Abstract
The storage, utilization, and control of the greenhouse (CO2) gas is a topic of interest for researchers in academia and society. The present review article is dedicating to cover the overall role of ionic liquid-modified hybrid materials in cycloaddition reactions. Special emphasis is on the synthesis of various cyclic carbonate using ionic liquid-based modified catalysts. Catalytic activity studies have discussed with respect to process conditions and their effects on conversion and product selectivity for the reaction of cycloaddition of CO2 with styrene oxide. The reaction temperature and the partial pressure of CO2 have found to play a key role in cyclic carbonate formation. The role of other influential parameter (solvent effect) is also discussed for the conversion of cyclic/aromatic oxides to polycarbonate production. Our own research work that deals with ionic liquid-based halide-modified mesoporous catalyst (MCM-41 type) derived from rice husk waste has also been discussed. Finally, the role of carbon dioxide activation and ring-opening mechanisms involved in the cyclic carbonate product formation from CO2 have been discussed.
1. Introduction
Greenhouse gas (carbon dioxide—CO2) in the atmosphere helps living things naturally by involving in photosynthesis [1]. About 32% of CO2 is being produced by hydrocarbon combustion and gasification process that raises concern over environmental pollution [2,3]. The transportation sector contributes nearly 30% to total carbon dioxide emissions [4]. Figure 1 shows the carbon cycle, CO2 storage, recycle and purification, and utilization mainly of fine chemicals formation by catalysis route. Figure 1 shows the global atmospheric CO2 concentration for about half a decade from 1958 with respect to continuous research reports carried by the Mauna Loa Observatory in Hawaii [3]. The concentration of atmospheric CO2 was 399.89 ppm till May 2013, and in 2020, it reached up to 412.78 ppm [5]. The continuous rise in greenhouse gas (CO2) concentration creates global warming issues and continues damage to the green environment. In recent years, smart technologies are developed to store and utilize CO2 reduction and which makes the pollution free atmosphere [6,7,8].
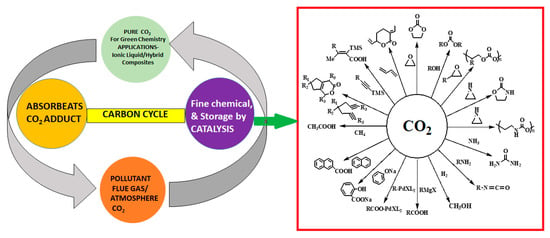
Figure 1.
Schematic of carbon cycle for CO2, recycle Storage and greenhouse gas utilization for possible fine chemicals production.
Carbon dioxide is recognized as a nonflammable [9], nontoxic [10], and inexpensive gas [11]. It is a renewable carbon source [12] and thermodynamically stable compound [13,14]. Interestingly, carbon oxide can replace the following toxic chemical compounds such as carbonyl dichloride known as phosgene (COCl2), carbon monoxide (CO), and isocyanides (R-NCO) for fine chemical applications. Carbon dioxide can be utilized as a mild oxygen source [15]. It can be used as an alternate medium or solvent [16], also work as a supercritical fluid (sc) [17], and act as a carbon source. It can be used based on its unique chemical properties to be incorporated with high “atom efficiency” such as in carboxylation synthesis or in catalyst synthesis [16]. Hutchings [15,17] used supercritical CO2 as an antisolvent for the preparation of Au/scCO2 and sc-VPO (vanadium phosphate) catalysts. Currently, CO2 has been used in various industrial applications such as chemical, pharmaceutical, foodstuff, laboratories and analysis, beverage, and pulp and paper industries [16]. The application of CO2 as C1 raw material in the chemical industry was started in past few decades. It has been reported that approximately 110 million metric tons of CO2 are currently used every year in the chemical industry. In the present decade, carbon dioxide utilization has reached around 110 million MT (metric tons) [18]. Carbon dioxide is also playing major role in the production of urea, [17] methanol [18], salicylic-acid [19], formic-acid [20], cyclic carbonates [21,22,23], copolymers, polymer building blocks, and fine chemicals [24,25,26,27]. Urea is one of the major fertilizers, and CO2 is the source for it [26]. The urea is prepared from ammonia and CO2 in fertilizer [28] and also in fabrication process of various types of polymers, such as melamine and urea-formaldehyde resin [29,30,31,32]. Salicylic acid is produced from phenol and CO2 via the Kolbe-Schmitt reaction [33]. The product is used to produce acetyl salicylic acid which is also known as aspirin, used mostly in healthcare applications [34,35,36]. Everyday monitoring of Carbon dioxide emission in atmosphere have shown in online website (www.co2.earth) to monitor the Keeling Curve of Atmospheric CO2 concentration emission between 1958 to 16 August 2020 [5,35].
The cyclic carbonates are odorless, colorless, and biodegradable [37,38]. The cyclic carbonates are used in industries as aprotic polar solvents [39], as a monomer for polymer synthesis, and as additives [40]. Besides, it is also been used in electrolytic materials such as secondary batteries (lithium batteries) [41], cosmetics, resins, and cleaning utensils [42]. Cyclic carbonates are utilized as an intermediate compound in the biomedical and pharma industries [12]. Cyclic carbonates also play a key role in herbicides and disinfectants synthesis [43] as well as are fuel additives [44].
Traditionally, phosgene with ethane-1,2-diol in dichloromethane solvent was utilized to produce cyclic carbonates, and one of the products is hydrochloric acid obtained as a by-product, which is harmful to human beings [43]. Scheme 1 shows the conventional synthesis of organic cyclic carbonates.
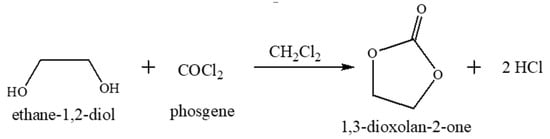
Scheme 1.
Synthesis of organic carbonate by the conventional route.
By considering the economic point of view and avoid toxicity generation, the CO2 is a natural choice to phosgene as an alternate and the other advantage is that CO2 can be incorporated into epoxides without side products [45]. However, due to the inert nature of CO2, various catalysts were adopted to activate the epoxide [11]. The cycloaddition of CO2 to epoxide is shown below (Scheme 2).
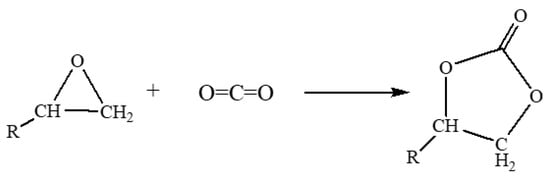
Scheme 2.
Cycloaddition of CO2 to epoxide forming cyclic carbonate.
To increase cyclic carbonates yield from cycloaddition of CO2 and epoxides, a different kind of catalyst has been adopted. In the past decades, the wide range of homogeneous and heterogeneous catalysts have been developed to catalyze the so-called CO2 fixation process. Homogeneous catalysts, such as CoCl2/onium salt [44], diimine Ru (II) complex [46], Al-salen-PEA [4], betaine-based quaternary ammonium ion and carboxylic acid [12], N,N-dimethyl formamide (DMF) [47,48], SnCl4-organic base [49], Au/Fe(OH)3-ZnBr2/Bu4NBr [50], ionic liquid-highly cross linked polymer [51], BrBu3PPEG600PBU3Br [52], cellulose/KI [53], and Au/R201 [54] have also been studied.
Several heterogeneous catalysts, such as metal oxides; MgO [55,56], Nb2O5 [43], Mg-Al oxide, guanidine-MCM-41 [57], Adeine-Pr-Al-SBA-15 [58], Cr-salen-SiO2 [13], Mn-salen-SiO2 [42], ClAlPC-MCM-41 [59], 3-(2-hydroxyl-ethyl)-1-propyl imidazolium bromide-SBA-15, and zeolite-based organic–inorganic hybrid catalysts have also been investigated [55,56,57,58,59,60,61,62,63,64,65,66].
