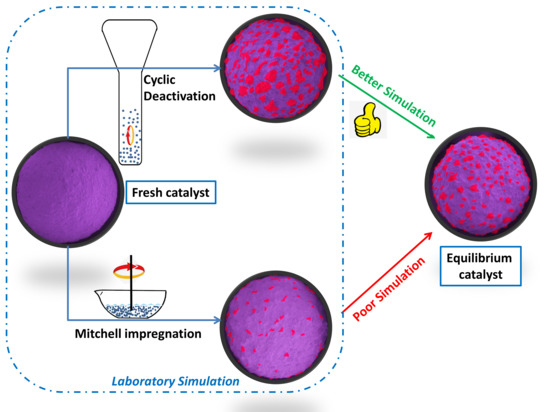
A Comparison of Laboratory Simulation Methods of Iron Contamination for FCC Catalysts
Abstract
1. Introduction
2. Results
2.1. Iron Deposited on the Catalyst Using Different Methods
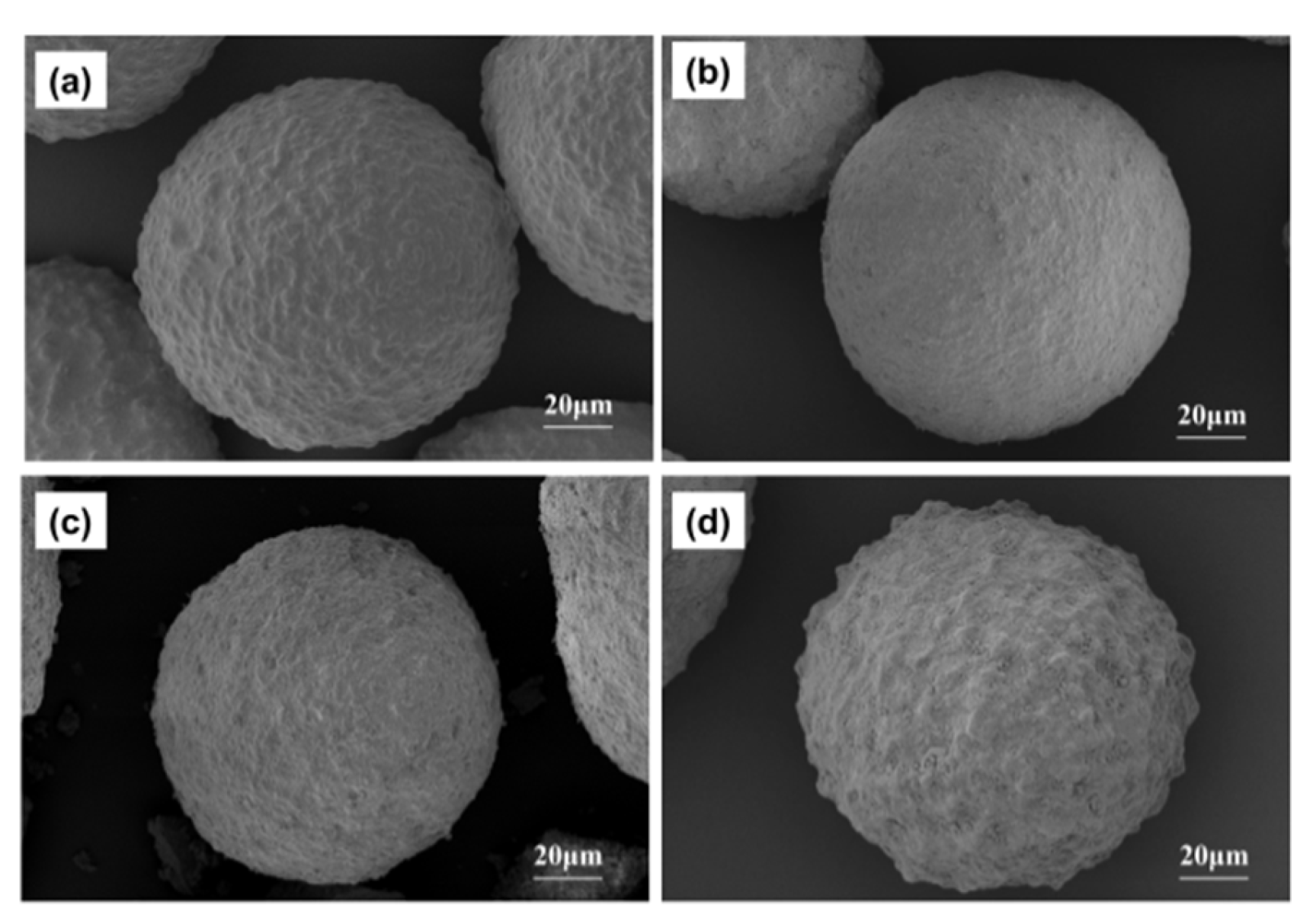
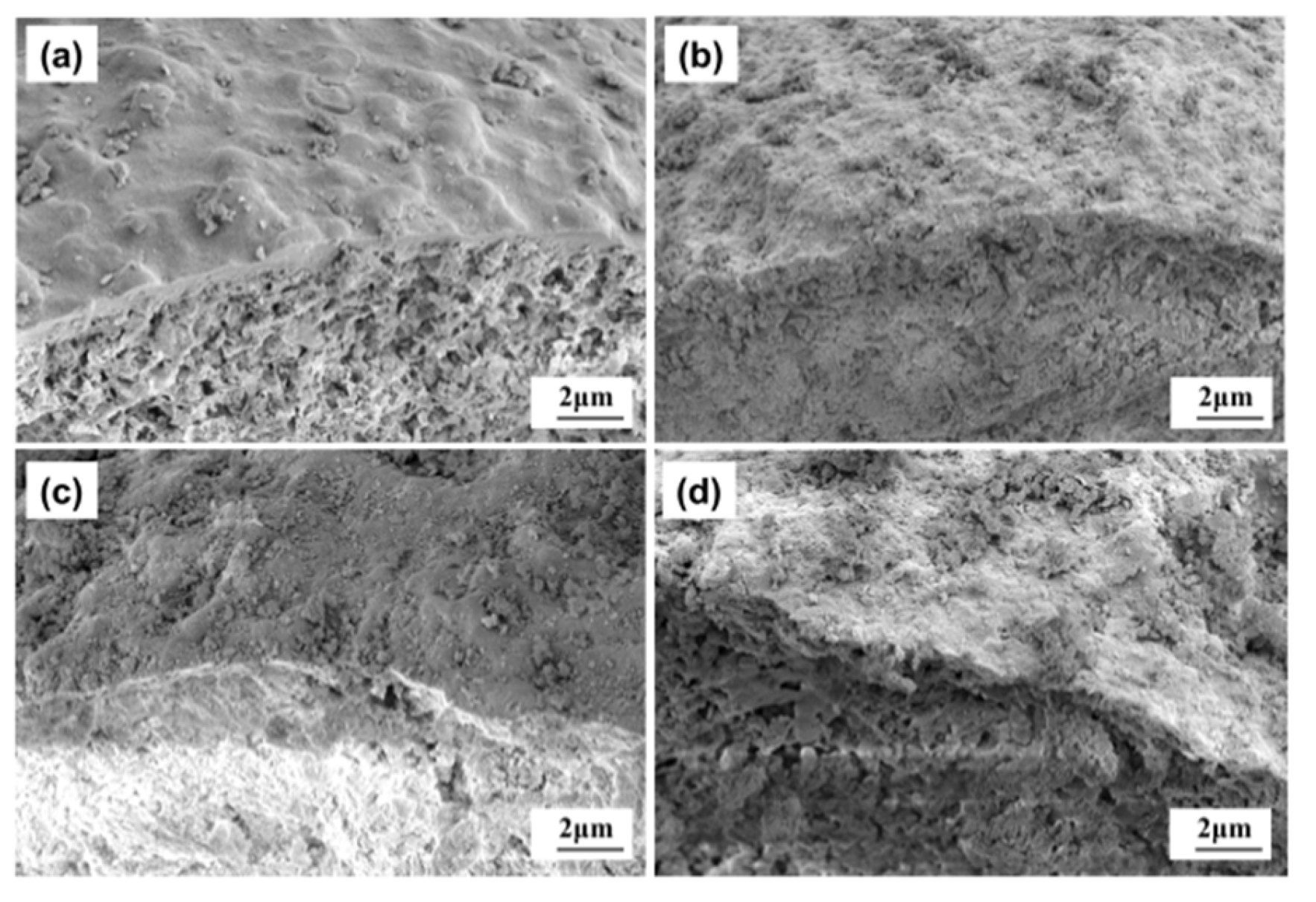
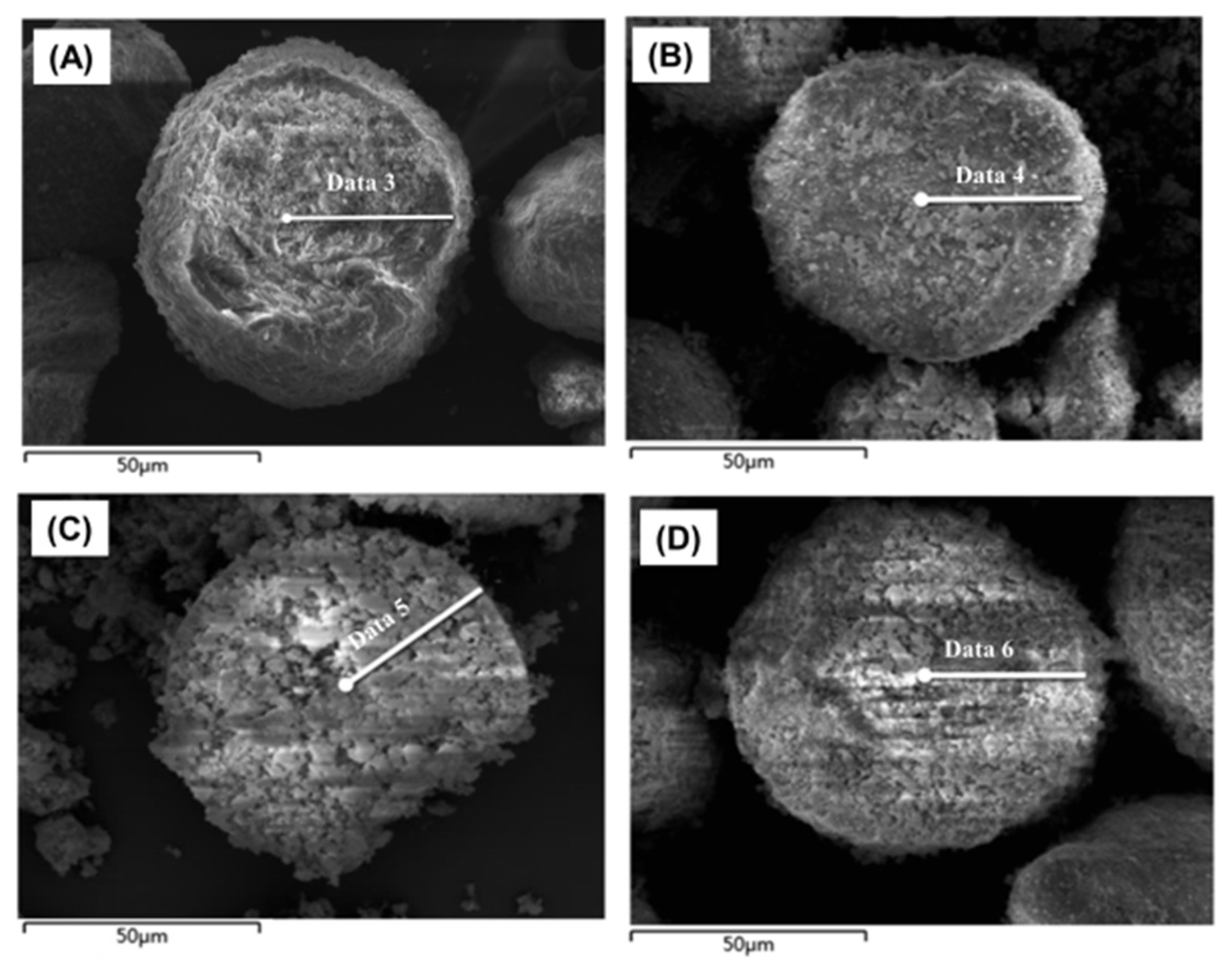
2.2. Iron-Contaminated Catalysts’ Morphology
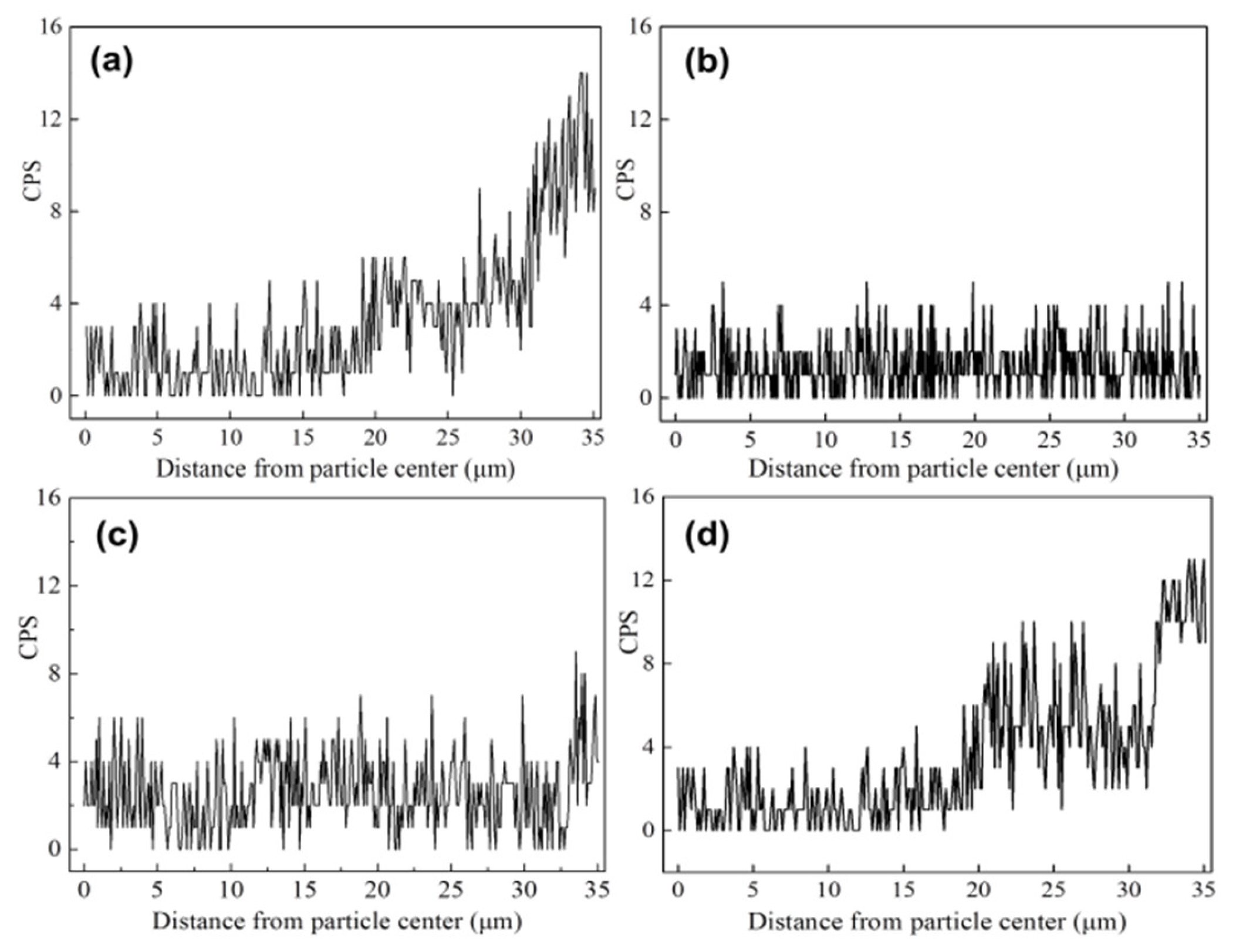
2.3. Iron Distribution on the Catalyst
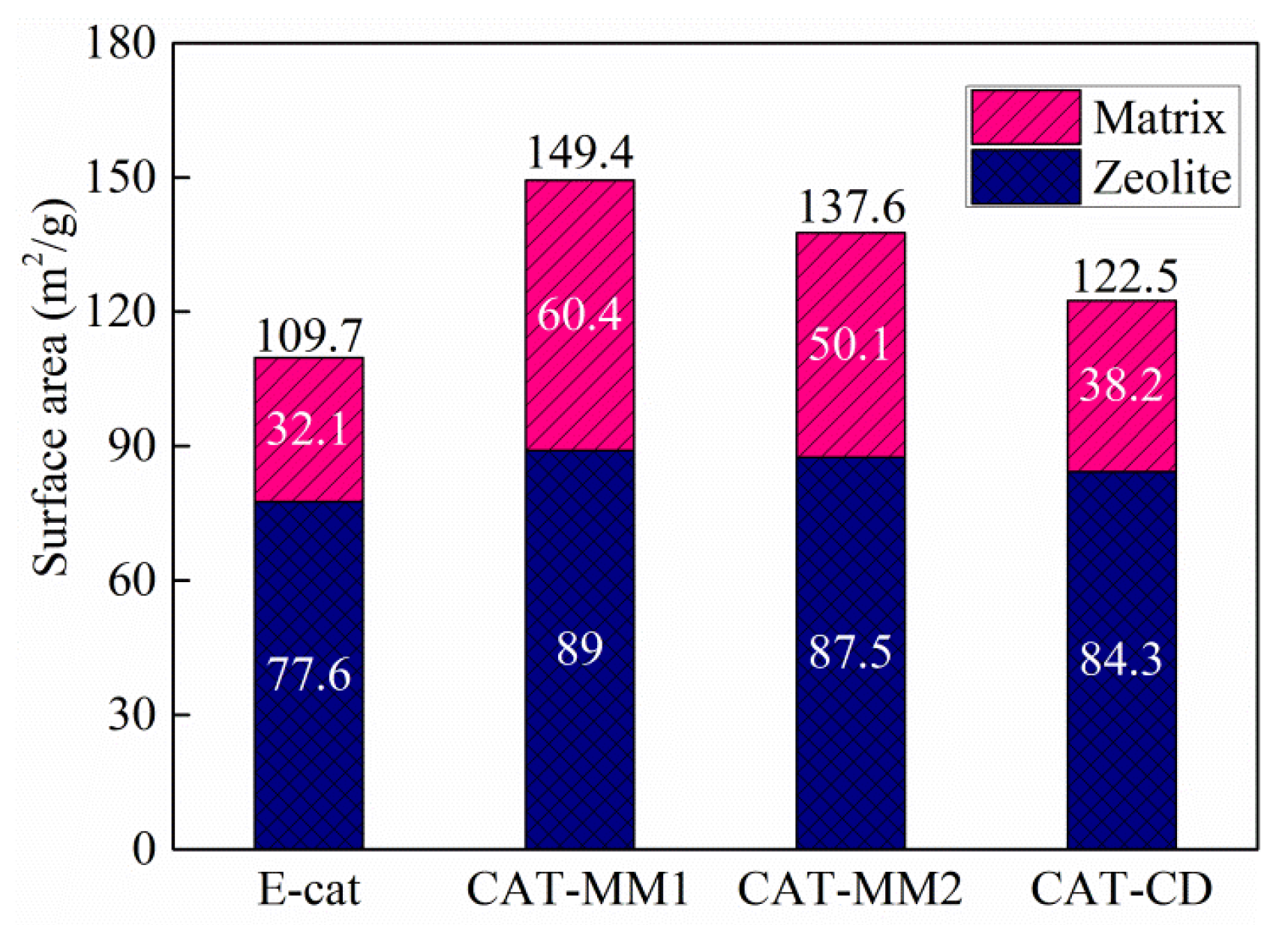
2.4. Specific Surface Area
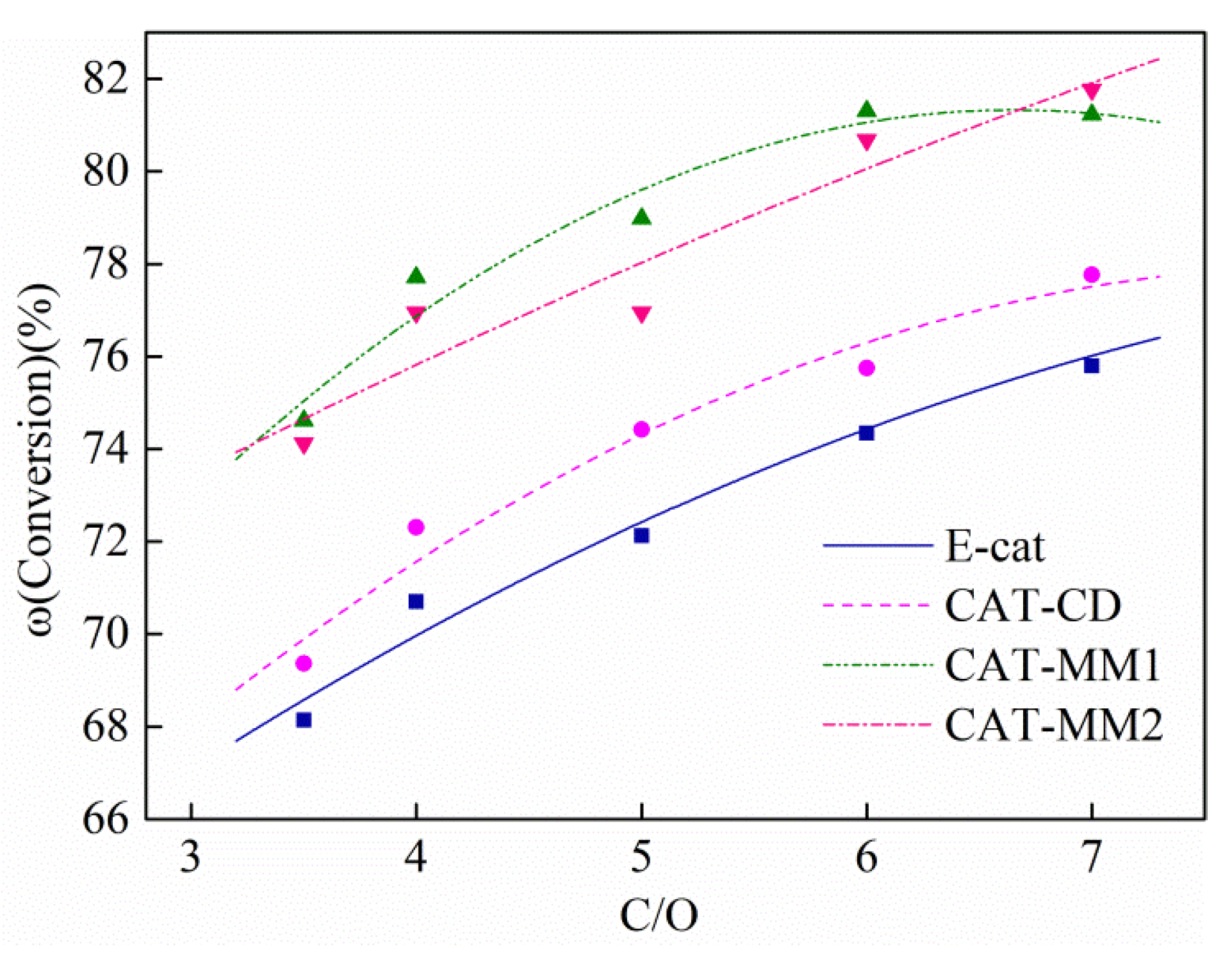
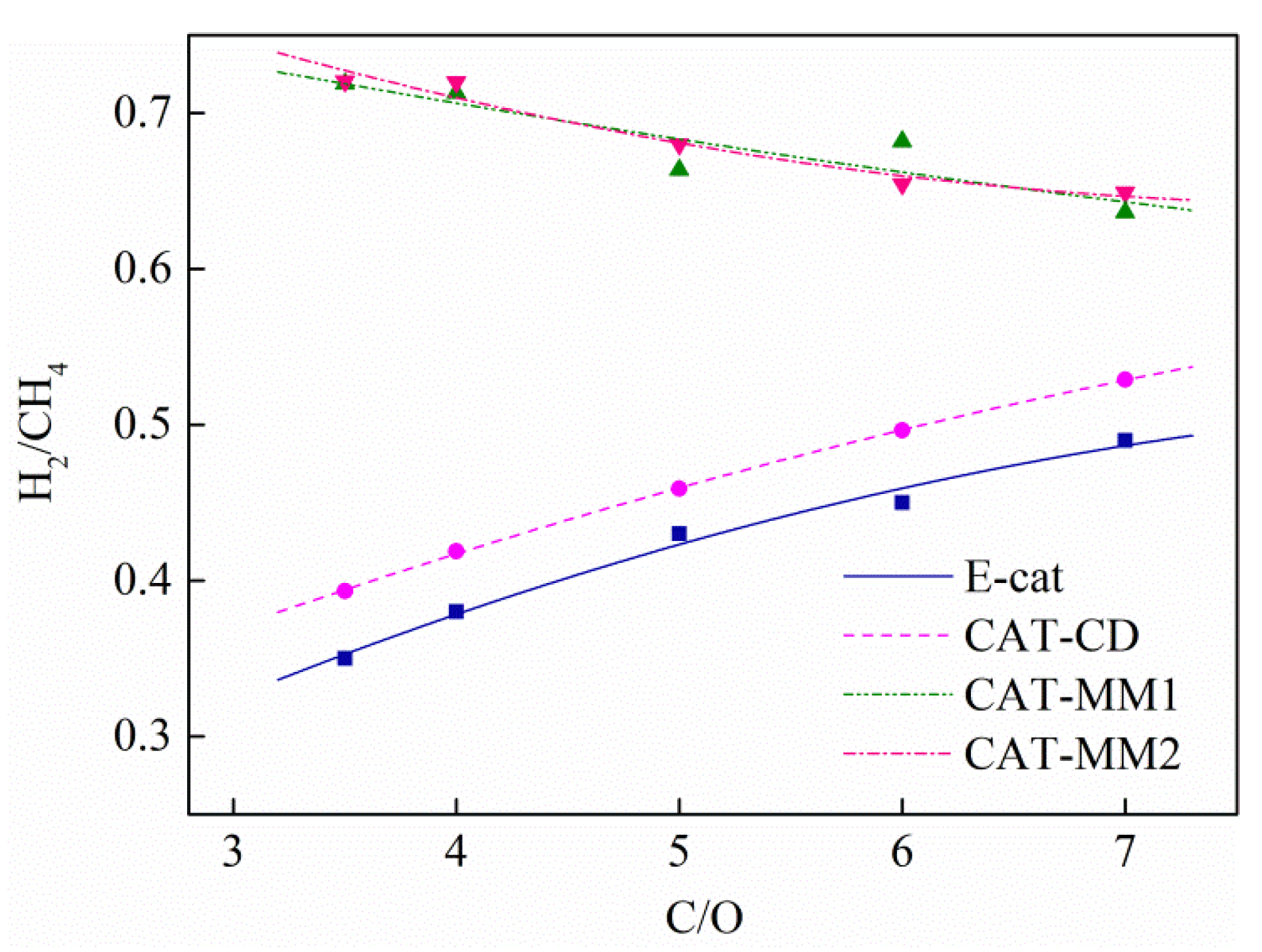
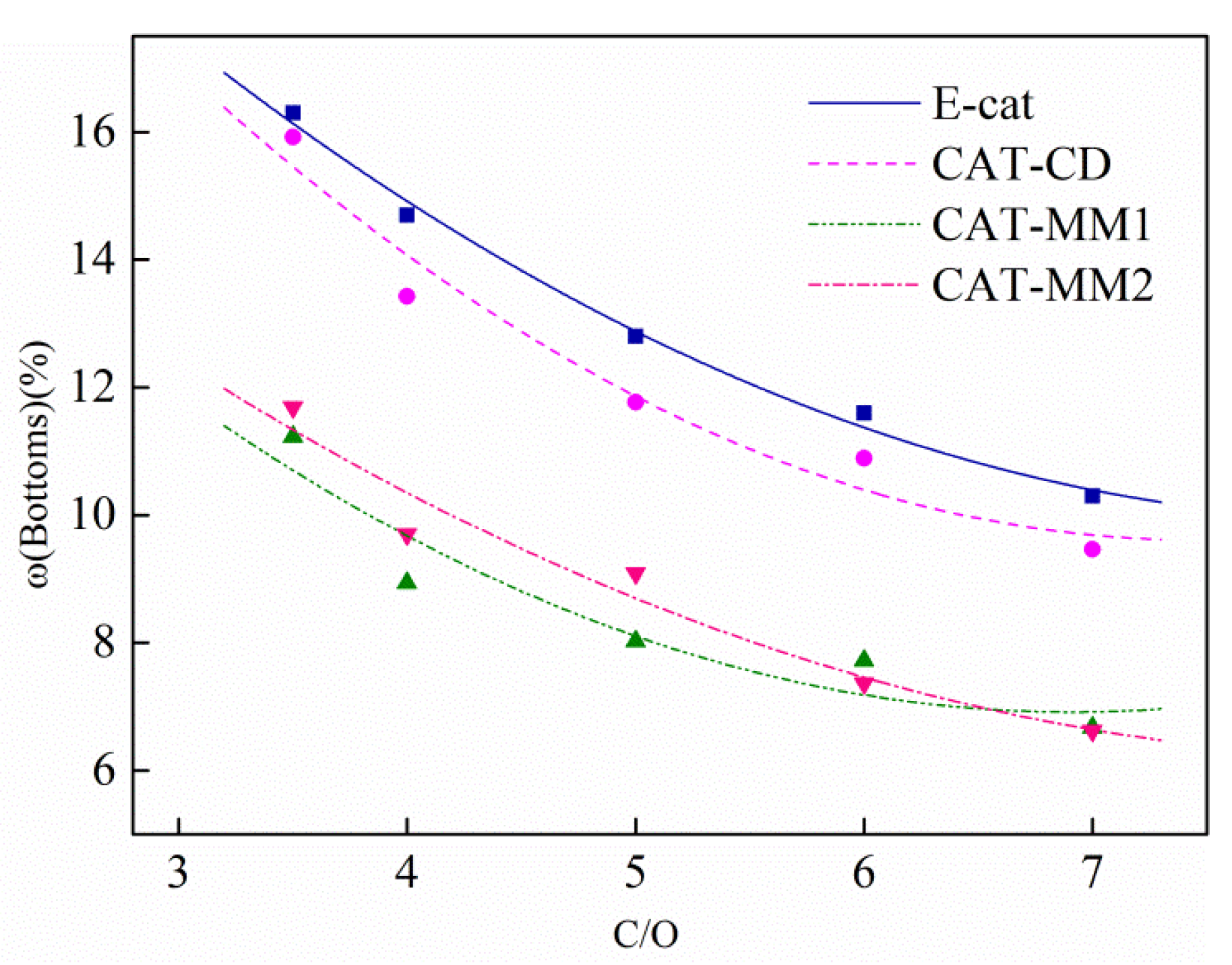
2.5. Iron Effects on the Catalyst Product Yields
2.6. NH3-TPD Analysis of Different Catalysts
2.7. SEM-EDS Analysis of the Iron Nodule Formation Mechanism
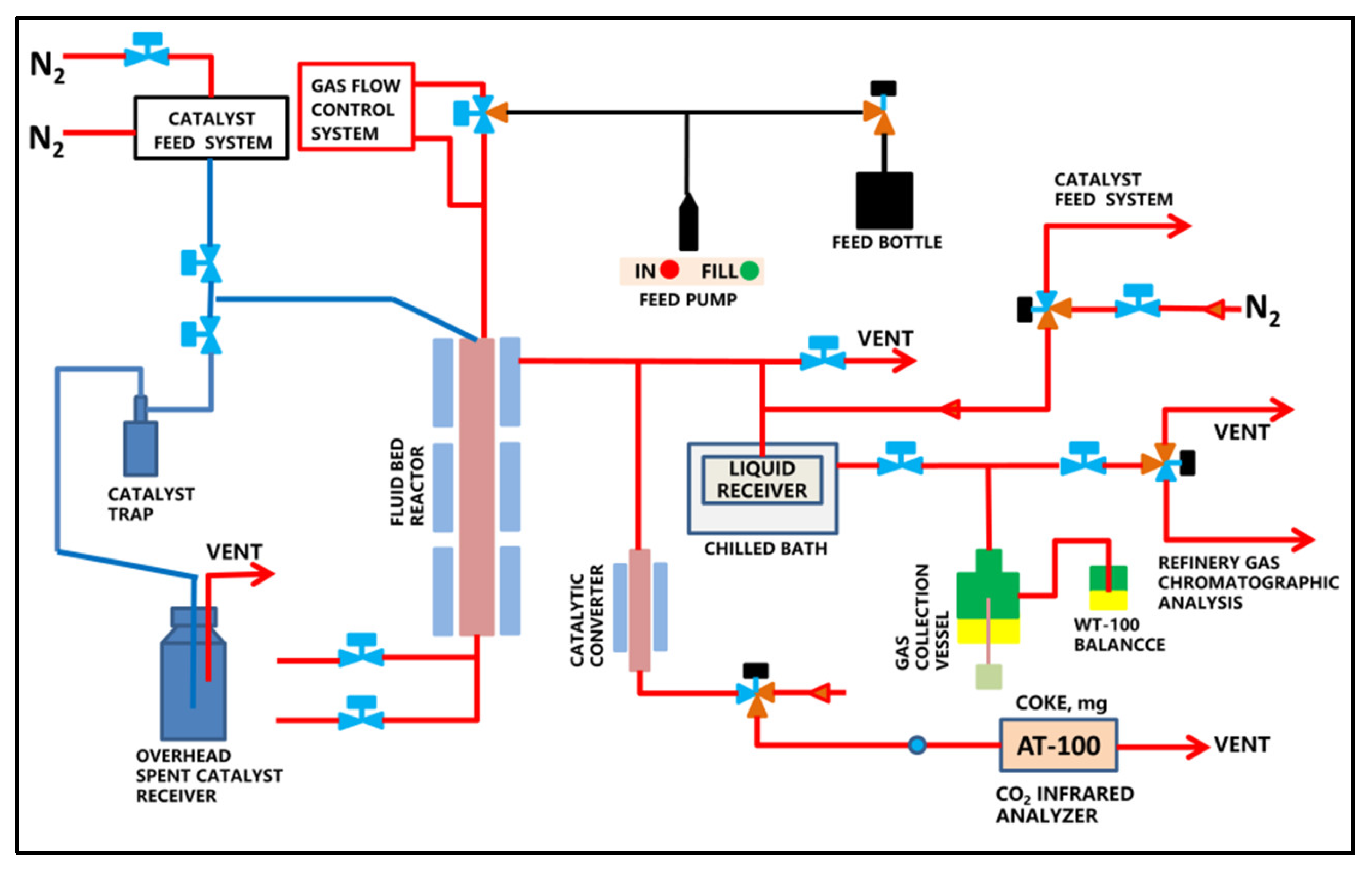
3. Materials and Methods
3.1. Materials
3.2. Methods
3.2.1. Mitchell Impregnation
3.2.2. Cyclic Deactivation
3.2.3. Catalyst Characterization
3.2.4. Catalyst Cracking Activity and Selectivity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rainer, D.R.; Rautiainen, E.; Imhof, P. Novel lab-scale deactivation method for FCC catalyst: Inducing realistic accessibility responses to iron poisoning. Appl. Catal. A Gen. 2003, 249, 69–80. [Google Scholar] [CrossRef]
- Vincz, C.; Rath, R.; Smith, G.M.; Yilmaz, B.; Mcguire, R. Dendritic nickel porphyrin for mimicking deposition of contaminant nickel on FCC catalysts. Appl. Catal. A Gen. 2015, 495, 39–44. [Google Scholar] [CrossRef]
