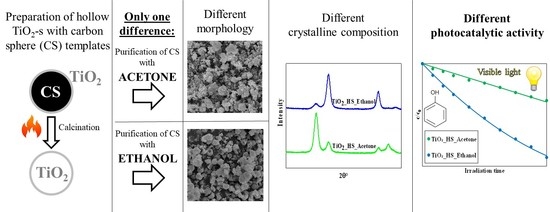
Unexpected Link between the Template Purification Solvent and the Structure of Titanium Dioxide Hollow Spheres
Abstract
1. Introduction
2. Results and Discussion
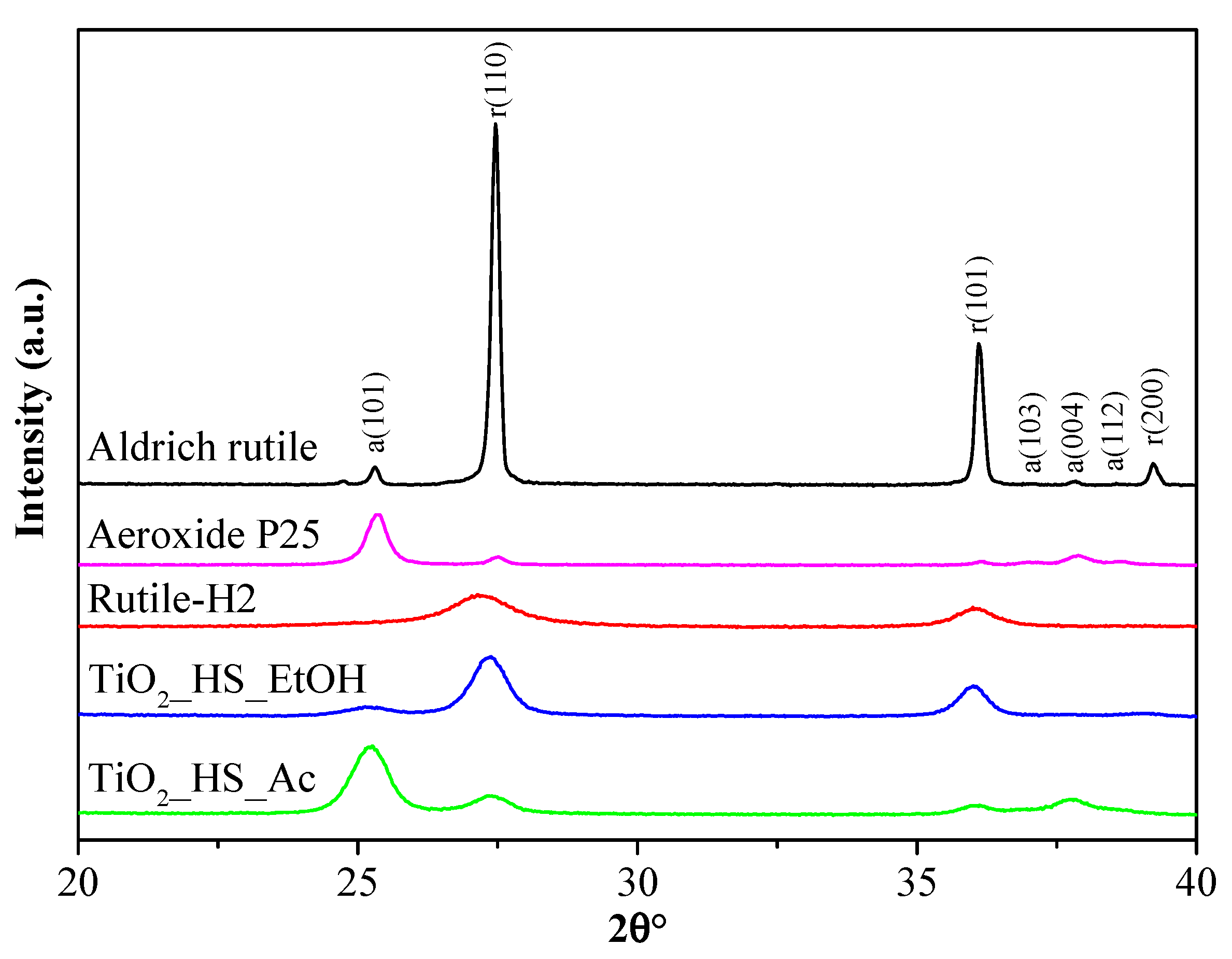
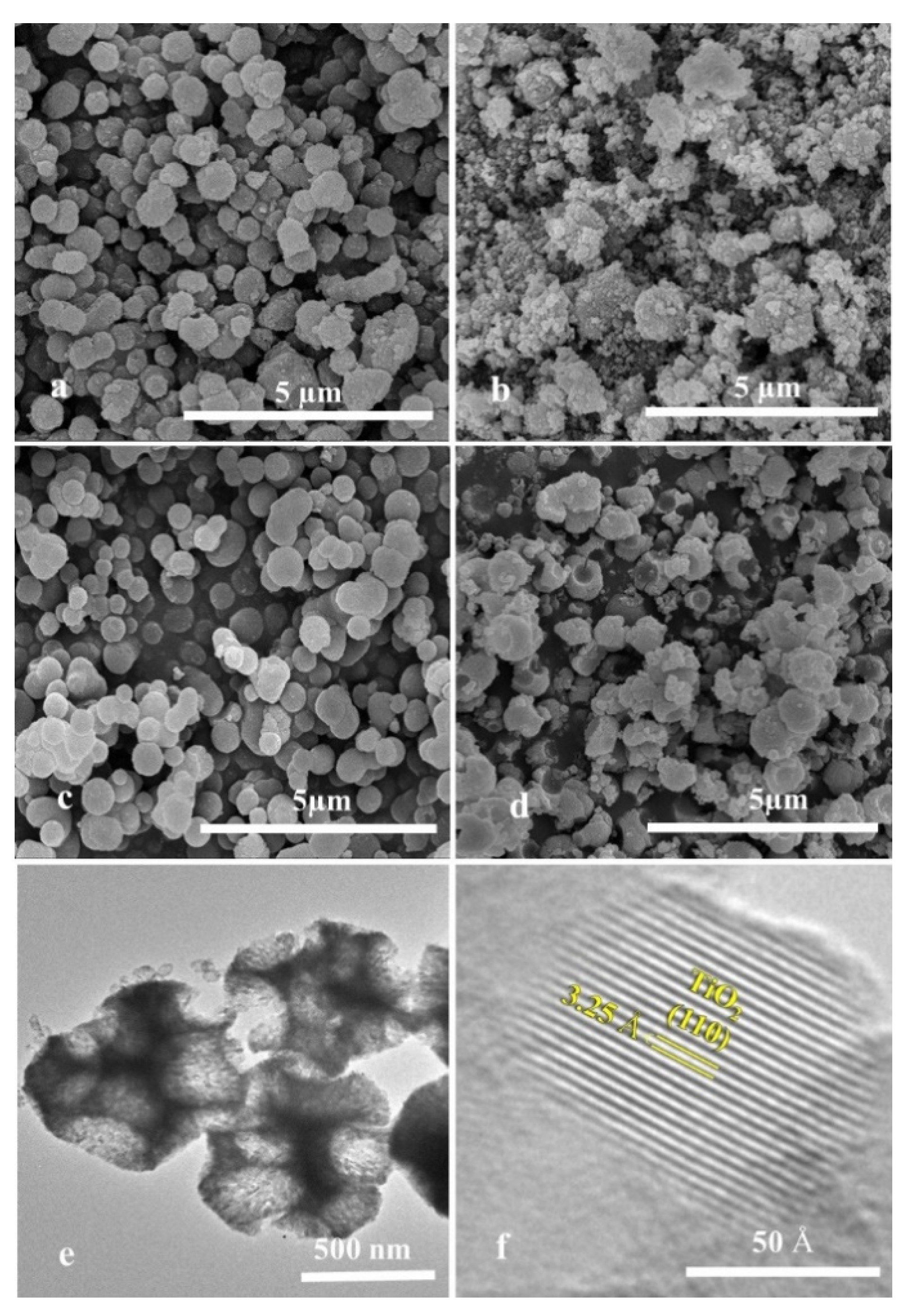
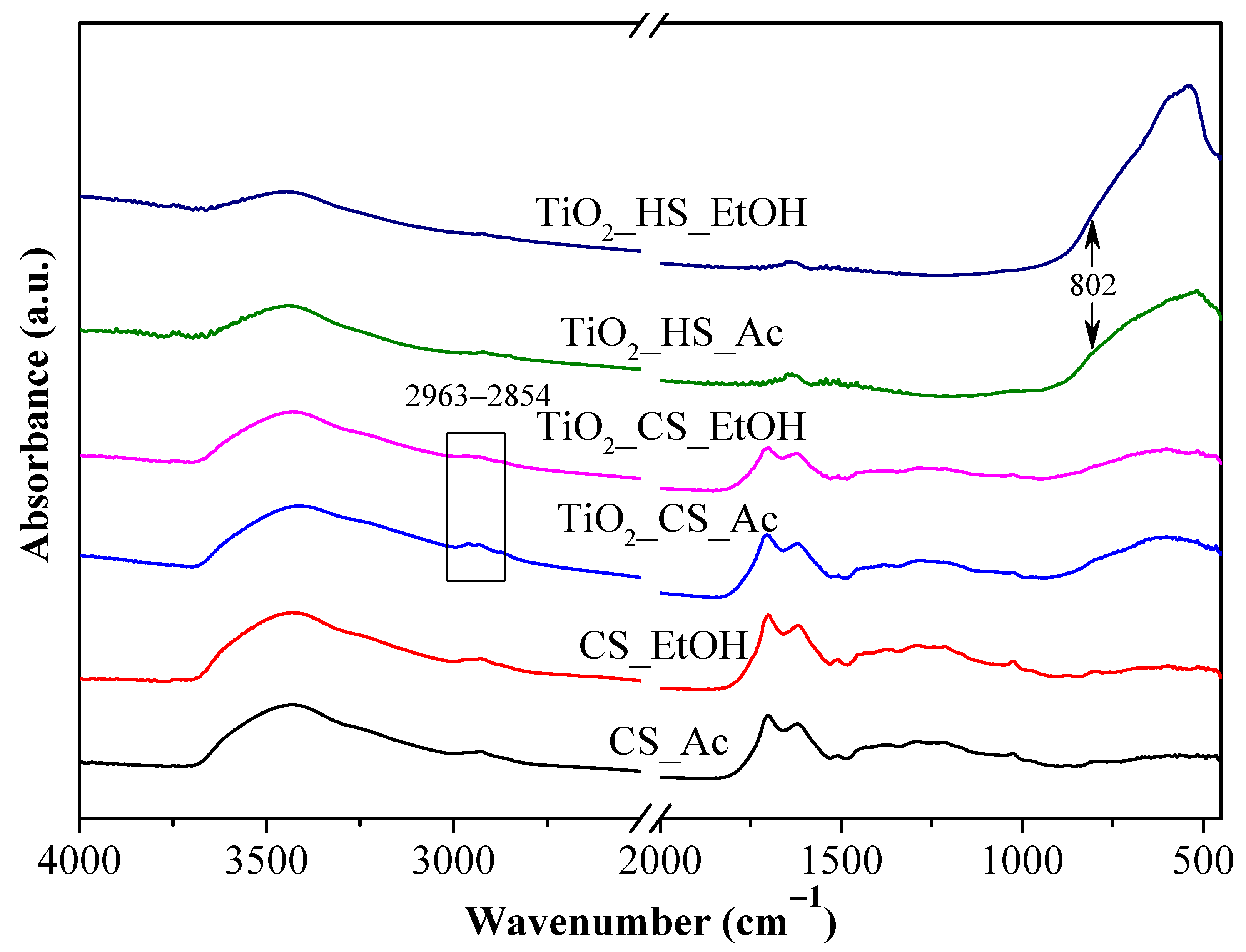
2.1. Textural Characterization
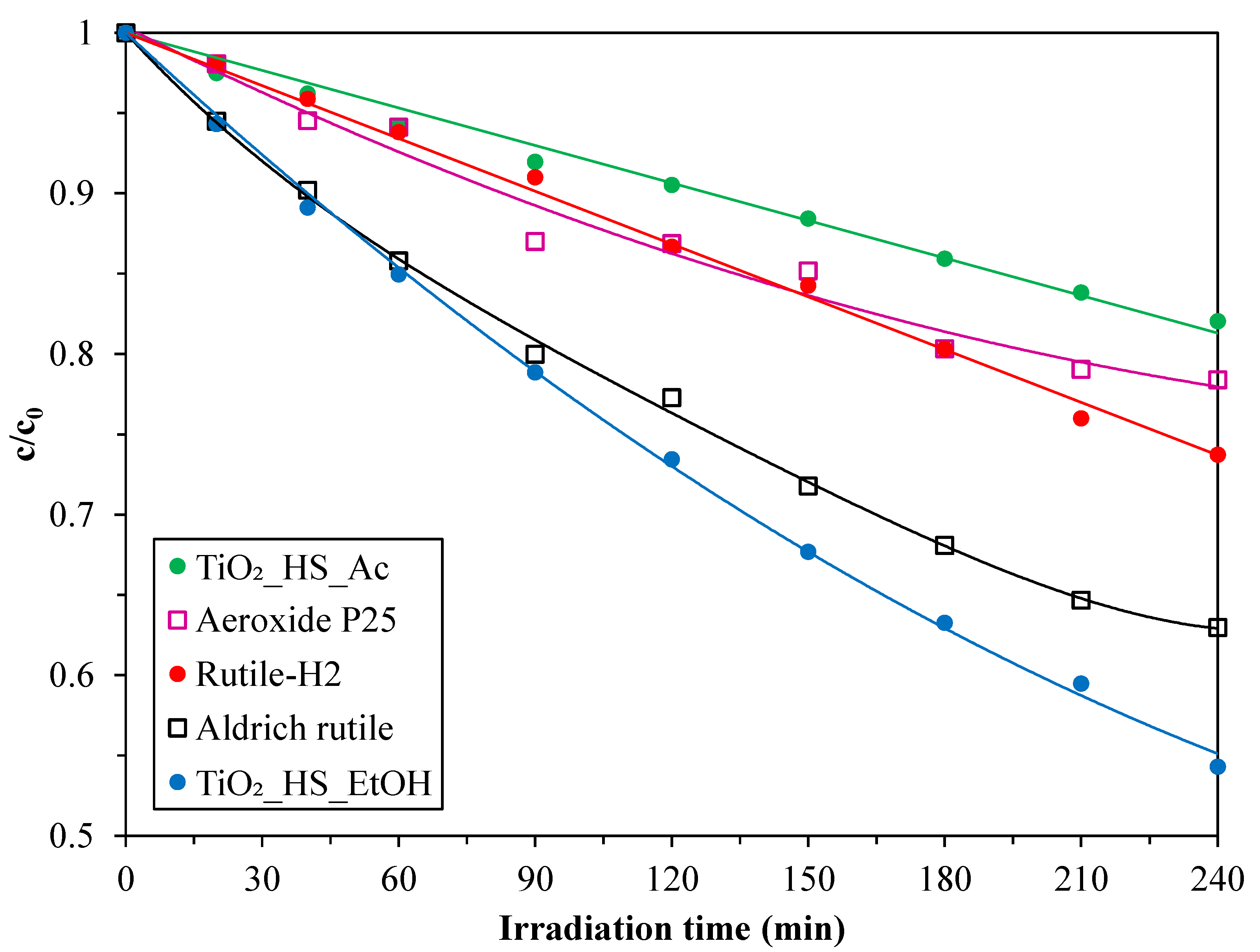
2.2. Photocatalytic Activity
3. Materials and Methods
3.1. Materials
3.2. Synthesis
3.3. Characterization Methods and Instrumentation
3.4. Photocatalytic Activity Experiments
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Herrmann, J.-M.; Matos, J.; Disdier, J.; Guillard, C.; Laine, J.; Malato, S.; Blanco, J. Solar photocatalytic degradation of 4-chlorophenol using the synergistic effect between titania and activated carbon in aqueous suspension. Catal. Today 1999, 54, 255–265. [Google Scholar] [CrossRef]
- Antoniou, M.G.; Dionysiou, D.D. Application of immobilized titanium dioxide photocatalysts for the degradation of creatinine and phenol, model organic contaminants found in NASA’s spacecrafts wastewater streams. Catal. Today 2007, 124, 215–223. [Google Scholar] [CrossRef]
- Veréb, G.; Manczinger, L.; Bozsó, G.; Sienkiewicz, A.; Forró, L.; Mogyorósi, K.; Hernádi, K.; Dombi, A. Comparison of the photocatalytic efficiencies of bare and doped rutile and anatase TiO2 photocatalysts under visible light for phenol degradation and E. coli inactivation. Appl. Catal. B Environ. 2013, 129, 566–574. [Google Scholar] [CrossRef]
