Selective Trimerization of α-Olefins with Immobilized Chromium Catalyst for Lubricant Base Oils
Abstract
1. Introduction
2. Results and Discussion
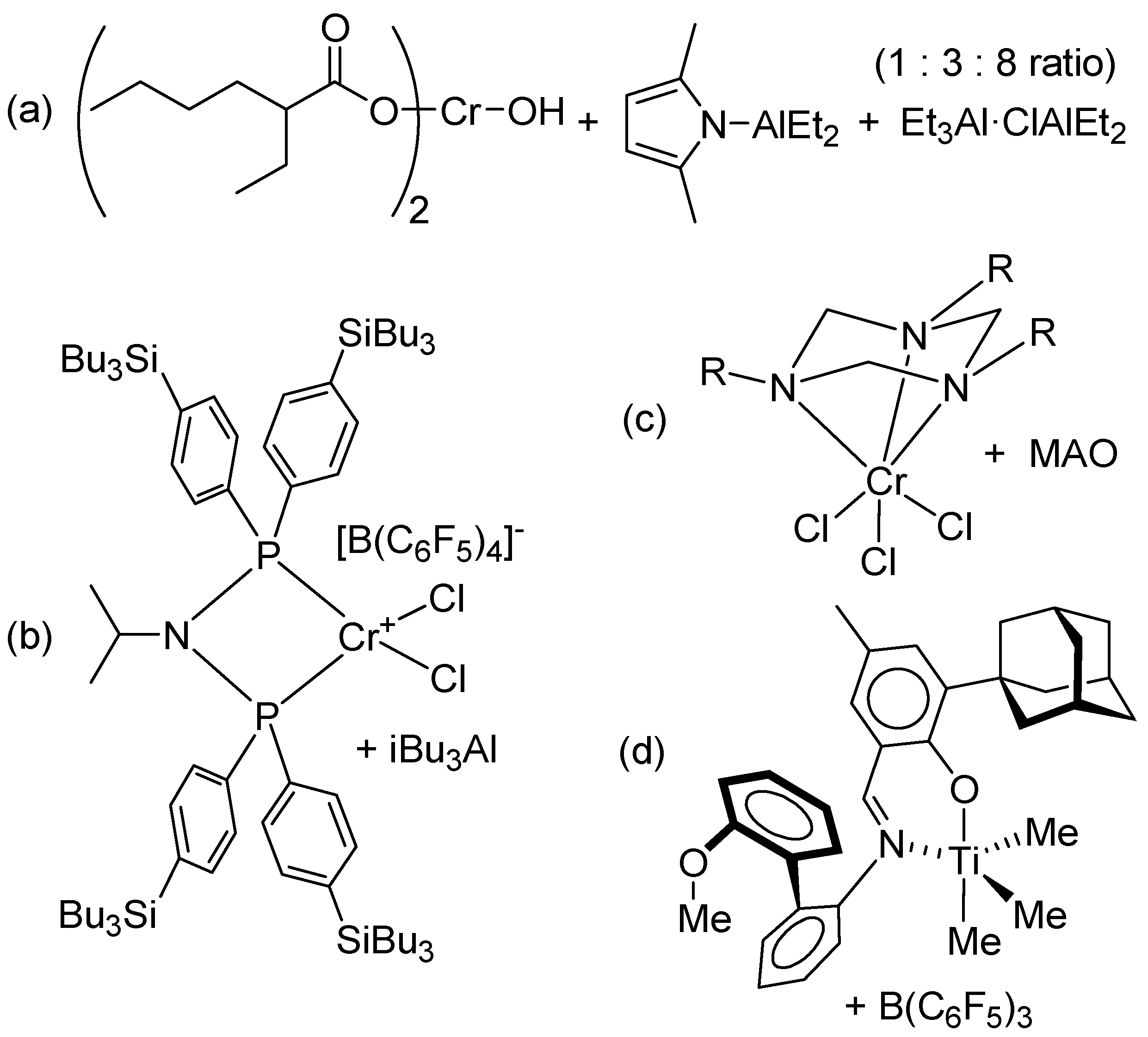
2.1. Catalyst Screening
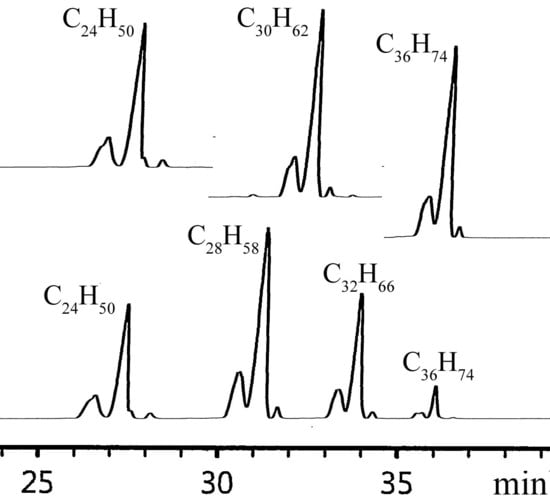
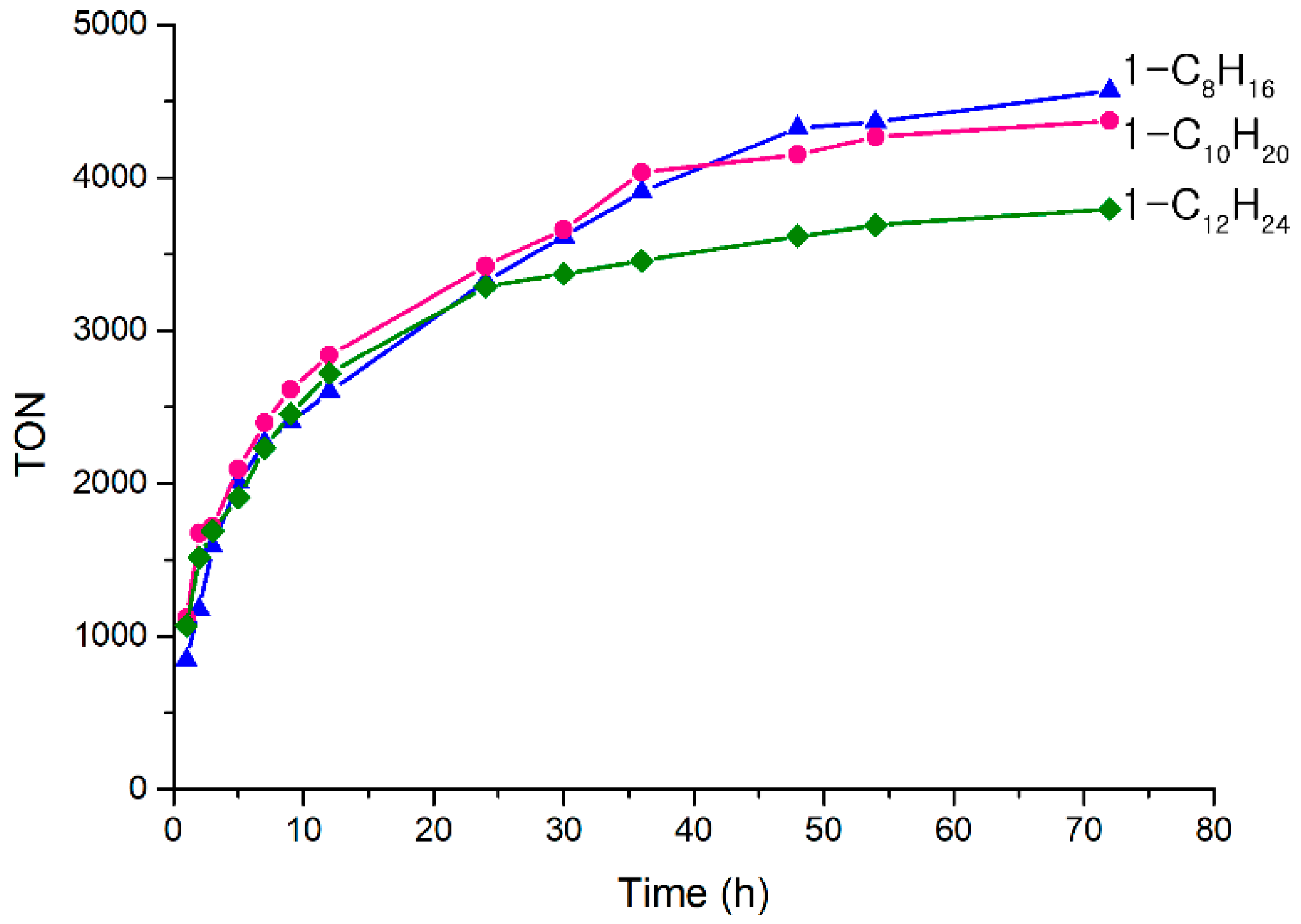
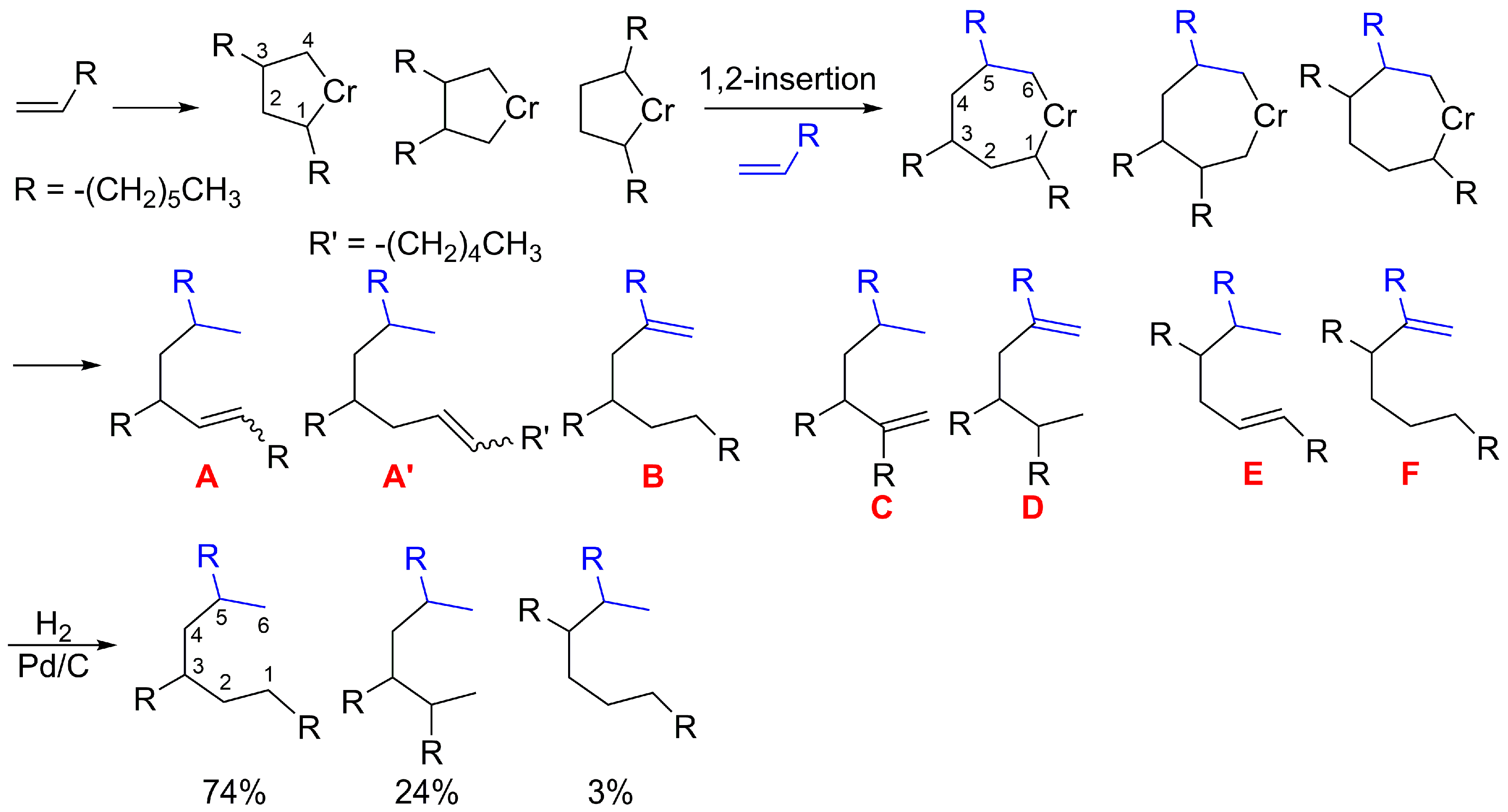
2.2. α-Olefin Oligomerization Studies with Supported Catalysts
2.3. Preparation of α-olefin Trimers and Lubricant Properties
3. Material and Methods
3.1. Materials
3.2. Preparation of Supported Catalyst
3.3. 1-Octene Trimerization on A Small Scale for the Data in Figure 2 and Figure 3
3.4. Preparation of α-Olefin Trimer on a Large Scale for the Samples in Table 1
3.5. SimDis GC Analysis
4. Conclusions
5. Patents
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Nifant’ev, I.; Ivchenko, P. Fair Look at Coordination Oligomerization of Higher α-Olefins. Polymers 2020, 12, 1082. [Google Scholar] [CrossRef] [PubMed]
- Saidi, M.Z.; Pasc, A.; El Moujahid, C.; Canilho, N.; Badawi, M.; Delgado-Sanchez, C.; Celzard, A.; Fierro, V.; Peignier, R.; Kouitat-Njiwa, R.; et al. Improved tribological properties, thermal and colloidal stability of poly-α-olefins based lubricants with hydrophobic MoS2 submicron additives. J. Colloid Interface Sci. 2020, 562, 91–101. [Google Scholar] [CrossRef] [PubMed]
- Soltanahmadi, S.; Charpentier, T.; Nedelcu, I.; Khetan, V.; Morina, A.; Freeman, H.M.; Brown, A.P.; Brydson, R.; Van Eijk, M.C.P.; Neville, A. Surface Fatigue Behavior of a WC/aC:H Thin-Film and the Tribochemical Impact of Zinc Dialkyldithiophosphate. ACS Appl. Mater. Interfaces 2019, 11, 41676–41687. [Google Scholar] [CrossRef]