Ionic liquid (IL) is recently explored as efficient catalysts with growing importance over the past decades [64]. Since 2003, ILs have widely been adopted in the chemical industry as a solvent as well as catalysts for many fine chemical productions [60,61,62,63,64,65]. Hence, a quaternary ammonium ion together with a halide anion, -OH, or a -COOH group with ionic liquid was considered as potential materials for heterogeneous catalysis.
2. Summary
The present review article described the effect of influential parameters such as temperature, pressure, and solvent on the conversion of cyclic epoxide into cyclic carbonate formation in presence of various ionic liquid modified hybrid catalysts. Another section describes the mechanisms insight into the activation of carbon dioxide and the ring-opening process in the process of substrate conversion and acidic/basic characteristics of the catalyst.
3. Results of Reaction Parameters and Influencing Factors for the Production of Cyclic Carbonates
The production of cyclic carbonates depends on various parameters including catalyst and reaction parameters such as solvents, temperature, and pressure condition. The optimization of all the above parameters could produce maximum product yield. Table 1 summarizes studies that were carried out for cycloaddition of greenhouse gas conversion to styrene oxide (SO) on different types of catalysts [66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99]. The produce yield was higher due to the presence of both acid and base bifunctional groups present on the catalyst and it synergistically activates the cycloaddition reactions.

Table 1.
Catalytic activity studies of cycloaddition of CO2 to styrene oxide using various homogeneous and heterogeneous catalysts.
3.1. Effect of Influence of Reaction Temperature for Cycloaddition of Epoxides with CO2
The reaction temperature is an important parameter in a catalytic reaction for an effective collision between molecules to enhance the bond-breaking step. Hence, the more molecular collision is the reason for the more yield of final products. Aresta et al. [26,41] reported the temperature effect on the production of styrene carbonate (SC) using Nb2O5 as a catalyst from styrene oxide by CO2 addition. Below 100 °C, the reaction did not yield any product. However, it provides 80% yield at temperature of 135 °C. Jutz et al. [40] studied the effect of reaction temperature on the performance of Mn (salen) Br. The highest yield was obtained at 160 °C, and with a further increase in temperature, the yield was dramatically reduced. This was attributed to changes in the phase distribution observed at higher reaction temperatures. Zhou et al. [11] reported that the reaction carried out at 140 °C results in the formation of propylene carbonate (PC) with the highest yield of 98%. Increase in the temperature from 140 to 150 °C dropped the yield up to 78% due to problems of side product generation at high-temperature conditions. Bai et al. [83,84] reported that in some instances, high-temperature conditions are causing the catalyst to decompose resulting in a decrease in propylene carbonate (PC) yield [84]. Qiao et al. [91] explained that styrene oxide (SO) is difficult to convert to styrene carbonate (SC) compared to all other epoxides due to the lower reactivity of β-carbon atom. They found that the temperature of 120 °C (98%) with suitable catalyst was the best-optimized condition than the high-temperature condition (130 °C (~80%) and 140 °C (~95%)).
Recently, Lee et al. [98] studied the cycloaddition reaction between ally glycidyl ether (AGE) and carbon dioxide using PS-hexyl-Methyl iodide at 12 bar of total pressure and different temperature conditions. They reported that the yield of allyl glycidyl carbonate (AGC) increases from 80 to 140 °C, and it decreased with a further increase in the temperature to 160 °C. The yield decreased was due to the generation of oligomers and other side products like 3-allyl oxy-1,2-propanediol. Zhong (2014) et al. [100] studied and reported the effect of temperature in the range between 120 and 160 °C for propylene carbonate formation. The yield of PC in the presence of 0.78 and 13.7 m/mol of DMF solvents for comparative purpose was studied. They found that the usage of a large amount of DMF was favorable to provide a higher yield at the lower reaction temperature.
3.2. Effect of Influence of Reaction Pressure Condition for Cycloaddition of Epoxides with CO2
The reaction pressure of the carbon dioxide insertion has been established as one of the most crucial and critical conditions for affecting the epoxide cycloaddition reaction [91,92,93,94,95,96,97,98]. The inserted CO2 acts as an important reactant for all catalytic transformations [42]. Two phases are established in the reaction system; the bottom phase is rich with epoxide and the top phase is enriched with CO2. According to Xie et al. (2007), the reactant CO2 favors the reaction when the bottom phase is under high pressure. However, the above condition is not favorable for the high-pressure reactions (120 bar) as concentration of epoxide for example propylene oxide [49].
Ghosh et al. [36] reported that at lower pressure (7 bar), the catalyst retains moderate reaction activity turn over frequency (TOF) of 312 h−1 and with increasing pressure up to 20 bar, increased TOF value of 351 h−1 was observed. However, the pressure more than 20 bar results in diminishes overall reactivity due to polarity and solubility problem of the catalysts. Qiao et al. [91] reported different pressure conditions, such as mild pressure (15 bar), medium pressure (80 bar), and supercritical pressure (140 bar), for the styrene oxide with CO2 cycloaddition reactions. This was due to changes in the phase from gas to supercritical fluid, where a part of styrene oxide (SO) dissolves in the supercritical condition. On the other hand, Wang, et. al (2017) [65] approach was for the same reaction but different perspective, i.e., introduction of high concentration of CO2 dissolves within a substrate or “liquefies” the formation of intermediate complex.
Xiang et al. (2009) [52] reported that the many oligomers were produced as the side products such as propylene oxide and styrene oxide due to insertion of CO2 at high pressure in a solvent-less condition. Jutz et al. [40,53] reported that a ratio of 1:4 (epoxide: CO2) was the best reactant ratio condition instead of 1:16 for conversion of both epoxides.
3.3. Effect of Influence of Solvent for Cycloaddition of Epoxides with CO2
A variety of solvents are adopted to synthesis cyclic carbonate from cycloaddition reaction. A solvent plays a key role in minimizing carbonaceous deposits on the catalyst surface. [55]. Aresta et al. [26,41] exploited that the N,N-dimethyl formamide (DMF) alone yields 34.7% of styrene carbonate at 50 bar pressure of CO2 and predict that amide group was a good promoter. Di-methyl acetamide (DMA) produced SC about 28% yield without the catalyst at 50 bar pressure and at temperature of 135 °C for 12 h. The role of DMF in the cycloaddition of epoxides mechanism and transformation is as follows (Scheme 3).
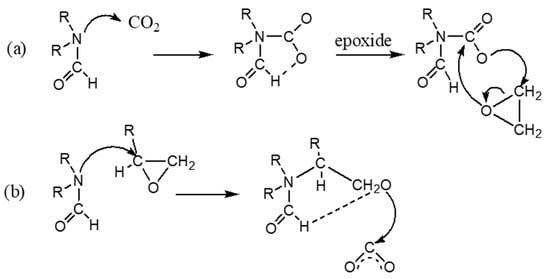
Scheme 3.
(a) Role of N,N-dimethyl formamide (DMF) ) (a) initial CO2 activation; (b) initial epoxide activation, in the cycloaddition of epoxides [43] (modified images and cited the related reference).
