Investigation of the Characteristics of Catalysis Synergy during Co-Combustion for Coal Gasification Fine Slag with Bituminous Coal and Bamboo Residue
Abstract
:1. Introduction
2. Results and Discussion
2.1. Physical and Chemical Characteristics of Samples
2.1.1. Basic Properties
2.1.2. Ash Chemical Compositions
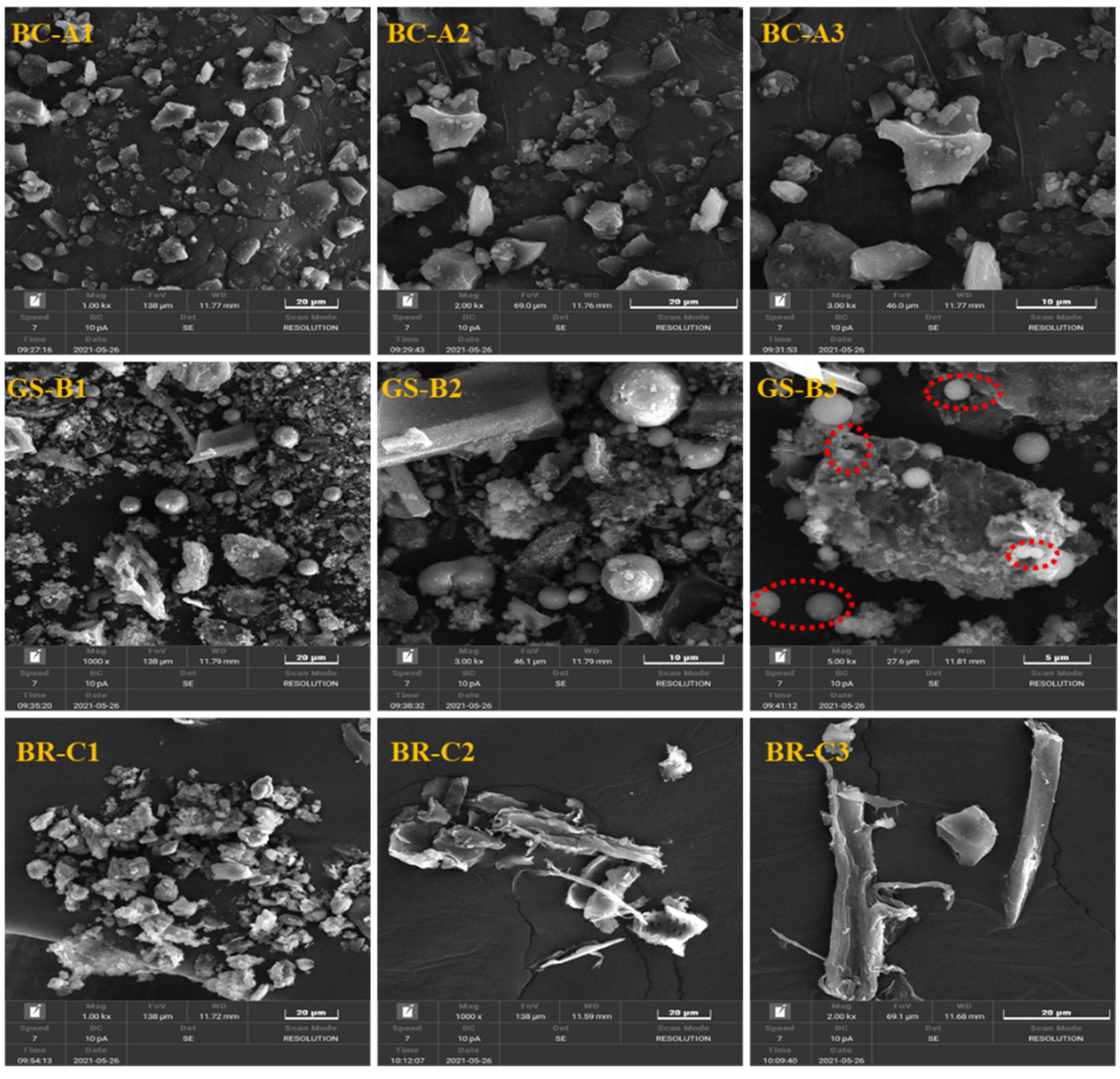
2.1.3. Micromorphology Structure
2.2. Combustion Characteristic Analysis
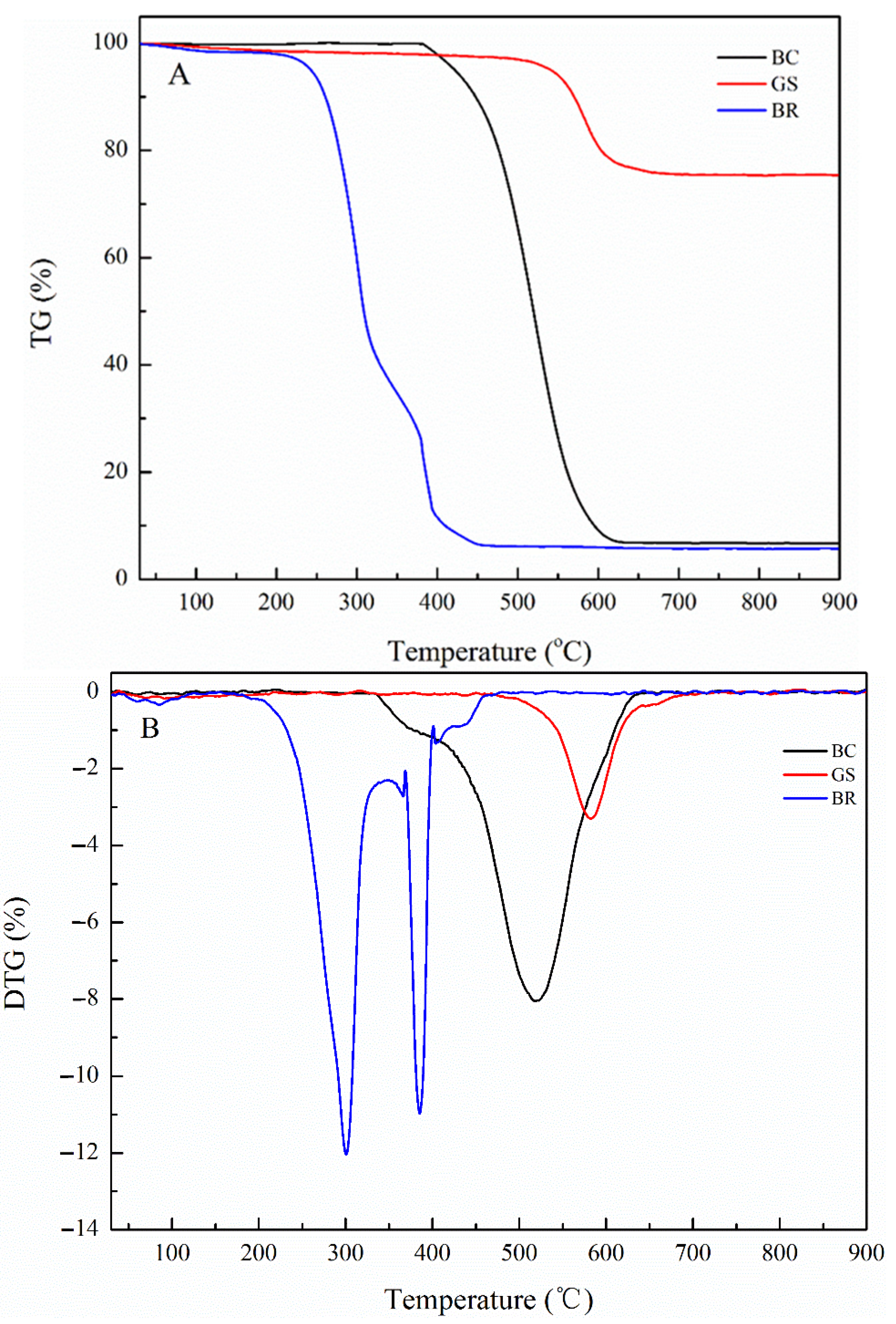
2.2.1. Combustion Behavior of Individual Fuel
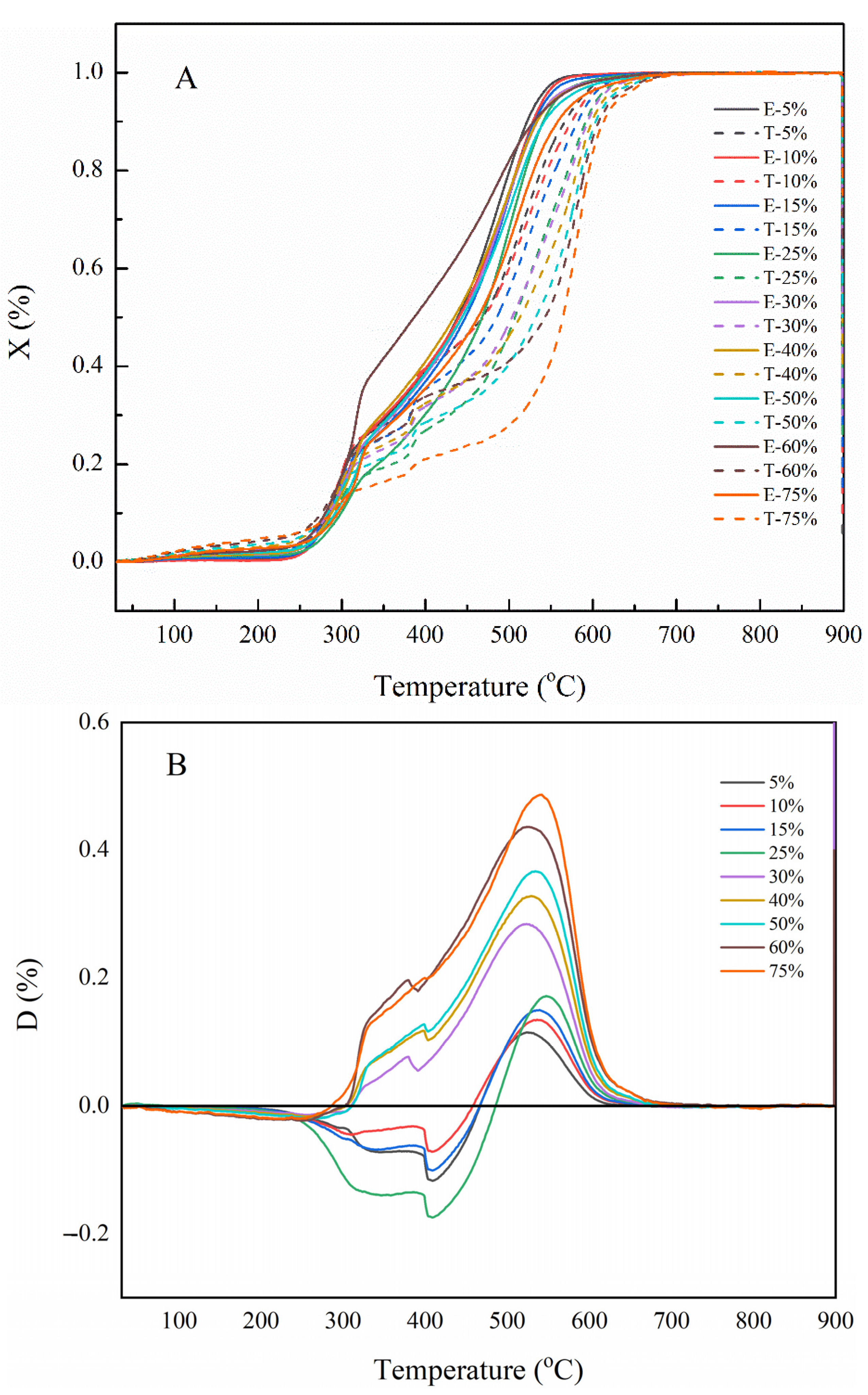
2.2.2. Tri-Fuel Co-Combustion Characteristic
2.3. Interaction and Synergistic Catalysis of Tri-Fuel Combustion
2.4. Kinetics Analysis
3. Materials and Methods
3.1. Material Preparation
3.2. Analysis Methods
3.2.1. Sample Properties
3.2.2. Ash Composition Analysis
3.2.3. Micromorphology Analysis
3.2.4. Thermogravimetric Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Guo, F.; Guo, Y.; Guo, Z.; Miao, Z.; Zhao, X.; Zhang, Y.; Li, J.; Wu, J. Recycling Residual Carbon from Gasification Fine Slag and Its Application for Preparing Slurry Fuels. ACS Sustain. Chem. Eng. 2020, 8, 8830–8839. [Google Scholar] [CrossRef]
- Miao, Z.; Wu, J.; Zhang, Y.; Zhao, X.; Guo, F.; Guo, Z.; Guo, Y. Physicochemical Characteristics of Mineral-Rich Particles Present in Fine Slag from Entrained-Flow Gasifiers. Energy Fuels 2019, 34, 616–623. [Google Scholar] [CrossRef]
- Guo, F.; Liu, H.; Zhao, X.; Guo, Y.; Guo, Z.; Miao, Z.; Zhang, Y.; Li, J.; Wu, J. Insights on water temporal-spatial migration laws of coal gasification fine slag filter cake during water removal process and its enlightenment for efficient dewatering. Fuel 2021, 292, 120274. [Google Scholar] [CrossRef]
- Zhu, D.; Miao, S.; Xue, B.; Jiang, Y.; Wei, C. Effect of Coal Gasification Fine Slag on the Physicochemical Properties of Soil. Water Air Soil Pollut. 2019, 230, 155. [Google Scholar] [CrossRef]
