The Effect of Ni-Modified LSFCO Promoting Layer on the Gas Produced through Co-Electrolysis of CO2 and H2O at Intermediate Temperatures
Abstract
1. Introduction
2. Results and Discussion
3. Experimental
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Acronym | Full Form |
| ASC | Anode-supporting cell |
| ASR | Area-specific resistance |
| a.u. | arbitrary unit |
| CGO | Ce0.9Gd0.1O2-δ |
| GC | Gas-chromatography |
| GDC | Gadolinia-doped ceria |
| LSC | La0.6Sr0.4CoO3 |
| LSFCO | La0.6Sr0.4Fe0.8Co0.2O3 |
| MIEC | Mixed ionic and electronic conductor |
| n.a. | data not available |
| OCV | Open circuit voltage |
| SOEC | Solid oxide electrolysis cell |
| SOFC | Solid oxide fuel cell |
| SOC | Solid oxide cell |
| YSZ | Yttria-stabilised zirconia |
References
- Marchand, R.D.; Koh, S.L.; Morris, J. Delivering energy efficiency and carbon reduction schemes in England: Lessons from Green Deal Pioneer Places. Energy Policy 2015, 84, 96–106. [Google Scholar] [CrossRef]
- Pettifor, H.; Wilson, C.; Chryssochoidis, G. The appeal of the green deal: Empirical evidence for the influence of energy efficiency policy on renovating homeowners. Energy Policy 2015, 79, 161–176. [Google Scholar] [CrossRef]
- Sikora, A. European Green Deal-legal and financial challenges of the climate change. ERA Forum 2021, 21, 681–697. [Google Scholar] [CrossRef]
- Lau, K.J.; Tokofsky, P.I.; Winick, S.D. What Goes Around Comes Around: The Circulation of Proverbs in Contemporary Life; Utah State University Press: Logan, UT, USA, 2004; pp. 1–19. [Google Scholar]
- Koytsoumpa, E.I.; Bergins, C.; Kakaras, E. The CO2 economy: Review of CO2 capture and reuse technologies. J. Supercrit. Fluids 2018, 132, 3–16. [Google Scholar] [CrossRef]
- Centi, G.; Perathoner, S. Green Carbon Dioxide: Advances in CO2 Utilization; Wiley: Hoboken, NJ, USA, 2014. [Google Scholar]
- Graves, C.R.; Ebbesen, S.D.; Mogensen, M.B.; Lackner, K.S. Sustainable hydrocarbon fuels by recycling CO 2 and H 2 O with renewable or nuclear energy. Renew. Sustain. Energy Rev. 2011, 15, 1–23. [Google Scholar] [CrossRef]
- Ioannidou, E.; Neophytides, S.; Niakolas, D.K. Experimental Clarification of the RWGS Reaction Effect in H2O/CO2 SOEC Co-Electrolysis Conditions. Catalysts 2019, 9, 151. [Google Scholar] [CrossRef]
