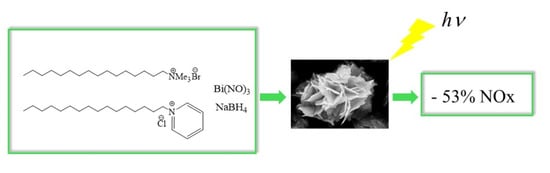
Bismuth Oxyhalides for NOx Degradation under Visible Light: The Role of the Chloride Precursor
Abstract
1. Introduction
2. Results and Discussion
2.1. XRPD and SEM
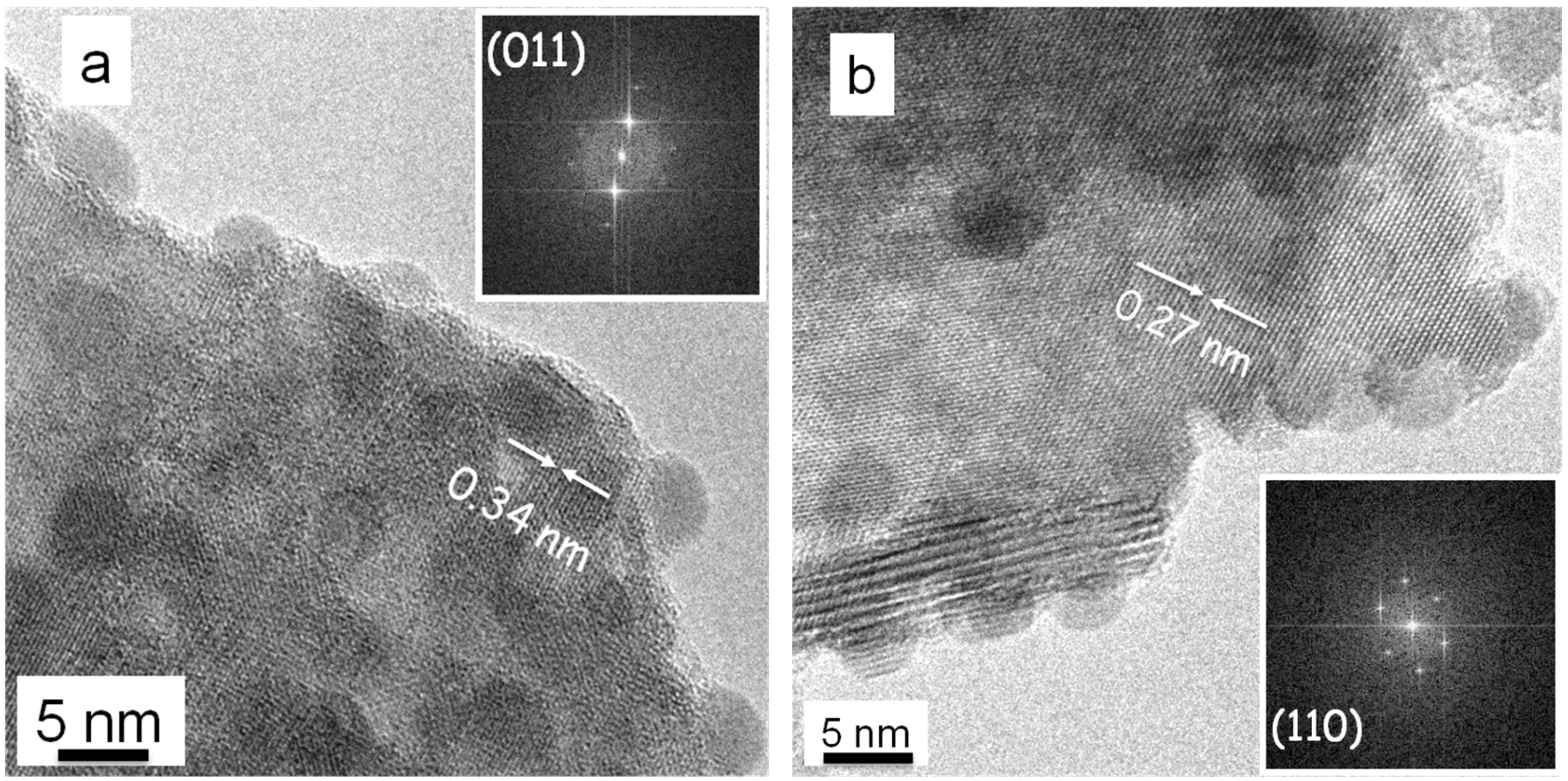
2.2. HRTEM
2.3. DRS
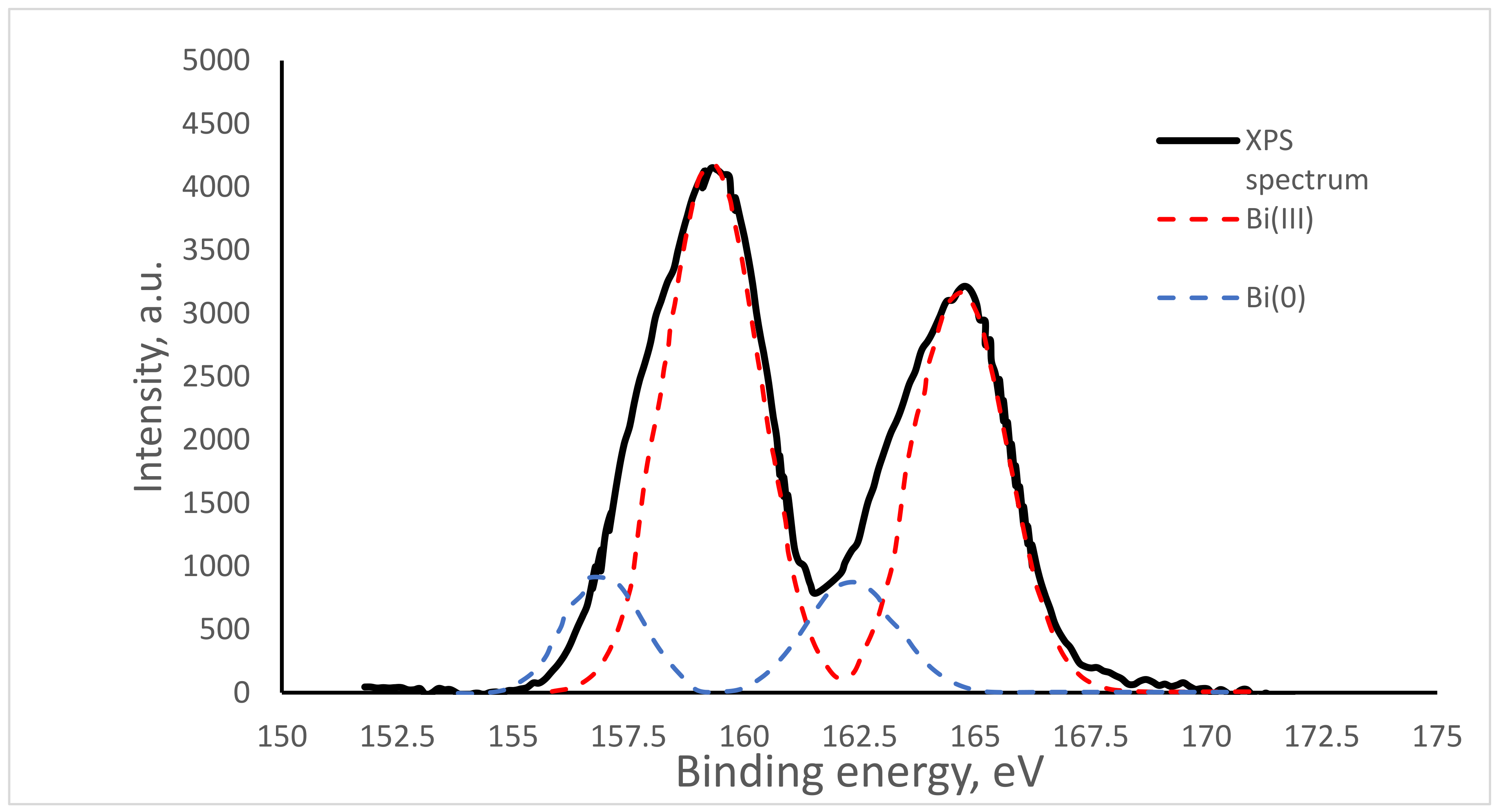
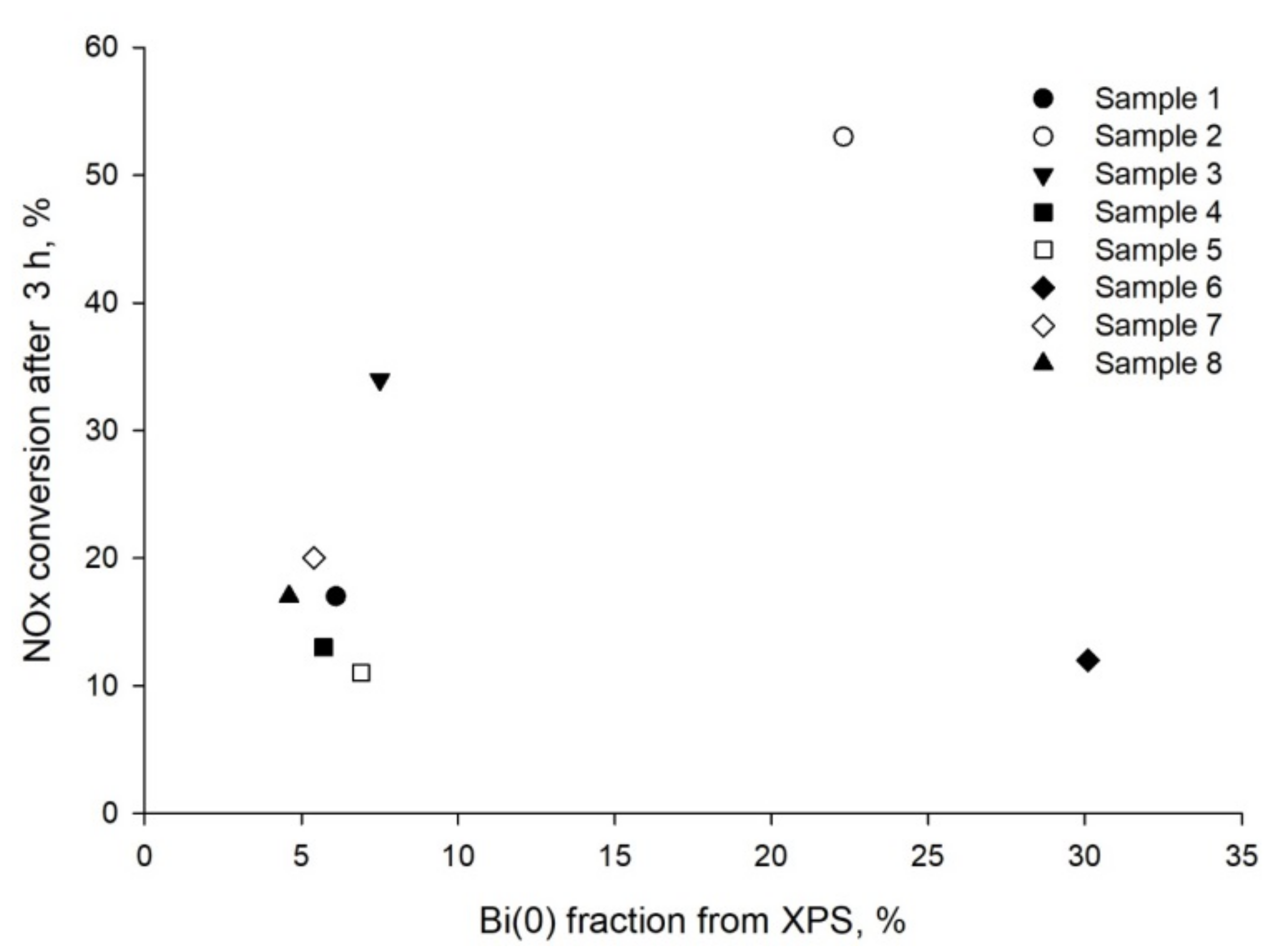
2.4. XPS
2.5. Catalytic Tests
3. Materials and Methods
3.1. Synthesis
3.2. Characterization
3.2.1. X-ray Powder Diffraction
3.2.2. Scanning Electron Microscopy and Transmission Electron Microscopy
3.2.3. UV–vis Diffuse Reflectance Spectroscopy
3.2.4. X-ray Photoelectron Spectroscopy
3.2.5. Photocatalytic Tests
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. Ambient Air Pollution: A Global Assessment of Exposure and Burden of Disease; WHO: Geneva, Switzerland, 2016. [Google Scholar]
- Schwarzenbach, R.P.; Egli, T.; Hofstetter, T.; von Gunten, U.; Wehrli, B. Global Water Pollution and Human Health. Annu. Rev. Environ. Res. 2010, 35, 109. [Google Scholar] [CrossRef]
- Stevens, G.; Mascarenhas, M.; Mathers, C. Global health risks: Progress and challenges. Bull. World Health Organ. 2009, 87, 646. [Google Scholar] [CrossRef]
- Elfiad, A.; Boffito, D.C.; Khemassia, S.; Galli, F.; Chegrouche, S.; Meddour-Boukhobza, L. Eco-friendly synthesis from industrial wastewater of Fe and Cu nanoparticles over NaX zeolite and activity in 4-nitrophenol reduction. Can. J. Chem. Eng. 2018, 96, 1566. [Google Scholar] [CrossRef]
- Bianchi, C.L.; Pirola, C.; Galli, F.; Vitali, S.; Minguzzi, A.; Stucchi, M.; Manenti, F.; Capucci, V. NOx degradation in a continuous large-scale reactor using full-size industrial photocatalytic tiles. Catal. Sci. Technol. 2016, 6, 2261. [Google Scholar] [CrossRef]