- Qamar, M.; Merzougui, B.; Anjum, D.; Hakeem, A.S.; Yamani, Z.H.; Bahnemann, D. Synthesis and photocatalytic activity of mesoporous nanocrystalline Fe-doped titanium dioxide. Catal. Today 2014, 230, 158–165. [Google Scholar] [CrossRef]
- Rózsa, G.; Kozmér, Z.; Alapi, T.; Schrantz, K.; Takács, E.; Wojnárovits, L. Transformation of Z-thiacloprid by three advanced oxidation processes: Kinetics, intermediates and the role of reactive species. Catal. Today 2017, 284, 187–194. [Google Scholar] [CrossRef]
- Alalm, M.G.; Tawfik, A.; Ookawara, S. Enhancement of photocatalytic activity of TiO2 by immobilization on activated carbon for degradation of pharmaceuticals. J. Environ. Chem. Eng. 2016, 4, 1929–1937. [Google Scholar] [CrossRef]
- Vidal, A.; Díaz, A.I.; El Hraiki, A.; Romero, M.; Muguruza, I.; Senhaji, F.; González, J. Solar photocatalysis for detoxification and disinfection of contaminated water: Pilot plant studies. Catal. Today 1999, 54, 283–290. [Google Scholar] [CrossRef]
- Malato, S.; Maldonado, M.I.; Fernández-Ibáñez, P.; Oller, I.; Polo, I.; Sánchez-Moreno, R. Decontamination and disinfection of water by solar photocatalysis: The pilot plants of the Plataforma Solar de Almeria. Mater. Sci. Semicond. Process. 2016, 42, 15–23. [Google Scholar] [CrossRef]
- Pelaez, M.; Nolan, N.T.; Pillai, S.C.; Seery, M.K.; Falaras, P.; Kontos, A.G.; Dunlop, P.S.M.; Hamilton, J.W.J.; Byrne, J.A.; O’Shea, K.; et al. A review on the visible light active titanium dioxide photocatalysts for environmental applications. Appl. Catal. B Environ. 2012, 125, 331–349. [Google Scholar] [CrossRef]