- Guo, M.; Ru, X.; Lin, Z.; Xiao, G.; Wang, J.-Q. Optimization Design of Rib Width and Performance Analysis of Solid Oxide Electrolysis Cell. Energies 2020, 13, 5468. [Google Scholar] [CrossRef]
- Glauche, A.; Betz, T.; Ise, M. Product Development for SOFC and SOE Applications. ECS Trans. 2011, MA2011-01, 157–165. [Google Scholar] [CrossRef]
- Aguilo-Rullan, A.; Atanasiu, M.; Biebuyck, B.; Lymperopoulos, N.; Marenco, C.; Tsimis, D. The Status of SOFC and SOEC R&D in the European Fuel Cell and Hydrogen Joint Undertaking Programme. ECS Trans. 2017, 78, 41–61. [Google Scholar]
- Küngas, R.; Blennow, P.; Heiredal-Clausen, T.; Nørby, T.H.; Rass-Hansen, J.; Primdahl, S.; Hansen, J.B. ECOs-A Commercial CO2 Electrolysis System Developed by Haldor Topsoe. ECS Trans. 2017, 78, 2879–2884. [Google Scholar] [CrossRef]
- Tsimis, D.; Aguilo-Rullan, A.; Atanasiu, M.; Zafeiratou, E.; Dirmiki, D. The Status of SOFC and SOEC R&D in the European Fuel Cell and Hydrogen Joint Undertaking Programme. ECS Trans. 2019, 91, 9–26. [Google Scholar]
- Küngas, R.; Blennow, P.; Heiredal-Clausen, T.; Nørby, T.H.; Rass-Hansen, J.; Hansen, J.B.; Moses, P.G. Progress in SOEC Development Activities at Haldor Topsøe. ECS Trans. 2019, 91, 215–223. [Google Scholar] [CrossRef]
- Redissi, Y.; Bouallou, C. Valorization of Carbon Dioxide by Co-Electrolysis of CO2/H2O at High Temperature for Syngas Production. Energy Procedia 2013, 37, 6667–6678. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, T.; Lei, L.; Chen, F. High temperature solid oxide H2O/CO2 co-electrolysis for syngas production. Fuel Process. Technol. 2017, 161, 248–258. [Google Scholar] [CrossRef]
- Maréchal, F.; Chen, M.; Küngas, R.; Lin, T.-E.; Diethelm, S.; Maréchal, F.; Van Herle, J. Power-to-fuels via solid-oxide electrolyzer: Operating window and techno-economics. Renew. Sustain. Energy Rev. 2019, 110, 174–187. [Google Scholar] [CrossRef]
- Kupecki, J.; Mastropasqua, L.; Motylinski, K.; Ferrero, D. Chapter 5-Multilevel modeling of solid oxide electrolysis. In Solid Oxide-Based Electrochemical Devices; Lo Faro, M., Ed.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 123–166. [Google Scholar]