- Mathieu, Y.; Corma, A.; EchardI, M.; Bories, M. Single and combined fluidized catalytic cracking (FCC) catalyst deactivation by iron and calcium metal–organic contaminants. Appl. Catal. A Gen. 2014, 469, 451–465. [Google Scholar] [CrossRef]
- Mehla, S.; Kukade, S.; Kumar, P.; Rao, P.V.C.; Sriganesh, G.; Ravishankar, R. Fine tuning h-transfer and β-scission reactions in VGO FCC using metal promoted dual functional ZSM-5. Fuel 2019, 242, 487–495. [Google Scholar] [CrossRef]
- Tangstad, E.; Andersen, A.; Myhrvold, E.M.; Myrstad, T. Catalytic behaviour of nickel and iron metal contaminants of an FCC catalyst after oxidative and reductive thermal treatments. Appl. Catal. A Gen. 2008, 346, 194–199. [Google Scholar] [CrossRef]
- Zhang, C.C.; Gao, X.; Yilmaz, B. Development of FTIR Spectroscopy Methodology for Characterization of Boron Species in FCC Catalysts. Catalysts 2020, 10, 1327. [Google Scholar] [CrossRef]
- Souza, N.L.A.; Tkach, I.; Morgado, E., Jr.; Krambrock, K. Vanadium poisoning of FCC catalysts: A quantitative analysis of impregnated and real equilibrium catalysts. Appl. Catal. A. Gen. 2018, 560, 206–214. [Google Scholar] [CrossRef]
- Long, J.; Zhu, Y.X.; Liu, Y.J.; Da, Z.J.; Zhou, H. Effects of vanadium oxidation number on desulfurization performance of FCC catalyst. Appl. Catal. A Genera 2005, 282, 295–301. [Google Scholar] [CrossRef]