- Qu, J.; Zhang, J.; Sun, Z.; Yang, C.; Shi, D.; Li, S.; Li, H. Research progress on comprehensive utilization of coal gasification slag. Clean Coal Technol. 2020, 26, 184–193. [Google Scholar] [CrossRef]
- Shang, X.; Ma, J.; Zhang, J. Research status and prospects of utilization technologies of slag from coal gasification. J. Environ. Eng. Technol. 2017, 7, 712–717. [Google Scholar] [CrossRef]
- Dai, G.; Zheng, S.; Wang, X.; Bai, Y.; Dong, Y.; Du, J.; Sun, X.; Tan, H. Combustibility analysis of high-carbon fine slags from an entrained flow gasifier. J. Environ. Manag. 2020, 271, 111009. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, F.; Zheng, Z.; Wang, H.; Wang, F. Combustion Performance and Kinetic Analysis of Shenhua Raw Coal Mixed Coal Gasification Slag. IOP Conf. Ser. Earth Environ. Sci. 2020, 545, 012039. [Google Scholar] [CrossRef]
- Guo, Y.; Guo, F.; Zhou, L.; Guo, Z.; Miao, Z.; Liu, H.; Zhang, X.; Wu, J.; Zhang, Y. Investigation on co-combustion of coal gasification fine slag residual carbon and sawdust char blends: Physiochemical properties, combustion characteristic and kinetic behavior. Fuel 2021, 292, 120387. [Google Scholar] [CrossRef]
- Gu, X.; Deng, X.; Liu, Y.; Zeng, Q.; Wu, X.; Ni, Y.; Liu, X.; Wu, T.; Fang, P.; Wang, B.; et al. Review on comprehensive utilization of bamboo residues. Trans. Chin. Soc. Agric. Eng. 2016, 23, 236–242. [Google Scholar] [CrossRef]
- Liu, Z.; Hu, W.; Jiang, Z.; Mi, B.; Fei, B. Investigating combustion behaviors of bamboo, torrefied bamboo, coal and their respective blends by thermogravimetric analysis. Renew. Energy 2016, 87, 346–352. [Google Scholar] [CrossRef]
- Chao, C.Y.; Kwong, P.C.; Wang, J.H.; Cheung, C.W.; Kendall, G. Co-firing coal with rice husk and bamboo and the impact on particulate matters and associated polycyclic aromatic hydrocarbon emissions. Bioresour. Technol. 2008, 99, 83–93. [Google Scholar] [CrossRef]