- Navasa, M.; Frandsen, H.L.; Skafte, T.L.; Sundén, B.; Graves, C. Localized carbon deposition in solid oxide electrolysis cells studied by multiphysics modeling. J. Power Sources 2018, 394, 102–113. [Google Scholar] [CrossRef]
- Aicart, J.; Usseglio-Viretta, F.; Laurencin, J.; Petitjean, M.; Delette, G.; Dessemond, L. Operating maps of high temperature H2O electrolysis and H2O+CO2 co-electrolysis in solid oxide cells. Int. J. Hydrog. Energy 2016, 41, 17233–17246. [Google Scholar] [CrossRef]
- Nelson, G.J.; Grew, K.N.; Izzo, J.R.; Lombardo, J.J.; Harris, W.M.; Faes, A.; Hessler-Wyser, A.; Van Herle, J.; Wang, S.; Chu, Y.S.; et al. Three-dimensional microstructural changes in the Ni–YSZ solid oxide fuel cell anode during operation. Acta Mater. 2012, 60, 3491–3500. [Google Scholar] [CrossRef]
- Iwanschitz, B.; Holzer, L.; Mai, A.; Schütze, M. Nickel agglomeration in solid oxide fuel cells: The influence of temperature. Solid State Ion. 2012, 211, 69–73. [Google Scholar] [CrossRef]
- Chen, B.; Hajimolana, Y.S.; Venkataraman, V.; Ni, M.; Aravind, P.V. Integration of reversible solid oxide cells with methane synthesis (ReSOC-MS) in grid stabilization: A dynamic investigation. Appl. Energy 2019, 250, 558–567. [Google Scholar] [CrossRef]
- Biswas, S.; Kulkarni, A.P.; Giddey, S.; Bhattacharya, S. A Review on Synthesis of Methane as a Pathway for Renewable Energy Storage with a Focus on Solid Oxide Electrolytic Cell-Based Processes. Front. Energy Res. 2020, 8. [Google Scholar] [CrossRef]
- Chen, B.; Xu, H.; Ni, M. Modelling of SOEC-FT reactor: Pressure effects on methanation process. Appl. Energy 2017, 185, 814–824. [Google Scholar] [CrossRef]
- Giglio, E.; Lanzini, A.; Santarelli, M.; Leone, P. Synthetic natural gas via integrated high-temperature electrolysis and methanation: Part I—Energy performance. J. Energy Storage 2015, 1, 22–37. [Google Scholar] [CrossRef]
- Frontera, P.; Macario, A.; Malara, A.; Antonucci, V.; Modafferi, V.; Antonucci, P.L. Simultaneous methanation of carbon oxides on nickel-iron catalysts supported on ceria-doped gadolinia. Catal. Today 2020, 357, 565–572. [Google Scholar] [CrossRef]