- Liñeira del Río, J.M.; López, E.R.; Fernández, J.; García, F. Tribological properties of dispersions based on reduced graphene oxide sheets and trimethylolpropane trioleate or PAO 40 oils. J. Mol. Liq. 2019, 274, 568–576. [Google Scholar] [CrossRef]
- Narita, K. Lubricants for Metal Belt Continuously Variable Transmissions. Lubricants 2014, 2, 11–20. [Google Scholar] [CrossRef]
- Ray, S.; Rao, P.V.C.; Choudary, N.V. Poly-α-olefin-based synthetic lubricants: A short review on various synthetic routes. Lubr. Sci. 2012, 24, 23–44. [Google Scholar] [CrossRef]
- Benda, R.; Bullen, J.; Plomer, A. Synthetics basics: Polyalphaolefins—Base fluids for high-performance lubricants. J. Synth. Lubr. 1996, 13, 41–57. [Google Scholar] [CrossRef]
- Sun, H.; Shen, B.; Wu, D.; Guo, X.; Li, D. Supported Al–Ti bimetallic catalysts for 1-decene oligomerization: Activity, stability and deactivation mechanism. J. Catal. 2016, 339, 84–92. [Google Scholar] [CrossRef]
- Wang, W.; Jiang, S.; Shen, Y.; Xia, S.; Xu, J. Synthesis of high viscosity index base stock and study on the lubricating properties. Ind. Lubr. Tribol. 2016, 68, 52–56. [Google Scholar] [CrossRef]
- Hogg, J.M.; Coleman, F.; Ferrer-Ugalde, A.; Atkins, M.P.; Swadźba-Kwaśny, M. Liquid coordination complexes: A new class of Lewis acids as safer alternatives to BF3 in synthesis of polyalphaolefins. Green Chem. 2015, 17, 1831–1841. [Google Scholar] [CrossRef]