- Galli, F.; Compagnoni, M.; Vitali, D.; Pirola, C.; Bianchi, C.L.; Villa, A.; Prati, L.; Rossetti, I. CO2 photoreduction at high pressure to both gas and liquid products over titanium dioxide. Appl. Catal. B Environ. 2017, 200, 386. [Google Scholar] [CrossRef]
- Pirola, C.; Boffito, D.C.; Vitali, S.; Bianchi, C.L. Photocatalytic coatings for building industry: Study of 1 year of activity in the NO x degradation. J. Coat. Technol. Res. 2012, 9, 453. [Google Scholar] [CrossRef]
- Ostad, M.I.; Shahrak, M.N.; Galli, F. Photocatalytic carbon dioxide reduction to methanol catalyzed by ZnO, Pt, Au, and Cu nanoparticles decorated zeolitic imidazolate framework-8. J. CO2 Util. 2020, 101373. [Google Scholar] [CrossRef]
- Stucchi, M.; Galli, F.; Bianchi, C.L.; Pirola, C.; Boffito, D.C.; Biasioli, F.; Capucci, V. Simultaneous photodegradation of VOC mixture by TiO2 powders. Chemosphere 2018, 193, 198. [Google Scholar] [CrossRef] [PubMed]
- Das, A.; Patra, M.; Wary, R.R.; Gupta, P.; Nair, R.G. Photocatalytic performance analysis of Degussa P25 under various laboratory conditions. IOP Conf. Ser. Mater. Sci. Eng. 2018, 377, 012101. [Google Scholar] [CrossRef]
- Ragaini, V.; Selli, E.; Bianchi, C.L.; Pirola, C. Sono-photocatalytic degradation of 2-chlorophenol in water: Kinetic and energetic comparison with other techniques. Ultrason. Sonochem. 2001, 8, 251. [Google Scholar] [CrossRef]
- Bianchi, C.L.; Pirola, C.; Galli, F.; Cerrato, G.; Morandi, S.; Capucci, V. Pigmentary TiO2: A challenge for its use as photocatalyst in NOx air purification. Chem. Eng. J. 2015, 261, 76. [Google Scholar] [CrossRef]