- Faro, M.L.; Vita, A.; Pino, L.; Aricò, A.S. Performance evaluation of a solid oxide fuel cell coupled to an external biogas tri-reforming process. Fuel Process. Technol. 2013, 115, 238–245. [Google Scholar] [CrossRef]
- Manenti, F.; Pelosato, R.; Vallevi, P.; Leon-Garzon, A.R.; Dotelli, G.; Vita, A.; Faro, M.L.; Maggio, G.; Pino, L.; Aricò, A.S. Biogas-fed solid oxide fuel cell (SOFC) coupled to tri-reforming process: Modelling and simulation. Int. J. Hydrog. Energy 2015, 40, 14640–14650. [Google Scholar] [CrossRef]
- Faro, M.L.; Trocino, S.; Zignani, S.; Aricò, A.S.; Maggio, G.; Italiano, C.; Fabiano, C.; Pino, L.; Vita, A. Study of a Solid Oxide Fuel Cell fed with n-dodecane reformate. Part I: Endurance test. Int. J. Hydrog. Energy 2016, 41, 5741–5747. [Google Scholar] [CrossRef]
- Faro, M.L.; Trocino, S.; Zignani, S.; Italiano, C.; Vita, A.; Aricò, A.S. Study of a solid oxide fuel cell fed with n-dodecane reformate. Part II: Effect of the reformate composition. Int. J. Hydrog. Energy 2017, 42, 1751–1757. [Google Scholar] [CrossRef]
- Faro, M.L.; Reis, R.M.; Saglietti, G.G.A.; Barcelos, M.R.D.S.; Ticianelli, E.A.; Zignani, S.C.; Aricò, A.S. Nickel-Copper/Gadolinium-Doped Ceria (CGO) Composite Electrocatalyst as a Protective Layer for a Solid-Oxide Fuel Cell Anode Fed with Ethanol. ChemElectroChem 2014, 1, 1395–1402. [Google Scholar] [CrossRef]
- Faro, M.L.; Reis, R.M.; Saglietti, G.G.A.; Zignani, S.C.; Trocino, S.; Frontera, P.; Antonucci, P.L.; Ticianelli, E.A.; Aricò, A.S. Investigation of Ni-based alloy/CGO electro-catalysts as protective layer for a solid oxide fuel cell anode fed with ethanol. J. Appl. Electrochem. 2015, 45, 647–656. [Google Scholar] [CrossRef]
- Faro, M.L.; Trocino, S.; Zignani, S.C.; Italiano, C.; Reis, R.M.; Ticianelli, E.A.; Aricò, A.S. Nickel-Iron/Gadolinium-Doped Ceria (CGO) Composite Electrocatalyst as a Protective Layer for a Solid-Oxide Fuel Cell Anode Fed with Biofuels. ChemCatChem 2016, 8, 648–655. [Google Scholar] [CrossRef]
- Faro, M.L.; Da Silva, W.O.; Barrientos, W.V.; Saglietti, G.; Zignani, S.; Ticianelli, E.; Antonucci, V.; Aricò, A. The role of CuSn alloy in the co-electrolysis of CO2 and H2O through an intermediate temperature solid oxide electrolyser. J. Energy Storage 2020, 27, 100820. [Google Scholar] [CrossRef]
