ZIF-67 Derived MnO2 Doped Electrocatalyst for Oxygen Reduction Reaction
Abstract
1. Introduction
2. Results and Discussion
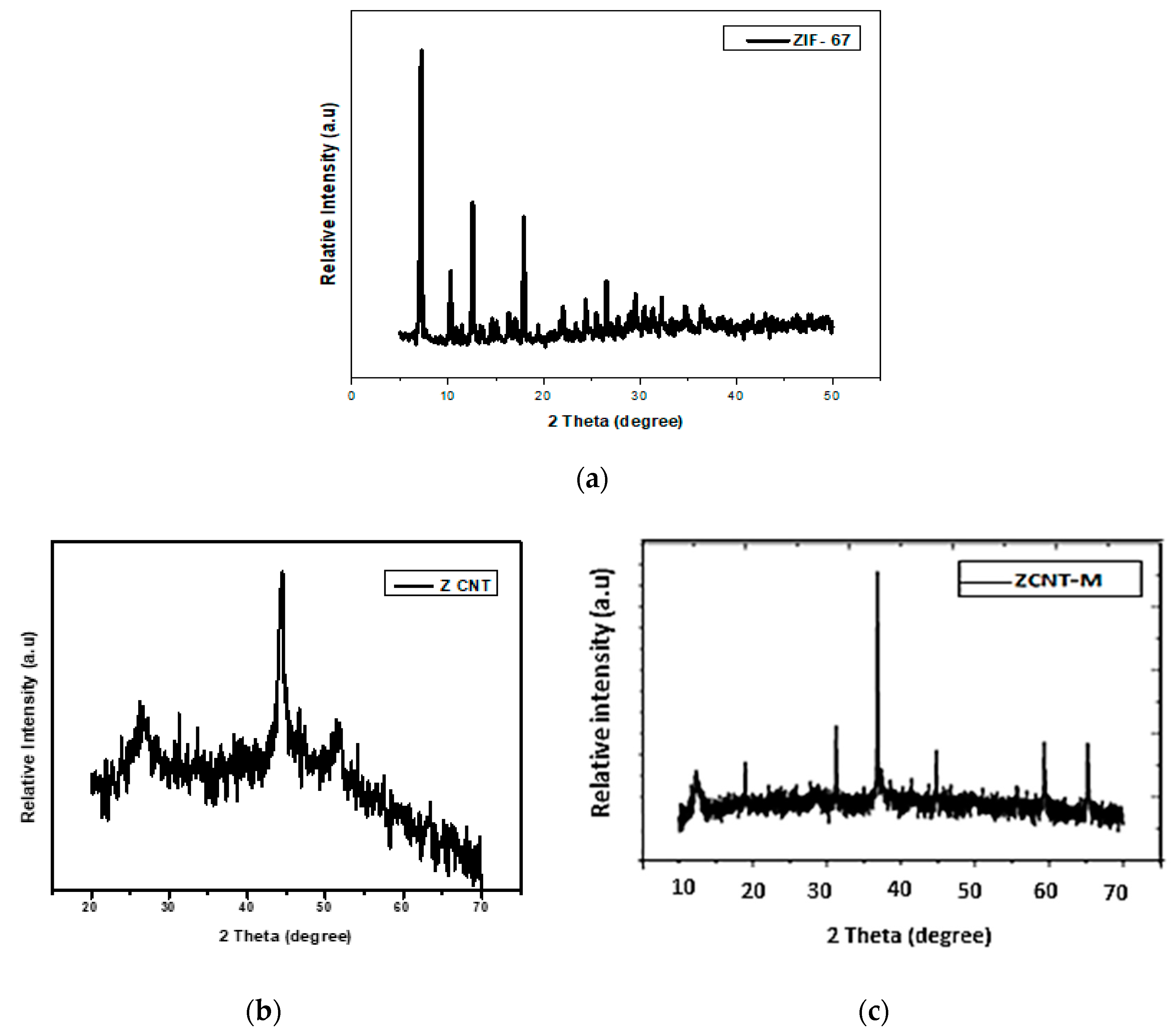
2.1. Characterization of Prepared Catalyst
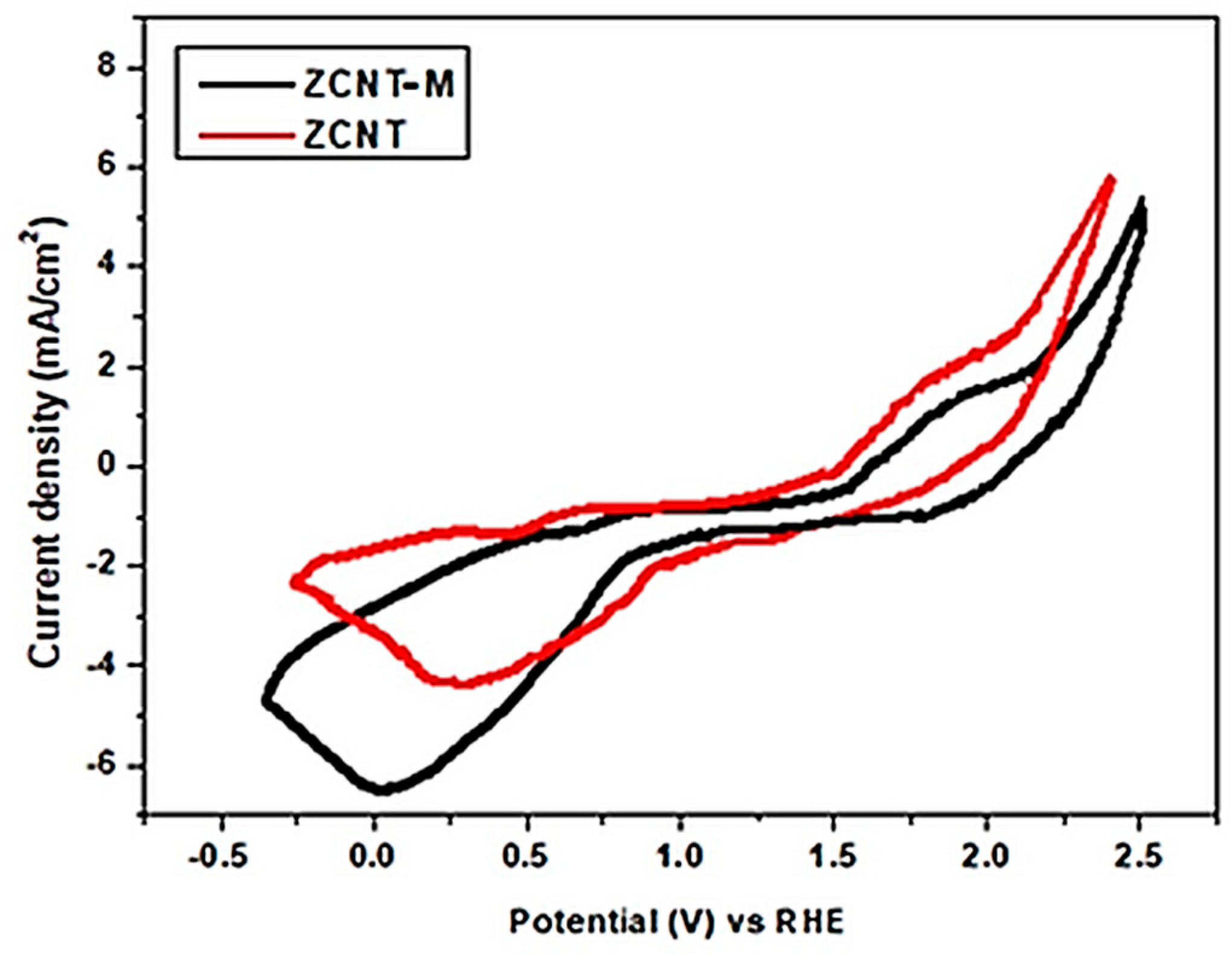
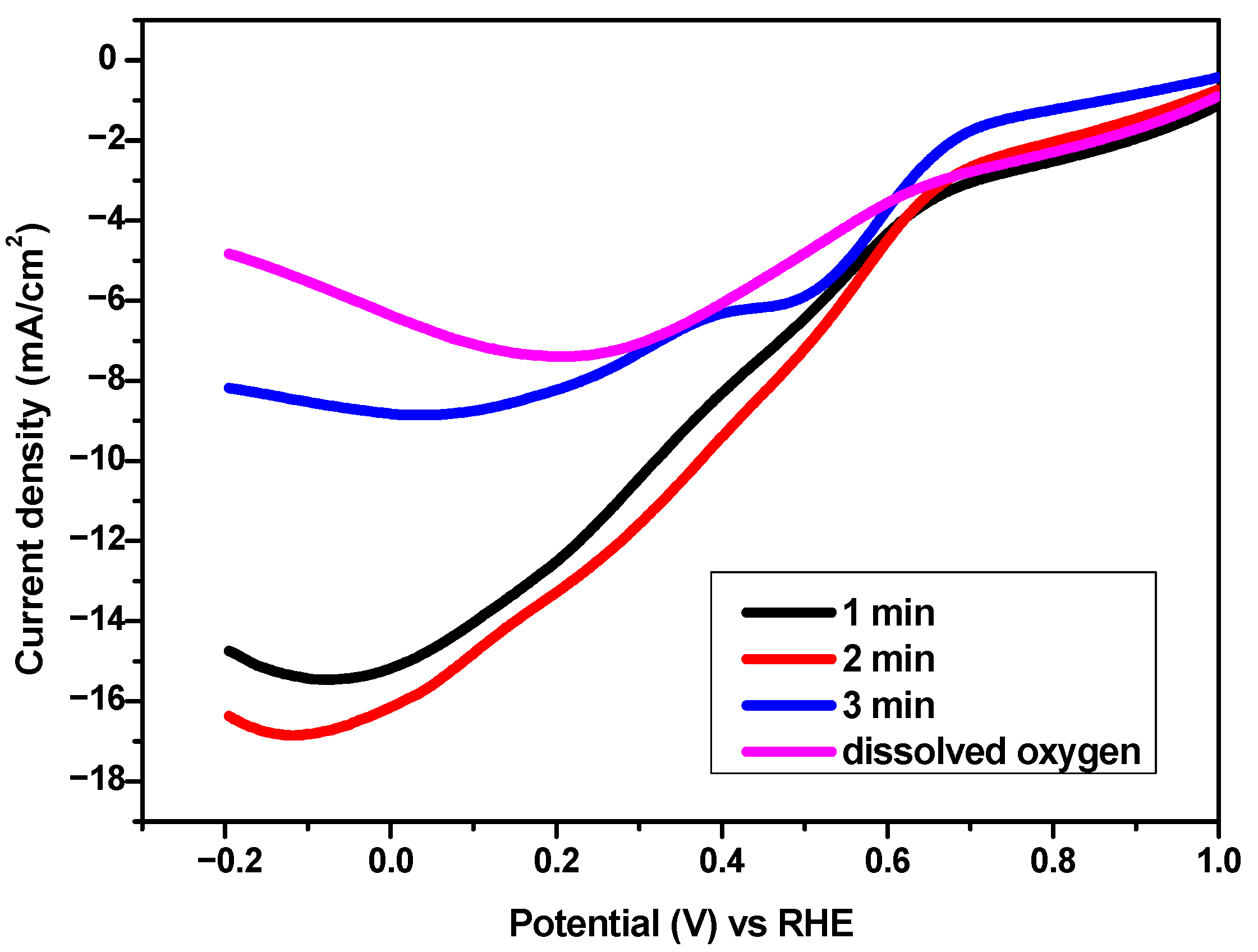
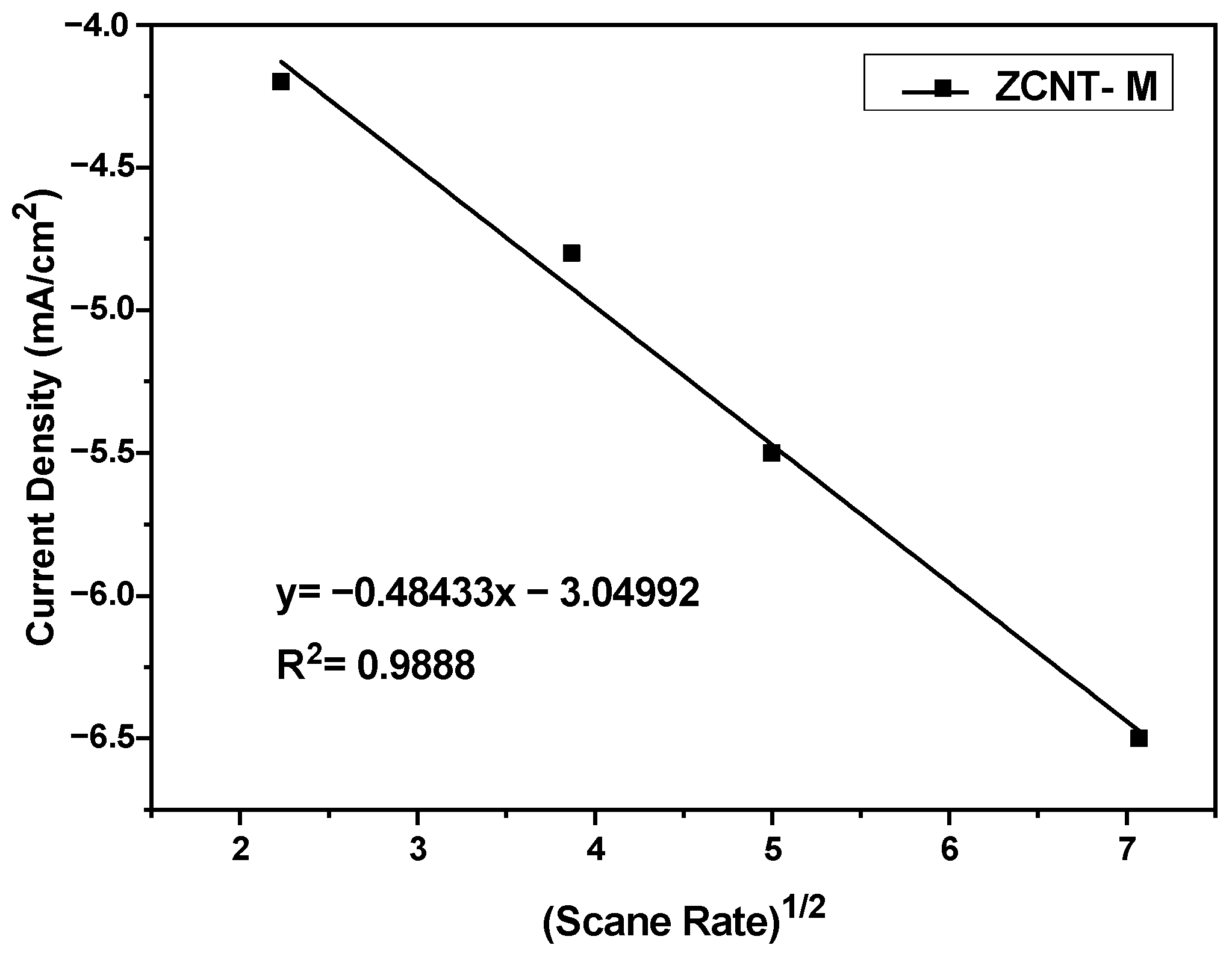
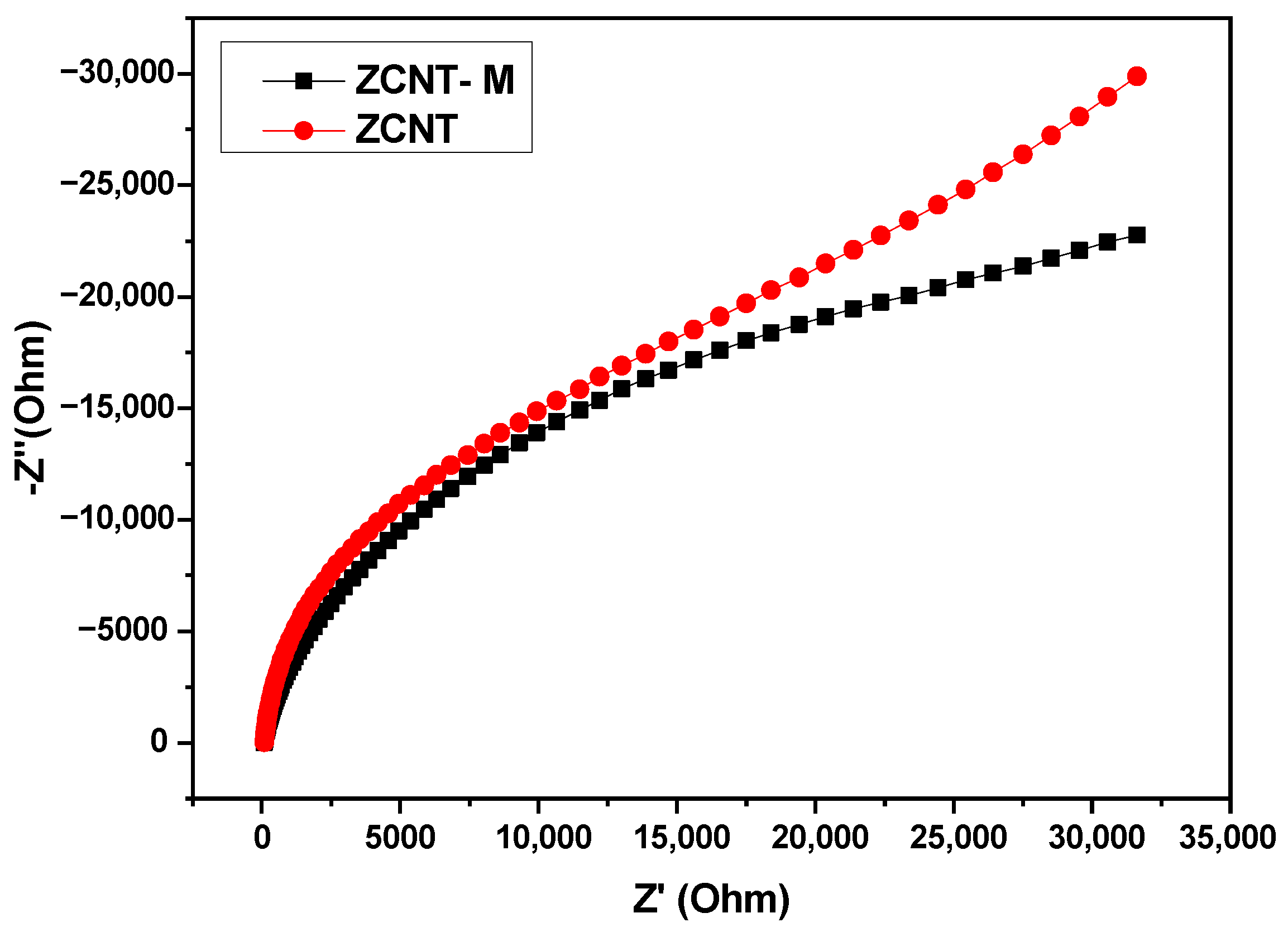
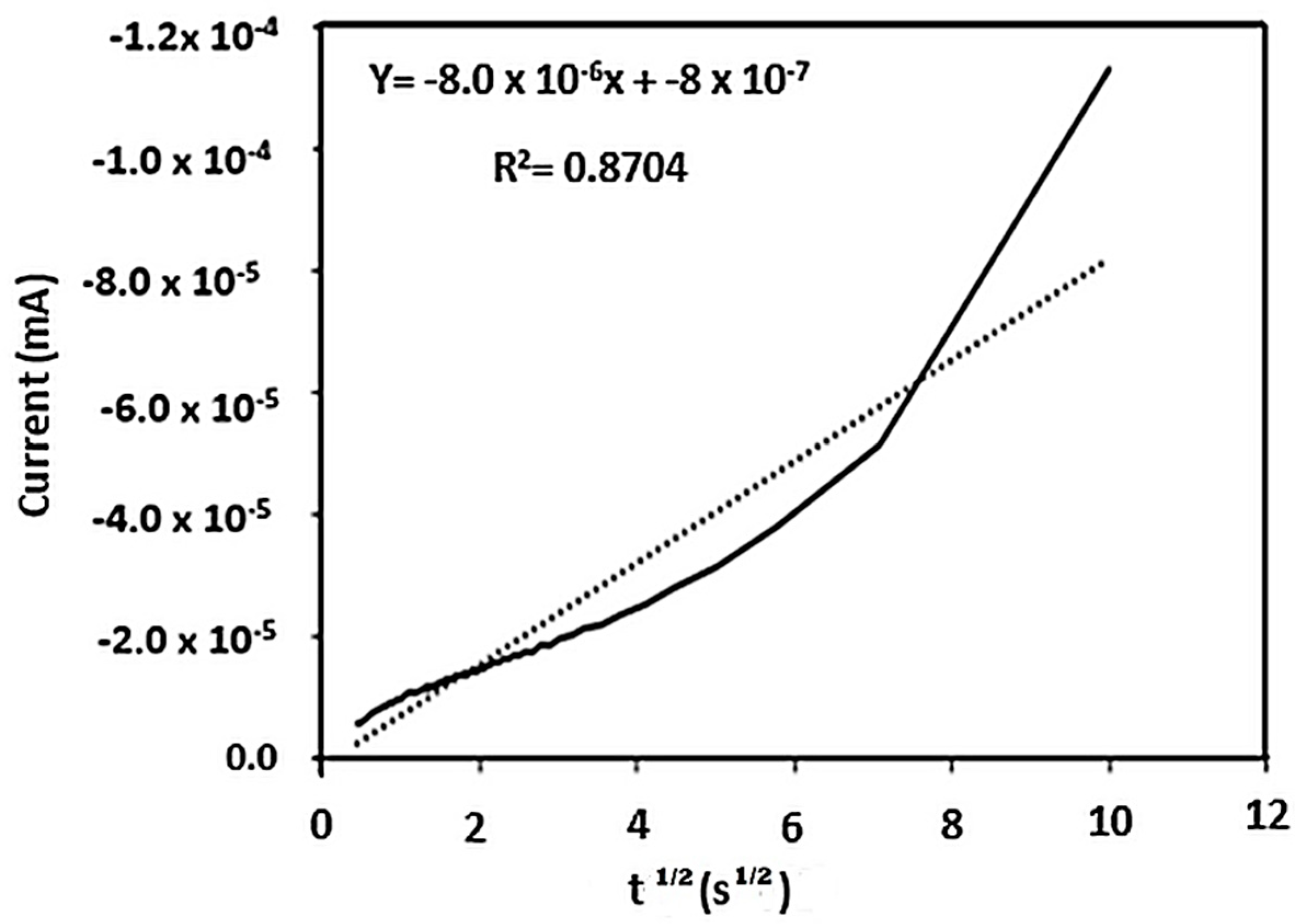
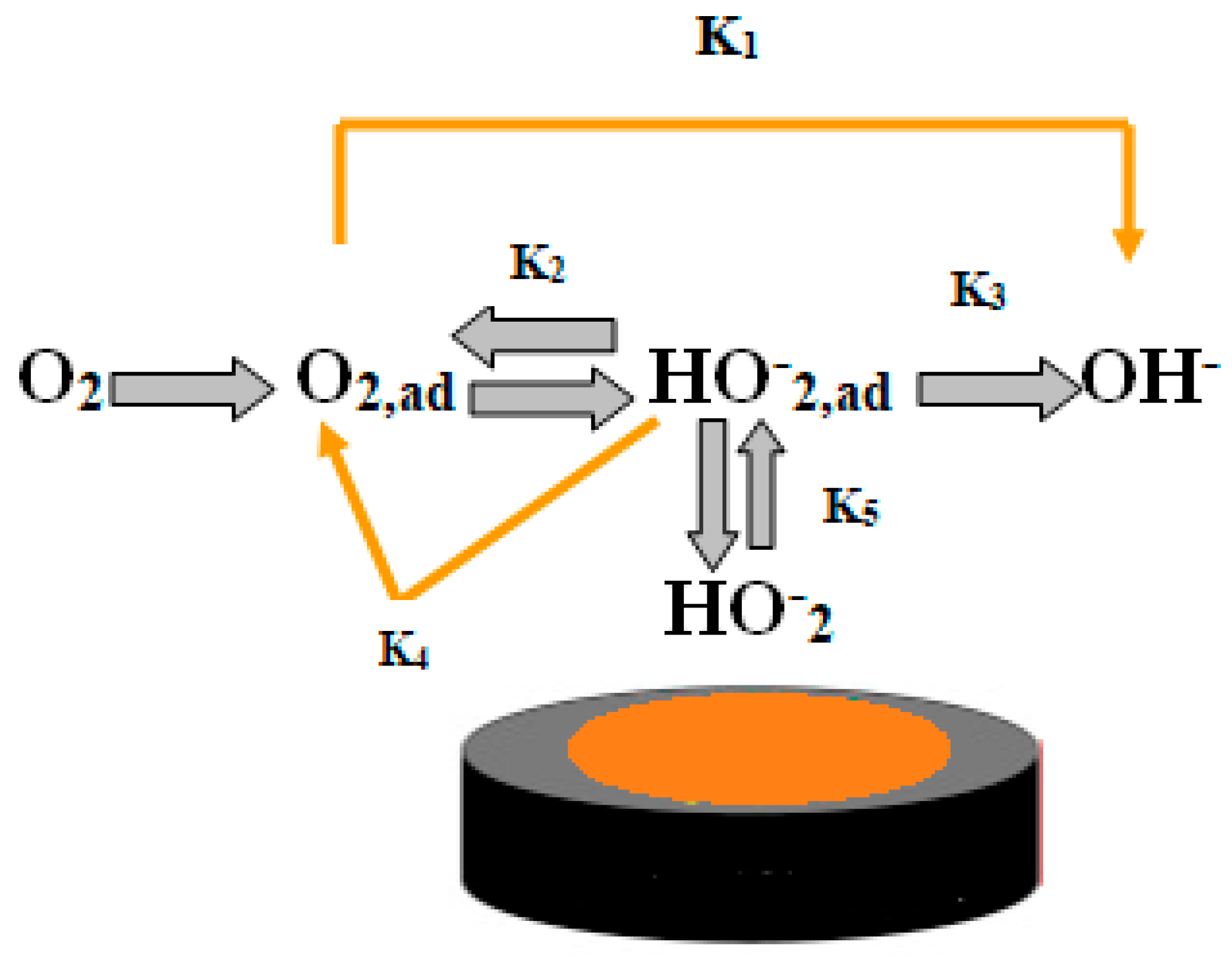
2.2. Electrochemical Analyses
2.3. Electrochemical Evaluation of Prepared Catalsyts
3. Experimental
3.1. Characterization
3.2. Synthesis of ZIF-67
3.3. Synthesis of Mesoporous Carbon
3.4. Synthesis of MnO2-Doped Mesoporous Carbon
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Pathak, S. Energy Crisis: A Review. Int. J. Eng. Res. Appl. 2014, 4, 845–851. [Google Scholar]
- Noor, T.; Ammad, M.; Zaman, N.; Iqbal, N.; Yaqoob, L.; Nasir, H. A highly efficient and stable copper BTC metal organic framework derived electrocatalyst for oxidation of methanol in DMFC application. Catal. Lett. 2019, 149, 3312–3327. [Google Scholar] [CrossRef]
- Shafiee, S.; Topal, E. When will fossil fuel reserves be diminished? Energy Policy 2009, 37, 181–189. [Google Scholar] [CrossRef]
- Qureshi, M.N. Energy Crisis in Pakistan: A Threat to National Security; ISSRA: Islamabad, Pakistan, 2009. [Google Scholar]
- Yaqoob, L.; Noor, T.; Iqbal, N.; Nasir, H.; Sohail, M.; Zaman, N.; Usman, M. Nanocomposites of cobalt benzene tricarboxylic acid MOF with rGO: An efficient and robust electocatalyst for oxygen evaluation reaction (OER). Renew. Energy 2020. [Google Scholar] [CrossRef]
- Baker, B.S.; Ghezel-Ayagh, H.G. Fuel Cell System. U.S. Patents 4532192A, 30 July 1985. [Google Scholar]