According to the mechanism, role of amide is to facilitate the nucleophilicity of the respective oxygen atoms in CO2 or epoxides. From the results, it is evident that the methylene chloride could contribute to stabilize the polar or ionic intermediates through the dipole effect, i.e., Cδ−-Hδ+ and Cδ+-Clδ−. The cooperative solvation effect occurred in the presence of tetrachloro methane and ethanol-like additive used along with DMF for cycloaddition reactions. Kawanami et al. (2000) [45] used supercritical condition (sc) to study the effect of DMF as a catalyst and solvent on epoxide formation, which is dissolved in DMF-scCO2. Recently, Zhong (2000) et al. [100] utilized DMF as cocatalyst with ZnBr2 as a catalyst for cycloaddition reaction of propylene oxide with carbon dioxide. In their study, they have observed that DMF acted as a solvent as well as carbon dioxide activator. Both high conversion and 100% selectivity was obtained at 150 °C and pressure condition of 30 bar with very quick reaction time of 10 min [100]. Alvaro (2004) et al. [12] used 0.4 mL dichloromethane (DMC) or dimethyl carbonate as a cosolvent to enhance product solubility in the supercritical medium and it serves as a cocatalyst as well. The results showed 70% conversion and 100% selectivity in presence of Cr-salen base catalyst under supercritical condition (100 bar, 80 °C, reactor volume = 50 m, 6 h reaction time).
A new approach was taken by Jiang et al. [46] in order to study the solvent effect for the synthesis of chloropropene carbonate from epichlorohydrin. Protic alcohols (methanol and ethanol) acted as good solvent for the formation of chloropropene carbonate with 90% and 82% yield. However, higher molecular weight alcohol such as benzyl alcohol was found to give less productivity yield (12%), whereas the same reaction carried out at 110 °C for 20 h with DMF as the solvent resulted in achieving for highest yield (f > 99%).
4. Discussion of the Mechanism Insight of Cycloaddition of Epoxides with CO2
4.1. Activation of CO2 for Cycloaddition of Epoxides
Lu et al. [59] reported that aluminum pthalocyanine complex formation on MCM-41 support for the cycloaddition reaction of epoxides They observed that CO2 activated through nucleophilic attack at the carbon atom of CO2 by the alcoholate group (-OCH2CH2Br). The weak interaction between the central metal ion of pthalocyanine complex and the lone pairs oxygen in CO2 makes synergistic mechanism. The halide intramolecular substitution facilitate the epoxide into cyclic carbonates. In another related study, Barbarini et al. [10] argued that the mechanism of CO2 activation through the formation of the zwitterion compound. Scheme 4 shows that the CO2 adds to the epoxide via nucleophilic attack.
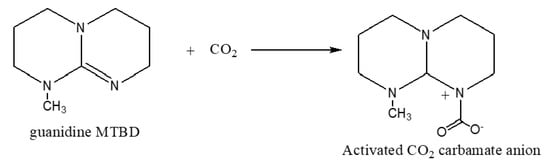
Scheme 4.
Hypothesized 7-Methyl-1,5,7-triazabicyclo [4.4.0]dec-5-ene (MTBD)-promoted CO2 activation [11].
Srivastava et al. 2005 [1] exploited at first regarding the physico-chemical properties of the model catalyst in the activation of CO2 molecules. Surface absorbing nature of CO2 on catalyst was studied by Ft-IR spectroscopy. The CO2 interacted with the amine functional groups in functionalized [SBA-15-pr-Ade(adenine) and Ti-SBA-15-pr-Ade(adenine)] was identified and confirmed by presence of the carbamate bands at 1609 and 1446 cm−1.
The efficient Epichlorohydrin conversion was obtained (62.3%) after functionalization of SBA-15 with adenine group compared to bulk SBA-15 (1.5%). This increased in conversion was related to the intensity of the band at 1609 cm−1 ascribed due to CO2 bonded with amine sites, which recognize the importance of such sites for CO2 activation. In a related study, Srivastava et al. [77,86] exploited and compared the importance of the basic sites present in the catalysts such as alkyl amines (-NH2), adenine (Ade), imidazole (Im) and guanine (Gua) to activation process of carbon dioxide. Different types of coordination modes of CO2 was discussed in detail in the past and its well known in the field of carbon dioxide chemistry [75].
Scheme 6 shows the stability of the activated CO2 complex formation occurs on the basic amine sites at the catalyst surface decreased as follows: primary > secondary > tertiary amines [75,77,86]. The formation of carbamate anions from different type of amine groups are demonstrated. The metal- electron deficient part of the catalyst facilitates the reaction rate for the formation of cyclic carbamate ions.
In Scheme 5 it is more clearly explained the role activation of CO2 for their efficient conversion towards carbon dioxide activation process.
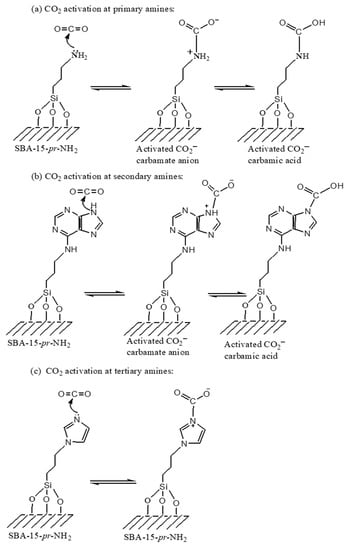
Scheme 5.
CO2 activation through primary, secondary, and tertiary amines [77,86].
From their point of view, Scheme 5 shows the stability of the activated CO2 complex formation that occurring on the basic amine sites at the surfaces of catalyst decreased as follow: primary > secondary > tertiary amines [75,77,86]. The formation of carbamate anions from different types of amine groups are demonstrated. The metal-electron deficient part of the catalyst facilitates the reaction rate for the formation of cyclic carbamate ions.
4.2. Ring Opening of Epoxide
The ring-opening mechanism of the epoxide is described in two ways such as (i) Lewis-acid catalyzed cleavage and (ii) Lewis-base catalyzed cleavage. Bu et al. (2007) [101] found that the ruthenium complex ((2,2′-bipy)RuCl3(CH3OH)) supported by cetyl-trimethyl ammonium chloride (CTAC) catalyzes propylene oxide (PO) in the presence of CO2 and achieved 100% yield for propylene carbonate (PC). In the above catalyst system, Ru acts as a Lewis-acid to activate the PO to form adduct of Ru-PO. CTAC addition enhances reaction rate and strikes the less sterically hindered carbon to break the epoxide ring while forming the oxy-anion species.
Bai et al. [83,96] reported that the bi-functional metal porphyrins M(TTMAPP)I4(X) (M = Co, Mn, Fe, and Cr; X = OAc, CCl3COO, CF3COO, OTs, I, Cl, and Br) were highly efficient catalysts for the respective cycloaddition (formation of propylene carbonate). In the above catalyst system, metal ion incorporation acts as a Lewis acid center to facilitate the catalytic reaction rate. The order of activity of the catalysts was Co > Mn > Fe > Cr. The catalytic activity of cobalt porphyrin decreased with different counter ions as follows: CH3COO− > I− > Cl− > Br− > OTs− > CF3COO− > CCl3COO−. Barbarini et al. [10] reported mesoporous silica (MCM-41) with hexagonal morphology in which Si-OH (hydroxyl and silanol functionalized)-supported guanidine catalysts are studied for cycloaddition reactions. The enhanced reactivity was obtained due to mechanisms involved in hydrogen bonding. Zhou et al. [11] studied the mechanism of cyclic carbonate formation in the presence of betaine (HBetX) and choline cation (ChoX) catalyst. They compared the anion effect and hydroxyl and carboxyclic acid group activation towards catalyst function. The order of reactivity for PC conversion and yield decreased as follows: Cl− > BF4− > PF6−. The role of leaving group ability has also been studied, and the activity follows in this order: I− > Br− > Cl−. Adopting better nucleophilic anions could improve the epoxide ring opening/breaking efficiency of the catalyst.
The carboxylic acid group is found to be best for ring-opening mechanism with respect to suitable halide anions. The reason behind the halide anions to activate the ring opening is due to presence of stronger BrøØnsted acid and thereby involved in hydrogen bonding. Scheme 6 shows the reaction mechanism for the cycloaddition reaction and its halide anion interaction.