- Faro, M.L.; Silva, W.; Barrientos, W.V.; Saglietti, G.; Zignani, S.; Antonucci, V.; Ticianelli, E.A.; Aricò, A.S. Enhanced production of methane through the use of a catalytic Ni–Fe pre-layer in a solid oxide co-electrolyser. Int. J. Hydrog. Energy 2020, 45, 5134–5142. [Google Scholar] [CrossRef]
- Yang, G.; Zhou, W.; Liu, M.; Shao, Z. Enhancing Electrode Performance by Exsolved Nanoparticles: A Superior Cobalt-Free Perovskite Electrocatalyst for Solid Oxide Fuel Cells. ACS Appl. Mater. Interfaces 2016, 8, 35308–35314. [Google Scholar] [CrossRef] [PubMed]
- Kwon, O.; Sengodan, S.; Kim, K.; Kim, G.; Jeong, H.Y.; Shin, J.; Ju, Y.-W.; Han, J.W.; Kim, G. Exsolution trends and co-segregation aspects of self-grown catalyst nanoparticles in perovskites. Nat. Commun. 2017, 8, 15967. [Google Scholar] [CrossRef] [PubMed]
- Faro, M.L.; Reis, R.M.; Saglietti, G.; Oliveira, V.; Zignani, S.; Trocino, S.; Maisano, S.; Ticianelli, E.; Hodnik, N.; Ruiz-Zepeda, F.; et al. Solid oxide fuel cells fed with dry ethanol: The effect of a perovskite protective anodic layer containing dispersed Ni-alloy @ FeOx core-shell nanoparticles. Appl. Catal. B Environ. 2018, 220, 98–110. [Google Scholar] [CrossRef]
- Faro, M.L.; Oliveira, V.L.; Reis, R.M.; Saglietti, G.G.A.; Zignani, S.C.; Trocino, S.; Ticianelli, E.A.; Aricò, A.S. Solid Oxide Fuel Cell Fed Directly with Dry Glycerol. Energy Technol. 2018, 7, 45–47. [Google Scholar] [CrossRef]
- Vecino-Mantilla, S.; Quintero, E.; Fonseca, C.; Gauthier, G.H.; Gauthier-Maradei, P. Catalytic Steam Reforming of Natural Gas over a New Ni Exsolved Ruddlesden-Popper Manganite in SOFC Anode Conditions. ChemCatChem 2020, 12, 1453–1466. [Google Scholar] [CrossRef]
- Nishikawa, R.; Kakinuma, K.; Nishino, H.; Brito, M.E.; Gopalan, S.; Uchida, H. Synthesis and Evaluation of Ni Catalysts Supported on BaCe0.5Zr0.3−xY0.2NixO3−δ with Fused-Aggregate Network Structure for the Hydrogen Electrode of Solid Oxide Electrolysis Cell. Catalysts 2017, 7, 223. [Google Scholar] [CrossRef]
- Rosen, B.A. Progress and Opportunities for Exsolution in Electrochemistry. Electrochemistry 2020, 1, 4. [Google Scholar] [CrossRef]