- Wahab, A.; Iqbal, N.; Noor, T.; Ashraf, S.; Raza, M.A.; Ahmad, A.; Khan, U.A. Thermally reduced mesoporous manganese MOF@ reduced graphene oxide nanocomposite as bifunctional electrocatalyst for oxygen reduction and evolution. RSC Adv. 2020, 10, 27728–27742. [Google Scholar] [CrossRef]
- Ren, Y.; Chia, G.H.; Gao, Z. Metal–organic frameworks in fuel cell technologies. Nano Today 2013, 8, 577–597. [Google Scholar] [CrossRef]
- Strahl, S.; Costa-Castelló, R. Temperature control of open-cathode PEM fuel cells. IFAC-PapersOnLine 2017, 50, 11088–11093. [Google Scholar] [CrossRef]
- Rizvi, S.A.M.; Iqbal, N.; Haider, M.D.; Noor, T.; Anwar, R.; Hanif, S. Synthesis and Characterization of Cu-MOF Derived Cu@ AC Electrocatalyst for Oxygen Reduction Reaction in PEMFC. Catal. Lett. 2019, 150, 1397–1407. [Google Scholar] [CrossRef]
- Zhang, P.; Sun, F.; Xiang, Z.; Shen, Z.; Yun, J.; Cao, D. ZIF-derived in situ nitrogen-doped porous carbons as efficient metal-free electrocatalysts for oxygen reduction reaction. Energy Environ. Sci. 2014, 7, 442–450. [Google Scholar] [CrossRef]
- Sarwar, E.; Noor, T.; Iqbal, N.; Mehmood, Y.; Ahmed, S.; Mehek, R. Effect of Co–Ni Ratio in Graphene Based Bimetallic Electro–catalyst for Methanol Oxidation. Fuel cells 2018, 18, 189–194. [Google Scholar] [CrossRef]
- Wang, L.; Zhao, X.; Lu, Y.; Xu, M.; Zhang, D.; Ruoff, R.S.; Stevenson, K.J.; Goodenough, J.B. CoMn2O4 spinel nanoparticles grown on graphene as bifunctional catalyst for lithium-air batteries. J. Electrochem. Soc. 2011, 158, A1379–A1382. [Google Scholar] [CrossRef]
