Low-Temperature Mineralisation of Titania-Siloxane Composite Layers
Abstract
1. Introduction
2. Results and Discussion
2.1. Viscosity of Siloxane Solutions
2.2. Surface Tension of Siloxane Solutions
2.3. Gel Permeation Chromatography
2.4. Specific Surface Area (SSA) and SEM of Siloxane/TiO2
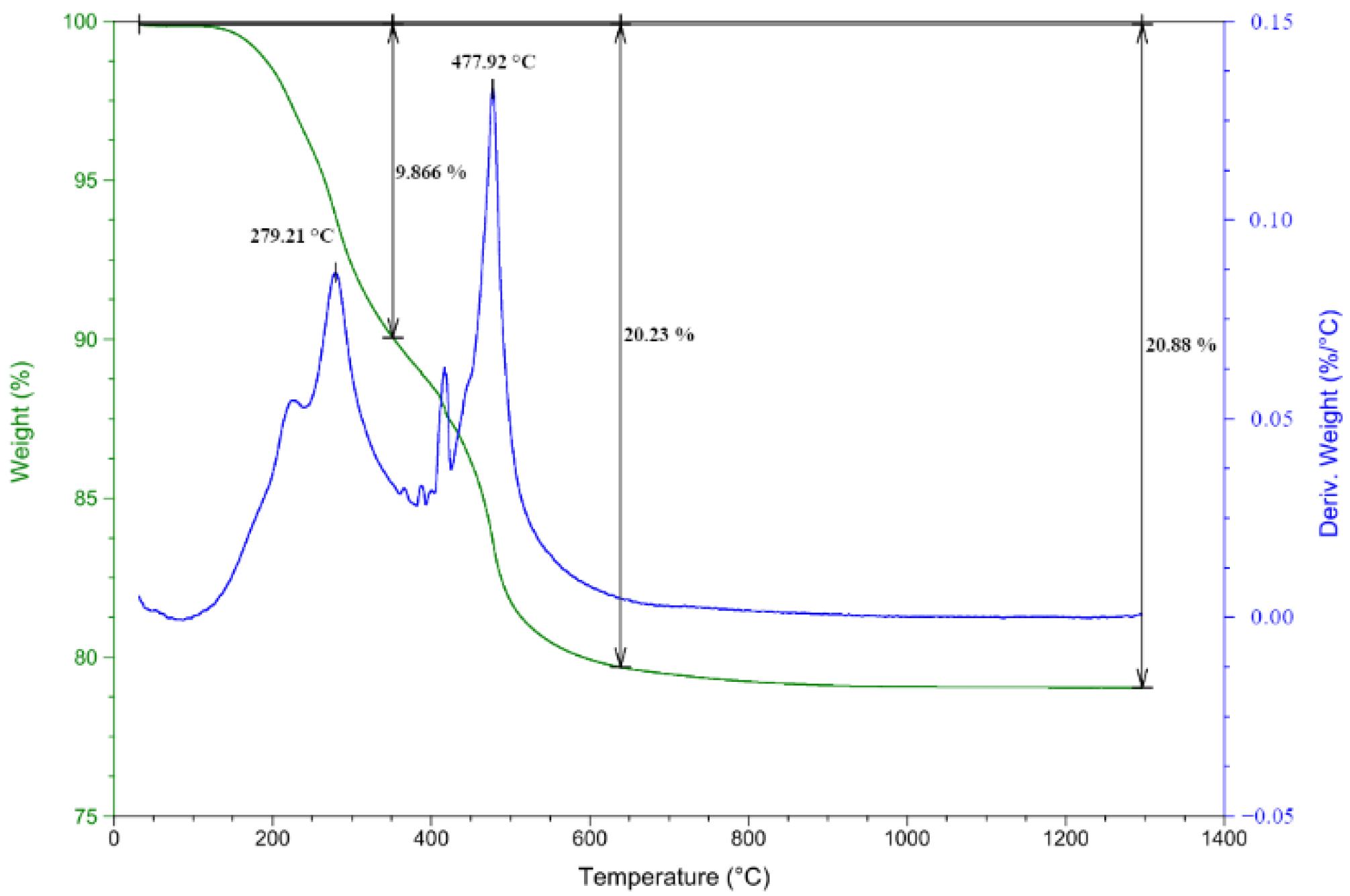
2.5. Thermal Treatment of TSC and TGA, DTG
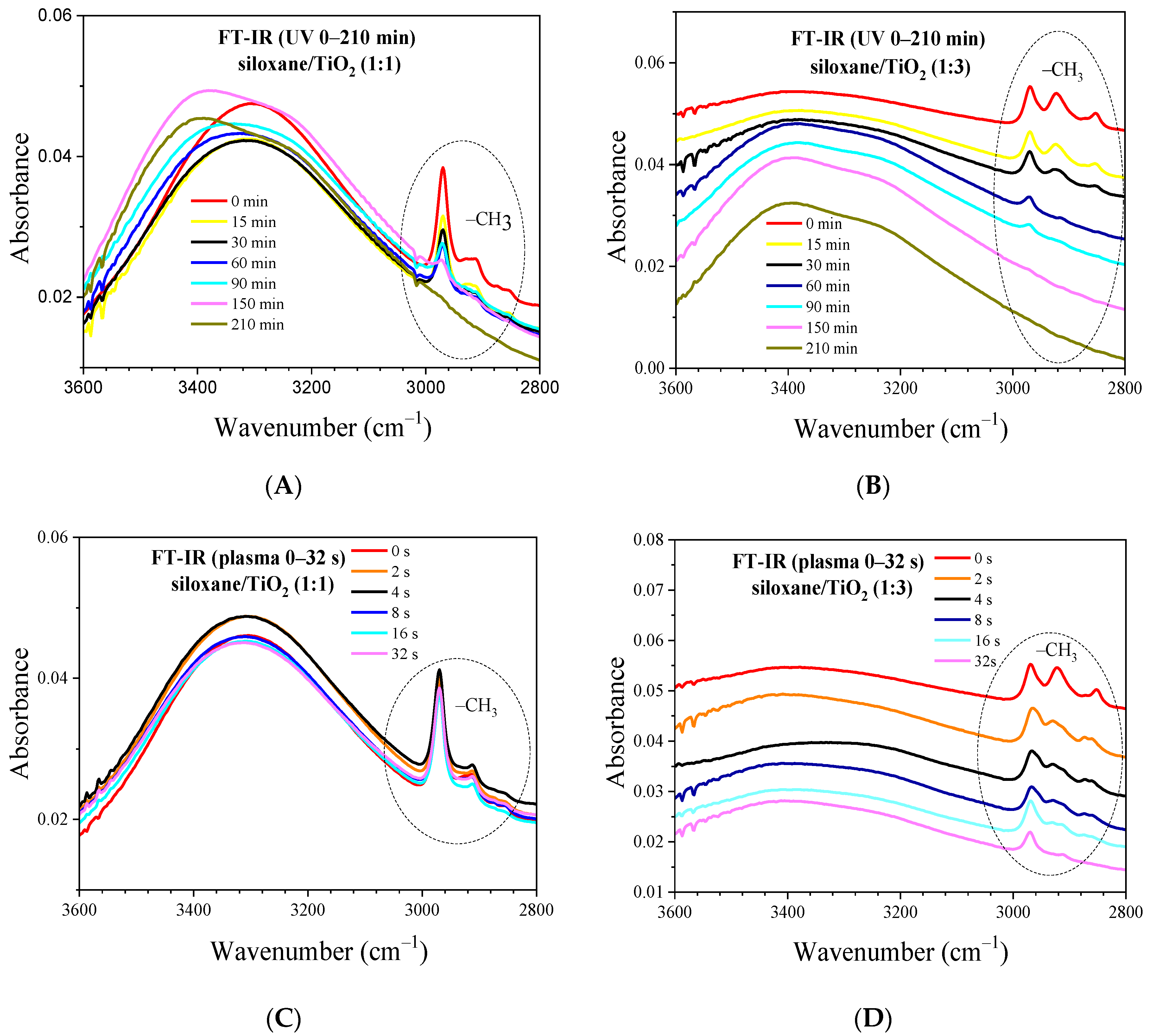
2.6. FT-IR of Nonthermal Curing TSC
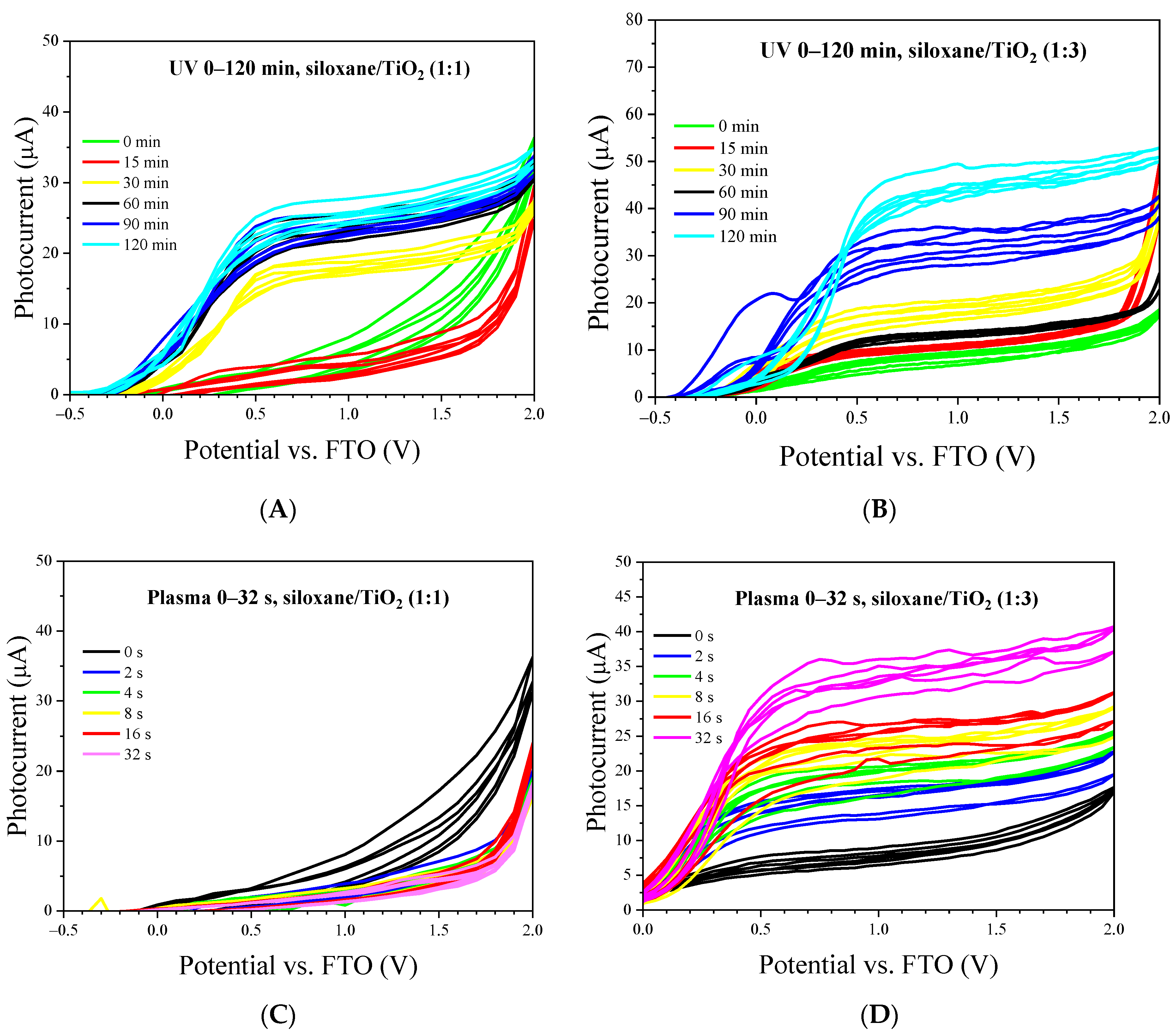
2.7. Voltammetric Measurements of Nonthermal Curing TSC
2.8. Photocatalytic Degradation of Acid Orange 7
3. Materials and Methods
3.1. Synthesis of Siloxane
3.2. Preparation of TSC
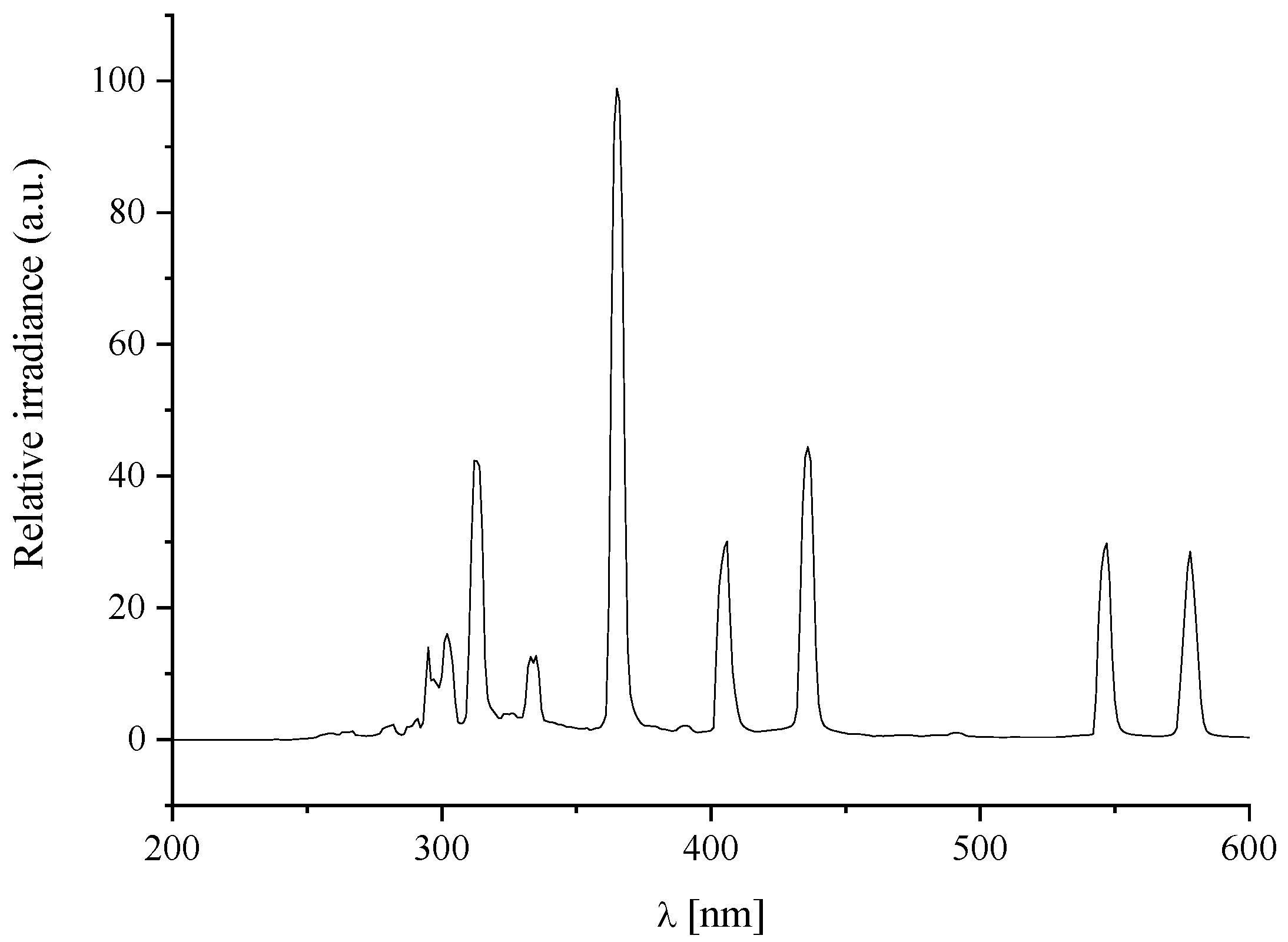
3.3. Mineralisation of the Printed TSC on the Substrate
3.4. Characterisation of Siloxane
3.5. Characterisation of TSC and Printed Layers on Glass
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Levchuk, I.; Sillanpää, M. Chapter 1—Titanium Dioxide–Based Nanomaterials for Photocatalytic Water Treatment. In Advanced Water Treatment: Advanced Oxidation Processes; Elsevier: Amsterdam, The Netherlands, 2020; pp. 1–56. ISBN 978-0-12-819225-2. [Google Scholar]
- Cha, B.J.; Saqlain, S.; Seo, H.O.; Kim, Y.D. Hydrophilic surface modification of TiO2 to produce a highly sustainable photocatalyst for outdoor air purification. Appl. Surf. Sci. 2019, 479, 31–38. [Google Scholar] [CrossRef]