- Hogg, J.M.; Ferrer-Ugalde, A.; Coleman, F.; Swadźba-Kwaśny, M. Borenium Ionic Liquids as Alternative to BF3 in Polyalphaolefins (PAOs) Synthesis. ACS Sustain. Chem. Eng. 2019, 7, 15044–15052. [Google Scholar] [CrossRef]
- Echaroj, S.; Santikunaporn, M.; Chavadej, S. Transformation of Bioderived 1-Decanol to Diesel-like Fuel and Biobased Oil via Dehydration and Oligomerization Reactions. Energy Fuels 2017, 31, 9465–9476. [Google Scholar] [CrossRef]
- Gee, J.C.; Small, B.L.; Hope, K.D. Behavior of protonated cyclopropyl intermediates during polyalphaolefin synthesis: Mechanism and predicted product distribution. J. Phys. Org. Chem. 2012, 25, 1409–1417. [Google Scholar] [CrossRef]
- Sadjadi, S.; Bahri-Laleh, N.; Nekoomanesh-Haghighi, M.; Ziaee, F.; Dehghani, S.; Shirbakht, S.; Rahbar, A.; Barough, M.S.; Mirmohammadi, S.A. Rationalizing chain microstructure in the polyα-olefins synthesized by cationic AlCl3/H2O catalytic system. Int. J. Polym. Anal. Charact. 2019, 24, 556–570. [Google Scholar] [CrossRef]
- Nifant’ev, I.E.; Vinogradov, A.A.; Vinogradov, A.A.; Sedov, I.V.; Dorokhov, V.G.; Lyadov, A.S.; Ivchenko, P.V. Structurally uniform 1-hexene, 1-octene, and 1-decene oligomers: Zirconocene/MAO-catalyzed preparation, characterization, and prospects of their use as low-viscosity low-temperature oil base stocks. Appl. Catal. A Gen. 2018, 549, 40–50. [Google Scholar] [CrossRef]
- Dong, S.Q.; Mi, P.K.; Xu, S.; Zhang, J.; Zhao, R.D. Preparation and Characterization of Single-Component Poly-α-olefin Oil Base Stocks. Energy Fuels 2019, 33, 9796–9804. [Google Scholar] [CrossRef]