- Yang, W.; Salim, J.; Ma, C.; Ma, Z.; Sun, C.; Li, J.; Chen, L.; Kim, Y. Flowerlike Co3O4 microspheres loaded with copper nanoparticle as an efficient bifunctional catalyst for lithium–air batteries. Electrochem. Commun. 2013, 28, 13–16. [Google Scholar] [CrossRef]
- Jin, C.; Yang, Z.; Cao, X.; Lu, F.; Yang, R. A novel bifunctional catalyst of Ba0.9Co0.5Fe0.4Nb0.1O3−δ perovskite for lithium–air battery. Int. J. Hydrogen Energy 2014, 39, 2526–2530. [Google Scholar] [CrossRef]
- Farrusseng, D.; Aguado, S.; Pinel, C. Metal–organic frameworks: Opportunities for catalysis. Angew. Chem. Int. Ed. 2009, 48, 7502–7513. [Google Scholar] [CrossRef] [PubMed]
- Haider, M.D.; Iqbal, N.; Rizvi, S.A.M.; Noor, T.; Hanif, S.; Anwar, R. ZIF-67 derived Cu doped electrocatalyst for oxygen reduction reaction. J. Electrochem. Energy Convers. Storage 2020, 18, 021001. [Google Scholar] [CrossRef]
- Xia, B.Y.; Yan, Y.; Li, N.; Wu, H.B.; Lou, X.W.D.; Wang, X. A metal–organic framework-derived bifunctional oxygen electrocatalyst. Nat. Energy 2016, 1, 15006. [Google Scholar] [CrossRef]
- Noor, T.; Zaman, N.; Nasir, H.; Iqbal, N.; Hussain, Z. Electro catalytic study of NiO-MOF/rGO composites for methanol oxidation reaction. Electrochim. Acta 2019, 307, 1–12. [Google Scholar] [CrossRef]
- Wang, R.; Dong, X.Y.; Du, J.; Zhao, J.Y.; Zang, S.Q. MOF-Derived bifunctional Cu3P nanoparticles coated by a N, P–codoped carbon shell for hydrogen evolution and oxygen reduction. Adv. Mater. 2018, 30, 1703711. [Google Scholar] [CrossRef]
- Yaqoob, L.; Noor, T.; Iqbal, N.; Nasir, H.; Zaman, N. Development of nickel-BTC-MOF-derived nanocomposites with rGO towards electrocatalytic oxidation of methanol and its product analysis. Catalysts 2019, 9, 856. [Google Scholar] [CrossRef]