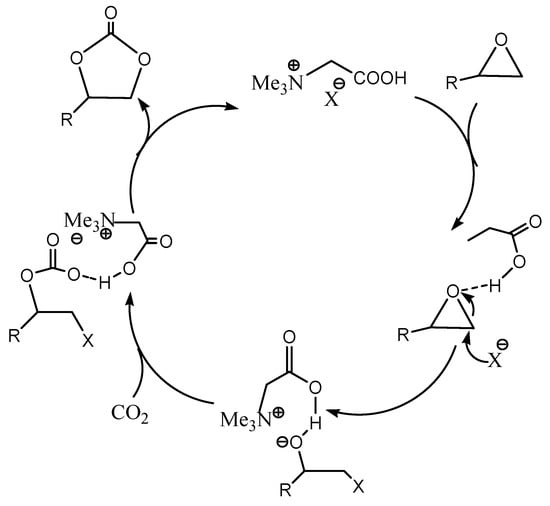
Scheme 6.
The plausible cycloaddition reaction mechanism for epoxide ring opening with CO2 by betaine (HBetX) catalysts [11].
Dai et al. [80,97] reported 3-(2-hydroxyl-ethyl)-1-propyl imidazolium bromide-SBA-15 (HEPIMBr), which is another type of mesoporous silica. The efficient synthesis of cyclic carbonates was achieved under mild conditions without solvent and in the absence of a co-catalyst.
Biopolymer chitosan-grafted quaternary phosphonium ionic liquid (CS-(BuPh3P)Br) was reported as an excellent catalyst [98]. The authors proposed that the bromide anion of the catalyst played a major role in epoxy ring opening activated by the hydroxyl groups and phosphonium cation interaction. The same type of catalysts was developed by reported hydroxyl, carboxyl, and amino-functionalized phosphonium-based ionic liquid catalyst. They observed that a similar mechanism as mentioned above for the opening of the epoxide ring via polarization of epoxide C-O bond [90,91,92,93,94,95,96,97,98]. Excellent selectivity and good yield were obtained for cyclic carbonates under suitable or optimizable reaction conditions [90,91,92,93,94,95,96,97,98,99,100,101]. The following yields were obtained for the each cyclic carbonates such as epichlorohydrine (97.0%),) glycidol (98.3%), styrene oxide (98.8%), phenyl glycidyl ether (96.7%) for allyl glycidyl ether (97.5%,) and 1,2-epoxyhexane (100% for) at the reaction time of three hours (3 h) [101,102].
Ramalingam et al. [102] and our group recently reported halide ion-modified mesoporous silica catalysts for solvent- free cycloaddition of styrene oxide with CO2. For above reaction, imidazole was first immobilized on MCM-41 (derived from biomass materials) using 3-chloropropyltriethoxysilane (CPTES) as the anchoring agent followed by alkylation with 1,2-dibromoethane at 110 °C. The prepared catalyst was mentioned as MCM-41-Imi/Br. The catalyst was used in the cycloaddition of styrene oxide, glycidol, epichlorohydrin and phenyl glycidyl ether, and allyl glycidyl ether.
The halide ion (Br) and the tertiary amine from imidazole anchored over mesoporous support (MCM-41-Imi/Br) involved in the ring opening and activation of CO2. In Scheme 7, the mechanism of ring opening of the epoxide carried out by a nucleophilic attack by the bromide ion at the less sterically hindered β-carbon resulted to the formation of haloalkoxy species.
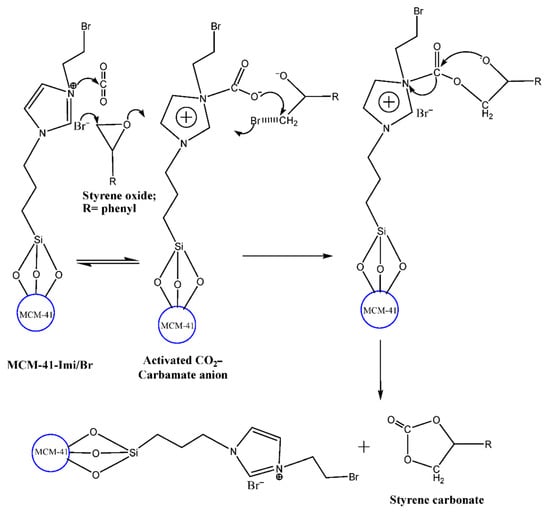
Scheme 7.
Mechanism of the halide ion (Br) and the tertiary amine from imidazole anchored over mesoporous support (MCM-41-Imi/Br) catalysts on cycloaddition of epoxide with CO2.
5. Conclusions
The present review explained the different types of porous and mesoporous solid acid-base and ionic liquid-modified mesoporous catalysts for the effective conversion for cycloaddition reaction of various epoxide with CO2. In addition, the influence of various parameters, such as reaction temperature, pressure, and usage of solvents or solvent-free conditions, is discussed.
Both excellent selectivity and good yield were obtained for cyclic carbonates under tuned reaction conditions by ionic liquid immobilized MCM-41 catalyst. The higher yields were obtained for the conversion of cyclic epoxides. The above higher conversion proves that the value of the development of ionic liquid-based mesoporous catalytic materials and their future applications. The mechanism insight of ring opening of epoxide at various catalyst systems has also been discussed. The Ft-IR spectroscopy is very useful to exploit the activation mechanism of CO2 for cycloaddition reaction using the various amine-functionalized solid catalyst. Hence, the development of a hybrid composite catalyst based on ionic liquid could be the potential material for direct usage of emerging greenhouse gas for various chemical processes.
Author Contributions
Conceptualization, R.J.R. and J.N.A., M.K.G.; methodology, R.J.R. and J.N.A.; validation, G.P., H.A.A.-L., P.A., investigation, R.A., F.A., M.D.W.; resources, R.J.R.; writing—original draft preparation, RJR and J.N.A.; M.K.G.; writing—R.J.R. and J.N.A., M.K.G.: project administration, R.J.R., F.A., P.A., R.A, H.A.A.-L.; funding acquisition R.J.R., G.P. All authors have read and agreed to the published version of the manuscript.
Funding
The author (JNA) thank and express gratitude to USM, Penang, Malaysia for the support of his post-doctoral research work. The authors are also thanks this research was funded by Deputyship for Research & Innovation, “Ministry of Education” in Saudi Arabia for funding this research work through the project number IFKSURP-335, Kingdom of Saudi Arabia.
Acknowledgments
The authors acknowledge the Deputyship for Research & Innovation, “Ministry of Education” in Saudi Arabia for funding this research work through the project number IFKSURP-335.
Conflicts of Interest
The authors declare that they have no known competing financial interest or personal relationships that could have appeared to influence the work reported in this paper.
References
- Srivastava, R.; Srinivas, D.; Ratnasamy, P. CO2 activation and synthesis of cyclic carbonates and alkyl/arylcarbamates over adenine-modified Ti-SBA-15 solid catalysts. J. Catal. 2005, 233, 1–15. [Google Scholar] [CrossRef]
- Luo, Y.; Ben, H.; Wu, Z.; Nie, K.; Han, G.; Jiang, W. Impact of CO2 on pyrolysis products of bituminous coal and platanus sawdust. Polymers 2019, 11, 1370. [Google Scholar] [CrossRef] [PubMed]
- Hong, D.; Si, T.; Li, X.; Guo, X. Reactive molecular dynamic simulations of the CO2 gasification effect on the oxy-fuel combustion of Zhundong coal char. Fuel Process. Technol. 2020, 199, 106305. [Google Scholar] [CrossRef]
- Alvaro, M.; Baleizao, C.; Carbonell, E.; El Ghoul, M.; García, H.; Gigante, B. Polymer-bound aluminium salen complex as reusable catalysts for CO2 insertion into epoxides. Tetrahedron 2005, 61, 12131–12139. [Google Scholar] [CrossRef]
- McGee, M. What the World Needs to Watch. Available online: http://co2now.org/ (accessed on 20 June 2020).