- Yoon, K.H.; Noh, J.S.; Kwon, C.H.; Muhammed, M. Photocatalytic behaviour of TiO2 thin films prepared by sol–gel process. Mater. Chem. Phys. 2006, 95, 79–83. [Google Scholar] [CrossRef]
- Hu, J.; Gao, Q.; Xu, L.; Wang, M.; Zhang, M.; Zhang, K.; Liu, W.; Wu, G. Functionalization of cotton fabrics with highly durable polysiloxane—TiO2 hybrid layers: Potential applications for photo-induced water–oil separation, UV shielding, and self-cleaning. J. Mater. Chem. A 2018, 6, 6085–6095. [Google Scholar] [CrossRef]
- Wang, M.; Zhang, M.; Zhang, M.; Aizezi, M.; Zhang, Y.; Hu, J.; Wu, G. In-situ mineralized robust polysiloxane–Ag@ZnO on cotton for enhanced photocatalytic and antibacterial activities. Carbohydr. Polym. 2019, 217, 15–25. [Google Scholar] [CrossRef]
- Gregori, D.; Benchenaa, I.; Chaput, F.; Thérias, S.; Gardette, J.-L.; Leonard, D.; Guillard, C.; Parola, S. Mechanically stable and photocatalytically active TiO2/SiO2 hybrid films on flexible organic substrates. J. Mater. Chem. A 2014, 2, 20096–20104. [Google Scholar] [CrossRef]
- Shan, A.Y.; Ghazi, T.I.M.; Rashid, S.A. Immobilisation of titanium dioxide onto supporting materials in heterogeneous photocatalysis: A review. Appl. Catal. A Gen. 2010, 389, 1–8. [Google Scholar] [CrossRef]
- Wang, M.; Zhang, M.; Pang, L.; Yang, C.; Zhang, Y.; Hu, J.; Wu, G. Fabrication of highly durable polysiloxane-zinc oxide (ZnO) coated polyethylene terephthalate (PET) fabric with improved ultraviolet resistance, hydrophobicity, and thermal resistance. J. Colloid Interface Sci. 2019, 537, 91–100. [Google Scholar] [CrossRef]