- Ghoshal, S.; Zaccarine, S.; Anderson, G.C.; Martinez, M.B.; Hurst, K.E.; Pylypenko, S.; Pivovar, B.S.; Alia, S.M. ZIF 67 Based Highly Active Electrocatalysts as Oxygen Electrodes in Water Electrolyzer. ACS Appl. Energy Mater. 2019, 2, 5568–5576. [Google Scholar] [CrossRef]
- Wang, H.; Wei, L.; Liu, J.; Shen, J. Hollow bimetal ZIFs derived Cu/Co/N co-coordinated ORR electrocatalyst for microbial fuel cells. Int. J. Hydrogen Energy 2020, 45, 4481–4489. [Google Scholar] [CrossRef]
- Yoon, H.; Lee, S.; Oh, S.; Park, H.; Choi, S.; Oh, M. Synthesis of Bimetallic Conductive 2D Metal-Organic Framework (CoxNiy-CAT) and Its Mass Production: Enhanced Electrochemical Oxygen Reduction Activity. Small 2019, 15, 1805232. [Google Scholar] [CrossRef]
- Wang, C.; Liu, D.; Lin, W. Metal–organic frameworks as a tunable platform for designing functional molecular materials. J. Am. Chem. Soc. 2013, 135, 13222–13234. [Google Scholar] [CrossRef] [PubMed]
- Hanif, S.; Iqbal, N.; Shi, X.; Noor, T.; Ali, G.; Kannan, A. NiCo-N-doped carbon nanotubes based cathode catalyst for alkaline membrane fuel cell. Renew. Energy 2020, 154, 508–516. [Google Scholar] [CrossRef]
- Wu, G.; Zelenay, P. Nanostructured nonprecious metal catalysts for oxygen reduction reaction. Acc. Chem. Res. 2013, 46, 1878–1889. [Google Scholar] [CrossRef] [PubMed]
- Palaniselvam, T.; Biswal, B.P.; Banerjee, R.; Kurungot, S. Zeolitic Imidazolate Framework (ZIF)-Derived, Hollow-Core, Nitrogen-Doped Carbon Nanostructures for Oxygen-Reduction Reactions in PEFCs. Chem. A Eur. J. 2013, 19, 9335–9342. [Google Scholar] [CrossRef]