- Sayes, C.M.; Wahi, R.; Kurian, P.A.; Liu, Y.; West, J.L.; Ausman, K.D.; Warheit, D.B.; Colvin, V.L. Correlating Nanoscale Titania Structure with Toxicity: A Cytotoxicity and Inflammatory Response Study with Human Dermal Fibroblasts and Human Lung Epithelial Cells. Toxicol. Sci. 2006, 92, 174. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, C.L.; Pirola, C.; Galli, F.; Stucchi, M.; Mofrandi, S.; Cerrato, G.; Capucci, V. Nano and micro-TiO2 for the photodegradation of ethanol: Experimental data and kinetic modelling. RSC Adv. 2015, 5, 53419. [Google Scholar] [CrossRef]
- Linsebigler, A.L.; Lu, G.; Yates, J.T. Photocatalysis on TiO2 Surfaces: Principles, Mechanisms, and Selected Results. Chem. Rev. 1995, 95, 735. [Google Scholar] [CrossRef]
- Schieppati, D.; Galli, F.; Peyot, M.L.; Yargeau, V.; Bianchi, C.L.; Boffito, D.C. An ultrasound-assisted photocatalytic treatment to remove an herbicidal pollutant from wastewaters. Ultrason. Sonochem. 2019, 54, 302. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Huang, F.; Lin, X. xBiOI–(1−x)BiOCl as efficient visible-light-driven photocatalysts. Scr. Mater. 2007, 56, 669. [Google Scholar] [CrossRef]
- Zhang, K.; Liu, C.; Huang, F.; Zheng, C.; Wang, W. Study of the electronic structure and photocatalytic activity of the BiOCl photocatalyst. Appl. Catal. B Environ. 2006, 68, 125. [Google Scholar] [CrossRef]
- Wang, W.; Huang, F.; Lin, X.; Yang, J. Visible-light-responsive photocatalysts xBiOBr–(1−x)BiOI. Catal. Commun. 2008, 9, 8. [Google Scholar] [CrossRef]
- Shenawi-Khalil, S.; Uvarov, V.; Kritsman, Y.; Menes, E.; Popov, I.; Sasson, Y. A new family of BiO(ClxBr1−x) visible light sensitive photocatalysts. Catal. Commun. 2011, 12, 1136. [Google Scholar] [CrossRef]