- Whitcombe, J.M.; Agranovski, I.E.; Braddock, R.D. Identification of Metal Contaminants on FCC Catalyst. Part. Part. Syst. Charact. 2005, 22, 268–275. [Google Scholar] [CrossRef]
- Tian, H.; Huang, C.; Fan, Z. Metals on a novel USY zeolite after hydrothermal aging. Stud. Surf. Sci. Catal. 2001, 139, S0167–S2991. [Google Scholar]
- Sandoval-Díaz, L.; Ruíz-Cardona, Y.; Trujillo, C. Amorphization of USY zeolite induced by sodium chloride and high temperature steaming. Microporous Mesoporous Mater. 2016, 224, 168–175. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, Z.; Liu, P.; Zhai, J.; Yang, C. Iron contamination mechanism and reaction performance research on FCC catalyst. J. Nanotechnol. 2015, 2015. [Google Scholar] [CrossRef][Green Version]
- Whitcombe, J.M.; Agranovski, I.E.; Braddock, R.D. Impact of metal ridging on the fluidization characteristics of FCC catalyst. Chem. Eng. Technol. 2002, 25, 981–987. [Google Scholar] [CrossRef]
- Yaluris, G.; Cheng, W.C.; Peters, M.; Mcdowell, L.T.; Hunt, L. Mechanism of fluid cracking catalysts deactivation by Fe. Stud. Surf. Sci. Catal. 2004, 149, 139–163. [Google Scholar] [CrossRef]
- Buurmans, I.L.; Soulimani, F.; Ruiz-Martínez, J.; van der Bij, H.E.; Weckhuysen, B.M. Structure and acidity of individual fluid catalytic cracking catalyst particles studied by synchrotron-based infrared micro-spectroscopy. Microporous Mesoporous Mater. 2013, 166, 86–92. [Google Scholar] [CrossRef]