- Hu, J.; Yan, Y.; Evrendilek, F.; Buyukada, M.; Liu, J. Combustion behaviors of three bamboo residues: Gas emission, kinetic, reaction mechanism and optimization patterns. J. Clean. Prod. 2019, 235, 549–561. [Google Scholar] [CrossRef]
- Du, J.; Dai, G.; Li, S.; Wang, X.; Sun, X.; Tan, H. Experimental study on the fundamental combustion characteristics of fine slag from gasification. Clean Coal Technol. 2019, 25, 83–88. [Google Scholar] [CrossRef]
- Wu, S.; Huang, S.; Wu, Y.; Gao, J. Characteristics and catalytic actions of inorganic constituents from entrained-flow coal gasification slag. J. Energy Inst. 2015, 88, 93–103. [Google Scholar] [CrossRef]
- Guo, Q.; Cheng, Z.; Chen, G.; Yan, B.; Hou, L.A.; Ronsse, F. Optimal strategy for clean and efficient biomass combustion based on ash deposition tendency and kinetic analysis. J. Clean. Prod. 2020, 271, 122529. [Google Scholar] [CrossRef]
- Hu, J.; Yan, Y.; Song, Y.; Liu, J.; Evrendilek, F.; Buyukada, M. Catalytic combustions of two bamboo residues with sludge ash, CaO, and Fe2O3: Bioenergy, emission and ash deposition improvements. J. Clean. Prod. 2020, 270, 122418. [Google Scholar] [CrossRef]
- Zhang, R.; Lei, K.; Ye, B.Q.; Cao, J.; Liu, D. Effects of alkali and alkaline earth metal species on the combustion characteristics of single particles from pine sawdust and bituminous coal. Bioresour. Technol. 2018, 268, 278–285. [Google Scholar] [CrossRef]
- Roni, M.S.; Chowdhury, S.; Mamun, S.; Marufuzzaman, M.; Lein, W.; Johnson, S. Biomass co-firing technology with policies, challenges, and opportunities: A global review. Renew. Sustain. Energy Rev. 2017, 78, 1089–1101. [Google Scholar] [CrossRef]
- Ram, S.; Tare, M.S.; Aswath, P.B.; Ralegaonkar, R.V. Potential of Co-Fired Fly Ashes as a Construction Material—A Review. Encycl. Renew. Sustain. Mater. 2020, 1, 674–685. [Google Scholar] [CrossRef]
- Wang, G.; Zhang, J.; Shao, J.; Ren, S. Characterisation and model fitting kinetic analysis of coal/biomass co-combustion. Thermochim. Acta 2014, 591, 68–74. [Google Scholar] [CrossRef]
