Abstract
Solution-processed perovskite quantum dots (QDs) have been intensively researched as next-generation photocatalysts owing to their outstanding optical properties. Even though the intrinsic physical properties of perovskite QDs have been significantly improved, the chemical stability of these materials remains questionable. Their low long-term chemical stability limits their commercial applicability in photocatalysis. In this study, we investigated the photodegradation mechanisms of perovskite QDs and their hybrids via photoluminescence (PL) by varying the excitation power and the ultraviolet (UV) exposure power. Defects in perovskite QDs and the interface between the perovskite QD and the co-catalyst influence the photo-stability of perovskite QDs. Consequently, we designed a stable perovskite QD film via an in-situ cross-linking reaction with amine-based silane materials. The surface ligand comprising 2,6-bis(N-pyrazolyl)pyridine nickel(II) bromide (Ni(ppy)) and 5-hexynoic acid improved the interface between the Ni co-catalyst and the perovskite QD. Then, ultrathin SiO2 was fabricated using 3-aminopropyltriethoxy silane (APTES) to harness the strong surface binding energy of the amine functional group of APTES with the perovskite QDs. The Ni co-catalyst content was further increased through Ni doping during purification using a short surface ligand (3-butynoic acid). As a result, stable perovskite QDs with rapid charge separation were successfully fabricated. Time-correlated single photon counting (TCSPC) PL study demonstrated that the modified perovskite QD film exhibited slow photodegradation owing to defect passivation and the enhanced interface between the Ni co-catalyst and the perovskite QD. This interface impeded the generation of hot carriers, which are a critical factor in photodegradation. Finally, a stable red perovskite QD was synthesized by applying the same strategy and the mixture between red and green QD/Ni(ppy)/SiO2 displayed an CO2 reduction capacity for CO (0.56 µmol/(g∙h)).
1. Introduction
Rapid industrialization and urbanization result in high energy consumption. The amount of energy required to support the modern lifestyle is increasing as the interactions between humans and devices increase through the Internet of Things. To generate the necessary energy, fossil fuels, nuclear energy, and natural energy sources such as solar, water, and wind power are used. Despite the global trend toward renewable energy, which generates energy via natural energy sources, the energy conversion efficiency of renewable energy systems is far behind those of fossil fuels and nuclear energy. Moreover, the by-products of energy conversion using fossil fuels and nuclear energy cause serious problems around the globe. In particular, the release of carbon dioxide (CO2) from fossil fuels gives rise to various global problems such as a rise in the greenhouse effect. To satisfy the demands of our age, namely a high energy consumption and lower environmental pollution, we need to develop both pollutant removal methods and renewable energy sources. Thus, renewable energy conversion via a carbon-neutral process is critical for next-generation energy systems.
Several researchers have studied CO2 conversion systems over the past decade. Among the various strategies proposed, photocatalytic CO2 conversion has received considerable attention, because it utilizes sunlight to convert pollutants into chemical fuels. This process not only converts solar energy into chemical fuels; however, it can reduce CO2 generation [1]. For photocatalytic CO2 conversion, the photocatalyst is the key material. Properties of the photocatalyst should include light absorption, exciton generation, charge separation, surface absorption/desorption, and surface redox reactions. Metal oxides, layered double hydroxides, metal–organic frameworks, and nano-dimensional materials have all been examined as photocatalysts [2]. Metal oxides such as TiO2, ZnO, NiO, and Al2O3 are considered as the standard owing to their high stability and low cost [3]. However, the large band gap of these material limits their performance owing to low energy absorption from sunlight.
Recently, perovskite materials have been intensively studied as photocatalysts owing to their strong light absorption, high absorption coefficients, low exciton binding energies, long charge-carrier diffusion lengths, multiexciton generation effects, and facile bandgap modulation [4,5,6]. However, their low stability in polar media limits their applicability as photocatalysts. Studies have attempted to enhance the stability of perovskites via templates. Kuang et al. improved the stability of perovskites by using graphene oxide as a template [7]. CsPbBr3 nanocrystals were placed on a graphene oxide substrate. Owing to the high mobility of graphene oxide, the charge separation of the CsPbBr3 nanocrystals was dramatically improved. Moreover, the graphene oxide acted as a template for the CsPbBr3 nanocrystals, thus preventing the aggregation of CsPbBr3 nanocrystals during the photo-reaction. To improve stability, the encapsulation of perovskite in a metal–organic framework (PCN-221(Fex)) was applied [8]. MAPbI3-PCN-221(Fex) demonstrated better stability and activity than pristine perovskite and PCN-221(Fex). Although several studies have demonstrated better stability and enhanced catalytic behavior by using various templates, the mechanism of photodegradation of the hybrid system is unclear. Thus, it is necessary to investigate the photodegradation behavior of the hybrid system. In addition, the development of perovskite quantum dots (QDs) with high stability in chemical environments is indispensable to the successful production of high-efficiency photocatalysts.
In this study, we investigated the photodegradation of encapsulated perovskite QDs. The design of the encapsulated material included an in-situ cross-linking reaction with 3-aminopropyltriethoxy silane (APTES). The surface ligand, which comprised 2,6-bis(N-pyrazolyl)pyridine nickel(II) bromide (Ni(ppy)) and the silane material, was carefully selected to preserve optoelectrical properties while driving the in-situ cross-linking reaction. This short surface ligand facilitated co-catalyst doping during the synthesis reaction and purification. In particular, the multi-π-electron-conjugated structure of Ni(ppy) improved the interface between the Ni complex and perovskite QD in addition to enhancing electron transfer and storage capacity during the photocatalytic reaction. Moreover, an ultrathin SiO2 coating formed through in-situ cross-linking of APTES effectively prevented ion migration and the vaporization of organic cations, resulting in better operational stability. A time-resolved photoluminescence (PL) study demonstrated that the modified perovskite QD film exhibited slow photodegradation because of rapid charge separation and defect passivation. As a result, our system achieved an CO2 reduction capacity for CO of 0.56 µmol/(g∙h).
2. Results
2.1. The Effect of Surface Lligand on Co-Catalyst Doping
The photodegradation of perovskite QDs is one of the main factors limiting their photocatalytic efficiency. To understand the photodegradation behavior, we investigated the photophysical properties of various types of perovskite QDs: Pristine QD, encapsulated perovskite QD (QD/SiO2), co-catalyst-doped perovskite QD (QD/Ni(ppy)), and encapsulated co-catalyst-doped perovskite QD (QD/Ni(ppy)/SiO2). Organic–inorganic perovskite QDs were used in this study because of their conduction band alignment with the CO2 reduction reaction [9].
Figure 1a is a schematic illustration of the synthesis of QD/Ni(ppy)/SiO2. The reaction conditions were modified from a previous ligand-assisted precipitation method [10]. The pristine QDs was synthesized using oleic acid and oleylamine as a surface ligand (method 3.2). The transmission electron microscopy (TEM) image of a pristine QD demonstrated a cubic shape of approximately 5 nm side length (Figure 1b). The photoluminescence quantum yield (PLQY) of this material was 71% with a full width at half maximum (FWHM) of approximately 27 nm (Figure 1c). For QD/SiO2 fabrication, the as-synthesized pristine QDs were re-dispersed in an APTES/toluene solution. Further, the 5 µL of ammonia was to ensure the silanization of the APTES (method 3.3) [11,12]. The QD/SiO2 demonstrated a PLQY of 69% and an FWHM of approximately 21 nm (Figure 1d). The PL peak position shifted from 511 to 516 nm after SiO2 coating. In addition, the position of the band edge in the ultraviolet–visible (UV–Vis) spectra changed from 520 nm for pristine QD to 529 nm for QD/SiO2. The TEM image in Figure 1e indicates that the size of QD/SiO2 increased to 15–30 nm. We determined the energy-dispersive X-ray spectroscopy (EDS) line profile for the TEM image in Figure 1f. The EDS line profiles of several QDs were determined and all displayed similar behaviors. Figure 1g indicates that QD/SiO2 has a core-shell structure consisting of a perovskite QD core and a SiO2 shell. The radius of the core was approximately 10 nm, which was larger than that of the pristine QD, and the SiO2 shell thickness was approximately 5 nm. The larger particle size of perovskite QD/SiO2 resulted in a redshift of the PL peak; however, the PLQY of QD/SiO2 did not significantly change compared with pristine QD. We predict that core-shell structure enhanced the quantum confinement of QD/SiO2, leading to a higher PLQY. Quantum confinement with size had a smaller impact on PLQY of QD/SiO2 [13].