- Faro, M.L.; La Rosa, D.; Nicotera, I.; Antonucci, V.; Aricò, A.S. Electrochemical investigation of a propane-fed solid oxide fuel cell based on a composite Ni–perovskite anode catalyst. Appl. Catal. B Environ. 2009, 89, 49–57. [Google Scholar] [CrossRef]
- Faro, M.L.; Zignani, S.; Aricò, A.S. Lanthanum Ferrites-Based Exsolved Perovskites as Fuel-Flexible Anode for Solid Oxide Fuel Cells. Materials 2020, 13, 3231. [Google Scholar] [CrossRef] [PubMed]
- Faro, M.L.; Modafferi, V.; Frontera, P.; Antonucci, P.; Aricò, A.S. Catalytic behavior of Ni-modified perovskite and doped ceria composite catalyst for the conversion of odorized propane to syngas. Fuel Process. Technol. 2013, 113, 28–33. [Google Scholar] [CrossRef]
- Faro, M.L.; Antonucci, V.; Antonucci, P.; Aricò, A. Fuel flexibility: A key challenge for SOFC technology. Fuel 2012, 102, 554–559. [Google Scholar] [CrossRef]
- Faro, M.L.; Aricò, A.S. Electrochemical behaviour of an all-perovskite-based intermediate temperature solid oxide fuel cell. Int. J. Hydrog. Energy 2013, 38, 14773–14778. [Google Scholar] [CrossRef]
- Faro, M.L.; Trocino, S.; Zignani, S.; Antonucci, V.; Aricò, A.S. Production of syngas by solid oxide electrolysis: A case study. Int. J. Hydrog. Energy 2017, 42, 27859–27865. [Google Scholar] [CrossRef]
- Faro, M.L.; Zignani, S.; Trocino, S.; Antonucci, V.; Aricò, A. New insights on the co-electrolysis of CO2 and H2O through a solid oxide electrolyser operating at intermediate temperatures. Electrochim. Acta 2019, 296, 458–464. [Google Scholar] [CrossRef]
- Bian, L.; Duan, C.; Wang, L.; Chen, Z.; Hou, Y.; Peng, J.; Song, X.; An, S.; O’Hayre, R. An all-oxide electrolysis cells for syngas production with tunable H2/CO yield via co-electrolysis of H2O and CO2. J. Power Sources 2021, 482, 228887. [Google Scholar] [CrossRef]
- Reisert, M.; Aphale, A.; Singh, P. Solid Oxide Electrochemical Systems: Material Degradation Processes and Novel Mitigation Approaches. Materials 2018, 11, 2169. [Google Scholar] [CrossRef]
- Zeng, K.; Zhang, D. Recent progress in alkaline water electrolysis for hydrogen production and applications. Prog. Energy Combust. Sci. 2010, 36, 307–326. [Google Scholar] [CrossRef]