- Leung, D.Y.C.; Caramanna, G.; Maroto-Valer, M.M. An overview of current status of carbon dioxide capture and storage technologies. Renew. Sustain. Energy Rev. 2014, 39, 426–443. [Google Scholar] [CrossRef]
- Takht Ravanchi, M.; Sahebdelfar, S. Carbon dioxide capture and utilization in petrochemical industry: Potentials and challenges. Appl. Petrochem. Res. 2014, 4, 63–77. [Google Scholar] [CrossRef]
- Al-Mamoori, A.; Krishnamurthy, A.; Rownaghi, A.A.; Rezaei, F. Carbon capture and utilization update. Energy Technol. 2017, 5, 834–849. [Google Scholar] [CrossRef]
- Liu, J.; Wang, A.; Jing, H. TiO2-based green heterogeneous catalysts for the cycloaddition of CO2 to epoxides. Chin. J. Catal. 2014, 35, 1669–1675. [Google Scholar] [CrossRef]
- Barbarini, A.; Maggi, R.; Mazzacani, A.; Mori, G.; Sartori, G.; Sartorio, R. Cycloaddition of CO2 to epoxides over both homogeneous and silica-supported guanidine catalysts. Tetrahedron Lett. 2003, 44, 2931–2934. [Google Scholar] [CrossRef]
- Zhou, Y.; Hu, S.; Ma, X.; Liang, S.; Jiang, T.; Han, B. Synthesis of cyclic carbonates from carbon dioxide and epoxides over betaine-based catalysts. J. Mol. Catal. A Chem. 2008, 284, 52–57. [Google Scholar] [CrossRef]
- Alvaro, M.; Baleizao, C.; Das, D.; Carbonell, E.; García, H. CO2 fixation using recoverable chromium salen catalysts: Use of ionic liquids as cosolvent or high-surface-area silicates as supports. J. Catal. 2004, 228, 254–258. [Google Scholar] [CrossRef]
- Noh, J.; Chang, J.-S.; Park, J.-N.; Lee, K.Y.; Park, S.-E. CO2 utilization for the formation of styrene from ethylbenzene over zirconia-supported iron oxide catalysts. Appl. Organomet. Chem. 2000, 14, 815–818. [Google Scholar] [CrossRef]
- Song, C. Global challenges and strategies for control, conversion and utilization of CO2 for sustainable development involving energy, catalysis, adsorption and chemical processing. Catal. Today 2006, 115, 2–32. [Google Scholar] [CrossRef]
- Hutchings, G. Catalyst Synthesis Using Supercritical Carbon Dioxide: A Green Route to High Activity Materials. Top. Catal. 2009, 52, 982–987. [Google Scholar] [CrossRef]
- Peters, M.; Köhler, B.; Kuckshinrichs, W.; Leitner, W.; Markewitz, P.; Müller, T.E. Chemical Technologies for Exploiting and Recycling Carbon Dioxide into the Value Chain. ChemSusChem 2011, 4, 1216–1240. [Google Scholar] [CrossRef]
- Koohestanian, E.; Sadeghi, J.; Mohebbi-Kalhori, D.; Shahraki, F.; Samimi, A. A novel process for CO2 capture from the flue gases to produce urea and ammonia. Energy 2018, 144, 279–285. [Google Scholar] [CrossRef]
- Huo, Z.; Hu, M.; Zeng, X.; Yun, J.; Jin, F. Catalytic reduction of carbon dioxide into methanol over copper under hydrothermal conditions. Catal. Today 2012, 194, 25–29. [Google Scholar] [CrossRef]
- Iijima, T.; Yamaguchi, T. Efficient regioselective carboxylation of phenol to salicylic acid with supercritical CO2 in the presence of aluminium bromide. J. Mol. Catal. A Chem. 2008, 295, 52–56. [Google Scholar] [CrossRef]
- Leitner, W. Carbon Dioxide as a Raw Material: The Synthesis of Formic Acid and Its Derivatives from CO2. Angew. Chem. Int. Ed. Engl. 1995, 34, 2207–2221. [Google Scholar] [CrossRef]
- Darensbourg, D.J.; Holtcamp, M.W.; Struck, G.E.; Zimmer, M.S.; Niezgoda, S.A.; Rainey, P.; Robertson, J.B.; Draper, J.D.; Reibenspies, J.H. Catalytic Activity of a Series of Zn (II) Phenoxides for the Copolymerization of Epoxides and Carbon Dioxide. J. Am. Chem. Soc. 1998, 121, 107–116. [Google Scholar] [CrossRef]
- Muthuraj, R.; Mekonnen, T. Recent progress in carbon dioxide (CO2) as feedstock for sustainable materials development: Co-polymers and polymer blends. Polymer 2018, 145, 348–373. [Google Scholar] [CrossRef]
- Ye, S.; Wang, S.; Lin, L.; Xiao, M.; Meng, Y. CO2 derived biodegradable polycarbonates: Synthesis, modification and applications. Adv. Ind. Eng. Polym. Res. 2019, 2, 143–160. [Google Scholar] [CrossRef]
- Forest, C.; Chaumont, P.; Cassagnau, P.; Swoboda, B.; Sonntag, P. Polymer nano-foams for insulating applications prepared from CO2 foaming. Prog. Polym. Sci. 2015, 41, 122–145. [Google Scholar] [CrossRef]
- Mazari, S.A.; Hossain, N.; Basirun, W.J.; Mubarak, N.M.; Abro, R.; Sabzoi, N.; Shah, A. An overview of catalytic conversion of CO2 into fuels and chemicals using metallic organic frameworks. Process. Saf. Environ. Prot. 2020. [Google Scholar] [CrossRef]
- Aresta, M.; Dibenedetto, A. Utilisation of CO2 as a chemical feedstock: Opportunities and challenges. Dalton Trans. 2007, 28, 2975–2992. [Google Scholar] [CrossRef]
- Tiwari, D.; Bhunia, H.; Bajpai, P.K. Adsorption of CO2 on KOH activated, N-enriched carbon derived from urea formaldehyde resin: Kinetics, isotherm and thermodynamic studies. Appl. Surf. Sci. 2018, 439, 760–771. [Google Scholar] [CrossRef]
- Yu, J.; Guo, M.; Muhammad, F.; Wang, A.; Zhang, F.; Li, Q.; Zhu, G. One-pot synthesis of highly ordered nitrogen-containing mesoporous carbon with resorcinol–urea–formaldehyde resin for CO2 capture. Carbon 2014, 69, 502–514. [Google Scholar] [CrossRef]
- Sajeeb, A.M.; Babu, C.S.; Arif, M.M. Evaluation of Mechanical Properties of Natural Fiber Reinforced Melamine Urea Formaldehyde (MUF) Resin Composites. Mater. Today: Proc. 2018, 5, 6764–6769. [Google Scholar] [CrossRef]
- Ma, C.-l.; Wang, Z.-r.; Hu, Z.-h.; Wang, Y.-h.; Zhao, Y.; Shi, J. Preparation of submicron monodisperse melamine resin microspheres and nitrogen-doped carbon microspheres derived from them. New Carbon Mater. 2020, 35, 269–285. [Google Scholar] [CrossRef]
- Kosugi, Y.; Imaoka, Y.; Gotoh, F.; Rahim, M.A.; Matsui, Y.; Sakanishi, K. Carboxylations of alkali metal phenoxides with carbon dioxide. Org. Biomol. Chem. 2003, 5, 817–821. [Google Scholar] [CrossRef]
- Tran, P.H.L.; Wang, T.; Yin, W.; Tran, T.T.D.; Nguyen, T.N.G.; Lee, B.-J.; Duan, W. Aspirin-loaded nanoexosomes as cancer therapeutics. Int. J. Pharm. 2019, 572, 118786. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, K.C.; Waterhouse, N.J.; Goldstein, J.C.; Schuler, M.; Green, D.R. Aspirin Induces Apoptosis through Release of Cytochrome c from Mitochondria. Neoplasia 2000, 2, 505–513. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Chiew, Y.C.; Lu, W.-D.; Kawi, S. Solubility of aspirin in supercritical carbon dioxide/alcohol mixtures. Fluid Phase Equilib. 2005, 237, 9–15. [Google Scholar] [CrossRef]
- The Keeling Curve. 2020. Available online: https://www.nationalgeographic.org/encyclopedia/keeling-curve/ (accessed on 20 November 2020).