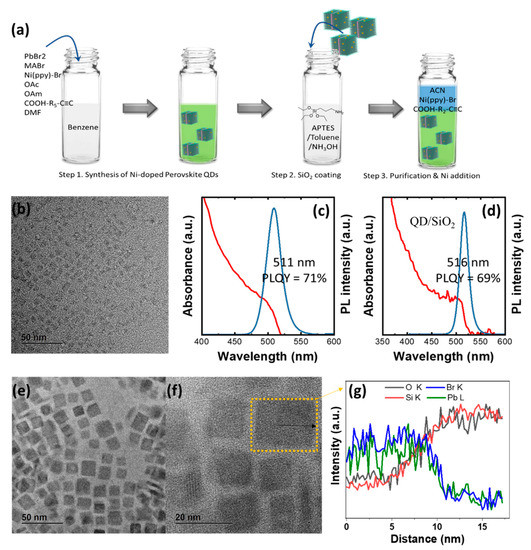
Figure 1.
(a) Schematic illustration of synthesis process of QD/Ni(ppy)/SiO2; (b) TEM image of pristine QD; UV-vis spectra; PL spectra of (c) pristine QD and (d) QD/SiO2; (e,f) TEM image of QD/SiO2 and (g) corresponding EDS line profile.
The synthesis process for QD/Ni(ppy) is similar to that of pristine QDs. For QD/Ni(ppy), a trace of Ni(ppy) was mixed with the other precursors. The 5-hexynoic acid was additionally inserted with original surface ligands (method 3.5). Additional Ni(ppy) was added during purification to increase the Ni(ppy) content. During purification, a short alkyl ligand (i.e., 3-butynoic acid) was combined with Ni(ppy) in methanol to facilitate ligand exchange [14] and the co-catalyst doping reaction (method 3.6). Without the Ni addition step during purification, QD/Ni(ppy) demonstrated a PLQY of 71% with a PL peak at 509 nm (FWHM ~26 nm in Figure 2a). The absorption band edge displayed blueshift. Ni(ppy) appeared to prevent the lattice growth of perovskite QDs during ligand-assisted precipitation [15]. However, when additional Ni(ppy) was introduced through ligand exchange during purification, the PL peak shifted to 511 nm (Figure 2b). However, the PLQY of QD/Ni(ppy) dropped to 52% with a FWHM of 26 nm because of the Ni(ppy)-facilitated charge separation in the perovskite QDs [16]. Finally, QD/Ni(ppy)/SiO2 was fabricated via a modified ligand-assisted precipitation method, described in method 3.8. Once QD/Ni(ppy) was obtained, the QD/Ni(ppy) was re-dispersed in the APTES/toluene solution. Subsequently, 5 µL of ammonia was added to silanize the APTES. Then, Ni(ppy) was additionally inserted through the purification process. The obtained QD/Ni(ppy)/SiO2 demonstrated a PLQY of 59% with a FWHM of 24 nm (Figure 2c).
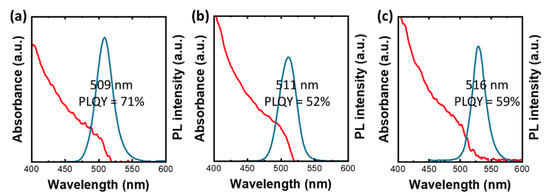
Figure 2.
UV-vis spectra and PL spectra of (a) QD/Ni(ppy) without Ni doping via purification process (P1-2), (b) QD/Ni(ppy) with Ni doping via purification process (P2-2), and (c) QD/Ni(ppy)/SiO2.
When we synthesized QD/Ni(ppy), we added Ni(ppy) with a short alkyl chain (i.e., 5-hexynoic acid) at the reaction pot. When QD/Ni(ppy) was fabricated without a short alkyl chain ligand (see process P1-1 in Figure 3a), Ni(ppy) could not effectively interact with the perovskite QDs because the densely-packed oleic acid and oleylamine prevented the diffusion of Ni(ppy) to the perovskite QD surface [17,18]. As a result, QD/Ni(ppy) formed during P1-1 displayed no significant Ni peak in the X-ray photoelectron spectroscopy (XPS) spectrum (Figure 3b). In contrast, when QD/Ni(ppy) was synthesized via a short alkyl chain ligand (P1-2 process in Figure 3a), we could distinguish various Ni components via XPS. The deconvoluted XPS Ni 2p spectra of P1-2 in Figure 3b revealed several oxidation states (i.e., Ni3+ at 861.1 eV, Ni(OH)2 at 857.5 and 878.9 eV, NiO at 855.6 and 875.5 eV, Ni(ppy) at 853.7 and 873.0 eV, and metallic Ni at 852 eV) [19]. Even though a Ni2+ precursor was used in the reaction, Ni(ppy) underwent oxidation and reduction. In particular, Ni3+ was formed when the Ni precursor reacted with the vacant positions of methylammonium (MA+) or Pb2+ cations in perovskite structure [20]. Thus, we obtained QD/Ni(ppy) with a high Ni3+ content as well as NiO via P1-2 owing to in-situ doping reaction.
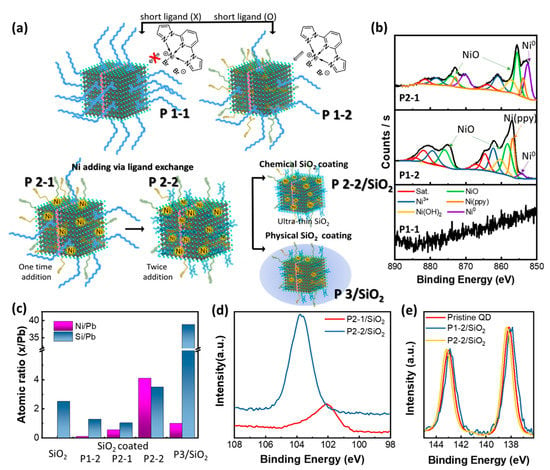
Figure 3.
(a) Schematic illustration of several different perovskite QDs; QD/Ni(ppy) without short surface ligand (P1-1), QD/Ni(ppy) via in-situ Ni doping without Ni doping during purification (P1-2), QD/Ni(ppy) via P1-2 with onetime Ni doping during purification (P2-1), QD/Ni(ppy) via P1-2 with twice Ni doping during purification (P2-2), QD/Ni(ppy) via P2-2 with SiO2 coating via spin coating (P3/SiO2); (b) XPS Ni 2p spectra of P1-1, P1-2, and P2-1; (c) Ni/Pb atomic ratio and Si/Pb atomic ratio of QD/SiO2, P1-2/SiO2, P2-1/SiO2, P2-2/SiO2, and P3/SiO2; (d) XPS Si 2p spectra of P2-1/SiO2 and P2-2/SiO2; (e) XPS Pb 4f spectra of pristine QD, P1-2/SiO2, and P2-2/SiO2.
To increase catalytic efficiency, the co-catalyst mobility should be improved. Mobility is enhanced when Ni is in the form of metallic Ni0 or Ni(ppy). We further developed the Ni(ppy) doping process via a ligand exchange strategy (P2-1 and P2-2 processes in Figure 3a). In P2-1, ligand exchange with Ni(ppy) was conducted once, while in P2-2, ligand exchange with Ni(ppy) was conducted twice. P2-1 and P2-2 were executed using perovskite QDs produced via P1-2. During ligand exchange, a ligand with a shorter alkyl chain (i.e., 3-butynoic acid, which is shorter than 5-hexynoic acid) was used to replace the bound oleic acid and oleylamine as much as possible. P2-1 perovskite QDs contained similar oxidation states to those in P1-2 perovskite QDs (Figure 3b). However, the intensity of the peak at 852 eV corresponding to metallic Ni0 dramatically increased, while the intensity of the peak at 861.1 eV corresponding to Ni3+ decreased after Ni(ppy) doping, indicating a co-catalyst composition conducive to charge separation. In addition, the total amount of Ni increased as the number of ligand exchange cycles increased (Table 1).

Table 1.
Ni/Pb atomic ratio and dominant chemical composition of the product obtained via P1-1, P1-2, P2-1, and P2-2.