- Hanifpour, A.; Bahri-Laleh, N.; Nekoomanesh-Haghighi, M.; Poater, A. Group IV diamine bis(phenolate) catalysts for 1-decene oligomerization. Mol. Catal. 2020, 493, 111047. [Google Scholar] [CrossRef]
- Shao, H.; Gu, X.; Wang, R.; Wang, X.; Jiang, T.; Guo, X. Preparation of Lubricant Base Stocks with High Viscosity Index through 1-Decene Oligomerization Catalyzed by Alkylaluminum Chloride Promoted by Metal Chloride. Energy Fuels 2020, 34, 2214–2220. [Google Scholar] [CrossRef]
- Zhao, R.; Mi, P.; Xu, S.; Dong, S. Structure and Properties of Poly-α-olefins Containing Quaternary Carbon Centers. ACS Omega 2020, 5, 9142–9150. [Google Scholar] [CrossRef]
- Park, J.H.; Jang, Y.E.; Jeon, J.Y.; Go, M.J.; Lee, J.; Kim, S.K.; Lee, S.-I.; Lee, B.Y. Preparation of ansa-metallocenes for production of poly(α-olefin) lubricants. Dalton Trans. 2014, 43, 10132–10138. [Google Scholar] [CrossRef]
- Mattran, D. ExxonMobil Chemical: SpectraSyn Elite™ 65 joins ExxonMobil chemical’s metallocene polyalphaolefin lineup. Tribol. Lubr. Technol. 2012, 68, 56–58. [Google Scholar]
- Wu, M.M.S.; Coker, C.L.; Walzer, J.P.J.; Jiang, P.; Rucker, S.P. Process to Produce High Viscosity Fluids. U.S. Patent 7989670B2, 2 August 2011. [Google Scholar]
- Köhn, R.D.; Haufe, M.; Kociok-Köhn, G.; Grimm, S.; Wasserscheid, P.; Keim, W. Selective Trimerization of α-Olefins with Triazacyclohexane Complexes of Chromium as Catalysts. Angew. Chem. Int. Ed. 2000, 39, 4337–4339. [Google Scholar] [CrossRef]