- Xiang, L.; Zhao, X. Wet-Chemical Preparation of TiO2-Based Composites with Different Morphologies and Photocatalytic Properties. Nanomaterials 2017, 7, 310. [Google Scholar] [CrossRef]
- Fernández-Ibáñez, P.; Polo-López, M.I.; Malato, S.; Wadhwa, S.; Hamilton, J.W.J.; Dunlop, P.S.M.; D’Sa, R.; Magee, E.; O’Shea, K.; Dionysiou, D.D.; et al. Solar photocatalytic disinfection of water using titanium dioxide graphene composites. Chem. Eng. J. 2015, 261, 36–44. [Google Scholar] [CrossRef]
- Balayeva, N.O.; Fleisch, M.; Bahnemann, D.W. Surface-grafted WO3/TiO2 photocatalysts: Enhanced visible-light activity towards indoor air purification. Catal. Today 2018, 313, 63–71. [Google Scholar] [CrossRef]
- Bao, Y.; Guo, R.; Gao, M.; Kang, Q.; Ma, J. Morphology control of 3D hierarchical urchin-like hollow SiO2@TiO2 spheres for photocatalytic degradation: Influence of calcination temperature. J. Alloys Compd. 2021, 853, 157202. [Google Scholar] [CrossRef]
- Ibhadon, A.; Fitzpatrick, P. Heterogeneous Photocatalysis: Recent Advances and Applications. Catalysts 2013, 3, 189–218. [Google Scholar] [CrossRef]
- Li, H.; Bian, Z.; Zhu, J.; Zhang, D.; Li, G.; Huo, Y.; Li, H.; Lu, Y. Mesoporous titania spheres with tunable chamber stucture and enhanced photocatalytic activity. J. Am. Chem. Soc. 2007, 129, 8406–8407. [Google Scholar] [CrossRef]