- Hanif, S.; Shi, X.; Iqbal, N.; Noor, T.; Anwar, R.; Kannan, A. ZIF derived PtNiCo/NC cathode catalyst for proton exchange membrane fuel cell. Appl. Catal. B Environ. 2019, 258, 117947. [Google Scholar] [CrossRef]
- Xia, W.; Zhu, J.; Guo, W.; An, L.; Xia, D.; Zou, R. Well-defined carbon polyhedrons prepared from nano metal–organic frameworks for oxygen reduction. J. Mater. Chem. A 2014, 2, 11606–11613. [Google Scholar] [CrossRef]
- Aijaz, A.; Masa, J.; Rösler, C.; Xia, W.; Weide, P.; Botz, A.J.; Fischer, R.A.; Schuhmann, W.; Muhler, M. Co@Co3O4 encapsulated in carbon nanotube-grafted nitrogen-doped carbon polyhedra as an advanced bifunctional oxygen electrode. Angew. Chem. Int. Ed. 2016, 55, 4087–4091. [Google Scholar] [CrossRef] [PubMed]
- Meng, F.; Zhong, H.; Bao, D.; Yan, J.; Zhang, X. In situ coupling of strung Co4N and intertwined N–C fibers toward free-standing bifunctional cathode for robust, efficient, and flexible Zn–air batteries. J. Am. Chem. Soc. 2016, 138, 10226–10231. [Google Scholar] [CrossRef]
- Ahmad, R.; Iqbal, N.; Baig, M.; Noor, T.; Ali, G.; Gul, I. ZIF-67 derived NCNT/S@Ni(OH)2 decorated Ni foam based electrode material for high-performance supercapacitors. Electrochem. Acta 2020, 364, 137147. [Google Scholar] [CrossRef]
- Yang, J.; Xu, J.J. Nanoporous amorphous manganese oxide as electrocatalyst for oxygen reduction in alkaline solutions. Electrochem. Commun. 2003, 5, 306–311. [Google Scholar] [CrossRef]
- Lima, F.H.; Calegaro, M.L.; Ticianelli, E.A. Investigations of the catalytic properties of manganese oxides for the oxygen reduction reaction in alkaline media. J. Electroanal. Chem. 2006, 590, 152–160. [Google Scholar] [CrossRef]