- Wasserscheid, P.; Grimm, S.; Köhn, R.D.; Haufe, M. Synthesis of Synthetic Lubricants by Trimerization of 1-Decene and 1-Dodecene with Homogeneous Chromium Catalysts. Adv. Synth. Catal. 2001, 343, 814–818. [Google Scholar] [CrossRef]
- Sattler, A.; Labinger, J.A.; Bercaw, J.E. Highly Selective Olefin Trimerization Catalysis by a Borane-Activated Titanium Trimethyl Complex. Organometallics 2013, 32, 6899–6902. [Google Scholar] [CrossRef]
- Sattler, A.; Aluthge, D.C.; Winkler, J.R.; Labinger, J.A.; Bercaw, J.E. Enhanced Productivity of a Supported Olefin Trimerization Catalyst. ACS Catal. 2016, 6, 19–22. [Google Scholar] [CrossRef]
- Coxon, A.G.N.; Köhn, R.D. Efficient 1-Hexene Trimerization with Triazacyclohexane Chromium Catalysts and Detailed Product Analysis by 13C NMR. ACS Catal. 2016, 6, 3008–3016. [Google Scholar] [CrossRef][Green Version]
- Keim, W. Oligomerization of Ethylene to α-Olefins: Discovery and Development of the Shell Higher Olefin Process (SHOP). Angew. Chem. Int. Ed. 2013, 52, 12492–12496. [Google Scholar] [CrossRef]
- Bollmann, A.; Blann, K.; Dixon, J.T.; Hess, F.M.; Killian, E.; Maumela, H.; McGuinness, D.S.; Morgan, D.H.; Neveling, A.; Otto, S.; et al. Ethylene Tetramerization: A New Route to Produce 1-Octene in Exceptionally High Selectivities. J. Am. Chem. Soc. 2004, 126, 14712–14713. [Google Scholar] [CrossRef]
- Sydora, O.L. Selective Ethylene Oligomerization. Organometallics 2019, 38, 997–1010. [Google Scholar] [CrossRef]
- Bariashir, C.; Huang, C.; Solan, G.A.; Sun, W.H. Recent advances in homogeneous chromium catalyst design for ethylene tri-, tetra-, oligo- and polymerization. Coord. Chem. Rev. 2019, 385, 208–229. [Google Scholar] [CrossRef]