- Lyu, J.; Zhou, L.; Shao, J.; Zhou, Z.; Gao, J.; Dong, Y.; Wang, Z.; Li, J. TiO2 hollow heterophase junction with enhanced pollutant adsorption, light harvesting, and charge separation for photocatalytic degradation of volatile organic compounds. Chem. Eng. J. 2020, 391, 123602. [Google Scholar] [CrossRef]
- Ao, Y.; Xu, J.; Fu, D.; Yuan, C. A simple method for the preparation of titania hollow sphere. Catal. Commun. 2008, 9, 2574–2577. [Google Scholar] [CrossRef]
- Zheng, M.; Cao, J.; Chang, X.; Wang, J.; Liu, J.; Ma, X. Preparation of oxide hollow spheres by colloidal carbon spheres. Mater. Lett. 2006, 60, 2991–2993. [Google Scholar] [CrossRef]
- Sun, X.; Li, Y. Colloidal carbon spheres and their core/shell structures with noble-metal nanoparticles. Angew. Chem. Int. Ed. Engl. 2004, 43, 597–601. [Google Scholar] [CrossRef]
- Mahyar, A.; Amani-Ghadim, A.R. Influence of solvent type on the characteristics and photocatalytic activity of TiO2 nanoparticles prepared by the sol–gel method. Micro Nano Lett. 2011, 6, 244. [Google Scholar] [CrossRef]
- Gyulavari, T.; Vereb, G.; Pap, Z.; Reti, B.; Baan, K.; Todea, M.; Magyari, K.; Szilagyi, I.M.; Hernadi, K. Utilization of Carbon Nanospheres in Photocatalyst Production: From Composites to Highly Active Hollow Structures. Materials 2019, 12, 2537. [Google Scholar] [CrossRef] [PubMed]
- Gyulavári, T.; Veréb, G.; Pap, Z.; Dombi, A.; Hernádi, K. Associating low crystallinity with peroxo groups for enhanced visible light active photocatalysts. Catal. Today 2018, 313, 231–238. [Google Scholar] [CrossRef]