We next measured the atomic ratio of Si and Ni after chemical SiO2 coating using APTES (Figure 3c). QD/Ni(ppy)/SiO2 was fabricated using QD/Ni(ppy) from three different processes, namely P1-2, P2-1, and P2-2. QD/SiO2 exhibited a Si content of 2.5%. When QD/Ni(ppy) was coated with SiO2, the Ni content reduced slightly. The P1-2 product contained 0.13% Ni without SiO2 coating, which reduced to 0.10% with SiO2 coating. The P2-1 and P2-2 products yielded similar results. The Ni content reduced from 0.62% to 0.54% for P2-1 and from 4.20% to 4.12% for P2-2 after SiO2 coating. Interestingly, QD/Ni(ppy)/SiO2 fabricated via P2-1 displayed a lower Si content than QD/Ni(ppy)/SiO2 fabricated via P2-2. This was attributed to the large solvated radii of the Ni(ppy)-rich QDs. Even though the Si content of QD/Ni(ppy)/SiO2 produced via P2-2 was higher than those of other types of QD/Ni(ppy)/SiO2, the Ni content was higher than the Si content, which is preferred for enhanced catalytic behavior. Finally, we examined the Ni and Si contents resulting from different Si coating methods, namely chemical (in-situ silanization) and physical (P3/SiO2 in Figure 3a). For P3/SiO2, QD/Ni(ppy) was fabricated via P2-2 and subsequently deposited on the desired substrate. Then, a solution of 10 µM of APTES in benzene was spin-coated onto the QD/Ni(ppy) film and annealed at 50 °C for 5 min in a vacuum. Unlike chemical silanization, the Ni content rapidly decreased to 1.09%, while the Si content increased to 35%, indicating that an effective shell coating was only achieved via chemical silanization. Moreover, we examined the XPS Si 2p spectra (Figure 3d) of QD/Ni(ppy)/SiO2 produced via the different processes (i.e., P2-1/SiO2, P2-2/SiO2) to confirm the chemical composition of the SiO2. A single peak appeared for both P2-1/SiO2 and P2-2/SiO2; however, the peak position shifted from a XPS binding energy (Ebinding) of 102 eV for P2-1/SiO2 to 104 eV for P2-2/SiO2. In general, Si 2p ½ and ⅓ peaks appear at 102 eV. The shape of the resultant peak should be asymmetric because of the overlapping of the Si 2p ½ and ⅓ peaks. However, the XPS Si 2p spectra of P2-1/SiO2 displayed a Gaussian shape, which indicated that the peak of the Si 2p spectra originated from the oxide form of Si [21]. Thus, the peak shift of the binding energies was attributed to the doping effect [22,23]. Figure 3d shows that the N-type doping became stronger as ligand exchange with Ni(ppy) increased (Ebinding (P2-1) < Ebinding (P2-2)). Moreover, we examined the XPS Pb 4f spectra of pristine QDs, P1-2/SiO2, and P2-2/SiO2 (Figure 3e). An n-type shift for P2-2/SiO2 and a p-type shift for P1-2/SiO2 relative to pristine QDs occurred. Both peak shifts were driven by strong chemical interactions between the perovskite QD, Ni(ppy), and the SiO2 shell, which contributed to the redistribution of charge in the perovskite QDs [24]. This confirms the PL results in Figure 2 where the wavelength of the peak for P1-2/SiO2 decreased and that of the peak for P2-2/SiO2 increased compared to pristine QD.
2.2. The Photodegradation Phenomina of Perovskite QD
Next, we investigated the photophysical properties of the four different perovskite QDs: Pristine QD, QD/SiO2, QD/Ni(ppy), and QD/Ni(ppy)/SiO2. Figure 4 demonstrates the photodegradation behavior of the QD solutions in ambient conditions. Each solution was exposed to UV light (1 mW), and a non-polar solvent, benzene, was used. The pristine QD displayed dramatic photodegradation as the duration of the exposure increased (Figure 4a). The PL intensity halved after 20 min of UV exposure and the peak width increased because of defect generation. The PL intensity was almost zero after 30 min. In contrast, QD/Ni(ppy) fabricated via P1-2 displayed slightly higher PL intensity (4%) after 5 min of UV treatment, and an 11% loss of the original PL intensity after 30 min of UV treatment (Figure 4b). Photodegradation appeared to have been suppressed by co-catalyst doping. We hypothesize that charge separation induced by the co-catalyst prevented hot-carrier generation and resulted in better photophysical stability [25].
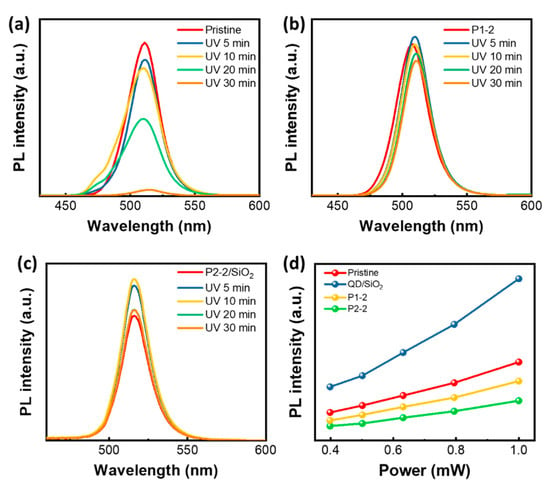
Figure 4.
Photodegradation of QD solution. PL spectra depending on the duration of UV exposure time with (a) pristine QD, (b) QD/Ni(ppy) via P1-2, and (c) QD/Ni(ppy)/SiO2 via silanization of P2-2; (d) Excitation power versus PL intensity plot for pristine QD, QD/SiO2, P1-2, and P2-2.
The SiO2 coating inhibited photodegradation in QD/Ni(ppy). Figure 4c shows the relationship between PL intensity and UV exposure time for QD/Ni(ppy)/SiO2 produced via P2-2. A 29% enhancement in PL intensity was observed after 10 min of UV treatment, however, the intensity decreased as the UV exposure time increased further. However, after 30 min of UV exposure, QD/Ni(ppy)/SiO2 still exhibited a higher PL intensity than that of the fresh sample. Interestingly, the PL peak of P1-2 was slightly red-shift as the UV exposure time increases, while no significant PL shift was observed for P2-2/SiO2. In general, the perovskite QD demonstrates the red-shifted PL with the larger size of QD. Under the UV exposure, perovskite QD underwent crystal reconstruction with the Ni content; the crystal reconstruction was facilitated without hot carrier generation, leading to the collision with the nearby QDs. On the other hand, the P2-2/SiO2 did not show the PL peak shift because the SiO2 coating prevented the collision behavior, but it only facilitated the crystal reconstruction within one QD.
To elucidate photostability enhancement due to the SiO2 coating, we conducted a PL-versus-power experiment. The results are shown in Figure 4d. As the excitation power increased from 0.3 to 1 mW, the PL intensity of pristine QDs at 2.43 eV increased at rate of 3.50. The PL intensity at 2.40 eV of QD/SiO2 increased more rapidly at a rate of 7.91. The PL intensity of pristine QDs rapidly plateaued with increasing excitation density. The numerous defects in pristine QDs limited the number of carriers available to generate excitons, resulting in a lower PL changes for pristine QDs compared with QD/SiO2. We hypothesize that the SiO2 coating plays an important role in passivating the surface defects of perovskite QDs [26]. In contrast, the PL changes of the QD/Ni(ppy) with excitation power decreased in inverse proportion to the amount of Ni(ppy) doping. The PL changes of QD/Ni(ppy) with excitation power produced via P1-2 and P2-2 were 2.65 and 1.87, respectively. This phenomenon was attributed to the limited number of minor carriers in QD/Ni(ppy). After Ni(ppy) doping, charge carriers in the QDs effectively moved to the Ni(ppy), resulting in a loss of PL and a slower PL intensity increase with increasing excitation power [27]. Next, we observed the photodegradation of pristine QDs, QD/SiO2, QD/Ni(ppy), and QD/Ni(ppy)/SiO2 in the form of films. First, the relationship between photodegradation and UV power was examined for pristine QDs (Figure 5a) and for QD/Ni(ppy)/SiO2 (Figure 5b). Pristine QDs only sustained PL up to 0.08 mW UV exposure. At 0.6 mW and 0.3 mW UV exposure, pristine QDs exhibited PL enhancement for the first 20 min; however, PL degraded with further UV exposure. The PL enhancement during short low-power UV exposure was because of defect repair via UV light [28]. However, SiO2 coating prevented the photodegradation of perovskite QDs. At 0.6 mW, 0.3 mW, and 0.08 mW UV exposure, QD/Ni(ppy)/SiO2 displayed PL enhancement for 40 min and the PL intensity was maintained.
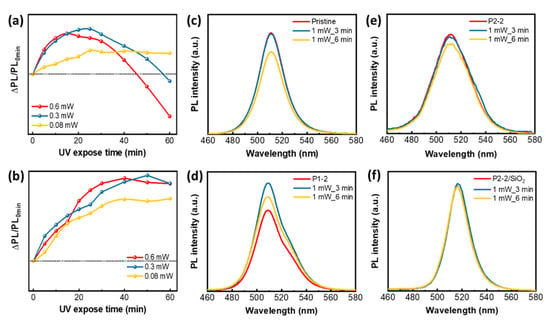
Figure 5.
Photodegradation of film. ΔPL /PL0 intensity depending on the UV exposure time of (a) pristine QD and (b) QD/Ni(ppy)/SiO2; PL intensity change depending on the 1mW UV exposure time of (c) pristine QD, (d) QD/Ni(ppy) via P1-2, (e) QD/Ni(ppy) via P2-2, and (f) QD/Ni(ppy)/SiO2 via P2-2 with silanization.