- Nguyen, T.H.; Ngo, P.T.; Tran, T.V.; Nguyen, S.; Vu, D.M.; Ha, Q.L.M.; Dao, X.T.T.; Dang, T.T. Effect of hydrothermal conditions on the catalytic deactivation of a fluid cracking catalyst. React. Kinet. Mech. Catal. 2013, 109, 563–574. [Google Scholar] [CrossRef]
- Etim, U.J.; Xu, B.; Bai, P.; Ullah, R.; Yan, Z. Role of nickel on vanadium poisoned FCC catalyst: A study of physiochemical properties. J. Energy Chem. 2016, 25, 667–676. [Google Scholar] [CrossRef]
- Etim, U.J.; Xu, B.; Ullah, R.; Yan, Z. Effect of vanadium contamination on the framework and micropore structure of ultra stable Y-zeolite. J. Colloid Interface Sci. 2016, 463, 188–198. [Google Scholar] [CrossRef]
- Chiranjeevi, T.; Gokak, D.T.; Ravikumar, V.; Viswanathan, P.S. Development of new deactivation method for simulation of fluid catalytic cracking equilibrium catalyst. J. Chem. Sci. 2014, 126, 353–360. [Google Scholar] [CrossRef]
- Wallenstein, D.; Farmer, D.; Knoell, J.; Fougret, C.M.; Brandt, S. Progress in the deactivation of metals contaminated FCC catalysts by a novel catalyst metallation method. Appl. Catal. A. Gen. 2013, 462, 91–99. [Google Scholar] [CrossRef]
- Psarras, A.C.; Iliopoulou, E.F.; Nalbandian, L.; Lappas, A.A.; Pouwels, C. Study of the accessibility effect on the irreversible deactivation of FCC catalysts from contaminant feed metals. Catal. Today 2007, 127, 44–53. [Google Scholar] [CrossRef]