- Zhang, J.; Palaniappan, A.; Su, X.; Tay, F.E. Mesoporous silica thin films prepared by argon plasma treatment of sol–gel-derived precursor. Appl. Surf. Sci. 2005, 245, 304–309. [Google Scholar] [CrossRef]
- Baskaran, S.; Liu, J.; Domansky, K.; Kohler, N.; Li, X.; Coyle, C.; Fryxell, G.E.; Thevuthasan, S.; Williford, R.E. Low Dielectric Constant Mesoporous Silica Films Through Molecularly Templated Synthesis. Adv. Mater. 2000, 14, 291–294. [Google Scholar] [CrossRef]
- Wirnsberger, G.; Yang, P.; Scott, B.J.; Chmelka, B.F.; Stucky, G.D. Mesostructured materials for optical applications: From low-k dielectrics to sensors and lasers. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2001, 57, 2049–2060. [Google Scholar] [CrossRef]
- Chen, J.Y.; Pan, F.M.; Chang, L.; Cho, A.T.; Chao, K.J. Thermal stability of trimethylsilylated mesoporous silica thin films as the ultralow-k dielectric for copper interconnects. J. Vac. Sci. Technol. B Microelectron. Nanometer Struct. 2005, 23, 2034. [Google Scholar] [CrossRef]
- Jung, S.-B.; Park, H.-H. Control of surface residual OH polar bonds in SiO2 aerogel film by silylation. Thin Solid Films 2002, 420, 503–507. [Google Scholar] [CrossRef]
- Zhao, C.; Pelaez, M.; Dionysiou, D.D.; Pillai, S.C.; Byrne, J.A.; O’Shea, K.E. UV and visible light activated TiO2 photocatalysis of 6-hydroxymethyl uracil, a model compound for the potent cyanotoxin cylindrospermopsin. Catal. Today 2014, 224, 70–76. [Google Scholar] [CrossRef]
