyst. Chem. Eng. Technol. 2012, 35, 2147–2154. [Google Scholar] [CrossRef]
- Stöber, W.; Fink, A.; Bohn, E. Controlled growth of monodisperse silica spheres in the micron size range. J. Colloid Interface Sci. 1968, 26, 62–69. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 1996, 54, 11169–11186. [Google Scholar] [CrossRef]
- Blöchl, P.E. Projector Augmented-Wave Method. Phys. Rev. B 1994, 50, 17953–17979. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef] [Green Version]
- Teter, M.P.; Payne, M.C.; Allan, D.C. Solution of Schrödinger’s Equation for Large Systems. Phys. Rev. B 1989, 40, 12255–12263. [Google Scholar] [CrossRef] [PubMed]








Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, Q.; Wang, Z.-Q.; Hong, W.; Lou, B.; Zou, S. Engineering Pt-Bi2O3 Interface to Boost Cyclohexanone Selectivity in Oxidative Dehydrogenation of KA-Oil. Catalysts 2021, 11, 1187. https://doi.org/10.3390/catal11101187
Zhou Q, Wang Z-Q, Hong W, Lou B, Zou S. Engineering Pt-Bi2O3 Interface to Boost Cyclohexanone Selectivity in Oxidative Dehydrogenation of KA-Oil. Catalysts. 2021; 11(10):1187. https://doi.org/10.3390/catal11101187
Chicago/Turabian StyleZhou, Qiuyue, Zhi-Qiang Wang, Wei Hong, Baohui Lou, and Shihui Zou. 2021. "Engineering Pt-Bi2O3 Interface to Boost Cyclohexanone Selectivity in Oxidative Dehydrogenation of KA-Oil" Catalysts 11, no. 10: 1187. https://doi.org/10.3390/catal11101187
APA StyleZhou, Q., Wang, Z.-Q., Hong, W., Lou, B., & Zou, S. (2021). Engineering Pt-Bi2O3 Interface to Boost Cyclohexanone Selectivity in Oxidative Dehydrogenation of KA-Oil. Catalysts, 11(10), 1187. https://doi.org/10.3390/catal11101187