- Laougé, Z.B.; Merdun, H. Investigation of thermal behavior of pine sawdust and coal during co-pyrolysis and co-combustion. Energy 2021, 231, 120895. [Google Scholar] [CrossRef]
- Namkung, H.; Lee, Y.-J.; Park, J.-H.; Song, G.-S.; Choi, J.W.; Choi, Y.-C.; Park, S.-J.; Kim, J.-G. Blending effect of sewage sludge and woody biomass into coal on combustion and ash agglomeration behavior. Fuel 2018, 225, 266–276. [Google Scholar] [CrossRef]
- Liang, F.; Wang, R.; Jiang, C.; Yang, X.; Zhang, T.; Hu, W.; Mi, B.; Liu, Z. Investigating co-combustion characteristics of bamboo and wood. Bioresour. Technol. 2017, 243, 556–565. [Google Scholar] [CrossRef]
- Dai, B.; Hoadley, A.; Zhang, L. Characteristics of high temperature co-gasification and ash slagging for Victorian brown coal char and bituminous coal blends. Fuel 2018, 215, 799–812. [Google Scholar] [CrossRef]
- Dai, B.; Hoadley, A.; Zhang, L. Characteristics of high temperature C-CO 2 gasification reactivity of Victorian brown coal char and its blends with high ash fusion temperature bituminous coal. Fuel 2017, 202, 352–365. [Google Scholar] [CrossRef]
- Wang, C.; Wang, C.A.; Jia, X.; Gao, X.; Wang, P.; Feng, Q.; Che, D. Experimental investigation on combustion characteristics and kinetics during Co-Firing bituminous coal with ultra-low volatile carbon-based solid fuels. J. Energy Inst. 2021, 95, 87–100. [Google Scholar] [CrossRef]
- Xinjie, L.; Singh, S.; Yang, H.; Wu, C.; Zhang, S. A thermogravimetric assessment of the tri-combustion process for coal, biomass and polyethylene. Fuel 2021, 287, 119355. [Google Scholar] [CrossRef]
- Boumanchar, I.; Chhiti, Y.; M’Hamdi Alaoui, F.E.; Elkhouakhi, M.; Sahibed-dine, A.; Bentiss, F.; Jama, C.; Bensitel, M. Investigation of (co)-combustion kinetics of biomass, coal and municipal solid wastes. Waste Manag. 2019, 97, 10–18. [Google Scholar] [CrossRef]