- Lee, M.; Park, I.J.; Jeong, H.; Kim, B.J.; Yun, Y.; Kim, H.J.; Cho, H.; Lee, S. Thermal-assisted photo-annealed TiO2 thin films for perovskite solar cells fabricated under ambient air. Appl. Surf. Sci. 2020, 530, 147221. [Google Scholar] [CrossRef]
- Kraus, F.; Cruz, S.; Müller, J. Plasma polymerized silicon organic thin films from HMDSN for capacitive humidity sensors. Sens. Actuators B Chem. 2003, 88, 300–311. [Google Scholar] [CrossRef]
- Gomez-Vega, J.M.; Teshima, K.; Hozumi, A.; Sugimura, H.; Takai, O. Mesoporous silica thin films produced by calcination in oxygen plasma. Surf. Coat. Technol. 2003, 169–170, 504–507. [Google Scholar] [CrossRef]
- Park, H.-H.; Jo, M.; Kim, H.; Hyun, S. Effect of Oxygen Plasma Treatment on SiO2 Aerogel Films. J. Mater. Sci. Lett. 1998, 17, 2083–2085. [Google Scholar] [CrossRef]
- Huang, J.; Ichinose, I.; Kunitake, T.; Nakao, A. Preparation of Nanoporous Titania Films by Surface Sol−Gel Process Accompanied by Low-Temperature Oxygen Plasma Treatment. Langmuir 2002, 18, 9048–9053. [Google Scholar] [CrossRef]
- Homola, T.; Dzik, P.; Veselý, M.; Kelar, J.; Černák, M.; Weiter, M. Fast and Low-Temperature (70 °C) Mineralization of Inkjet Printed Mesoporous TiO2 Photoanodes Using Ambient Air Plasma. ACS Appl. Mater. Interfaces 2016, 8, 33562–33571. [Google Scholar] [CrossRef]
- Lidmila, B.; Marie, Š. Physical Chemistry of Surfaces and Colloidal Systems [In Czech: Fyzikální chemie povrchů a koloidních soustav], 4th ed.; VŠCHT: Prague, Czech Republic, 2002; pp. 51–56. ISBN 80-708-0475-0. [Google Scholar]