Photodegradation due to high-power UV exposure was significantly different even for short exposure times. Pristine QDs exhibited a 19% PL loss after only 6 min at 1 mW UV light exposure (Figure 5c). QD/Ni(ppy) produced via P1-2 displayed the opposite tendency (Figure 5d), namely an increase in PL at 1 mW UV light exposure up to 3 min. Compared to the PL spectra of the solution, the PL spectra of the film became broader because the stacked QD/Ni(ppy) in the film facilitated intermolecular charge transport between the QD and the Ni co-catalyst [29]. In contrast to PL enhancement during short low-power UV exposure, only the P1-2 sample displayed PL enhancement with high-power UV exposure, which was attributed to defect repair via UV light. We hypothesize that the effective interaction between the perovskite QD and Ni(ppy) in the P1-2 sample inhibited hot-carrier generation under high-power UV light and enhanced PL intensity. In addition, the two discernible PL peaks appeared for P1-2 sample due to the crystal reconstruction and collision behavior under UV exposure, as we mentioned. Hence, QD/Ni(ppy) produced via P2-2 did not show PL enhancement with 1 mW UV light exposure (Figure 5e), but it demonstrated a smaller PL loss (10%) than that of pristine QDs. Finally, QD/Ni(ppy)/SiO2 produced via P2-2 displayed no significant change during 1 mW.
To demonstrate the importance of the interface between the QD and the Ni complex, we used NiBr2 as the Ni doping precursor. In contrast to Ni(ppy), the PL intensity did not decrease as the amount of Ni doping increased (Figure 6a). The PL intensity of QD/NiBr2 increased by 20% after a single ligand exchange and by 28% after a double ligand exchange. However, QD/Ni(ppy) displayed excellent PL quenching behavior because of the strong interaction between the QDs and Ni(ppy) and the multi-electron-conjugated structure of Ni(ppy), which enhanced the electron transfer and storage ability of the Ni co-catalyst [30]. Interestingly, when we conducted the P2-1 process without P1-2 pre-treatment, the resultant QD/Ni(ppy) displayed significantly lower PL quenching behavior because of the small amount of immobilized Ni(ppy) (Figure 6b), but still exhibited a 6% PL loss with 20 μM Ni(ppy) addition. With P1-2 pre-treatment, the PL intensity dramatically decreased as the amount of Ni doping increased because of effective charge separation (Figure 6c), indicating that P1-2 promoted interactions between Ni(ppy) and QD during Ni doping via the ligand exchange processes (i.e., P2-1 and P2-2).
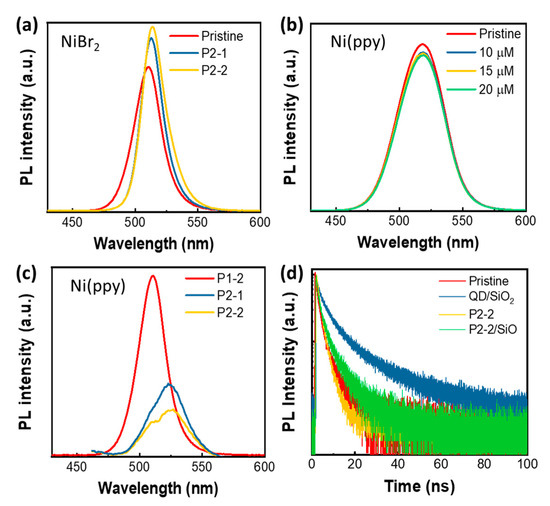
Figure 6.
(a) PL Spectrum of pristine QD, QD/NiBr2 via P2-1, and QD/NiBr2 via P2-2; (b) PL Spectrum of QD/Ni(ppy) with different Ni(ppy) concentrations. The QD/Ni(ppy) is synthesized via P2-1 without P1-2 process; (c) PL Spectrum of QD/Ni(ppy) via P1-2, QD/Ni(ppy) via P2-1, and QD/Ni(ppy) via P2-2; (d) TCSPC of pristine QD, QD/SiO2, QD/Ni(ppy) via P2-2, QD/Ni(ppy)/SiO2 via P2-2.
The TCSPC PL spectra supported this result (Figure 6d). The PL lifetimes of pristine QD, QD/Ni(ppy) via P2-2, QD/SiO2, and QD/Ni(ppy)/SiO2 were 6.80 ns (χ2 = 1.031), 4.87 ns (χ2 = 1.007), 13.60 ns (χ2 = 1.010), and 6.15 ns (χ2 = 0.984), respectively; χ2 is the reduced chi-squared value. After Ni(ppy) doping, the PL lifetime of QD/Ni(ppy) decreased to less than that of pristine QD because of charge separation. In contrast, the PL lifetime of QD/SiO2 dramatically increased because of defect passivation. As a result, QD/Ni(ppy)/SiO2 produced via P2-2 demonstrated a longer lifetime than QD/Ni(ppy) produced via P2-2 but demonstrated a significantly shorter lifetime than QD/SiO2, indicating that PL quenching via Ni(ppy) still occurred after SiO2 coating.
2.3. Catalytic Behavior of Perovskite QD
To increase the catalytic selectivity for CO2 conversion, the conduction band of the photocatalyst should be close to the CO2 reduction potential. Red perovskite QDs are known to have excellent band alignment with the CO2 reduction potential. However, red perovskite QDs are impractical to use as photocatalysts for CO2 conversion because of their rapid photodegradation. We improved the photo-stability of perovskite QDs via SiO2 encapsulation to produce a red/green perovskite QD/Ni(ppy)/SiO2 for use as a CO2 conversion photocatalyst. Figure 7a shows the schematic band alignment of red/green perovskite QD/Ni(ppy)/SiO2. The solar simulator has a continuous light spectrum from 400 to 1100 nm, with 400–700 nm light contributing approximately 40% of the total irradiance. The red/green perovskite QDs in this study can absorb a wider range of light compared with the green perovskite QDs. Moreover, red/green perovskite QDs facilitated photocarrier generation efficiency. Higher energies above 500 nm generated multiexcitons in red and green QDs. In particular, the excitons from green QDs were delivered to red QDs through energy transfer and charge transfer, dramatically increasing photocarrier generation in the red QDs. The photo-induced electrons transferred to the Ni complex for use in CO2 reduction. Figure 7b shows the PL intensities of various materials. The pristine green and red perovskite QDs that were used to synthesize red/green perovskite QD/Ni(ppy) demonstrated a 78% PLQY and 41% PLQY, respectively. The red/green perovskite QD/Ni(ppy) was fabricated using different amounts of Ni(ppy) doping. When the red perovskite QD/Ni(ppy) and green perovskite QD/Ni(ppy) were mixed, the PL intensities of both reduced significantly even at a low Ni(ppy) content. This PL quenching is evidence of energy transfer and charge transfer between the green and red perovskite QDs. Next, we measured the PL intensities at different Ni(ppy) contents. The PL intensities of both the green QDs and red QDs decreased in inverse proportion to the Ni(ppy) content. Finally, red/green perovskite QD/Ni(ppy)/SiO2 was synthesized. The TEM image of red/green perovskite QD/Ni(ppy)/SiO2 indicated a strong interaction between the red and green perovskite QDs (Figure 7c,d). Small green perovskite QDs attached to the surface of large red perovskite QDs. Interestingly, we observed many small perovskite QDs (~10 nm in diameter) similar to the original green perovskite QDs. However, we identified mid-sized perovskite QDs (20–30 nm in diameter) on the surface of the red perovskite QDs. Pristine green QDs normally exhibit diameters less than 10 nm. In the red/green perovskite QD/Ni(ppy)/SiO2, mid-sized perovskite QDs were fabricated via mixing of red and green perovskite QDs during the silanization reaction [31]. Rapid silanization prevents a large number of mid-sized QD formation because the red and green perovskite QD experience less nanocrystal confusion through rapid silanization. There were significantly fewer mid-sized perovskite QDs than small perovskite QDs. In addition, we observed an ultrathin SiO2 coating at the edge of the red/green perovskite QD/Ni(ppy)/SiO2.
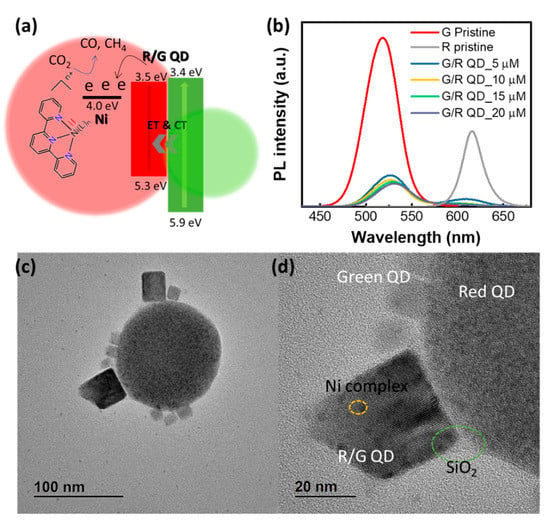
Figure 7.