- Masa, J.; Xia, W.; Sinev, I.; Zhao, A.; Sun, Z.; Grützke, S.; Weide, P.; Muhler, M.; Schuhmann, W. MnxOy/NC and CoxOy/NC nanoparticles embedded in a nitrogen-doped carbon matrix for high-performance bifunctional oxygen electrodes. Angew. Chem. Int. Ed. 2014, 53, 8508–8512. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Wang, H.; Zhou, J.; Li, Y.; Wang, J.; Regier, T.; Dai, H. Covalent hybrid of spinel manganese–cobalt oxide and graphene as advanced oxygen reduction electrocatalysts. J. Am. Chem. Soc. 2012, 134, 3517–3523. [Google Scholar] [CrossRef] [PubMed]
- Noor, T.; Raffi, U.; Iqbal, N.; Yaqoob, L.; Zaman, N. Kinetic evaluation and comparative study of cationic and anionic dyes adsorption on Zeolitic imidazolate frameworks based metal organic frameworks. Mater. Res. Express 2019, 6, 125088. [Google Scholar] [CrossRef]
- Shi, X.; Iqbal, N.; Kunwar, S.; Wahab, G.; Kasat, H.; Kannan, A.M. PtCo@ NCNTs cathode catalyst using ZIF-67 for proton exchange membrane fuel cell. Int. J. Hydrogen Energy 2018, 43, 3520–3526. [Google Scholar] [CrossRef]
- Fan, Q.; Guo, Z.; Li, Z.; Wang, Z.; Yang, L.; Chen, Q.; Liu, Z.; Wang, X. Atomic layer deposition of cobalt carbide thin films from cobalt amidinate and hydrogen plasma. ACS Appl. Electron. Mater. 2019, 1, 444–453. [Google Scholar] [CrossRef]
- Yang, Z.; Wang, S.; Liu, Y.; Lei, X. Cobalt oxide microtubes with balsam pear-shaped outer surfaces as anode material for lithium ion batteries. Ionics 2015, 21, 2423–2430. [Google Scholar] [CrossRef]
- Johnson, C.; Dees, D.; Mansuetto, M.; Thackeray, M.; Vissers, D.; Argyriou, D.; Loong, C.-K.; Christensen, L. Structural and electrochemical studies of α-manganese dioxide (α-MnO2). J. Power Sources 1997, 68, 570–577. [Google Scholar] [CrossRef]