- Ndou, N.R.; Bada, S.O.; Falcon, R.M.S.; Weiersbye, I.M. Co-combustion of Searsia lancea and Tamarix usneoides with high ash coal. Fuel 2020, 267, 117282. [Google Scholar] [CrossRef]
- Xiao, H.; Ma, X.; Liu, K. Co-combustion kinetics of sewage sludge with coal and coal gangue under different atmospheres. Energy Convers. Manag. 2010, 51, 1976–1980. [Google Scholar] [CrossRef]
- Li, J. Investigation on the occurrence of carbon in gasification filter cake and its feasibility of circulating combustion. Clean Coal Technol. 2020, 26, 224–228. [Google Scholar] [CrossRef]
- Wang, C.; Cui, H.; Di, H.; Guo, Q.; Huang, F. Experimental Investigation on the Multi-cycle Performance of Coal/Straw Chemical Loop Combustion with α-Fe2O3 as the Oxygen Carrier. Energy Fuels 2014, 28, 4162–4166. [Google Scholar] [CrossRef]
- Deng, S.; Wang, X.; Zhang, J.; Liu, Z.; Mikulčić, H.; Vujanović, M.; Tan, H.; Duić, N. A kinetic study on the catalysis of KCl, K2SO4, and K2CO3 during oxy-biomass combustion. J. Environ. Manag. 2018, 218, 50–58. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Yu, W.; Zhnag, T.; Bai, Y.; Liu, L.; Shi, Z.; Yin, R.; Tan, H. Characteristic analysis and utilization of coal gasification fine slag based on particle size classification. Clean Coal Technol. 2021, 27, 61–69. [Google Scholar] [CrossRef]
- Guo, F.; Zhao, X.; Guo, Y.; Zhang, Y.; Wu, J. Fractal analysis and pore structure of gasification fine slag and its flotation residual carbon. Colloids Surf. A Physicochem. Eng. Asp. 2020, 585, 124148. [Google Scholar] [CrossRef]
- Huang, L.; Liu, J.; He, Y.; Sun, S.; Chen, J.; Sun, J.; Chang, K.; Kuo, J.; Ning, X.A. Thermodynamics and kinetics parameters of co-combustion between sewage sludge and water hyacinth in CO2/O2 atmosphere as biomass to solid biofuel. Bioresour. Technol. 2016, 218, 631–642. [Google Scholar] [CrossRef]
- Álvarez, A.; Pizarro, C.; García, R.; Bueno, J.L.; Lavín, A.G. Determination of kinetic parameters for biomass combustion. Bioresour. Technol. 2016, 216, 36–43. [Google Scholar] [CrossRef]