(a) Energy diagram of red/green perovskite QD/Ni(ppy)/SiO2.; (b) PL Spectrum of green pristine QD, red pristine QD, and red/green perovskite QD/Ni(ppy) with different Ni(ppy) concentrations. (c,d) TEM image of red/green perovskite QD/Ni(ppy)/SiO2.
Finally, the catalytic behavior of different types of perovskite QDs is presented in Figure 8: pristine QD, perovskite QD/SiO2, and red/green perovskite QD/Ni(ppy)/SiO2. We firstly investigated the photocatalytic activity in CO2-saturated water (4%). The pristine QDs demonstrated a low CO2 reduction catalytic activity of approximately 3 µmol/g in Figure 8a. The CO2 reduction rate of pristine QD was higher over a 6 h period than over a 24 h period because QD catalytic activity reduced and finally stopped during the CO2 reduction. Interestingly, the amount of generated H2 gas was also reduced after 18 h. It indicated the fast degradation behavior of pristine QD. The perovskite QD/SiO2 demonstrated significantly lower catalytic activity because the SiO2 coating impaired efficient charge separation for CO2 reduction (Figure 8b). The catalytic behavior of red/green perovskite QD/Ni(ppy)/SiO2 was improved. The photocatalytic hydrogen reduction activity of QD/Ni(ppy)/SiO2 improved to 72 μmol/g, compared to that of pristine QD (16 μmol/g) in Figure 8c. The perovskite QD/Ni(ppy)/SiO2 exhibited more consistent activity, and its catalytic behavior still sustained until 27 h, resulting in enhanced catalytic stability after SiO2 coating. However, the CO conversion rate of this material was inferior (Figure 8d). To improve the CO2 reduction rate, we optimized the hole scavenger and the ratio between red and green QDs. The CO2-saturated ethyl acetate in the presence of water (1%) showed a 15-fold CO2 reduction rate compared to ~4% H2O hole scavenger (Figure 8e). Finally, the 1:2 ratio of red/green perovskite QD/Ni(ppy)/SiO2 demonstrated catalytic activity of approximately 0.56 μmol/g·h for CO (Figure 8f).
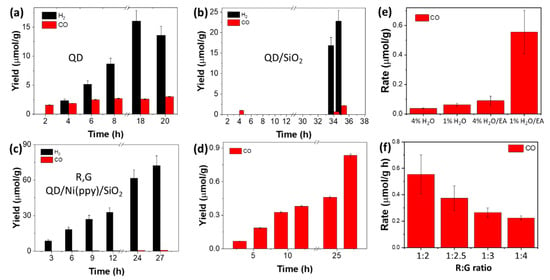
Figure 8.
Photocatalytic behavior of (a) green pristine QD, (b) green perovskite QD/SiO2, and (c,d) red/green perovskite QD/Ni(ppy)/SiO2 with 4% H2O hole scavengers. Photocatalytic behavior of (e) red/green perovskite QD/Ni(ppy)/SiO2 with different hole scavengers and (f) red/green perovskite QD/Ni(ppy)/SiO2 with different red/green QD ratio.
The photodegradation of perovskite QD/Ni(ppy) and perovskite QD/Ni(ppy)/SiO2 was investigated via a TCSPC PL study. The PL lifetime of pristine red/green QD mixtures without SiO2 coating is difficult to observe because of nanocrystal confusion and defect generation over time. Thus, we measured the TCSPC PL of green perovskite QD/Ni(ppy) (Figure 9a) and red perovskite QD/ Ni(ppy) (Figure 9b) separately. The TCSPC PL of green perovskite QD/Ni(ppy) at different excitation power levels revealed significant photon losses as the excitation power increased. The average PL lifetime reduced from 4.80 to 4.09 ns when the TCSPC tests were conducted at the same spot in the film. The red perovskite QD/Ni(ppy) demonstrated a reduction in average PL lifetime as the excitation power increased but to a lesser degree than the green perovskite QD/Ni(ppy), even though the stability of green perovskite QDs was better than that of red perovskite QDs. We hypothesize that good alignment of the red perovskite QD and Ni(ppy) facilitated charge transport between them and prevented a photoinduced redox reaction in the perovskite QDs. In contrast, red/green perovskite QD/Ni(ppy)/SiO2 displayed no significant change at different excitation power levels, indicating high stability. The PL lifetimes at 511 nm (Figure 9c) and 612 nm (Figure 9d) were measured at the same spot in the film of the red/green perovskite QD/Ni(ppy)/SiO2 sample. Both red and green PL were detected and their lifetimes were constant within the same excitation power range used for the pristine QDs. The SiO2 coating appeared to effectively minimize photodegradation via defect passivation behavior.
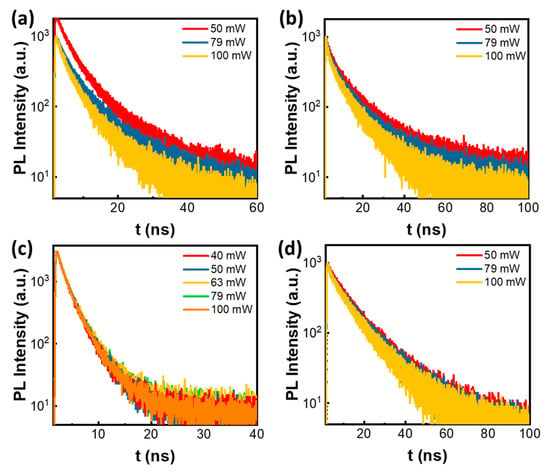
Figure 9.
Photodegradation behavior of QD/Ni(ppy) and red/green perovskite QD/Ni(ppy)/SiO2. Time-resolved PL depending on excitation power for (a) green QD/Ni(ppy) and (b) red QD/Ni(ppy). Time-resolved PL depending on excitation power of red/green perovskite QD/Ni(ppy)/SiO2 at (c) 511 nm and (d) 612 nm emission wavelength.
3. Materials and Methods
3.1. Materials
Methylammoniumbromide (CH3NH3Br), leadbromide (PbBr2), leadiodine (PbI2), oleylamine (98%), oleic acid (90%), 2,6-bis(N-pyrazolyl)pyridine nickel(II) bromide (Ni(ppy)), Nickel(II) bromide (NiBr2), 3-aminopropyltriethoxy silane (APTES), butynoic acid, hexynoic acid, and N,N-dimethylformamide (DMF) were purchased from Sigma-Aldrich, Seoul, Korea. Benzene, hexane, toluene, acetonitrile, methanol, and methyl acetate (HPCL grade) were purchased from TCI, Seoul, Korea. All chemicals were used without further purification.
3.2. Preparation of Pristine Perovskite QD
The green perovskite QDs were synthesized by a well-known precipitation method [32]. All solvents are used in an anhydrate state. The 33.56 mg of CH3NH3Br and 146.8 mg of PbBr2 are dissolved in 10 mL of DMF. Then, 0.1 mL of oleylamine and 1 mL of oleic acid are added into the above solution (Precursor A). The 0.5 mL of ‘Precursor A’ solution is added into 5 mL benzene at 45°C under vigorous stirring. To gather the green emissive perovskite QDs, the solution is centrifuged at 8 krpm for 5 min. The precipitated material is re-dispersed in toluene (4 mg/mL) and the solution is centrifuged again at 10 krpm 5 min after 0.3 mL of ACN adding. The final product is re-dispersed in hexane.
3.3. Preparation of Perovskite QD/SiO2
The perovskite QD/SiO2 is synthesized by a modified encapsulation method [33]. Perovskite QD is firstly fabricated with the same process with pristine QD (method 3.2). The 8 mg of as-synthesized QD is re-dispersed in 10 mL of toluene with 10 μL of APTES. The 5 μL of ammonia is added and vigorously stirred for an hour to conduct the silanization of APTES. The solution is centrifuged at 10 krpm for 5 min after 0.3 mL of ACN being added. The final product is re-dispersed in hexane.
3.4. Preparation of Perovskite QD/Ni(ppy) via P1-1 (No Short Ligand)
Perovskite QD is fabricated with the modified synthesis method of pristine QD (method 3.2). All solvents are used in an anhydrate state. The 33.56 mg of CH3NH3Br, 146.8 mg of PbBr2, and 1 mg of of Ni(ppy) are dissolved in 10 mL of DMF. Then, 0.1 mL of oleylamine and 1 mL of oleic acid are added into the above solution (Precursor A). The 0.5 mL of ‘Precursor A’ solution is added into 5 mL benzene at 45 °C under vigorous stirring. To gather the green emissive perovskite QDs solution, the solution is centrifuged at 8 krpm for 5 min with 0.3 mL of ACN anti-solvent. The precipitated perovskite QD is re-dispersed in toluene (4 mg/mL) and 60 μL of 1 mM of Ni(ppy) in methanol is added. The solution is stirred for 10 min. Then, the solution is centrifuged again at 10 krpm for 5 min after 0.3 mL of ACN being added. The final product is re-dispersed in hexane.