- Gnayem, H.; Sasson, Y. Hierarchical nanostructured 3D flowerlike BiOClxBr1-x semiconductors with exceptional visible light photocatalytic activity. ACS Catal. 2013, 3, 186. [Google Scholar] [CrossRef]
- Gnayem, H.; Sasson, Y. Nanostructured 3D Sunflower-like Bismuth Doped BiOClxBr1-x Solid Solutions with Enhanced Visible Light Photocatalytic Activity as a Remarkably Efficient Technology for Water Purification. J. Phys. Chem. C 2015, 119, 19201. [Google Scholar] [CrossRef]
- Dandapat, A.; Horovitz, I.; Gnayem, H.; Sasson, Y.; Avisar, D.; Luxbacher, T.; Mamane, H. Solar Photocatalytic Degradation of Trace Organic Pollutants in Water by Bi(0)-Doped Bismuth Oxyhalide Thin Films. ACS Omega 2018, 3, 10858. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Ambient (Outdoor) Air Pollution; WHO: Geneva, Switzerland, 2018. [Google Scholar]
- Dong, F.; Xiong, T.; Sun, Y.; Zhao, Z.; Zhou, Y.; Feng, X.; Wu, Z. A semimetal bismuth element as a direct plasmonic photocatalyst. Chem. Commun. 2014, 50, 10386. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Sun, Y.; Xiong, T.; Jiang, G.; Zhang, Y.; Wu, Z.; Dong, F. Activation of amorphous bismuth oxide via plasmonic Bi metal for efficient visible-light photocatalysis. J. Catal. 2017, 352, 102. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, Q.; Dong, F. Visible-light photocatalytic removal of NO in air over BiOX (X = Cl, Br, I) single-crystal nanoplates prepared at room temperature. Ind. Eng. Chem. Res. 2013, 52, 6740. [Google Scholar] [CrossRef]
- Jacob, D.J. Introduction to Atmospheric Chemistry; Princeton University Press: Princeton, NJ, USA, 1999. [Google Scholar]