- Ahmadian, H.; Veisi, H.; Karami, C.; Sedrpoushan, A.; Nouri, M.; Jamshidi, F.; Alavioon, I. Cobalt manganese oxide nanoparticles as recyclable catalyst for efficient synthesis of 2-aryl-1-arylmethyl-1H-1, 3-benzimidazoles under solvent-free conditions. Appl. Organomet. Chem. 2015, 29, 266–269. [Google Scholar] [CrossRef]
- Rapson, T.D.; Kusuoka, R.; Butcher, J.; Musameh, M.; Dunn, C.J.; Church, J.S.; Warden, A.C.; Blanford, C.F.; Nakamura, N.; Sutherland, T.D. Bioinspired electrocatalysts for oxygen reduction using recombinant silk films. J. Mater. Chem. A 2017, 5, 10236–10243. [Google Scholar] [CrossRef]
- Wang, W.; Geng, J.; Kuai, L.; Li, M.; Geng, B. Porous Mn2O3: A Low-Cost Electrocatalyst for Oxygen Reduction Reaction in Alkaline Media with Comparable Activity to Pt/C. Chem. Eur. J. 2016, 22, 9909–9913. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.; Zhang, X.; Cai, J.; Liu, W.; Lin, S. A novel MnO2/rGO composite prepared by electrodeposition as a non-noble metal electrocatalyst for ORR. J. Appl. Electrochem. 2019, 49, 767–777. [Google Scholar] [CrossRef]
- Chhetri, B.P.; Parnell, C.M.; Wayland, H.; RanguMagar, A.B.; Kannarpady, G.; Watanabe, F.; Albkuri, Y.M.; Biris, A.S.; Ghosh, A. Chitosan-derived NiO-Mn2O3/C nanocomposites as non-precious catalysts for enhanced oxygen reduction reaction. ChemistrySelect 2018, 3, 922–932. [Google Scholar] [CrossRef]
- Atabaki, M.M.; Kovacevic, R. Graphene composites as anode materials in lithium-ion batteries. Electron. Mater. Lett. 2013, 9, 133–153. [Google Scholar] [CrossRef]