- Dzik, P.; Svoboda, T.; Kaštyl, J.; Veselý, M. Modification of photocatalyst morphology by ball milling and its impact on the physicochemical properties of wet coated layers. Catal. Today 2019, 328, 65–70. [Google Scholar] [CrossRef]
- Ghosh, M.; Lohrasbi, M.; Chuang, S.S.C.; Jana, S.C. Mesoporous Titanium Dioxide Nanofibers with a Significantly Enhanced Photocatalytic Activity. ChemCatChem 2016, 8, 2525–2535. [Google Scholar] [CrossRef]
- Dzik, P.; Veselý, M.; Pachovská, M.; Neumann-Spallart, M.; Buršíková, V.; Homola, T. The influence of curing methods on the physico-chemical properties of printed mesoporous titania patterns reinforced by methylsilica binder. Catal. Today 2018, 313, 26–32. [Google Scholar] [CrossRef]
- Homola, T.; Ďurašová, Z.; Shekargoftar, M.; Souček, P.; Dzik, P. Optimization of TiO2 Mesoporous Photoanodes Prepared by Inkjet Printing and Low-Temperature Plasma Processing. Plasma Chem. Plasma Process. 2020, 40, 1311–1330. [Google Scholar] [CrossRef]
- Junlabhut, P.; Boonruang, S.; Mekprasart, W.; Pecharapa, W. Ag nanoparticle-doped SiO2/TiO2 hybrid optical sensitive thin film for optical element applications. Surf. Coat. Technol. 2016, 306, 262–266. [Google Scholar] [CrossRef]
- Product Specification: TiO2. Sigma-Aldrich. Available online: https://api.sigmaaldrich.com/deepweb/assets/sigmaaldrich/quality/spec/440/011/637254-BULK_______ALDRICH__.pdf (accessed on 2 December 2020).