- Ghosh, A.; Ramidi, P.; Pulla, S.; Sullivan, S.Z.; Collom, S.L.; Gartia, Y.; Munshi, P.; Biris, A.S.; Noll, B.C.; Berry, B.C. Cycloaddition of CO2 to Epoxides Using a Highly Active Co (III) Complex of Tetraamidomacrocyclic Ligand. Catal. Lett. 2010, 137, 1–7. [Google Scholar] [CrossRef]
- Khoshro, H.; Zare, H.R.; Namazian, M.; Jafari, A.A.; Gorji, A. Synthesis of cyclic carbonates through cycloaddition of electrocatalytic activated CO2 to epoxides under mild conditions. Electrochim. Acta 2013, 113, 263–268. [Google Scholar] [CrossRef]
- Dharman, M.M.; Choi, H.-J.; Park, S.-W.; Park, D.-W. Microwave Assisted Synthesis of Cyclic Carbonate Using Homogeneous and Heterogeneous Ionic Liquid Catalysts. Top. Catal. 2010, 53, 462–469. [Google Scholar] [CrossRef]
- Zalomaeva, O.V.; Maksimchuk, N.V.; Chibiryaev, A.M.; Kovalenko, K.A.; Fedin, V.P.; Balzhinimaev, B.S. Synthesis of cyclic carbonates from epoxides or olefins and CO2 catalyzed by metal-organic frameworks and quaternary ammonium salts. J. Energy Chem. 2013, 22, 130–135. [Google Scholar] [CrossRef]
- Jutz, F.; Grunwaldt, J.-D.; Baiker, A. Mn (III)(salen)-catalyzed synthesis of cyclic organic carbonates from propylene and styrene oxide in “supercritical” CO2. J. Mol. Catal. A Chem. 2008, 279, 94–103. [Google Scholar] [CrossRef]
- Aresta, M.; Dibenedetto, A.; Gianfrate, L.; Pastore, C. Nb (V) compounds as epoxides carboxylation catalysts: The role of the solvent. J. Mol. Catal. A Chem. 2003, 204–205, 245–252. [Google Scholar] [CrossRef]
- Sibaouih, A.; Ryan, P.; Leskela, M.; Rieger, B.; Repo, T. Facile synthesis of cyclic carbonates from CO2 and epoxides with cobalt (II)/onium salt based catalysts. Appl. Catal. A Gen. 2009, 365, 194–198. [Google Scholar] [CrossRef]
- Du, Y.; Cai, F.; Kong, D.I.; He, I.N. Organic solvent-free process for the synthesis of propylene carbonate from supercritical carbon dioxide and propylene oxide catalyzed by insoluble ion exchange resins. Green Chem. 2005, 7, 518–523. [Google Scholar] [CrossRef]
- Ulusoy, M.; Cetinkaya, E.; Cetinkaya, B. Conversion of carbon dioxide to cyclic carbonates using diimine Ru (II) complexes as catalysts. Appl. Organomet. Chem. 2009, 23, 68–74. [Google Scholar] [CrossRef]
- Kawanami, H.; Ikushima, Y. Chemical fixation of carbon dioxide to styrene carbonate under supercritical conditions with DMF in the absence of any additional catalysts. Chem. Commun. 2000. [Google Scholar] [CrossRef]
- Jiang, J.-L.; Hua, R. Efficient DMF Catalyzed Coupling of Epoxides with CO2 under Solvent Free Conditions to Afford Cyclic Carbonates. Synth. Commun. 2006, 36, 3141–3148. [Google Scholar] [CrossRef]
- Jing, H.; Nguyen, S.T. SnCl4-organic base: Highly efficient catalyst system for coupling reaction of CO2 and epoxides. J. Mol. Catal. A Chem. 2007, 261, 12–15. [Google Scholar] [CrossRef]
- Wang, Y.; Sun, J.; Xiang, D.; Wang, L.; Sun, J.; Xiao, F.-S. A Facile, Direct Synthesis of Styrene Carbonate from Styrene and CO2 Catalyzed by Au/Fe (OH)3—ZnBr2 /Bu4NBr System. Catal. Lett. 2009, 129, 437–443. [Google Scholar] [CrossRef]
- Xie, Y.; Zhang, Z.; Jiang, T.; He, J.; Han, B.; Wu, T.; Ding, K. CO2 Cycloaddition Reactions Catalyzed by an Ionic Liquid Grafted onto a Highly Cross-Linked Polymer Matrix. Angew. Chem. 2007, 119, 7393–7396. [Google Scholar] [CrossRef]
- He, L.-N.; Wang, J.-Q.; Wang, J.-L. Carbon dioxide chemistry: Examples and challenges in chemical utilization of carbon dioxide. Pure Appl. Chem. 2009, 81, 2069–2080. [Google Scholar] [CrossRef]
- Liang, S.; Liu, H.; Jiang, T.; Song, J.; Yang, G.; Han, B. Highly efficient synthesis of cyclic carbonates from CO2 and epoxides over Cellulose/KI. Chem. Commun. 2011, 47, 2131. [Google Scholar]
- Xiang, D.; Liu, X.; Sun, J.; Xiao, F.-S.; Sun, J. A novel route for synthesis of styrene carbonate using styrene and CO2 as substrates over basic resin R201 supported Au catalyst. Catal. Today 2009, 148, 383–388. [Google Scholar] [CrossRef]
- Jutz, F.; Andanson, J.-M.; Baiker, A. Ionic Liquids and Dense Carbon Dioxide: A Beneficial Biphasic System for Catalysis. Chem. Rev. 2010, 111, 322–353. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.-Z.; Zhao, Y.-N.; He, L.-N. CO2 chemistry: Task-specific ionic liquids for CO2 capture/activation and subsequent conversion. RSC Adv. 2011, 1, 545–567. [Google Scholar] [CrossRef]
- Cheng, W.; Xiao, B.; Sun, J.; Dong, K.; Zhang, P.; Zhang, S.; Ng, F.T.T. Effect of hydrogen bond of hydroxyl-functionalized ammonium ionic liquids on cycloaddition of CO2. Tetrahedron Lett. 2015, 56, 1416–1419. [Google Scholar] [CrossRef]
- Guglielmero, L.; Mezzetta, A.; Pomelli, C.S.; Chiappe, C.; Guazzelli, L. Evaluation of the effect of the dicationic ionic liquid structure on the cycloaddition of CO2 to epoxides. J. CO2 Util. 2019, 34, 437–445. [Google Scholar] [CrossRef]
- Ji, L.; Luo, Z.; Zhang, Y.; Wang, R.; Ji, Y.; Xia, F.; Gao, G. Imidazolium ionic liquids/organic bases: Efficient intermolecular synergistic catalysts for the cycloaddition of CO2 and epoxides under atmospheric pressure. Mol. Catal. 2018, 446, 124–130. [Google Scholar] [CrossRef]
- Liu, D.; Li, G.; Liu, H. Functionalized MIL-101 with imidazolium-based ionic liquids for the cycloaddition of CO2 and epoxides under mild condition. Appl. Surf. Sci. 2018, 428, 218–225. [Google Scholar] [CrossRef]
- Liu, M.; Liang, L.; Liang, T.; Lin, X.; Shi, L.; Wang, F.; Sun, J. Cycloaddition of CO2 and epoxides catalyzed by dicationic ionic liquids mediated metal halide: Influence of the dication on catalytic activity. J. Mol. Catal. A Chem. 2015, 408, 242–249. [Google Scholar] [CrossRef]