- Almas, Q.; Naeem, M.A.; Baldanza, M.A.S.; Solomon, J.; Kenvin, J.C.; Christoph, R.; Müller, C.R.; da Silva Teixeira, V.; Jones, C.W.; Sievers, C. Transformations of FCC catalysts and carbonaceous deposits during repeated reaction-regeneration cycles. Catal. Technol. 2019, 9, 6977–6992. [Google Scholar] [CrossRef]
- Rainer, D.R.; Rautiainen, E.; Nelissen, B.; Imhof, P.; Vadovic, C. Simulating iron-induced FCC accessibility losses in lab-scale deactivation. Stud. Surf. Sci. Catal. 2004, 149, 165–176. [Google Scholar] [CrossRef]
- Brandt, S.; Knöll, J.; Jiang, H.; Saar, J.; Fougret, C. The role of magnetic susceptibility in detecting iron poisoning in FCC equilibrium catalyst samples and its combination with other macroscopic bulk analysis techniques. Ind. Eng. Chem. Res. 2019, 58, 20528–20535. [Google Scholar] [CrossRef]
- Zhu, Y.X.; Du, Q.S.; Lin, W.; Tang, L.W.; Jun, L. Chapter 13 studies of iron effects on fcc catalysts. Stud. Surf. Sci. Catal. 2007, 166, 201–212. [Google Scholar] [CrossRef]
- Jiang, H.; Livi, K.J.; Kundu, S.; Cheng, W.C. Characterization of iron contamination on equilibrium fluid catalytic cracking catalyst particles. J. Catal. 2018, 361, 126–134. [Google Scholar] [CrossRef]
- Souza, N.L.; Paniago, R.; Ardisson, J.D.; Morgado, E., Jr.; Krambrock, K. Iron contamination of FCC catalysts: Quantification of different crystalline phases and valence states. Appl. Catal. A Gen. 2019, 569, 57–65. [Google Scholar] [CrossRef]