- Homola, T.; Wu, L.Y.L.; Černák, M. Atmospheric Plasma Surface Activation of Poly(Ethylene Terephthalate) Film for Roll-To-Roll Application of Transparent Conductive Coating. J. Adhes. 2014, 90, 296–309. [Google Scholar] [CrossRef]
- Homola, T.; Matoušek, J.; Kormunda, M.; Wu, L.Y.L.; Černák, M. Plasma Treatment of Glass Surfaces Using Diffuse Coplanar Surface Barrier Discharge in Ambient Air. Plasma Chem. Plasma Process. 2013, 33, 881–894. [Google Scholar] [CrossRef]
- George, S. Infrared and Raman Characteristic Group Frequencies: Tables and Charts, 3rd ed.; John Wiley & Sons: Hoboken, NJ, USA, 2004; ISBN 978-0-470-09307-8. [Google Scholar]
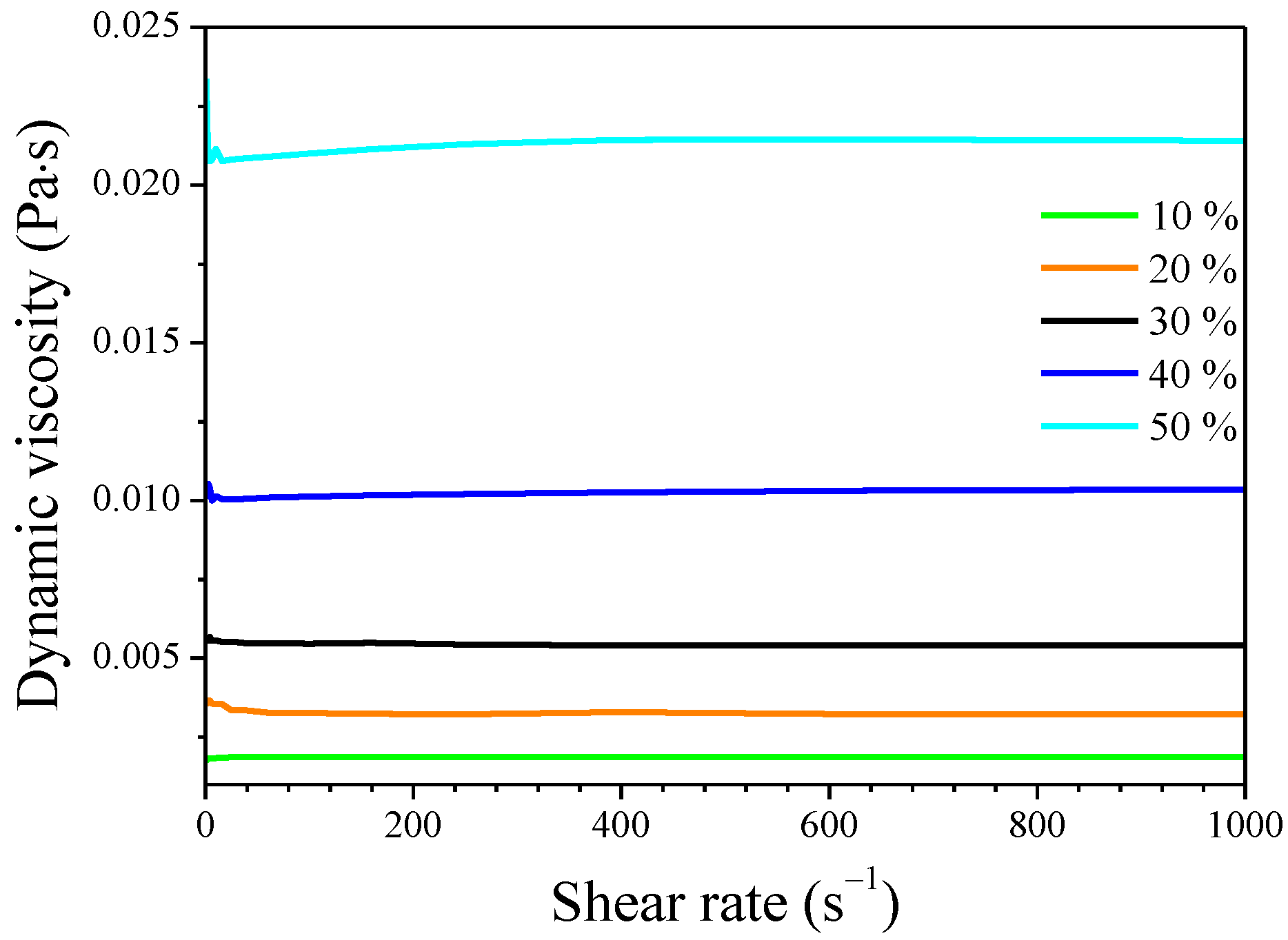
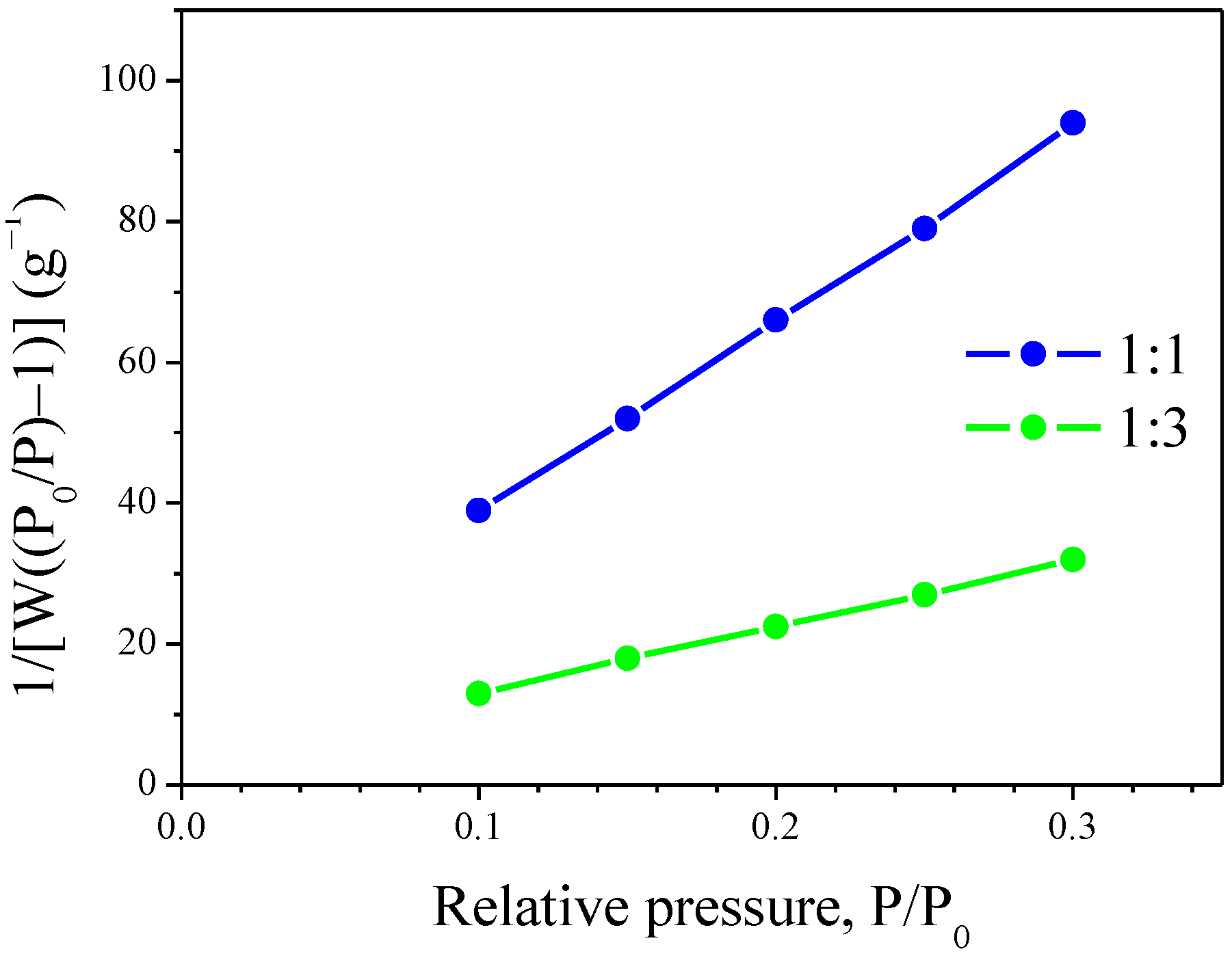
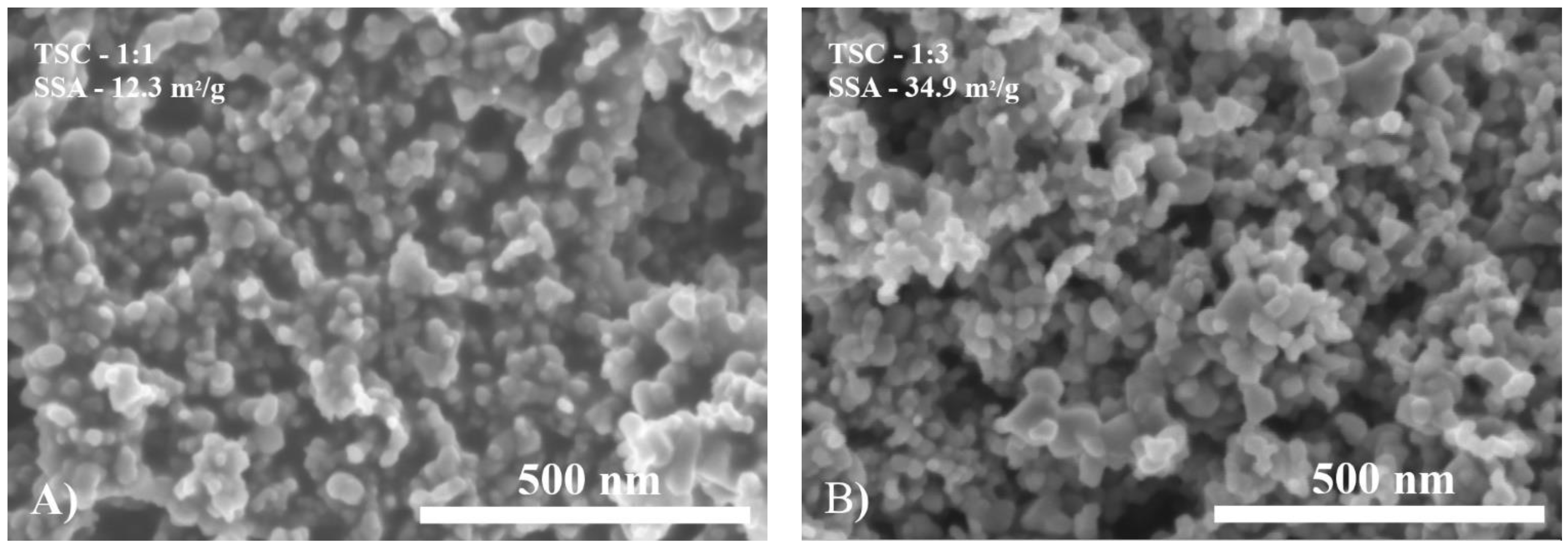
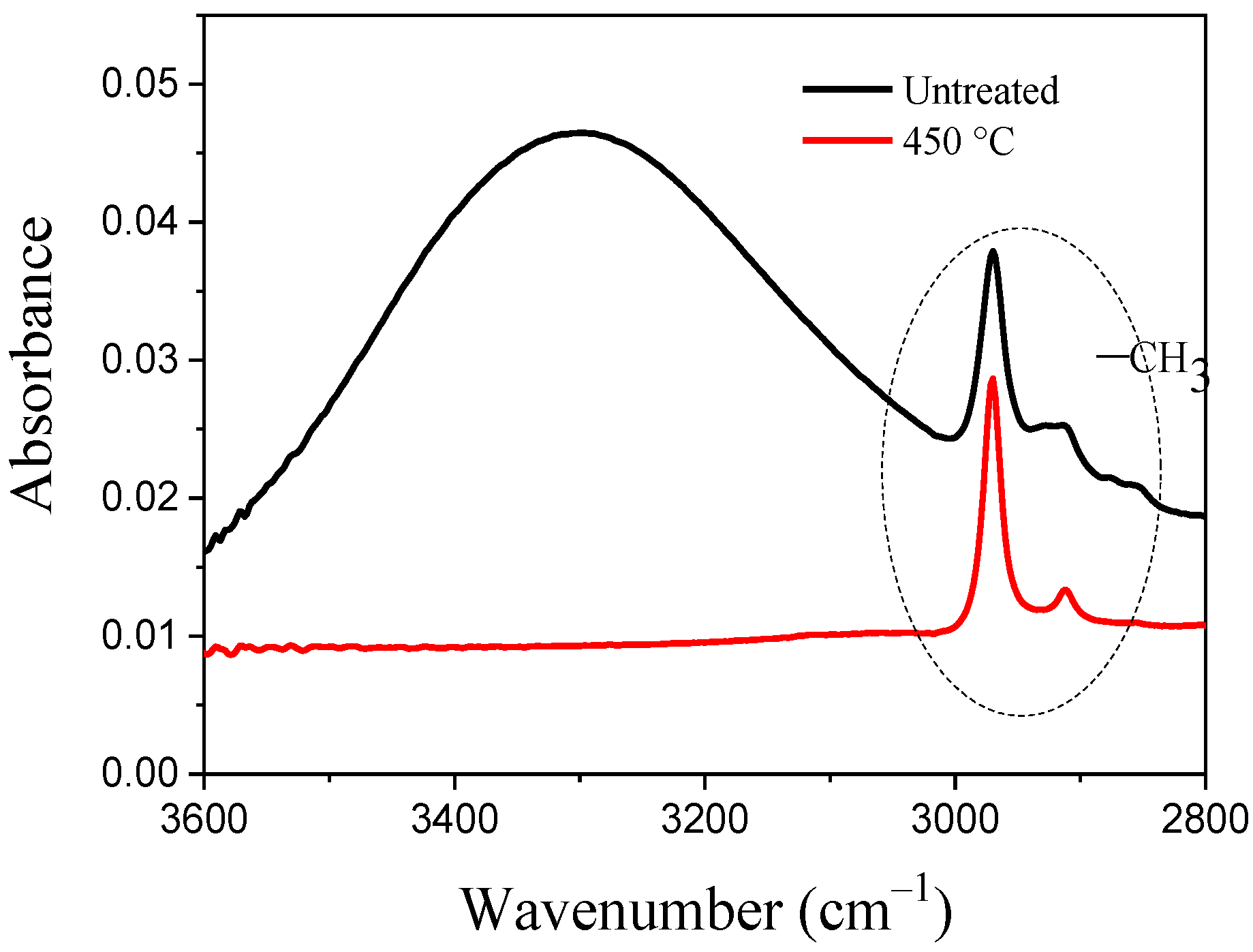