- Noh, J.; Yi, M.; Hwang, S.; Im, K.M.; Yu, T.; Kim, J. A facile synthesis of rutile-rich titanium oxide nanoparticles using reverse micelle method and their photocatalytic applications. J. Ind. Eng. Chem. 2016, 33, 369–373. [Google Scholar] [CrossRef]
- Orlikowski, J.; Tryba, B.; Ziebro, J.; Morawski, A.W.; Przepiórski, J. A new method for preparation of rutile phase titania photoactive under visible light. Catal. Commun. 2012, 24, 5–10. [Google Scholar] [CrossRef]
- Ye, X.; Zheng, C.; Ma, L.; Huang, X. Microemulsion-assisted hydrothermal preparation and infrared radiation property of TiO2 nanomaterials with tunable morphologies and crystal form. Mater. Sci. Semicond. Process. 2015, 31, 295–301. [Google Scholar] [CrossRef]
- Busani, T.; Devine, R.A.B. Dielectric and infrared properties of TiO2 films containing anatase and rutile. Semicond. Sci. Technol. 2005, 20, 870–875. [Google Scholar] [CrossRef]
- Talari, A.C.S.; Martinez, M.A.G.; Movasaghi, Z.; Rehman, S.; Rehman, I.U. Advances in Fourier transform infrared (FTIR) spectroscopy of biological tissues. Appl. Spectrosc. Rev. 2016, 52, 456–506. [Google Scholar] [CrossRef]
- Etacheri, V.; Michlits, G.; Seery, M.K.; Hinder, S.J.; Pillai, S.C. A highly efficient TiO2−xCx nano-heterojunction photocatalyst for visible light induced antibacterial applications. ACS Appl. Mater. Interfaces 2013, 5, 1663–1672. [Google Scholar] [CrossRef]
- Shao, P.; Tian, J.; Zhao, Z.; Shi, W.; Gao, S.; Cui, F. Amorphous TiO2 doped with carbon for visible light photodegradation of rhodamine B and 4-chlorophenol. Appl. Surf. Sci. 2015, 324, 35–43. [Google Scholar] [CrossRef]