3.5. Preparation of Perovskite QD/Ni(ppy) via P1-2
The 33.56 mg of CH3NH3Br and 146.8 mg of PbBr2 are mixed with 1 mg of Ni(ppy), 0.6 mL of oleic acid, 0.4 mL of 5-hexynoic acid, and 0.1 mL of oleylamine altogether in 10 mL of DMF (Precursor A). The 0.4 mL of ‘Precursor A’ solution is added into 5 mL benzene at 50 °C under vigorous stirring. To gather the green emissive perovskite QDs, the solution is centrifuged at 8 krpm for 5 min. The precipitated material is re-dispersed in toluene (4 mg/mL), and the solution is centrifuged again at 10 krpm for 5 min after 0.3 mL of ACN is added. The final product is re-dispersed in hexane.
3.6. Preparation of Perovskite QD/Ni(ppy) via P2-1
The 33.56 mg of CH3NH3Br and 146.8 mg of PbBr2 are mixed with 1 mg of Ni(ppy), 0.6 mL of oleic acid, 0.4 mL of 5-hexynoic acid, and 0.1 mL of oleylamine altogether in 10 mL of DMF (Precursor A). The 0.4 mL of ‘Precursor A’ solution is added into 5 mL benzene at 50 °C under vigorous stirring. To gather the green emissive perovskite QDs, the solution is centrifuged at 8 krpm for 5 min with 0.3 mL of ACN anti-solvent. The precipitated perovskite QD is re-dispersed in toluene (4 mg/mL) and 30 μL of 10 mM of 3-Butynoic acid in methanol is added. The solution is stirred for 10 min and the 60 μL of 1 mM of Ni(ppy) in methanol is additionally injected. The solution is stirred for another 10 min. Then, the solution is centrifuged again at 10 krpm 5 min after 0.3 mL of ACN adding. The final product is re-dispersed in hexane.
3.7. Preparation of Perovskite QD/Ni(ppy) via P2-2
The procedure is exactly the same as the P2-1. For P2-2, the product is centrifuged twice. For the first centrifugation, the precipitated perovskite QD is re-dispersed in toluene (4 mg/mL) and 30 μL of 10 mM of 3-Butynoic acid in methanol and the 60 μL of 1 mM of Ni(ppy) in methanol is added. The solution is stirred for 5 min and the solution is centrifuged again at 10 krpm 5 min after 0.3 mL of ACN adding. The precipitated perovskite QD is re-dispersed again in toluene (4 mg/mL) and 30 μL of 10 mM of 3-Butynoic acid in methanol is added again. The solution is stirred for 10 min and the 60 μL of 1 mM of Ni(ppy) in methanol is additionally injected. The solution is stirred for another 10 min. Then, the solution is centrifuged at 10 krpm 5 min with 0.3 mL ACN adding. The final product is re-dispersed in hexane.
3.8. Preparation of Perovskite QD/Ni(ppy)/SiO2 via P2-2
Perovskite QD is fabricated with the same process with QD/Ni(ppy) via P1-2 (method 3.7). The 40 mg of as-synthesized QD/Ni(ppy) is re-dispersed in APTES/Toluene solution (10 μL/10 mL). The 5 μL of ammonia is added and vigorously stirred for 40 min to conduct the silanization of APTES (Solution B). The 30 μL of 10 mM of 3-Butynoic acid in methanol is added in to ‘Solution B’. The solution is stirred for 10 min and the 60 μL of 1 mM of Ni(ppy) in methanol is added. The solution is stirred for another 30 min. Then, the solution is centrifuged at 10 krpm 5 min after 0.3 mL of ACN. The precipitated perovskite QD is re-dispersed in hexane (4 mg/mL). The 30 μL of 10 mM of 3-Butynoic acid in methanol is added, and the solution is stirred for 10 min. The 60 μL of 1 mM of Ni(ppy) in methanol is added again. The solution is stirred for another 30 min. Then, the solution is centrifuged at 10 krpm 5 min after 0.3 mL of ACN is added. The final product is re-dispersed in hexane.
3.9. Preparation of Perovskite QD/NiBr2 via P2-1 and P2-2
The 33.56 mg of CH3NH3Br and 146.8 mg of PbBr2 are mixed with 1 mg of NiBr2, 0.6 mL of oleic acid, 0.4 mL of 5-hexynoic acid, and 0.1 mL of oleylamine in 10 mL of DMF solution (Precursor A). The 0.4 mL of ‘Precursor A‘solution is added into 5 mL benzene at 50 °C under vigorous stirring. To gather the green emissive perovskite QDs, the solution is centrifuged at 8 krpm for 5 min. The precipitated QD is re-dispersed in toluene (4 mg/mL) and 30 μL of 10 mM of 3-Butynoic acid in methanol is added. The solution is stirred for 10 min and then centrifuged again at 10 krpm for 5 min after 0.3 mL of ACN is added. For P2-1, the precipitated perovskite QD is re-dispersed again in toluene (4 mg/mL) and 30 μL of 10 mM of 3-Butynoic acid in methanol is added again. The solution is stirred for 10 min and 60 μL of 1 mM of NiBr2 in methanol is additionally injected. The solution is stirred for another 10 min. Then, the solution is centrifuged at 10 krpm for 5 min with 0.3 mL ACN being added.
For P2-2, the precipitated perovskite QD is re-dispersed in toluene (4 mg/mL) and 30 μL of 10 mM of 3-Butynoic acid in methanol is added. The solution is stirred for 10 min and the solution is centrifuged again at 10 krpm for 5 min after 0.3 mL of ACN is added. The precipitated perovskite QD is re-dispersed again in toluene (4 mg/mL) and 30 μL of 10 mM of 3-Butynoic acid in methanol and 60 μL of 1 mM of NiBr2 in methanol are added again. The solution is stirred for 5 min and the solution is centrifuged again at 10 krpm for 5 min after 0.3 mL of ACN is added. The precipitated perovskite QD is re-dispersed again in toluene (4 mg/mL) and 30 μL of 10 mM of 3-Butynoic acid in methanol is added again. The solution is stirred for 10 min and 60 μL of 1 mM of NiBr2 in methanol is additionally injected. The solution is stirred for another 10 min. Then, the solution is centrifuged at 10 krpm 5 min with 0.3 mL ACN being added. The final product is re-dispersed in hexane.
3.10. Preparation of Red Perovskite QD
The red perovskite QD is synthesized by a modified precipitation method [32]. All solvents are used in an anhydrate state. The 5 mg of CH3NH3Br, 45 mg of CH3NH3I, and 100 mg of PbI2 are dissolved in 2mL of DMF. Then, 0.15 mL of oleylamine and 1 mL of oleic acid are added into the above solution (Precursor A). The 0.1 mL of ‘precursor A’ solution is added into 5 mL benzene at 40 °C under vigorous stirring. To gather the red emissive perovskite QDs, the solution is centrifuged at 8 krpm for 5 min with 1 mL of methyl acetate anti-solvent. The precipitated perovskite QD is re-dispersed in toluene (4 mg/mL). The final product is re-dispersed in hexane.
3.11. Preparation of Red Perovskite QD/Ni(ppy)/SiO2
The 5 mg of CH3NH3Br, 45 mg of CH3NH3I, and 100 mg of PbI2 are dissolved in 2 mL of DMF. Then, a trace of Ni(ppy) (1 mg), 0.6mL of oleic acid, 0.4 mL of 5-hexynoic acid, and 0.15 mL of oleylamine is added into above solution (Precursor A). The 0.1 mL of ‘precursor A’ solution is added into 5 mL benzene at 45 °C under vigorous stirring. To gather the red emissive perovskite QDs, the solution is centrifuged at 8 krpm for 5 min with 1 mL of methyl acetate anti-solvent. The precipitated perovskite QD is re-dispersed in toluene (4 mg/mL). Then, 10 μL APTES is added into 10 mL of QD solution and vigorously stirred for an hour to conduct the silanization of APTES (Solution B). The 30 μL of 10 mM of 3-Butynoic acid in methanol is added in to ‘Solution B’. The solution is stirred for 10 min and 60 μL of 1 mM of Ni(ppy) in methanol is added. The solution is stirred for another 30 min. Then, the solution is centrifuged at 10 krpm 5 min after 1 mL of methyl acetate adding. The precipitated perovskite QD is re-dispersed in hexane (4 mg/mL). The 30 μL of 10 mM of 3-Butynoic acid in methanol is added, and the solution is stirred for 10 min. The 60 μL of 1 mM of Ni(ppy) in methanol is added again. The solution is stirred for another 30 min. Then, the solution is centrifuged at 10 krpm for 5 min after 1 mL of methyl acetate is added. The final product is re-dispersed in hexane.