- Kim, J.S.; Lee, J.Y.; Swinnea, J.S.; Steinfink, H.; Reiff, W.M.; Lightfoot, P.; Pei, S.; Jorgensen, J.D. Ruddlesden-Popper Phases An+1MnO3n+1: Structures and Properties; NIST Special Publication: Jackson, WY, USA, 1991; pp. 301–306. [Google Scholar]
- Lee, D.; Lee, H.N. Controlling Oxygen Mobility in Ruddlesden–Popper Oxides. Materials 2017, 10, 368. [Google Scholar] [CrossRef] [PubMed]
- Smith, R.D.L.; Prévot, M.S.; Fagan, R.D.; Zhang, Z.; Sedach, P.A.; Siu, M.K.J.; Trudel, S.; Berlinguette, C.P. Photochemical route for accessing amorphous metal oxide materials for water oxidation catalysis. Science 2013, 340, 60–63. [Google Scholar] [CrossRef] [PubMed]
- Smith, R.D.L.; Prévot, M.S.; Fagan, R.D.; Trudel, S.; Berlinguette, C.P. Water oxidation catalysis: Electrocatalytic response to metal stoichiometry in amorphous metal oxide films containing Iron, Cobalt, and Nickel. J. Am. Chem. Soc. 2013, 135, 11580–11586. [Google Scholar] [CrossRef] [PubMed]
- Torrell, M.; García-Rodríguez, S.; Morata, A.; Penelas, G.; Tarancón, A. Co-electrolysis of steam and CO2 in full-ceramic symmetrical SOECs: A strategy for avoiding the use of hydrogen as a safe gas. Faraday Discuss. 2015, 182, 241–255. [Google Scholar] [CrossRef]
- Hou, S.; Xie, K. Enhancing the performance of high-temperature H2O/CO2 co-electrolysis process on the solid oxide Sr2Fe1.6Mo0.5O6-δ-SDC/LSGM/Sr2Fe1.5Mo0.5O6-δ-SDC cell. Electrochim. Acta 2019, 301, 63–68. [Google Scholar] [CrossRef]
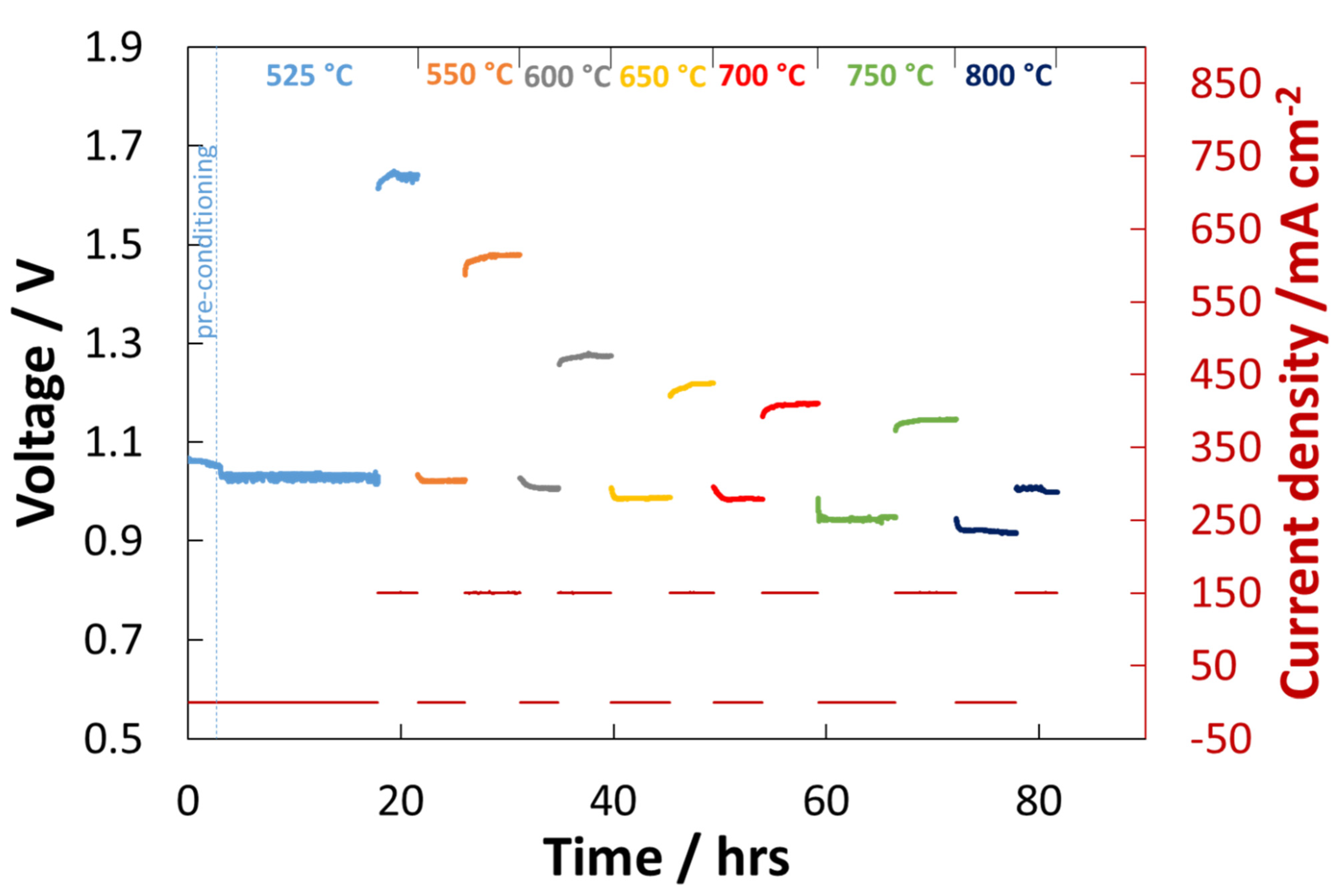
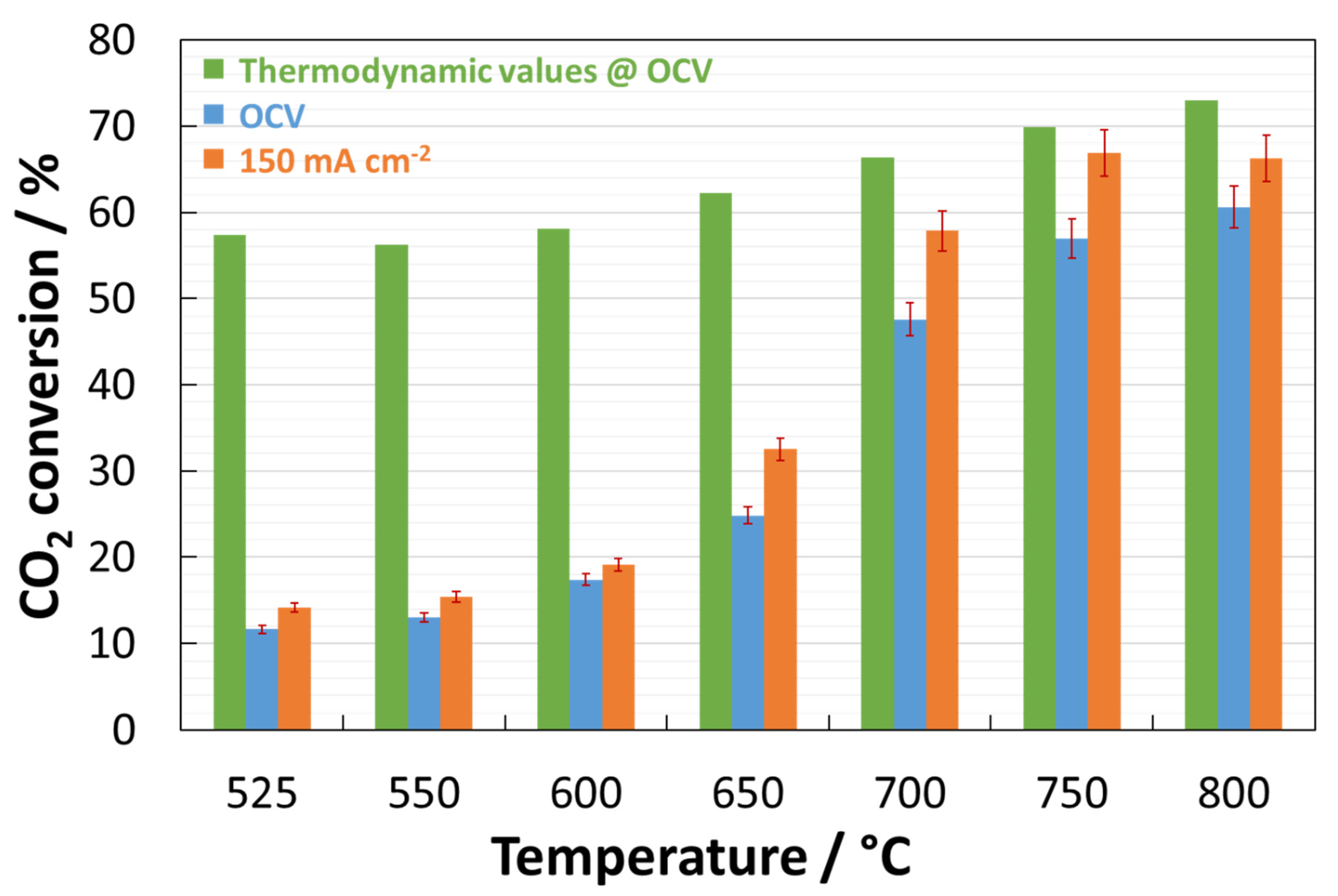
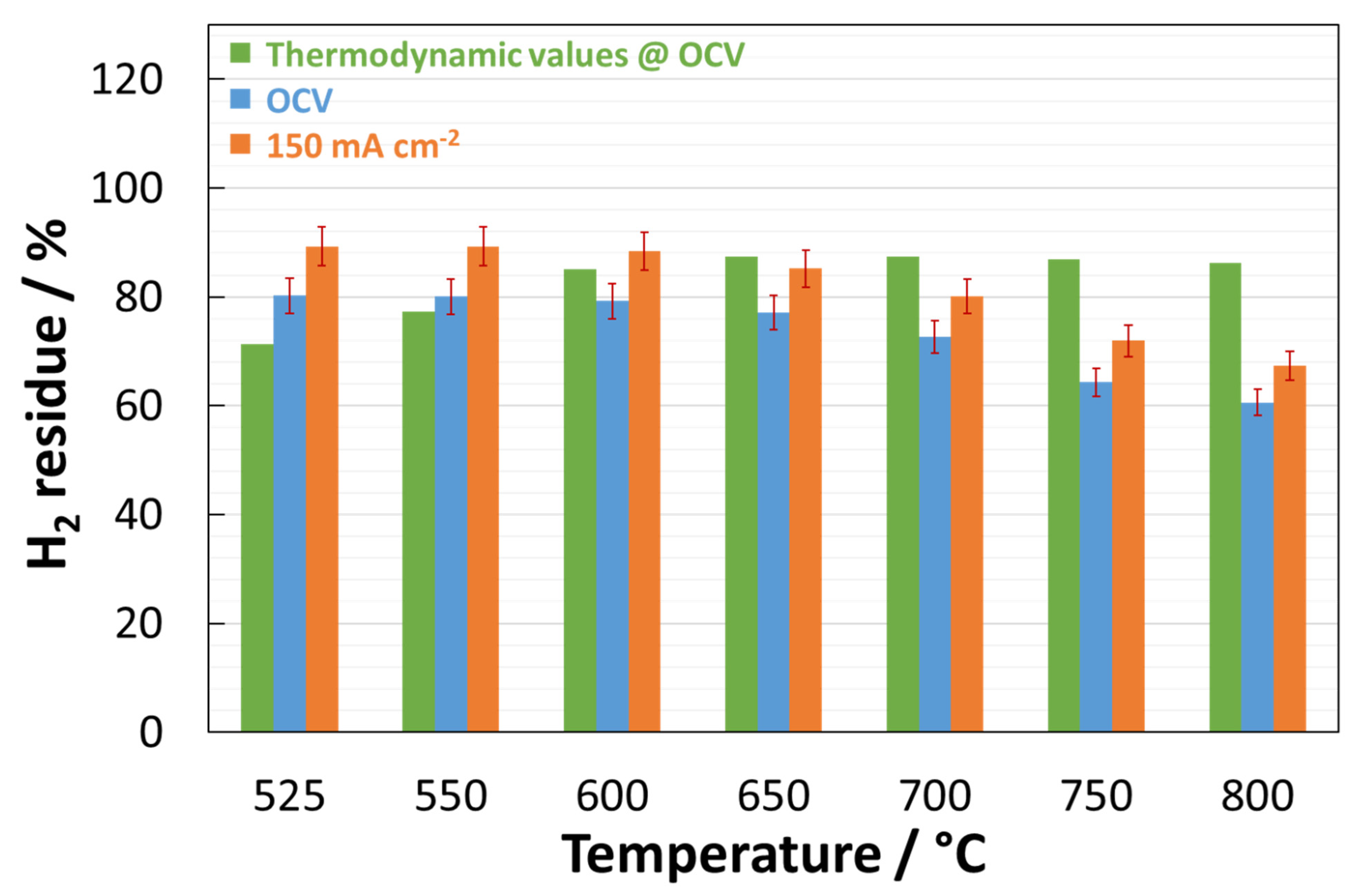

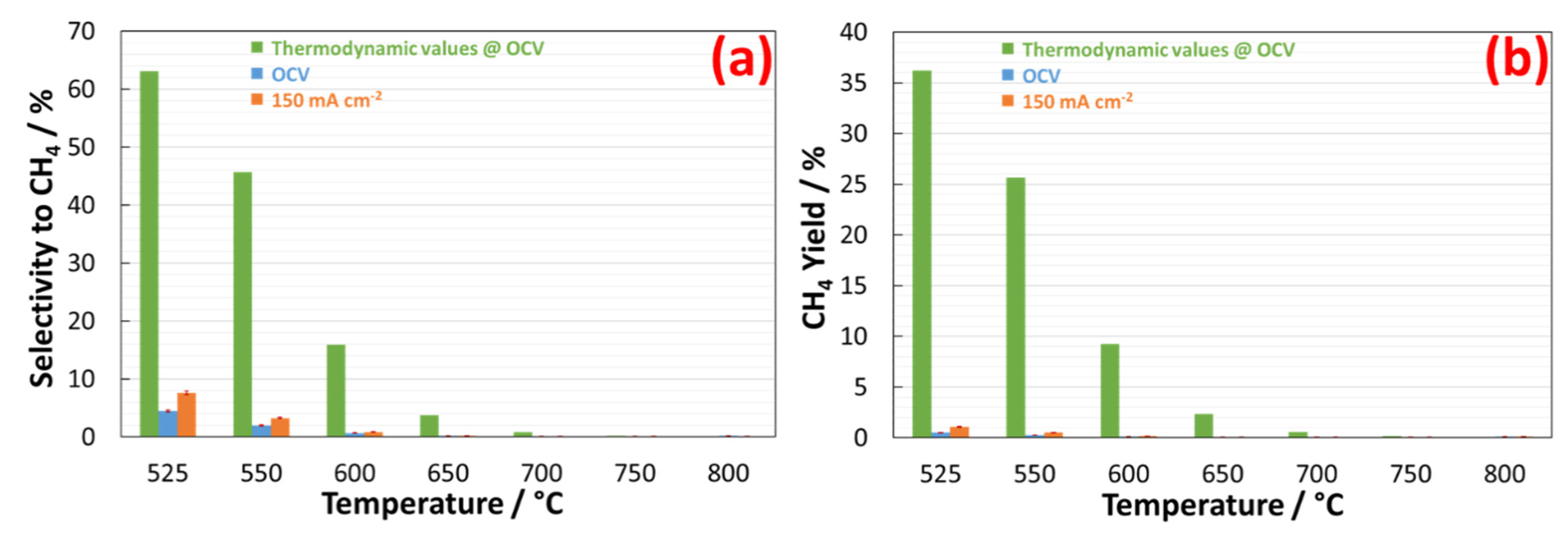

| Temperature | ||||||||
|---|---|---|---|---|---|---|---|---|
| 525 °C | 550 °C | 600 °C | 650 °C | 700 °C | 750 °C | 800 °C | ||
| Cell Voltage at 150 mA cm−2 | Coated cell | 1.88 V | 1.48 V | 1.24 V | 1.24 V | 1.24 V | 1.11 V | 1.00 V |
| Bare cell Ref. [50] | 1.15 V | 1.07 V | 0.99 V | 0.94 V | 0.91 | n.a. | n.a. | |
| Area Specific Resistance at 150 mA cm−2 | Coated cell | >16 Ω cm2 | 3.51 Ω cm2 | 2.07 Ω cm2 | 1.80 Ω cm2 | 1.72 Ω cm2 | 1.33 Ω cm2 | 0.69 Ω cm2 |
| Bare cell Ref. [50] | 2.89 Ω cm2 | 2.25 Ω cm2 | 1.24 Ω cm2 | 0.81 Ω cm2 | 0.76 Ω cm2 | n.a. | n.a. | |
| Temperature | ||||||||