- Britovsek, G.J.P.; Malinowski, R.; McGuinness, D.S.; Nobbs, J.D.; Tomov, A.K.; Wadsley, A.W.; Young, C.T. Ethylene Oligomerization beyond Schulz–Flory Distributions. ACS Catal. 2015, 5, 6922–6925. [Google Scholar] [CrossRef]
- Park, H.S.; Kim, T.H.; Baek, J.W.; Lee, H.J.; Kim, T.J.; Ryu, J.Y.; Lee, J.; Lee, B.Y. Extremely Active Ethylene Tetramerization Catalyst Avoiding the Use of Methylaluminoxane: [iPrN{P(C6H4-p-SiR3)2}2CrCl2]+[B(C6F5)4]−. ChemCatChem 2019, 11, 4351–4359. [Google Scholar] [CrossRef]
- Kim, T.H.; Lee, H.M.; Park, H.S.; Kim, S.D.; Kwon, S.J.; Tahara, A.; Nagashima, H.; Lee, B.Y. MAO-free and extremely active catalytic system for ethylene tetramerization. Appl. Organomet. Chem. 2019, 33, e4829. [Google Scholar] [CrossRef]
- Kim, E.H.; Lee, H.M.; Jeong, M.S.; Ryu, J.Y.; Lee, J.; Lee, B.Y. Methylaluminoxane-Free Chromium Catalytic System for Ethylene Tetramerization. ACS Omega 2017, 2, 765–773. [Google Scholar] [CrossRef] [PubMed]
- Bagheri, V.; Eisenber, D.C.; Ratliff, K.S.; Benda, R.; Lanier, C.W. Oligomer Oils and their Manufacture. U.S. Patent 6548724B2, 15 April 2003. [Google Scholar]
- Suzuki, Y.; Kinoshita, S.; Shibahara, A.; Ishii, S.; Kawamura, K.; Inoue, Y.; Fujita, T. Trimerization of Ethylene to 1-Hexene with Titanium Complexes Bearing Phenoxy−Imine Ligands with Pendant Donors Combined with MAO. Organometallics 2010, 29, 2394–2396. [Google Scholar] [CrossRef]