- Hochheiser, T.; Tang, Y.; Allahverdi, M.; Graaf, B.D. FCC Additive Improves Residue Processing Economics with High Iron Feeds. In Proceedings of the AFPM Annual Meeting, Orlando, FL, USA, 23–25 March 2014. [Google Scholar]
- Meirer, F.; Morris, D.T.; Kalirai, S.; Liu, Y.; Andrews, J.C.; Weckhuysen, B.M. Mapping metals incorporation of a whole single catalyst particle using element specific X-ray nanotomography. J. Am. Chem. Soc. 2015, 137, 102–105. [Google Scholar] [CrossRef] [PubMed]
- Pan, S.S.; Lin, L.T.X.; Komvokis, V.; Spann, A.; Clough, M.; Yilmaz, B. Nanomaterials Fueling the World. In Nanomaterials for Sustainable Energy; American Chemical Society: Washington, DC, USA, 2015; Volume 1213, pp. 3–18. [Google Scholar] [CrossRef]
- Cristiano-Torres, D.V.; Osorio-Pérez, Y.; Palomeque-Forero, L.A.; Sandoval-Díaz, L.E.; Trujillo, C.A. The action of vanadium over Y zeolite in oxidant and dry atmosphere. Appl. Catal. A. Gen. 2008, 346, 104–111. [Google Scholar] [CrossRef]
- Wise, A.M.; Weker, J.N.; Kalirai, S.; Farmand, M.; Shapiro, D.A.; Meirer, F.; Weckhuysen, B.M. Nanoscale chemical imaging of an individual catalyst particle with soft x-ray ptychography. Acs Catal. 2016, 6, 2178–2181. [Google Scholar] [CrossRef] [PubMed]
- Ihli, J.; Ferreira Sanchez, D.; Rosh, J.; Cuartero, V.; Mathon, O.; Krumeich, F.; Borca, C.; Huthwelker, T.; Cheng, W.-C.; Shu, Y.Y.; et al. Localization and speciation of iron impurities within a fluid catalytic cracking catalyst. Angew. Chem. 2017, 129, 14219–14223. [Google Scholar] [CrossRef]