- Li, W.; Xu, Y.; Dong, Y.; Wu, Y.; Zhang, C.; Zhou, M.; Fu, Q.; Wu, M.; Lei, Y. Bismuth oxychloride nanoflake assemblies as a new anode for potassium ion batteries. Chem. Commun. 2019, 55, 6507. [Google Scholar] [CrossRef]
- Yan, Y.; Yang, H.; Yi, Z.; Xian, T. NaBH4-reduction induced evolution of Bi nanoparticles from BiOCl nanoplates and construction of promising Bi@BiOCl hybrid photocatalysts. Catalysts 2019, 9, 795. [Google Scholar] [CrossRef]
- Murphy, A.B. Band-gap determination from diffuse reflectance measurements of semiconductor films, and application to photoelectrochemical water-splitting. Sol. Energy Mater. Sol. Cells 2007, 91, 1326. [Google Scholar] [CrossRef]
- Tauc, J. Optical properties and electronic structure of amorphous Ge and Si. Mater. Res. Bull. 1968, 3, 37. [Google Scholar] [CrossRef]
- Cerrato, G.; Galli, F.; Boffito, D.C.; Operti, L.; Bianchi, C.L. Correlation preparation parameters/activity for microTiO2 decorated with SilverNPs for NOx photodegradation under LED light. Appl. Catal. B Environ. 2019, 253, 218. [Google Scholar] [CrossRef]
- Wang, L.; Xu, K.; Cui, W.; Lv, D.; Wang, L.; Ren, L.; Xu, X.; Dong, F.; Dou, S.-X.; Hao, W.; et al. Monolayer epitaxial heterostructures for selective visible-light-driven photocatalytic NO oxidation. Adv. Func. Mater. 2019, 29, 1808084. [Google Scholar] [CrossRef]
- Xu, M.; Wang, Y.; Geng, J.; Jing, D. Photodecomposition of NOx on Ag/TiO2 composite catalysts in a gas phase reactor. Chem. Eng. J. 2017, 307, 181. [Google Scholar] [CrossRef]





| Sample | Chloride Source | Nominal Percentage of Metallic Bi |
|---|---|---|
| 1 | DTAC | 0.85 |
| 2 | CPC | 0.85 |
| 3 | CTAC | 1.0 |
| 4 | CPC | 1.2 |
| 5 | BTAC | 1.1 |
| 6 | CTAC 25% water | 1.2 |