- Deckers, P.; Hessen, B.; Teuben, J. Switching a Catalyst System from Ethene Polymerization to Ethene Trimerization with a Hemilabile Ancillary Ligand. Angew. Chem. Int. Ed. 2001, 40, 2516–2519. [Google Scholar] [CrossRef]
- Jeon, J.Y.; Park, D.S.; Lee, D.H.; Eo, S.C.; Park, S.Y.; Jeong, M.S.; Kang, Y.Y.; Lee, J.; Lee, B.Y. A chromium precursor for the Phillips ethylene trimerization catalyst: (2-ethylhexanoate)2CrOH. Dalton Trans. 2015, 44, 11004–11012. [Google Scholar] [CrossRef]
- Sun, H.; Lei, X.; Tan, J.; Sun, Z.; Han, X.; Liu, J.; Zhao, J.; Shen, B.; Zhang, X.; Guo, X.; et al. Tuning the Catalytic Activity and Stability of Al-Ti Bimetallic Species Immobilized on MgO-Al2O3-SiO2 for 1-Decene Oligomerization. Ind. Eng. Chem. Res. 2018, 57, 6664–6672. [Google Scholar] [CrossRef]
- Lee, B.Y. Catalyst for Trimerization of Alpha-Olefin, Method for Trimerization of Alpha-Olefin Comprising Using the Same and Method for Preparing Lubricating Oil Comprising Using the Same. Kr. Patent 101449474B1, 13 October 2014. [Google Scholar]
- Bashir, M.A.; Vancompernolle, T.; Gauvin, R.M.; Delevoye, L.; Merle, N.; Monteil, V.; Taoufik, M.; McKenna, T.F.L.; Boisson, C. Silica/MAO/(n -BuCp)2ZrCl2 catalyst: Effect of support dehydroxylation temperature on the grafting of MAO and ethylene polymerization. Catal. Sci. Technol. 2016, 6, 2962–2974. [Google Scholar] [CrossRef]
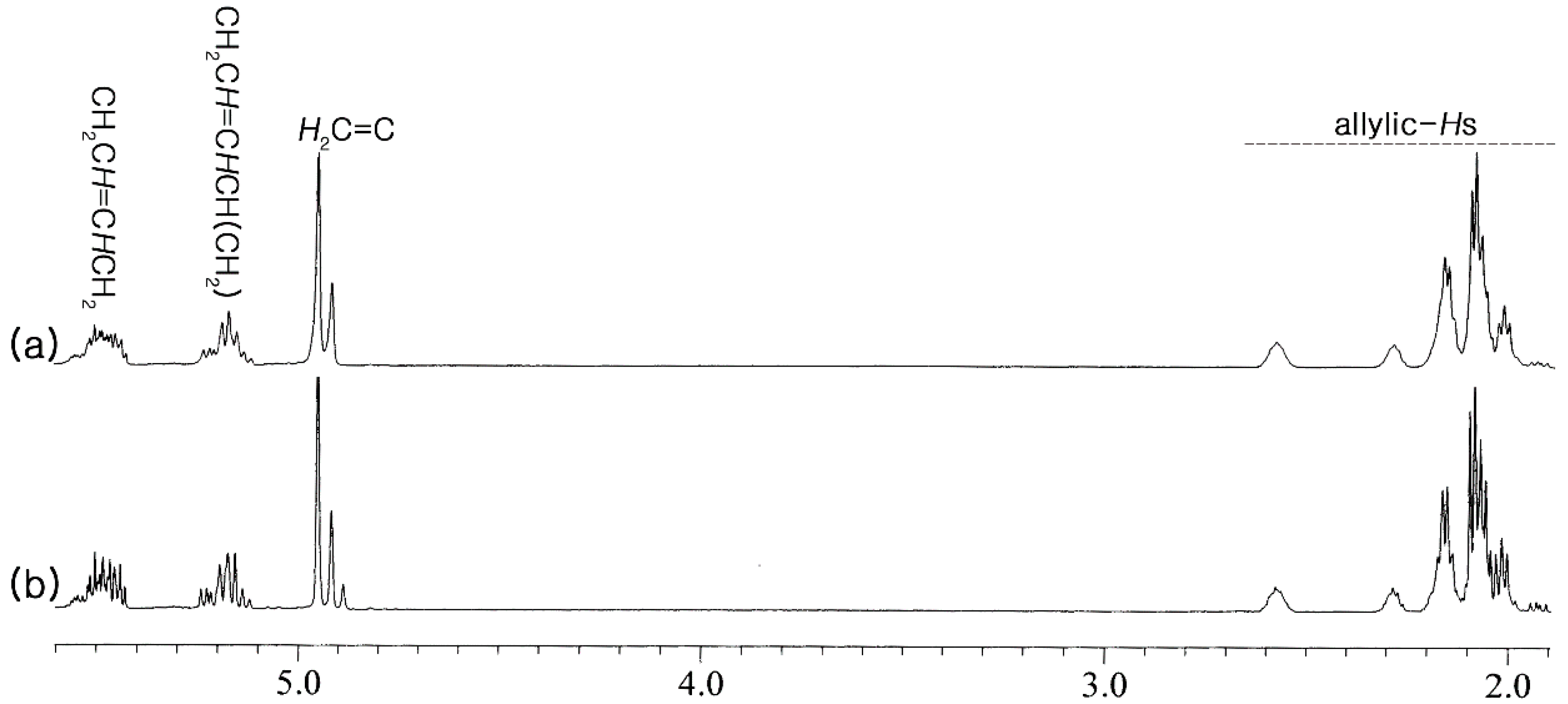
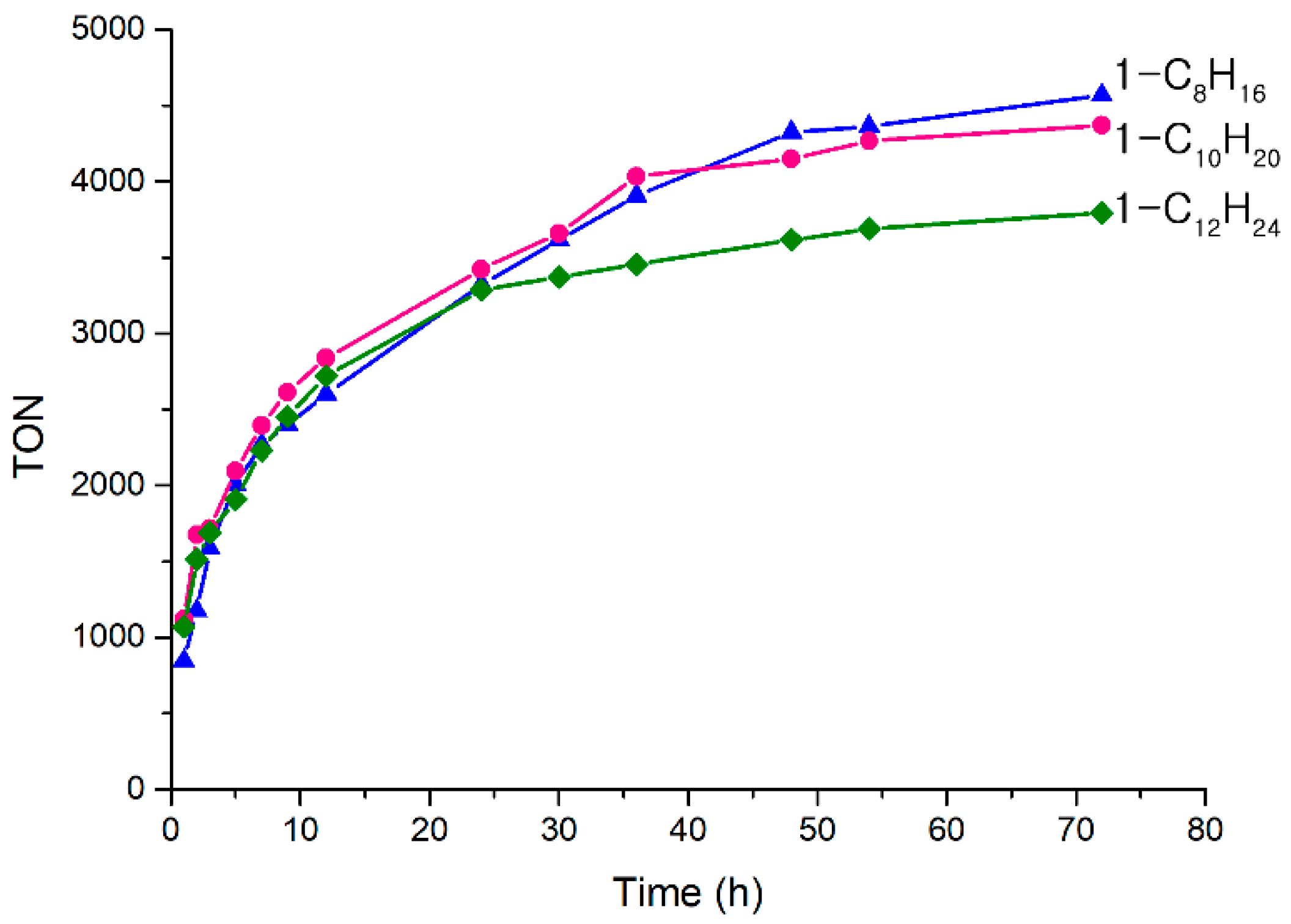
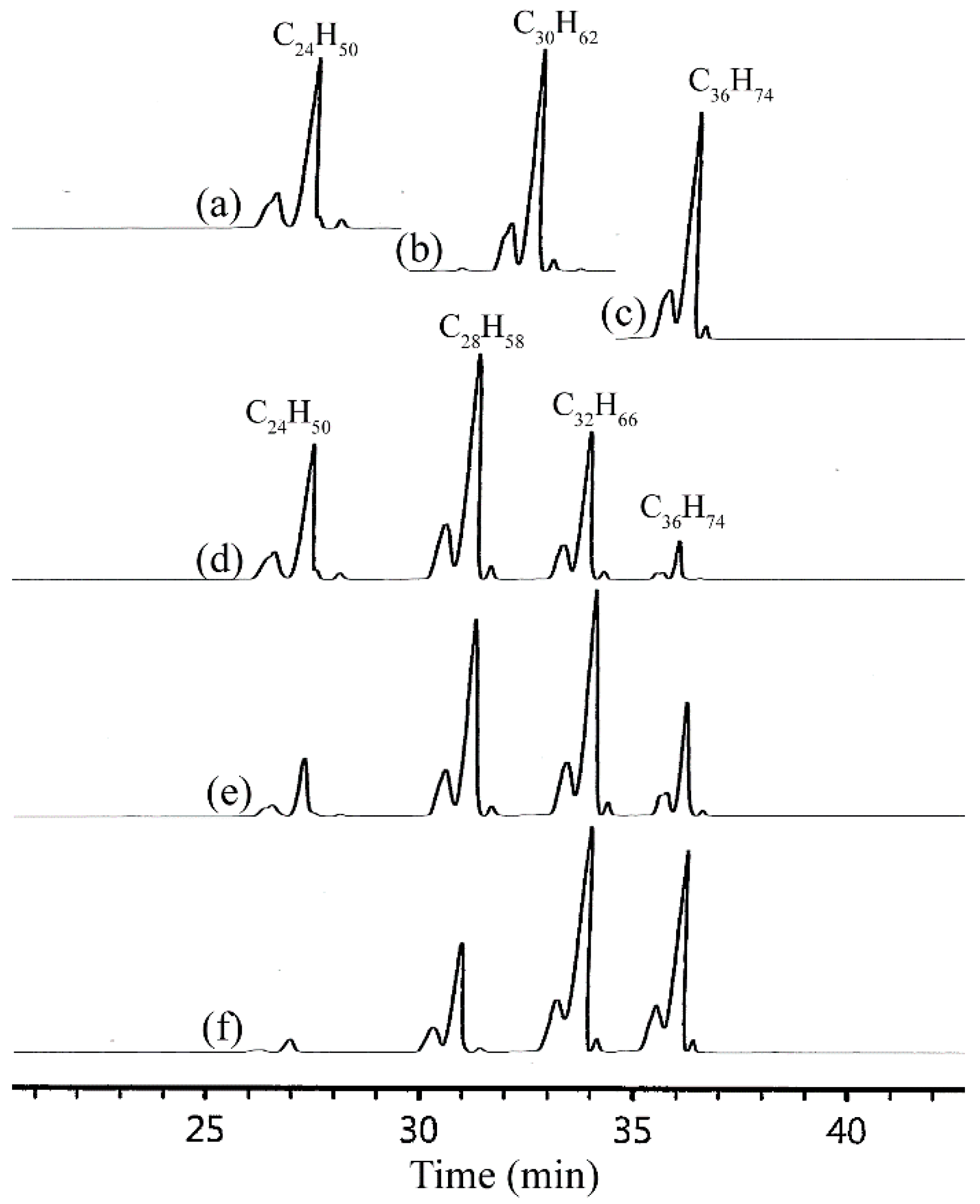
| Entry | Monomer | Kinematic Viscosity at 40 °C (cSt) | Kinematic Viscosity at 100 °C (cSt) | Viscosity Index | Pour Point (°C) |
|---|---|---|---|---|---|