- Senter, M.; Mastry, M.C.; Mannion, A.M.; McGuire, R.; Houtz, D.; Yilmaz, B. Quantitative visual characterization of contaminant metals and their mobility in fluid catalytic cracking catalysts. Catalysts 2019, 9, 831. [Google Scholar] [CrossRef]
- Alkhlel, A.; de Lasa, H. Catalyst/Feedstock Ratio Effect on FCC Using Different Catalysts Samples. Catalysts 2019, 9, 542. [Google Scholar] [CrossRef]
- Chen, J.W.; Xu, Y.H. Fluid Catalytic Cracking Process and Engineering; Sinopec Press: Beijing, China, 2018; pp. 392–395. [Google Scholar]
- Bai, P.; Etim, U.J.; Yan, Z.; Mintova, S.; Zhang, Z.; Zhong, Z.; Gao, X. Fluid catalytic cracking technology: Current status and recent discoveries on catalyst contamination. Catal. Rev. 2019, 61, 333–405. [Google Scholar] [CrossRef]


| Catalysts | ω (Na2O) (%) | ω (Al2O3) (%) | S.A. (m2·g−1) | P.V.total (cm3·g−1) | Unit Cell Size (nm) | Fe on Catalyst (μg·g−1) |
|---|---|---|---|---|---|---|
| CAT-BASE | 0.16 | 48.6 | 269 | 0.42 | 2.462 | 1828 |
| CAT-MM1 | 0.16 | 48.6 | 149.4 | 0.21 | 2.440 | 13,308 |
| CAT-MM2 | 0.16 | 48.6 | 137.6 | 0.20 | 2.439 | 13,268 |
| CAT-CD | 0.16 | 48.6 | 122.5 | 0.16 | 2.436 | 13,518 |
| E-cat | 0.14 | 47.4 | 109.7 | 0.15 | 2.437 | 10,471 |
| ω(Yields) (%) | E-Cat | CAT-CD | CAT-MM1 | CAT-MM2 |
|---|---|---|---|---|
| Coke | 9.18 | 9.33 | 10.29 | 9.87 |
| Dry gas | 3.28 | 3.22 | 3.24 | 3.06 |
| H2 | 0.46 | 0.51 | 0.69 | 0.67 |
| Gasoline | 44.56 | 44.87 | 43.00 | 43.19 |