3.12. Preparation of Red & Green Perovskite QD/SiO2
The red perovskite QD/Ni(ppy)/SiO2 (method 3.11) and green perovskite QD/Ni(ppy)/SiO2 (method 3.8) are separately synthesized. The solution with a specific ratio is mixed in hexane and is stirred for 30 min at RT in ambient condition for hydrolysis of unchained SiO2.
3.13. Preparation of Photocatalytic CO2 Reduction Reaction
Photocatalytic CO2 reduction experiments are set up as shown in Figure 10. The catalyst film is fabricated on a glass membrane substrate (Ø 25 mm). The catalyst-covered substrate is then placed in a stainless steel home-made photoreactor (Volume = 50 cm3) equipped with a quartz window. H2O or EA/H2O is added into the chamber and CO2 gas is continuously flowed over 30 min under dark conditions to remove unknown gases inside the chamber. Then the chamber is filled with CO2 gas and the whole system is sealed up. The chamber is irradiated with a Xe lamp with an AM 1.5G filter to simulate the solar light spectrum (100 mW/cm2); A Si reference cell (BS-520BK, S/N 568, Bunkoukeiki Co., Ltd., Tokyo, Japan) is used for calibration. The 250 μL of gases are collected using a gas-tight syringe and analyzed by gas chromatography (YL 6500GC system, YL Instrument Co, Ltd., Anyang-si, Korea).

Figure 10.
Experimental setup used for photocatalytic CO2 reduction.
3.14. TCSPC Measurement
Optical properties of perovskite QDs: The first excitonic peak position of perovskite QDs ink is measured using the UV-Vis spectrometer (JASCO V-650 spectrophotometer, Seoul, Korea). Steady-state photoluminescence (PL) spectra are obtained using two monochromators (SP-2150i and SP-2300i, Acton, MA, USA) systems equipped with a photomultiplier tube (PMT, Acton PD471, MA, USA) and a Xenon lamp as an excitation light source. The PLQY of perovskite QDs solution is obtained relative to a Coumarin 500 (PLQY ≅ 47% in ethanol). The PL decay of perovskite QDs in solution and film is investigated using a time-correlated single-photon counting (TCSPC) measurement. A pulsed diode-laser head (LDH-P-C-378 nm, PicoQuant, Berlin, Germany) coupled with a laser-diode driver (PDL 800-B, PicoQuant, Berlin, Germany) is used as the excitation source with a repetition rate of 10MHz. The excitation wavelength is 405 nm. The PL emission is spectrally resolved using collection optics and a monochromator (SP-2150i, Acton, MA, USA). A TCSPC module (PicoQuant, PicoHarp 300, Berlin, Germany) with a MCP-PMT (Hamamatsu, R3809U-59, Shizuoka, Japan) is used for ultrafast detection. The total instrument response function (IRF) is less than ~140 ps, and the temporal resolution is ~8 ps. The deconvolution of PL decay curve, which separates the IRF and actual PL decay signal, is performed using fitting software (FluoFit, PicoQuant, Berlin, Germany) to deduce the time constant associated with each exponential decay curve.
3.15. TEM and EDS Measurement
The transmission electron microscopy (Tecnai G2 F30 S-Twin 300 kV, GA, USA) equipped with an energy-dispersive X-ray spectrometer is used to define size. The samples are prepared on the carbon-coated Cu grid (product# 01840-F, TED PELLA, INC., Redding, CA, USA). The chemical composition of perovskite QD is detected by the octane Elite EDS System. In addition, TEM is also used to observe the morphology of perovskite QD photocatalysts.
4. Conclusions
We designed a red/green perovskite QD/Ni(ppy)/SiO2 to increase the catalytic behavior of the perovskite QD photocatalyst. A co-catalyst, Ni(ppy), was incorporated into the perovskite QDs. The Ni precursor was mixed with a ligand selected for strong interaction with the surface of the perovskite QDs. The Ni complex was successfully doped via a ligand exchange process. The Ni content was detected by XPS and increased as the concentration of the Ni precursor increased. When the number of ligand exchange cycles increased, the Ni atomic ratio increased simultaneously. The red/green perovskite QD/Ni(ppy) underwent in-situ silanization to produce an ultrathin SiO2 coating. The photodegradation of red/green perovskite QD/Ni(ppy)/SiO2 dramatically improved after SiO2 coating. We studied the photodegradation of various types of perovskite QDs during time-resolved PL tests. Pristine QDs/Ni(ppy) demonstrated significant PL degradation as the power of the light source increased. Time-resolved PL spectra indicated that trap-assisted recombination increased after light exposure, leading to photodegradation. With the addition of the SiO2 shell, the photodegradation of perovskite significantly reduced. SiO2 passivated the defects in the perovskite QDs, preventing ion migration triggered by the high-power light source. As a result, the SiO2-coated perovskite QDs demonstrated slower photodegradation than pristine QDs at the same power level. Moreover, the SiO2 coating enhanced catalytic stability. In general, the catalytic behavior gradually decreased as the degree of contamination in the catalyst increased. During the absorption/desorption reaction, by-products and/or remaining molecules act as contaminants, sometimes changing the chemical composition of the catalyst, which is an irreversible reaction. Pristine QDs have exposed defects and easily undergo unwanted reactions with neighboring molecules. Thus, the photocatalytic activity of pristine QDs varied with time, while the red/green perovskite QD/Ni(ppy)/SiO2 effectively prevented ion migration and defect contamination, resulting in better operational stability. Our system achieved a CO2 reduction capacity for CO of 0.56 µmol/(g∙h). We hypothesize that developing an effective hole scavenger for red/green perovskite QD/Ni(ppy)/SiO2 can further improve the catalytic behavior in the future.
Author Contributions
Conceptualization, H.L. (Hanleem Lee); methodology, H.L. (Hanleem Lee); formal analysis, H.L (Hanleem Lee) and M.K.; investigation, H.L. (Hanleem Lee) and M.K.; resources, H.L. (Hanleem Lee); data curation, H.L. (Hanleem Lee); writing—original draft preparation, H.L. (Hanleem Lee) and M.K.; writing—review and editing, H.L. (Hyoyoung Lee) and H.L. (Hanleem Lee); visualization, H.L.; supervision, H.L. (Hyoyoung Lee); project administration, H.L. (Hyoyoung Lee); funding acquisition, H.L. (Hanleem Lee).These authors contributed equally. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Acknowledgments
This research was supported by Basic Science Research Program through the National Research Foundation of Korea(NRF) funded by the Ministry of Education (NRF-2020R1A6A3A01099729) and the Institute for Basic Science (No. IBS-R011-D1), Korea evaluation institute of industrial technology (20004627). We also thank for APRI (Advanced Photonics Research Institute) in GIST for using TCSPC.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Shen, H.; Peppel, T.; Strunk, J.; Sun, Z. Photocatalytic Reduction of CO2 by Metal-Free-Based Materials: Recent Advances and Future Perspective. Sol. RRL 2020, 4, 1900546. [Google Scholar] [CrossRef]
- Razzaq, A.; Ali, S.; Asif, M.; In, S.-I. Layered Double Hydroxide (LDH) Based Photocatalysts: An Outstanding Strategy for Efficient Photocatalytic CO2 Conversion. Catalysts 2020, 10, 1185. [Google Scholar] [CrossRef]
- Mo, S.; Ching, W. Electronic and optical properties of three phases of titaniumdioixde: Rutile, anatase, and brookite. Phys. Rev. 1995, 51, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Herz, L.M. Charge-Carrier Dynamics in Organic-Inorganic Metal Halide Perovskites. Annu. Rev. Phys. Chem. 2016, 67, 65–89. [Google Scholar] [CrossRef] [PubMed]