| 1 | 1-C8H16 | 8.13 | 2.4 | 110 | <−57 |
| 2 | 1-C10H20 | 15.1 | 3.9 | 161 | <−57 |
| 3 | 1-C12H24 | 21.6 | 5.0 | 171 | −39 |
| 4 | 1-C8/1-C12 (2 : 1) | 11.5 | 3.0 | 122 | <−57 |
| 5 | 1-C8/1-C12 (1 : 1) | 14.7 | 3.6 | 137 | <−57 |
| 6 | 1-C8/1-C12 (1 : 2) | 16.5 | 4.1 | 160 | −51 |
| 7 | PAO-2.0 (1-C10) | 4.98 | 1.7 | 93 | <−57 |
| 8 | PAO-2.5 (1-C10) | 7.71 | 2.3 | 109 | −51 |
| 9 | PAO-4.0 (1-C10) | 17.4 | 3.9 | 123 | <−57 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baek, J.W.; Hyun, Y.B.; Lee, H.J.; Lee, J.C.; Bae, S.M.; Seo, Y.H.; Lee, D.G.; Lee, B.Y. Selective Trimerization of α-Olefins with Immobilized Chromium Catalyst for Lubricant Base Oils. Catalysts 2020, 10, 990. https://doi.org/10.3390/catal10090990
Baek JW, Hyun YB, Lee HJ, Lee JC, Bae SM, Seo YH, Lee DG, Lee BY. Selective Trimerization of α-Olefins with Immobilized Chromium Catalyst for Lubricant Base Oils. Catalysts. 2020; 10(9):990. https://doi.org/10.3390/catal10090990
Chicago/Turabian StyleBaek, Jun Won, Young Bin Hyun, Hyun Ju Lee, Jong Chul Lee, Sung Moon Bae, Yeong Hyun Seo, Dong Geun Lee, and Bun Yeoul Lee. 2020. "Selective Trimerization of α-Olefins with Immobilized Chromium Catalyst for Lubricant Base Oils" Catalysts 10, no. 9: 990. https://doi.org/10.3390/catal10090990
APA StyleBaek, J. W., Hyun, Y. B., Lee, H. J., Lee, J. C., Bae, S. M., Seo, Y. H., Lee, D. G., & Lee, B. Y. (2020). Selective Trimerization of α-Olefins with Immobilized Chromium Catalyst for Lubricant Base Oils. Catalysts, 10(9), 990. https://doi.org/10.3390/catal10090990