- Bae, S.H.; Kim, J.E.; Randriamahazaka, H.; Moon, S.Y.; Park, J.Y.; Oh, I.K. Seamlessly conductive 3D nanoarchitecture of core-shell Ni-Co nanowire network for highly efficient oxygen evolution. Adv. Energy Mater. 2017, 7, 1601492. [Google Scholar] [CrossRef]
- Lin, X. The Kinetic and Mechanism of the Oxygen Reduction Reaction on Pt, Au, Cu, PtCu/C and CuAu/C in Alkaline Media. Master’s Thesis, The Ohio State University, Columbus, OH, USA, 2016. [Google Scholar]
- Ahmad, R.; Iqbal, N.; Noor, T. Development of ZIF-Derived Nanoporous Carbon and Cobalt Sulfide-Based Electrode Material for Supercapacitor. Materials 2019, 12, 2940. [Google Scholar] [CrossRef]






| Sample Element | ZCNT | ZCNT-M |
|---|---|---|
| C wt % | 50.02 | 15.98 |
| O wt % | 19.07 | 47.91 |
| Co wt% | 30.91 | 9.99 |
| Mn wt% | - | 24.80 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salahuddin, U.; Iqbal, N.; Noor, T.; Hanif, S.; Ejaz, H.; Zaman, N.; Ahmed, S. ZIF-67 Derived MnO2 Doped Electrocatalyst for Oxygen Reduction Reaction. Catalysts 2021, 11, 92. https://doi.org/10.3390/catal11010092
Salahuddin U, Iqbal N, Noor T, Hanif S, Ejaz H, Zaman N, Ahmed S. ZIF-67 Derived MnO2 Doped Electrocatalyst for Oxygen Reduction Reaction. Catalysts. 2021; 11(1):92. https://doi.org/10.3390/catal11010092
Chicago/Turabian StyleSalahuddin, Usman, Naseem Iqbal, Tayyaba Noor, Saadia Hanif, Haider Ejaz, Neelam Zaman, and Safeer Ahmed. 2021. "ZIF-67 Derived MnO2 Doped Electrocatalyst for Oxygen Reduction Reaction" Catalysts 11, no. 1: 92. https://doi.org/10.3390/catal11010092
APA StyleSalahuddin, U., Iqbal, N., Noor, T., Hanif, S., Ejaz, H., Zaman, N., & Ahmed, S. (2021). ZIF-67 Derived MnO2 Doped Electrocatalyst for Oxygen Reduction Reaction. Catalysts, 11(1), 92. https://doi.org/10.3390/catal11010092