- Gil, M.V.; Casal, D.; Pevida, C.; Pis, J.J.; Rubiera, F. Thermal behaviour and kinetics of coal/biomass blends during co-combustion. Bioresour. Technol. 2010, 101, 5601–5608. [Google Scholar] [CrossRef] [Green Version]
- Alshehri, S.M.; Monshi, M.A.S.; El-Salam, N.M.A.; Mahfouz, R.M. Kinetics of the thermal decomposition of γ-irradiated cobaltous acetate. Thermochim. Acta 2000, 363, 61–70. [Google Scholar] [CrossRef]
- Ren, X.; Hu, Q.; Yang, H.; Wang, X.; Zhnag, S.; Chen, H. Effect of alkali/alkaline earth metals on combustion characteristics and reaction kinetics of biochar. Acta Energ. Sol. Sin. 2021, 42, 484–487. [Google Scholar] [CrossRef]
- Zhang, F.; Xu, D.; Wang, Y.; Argyle, M.D.; Fan, M. CO2 gasification of Powder River Basin coal catalyzed by a cost-effective and environmentally friendly iron catalyst. Appl. Energy 2015, 145, 295–305. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.; Zhu, M.; Zhang, Y.; Setyawan, H.Y.; Li, J.; Zhang, D. Ignition and combustion characteristics of single particles of Zhundong lignite: Effect of water and acid washing. Proc. Combust. Inst. 2017, 36, 2139–2146. [Google Scholar] [CrossRef]


| Samples | Proximate Analysis (wt%) | Ultimate Analysis (wt%) | HHV (ad, MJ/kg) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mad | Ad | Vd | FCd | Cdaf | Hdaf | Odaf * | Ndaf | St,d | ||
| BC | 1.62 | 9.33 | 21.89 | 68.77 | 89.23 | 4.78 | 3.85 | 1.48 | 0.61 | 32.83 |
| GS | 3.48 | 71.95 | 3.81 | 24.23 | 93.54 | 0.81 | 3.07 | 0.33 | 0.63 | 9.31 |
| BR | 6.57 | 3.35 | 80.67 | 15.98 | 54.84 | 5.74 | 38.81 | 0.54 | 0.07 | 21.47 |
| Samples | K2O | Na2O | SiO2 | Al2O3 | Fe2O3 | CaO | MgO | SO3 | TiO2 | MnO2 | P2O5 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| BC | 0.75 | 0.28 | 52.52 | 37.03 | 3.45 | 1.56 | 0.66 | 1.66 | 1.66 | 0.01 | 0.16 |
| GS | 1.26 | 1.93 | 46.90 | 18.81 | 11.46 | 11.31 | 3.88 | 2.44 | 0.80 | 0.21 | 0.15 |