|---|---|---|---|---|---|---|---|---|
| 525 °C | 550 °C | 600 °C | 650 °C | 700 °C | 750 °C | 800 °C | ||
| Coated cell -@ 150 mA cm−2- | CO2conversion | 14.1% | 15.4% | 19.1% | 32.5% | 57.9% | 66.8% | 66.3% |
| H2residue | 89.2% | 89.2% | 88.4% | 85.2% | 80.11% | 71.9% | 67.4% | |
| Selectivity to CO | 92.4% | 96.7% | 99.1% | 99.8% | 99.9% | 99.9% | 99.8% | |
| CO yield | 13.1% | 14.9% | 19.0% | 32.5% | 57.8% | 66.8% | 66.1% | |
| Selectivity to CH4 | 7.6% | 3.2% | 0.8% | 0.2% | 0.1% | 0.1% | 0.2% | |
| CH4yield | 1.1% | 0.5% | 0.2% | 0.1% | 0.1% | 0.1% | 0.1% | |
| Bare cell -@ 150 mA cm−2- Ref. [50] | CO2conversion | 25.9% | 26.9% | 29.8% | 30.3% | 31% | n.a. | n.a. |
| H2residue | 107% | 108.1% | 108.2% | 103.7% | 98.9% | n.a. | n.a. | |
| Selectivity to CO | 97.9% | 99.2% | 99.9% | 100% | 100% | n.a. | n.a. | |
| CO yield | 25.4% | 26.7% | 29.7% | 30.3% | 30.9% | n.a. | n.a. | |
| Selectivity to CH4 | 2.1% | 0.8% | 0.1% | 0% | 0% | n.a. | n.a. | |
| CH4yield | 0.5% | 0.2% | traces | traces | traces | n.a. | n.a. | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lo Faro, M.; Campagna Zignani, S.; Antonucci, V.; Aricò, A.S. The Effect of Ni-Modified LSFCO Promoting Layer on the Gas Produced through Co-Electrolysis of CO2 and H2O at Intermediate Temperatures. Catalysts 2021, 11, 56. https://doi.org/10.3390/catal11010056
Lo Faro M, Campagna Zignani S, Antonucci V, Aricò AS. The Effect of Ni-Modified LSFCO Promoting Layer on the Gas Produced through Co-Electrolysis of CO2 and H2O at Intermediate Temperatures. Catalysts. 2021; 11(1):56. https://doi.org/10.3390/catal11010056
Chicago/Turabian StyleLo Faro, Massimiliano, Sabrina Campagna Zignani, Vincenzo Antonucci, and Antonino Salvatore Aricò. 2021. "The Effect of Ni-Modified LSFCO Promoting Layer on the Gas Produced through Co-Electrolysis of CO2 and H2O at Intermediate Temperatures" Catalysts 11, no. 1: 56. https://doi.org/10.3390/catal11010056
APA StyleLo Faro, M., Campagna Zignani, S., Antonucci, V., & Aricò, A. S. (2021). The Effect of Ni-Modified LSFCO Promoting Layer on the Gas Produced through Co-Electrolysis of CO2 and H2O at Intermediate Temperatures. Catalysts, 11(1), 56. https://doi.org/10.3390/catal11010056