| 7 | CPC | 2.0 |
| 8 | CPC | 10 |
| Sample | Chloride Source | Bi Atomic % | % Br | % Cl | Eg (eV) | NOx Conv (t = 3 h) |
|---|---|---|---|---|---|---|
| 1 | DTAC | 6 | 5 | 29 | 3.03 | 17 |
| 2 | CPC | 22 | 6 | 25 | 2.98 | 53 |
| 3 | CTAC | 7.5 | 3 | 15 | 3.01 | 34 |
| 4 | CPC | 5.7 | 4 | 16 | 2.95 | 13 |
| 5 | BTAC | 6.9 | 4 | 23 | 3.05 | 11 |
| 6 | CTAC 25% water | 30 | 2 | 13 | 3.07 | 12 |
| 7 | CPC | 5.4 | 2 | 15 | 2.81 | 20 |
| 8 | CPC | 4.6 | 3 | 15 | 3.00 | 17 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tessore, F.; Galli, F.; Schieppati, D.; Boffito, D.C.; Di Michele, A.; Demartin, F.; Cerrato, G.; Bianchi, C.L. Bismuth Oxyhalides for NOx Degradation under Visible Light: The Role of the Chloride Precursor. Catalysts 2021, 11, 81. https://doi.org/10.3390/catal11010081
Tessore F, Galli F, Schieppati D, Boffito DC, Di Michele A, Demartin F, Cerrato G, Bianchi CL. Bismuth Oxyhalides for NOx Degradation under Visible Light: The Role of the Chloride Precursor. Catalysts. 2021; 11(1):81. https://doi.org/10.3390/catal11010081
Chicago/Turabian StyleTessore, Francesca, Federico Galli, Dalma Schieppati, Daria C. Boffito, Alessandro Di Michele, Francesco Demartin, Giuseppina Cerrato, and Claudia L. Bianchi. 2021. "Bismuth Oxyhalides for NOx Degradation under Visible Light: The Role of the Chloride Precursor" Catalysts 11, no. 1: 81. https://doi.org/10.3390/catal11010081
APA StyleTessore, F., Galli, F., Schieppati, D., Boffito, D. C., Di Michele, A., Demartin, F., Cerrato, G., & Bianchi, C. L. (2021). Bismuth Oxyhalides for NOx Degradation under Visible Light: The Role of the Chloride Precursor. Catalysts, 11(1), 81. https://doi.org/10.3390/catal11010081