- Sakthivel, S.; Kisch, H. Daylight photocatalysis by carbon-modified titanium dioxide. Angew. Chem. Int. Ed. Engl. 2003, 42, 4908–4911. [Google Scholar] [CrossRef]
- Matthews, A. The crystallization of anatase and rutile from amorphous titanium dioxide under hydrothermal condition. Am. Mineral. 1976, 61, 419–424. [Google Scholar]
- Buxton, G.V.; Greenstock, C.L.; Helman, W.P.; Ross, A.B. Critical Review of rate constants for reactions of hydrated electrons, hydrogen atoms and hydroxyl radicals (OH/O− in Aqueous Solution. J. Phys. Chem. Ref. Data 1988, 17, 513–886. [Google Scholar] [CrossRef]
- Merouani, S.; Hamdaoui, O.; Saoudi, F.; Chiha, M.; Petrier, C. Influence of bicarbonate and carbonate ions on sonochemical degradation of Rhodamine B in aqueous phase. J. Hazard. Mater. 2010, 175, 593–599. [Google Scholar] [CrossRef] [PubMed]
- Réti, B.; Kiss, G.I.; Gyulavári, T.; Baan, K.; Magyari, K.; Hernadi, K. Carbon sphere templates for TiO2 hollow structures: Preparation, characterization and photocatalytic activity. Catal. Today 2017, 284, 160–168. [Google Scholar] [CrossRef]
- Gyulavári, T.; Pap, Z.; Kovács, G.; Baia, L.; Todea, M.; Hernádi, K.; Veréb, G. Peroxo group enhanced nanorutile as visible light active photocatalyst. Catal. Today 2017, 284, 129–136. [Google Scholar] [CrossRef]
| Titanium-Dioxide | Anatase | Rutile | ||||
|---|---|---|---|---|---|---|