| LCO | 14.06 | 13.89 | 14.21 | 14.12 |
| Bottoms | 11.94 | 12.11 | 11.79 | 11.88 |
| LPG | 16.88 | 16.58 | 17.47 | 17.88 |
| Location | Scanning Area | Si | Al | Fe | Na | Ca |
|---|---|---|---|---|---|---|
| Raised area on surface | Area scan 1 (%) | 34.16 | 48.89 | 15.18 | 1.77 | 0 |
| Area scan 2 (%) | 33.55 | 43.62 | 18.29 | 4.53 | 0 | |
| Area scan 3 (%) | 38.91 | 43.42 | 16.36 | 1.31 | 0 | |
| Valley area on surface | Area scan 4 (%) | 45.94 | 40.94 | 11.86 | 1.26 | 0 |
| Area scan 5 (%) | 43.91 | 42.69 | 11.59 | 1.81 | 0 | |
| Area scan 6 (%) | 43.60 | 41.35 | 11.41 | 3.64 | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liao, Y.; Liu, T.; Zhao, H.; Gao, X. A Comparison of Laboratory Simulation Methods of Iron Contamination for FCC Catalysts. Catalysts 2021, 11, 104. https://doi.org/10.3390/catal11010104
Liao Y, Liu T, Zhao H, Gao X. A Comparison of Laboratory Simulation Methods of Iron Contamination for FCC Catalysts. Catalysts. 2021; 11(1):104. https://doi.org/10.3390/catal11010104
Chicago/Turabian StyleLiao, Yitao, Tao Liu, Huihui Zhao, and Xionghou Gao. 2021. "A Comparison of Laboratory Simulation Methods of Iron Contamination for FCC Catalysts" Catalysts 11, no. 1: 104. https://doi.org/10.3390/catal11010104
APA StyleLiao, Y., Liu, T., Zhao, H., & Gao, X. (2021). A Comparison of Laboratory Simulation Methods of Iron Contamination for FCC Catalysts. Catalysts, 11(1), 104. https://doi.org/10.3390/catal11010104