- Liu, M.; Zhao, P.; Gu, Y.; Ping, R.; Gao, J.; Liu, F. Squaramide functionalized ionic liquids with well-designed structures: Highly-active and recyclable catalyst platform for promoting cycloaddition of CO2 to epoxides. J. CO2 Util. 2020, 37, 39–44. [Google Scholar] [CrossRef]
- Mao, P.; Dai, W.; Yang, W.; Luo, S.; Zhang, Y.; Mao, J.; Luo, X.; Zou, J. Polymer nanoparticles grafted zinc-containing ionic liquids: A highly efficient and recyclable catalyst for cooperative cycloaddition of CO2 with epoxides. J. CO2 Util. 2018, 28, 96–106. [Google Scholar] [CrossRef]
- Muniandy, L.; Adam, F.; Rahman, N.R.A.; Ng, E.-P. Highly selective synthesis of cyclic carbonates via solvent free cycloaddition of CO2 and epoxides using ionic liquid grafted on rice husk derived MCM-41. Inorg. Chem. Commun. 2019, 104, 1–7. [Google Scholar] [CrossRef]
- Shang, Y.; Gong, Q.; Zheng, M.; Zhang, H.; Zhou, X. An efficient morpholinium ionic liquid based catalyst system for cycloaddition of CO2 and epoxides under mild conditions. J. Mol. Liq. 2019, 283, 235–241. [Google Scholar] [CrossRef]
- Tharun, J.; Kathalikkattil, A.C.; Roshan, R.; Kang, D.-H.; Woo, H.-C.; Park, D.-W. Microwave-assisted, rapid cycloaddition of allyl glycidyl ether and CO2 by employing pyridinium-based ionic liquid catalysts. Catal. Commun. 2014, 54, 31–34. [Google Scholar] [CrossRef]
- Wang, T.; Zheng, D.; Ma, Y.; Guo, J.; He, Z.; Ma, B.; Liu, L.; Ren, T.; Wang, L.; Zhang, J. Benzyl substituted imidazolium ionic liquids as efficient solvent-free catalysts for the cycloaddition of CO2 with epoxides: Experimental and Theoretic study. J. CO2 Util. 2017, 22, 44–52. [Google Scholar] [CrossRef]
- Wang, T.; Zhu, X.; Mao, L.; Liu, Y.; Ren, T.; Wang, L.; Zhang, J. Synergistic cooperation of bi-active hydrogen atoms in protic carboxyl imidazolium ionic liquids to push cycloaddition of CO2 under benign conditions. J. Mol. Liq. 2019, 296, 111936. [Google Scholar] [CrossRef]
- Wu, X.; Wang, M.; Xie, Y.; Chen, C.; Li, K.; Yuan, M.; Zhao, X.; Hou, Z. Carboxymethyl cellulose supported ionic liquid as a heterogeneous catalyst for the cycloaddition of CO2 to cyclic carbonate. Appl. Catal. A Gen. 2016, 519, 146–154. [Google Scholar] [CrossRef]
- Yang, C.; Liu, M.; Zhang, J.; Wang, X.; Jiang, Y.; Sun, J. Facile synthesis of DBU-based ionic liquids cooperated with ZnI2 as catalysts for efficient cycloaddition of CO2 to epoxides under mild and solvent-free conditions. Mol. Catal. 2018, 450, 39–45. [Google Scholar] [CrossRef]
- Zhang, Y.; Tan, Z.; Liu, B.; Mao, D.; Xiong, C. Coconut shell activated carbon tethered ionic liquids for continuous cycloaddition of CO2 to epichlorohydrin in packed bed reactor. Catal. Commun. 2015, 68, 73–76. [Google Scholar] [CrossRef]
- Zheng, D.; Wang, L.; Jiang, J.; Liu, F.; Zhang, J. Effect of cluster of protic pyrazolium ionic liquids or epoxides on the cycloaddition of CO2. J. Mol. Liq. 2019, 295, 111652. [Google Scholar] [CrossRef]
- Zhu, J.; Wang, S.; Gu, Y.; Xue, B.; Li, Y. A new and efficient method of graphene oxide immobilized with ionic liquids: Promoted catalytic activity for CO2 cycloaddition. Mater. Chem. Phys. 2018, 208, 68–76. [Google Scholar] [CrossRef]
- Bhanage, B.M.; Fujita, S.-i.; Ikushima, Y.; Arai, M. Synthesis of dimethyl carbonate and glycols from carbon dioxide, epoxides, and methanol using heterogeneous basic metal oxide catalysts with high activity and selectivity. Appl. Catal. A Gen. 2001, 219, 259–266. [Google Scholar] [CrossRef]
- Yano, T.; Matsui, H.; Koike, T.; Ishiguro, H.; Fujihara, H.; Yoshihara, M.; Maeshima, T. Magnesium oxide-catalysed reaction of carbon dioxide with an epoxide with retention of stereochemistry. Chem. Commun. 1997, 1129–1130. [Google Scholar] [CrossRef]
- Yamaguchi, K.; Ebitani, K.; Yoshida, T.; Yoshida, H.; Kaneda, K. Mg-Al Mixed Oxides as Highly Active Acid−Base Catalysts for Cycloaddition of Carbon Dioxide to Epoxides. J. Am. Chem. Soc. 1999, 121, 4526–4527. [Google Scholar] [CrossRef]
- Srivastava, R.; Srinivas, D.; Ratnasamy, P. Sites for CO2 activation over amine-functionalized mesoporous Ti(Al)-SBA-15 catalysts. Microporous Mesoporous Mater. 2006, 90, 314–326. [Google Scholar] [CrossRef]
- Lu, X.-B.; Wang, H.; He, R. Aluminum phthalocyanine complex covalently bonded to MCM-41 silica as heterogeneous catalyst for the synthesis of cyclic carbonates. J. Mol. Catal. A Chem. 2002, 186, 33–42. [Google Scholar] [CrossRef]
- Srivastava, R.; Srinivas, D.; Ratnasamy, P. Syntheses of polycarbonate and polyurethane precursors utilizing CO2 over highly effcient, solid as-synthesized MCM-41 catalyst. Tetrahedron Lett. 2006, 47, 4213–4217. [Google Scholar] [CrossRef]
- Qiao, K.; Ono, F.; Bao, Q.; Tomida, D.; Yokoyama, C. Efficient synthesis of styrene carbonate from CO2 and styrene oxide using zinc catalysts immobilized on soluble imidazolium–styrene copolymers. J. Mol. Catal. A Chem. 2009, 303, 30–34. [Google Scholar] [CrossRef]
- Jing, H.; Tao, C.; Lili, J.; Mei, W.; Wenyuan, Q. Ruthenium Salen/phenyltrimethylammonium tribromide catalyzed coupling reaction of carbon dioxide and epoxides. Catal. Commun. 2007, 8, 1630–1634. [Google Scholar] [CrossRef]
- Dai, W.-L.; Chen, L.; Yin, S.-F.; Luo, S.-L.; Au, C.-T. 3-(2-Hydroxyl-Ethyl)-1-Propylimidazolium Bromide Immobilized on SBA-15 as Efficient Catalyst for the Synthesis of Cyclic Carbonates via the Coupling of Carbon Dioxide with Epoxides. Catal. Lett. 2010, 135, 295–304. [Google Scholar] [CrossRef]