| wt% | Particle Size (nm) | wt% | Particle Size (nm) | Specific Surface Area (m2·g−1) | r0,phenol (10−9 M·s−1) | |
| TiO2_HS_Ac | 79 | 12.3 | 21 | 11.7 | 51 | 1.2 |
| TiO2_HS_EtOH | 14 | 10.4 | 86 | 13.1 | 30 | 3.9 |
| Rutile-H2 | <1 | - | >99 | 7.0 | 237 | 1.9 |
| Aeroxide P25 | 90 | 25.4 | 10 | 40.0 | 49 | 1.6 |
| Aldrich rutile | 4 | 315.0 | 96 | 315.0 | 3 | 3.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gyulavári, T.; Kovács, K.; Magyari, K.; Baán, K.; Szabó, A.; Veréb, G.; Pap, Z.; Hernadi, K. Unexpected Link between the Template Purification Solvent and the Structure of Titanium Dioxide Hollow Spheres. Catalysts 2021, 11, 112. https://doi.org/10.3390/catal11010112
Gyulavári T, Kovács K, Magyari K, Baán K, Szabó A, Veréb G, Pap Z, Hernadi K. Unexpected Link between the Template Purification Solvent and the Structure of Titanium Dioxide Hollow Spheres. Catalysts. 2021; 11(1):112. https://doi.org/10.3390/catal11010112
Chicago/Turabian StyleGyulavári, Tamás, Kata Kovács, Klára Magyari, Kornélia Baán, Anna Szabó, Gábor Veréb, Zsolt Pap, and Klara Hernadi. 2021. "Unexpected Link between the Template Purification Solvent and the Structure of Titanium Dioxide Hollow Spheres" Catalysts 11, no. 1: 112. https://doi.org/10.3390/catal11010112
APA StyleGyulavári, T., Kovács, K., Magyari, K., Baán, K., Szabó, A., Veréb, G., Pap, Z., & Hernadi, K. (2021). Unexpected Link between the Template Purification Solvent and the Structure of Titanium Dioxide Hollow Spheres. Catalysts, 11(1), 112. https://doi.org/10.3390/catal11010112