| BR | 58.97 | 0.46 | 24.17 | 2.26 | 3.11 | 8.47 | 4.11 | 4.42 | 0.17 | 0.64 | 4.07 |
| Samples | 5% | 10% | 15% | 25% | 30% | 40% | 50% | 60% | 75% |
|---|---|---|---|---|---|---|---|---|---|
| BC + GS | 1.71 | 2.79 | 3.93 | 6.21 | 7.24 | 9.42 | 11.64 | 13.77 | 17.18 |
| BR | 30.37 | 30.37 | 26.58 | 18.98 | 22.78 | 22.78 | 18.98 | 22.78 | 11.39 |
| Samples | Ti (°C) | Tb (°C) | Tmax (°C) | (dw/dt)max (%/min) | (dw/dt)mean (%/min) | S (10−7) | |
|---|---|---|---|---|---|---|---|
| Stage 1 | Stage 2 | ||||||
| BC | 458.64 | 571.52 | 520.16 | / | 8.07 | 3.25 | 2.18 |
| GS | 540.53 | 614.23 | 580.84 | / | 2.99 | 1.12 | 0.19 |
| BR | 267.59 | 401.85 | 301.29 | 385.33 | 12.8 | 2.93 | 13.03 |
| Samples | Ti (°C) | Tb (°C) | Tmax (°C) | (dw/dt)max (%/min) | (dw/dt)mean (%/min) | S (10−7) | |
|---|---|---|---|---|---|---|---|
| Stage 1 | Stage 2 | ||||||
| 5% GS | 264.17 | 534.25 | 290.93 | 487.98 | 4.81 | 2.17 | 2.84 |
| 10% GS | 260.08 | 542.61 | 299.52 | 503.74 | 4.38 | 1.83 | 2.18 |
| 15% GS | 264.99 | 544.59 | 296.61 | 495.54 | 4.51 | 1.80 | 2.12 |
| 25% GS | 269.54 | 553.20 | 301.81 | 499.58 | 4.67 | 1.40 | 1.63 |
| 30% GS | 268.68 | 544.74 | 303.65 | 493.78 | 3.78 | 1.59 | 1.50 |
| 40% GS | 266.40 | 542.04 | 308.88 | 498.33 | 3.07 | 1.27 | 1.02 |
| 50% GS | 286.61 | 557.68 | 316.39 | 505.07 | 2.94 | 1.21 | 0.78 |
| 60% GS | 288.27 | 545.70 | 318.10 | 489.40 | 1.77 | 1.39 | 0.54 |
| 75% GS | 298.37 | 565.63 | 319.89 | 508.61 | 2.10 | 0.92 | 0.38 |
| Mechanism and Model | g(x) |
|---|---|
| Reaction order | |
| O1 | |
| O2 | |
| O3 | |
| Phase boundary controlled reaction | |
| R2 | |
| R3 | |
| Diffusion models | |
| D1 | |
| D2 | |
| D3 | |
| D4 |
| Samples | Stage | E (kJ/mol) | g(x) | R2 |
|---|---|---|---|---|
| BC | S1 | 43.68 | O1 | 0.9941 |
| GC | S1 | 130.87 | D3 | 0.9718 |
| BR | S1 | 18.14 | O1 | 0.9807 |
| S2 | 149.37 | O3 | 0.9595 |
| Samples | Stage 1 | Stage 2 | ||||
|---|---|---|---|---|---|---|
| g(x) | E (kJ/mol) | R2 | g(x) | E (kJ/mol) | R2 | |