- Jagtap, S.; Bhanushali, M.; Panda, A.; Bhanage, B. Synthesis of cyclic carbonates from carbon dioxide and epoxides using alkali metal halide supported liquid phase catalyst. Catal. Lett. 2006, 112, 51–55. [Google Scholar] [CrossRef]
- Paddock, R.L.; Hiyama, Y.; McKay, J.M.; Nguyen, S.T. Co (III) porphyrin/DMAP: An efficient catalyst system for the synthesis of cyclic carbonates from CO2 and epoxides. Tetrahedron Lett. 2004, 45, 2023–2026. [Google Scholar] [CrossRef]
- Bai, D.; Wang, Q.; Song, Y.; Li, B.; Jing, H. Synthesis of cyclic carbonate from epoxide and CO2 catalyzed by magnetic nanoparticle-supported porphyrin. Catal. Commun. 2011, 12, 684–688. [Google Scholar] [CrossRef]
- Bai, D.; Wang, X.; Song, Y.; Li, B.; Zhang, L.; Yan, P.; Jing, H. Bifunctional Metalloporphyrins-Catalyzed Coupling Reaction of Epoxides and CO2 to Cyclic Carbonates. Chin. J. Catal. 2010, 31, 176–180. [Google Scholar]
- Jin, L.; Jing, H.; Chang, T.; Bu, X.; Wang, L.; Liu, Z. Metal porphyrin/phenyltrimethylammonium tribromide: High efficient catalysts for coupling reaction of CO2 and epoxides. J. Mol. Catal. A Chem. 2007, 261, 262–266. [Google Scholar] [CrossRef]
- Srivastava, R.; Srinivas, D.; Ratnasamy, P. Zeolite-based organic–inorganic hybrid catalysts for phosgene-free and solvent-free synthesis of cyclic carbonates and carbamates at mild conditions utilizing CO2. Appl. Catal. A Gen. 289 2005, 289, 128–134. [Google Scholar] [CrossRef]
- Jing-Xian, C.; Bi, J.; Wei-Li, D.; Sen-Lin, D.; Liu-Ren, C.; Zong-Jie, C.; Sheng-Lian, L.; Xu-Biao, L.; Xin-Man, T.; Chak-Tong, A. Catalytic fixation of CO2 to cyclic carbonates over biopolymer chitosan-grafted quarternary phosphonium ionic liquid as a recylable catalyst. Appl. Catal. A Gen. 2014, 484, 26–32. [Google Scholar] [CrossRef]
- Wei-Li, D.; Bi, J.; Sheng-Lian, L.; Xu-Biao, L.; Xin-Man, T.; Chak-Tong, A. Functionalized phosphonium-based ionic liquids as efficient catalysts for the synthesis of cyclic carbonate from expoxides and carbon dioxide. Appl. Catal. A Gen. 2014, 470, 183–188. [Google Scholar] [CrossRef]
- Yang, Z.; Sun, J.; Liu, X.; Su, Q.; Liu, Y.; Li, Q.; Zhang, S. Nano-sized polydopamine-based biomimetic catalyst for the efficient synthesis of cyclic carbonates. Tetrahedron Lett. 2014, 55, 3239–3243. [Google Scholar] [CrossRef]
- Wei-Li, D.; Bi, J.; Sheng-Lian, L.; Xu-Biao, L.; Xin-Man, T.; Chak-Tong, A. Polymer grafted with asymmetrical dication ionic liquid as efficient and reusable catalysts for the synthesis of cyclic carbonates from CO2 and expoxides. Catal. Today 2014, 233, 92–99. [Google Scholar] [CrossRef]
- Cheng, W.; Chen, X.; Sun, J.; Wang, J.; Zhang, S. SBA-15 supported triazolium-based ionic liquids as highly efficient and recyclable catalysts for fixation of CO2 with epoxides. Catal. Today 2013, 200, 117–124. [Google Scholar] [CrossRef]
- Sankar, M.; Ajithkumar, T.G.; Sankar, G.; Manikandan, P. Supported imidazole as heterogeneous catalyst for the synthesis of cyclic carbonates from epoxides and CO2. Catal. Commun. 2015, 59, 201–205. [Google Scholar] [CrossRef]
- Xie, Y.; Yang, R.-X.; Huang, N.-Y.; Luo, H.-J.; Deng, W.-Q. Efficient fixation of CO2 at mild conditions by a Cr-conjugated microporous polymer. J. Energy Chem. 2014, 23, 22–28. [Google Scholar] [CrossRef]
- Song, B.; Guo, L.; Zhang, R.; Zhao, X.; Gan, H.; Chen, C.; Chen, J.; Zhu, W.; Hou, Z. The polymeric quaternary ammonium salt supported on silica gel as catalyst for the efficient synthesis of cyclic carbonate. J. CO2 Util. 2014, 6, 62–68. [Google Scholar] [CrossRef]
- Motokura, K.; Itagaki, S.; Iwasawa, Y.; Miyaji, A.; Baba, T. Zinc-Accelerated Cycloaddition of Carbon Dioxide to Styrene Oxide Catalyzed by Pyrrolidinopyridinium Iodides. Top. Catal. 2014, 57, 953–959. [Google Scholar] [CrossRef]
- Baj, S.; Krawczyk, T.; Jasiak, K.; Siewniak, A.; Pawlyta, M. Catalytic coupling of epoxides and CO2 to cyclic carbonates by carbon nanotube-supported quaternary ammonium salts. Appl. Catal. A Gen. 2014, 488, 96–102. [Google Scholar] [CrossRef]
- Dai, W.-L.; Jin, B.; Luo, S.-L.; Yin, S.-F.; Luo, X.-B.; Au, C.-T. Cross-linked polymer grafted with functionalized ionic liquid as reusable and efficient catalyst for the cycloaddition of carbon dioxide to epoxides. J. CO2 Util. 2013, 3–4, 7–13. [Google Scholar] [CrossRef]
- Lee, S.-D.; Kim, B.-M.; Kim, D.-W.; Kim, M.-I.; Roshan, K.R.; Kim, M.-K.; Won, Y.-S.; Park, D.-W. Synthesis of cyclic carbonate from carbon dioxide and epoxides with polystyrene-supported quaternized ammonium salt catalysts. Appl. Catal. A Gen. 2014, 486, 69–76. [Google Scholar] [CrossRef]
- Siewniak, A.; Jasiak, K.; Baj, S. An efficient method for the synthesis of cyclic carbonates from CO2 and epoxides using an effective two-component catalyst system: Polymer-supported quaternary onium salts and aqueous solutions of metal salts. Appl. Catal. A Gen. 2014, 482, 266–274. [Google Scholar] [CrossRef]
- Zhong, S.; Liang, L.; Liu, B.; Sun, J. ZnBr2/DMF as simple and highly active Lewis acid–base catalysts for the cycloaddition of CO2 to propylene oxide. J. CO2 Util. 2014, 6, 75–79. [Google Scholar] [CrossRef]
- Bu, Z.; Qin, G.; Cao, S. A ruthenium complex exhibiting high catalytic efficiency for the formation of propylene carbonate from carbon dioxide. J. Mol. Catal. A Chem. 2007, 277, 35–39. [Google Scholar] [CrossRef]
- Ramalingam, R.J.; Appaturi, J.N.; Pulingam, T.; Ibrahim, S.N.; Al-Lohedan, H.A. Synthesis, characterization and catalytic activity of ionic liquid mimic halides modified MCM-41 for solvent free synthesis of phenyl glycidyl carbonate. Mater. Chem. Phys. 2019, 233, 79–88. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).