| Concentration (%) | 10 | 20 | 30 | 40 | 50 |
|---|---|---|---|---|---|
| Surface tension (mN∙m−1) | 22.58 | 22.96 | 23.52 | 24.08 | 24.75 |
| Dilution of 10% Solution | Injection Volume (μL) | MW (kDa) | Polydispersity (MW/MN) | Radius of Gyration (nm) |
|---|---|---|---|---|
| 1:1 | 100 | 1.497 | 1.019 | 12.8 |
| 1:1 | 100 | 1.534 | 1.036 | 11.9 |
| 1:1 | 100 | 1.475 | 1.033 | 12.0 |
| 1:1 | 100 | 1.506 | 1.046 | 12.5 |
| Diameter MW (kDa) | 1.52 | 1.032 | 13 | |
| Selective standard deviation | 0.04 | 0.009 | 2 |
| Composition | Siloxane (20% in Ethanol) | TiO2 (20% P25 in Dowanol) | Hexanol |
|---|---|---|---|
| E38-9AD (1:1) | 4 mL | 4 mL | 20 mL |
| E38-10AD (1:3) | 2 mL | 6 mL | 20 mL |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Svoboda, T.; Veselý, M.; Bartoš, R.; Homola, T.; Dzik, P. Low-Temperature Mineralisation of Titania-Siloxane Composite Layers. Catalysts 2021, 11, 50. https://doi.org/10.3390/catal11010050
Svoboda T, Veselý M, Bartoš R, Homola T, Dzik P. Low-Temperature Mineralisation of Titania-Siloxane Composite Layers. Catalysts. 2021; 11(1):50. https://doi.org/10.3390/catal11010050
Chicago/Turabian StyleSvoboda, Tomáš, Michal Veselý, Radim Bartoš, Tomáš Homola, and Petr Dzik. 2021. "Low-Temperature Mineralisation of Titania-Siloxane Composite Layers" Catalysts 11, no. 1: 50. https://doi.org/10.3390/catal11010050
APA StyleSvoboda, T., Veselý, M., Bartoš, R., Homola, T., & Dzik, P. (2021). Low-Temperature Mineralisation of Titania-Siloxane Composite Layers. Catalysts, 11(1), 50. https://doi.org/10.3390/catal11010050