| 5% GS | O1 | 22.88 | 0.9946 | D3 | 37.45 | 0.9966 |
| 10% GS | OI | 22.81 | 0.9935 | D3 | 44.46 | 0.9906 |
| 15% GS | O1 | 21.37 | 0.9960 | D3 | 37.23 | 0.9840 |
| 25% GS | O1 | 17.37 | 0.9990 | D3 | 41.82 | 0.9865 |
| 30% GS | O1 | 16.02 | 0.9826 | D3 | 34.42 | 0.9909 |
| 40% GS | O1 | 17.96 | 0.9980 | D3 | 30.87 | 0.9838 |
| 50% GS | O1 | 16.15 | 0.9991 | D3 | 31.32 | 0.9843 |
| 60% GS | O1 | 17.88 | 0.9965 | D3 | 20.44 | 0.9946 |
| 75% GS | O1 | 13.56 | 0.9967 | D3 | 35.73 | 0.9909 |
| GS Mass Fraction/% | BR Mass Fraction/% | BC Mass Fraction/% |
|---|---|---|
| 5 | 40 | 55 |
| 10 | 40 | 50 |
| 15 | 35 | 50 |
| 25 | 25 | 50 |
| 30 | 30 | 40 |
| 40 | 30 | 30 |
| 50 | 25 | 25 |
| 60 | 30 | 10 |
| 75 | 15 | 10 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Jia, W.; Wang, R.; Guo, Y.; Guo, F.; Wu, J.; Dai, B. Investigation of the Characteristics of Catalysis Synergy during Co-Combustion for Coal Gasification Fine Slag with Bituminous Coal and Bamboo Residue. Catalysts 2021, 11, 1152. https://doi.org/10.3390/catal11101152
Zhang Y, Jia W, Wang R, Guo Y, Guo F, Wu J, Dai B. Investigation of the Characteristics of Catalysis Synergy during Co-Combustion for Coal Gasification Fine Slag with Bituminous Coal and Bamboo Residue. Catalysts. 2021; 11(10):1152. https://doi.org/10.3390/catal11101152
Chicago/Turabian StyleZhang, Yixin, Wenke Jia, Rumeng Wang, Yang Guo, Fanhui Guo, Jianjun Wu, and Baiqian Dai. 2021. "Investigation of the Characteristics of Catalysis Synergy during Co-Combustion for Coal Gasification Fine Slag with Bituminous Coal and Bamboo Residue" Catalysts 11, no. 10: 1152. https://doi.org/10.3390/catal11101152
APA StyleZhang, Y., Jia, W., Wang, R., Guo, Y., Guo, F., Wu, J., & Dai, B. (2021). Investigation of the Characteristics of Catalysis Synergy during Co-Combustion for Coal Gasification Fine Slag with Bituminous Coal and Bamboo Residue. Catalysts, 11(10), 1152. https://doi.org/10.3390/catal11101152