- Wehrenfennig, C.; Eperon, G.E.; Johnston, M.B.; Snaith, H.J.; Herz, L.M. High Charge Carrier Mobilities and Lifetimes in Organolead Trihalide Perovskites. Adv. Mater. 2014, 26, 1584–1589. [Google Scholar] [CrossRef]
- Jin, H.; Debroye, E.; Keshavarz, M.; Scheblykin, I.G.; Roeffaers, M.B.J.; Hofkens, J.; Steele, J.A. It’s a trap! On the nature of localised states and charge trapping in lead halide perovskites. Mater. Horiz. 2020, 7, 397–410. [Google Scholar] [CrossRef]
- Wang, Q.; Tao, L.; Jiang, X.; Wang, M.; Shen, Y. Graphene oxide wrapped CH3NH3PbBr3 perovskite quantum dots hybrid for photoelectrochemical CO2 reduction in organic solvents. Appl. Surf. Sci. 2019, 465, 607–613. [Google Scholar] [CrossRef]
- Wu, L.-Y.; Mu, Y.-F.; Guo, X.-X.; Zhang, W.; Zhang, Z.-M.; Zhang, M.; Lu, T.-B. Encapsulating Perovskite Quantum Dots in Iron-Based Metal–Organic Frameworks (MOFs) for Efficient Photocatalytic CO2 Reduction. Angew. Chem. Int. Ed. 2019, 58, 9491–9495. [Google Scholar] [CrossRef]
- Huang, H.; Pradhan, B.; Hofkens, J.; Roeffaers, M.B.J.; Steele, J.A. Solar-Driven Metal Halide Perovskite Photocatalysis: Design, Stability, and Performance. ACS Energy Lett. 2020, 5, 1107–1123. [Google Scholar] [CrossRef]
- Deng, W.; Fang, H.; Jin, X.; Zhang, X.; Zhang, X.; Jie, J. Organic–inorganic hybrid perovskite quantum dots for light-emitting diodes. J. Mater. Chem. C 2018, 6, 4831–4841. [Google Scholar] [CrossRef]
- Huang, S.; Li, Z.; Kong, L.; Zhu, N.; Shan, A.; Li, L. Enhancing the Stability of CH3NH3PbBr3 Quantum Dots by Embedding in Silica Spheres Derived from Tetramethyl Orthosilicate in “Waterless” Toluene. J. Am. Chem. Soc. 2016, 138, 5749–5752. [Google Scholar] [CrossRef] [PubMed]
- Song, W.; Wang, Y.; Wang, B.; Yao, Y.; Wang, W.; Wu, J.; Shen, Q.; Luo, W.; Zou, Z. Super stable CsPbBr3@SiO2 tumor imaging reagent by stress-response encapsulation. Nano Res. 2020, 13, 795–801. [Google Scholar] [CrossRef]
- Jang, H.M.; Kim, J.-S.; Heo, J.-M.; Lee, T.-W. Enhancing photoluminescence quantum efficiency of metal halide perovskites by examining luminescence-limiting factors. APL Mater. 2020, 8, 020904. [Google Scholar] [CrossRef]
- Lee, H.; Jeong, J.W.; So, M.G.; Jung, G.Y.; Lee, C.-L. Chemically Stable Organic Perovskite Quantum Dots through Kinetic Control of Cross-linkable Ligand System for Microsize Pattern Light Emitting Diodes. Adv. Mater. 2021. under review. [Google Scholar]
- Nag, A.; Kundu, J.; Hazarika, A. Seeded-growth, nanocrystal-fusion, ion-exchange and inorganic-ligand mediated formation of semiconductor-based colloidal heterostructured nanocrystals. Cryst. Eng. Comm. 2014, 16, 9391–9407. [Google Scholar] [CrossRef]
- Chen, Z.; Hu, Y.; Wang, J.; Shen, Q.; Zhang, Y.; Ding, C.; Bai, Y.; Jiang, G.; Li, Z.; Gaponik, N. Boosting Photocatalytic CO2 Reduction on CsPbBr3 Perovskite Nanocrystals by Immobilizing Metal Complexes. Chem. Mater. 2020, 32, 1517–1525. [Google Scholar] [CrossRef]
- Yu, S.; Fan, X.-B.; Wang, X.; Li, J.; Zhang, Q.; Xia, A.; Wei, S.; Wu, L.-Z.; Zhou, Y.; Patzke, G.R. Efficient photocatalytic hydrogen evolution with ligand engineered all-inorganic InP and InP/ZnS colloidal quantum dots. Nat. Commun. 2018, 9, 4009. [Google Scholar] [CrossRef]
- Bang, J.; Das, S.; Yu, E.-J.; Kim, K.; Lim, H.; Kim, S.; Hong, J.W. Controlled Photoinduced Electron Transfer from InP/ZnS Quantum Dots through Cu Doping: A New Prototype for the Visible-Light Photocatalytic Hydrogen Evolution Reaction. Nano Lett. 2020, 20, 6263–6271. [Google Scholar] [CrossRef]
- Dutta, S.; Indra, A.; Feng, Y.; Song, T.; Paik, U. Self-Supported Nickel Iron Layered Double Hydroxide-Nickel Selenide Electrocatalyst for Superior Water Splitting Activity. ACS Appl. Mater. Interfaces 2017, 9, 33766–33774. [Google Scholar] [CrossRef]
- Salunkhe, P.; Ali, M.; Kekuda, D. Investigation on tailoring physical properties of Nickel Oxide thin films grown by dc magnetron sputtering. Mater. Res. Express 2020, 7, 016427. [Google Scholar] [CrossRef]
- Koshizaki, N.; Umehara, H.; Oyama, T. XPS characterization and optical properties of Si/SiO2, Si/Al2O3 and Si/MgO co-sputtered films. Thin Solid Films 1998, 325, 130–136. [Google Scholar] [CrossRef]
- Roberts, M.W.; Smart, R.S.C. The defect structure of nickel oxide surfaces as revealed by photoelectron spectroscopy. J. Chem. Soc. Faraday Trans. 1 Phys. Chem. Condens. Phases 1984, 80, 2957–2968. [Google Scholar] [CrossRef]
- Uhlenbrock, S.; Scharfschwerdt, C.; Neumann, M.; Illing, G.; Freund, H.J. The influence of defects on the Ni 2p and O 1s XPS of NiO. J. Phys. Condens. Matter 1992, 4, 7973–7978. [Google Scholar] [CrossRef]
- Zhidkov, I.S.; Poteryaev, A.I.; Kukharenko, A.I.; Finkelstein, L.D.; Cholakh, S.O.; Akbulatov, A.F.; Troshin, P.A.; Chueh, C.-C.; Kurmaev, E.Z. XPS evidence of degradation mechanism in CH3NH3PbI3 hybrid perovskite. J. Phys. Condens. Matter 2019, 32, 095501. [Google Scholar] [CrossRef]
- Kim, M.; Lin, M.; Son, J.; Xu, H.; Nam, J.-M. Hot-Electron-Mediated Photochemical Reactions: Principles, Recent Advances, and Challenges. Adv. Opt. Mater. 2017, 5, 1700004. [Google Scholar] [CrossRef]
- Zhang, F.; Shi, Z.-F.; Ma, Z.-Z.; Li, Y.; Li, S.; Wu, D.; Xu, T.-T.; Li, X.-J.; Shan, C.-X.; Du, G.-T. Silica coating enhances the stability of inorganic perovskite nanocrystals for efficient and stable down-conversion in white light-emitting devices. Nanoscale 2018, 10, 20131–20139. [Google Scholar] [CrossRef]
- Kawazu, T.; Noda, T.; Sakuma, Y.; Sakaki, H. Excitation power dependence of photoluminescence spectra of GaSb type-II quantum dots in GaAs grown by droplet epitaxy. AIP Adv. 2016, 6, 045312. [Google Scholar] [CrossRef]
- Wei, Y.; Li, X.; Chen, Y.; Cheng, Z.; Xiao, H.; Li, X.; Ding, J.; Lin, J. In Situ Light-Initiated Ligands Cross-Linking Enables Efficient All-Solution-Processed Perovskite Light-Emitting Diodes. J. Phys. Chem. Lett. 2020, 11, 1154–1161. [Google Scholar] [CrossRef]
- Li, Z.-J.; Fan, X.-B.; Li, X.-B.; Li, J.-X.; Zhan, F.; Tao, Y.; Zhang, X.; Kong, Q.-Y.; Zhao, N.-J.; Zhang, J.-P.; et al. Direct synthesis of all-inorganic heterostructured CdSe/CdS QDs in aqueous solution for improved photocatalytic hydrogen generation. J. Mater. Chem. A 2017, 5, 10365–10373. [Google Scholar] [CrossRef]
- Teh, Y.W.; Chee, M.K.T.; Kong, X.Y.; Yong, S.-T.; Chai, S.-P. An insight into perovskite-based photocatalysts for artificial photosynthesis. Sustain. Energy Fuels 2020, 4, 973–984. [Google Scholar] [CrossRef]
- Li, Y.-F.; Feng, J.; Sun, H.-B. Perovskite quantum dots for light-emitting devices. Nanoscale 2019, 11, 19119–19139. [Google Scholar] [CrossRef] [PubMed]
- Woo Choi, J.; Woo, H.C.; Huang, X.; Jung, W.-G.; Kim, B.-J.; Jeon, S.-W.; Yim, S.-Y.; Lee, J.-S.; Lee, C.-L. Organic–inorganic hybrid perovskite quantum dots with high PLQY and enhanced carrier mobility through crystallinity control by solvent engineering and solid-state ligand exchange. Nanoscale 2018, 10, 13356–13367. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Zhang, Y.; Fan, Y.; Chen, Z.; Tang, Z.; Zhao, J.; Lv, Y.; Lin, J.; Guo, X.; Zhang, J.; et al. Toward Highly Luminescent and Stabilized Silica-Coated Perovskite Quantum Dots through Simply Mixing and Stirring under Room Temperature in Air. ACS Appl. Mater. Interfaces 2018, 10, 13053–13061. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).